Abstract
Background: It has been hypothesized that monosodium glutamate (MSG), a flavor enhancer, is positively associated with weight gain, which influences energy balance through the disruption of the hypothalamic signaling cascade of leptin action.
Objective: The objective was to examine the longitudinal association between MSG consumption and incidence of overweight.
Design: Data were collected from the China Health and Nutrition Survey (CHNS), a prospective open-cohort, ongoing nationwide health and nutrition survey, consisting of 10,095 apparently healthy Chinese adults aged 18–65 y at entry from 1991 to 2006. Diet, including MSG and other condiments, was assessed with a weighed food inventory in combination with three 24-h recalls. Incident overweight was defined as a body mass index (BMI; in kg/m2) ≥ 25 or ≥23 based on World Health Organization recommendations for Asian populations. Multilevel mixed-effects models were constructed to estimate change in BMI, and Cox regression models with gamma shared frailty were used to determine the incidence of overweight.
Results: The mean follow-up was 5.5 y. The cumulative mean (±SD) MSG intake of 2.2 ± 1.6 g/d was positively associated with BMI after adjustment for potential confounders and cluster effects at different levels (individual, household, and community). The adjusted hazard ratio of overweight was 1.33 (95% CI: 1.01, 1.75; P for trend < 0.01) for participants in the highest quintile of MSG intake compared with those in the lowest quintile after adjustment for age, physical activity, total energy intake, and other major lifestyle factors.
Conclusions: MSG consumption was positively, longitudinally associated with overweight development among apparently healthy Chinese adults. Additional studies are needed to elucidate mechanisms of action and to establish causal inference.
INTRODUCTION
As a flavor enhancer, monosodium glutamate (MSG), also called umami, has been used for 100 y in household food preparation and commercially processed foods. For decades, it was believed that MSG was only used in Asian cuisine. Today, MSG has become one of the world's most widely used food additives. It exists in most processed foods, but may be hidden on ingredient labels and listed under other names (1, 2). According to the available data, large variations exist in MSG consumption within and across populations. The US Food and Drug Administration's (FDA's) Generally Recognized as Safe (GRAS) Committee reported a mean daily intake of MSG per capita of 550 mg/d in the United States in 1979 (3). A survey published in 1991 found an average intake of 580 mg/d for the general population and 4.68 g/d for extreme users (97.5th percentile consumers) in the United Kingdom (4). In Japan and Korea, the estimated average MSG intake in the 1990s was 1.2–1.7 g/d (5). It is speculated, however, that the average daily MSG intake may be up to 10 g/d (5, 6). Of note, MSG intakes in previous studies were likely underestimated because of missing data on MSG content in processed foods.
Concern has been raised about MSG as a risk factor for epidemic obesity because data from both animal models (7–10) and human studies (11, 12) suggest a possible link between MSG and overweight/obesity. Potential mechanisms for the MSG-obesity link include the possible influence of MSG on energy balance by increasing palatability (13, 14) and by disrupting the hypothalamic signaling cascade of leptin action (15, 16). The longitudinal association between MSG consumption and incidence of overweight/obesity, however, is unclear. Therefore, we prospectively examined MSG intake in relation to incidence of overweight and change in body mass index (BMI) in 10,095 apparently healthy Chinese adults from the China Health and Nutrition Survey (CHNS)—a longitudinal open-cohort, ongoing, nationwide survey that has available MSG data. The primary purpose of collecting MSG data in the CHNS was to study association between sodium intake and hypertension.
SUBJECTS AND METHODS
Study sample
Details of the CHNS are described elsewhere (17). Briefly, this cohort was established in 1989 and includes participants from 9 provinces, which vary substantially in geography, stage of economic development, and health status. Four counties within each province (1 low-, 2 middle-, and 1 high-income based on per-capita income reported by the National Bureau of Statistics) were randomly selected by using a weighted sampling scheme. In addition, a large higher-income city along with a lower-income city was selected. Villages and townships within the counties and urban and suburban neighborhoods within the cities were selected randomly, for a total of 190 primary sampling units at baseline. Twenty randomly selected households were surveyed within each unit, and all individuals within a household were interviewed. The CHNS was conducted in 1989, 1991, 1993, 1997, 2000, 2004, and 2006.
In the present study, data from the 1989 survey were not used because only children younger than 7 y and young adults aged 20–45 y were surveyed in that year because of funding constraints. From the 1991–2006 surveys, 10,185 participants aged 18–65 y when they entered into the survey had MSG data available. Of them, we excluded pregnant women (n = 84) and those who had an implausible daily MSG intake (>10 g/d; n = 6). A total of 10,095 Chinese men and women remained in the analyses.
Survey protocols, instruments, and the process for obtaining informed consent for this study were approved by the institutional review committees of the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention. All participants gave their written informed consent.
Dietary measurement
Dietary data were collected at both the household and individual levels. Household food consumption was determined by conducting a detailed examination of changes in inventory from the beginning to the end of each day for 3 consecutive days (2 weekdays and 1 weekend day) in combination with a weighing technique (18, 19). Digital diet and kitchen scales with a maximum limit of 3 kg and a minimum of 1 g were used to weigh the food. All purchases, home production, and processed snack foods were weighed and recorded. Preparation waste (eg, spoiled rice or discarded cooked meals fed to pets or animals) was estimated when weighing was not possible. At the end of the survey, all remaining foods were again weighed and recorded.
Dietary intake at the individual level was assessed by using 24-h recalls for the same 3 consecutive days. Individuals were asked to report all food consumed away from home and at home in a 24-h recall day. We linked individual food consumption with household consumption based on the percentage of individual consumption of any food as a proportion of what the household consumed. Also, we adjusted individual reported consumption by the amount weighed at the household level if there was a large difference in consumption between the household and individual levels (18).
MSG intake assessment and validation
In the CHNS, MSG was directly measured as part of the very detailed, in-depth dietary data collection. In each household, MSG, soy sauce, and all other condiments were weighed before and after the 24-h recall. The household MSG consumption was calculated as the difference between the 2 weights. MSG intake for each household member was estimated based on the proportion of each individual's food consumption.
The estimation of MSG intake for each household has been found to be accurate because we directly weighed consumed MSG and soy sauce. To evaluate the accuracy of MSG intake at the individual level, we conducted a validation study using riboflavin as an adherence marker (20) of MSG intake to estimate the proportion of MSG consumed by each household member. Six households were selected from one community in Zhenjiang, Jiangsu Province, for validating MSG intake. To ensure that all participants were saturated with riboflavin, selected participants were given 5 mg of over-the-counter riboflavin once per day for 5 d before the validation study. The MSG container was weighed before and after each of the three 24-h recalls. In addition, 24-h urine samples were collected for all participants in these 3 d. MSG intakes for individuals were estimated based on self-reported proportion of food consumption in the recalls and were also calculated based on the proportion of urinary riboflavin excretion among household members. The Spearman's correlation coefficient between individual MSG intake assessed from three 24-h recalls combined with a detailed weighing and MSG measurement by using urinary riboflavin marker was 0.82 (P < 0.01). This validation study suggests that individual MSG intake assessed by using three 24-h recalls and direct weighing in the CHNS is reliable and informative at least for ranking participants and estimating relative risks. To reduce the effect of random within-person variation, the mean of 3-d MSG intake was used in the analysis.
Validation of total energy intake
The total energy intake estimated by the methods of directly weighing foods and three 24-h recalls was evaluated previously in 73 weight-stable Chinese men and women aged 35–49 y (21). The reported total energy intake calculated from the 3-d weighed food intake was compared with the total energy expenditure determined with the use of the doubly labeled water method. The correlation coefficient was 0.56 (P < 0.01) for men and 0.60 (P < 0.01) for women (21). The findings from this validation study indicate that the measurement on total energy intake in the CHNS was as good as or better than other established diet measurement instruments used in the United States.
Measurements of physical activity and other covariates
The CHNS has comprehensive data on physical activity (22–25). Occupational activity was measured by using self-reported occupation(s) and the average number of hours spent working per week in the past year for up to 2 market-sector jobs as well as hours worked from home. Participants were also asked about the activity levels of their occupations, which interviewers categorized into 5 levels (very light, light, moderate, heavy, and very heavy) based on respondents’ job descriptions and the time spent sitting, standing, walking, and lifting heavy loads during an average work day. Reported occupations were then cross-tabulated against these occupational activity levels, and specific metabolic equivalent (MET) values were assigned based on how most of the respondents reported the activity level of their occupation. Time spent traveling by each mode and time in inactive and active leisure activities were collected in detail. In addition, home activity was measured on the basis of 4 activities: time spent preparing food, buying food, doing laundry, and on childcare. All activities were reported in average hours per week spent in the past year. Time spent in each activity was multiplied by specific MET values based on the Compendium of Physical Activities (26). High-quality publications using the CHNS physical activity measurement method have been generated (22–24, 27, 28). Each measure of activity has been predictive of weight gain and incident obesity.
Other demographic and lifestyle covariates, including age, sex, urban residence, region (south or north area), smoking status, alcohol consumption, education level, and individual income were measured by questionnaire-based interview in the surveys.
Overweight definition
In accordance with recommendations of the World Health Organization (WHO) (29) and the International Association for the Study of Obesity for Asian populations (30), overweight was defined as a BMI (in kg/m2) ≥ 25.0 in the main analysis and as a BMI ≥ 23.0 in the sensitivity analyses.
Statistical analysis
We used chi-square tests (categorical variables) and analysis of variance and nonparametric Kruskal-Wallis tests (continuous variables) to compare the baseline characteristics of participants according to quintiles of MSG intake. To examine the association of MSG intake with overweight across households and communities, we used the statistical software MLwiN 2.20 to construct multilevel (4 levels) mixed-effects linear regression models (BMI) and logistic regression models (overweight) with measurement occasions (level one) nested within individuals (level 2), household (level 3), and community (level 4). These models enabled us to assess the effects of covariates measured at different levels of a hierarchy on the outcome. Because the multilevel model accounts for the clustering of data, it can correct biases in parameter estimates and provide accurate SEs and thus produce correct CIs and significance tests (31). The multilevel models also allow for incomplete outcome data under the missing at random (MAR) assumption (31). β Regression coefficients (SEs) in the linear regression and prospective odds ratios (and 95% CIs) in the logistic regression were calculated for BMI and overweight, respectively. In addition, we used Cox regression models with a gamma-distributed random effect representing unobserved heterogeneity of frailty over household level to examine the association between MSG intake and incidence of overweight by using the statistical software STATA 11.0 (StataCorp, College Station, TX). We considered 2 sequential models in the aforementioned analyses: model 1 adjusted for age, sex, urban residence, and region (south area or north area), and model 2 additionally adjusted for smoking status, alcohol consumption, individual income inflated to 2006, education, physical activity, and intakes of total energy, sodium, potassium, and calcium. Other potential dietary and nondietary confounders were also considered in sensitivity analyses. In addition, propensity scores were used to further ensure that the comparison groups are comparable with multivariable adjustment (32). Furthermore, a general linear regression model was used to examine the cross-sectional relation between MSG intake and serum leptin concentrations in a subcohort. Spearman's rank correlation coefficient was computed for the MSG intake validation study. Two-sided tests were used, and P ≤ 0.05 was considered statistically significant.
RESULTS
The mean duration of follow-up was 5.5 y (48,857 person-years). The median cumulative MSG intake was 1.8 g/d (mean ± SD: 2.2 ± 1.6 g/d). Men had a slightly higher MSG intake than did the women (median: 1.9 compared with 1.7 g/d; P < 0.01). In general, participants with a high MSG intake had a higher BMI and individual income, higher intakes of total energy and sodium, and lower physical activity levels (Table 1).
TABLE 1.
Characteristics of the study population from the China Health and Nutrition Survey by quintile (Q) of monosodium glutamate (MSG) intake and survey year1
| Characteristics | 1991 |
1993 |
1997 |
2000 |
2004 |
2006 |
||||||||||||
| Q1 | Q5 | P | Q1 | Q5 | P | Q1 | Q5 | P | Q1 | Q5 | P | Q1 | Q5 | P | Q1 | Q5 | P | |
| No. of participants | 518 | 518 | 470 | 470 | 523 | 521 | 677 | 677 | 882 | 882 | 886 | 886 | ||||||
| MSG intake (g/d) | ||||||||||||||||||
| Median | 0.4 | 4.2 | 0.4 | 4.6 | 0.5 | 5.4 | 0.6 | 5.9 | 0.5 | 4.7 | 0.5 | 4.7 | ||||||
| Interquartile range | 0.2–0.5 | 3.4–5.6 | 0.1–0.6 | 3.8–6.2 | 0.3–0.6 | 4.5–6.9 | 0.4–0.8 | 5.1–7.3 | 0.3–0.6 | 3.6–6.1 | 0.3–0.6 | 3.8–6.0 | ||||||
| BMI (kg/m2) | 21.5 ± 2.72 | 22.5 ± 3.1 | <0.01 | 21.7 ± 3.0 | 22.9 ± 3.2 | <0.01 | 22.1 ± 2.9 | 22.9 ± 3.2 | <0.01 | 22.5 ± 3.1 | 23.8 ± 3.5 | <0.01 | 23.0 ± 3.5 | 23.7 ± 3.6 | <0.01 | 23.1 ± 3.4 | 23.8 ± 3.6 | 0.83 |
| Age (y) | 38.5 ± 13.1 | 39.1 ± 12.1 | 0.52 | 40.1 ± 13.4 | 40.6 ± 11.7 | 0.80 | 41.3 ± 12.7 | 43.5 ± 13.1 | 0.01 | 43.7 ± 12.6 | 45.1 ± 12.5 | <0.01 | 45.7 ± 12.4 | 47.2 ± 11.8 | 0.11 | 45.7 ± 12.7 | 50.2 ± 12.3 | <0.01 |
| Female (%) | 53.1 | 51.9 | 0.15 | 55.5 | 51.2 | 0.75 | 55.1 | 50.7 | 0.26 | 55.2 | 47.6 | 0.04 | 55.7 | 49.6 | 0.11 | 56.7 | 50.3 | 0.08 |
| Current smoker (%) | 32.3 | 39.2 | <0.01 | 30.8 | 35.7 | 0.34 | 29.0 | 32.6 | 0.40 | 28.8 | 34.8 | 0.19 | 28.9 | 33.1 | 0.38 | 27.6 | 30.5 | 0.07 |
| Alcohol consumption (%) | 7.1 | 8.7 | 0.76 | 8.3 | 8.3 | 0.13 | 12.1 | 5.4 | <0.01 | 9.3 | 12.0 | 0.049 | 9.0 | 9.4 | 0.32 | 8.8 | 8.6 | 0.16 |
| Education (y) | 6.0 ± 4.2 | 6.9 ± 3.9 | <0.01 | 6.4 ± 3.9 | 7.4 ± 4.0 | <0.01 | 6.6 ± 3.9 | 6.9 ± 4.1 | 0.38 | 6.9 ± 4.0 | 8.0 ± 3.8 | <0.01 | 7.6 ± 3.9 | 8.1 ± 3.8 | 0.13 | 7.8 ± 4.3 | 7.5 ± 4.1 | 0.42 |
| Individual income (RMB)3 | ||||||||||||||||||
| Median | 2861 | 3190 | 0.04 | 3211 | 3845 | <0.01 | 3742 | 4485 | <0.01 | 4603 | 6561 | <0.01 | 5889 | 6943 | 0.12 | 7316 | 6855 | 0.20 |
| Interquartile range | 1605–4333 | 2078–4750 | 1876–5550 | 2517–6278 | 1939–6397 | 2365–7986 | 1950–8099 | 3365–10,254 | 2149–10,839 | 2722–11,778 | 2941–13,206 | 2451–12,944 | ||||||
| Physical activities (MET-h/wk)4 | ||||||||||||||||||
| Median | 362.3 | 259.9 | <0.01 | 330.8 | 233.2 | <0.01 | 313.4 | 224.6 | <0.01 | 315.5 | 182.5 | <0.01 | 194.0 | 150.9 | <0.01 | 208.2 | 166.1 | 0.02 |
| Interquartile range | 170.5–583.6 | 130.8–453.5 | 144.0–523.7 | 107.3–420.0 | 136.1–537.9 | 100.7–462.1 | 126.9–481.1 | 100.0–377.8 | 81.4–407.8 | 57.2–358.9 | 78.8–390.5 | 44.6–395.6 | ||||||
| Total energy intake (kcal) | ||||||||||||||||||
| Median | 2448 | 2563 | <0.01 | 2341 | 2423 | <0.01 | 2238 | 2434 | <0.01 | 2195 | 2425 | <0.01 | 2111 | 2219 | <0.01 | 2000 | 2262 | <0.01 |
| Interquartile range | 2129–2830 | 2210–3006 | 1993–2702 | 2061–2855 | 1834–2727 | 1986–2995 | 1780–2656 | 1985–2877 | 1722–2554 | 1734–2690 | 1578–2474 | 1791–2760 | ||||||
| Daily nutrient intake | ||||||||||||||||||
| Sodium (g/1000 kcal) | 2.3 ± 1.2 | 3.2 ± 1.5 | <0.01 | 2.3 ± 1.1 | 3.3 ± 1.5 | <0.01 | 1.9 ± 1.1 | 3.2 ± 1.7 | <0.01 | 2.2 ± 1.2 | 3.2 ± 1.8 | <0.01 | 2.0 ± 1.1 | 3.1 ± 1.5 | <0.01 | 2.0 ± 1.1 | 2.8 ± 1.4 | <0.01 |
| Potassium (mg/1000 kcal) | 682.7 ± 158.0 | 716.2 ± 194.8 | <0.01 | 690.0 ± 169.3 | 716.8 ± 180.9 | 0.32 | 683.0 ± 182.3 | 681.9 ± 200.2 | <0.01 | 707.8 ± 270.1 | 750.9 ± 252.6 | <0.01 | 729.6 ± 228.3 | 734.7 ± 236.8 | 0.08 | 806.2 ± 236.9 | 771.2 ± 237.0 | <0.01 |
| Phosphorus (mg/1000 kcal) | 429.6 ± 70.9 | 456.3 ± 82.6 | <0.01 | 438.1 ± 77.8 | 451.6 ± 82.6 | 0.02 | 417.0 ± 75.8 | 425.6 ± 93.8 | <0.01 | 426.8 ± 101.2 | 425.5 ± 110.2 | <0.01 | 451.3 ± 93.0 | 441.6 ± 85.8 | 0.31 | 464.7 ± 83.3 | 457.9 ± 88.9 | <0.01 |
| Carbohydrate (g/1000 kcal) | 159.3 ± 23.7 | 148.8 ± 26.5 | <0.01 | 157.6 ± 25.5 | 142.5 ± 28.2 | <0.01 | 152.7 ± 30.4 | 137.7 ± 33.5 | <0.01 | 147.9 ± 28.3 | 133.5 ± 30.8 | <0.01 | 149.9 ± 29.6 | 139.5 ± 32.0 | <0.01 | 147.6 ± 29.6 | 138.6 ± 30.4 | <0.01 |
| Total fat (g/1000 kcal) | 26.3 ± 10.0 | 30.9 ± 11.5 | <0.01 | 26.9 ± 10.8 | 33.4 ± 11.8 | <0.01 | 29.4 ± 12.3 | 36.5 ± 15.2 | <0.01 | 31.3 ± 11.5 | 37.0 ± 13.4 | <0.01 | 29.4 ± 11.7 | 34.5 ± 13.3 | <0.01 | 30.4 ± 12.2 | 34.7 ± 13.5 | <0.01 |
| Fiber (g/1000 kcal) | 4.5 ± 2.1 | 4.2 ± 1.9 | 0.22 | 4.4 ± 2.3 | 4.2 ± 1.7 | 0.19 | 4.7 ± 3.8 | 4.2 ± 2.6 | 0.16 | 4.9 ± 4.8 | 5.2 ± 10.8 | 0.47 | 5.1 ± 3.0 | 4.9 ± 3.0 | 0.10 | 5.4 ± 2.4 | 4.9 ± 2.5 | <0.01 |
| Total protein (g/1000 kcal) | 29.5 ± 5.3 | 29.9 ± 5.9 | 0.10 | 29.3 ± 5.9 | 30.6 ± 6.4 | <0.01 | 29.1 ± 5.9 | 28.9 ± 6.5 | 0.03 | 29.3 ± 6.7 | 30.8 ± 7.8 | <0.01 | 30.9 ± 6.8 | 29.6 ± 7.4 | <0.01 | 31.4 ± 6.4 | 30.9 ± 6.7 | 0.02 |
MET-h, metabolic equivalent hours; RMB, Chinese dollar. Differences across all quintiles were obtained by using ANOVA, the Kruskal-Wallis test, or the chi-square test.
Mean ± SD (all such values).
Individual income refers to total individual income inflated to 2006.
Physical activity included 4 aspects: occupational activity, home activity, leisure activity, and transportation activity. The former 2 aspects were measured in all surveys from 1991 to 2006, and the latter 2 aspects were measured since 1997.
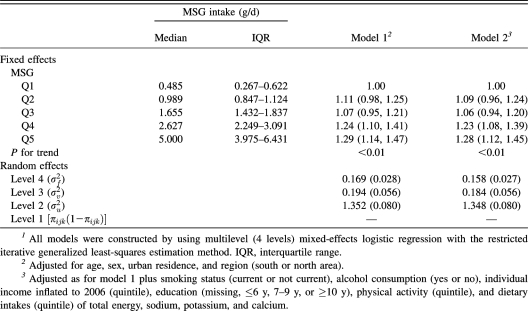
MSG consumption was significantly, positively associated with BMI in a dose-response manner (P for trend <0.01) after adjustment for potential confounders and control for clustering of data at multiple levels (individual, household, and community) (Table 2). For participants in the highest compared with the lowest quintile of MSG intake, the prospective odds ratio of overweight was 1.28 (95% CI: 1.12, 1.45; P for trend <0.01) after baseline BMI and other potential confounders were adjusted for (Table 3).
TABLE 2.
Multilevel-adjusted associations between quintile (Q) of monosodium glutamate (MSG) intake and BMI (n = 10,095)1
| Fixed effects | MSG intake (g/d) |
β ± SE |
MSG intake (g/d) |
|||||||
| Median | IQR | Model 12 | Model 23 | Random effects4 | Intercept | Q2 | Q3 | Q4 | Q5 | |
| MSG | ||||||||||
| Q1 | 0.485 | 0.267–0.622 | 0 | 0 | Intercept | 0.483 (0.094) | ||||
| Q2 | 0.989 | 0.847–1.124 | 0.097 ± 0.060 | 0.076 ± 0.060 | MSG, Q2 | −0.076 | 0.084 (0.060) | |||
| Q3 | 1.655 | 1.432–1.837 | 0.101 ± 0.060 | 0.090 ± 0.061 | MSG, Q3 | −0.098 | 0.115 | 0.118 (0.066) | ||
| Q4 | 2.627 | 2.249–3.091 | 0.235 ± 0.069 | 0.212 ± 0.070 | MSG, Q4 | −0.101 | 0.128 | 0.233 | 0.297 (0.091) | |
| Q5 | 5.000 | 3.975–6.431 | 0.248 ± 0.063 | 0.227 ± 0.064 | MSG, Q5 | −0.114 | 0.062 | 0.089 | 0.080 | 0.111 (0.072) |
| P for trend | <0.001 | <0.001 | ||||||||
All of the models were constructed by using multilevel (4 levels) mixed-effects linear regression with the iterative generalized least-squares estimation method. IQR, interquartile range.
Adjusted for age, sex, urban residence, and region (south or north area).
Adjusted as for model 1 plus smoking status (current or not current), alcohol consumption (yes or no), individual income inflated to 2006 (quintile), education (missing, ≤6 y, 7–9 y, or ≥10 y), physical activity (quintile), and dietary intakes (quintile) of total energy, sodium, potassium, and calcium.
Correlation matrix of community level (level 4) with variance on diagonal (SE) based on model 2; random intercept for levels 3, 2, and 1 (SE): 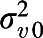 =0.749 (0.103),
=0.749 (0.103), 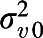 =5.541 (0.141),
=5.541 (0.141), 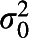 =3.472 (0.050); −2 log-likelihood (iterative generalized least-squares deviance) = 95,413.832.
=3.472 (0.050); −2 log-likelihood (iterative generalized least-squares deviance) = 95,413.832.
TABLE 3.
Multilevel multivariable-adjusted prospective odds ratios (and 95% CIs) of overweight according to quintile (Q) of monosodium glutamate (MSG) intake (n = 10,095)1
To examine the association between MSG intake and incidence of overweight, we excluded participants who were overweight or obese at baseline and who had no follow-up data (ie, enrolled in 2006). A total of 824 incident cases of overweight were documented among 7192 normal-weight participants at baseline. Compared with those who were in the lowest quintile of MSG intake, participants in the highest quintile were 33% more likely to develop overweight [hazard ratio (HR): 1.33; 95% CI: 1.01, 1.75; P for trend <0.01] independent of baseline BMI and other potential confounders (Table 4).
TABLE 4.
Multilevel multivariable-adjusted hazard ratios (and 95% CIs) of overweight according to quintile (Q) of monosodium glutamate (MSG) intake (n = 7192)1
| MSG intake |
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend | |
| No. of participants | 1438 | 1439 | 1438 | 1439 | 1438 | |
| Median of MSG intake (g/d) | 0.63 | 1.18 | 1.76 | 2.57 | 4.19 | |
| IQR of MSG intake (g/d) | 0.42–0.78 | 1.04–1.33 | 1.63–1.94 | 2.34–2.84 | 3.58–5.58 | |
| No. of overweight participants | 86 | 159 | 162 | 208 | 209 | |
| Model 12 | 1.00 | 1.05 (0.80, 1.39) | 0.88 (0.67, 1.17) | 1.04 (0.79, 1.36) | 1.40 (1.07, 1.84) | <0.01 |
| Model 23 | 1.00 | 1.00 (0.75, 1.32) | 0.83 (0.63, 1.10) | 0.96 (0.73, 1.27) | 1.33 (1.01, 1.75) | <0.01 |
All models were constructed by using Cox regression with gamma shared frailty over the household level. IQR, interquartile range.
Adjusted for age, sex, urban residence, and region (south or north area).
Adjusted as for model 1 plus smoking status (current or not current), alcohol consumption (yes or no), individual income inflated to 2006 (quintile), education (missing, ≤6 y, 7–9 y, or ≥10 y), physical activity (quintile), and dietary intakes (quintile) of total energy, sodium, potassium, and calcium.
The observed associations remained after control for propensity scores. For instance, the HR in the highest quintile of MSG intake group related to the first quintile was 1.37 (95% CI: 1.04, 1.80) with adjustment for propensity scores, which were derived from the same covariates in the multivariable analysis; the corresponding HR was essentially similar when adjusted for propensity scores derived from the covariates in the final model with additional dietary variables such as fiber, carbohydrates, and total fat. Because men and people in urban areas are more likely to eat outside, we stratified data by sex and residence. The associations were generally consistent across subgroups. In addition, our findings remained when we used BMI ≥ 23.0 to define overweight.
To explore the potential mechanism, we conducted a pilot study to examine the association between MSG consumption and serum leptin concentrations among 669 Chinese adults who participated in the 2006 survey and provided fasting blood specimens. The median leptin concentration was 7.2 ng/mL (mean ± SD: 9.92 ± 9.15 ng/mL). MSG intake was positively related to serum leptin concentrations. After control for the potential confounders listed in model 2 (Table 2), the serum leptin concentration increased by 0.45 ng/mL (SE = 0.16, P < 0.01) with every 1-g increment increase in MSG intake.
DISCUSSION
In this diverse, large cohort of Chinese men and women, we found that MSG consumption was positively, longitudinally related to risk of overweight with adjustment for baseline BMI, physical activity, total energy intake, and other potential confounders, and accounting for clustering data at individual, household, and community levels.
Strengths and limitations of the study
The major strengths of this study include the large nationwide, diverse population, prospective follow-up study design, and wide variation of MSG intake both spatially and temporally. Also, our findings are further strengthened by a few supportive pilot studies including the MSG intake validation study, total energy intake validation study, and exploratory study on the hypothesized MSG–leptin resistance–obesity pathway. Because of ethical considerations and other methodologic constraints (eg no proper placebo), we may not be able to conduct a large-scale, randomized, placebo-controlled clinical trial. Thus, data from a large cohort study may be very important to advance our knowledge.
In addition to some common weaknesses of any observational study, eg, confounding from unknown factors, some other limitations also need to be considered. One major concern is the accuracy of the MSG measurement. Similar to other food additives (eg, salt), MSG intake is difficult to be captured accurately by any available dietary assessment instruments such as food frequency questionnaires. In the present study, however, household MSG consumption was considered accurate because it was measured by direct weighing of the MSG container before and after each of three 24-h recalls. The accuracy of MSG intake for individuals was evaluated using riboflavin as an adherence marker. Thus, the estimation of MSG intake is unlikely to be substantially biased, although measurement errors are inevitable and likely to be nondifferential. For example, data on MSG content in processed foods are missing. Nevertheless, MSG from processed foods in Chinese population may be a relatively small portion as compared with added MSG including MSG from soy sauce in food preparation. Also, our ability to examine the relation of MSG intake with obesity was limited by the relatively small number of participants with BMI ≥ 30. Presumably, a positive association between MSG intake and risk of obesity exists given the linear relation of MSG and BMI. In addition, loss to follow-up might bias our results, although it is unlikely that the cases of loss to follow-up are associated with both MSG intake and overweight.
A recent study found no significant association between MSG consumption and weight gain among 1282 Chinese men and women in Jiangsu province, China (33). In addition to the different study population and sample size, its outcome of interest is 5% weight gain rather than BMI, overweight, or obesity. Of note, MSG intake and weight were only measured twice 5 y apart in the study and no results on the association of MSG intake with incidence of overweight/obesity were reported. Detailed criticism on its methodologic flaws was published (34). Our study may provide more solid evidence given the large, diverse population in terms of geography, stage of economic development, and health status.
Recently, debate has arisen over whether MSG intake is a risk factor for being overweight or obese (35). Researchers from MSG industry reported that MSG intake would suppress weight gain in animal models (36). Whereas glutamate industry sponsored studies have been questioned previously (37, 38), further studies are clearly warranted.
Potential mechanisms
Whereas the mechanisms are not completely understood, a hypothesis has been made that chronic MSG intake may induce pathologic changes of the arcuate nucleus neurons and disrupt the hypothalamic signaling cascade of leptin action, causing leptin resistance related to overweight and obesity (15, 16). The arcuate nucleus is the major site of glutamic acid–induced neuronal damage in the hypothalamus (39). In addition, leptin is produced in the adipose tissue, crosses the blood-brain barrier by active transport systems, and stimulates a specific signaling cascade (40). The hypothalamic arcuate nucleus is essential for mediating the anorectic effects of leptin influencing energy balance (8, 9). Studies suggest that MSG may cause leptin resistance. For instance, leptin production is increased in animal models of obesity associated with experimentally induced hypothalamic damage, including the MSG-treated model (41). Also, it was reported that leptin suppressed body weight gain in controls but did not suppress weight gain in MSG-treated rats (8), and that leptin significantly inhibited food intake and caused weight loss in control rats whereas MSG-treated rats were unresponsive to leptin treatment (9).
Injected compared with orally administered MSG
Because animal studies suggest that injection or oral administration of MSG may induce neuronal necrosis in the hypothalamus (7, 42–47), concerns have arisen that ingestion of MSG by humans may contribute to occurrence of neuroendocrinopathies (48, 49). Whether MSG ingestion causes hypothalamic damage, however, has been debated for some time because most of the evidence on hypothalamic lesions and appetite regulation has been derived from studies where animals were injected with a large dose of MSG. Nevertheless, some studies have examined the effects of orally administered MSG (14, 50–53) and found that oral MSG use at a level similar to the amount typically added to food had significant potential of damaging the hypothalamic regulation of appetite (14). In addition, we conducted a pilot study on MSG intake and serum leptin concentrations and observed a positive correlation between these 2 variables. Our pilot data further support the hypothesis that MSG intake may reduce the sensitivity of leptin or cause leptin resistance. Further studies are warranted.
Safety compared with health
MSG is considered as a food ingredient that is “generally recognized as safe” by US FDA. Other health organizations, including the Federation of American Societies for Experimental Biology (FASEB) and the World Health Organization (WHO), have also concluded that MSG is safe as a food additive (54, 55). None of these evaluations, however, discussed or investigated MSG as a risk factor for overweight and obesity. Concern about health impact is different from the issue of safety. Sugar is safe, but it may not be healthy (56, 57). trans Fat is not toxic, but sufficient evidence supports that it is a risk factor for heart disease (58).
Natural compared with processed free glutamic acid
Glutamic acid is one of the most common amino acids and is recognized as the physiologic ligand of the taste receptor umami, which is responsible for the immediate sensory effect of MSG on the palatability of food. One key point that the MSG industry promotes is that MSG is identical to the glutamic acid in intact protein and in higher organisms. Of note, there are 2 forms of glutamic acid found in nature: l-glutamic acid and d-glutamic acid. Although these 2 enantiomers appear to be identical, chiral molecules are fundamentally different. The glutamic acid found in protein is l-glutamic acid only, whereas d-glutamic acid is not found naturally in higher organisms but only in the cell walls of certain bacteria (59–61). Manufactured or processed free glutamic acid always contains some d-glutamic acid (59, 62, 63), which is unavailable for peptide and protein synthesis and may even inhibit enzymes. Thus, d-configured amino acids should not be consumed (64). In addition, MSG—the manufactured salt of glutamic acid—is a single molecule. It does not need the digestion process and can be absorbed immediately into circulation. In contrast, glutamic acid in unprocessed or natural foods predominantly exists as a part of protein (polypeptide molecules) or is connected as peptides. Similar to complex carbohydrates, the human digestive system is needed to break down glutamic acid to free amino acids before the protein or polypeptides can pass through the stomach and intestines. Because it takes longer for the digestive system to break down these proteins or peptides into free amino acids, it may take longer to get hungry again. Certainly, the potential health impact of this difference needs to be further investigated. In addition, a few studies suggest that some natural foods also contain a tiny amount of free glutamic acid (65). For example, 100 g cod fish contains 2101 mg bound glutamic acid but only 9 mg free glutamic acid. Also, 100 g cow milk contains 819 mg bound glutamic acid but only 2 mg free glutamic acid (65). Despite the minute amount of free glutamic acid in natural foods, this does not necessarily mean that the free glutamic acid or MSG is healthy. A similar example is trans fatty acid—an established risk factor for heart disease. Although trans fatty acids are largely produced in processed foods (eg, in foods containing hydrogenated fat or in deep fried foods), small amounts of trans fatty acids occur naturally in some meat and dairy products, including beef, lamb, and butter, although it is not clear whether these naturally occurring trans fatty acids have the same adverse effects on human health (66). Thus, naturally occurring, small amounts of free glutamic acid do not offer sufficient support that MSG is healthy.
Why are Asians relatively lean compared with Westerners if MSG is more popular in Asian cuisine?
Data on MSG consumption across countries or populations are limited. It is generally believed that Asians have higher MSG consumption because MSG is more popular in Asian cuisine; in particular, many more Asians than Westerners add MSG in food preparation. Body weight is a multiinfluenced phenotype, however, related to lifestyle, environment, and genome and not determined by a single factor. MSG may be one of numerous known and unknown risk factors of obesity. The potential adverse effect of MSG intake on body weight in Asians may be attenuated by other lifestyle factors, eg, greater physical activity and lower calorie intake or lower energy density of foods. In addition, Asians BMIs, waist circumferences, and particularly their visceral fat are rising rapidly (67). At the same time, MSG consumption has risen as have many other possibly obesogenic changes have occurred. Notably, people in Western countries may also consume substantial amount of MSG by adding seasonings and other condiments with hidden MSG.
Conclusions
In this large, open-cohort study, we found that MSG intake was positively, longitudinally associated with BMI and incidence of overweight independent of baseline BMI, physical activity, total energy intake, and other potential confounders among apparently healthy Chinese adults. Although the magnitude of weight gain in MSG users compared with nonusers is modest, it is of great public health significance if the weight gain is solely caused by MSG intake given that MSG is increasingly used worldwide. Evidently, further studies are needed to confirm our findings, to elucidate the mechanisms of action, and to establish causal inference. For example, an MSG cessation intervention may be warranted.
Acknowledgments
We thank all of our colleagues in China and the United States who are involved in the China Health and Nutrition Survey and in the related pilot studies, particularly Bing Zhang, Hua Wang, Guangli He, Baojun Yuan, Zumin Shi, and Yan Liu. We also thank John Anderson, Jim Terry, and Philip Bardsley for their comments and technical support and all of the study participants for their continuous support and contribution.
The authors’ responsibilities were as follows—KH: conception and design of the study and draft of the manuscript; PX and KH: data analysis and interpretation; and KH, PX, SD, SS, HW, FZ, and BP: critical revision of the manuscript for content and approval of the final version of the manuscript. None of the authors reported any conflicts of interest.
REFERENCES
- 1.Ito M. Domestic and overseas food products business FY 2008-2010 medium term plan. Available from: http://www.ajinomoto.com/ir/pdf/08July02-food-E.pdf (cited 12 September 2009)
- 2.Erb JE, Erb TM. The slow poisoning of America. Virginia Beach:,VA Paladins Press, 2003 [Google Scholar]
- 3.GRAS Committee on GRAS List Survey. Estimating distributions of daily intake of monosodium glutamate (MSG). Washington, DC: Food and Nutrition Board, Division of Biological Sciences, Assembly of Life Sciences, 1979 [Google Scholar]
- 4.Rhodes J, Titherley AC, Norman JA, Wood R, Lord DW. A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutamate. Food Addit Contam 1991;8:663–72 [DOI] [PubMed] [Google Scholar]
- 5.Beyreuther K, Biesalski HK, Fernstrom JD, et al. Consensus meeting: monosodium glutamate - an update. Eur J Clin Nutr 2007;61:304–13 [DOI] [PubMed] [Google Scholar]
- 6.Collison KS, Maqbool Z, Saleh SM, et al. Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J Lipid Res 2009;50:1521–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969;164:719–21 [DOI] [PubMed] [Google Scholar]
- 8.Dawson R, Pelleymounter MA, Millard WJ, Liu S, Eppler B. Attenuation of leptin-mediated effects by monosodium glutamate-induced arcuate nucleus damage. Am J Physiol 1997;273:E202–6 [DOI] [PubMed] [Google Scholar]
- 9.Tang-Christensen M, Holst JJ, Hartmann B, Vrang N. The arcuate nucleus is pivotal in mediating the anorectic effects of centrally administered leptin. Neuroreport 1999;10:1183–7 [DOI] [PubMed] [Google Scholar]
- 10.Bergen HT, Mizuno TM, Taylor J, Mobbs CV. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology 1998;139:4483–8 [DOI] [PubMed] [Google Scholar]
- 11.He K, Zhao L, Daviglus ML, et al. Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP Study. Obesity (Silver Spring) 2008;16:1875–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeomans MR, Gould NJ, Mobini S, Prescott J. Acquired flavor acceptance and intake facilitated by monosodium glutamate in humans. Physiol Behav 2008;93:958–66 [DOI] [PubMed] [Google Scholar]
- 13.Bellisle F, Monneuse MO, Chabert M, Larue-Achagiotis C, Lanteaume MT, Louis-Sylvestre J. Monosodium glutamate as a palatability enhancer in the European diet. Physiol Behav 1991;49:869–73 [DOI] [PubMed] [Google Scholar]
- 14.Hermanussen M, Garcia AP, Sunder M, Voigt M, Salazar V, Tresguerres JA. Obesity, voracity, and short stature: the impact of glutamate on the regulation of appetite. Eur J Clin Nutr 2006;60:25–31 [DOI] [PubMed] [Google Scholar]
- 15.Hermanussen M, Tresguerres JA. Does high glutamate intake cause obesity? J Pediatr Endocrinol Metab 2003;16:965–8 [DOI] [PubMed] [Google Scholar]
- 16.Hermanussen M, Tresguerres JA. Does the thrifty phenotype result from chronic glutamate intoxication? A hypothesis. J Perinat Med 2003;31:489–95 [DOI] [PubMed] [Google Scholar]
- 17.Popkin BM, Du S, Zhai F, Zhang B. The China Health and Nutrition Survey: monitoring and understanding socioeconomic and health change in China 1989-2011. Int J Epidemiol 2010;39:1435–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai F, Guo X, Popkin BM, et al. Evaluation of the 24-hour individual recall method in China. Tokyo, Japan: United Nations University; 1996 [Google Scholar]
- 19.Popkin BM, Lu B, Zhai F. Understanding the nutrition transition: measuring rapid dietary changes in transitional countries. Public Health Nutr 2002;5(6A):947–53 [DOI] [PubMed] [Google Scholar]
- 20.Switzer BR, Stark AH, Atwood JR, Ritenbaugh C, Travis RG, Wu HM. Development of a urinary riboflavin adherence marker for a wheat bran fiber community intervention trial. Cancer Epidemiol Biomarkers Prev 1997;6:439–42 [PubMed] [Google Scholar]
- 21.Yao M, McCrory MA, Ma G, et al. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr 2003;77:1409–16 [DOI] [PubMed] [Google Scholar]
- 22.Monda KL, Adair LS, Zhai F, Popkin BM. Longitudinal relationships between occupational and domestic physical activity patterns and body weight in China. Eur J Clin Nutr 2008;62:1318–25 [DOI] [PubMed] [Google Scholar]
- 23.Bell AC, Ge K, Popkin BM. Weight gain and its predictors in Chinese adults. Int J Obes Relat Metab Disord 2001;25:1079–86 [DOI] [PubMed] [Google Scholar]
- 24.Bell AC, Ge K, Popkin BM. The road to obesity or the path to prevention: motorized transportation and obesity in China. Obes Res 2002;10:277–83 [DOI] [PubMed] [Google Scholar]
- 25.Paeratakul S, Popkin BM, Keyou G, Adair LS, Stevens J. Changes in diet and physical activity affect the body mass index of Chinese adults. Int J Obes Relat Metab Disord 1998;22:424–31 [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(suppl):S498–504 [DOI] [PubMed] [Google Scholar]
- 27.Monda KL, Gordon-Larsen P, Stevens J, Popkin BM. China's transition: the effect of rapid urbanization on adult occupational physical activity. Soc Sci Med 2007;64:858–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991-2006 China Health and Nutrition Surveys. Soc Sci Med 2009;68:1305–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63 [DOI] [PubMed] [Google Scholar]
- 30.International Obesity Task Force (on behalf of the Steering Committee). The Asia-Pacific perspective: redefining obesity and its treatment. Western Pacific Region. Sydney, Australia: Health Communications Australia Pty Ltd, 2002 [Google Scholar]
- 31.Von Korff M, Koepsell T, Curry S, Diehr P. Multi-level analysis in epidemiologic research on health behaviors and outcomes. Am J Epidemiol 1992;135:1077–82 [DOI] [PubMed] [Google Scholar]
- 32.Greenland S. Randomization, statistics, and causal inference. Epidemiology 1990;1:421–9 [DOI] [PubMed] [Google Scholar]
- 33.Shi Z, Luscombe-Marsh ND, Wittert GA, et al. Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: findings from the Jiangsu Nutrition Study of Chinese adults. Br J Nutr 2010;104:457–63 [DOI] [PubMed] [Google Scholar]
- 34.Samuels A. Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: findings from the Jiangsu Nutrition Study of Chinese adults—comments by Samuels. Br J Nutr 2010;104:1729. [DOI] [PubMed] [Google Scholar]
- 35.Ebert AG. Evidence that MSG does not induce obesity. Obesity (Silver Spring) 2009;17:629–30 [DOI] [PubMed] [Google Scholar]
- 36.Kondoh T, Torii K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague-Dawley rats. Physiol Behav 2008;95:135–44 [DOI] [PubMed] [Google Scholar]
- 37.Samuels A. The toxicity/safety of processed free glutamic acid (MSG): a study in suppression of information. Account Res 1999;6:259–310 [DOI] [PubMed] [Google Scholar]
- 38.Samuels A. Monosodium L-glutamate: a double-blind study and review. Food Chem Toxicol 1995;33:69–78 [DOI] [PubMed] [Google Scholar]
- 39.Olney JW, Ho OL, Rhee V. Brain-damaging potential of protein hydrolysates. N Engl J Med 1973;289:391–5 [DOI] [PubMed] [Google Scholar]
- 40.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 2002;967:379–88 [DOI] [PubMed] [Google Scholar]
- 41.Frederich RC, Lollmann B, Hamann A, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 1995;96:1658–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science 1969;166:386–8 [DOI] [PubMed] [Google Scholar]
- 43.Arees EA, Mayer J. Monosodium glutamate-induced brain lesions: electron microscopic examination. Science 1970;170:549–50 [DOI] [PubMed] [Google Scholar]
- 44.Pizzi WJ, Barnhart JE, Fanslow DJ. Monosodium glutamate admlinistration to the newborn reduces reproductive ability in female and male mice. Science 1977;196:452–4 [DOI] [PubMed] [Google Scholar]
- 45.Simson EL, Gold RM, Standish LJ, Pellett PL. Axon-sparing brain lesioning technique: the use of monosodium-L-glutamate and other amino acids. Science 1977;198:515–7 [DOI] [PubMed] [Google Scholar]
- 46.Olney JW, Ho OL. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature 1970;227:609–11 [DOI] [PubMed] [Google Scholar]
- 47.Olney JW. Brain damage and oral intake of certain amino acids. New York, NY: Plenum, 1976 [DOI] [PubMed] [Google Scholar]
- 48.Olney JW. Excitotoxic food additives—relevance of animal studies to human safety. Neurobehav Toxicol Teratol 1984;6:455–62 [PubMed] [Google Scholar]
- 49.Olney JW. Food additives, excitotoxic. Boston, MA: Birkhauser, 1987 [Google Scholar]
- 50.Monno A, Vezzani A, Bastone A, Salmona M, Garattini S. Extracellular glutamate levels in the hypothalamus and hippocampus of rats after acute or chronic oral intake of monosodium glutamate. Neurosci Lett 1995;193:45–8 [DOI] [PubMed] [Google Scholar]
- 51.Olney JW, Labruyere J, de Gubareff T. Brain damage in mice from voluntary ingestion of glutamate and aspartate. Neurobehav Toxicol 1980;2:125–9 [PubMed] [Google Scholar]
- 52.Olney JW, Sharpe LG, Feigin RD. Glutamate-induced brain damage in infant primates. J Neuropathol Exp Neurol 1972;31:464–88 [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Tresguerres Hernandez JA. [Effect of monosodium glutamate given orally on appetite control (a new theory for the obesity epidemic).] An R Acad Nac Med (Madr) 2005;122(2):341–55; discussion 55–60 (in Spanish) [PubMed] [Google Scholar]
- 54.FASEB Analysis of adverse reactions to monosodium glutamate (MSG). Washington, DC: Life Sciences Research Office, Federation of American Societies for Experimental Biology, 1995 [Google Scholar]
- 55.WHO Toxicological evaluation of certain food additives. Food Additive Series, 22. Cambridge, United Kingdom: Cambridge University Press, 1988 [Google Scholar]
- 56.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 57.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601–13 [DOI] [PubMed] [Google Scholar]
- 59.Rundlett KL, Armstrong DW. Evaluation of free D-glutamate in processed foods. Chirality 1994;6:277–82 [DOI] [PubMed] [Google Scholar]
- 60.Maga JA. Flavor potentiators. Crit Rev Food Sci Nutr 1983;18:231–312 [DOI] [PubMed] [Google Scholar]
- 61.Konno R, Oowada T, Ozaki A, et al. Origin of D-alanine present in urine of mutant mice lacking D-amino-acid oxidase activity. Am J Physiol 1993;265:G699–703 [DOI] [PubMed] [Google Scholar]
- 62.Cram DJ, Cram JM. Host-Guest Chemistry: complexes between organic compounds simulate the substrate selectivity of enzymes. Science 1974;183:803–9 [DOI] [PubMed] [Google Scholar]
- 63.Man EH, Bada JL. Dietary D-amino acids. Annu Rev Nutr 1987;7:209–25 [DOI] [PubMed] [Google Scholar]
- 64.Rerucha J. The Nutrition Encyclopedia. Available from: http://www.nutripedia.com/Amino_Acids/ (cited 21 September 2009)
- 65.Giacometti T. Free and bound glutamate in natural products Filer LJ, Garattini MR, Kare MR, Reynolds WA, Wurtman RJ, eds Glutamic acid, advances in biochemistry and physiology. New York, NY: Raven Press, 1979 [Google Scholar]
- 66.American Heart Association. Available from: http://www.americanheart.org/presenter.jhtml?identifier=3045792#def_trans_fat (cited 16 September 2009)
- 67.Jones-Smith J, Gordon-Larsen P, Siddiqi A, Popkin BM. Cross-national comparisons of time trends in overweight inequality by socioeconomic status among women using repeated cross-sectional surveys from 37 developing countries, 1989-2007. Am J Epidemiol 2001;173:667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]