Abstract
Background: The associations between different sources of dietary n−3 (omega-3) and n−6 (omega-6) fatty acids and the risk of depression have not been prospectively studied.
Objective: The objective was to examine the relation between different n−3 and n−6 types with clinical depression incidence.
Design: We prospectively studied 54,632 US women from the Nurses' Health Study who were 50–77 y of age and free from depressive symptoms at baseline. Information on diet was obtained from validated food-frequency questionnaires. Clinical depression was defined as reporting both physician-diagnosed depression and regular antidepressant medication use.
Results: During 10 y of follow-up (1996–2006), 2823 incident cases of depression were documented. Intake of long-chain n−3 fatty acids from fish was not associated with depression risk [relative risk (RR) for 0.3-g/d increment: 0.99; 95% CI: 0.88, 1.10], whereas α-linolenic acid (ALA) intake was inversely associated with depression risk (multivariate RR for 0.5-g/d increment: 0.82; 95% CI: 0.71, 0.94). The inverse association between ALA and depression was stronger in women with low linoleic acid (LA) intake (P for interaction = 0.02): a 0.5-g/d increment in ALA was inversely associated with depression in the first, second, and third LA quintiles [RR (95% CI): 0.57 (0.37, 0.87), 0.62 (0.41, 0.93), and 0.68 (0.47, 0.96), respectively] but not in the fourth and fifth quintiles.
Conclusions: The results of this large longitudinal study do not support a protective effect of long-chain n−3 from fish on depression risk. Although these data support the hypothesis that higher ALA and lower LA intakes reduce depression risk, this relation warrants further investigation.
INTRODUCTION
Major depressive disorder (MDD) is a chronic and recurrent illness that affects 2 times as many women as men (1). In the United States, ≈1 in 5 women will be affected during their lifetime (2, 3). Lowered intake of n−3 relative to n−6 fatty acids has been implicated in the pathogenesis of depression (4, 5). Substantial evidence from randomized controlled trials indicates that long-chain n−3 from fish is associated with improvement of depressive symptoms (6, 7). However, the associations between different sources of dietary n−3 and n−6 fatty acids, and their ratio, and the risk of depression have not been prospectively studied.
Linoleic acid (LA) and α-linolenic acid (ALA), which represent ≈88.5% (≈15 g/d) and ≈8.5% (≈1.5 g/d), respectively, of polyunsaturated fatty acid (PUFA) intake in the United States, are the primary sources of fatty acids in human tissues (8, 9). Because they require the same metabolic enzymes to form derivative long-chain n−6 and n−3 fatty acids, experimental studies have raised concerns that LA intake may reduce ALA incorporation in tissues and/or its conversion to long-chain n−3 (10–12). Therefore, a potential competition effect of LA may be more applicable to ALA than to long-chain n−3 intake [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)].
To date, the link between n−3 fatty acids intake and the risk of developing new-onset depression has been analyzed in only 3 prospective cohorts (13–15). Results have been inconsistent, perhaps due to a number of limitations in the study designs such as the following: use of a single dietary intake assessment at baseline, small sample size, definition of depression, and short follow-up. We therefore accessed data from the Nurses' Health Study to examine the relation between different n−3 and n−6 fatty acids and the risk of incident clinical depression. Our analysis improved on previous work in terms of sample size, repeated measurements of dietary intake, adjustment for updated lifestyle risk factors, and a more rigorous definition of clinical depression.
SUBJECTS AND METHODS
Study population
Established in 1976, the Nurses' Health Study is a prospective cohort of 121,700 registered female nurses aged 30–55 y at enrollment and residing in 11 states of the United States. The cohort has been contacted every 2 y with mailed questionnaires about lifestyle and medical history, which update exposure information and inquire about newly diagnosed medical illnesses. The analysis excluded women who left ≥11 food items blank in food-frequency questionnaires, reported implausible energy intake (<600 or >3500 kcal/d), or did not return or answer the long-form questionnaires in 1996, 1998, or 2000. Of the 76,516 remaining women, we sequentially excluded those who reported physician-diagnosed depression in 1996 (n = 4286) or with an unknown start date (n = 168), those who reported taking regular antidepressant medication in 1996 (n = 2636), and those who presented severe depressive symptoms in 1992 (n = 2799) or 1996 (n = 2381) [ie, score of ≤52 on the Mental Health Index (MHI-5), a 5-item subscale of the Short-Form 36 Health Status Survey (16, 17)]. A lower score (≤52) on the MHI-5 has been associated with high sensitivity and specificity for MDD (18). After excluding those who had missing values for exposure variables (n = 9614), the final 1996 baseline population comprised 54,632 women. The Institutional Review Boards of Brigham and Women's Hospital and the Harvard School of Public Health approved the study protocol.
Dietary assessment
In 1980, a 61-item food-frequency questionnaire containing a single question on fish intake was administered to assess the intake of specific fats and other nutrients (19). In 1984, this food-frequency questionnaire was revised to incorporate questions on 116 individual foods that included 4 fish and seafood items (dark-meat fish, canned tuna, other fish and shellfish). Food-frequency questionnaire reproducibility and validity (20), and calculation of intake of EPA+DHA fatty acids have been reported elsewhere (21). Because the subsequent questionnaires contained food items that were critical for the assessment of n−3 and n−6 intake, we considered the 1984 questionnaire as the starting point for dietary information. These expanded questionnaires incorporate additional questions regarding types of fats used in cooking and the brand of margarine. Questions were also asked about frequency of utilization of mayonnaise or other creamy salad dressing and oil and vinegar (eg, Italian) salad dressing. Salad dressing and mayonnaise fatty acid compositions have been imputed from soybean oil, which accounted for all (salad dressing) or most (mayonnaise) of the market until recently. Composition values for ALA and other nutrients were obtained from the Harvard University Food Composition Database, which is derived from US Department of Agriculture sources (22), and complemented by direct analysis of food samples obtained from Boston area grocery stores and fast-food restaurants. More details have been published elsewhere (23). Food contributors to the overall intake of ALA were also previously published (24).
All nutrient intakes were adjusted for total energy intake according to the residual model (25). As a proxy measure of long-term dietary exposure, we took the cumulative average of the 4 dietary assessments (1984, 1986, 1990, and 1994) before our baseline (1996). This average better represents the long-term dietary intakes than does a single baseline assessment and is less likely to be affected by reverse causation than the more recent dietary intake. Information on fish-oil consumption was available only in 1990 and 1994.
Case ascertainment
Clinical depression was defined as reporting both physician-diagnosed depression and regular antidepressant medication use. Because questions relating to clinical depression were first addressed in 1996, we considered that year's questionnaire cycle as the baseline for follow-up. The question of regular antidepressant medication use was first asked in 1996, and this information was updated biennially through 2006. In 2000, nurses were asked to report the year of their first physician-diagnosed depression (1996 or before, 1997, 1998, 1999, or 2000). Thereafter, this information was updated biennially through 2006. Deaths were identified from the National Death Index, by next of kin, or by the postal system. Combining all sources, we estimated that follow-up for deaths was >98% complete (26).
Statistical analysis
Among women who were free of significant depressive symptoms at baseline, person-years of follow-up were calculated from the date of return of the 1996 questionnaire to the first endpoint—death, 1 June 2006, or the date of return of their last questionnaire, whichever came first. The age-standardized baseline characteristics of participants according to quintiles of ALA and EPA+DHA are presented for descriptive purposes (Table 1). Cox proportional hazards models, stratified on age in months and questionnaire cycle, served to estimate the relative risks (RRs) and their 95% CIs of developing clinical depression. The choice of units to express the RRs for continuous n−3 and n−6 intakes was based on differences between the 90th and 10th percentiles of their cumulative average intakes. Multivariate analyses were adjusted for nondietary covariates, including hormonal status (postmenopausal without and with hormonal therapy, premenopausal); ethnicity (white, yes or no); obesity [body mass index (BMI; in kg/m2) ≥30, yes or no]; smoking status (never smoked; past smoker; currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d); physical activity (quintiles); reported diagnosis of diabetes, cancer, or myocardial infarction or angina; and multivitamin use (yes or no) (model 1). All nondietary covariates were updated biennially. We further adjusted for cumulative average intake of dietary covariates (all continuous), including energy (kcal/d), alcohol (g/d), protein (g/d), trans fatty acids (g/d), saturated fatty acids (g/d), monounsaturated fatty acids (g/d), other n−3 and n−6 PUFAs (g/d), and fish-oil consumption (never, 1990 only, 1994 only, or both 1990 and 1994) (model 2). In sensitivity analysis (model 3), 691 cases of clinical depression observed before June 1998 were excluded. We performed further sensitivity analyses with the exclusion of 11,462 women who reported general medical conditions that could cause mood symptoms according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (27).
TABLE 1.
Baseline (1996) characteristics of participants in the Nurses' Health Study according to quintiles (Q) of n−3 intake1
| Quintiles of ALA intake (median g/d) |
Quintiles of EPA+DHA intake (median g/d) |
|||||||||
| Variable | Q1 (0.75) | Q2 (0.86) | Q3 (0.94) | Q4 (1.04) | Q5 (1.20) | Q1 (0.08) | Q2 (0.13) | Q3 (0.18) | Q4 (0.25) | Q5 (0.37) |
| n | 10,975 | 10,907 | 10,877 | 10,957 | 10,916 | 10,862 | 10,923 | 11,061 | 10,873 | 10,913 |
| Age (y) | 63.1 ± 7.12 | 62.7 ± 7.1 | 62.6 ± 7.0 | 62.8 ± 7.0 | 63.0 ± 7.0 | 62.3 ± 7.3 | 62.3 ± 7.1 | 62.7 ± 7.0 | 63.1 ± 6.9 | 63.8 ± 6.6 |
| General medical conditions (%)3 | 21.3 | 21.7 | 20.9 | 20.7 | 20.5 | 21.0 | 21.2 | 21.2 | 20.7 | 21.0 |
| White (%) | 96.5 | 97.8 | 98.1 | 98.4 | 98.4 | 98.7 | 98.8 | 98.3 | 97.4 | 96.1 |
| Hormonal status (%) | ||||||||||
| Postmenopausal, no hormones | 30.6 | 29.8 | 30.3 | 29.7 | 30.6 | 32.2 | 31.7 | 29.6 | 28.5 | 29.1 |
| Postmenopausal, with hormones | 59.6 | 60.0 | 60.3 | 60.8 | 60.0 | 57.8 | 58.6 | 61.0 | 61.8 | 61.1 |
| Premenopausal | 9.8 | 10.2 | 9.4 | 9.5 | 9.4 | 10.0 | 9.7 | 9.4 | 9.7 | 9.8 |
| Reported diagnosis of (%) | ||||||||||
| Myocardial infarction or angina | 3.8 | 4.0 | 3.5 | 3.8 | 3.5 | 3.4 | 3.6 | 3.7 | 3.8 | 4.1 |
| Diabetes | 3.6 | 4.4 | 4.5 | 4.4 | 5.0 | 4.3 | 4.1 | 4.3 | 4.6 | 4.5 |
| Cancer | 7.0 | 6.0 | 6.3 | 6.3 | 6.3 | 5.7 | 6.3 | 6.5 | 6.9 | 6.6 |
| BMI (in kg/m2) ≥30 (%) | 16.1 | 18.7 | 19.3 | 20.9 | 22.1 | 18.4 | 18.4 | 19.3 | 20.0 | 21.1 |
| Physical activity (MET-h/wk) | 19.4 ± 23.9 | 18.5 ± 22.0 | 18.7 ± 21.1 | 18.3 ± 21.7 | 18.0 ± 21.1 | 15.7 ± 21.6 | 17.0 ± 20.0 | 18.3 ± 20.7 | 19.6 ± 22.2 | 22.4 ± 24.7 |
| Current smoking (%) | 10.0 | 10.2 | 10.4 | 10.6 | 12.2 | 12.2 | 11.6 | 10.5 | 10.2 | 8.9 |
| Aspirin use (≥7/wk) (%) | 16.5 | 16.0 | 15.4 | 15.8 | 15.6 | 15.8 | 15.8 | 16.1 | 15.7 | 16.0 |
| Multivitamin use (%) | 55.3 | 54.0 | 52.9 | 51.0 | 48.5 | 49.0 | 50.4 | 51.9 | 53.6 | 56.8 |
| Fish-oil use (%) | 2.8 | 3.3 | 3.7 | 3.9 | 4.6 | 2.8 | 3.1 | 3.4 | 4.1 | 5.0 |
| Daily intake (1984–1994)4 | ||||||||||
| Energy (kcal) | 1706 ± 446 | 1753 ± 440 | 1786 ± 441 | 1781 ± 436 | 1737 ± 449 | 1754 ± 457 | 1764 ± 445 | 1773 ± 449 | 1763 ± 430 | 1706 ± 432 |
| Alcohol (g) | 6.3 ± 10.6 | 5.7 ± 8.8 | 5.6 ± 8.4 | 5.7 ± 8.3 | 5.9 ± 8.5 | 4.7 ± 9.0 | 5.5 ± 8.8 | 6.1 ± 9.2 | 6.4 ± 9.0 | 6.5 ± 8.7 |
| Vitamin D (IU) | 371 ± 210 | 354 ± 197 | 347 ± 190 | 336 ± 188 | 325 ± 196 | 295 ± 188 | 310 ± 183 | 331 ± 181 | 362 ± 186 | 436 ± 211 |
| LA (g) | 7.5 ± 1.6 | 8.3 ± 1.4 | 9.0 ± 1.4 | 9.5 ± 1.5 | 10.9 ± 2.0 | 9.2 ± 2.1 | 9.2 ± 1.9 | 9.1 ± 1.9 | 9.0 ± 1.9 | 8.8 ± 1.9 |
| AA (g) | 0.13 ± 0.04 | 0.13 ± 0.04 | 0.13 ± 0.03 | 0.13 ± 0.04 | 0.14 ± 0.04 | 0.11 ± 0.03 | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.16 ± 0.04 |
| ALA (g) | 0.73 ± 0.07 | 0.86 ± 0.03 | 0.94 ± 0.02 | 1.04 ± 0.03 | 1.24 ± 0.16 | 0.95 ± 0.20 | 0.96 ± 0.19 | 0.96 ± 0.19 | 0.96 ± 0.19 | 0.97 ± 0.20 |
| EPA+DHA (g) | 0.21 ± 0.14 | 0.21 ± 0.13 | 0.21 ± 0.13 | 0.21 ± 0.13 | 0.21 ± 0.14 | 0.07 ± 0.02 | 0.13 ± 0.01 | 0.18 ± 0.02 | 0.25 ± 0.02 | 0.41 ± 0.13 |
| ALA:LA ratio | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 |
| n−3:n−6 ratio | 0.13 ± 0.04 | 0.13 ± 0.03 | 0.13 ± 0.03 | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.17 ± 0.04 |
All characteristics are age-standardized with the exception of age. Percentages may not total 100% because of rounding and missing data. Intake of vitamin D includes both dietary and supplement sources. AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; MET, metabolic equivalents of task.
Mean ± SD (all such values).
Percentages are for the entire follow-up period. General medical conditions associated with mood disorder according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (27): neurological conditions (Parkinson disease, Huntington disease), cerebrovascular condition (stroke), metabolic conditions (pernicious anemia or vitamin B-12 deficiency), endocrine conditions (hyper- and hypothyroidism, hyper- and hypoparathyroidism), autoimmune conditions (systemic lupus erythematosus), and pancreatic carcinoma.
Computed as the cumulative average of 1984 through 1994 (see Subjects and Methods). Except for ALA:LA and n−3:n−6 ratios, all nutrient intakes were adjusted for total energy intake with the residual model.
We also calculated the cumulative average of total fish intake from 1984 through 1994. Multivariate Cox proportional hazards models were also adopted to estimate the RRs (95% CIs) of developing clinical depression according to fish consumption frequency. Participants were classified into 5 groups: <1 time/mo (reference group), 1–3 times/mo, 1 time/wk, 2–4 times/wk, and ≥5 times/wk.
Interaction between ALA and LA intake was assessed by using a cross-product term, with both elements as continuous variables. ALA and LA interaction was found to be positively significant for clinical depression risk (P = 0.02). Therefore, the association between ALA intake and the risk of clinical depression was also assessed separately within each quintile of LA and vice versa. All analyses were performed with SAS software, version 9.1 (2003; SAS Institute Inc, Cary, NC). All P values reported are 2-sided.
RESULTS
Among 54,632 women who were free from depressive symptoms at baseline, 2823 had experienced incident clinical depression during 10 y of follow-up (495,829 person-years). The age-adjusted baseline characteristics of participants by quintiles of ALA and EPA+DHA intakes are shown in Table 1.
Dietary n−3 intake from plant sources, ALA, was not associated with the risk of depression in age-adjusted or nondietary multivariate models (model 1) (Table 2). However, when we further adjusted for dietary factors (model 2), the RR of clinical depression was 0.82 (95% CI: 0.71, 0.94) for each 0.5-g/d increment of ALA. Dietary intake of EPA+DHA from fish (Table 2) or fish consumption frequency (data not shown) was not associated with the risk of clinical depression. Compared with women who ate fish <1 time/mo, RRs (95% CIs) were 0.95 (0.79, 1.15) for those who ate fish 1–3 times/mo, 0.96 (0.81, 1.15) for those who ate fish 1 time/wk, 0.96 (0.78, 1.19) for those who ate fish 2–4 times/wk, and 1.07 (0.74, 1.55) for those who ate fish ≥5 times/wk. When examined separately, neither fatty nor lean fish intake was associated with depression risk. Compared with nonusers of fish-oil supplements, the risk of clinical depression was unexpectedly high in the small fraction of women (n = 689) who reported fish-oil consumption in 1990 only (RR: 1.40; 95% CI: 1.05, 1.87), but no association was found in the 841 women who reported fish-oil use in 1994 only (RR: 1.09; 95% CI: 0.81, 1.46) or in the 475 women who reported use in both 1990 and 1994 (RR: 0.98; 95% CI: 0.64, 1.50) (model 2; data not shown).
TABLE 2.
Relative risks (RRs) of clinical depression according to n−3 and n−6 intakes in women from the Nurses' Health Study cohort1
| Continuous analysis |
||
| RR (95% CI) | P value | |
| ALA (each increase of 0.5 g) | ||
| Age-adjusted2 | 0.99 (0.90, 1.09) | 0.86 |
| Multivariate model 13 | 0.96 (0.87, 1.05) | 0.37 |
| Multivariate model 24 | 0.82 (0.71, 0.94) | 0.005 |
| Multivariate model 35 | 0.81 (0.69, 0.95) | 0.009 |
| EPA+DHA (each increase of 0.3 g) | ||
| Age-adjusted2 | 1.05 (0.97, 1.15) | 0.21 |
| Multivariate model 13 | 1.06 (0.97, 1.16) | 0.17 |
| Multivariate model 24 | 0.99 (0.88, 1.10) | 0.82 |
| Multivariate model 35 | 0.96 (0.84, 1.10) | 0.57 |
| LA (each increase of 5 g) | ||
| Age-adjusted2 | 1.12 (1.02, 1.23) | 0.02 |
| Multivariate model 13 | 1.07 (0.97, 1.17) | 0.19 |
| Multivariate model 24 | 1.26 (1.07, 1.49) | 0.006 |
| Multivariate model 35 | 1.33 (1.10, 1.61) | 0.003 |
| AA (each increase of 0.1 g) | ||
| Age-adjusted2 | 1.22 (1.10, 1.34) | <0.001 |
| Multivariate model 13 | 1.15 (1.04, 1.27) | 0.007 |
| Multivariate model 24 | 1.02 (0.88, 1.19) | 0.80 |
| Multivariate model 35 | 1.06 (0.89, 1.26) | 0.54 |
| ALA:LA (each increase of 0.05 U) | ||
| Age-adjusted2 | 0.81 (0.74, 0.90) | <0.001 |
| Multivariate model 13 | 0.84 (0.76, 0.92) | <0.001 |
| Multivariate model 24 | 0.78 (0.69, 0.88) | <0.001 |
| Multivariate model 35 | 0.77 (0.66, 0.88) | <0.001 |
| n−3:n−6 (each increase of 0.1 U) | ||
| Age-adjusted2 | 0.85 (0.75, 0.98) | 0.02 |
| Multivariate model 13 | 0.89 (0.78, 1.02) | 0.10 |
| Multivariate model 24 | 0.78 (0.66, 0.92) | 0.004 |
| Multivariate model 35 | 0.74 (0.61, 0.90) | 0.003 |
Values are RRs (95% CIs) from Cox proportional hazards models. The RR is for each unit increment of fatty acids or ratios. AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
Adjusted for age (continuous) and time interval.
Further adjusted for hormonal status (postmenopausal without or with hormonal therapy, premenopausal), white race (binary), obesity (binary), smoking status (never smoked; past smoker; currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d), physical activity (quintiles), reported diagnosis of diabetes (binary), cancer (binary), myocardial infarction or angina (binary), and multivitamin use (binary).
Further adjusted for cumulative average intake (1984–1994) of energy (kcal/d), protein (g/d), trans fatty acids (g/d), saturated fatty acids (g/d), monounsaturated fatty acids (g/d), and alcohol (g/d) (all continuous). For ALA, model 2 was further adjusted for EPA+DHA, LA, and AA (all continuous) and fish-oil use (never, 1990 only, 1994 only, both 1990 and 1994). For EPA+DHA, the model was further adjusted for ALA, LA, AA, and fish-oil use. For LA, the model was further adjusted for ALA, EPA+DHA, AA, and fish-oil use. For AA, the model was further adjusted for ALA, EPA+DHA, LA, and fish-oil use. For the ALA:LA ratio, the model was further adjusted for EPA+DHA, AA, and fish-oil use.
Adjusted as in model 2 but excludes cases observed before June 1998 (n = 691).
The risk of clinical depression increased with increasing LA intake (RR for each 5-g/d increment: 1.26; 95% CI: 1.07, 1.49) (model 2, Table 2) and decreased with increasing ALA:LA (P < 0.001 for trend) and n−3:n−6 ratios (P = 0.004 for trend). Arachidonic acid (AA) intake was not significantly associated (P = 0.80) with the risk of depression in multivariate model 2. The results obtained after excluding women with general medical conditions were almost identical to those for all women (data not included). When we excluded clinical depression cases observed before June 1998, the results for n−3 and n−6 were similar (model 3, Table 2).
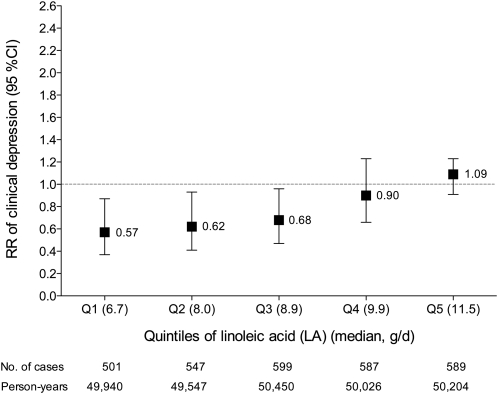
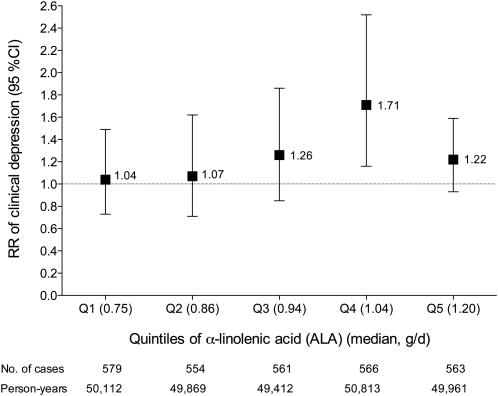
We assessed the multivariable-adjusted risk of clinical depression for ALA and LA intakes within quintiles of LA and ALA, respectively. For each 0.5-g/d increment of ALA, the RR (95% CI) of clinical depression was 0.57 (0.37, 0.87) in the first, 0.62 (0.41, 0.93) in the second, 0.68 (0.47, 0.96) in the third, 0.90 (0.66, 1.23) in the fourth, and 1.09 (0.91, 1.32) in the fifth quintiles of LA (Figure 1). For each 5-g/d increment of LA, the RR (95% CI) of clinical depression was 1.04 (0.73, 1.49) in the first, 1.07 (0.71, 1.62) in the second, 1.26 (0.85, 1.86) in the third, 1.71 (1.16, 2.52) in the fourth, and 1.22 (0.93, 1.59) in the fifth quintiles of ALA (Figure 2).
FIGURE 1.
Multivariate relative risks (RR; 95% CIs) of clinical depression with increased intake of α-linolenic acid (ALA) according to stratification by quintiles (Q) of linoleic acid (LA). Values are RRs from Cox proportional hazards models. The RR is for each 0.5-g/d increase of ALA. The ALA × LA interaction was significant for clinical depression risk (P = 0.02). Multivariate analysis included the following covariates: age (continuous); time interval; hormonal status (postmenopausal without or with hormonal therapy, premenopausal); white race (binary); obesity (binary); smoking status (never smoked; past smoker; currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d); physical activity (quintiles); reported diagnosis of diabetes (binary), cancer (binary), or myocardial infarction or angina (binary); multivitamin use (binary); and cumulative average intake (all continuous) of energy (kcal/d), protein (g/d), trans fatty acids (g/d), saturated fatty acids (g/d), monounsaturated fatty acids (g/d), alcohol (g/d), eicosapentaenoic acid plus docosahexaenoic acid (g/d), arachidonic acid (g/d), and fish oil (never, 1990 only, 1994 only, or both 1990 and 1994).
FIGURE 2.
Multivariate relative risks (RR; 95% CI) of clinical depression with increased intake of linoleic acid (LA) according to stratification by quintiles (Q) of α-linolenic acid (ALA). Values are RRs from Cox proportional hazards models. The RR is for each 5-g/d increase of LA. The ALA × LA interaction was significant for clinical depression risk (P = 0.02). Multivariate analysis included the following covariates: age (continuous); time interval; hormonal status (postmenopausal without or with hormonal therapy, premenopausal); white race (binary); obesity (binary); smoking status (never smoked; past smoker; currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d); physical activity (quintiles); reported diagnosis of diabetes (binary), cancer (binary), or myocardial infarction or angina (binary); multivitamin use (binary); and cumulative average intake (all continuous) of energy (kcal/d), protein (g/d), trans fatty acids (g/d), saturated fatty acids (g/d), monounsaturated fatty acids (g/d), alcohol (g/d), eicosapentaenoic acid plus docosahexaenoic acid (g/d), arachidonic acid (g/d), and fish oil (never, 1990 only, 1994 only, or both 1990 and 1994).
DISCUSSION
In this large prospective cohort of women, we found that higher dietary intake of vegetable n−3, ALA, was significantly associated with a lower risk of clinical depression, especially among those who had the lowest intake of LA. We did not observe any association between the risk of clinical depression and fish consumption frequency or EPA+DHA intake from fish. A novel aspect of our study is the more complete analysis of n−3 and n−6 PUFAs and their effect on depression risk and, consequently, the significant interaction noted between LA and ALA. Stratification analyses within quintiles of LA revealed that ALA was associated with a significantly lower risk of clinical depression among women in the first 3 quintiles of LA intake but not among those in the fourth and fifth quintiles (Figure 1).
Because ALA and LA require common metabolic enzymes, when LA intake is low, up to 11.5% of ALA is converted to EPA (10). Therefore, the ability of ALA to slightly raise long-chain n−3, mainly EPA, and to a lesser extent docosapentaenoic acid, might partially explain our results. Evidence from randomized trials indicates that the combination of EPA+DHA improves depressive symptoms when administered as an adjunct to antidepressant medication (6, 7, 28). However, significant heterogeneity between studies and publication bias are noted (7, 28). Some authors have suggested that EPA alone or a higher EPA/DHA ratio is associated with better outcomes than supplementation with DHA alone (6, 29). A protective effect of EPA would be consistent with the inverse correlation that we observed with intake of ALA, of which very little is converted to DHA (no greater than 0.5%) (30). An argument against effects of ALA from its higher conversion to EPA is the absence of relation between EPA+DHA intake and depression in our results. However, EPA intake among our participants was low (median: 58 mg/d) and therefore EPA formation from dietary ALA acid might exceed EPA intake. It is also possible that long-term dietary ALA intake may play a physiologic role in clinical depression that is independent of EPA+DHA.
Despite adjustment for covariates, including ALA, each 5-g increase in LA was associated with a 26% (95% CI: 7%, 49%) higher risk of clinical depression (Table 2). Again, this might be due to the significant interaction between LA and ALA. Stratification analyses within quintiles of ALA indicated that augmented intake of LA was linked with a significantly higher risk of clinical depression only among women in the fourth quintile of ALA (Figure 2). This significantly heightened risk was probably due to chance, because elevated LA intake was not associated with excess risk in the lowest quintiles of ALA (in which the risk might be hypothesized to be the greatest). Therefore, LA might not have a direct detrimental effect on depression but rather a potential biological interaction with ALA. However, an adverse effect of LA intake on risk of depression in susceptible individuals cannot be excluded.
The results of previous longitudinal studies on n−3 intake and the risk of depression have been inconsistent. In a Finnish cohort of 29,133 men aged 50–69 y and followed for 9 y, no associations were noted between dietary intake of EPA+DHA from fish or fish consumption and proxy depression measures (13). This cohort was the only one in which the relation between ALA intake and the risk of depression was analyzed, and no association was found. Among 7903 Spanish participants in the SUN (Seguimiento Universidad de Navarra) cohort, a lower risk of mental disorder was noted only for the second and fourth quintiles of long-chain n−3 intake after a median follow-up of 27.5 mo compared with the first quintile of intake (14). However, no linear trend was observed, and most of these cases (≈67%) were of anxiety disorder. In a US cohort of 3317 men and women, the odds of depressive symptoms were not significantly different in higher quintiles of baseline long-chain n−3 or fish intake after 3 y of follow-up (15). However, an inverse association between long-chain n−3 and the number of visits with depressive symptoms was encountered in women only (15).
The use of 4 dietary assessments over a period of 10 y was a unique strength of our study. Indeed, other cohorts completed only one baseline food-frequency questionnaire as a measure of exposure. This approach is less accurate than ours as it assumes that dietary intake measured once at baseline is representative of usual diet that remains unchanged for the entire follow-up period. In addition, our assessment of ALA and LA has been validated against the amounts of these fatty acids in adipose tissue [r = 0.34 (P < 0.001) for ALA and r = 0.37 (P < 0.001) for LA] (23). However, because LA and ALA are largely contributed by the same or similar food items, complete disentangling of LA from ALA intake is not possible, and some degree of misclassification and bias due to residual confounding is inevitable (31). Furthermore, due to the high positive correlation between LA and ALA intake in the US diet, the LA:ALA ratio displays only modest variability; it would thus be of interest to examine the relation between specific fatty acid intakes and depression in populations with dietary patterns that allow better discrimination of ALA from LA. Statistical limitations common to any study with multiple comparisons also apply to the present study.
Two additional concerns are reverse causation and confounding by indication. Reverse causation may derive from an effect of depressive symptoms on diet. For example, a spurious inverse association between n−3 intake and the risk of depression could be observed if women with depressed mood reduced their n−3 intake. To minimize bias from this source, we excluded at baseline 12,270 women with severe depressive symptoms, and we considered the cumulative average of n−3 and n−6 intake assessed between 1984 and 1994 to predict the occurrence of depression between 1996 and 2006. Furthermore, we confirmed the results in sensitivity analyses in which we excluded 691 women with depression before June 1998, and 11,462 women who had general medical conditions associated with mood disorder. Neither of these exclusions altered our findings. Confounding by indication, on the other hand, could obfuscate a true protective effect of n−3 or even induce a false-positive association between the use of fish-oil supplements and the risk of depression. This may happen if women with depressed mood started taking fish oil in an attempt to improve their symptoms, an occurrence that would be consistent with the 40% increased risk noted among the small group of women (1.26%) who consumed fish oil only in 1990. However, considering that no significant risks were discerned for women who used fish oil in 1994 and for those who ingested it in both 1990 and 1994, the heightened risk among such consumers in 1990 only may simply reflect random variation.
Finally, some outcome misclassification is inevitable because of a combination of errors in reporting depression or the intake of antidepressant medications, low recognition of depression by physicians (32), undertreatment of depression (33), and the prescription of antidepressant medications for indications other than depression. We tried to maximize the specificity of case definition, accepting as incident cases of depression only those women who reported both a diagnosis of depression and the use of antidepressant medications. A significant percentage of diagnosed depressed cases did not receive antidepressant medication during the follow-up. Thus, our strict definition of depression (ie, diagnosis of depression plus use of antidepressant medication) may cause inclusion of only relatively more severe cases. However, we were unable to distinguish between bipolar and unipolar depression. To the extent that the probability of correctly classifying women with an incident case of depression is independent from their dietary habits (nondifferential misclassification of outcome), the low sensitivity of this strict case definition should not bias RR estimates (34). Over 10 y of follow-up, 5.2% of the women in our cohort developed clinical depression. This incidence is not directly comparable to that observed in unselected populations because, to minimize reverse causation, we excluded women with severe depressive symptoms at baseline, thus eliminating a group of women at higher risk of depression. Thus, unlike most previous studies, our analyses of the Nurses' Health Study address the relation between n−3 and n−6 fatty acids and new onset of relatively severe depression—that is, depression severe enough to be diagnosed as such and treated with antidepressant medication.
Several biological mechanisms might potentially explain the effect of ALA in depression. Dietary ALA deficiency has been linked with altered brain biochemistry, such as membrane structure and fluidity, ion channels, second messengers, reduced cyclic AMP response element-binding protein transcription factor activity and brain-derived neurotrophic factor expression, and increased expression of cytosolic and secretory phospholipase A2 and cyclooxygenase-2 (35–37). Animal studies have also indicated that ALA deficiency changes serotoninergic and dopaminergic neurotransmission in the frontal cortex (38, 39), and its augmented intake might influence neurogenesis as well as key proteins involved in synaptic functions (40). Inflammatory processes and endothelial dysfunction are often involved in depression and cardiovascular disease (3). A higher dietary intake of ALA has been related to lower plasma inflammatory biomarkers and endothelial activation among women in the Nurses' Health Study (41). However, the mechanism of action of dietary n−3 and n−6 in depression requires further exploration in humans.
In conclusion, the results of this large longitudinal study do not support a protective effect of long-chain n−3 fatty acids or fish intake on the risk of depression. Higher ALA and lower LA intakes were associated with a reduced risk of clinical depression, but this association should be interpreted cautiously because of the difficulty of separating the effects of correlated nutrient intakes.
Acknowledgments
We are indebted to the participants in the Nurses' Health Study for their continuing outstanding support and to colleagues working in the study for their valuable help. We thank Dariush Mozaffarian, Department of Epidemiology, Harvard School of Public Health, for his comments on the manuscript.
The authors' responsibilities were as follows: AA and WCW: obtained funding and were investigators in the Nurses' Health Study; ML, FM, EJO, AP, WCW, IK, KK, and AA: collected data, created the concept for the current analysis, and provided statistical expertise; ML, FM, EJO, and AA: analyzed the data; and ML: wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and to critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. The authors had no conflicts of interest to declare.
REFERENCES
- 1.Kessler RC. Epidemiology of women and depression. J Affect Disord 2003;74:5–13 [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105 [DOI] [PubMed] [Google Scholar]
- 3.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008;358:55–68 [DOI] [PubMed] [Google Scholar]
- 4.Hibbeln JR, Salem NJ. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr 1995;62:1–9 [DOI] [PubMed] [Google Scholar]
- 5.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 2006;60:502–7 [DOI] [PubMed] [Google Scholar]
- 6.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006;67:1954–67 [DOI] [PubMed] [Google Scholar]
- 7.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n−3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010;91:757–70 [DOI] [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 2000;71(suppl):179s–88s [DOI] [PubMed] [Google Scholar]
- 9.National Health and Nutrition Examination Survey (NHANES) 2005–2006 [database on the Internet] What we eat in America. Beltsville, MD: USDA, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD and Hyattsville, MD), US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Available from: http://www.ars.usda.gov/ (cited March 2010) [Google Scholar]
- 10.Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of α-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr 2006;84:44–53 [DOI] [PubMed] [Google Scholar]
- 11.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n−3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr 2007;137:945–52 [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Novak EM, Innis SM. Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 alpha-linolenic acid ratios in normal, fat-fed pigs. Am J Physiol Heart Circ Physiol 2007;293:H2919–27 [DOI] [PubMed] [Google Scholar]
- 13.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lonnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry 2004;161:567–9 [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr 2007;46:337–46 [DOI] [PubMed] [Google Scholar]
- 15.Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition 2009;25:1011–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83 [PubMed] [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: manual & interpretation guide. Lincoln, RI: QualityMetric Incorporated, 2000 [Google Scholar]
- 18.Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care 1991;29:169–76 [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–99 [DOI] [PubMed] [Google Scholar]
- 21.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 2001;285:304–12 [DOI] [PubMed] [Google Scholar]
- 22.US Department of Agriculture Composition of foods: raw, processed, and prepared, 1963–1988. Agriculture handbook no.8. Washington, DC: US Government Printing Office, 1989 [Google Scholar]
- 23.Garland M, Sacks FM, Colditz GA, et al. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr 1998;67:25–30 [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Stampfer MJ, Manson JE, et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr 1999;69:890–7 [DOI] [PubMed] [Google Scholar]
- 25.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27 [DOI] [PubMed] [Google Scholar]
- 26.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol 1984;119:837–9 [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association Mood disorder due to a general medical condition (293.83). : Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association, 1994 [Google Scholar]
- 28.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 2007;68:1056–61 [DOI] [PubMed] [Google Scholar]
- 29.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 2009;28:525–42 [DOI] [PubMed] [Google Scholar]
- 30.Plourde M, Cunnane S. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab 2007;32:619–34 [DOI] [PubMed] [Google Scholar]
- 31.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for measurement error: the case of multiple covariates measured with error. Am J Epidemiol 1990;132:734–45 [DOI] [PubMed] [Google Scholar]
- 32.Lowe B, Spitzer RL, Grafe K, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. J Affect Disord 2004;78:131–40 [DOI] [PubMed] [Google Scholar]
- 33.Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA 2004;291:2581–90 [DOI] [PubMed] [Google Scholar]
- 34.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins, 1998 [Google Scholar]
- 35.Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions. J Neurosci Res 1999;56:565–70 [DOI] [PubMed] [Google Scholar]
- 36.Haag M. Essential fatty acids and the brain. Can J Psychiatry 2003;48:195–203 [DOI] [PubMed] [Google Scholar]
- 37.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids 2007;77:251–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delion S, Chalon S, Hérault J, Guilloteau D, Besnard J-C, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr 1994;124:2466–76 [DOI] [PubMed] [Google Scholar]
- 39.de la Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J Nutr 1999;129:2088–93 [DOI] [PubMed] [Google Scholar]
- 40.Blondeau N, Nguemeni C, Debruyne DN, et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology 2009;34:2548–59 [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Garcia E, Schulze MB, Manson JE, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 2004;134:1806–11 [DOI] [PubMed] [Google Scholar]