Abstract
Background: Zinc deficiency is a cause of immune dysfunction and infection. Previous human studies have shown that the activation of the acute phase response alters zinc metabolism. Whether the alteration in zinc metabolism is predictive of disease severity in the setting of critical illness is unclear.
Objective: We sought to determine whether differences occur in zinc metabolism at the onset of critical illness between infected (septic) and noninfected subjects.
Design: We conducted this prospective study in an adult medical intensive care unit (MICU) at a tertiary care hospital. Subjects were enrolled within 24 h of intensive care unit admission. Subjects who did not meet sepsis criteria were considered for the critically ill control (CIC) arm. After patient consent, blood was immediately collected to measure plasma zinc and cytokine concentrations and zinc transporter gene expression in peripheral blood monocytes. Clinical data during the MICU stay were also recorded.
Results: A total of 56 patients were evaluated (22 septic, 22 CIC, and 12 healthy subjects). Plasma zinc concentrations were below normal in CIC patients and further reduced in the septic cohort (57.2 ± 18.2 compared with 45.5 ± 18.1 μg/dL). Cytokine concentrations increased with decreasing plasma zinc concentrations (P = 0.05). SLC39A8 gene expression was highest in patients with the lowest plasma zinc concentrations and the highest severity of illness.
Conclusions: The alteration of zinc metabolism was more pronounced in septic patients than in noninfected critically ill patients. Specifically, sepsis was associated with lower plasma zinc concentrations and higher SLC39A8 mRNA expression, which correlated with an increased severity of illness, including cardiovascular dysfunction.
INTRODUCTION
Zinc deficiency is associated with immune dysfunction, higher rates of infection, and increased morbidity and mortality after infection (1–8). Mild to moderate zinc deficiency is often undetected and not considered during the early stages of patient care after admission to the intensive care unit (9). Thus, the true incidence of zinc deficiency in the setting of critical illness is underappreciated as it is in many other populations (10). Relevant to this investigation, zinc deficiency is more common in the elderly (7) and in individuals with chronic medical conditions (2), who are the same populations that have increased risk of developing sepsis. With respect to the zinc metabolic response, it is well established that plasma zinc concentrations rapidly decline at the onset of the acute phase response because of the redistribution of zinc into the cellular compartment (8, 11). Whether zinc metabolism differs between adult septic subjects and noninfected subjects is unknown. If differences exist, the assessment of zinc metabolism may be beneficial in determination of the host disposition, treatment strategies, and outcome. Recently, we reported that moderate zinc deficiency significantly increased mortality during the early stages of infection compared with that of zinc sufficient septic controls in a murine model of polymicrobial sepsis (12). Therefore, it is plausible that the alteration of zinc metabolism during a critical illness as a consequence of infection may be an important determinant in predicting the host response and outcome (13). On the basis of these considerations and the difficulty of assessing the nutritional status in critically ill subjects, we chose to conduct a prospective observational human study to determine whether zinc metabolic status correlated with inflammation and the severity of illness [as determined by the Simplified Acute Physiology Score (SAPS) II or Sequential Organ Failure Assessment (SOFA)] in critically ill infected and noninfected patients. In particular, we also determined whether changes in plasma zinc concentrations during the initial stages of critical illness were associated with alterations in the expression of zinc transporters, which are key regulators of cellular zinc homeostasis. Compared with previous human studies (14, 15), we believe that the current study was the first adult human study to compare septic and critically ill, noninfected subjects within the early stages of critical illness in a medical intensive care unit (MICU) setting.
SUBJECTS AND METHODS
Participants
Patients in this prospective study were admitted to the MICU at The Ohio State University Medical Center, which is an academic tertiary care hospital, over a 4-mo period. The study was approved by the hospital internal review board, and enrollment began before 1 July 2008. Patients were screened for sepsis by consensus criteria, and inclusion criteria (Table 1) were determined by a chart review. A single blood sample was obtained in ≤24 h of sepsis onset (septic group) or MICU admission [critically ill control (CIC) group] (average time to blood collection after meeting inclusion criteria: 15.4 h). Patients admitted to the MICU without criteria for sepsis were eligible for the CIC arm (Table 1).
TABLE 1.
Inclusion and exclusion criteria1
| Participant criteria |
| Critically ill sepsis arm |
| Inclusion criteria2 |
| Known or suspected infection and ≥2 of 4 SIRS criteria |
| 1) Temperature ≤36°C or ≥38°C |
| 2) Heart rate ≥90 beats/min |
| 3) Respiratory rate ≥20 breaths/min or PaCO2 <32 mm Hg |
| 4) White blood cell count <4000 or >12,000 or >10% bands |
| Exclusion criteria |
| 1) Consent not available or patient declined |
| 2) Prisoner |
| 3) Patient died before blood was collected |
| 4) Patient met criteria for sepsis onset at an outside hospital >24 h before transfer to our medical center |
| Critically ill control arm |
| Inclusion criteria |
| 1) ≤24 h since medical intensive care unit admission |
| 2) No known or suspected infection on the basis of review of the assessment of the treating physicians |
| 3) Lack of antibiotic therapy (except for prophylactic therapy) |
| 4) No known microbiologic and radiographic signs of infection |
| Exclusion criteria |
| 1) Consent not available or patient declined |
| 2) Prisoner |
| 3) Patient died before blood was collected |
| 4) Patient critically ill in a medical intensive care unit >24 h |
SIRS, systemic inflammatory response syndrome; PaCO2, partial pressure of carbon dioxide in arterial blood.
Blood was collected within 24 h of sepsis onset (regardless of the patient location when inclusion criteria were first met).
All samples were processed by individuals blinded to the group assignment of patients and to clinical information. Blood was also collected from 12 healthy adult donors during the same time period and processed in the same manner for comparison with the sepsis and CIC patient groups.
Procedures
After patient consent, baseline demographic data were recorded and 20 mL blood was drawn. Fifteen milliliters of blood was collected in evacuated tubes containing EDTA for RNA analysis, and 5 mL blood was collected in vacuum tubes containing heparin for zinc, metallothionein, and cytokine analysis. All tubes were immediately placed on ice at the time of collection.
Monocyte purification
Monocytes were isolated immediately after a blood draw with Histopaque-1077 density gradient centrifugation (Sigma-Aldrich, St Louis, MO) at 2000 rpm for 20 min at room temperature. The mononuclear layer was removed and washed twice in RPMI 1640 medium (BioWhittaker, Walkersville, MD). Monocytes were isolated by a positive selection with anti-CD14–coated magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. This method consistently yielded a ≥ 98% pure population of CD14+ monocytes as confirmed by flow-cytometry analysis (data not shown). Zinc contamination was minimized by using conical tubes and isolation medium without the detectable presence of zinc (per manufacturer reporting). Monocytes were immediately resuspended in TRIzol reagent (Invitrogen, Carlsbad, CA), for RNA related studies within 4 h of blood collection and frozen at −80°C.
RNA isolation
RNA was extracted with TRizol reagent (Invitrogen) as per the standard operating procedure, and 1 μg total RNA was reverse transcribed to generate complementary DNA by ThermoScript RNase H-Reverse Transcriptase (Invitrogen Life Technologies, Carlsbad, CA) followed by dilution (1:10) with RNase-free water. The complementary DNA was subjected to quantitative polymerase chain reaction (PCR) analysis by using SYBR Green PCR Master Mix and a PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA). ΔCt values were first used for all statistical analyses and then transformed to a relative copy number (RCN) value to facilitate the ease of data interpretation. The RCN of selected genes were determined by normalization to the expression of the 2 housekeeping genes GAPDH and cyclic AMP-accessory protein (CAP-1), and calculated with the equation:
where E denotes the efficiency of the PCR, and ΔCt denotes the Ct target minus the Ct reference (the average of 2 housekeeping genes) (16). PCR primer pairs, including those for all known human zinc transporters, were previously validated and reported (13).
Plasma cytokine measurements
Heparinized blood was centrifuged at 2000 rpm for 10 min at 4°C, and the plasma was carefully removed. Fifty microliters of plasma was analyzed to simultaneously quantify cytokine or chemokine protein concentrations with a Bio-Plex Multiplex Cytokine Assay (BioRad, Hercules, CA) per the manufacturer's instructions.
Plasma zinc and red blood cell metallothionein concentrations
Plasma was also assessed for the total zinc content by using atomic absorption spectrophotometry (Analyst 400; PerkinElmer, Waltham, MA). Two hundred microliters of plasma was diluted 1:15 with deionized water and compared with zinc standards (PerkinElmer). All patient samples were analyzed in triplicate. Total red blood cell metallothionein (RBCMT) concentrations were obtained by using the silver binding assay (17).
Statistical analysis
Patient information at the time of blood collection was used to calculate the SAPS II and SOFA scores if available. If not available, the worst reported values of patients within the 12 h before or after the blood draw were used. The patient disposition at the time of hospital discharge and in-hospital mortality were also recorded. Once collected, data were deidentified and entered into a secure data-management program. Statistical analysis was performed with JMP 6.0 (SAS Institute, Cary, NC) and STATA 6.0 (Stata Corp, College Station, TX) software in collaboration with a biostatistician at The Ohio State University Center for Biostatistics. An analysis of variance determined that the P value was significant for the assessment of differences in zinc concentrations in the 3 groups; differences between individual groups were then evaluated by using the 2-sample t test. A univariate linear regression was used to evaluate plasma zinc concentrations that were adjusted for clinical variables that may have affected zinc, including age or insulin infusion. Cytokine values were log transformed to stabilize the variance and provide a normal distribution before further analysis. A multivariate analysis was used to evaluate the relation between cytokine concentrations and plasma zinc concentrations in the combined CIC and sepsis groups. A multiple comparison analysis was adjusted for by using Holm's procedure to control for type I error.
RESULTS
Patients
Over a 4-mo period, 296 MICU patients were screened for our study (see supplemental Table 1 under “Supplemental data” in the online issue). A total of 45 patients (23 CIC and 22 septic patients) met criteria and gave consent for enrollment. Because of an inadequate plasma sample in one CIC patient, data were analyzed for 22 patients in that group. Plasma from 12 normal, adult control subjects was also compared in this study. Overall, the CIC and sepsis cohorts were well matched on the basis of demographic and laboratory values (Table 2). The comparison of the SAPS II was also similar between the 2 groups, whereas the septic group had a higher SOFA score (P = 0.03). SAPS II and SOFA scores incorporated an algorithm of physiologic and laboratory variables of the critically ill patient to predict the severity of illness and hospital mortality or to assess the degree of organ dysfunction, respectively. Most patients in both groups survived their hospital stay and were discharged to their homes or to an acute rehabilitation facility. Two septic patients died before being discharged from the hospital (one patient died from an abdominal infection, and the other patient died of pneumonia). The source of infection was confirmed by culture in 16 of 22 septic patients and included Candida glabratta, Candida albicans, methicillin sensitive and methicillin resistant Staphylococcus areus, Escherichia. coli, Clostridium difficile, α-hemolytic Streptococcus, Pseudomonas aeruginosa, and Enterococcus fecalis. Cultures were negative in all CIC patients, and one patient died after a subarachnoid hemorrhage. Primary diagnoses and comorbid conditions for patients are summarized in Table 3.
TABLE 2.
Demographic characteristics and laboratory data1
| CIC (n = 22) | Sepsis (n = 22) | P | |
| Demographic characteristics | |||
| Age (y) | 21–81 (55)2 | 23–72 (51) | 0.43 |
| Sex (no. of F, M) | 11, 11 | 13, 9 | — |
| BMI (kg/m2) | 16.2–47.1 (31) | 10.6–48.7 (32.5) | 0.6 |
| Required a ventilator (n) | 7 | 8 | — |
| Required vasopressors (n) | 4 | 10 | — |
| Laboratory measures | |||
| Lactate (mmol/L) | 0.6–5.2 (2.5) | 0.6–8.9 (2.5) | 0.96 |
| PaO2/FiO2 | 48–558 (313) | 67–578 (230) | 0.22 |
| Platelets (K/μL) | 36–496 (226) | 40–399 (181) | 0.19 |
| Creatinine (mg/dL) | 0.51–6.44 (1.3) | 0.47–4.23 (1.9) | 0.16 |
| Total bilirubin (mg/dL) | 0.2–7.2 (1.5) | 0.5–27.5 (2.5) | 0.53 |
| WBC (K/μL) | 4.3–87.9 (13.8) | 1.8–22.1 (12.6) | 0.75 |
| SAPS II | 13–60 (33.7) | 11.0–54 (33.9) | 0.78 |
| SOFA score | 0–13 (3.3) | 0–16 (5.8) | 0.03 |
| Discharged home or to an acute rehabilitation facility (n) | 19 | 13 | — |
| Deceased or hospice (n) | 1 | 4 | — |
CIC, critically ill control; PaO2/FiO2, ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen; WBC, white blood cells; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; K/μL, thousand per cubic milliliter of blood.
Range; mean in parentheses (all such values).
TABLE 3.
Subject diagnoses1
| CIC (n = 22) | Sepsis (n = 22) | |
| Primary admitting diagnosis (n) | ||
| Respiratory distress | 3 | 4 |
| Overdose | 1 | 0 |
| Altered mental status or seizures | 4 | 1 |
| Gastrointestinal bleed | 6 | 3 |
| CVA/SAH | 3 | 1 |
| Sepsis or septic shock | 0 | 11 |
| Postoperative | 1 | 0 |
| Cellulitis | 1 | 0 |
| Pneumonia | 0 | 2 |
| Diabetic ketoacidosis | 1 | 0 |
| Other | 1 | 1 |
| Comorbid conditions2 (n) | ||
| COPD | 3 | 3 |
| Alcohol abuse | 2 | 1 |
| Diabetes | 5 | 6 |
| Pancreatitis | 2 | 0 |
| Coronary artery disease | 2 | 1 |
| Cirrhosis | 2 | 2 |
| Cancer | 4 | 2 |
| End-stage renal disease | 1 | 1 |
| Crohn disease | 1 | 0 |
| Other inflammatory | 1 | 5 |
CIC, critically ill control; CVA, cerebral vascular accident; SAH, subarachnoid hemorrhage; COPD, chronic obstructive pulmonary disease.
Can affect nutritional status.
Zinc and metallothionein analyses
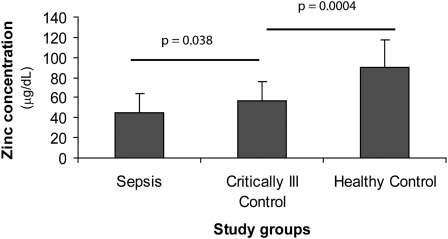
Twenty out of 22 septic patients and 16 out of 22 CIC patients had below normal plasma zinc concentrations recorded in the first 24 h of sepsis onset or admission to the MICU (normal range of zinc concentrations: 70–100 μg/dL) (18). Zinc concentrations of patients before admission to the intensive care unit were not obtainable. One of 12 healthy control subjects also had a low zinc concentration, but the concentration was within 1 SD of the normal concentration. A comparison between groups revealed significant lower plasma zinc concentrations in the CIC cohort (P = 0.0004) than in healthy control subjects, and plasma zinc concentrations were even lower in the septic group than in CIC subjects (P = 0.038) (Figure 1). A similar effect was observed between CIC and sepsis groups even after adjustment for age, sex, use of an insulin drip, or the use of propofol infusion (P = 0.03). Some propofol formulations contain edetate disodium, which is known to decrease zinc concentrations in patients (19).
FIGURE 1.
Evaluation of mean (±SD) plasma zinc concentrations in septic (n = 22) and critically ill ( n = 22) intensive care unit patients in the first 24 h of intensive care unit admission compared with mean plasma zinc concentrations in healthy, adult, nonhospitalized control subjects (n = 12) (plasma zinc concentrations: 45.4 ± 18.1, 57.2 ± 18.2, and 89.6 ± 27.4 μg/dL, respectively). Plasma zinc concentrations were determined by using atomic absorption spectrophotometry. Samples were run in triplicate. ANOVA followed by a 2-sample t test showed significant differences between the 3 groups. P values were adjusted for multiple comparisons by using Holm's method.
Plasma zinc concentrations were also evaluated in CIC and sepsis subjects in association with 5 additional variables known to potentially affect plasma zinc concentrations that included age, SAPS II, a diagnosis of diabetes mellitus, body mass index, and a concomitant propofol infusion. Results were suggestive of an association but did not achieve statistical significance (data not shown).
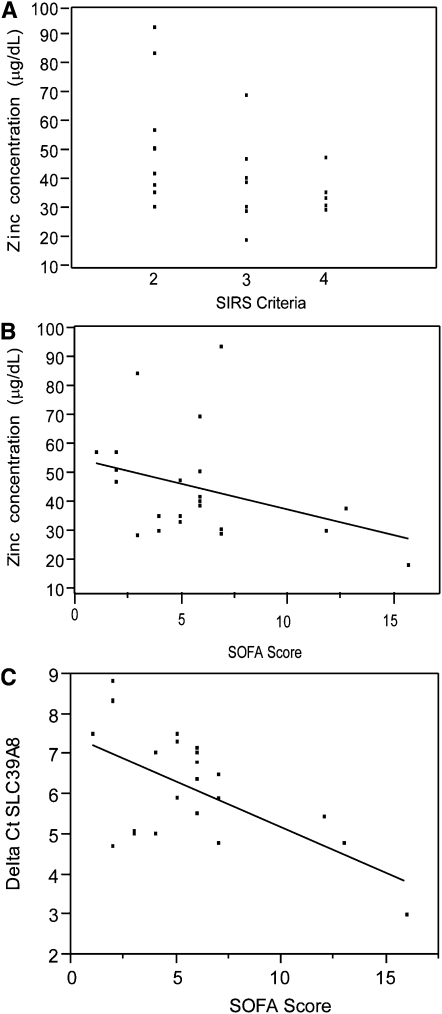
Additional analysis of the sepsis cohort revealed an inverse correlation between plasma zinc concentrations and sepsis severity as determined by systemic inflammatory response syndrome criteria (P = 0.02) and SOFA scores (P = 0.05) (Figure 2). These findings suggested that plasma zinc concentrations declined further with increasing sepsis severity and organ failure. Upon the observation of an inverse relation between plasma zinc concentrations and SOFA scores, we determined whether specific components of the SOFA score (vasopressor use, mean arterial pressure, acute lung injury ratio [ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FiO2)], platelet count, and serum creatinine) were more predictive of plasma zinc concentrations. Strikingly, plasma zinc concentrations were most closely associated with changes in cardiovascular variables. Plasma zinc concentrations were lower in subjects who required the use of a vasopressor medication to support their blood pressure during hypotension than the concentrations in subjects who did not require the use of a vasopressor medication to support their blood pressure during hypotension (P = 0.008). In addition, zinc concentrations were lower in subjects who required a higher dose of vasopressor medication as observed by the SOFA cardiovascular category [rank: 0 (no requirement) to 4 (most requirement)] (P = 0.039) (Table 4), whereas no significant correlation was observed with the other variables. Total RBCMT concentrations were also analyzed during the interim analysis of the first 37 patients enrolled in the study. No significant difference in RBCMT concentrations was shown between CIC and septic groups (P = 0.94) (data not shown).
FIGURE 2.
Relations between plasma zinc concentrations or zinc transporter expression compared with severity-of-illness scores in septic patients (n = 22). A: Relation between plasma zinc concentrations (mean ± SD: 45.4 ± 18.1 μg/dL) and systemic inflammatory response syndrome (SIRS) criteria (mean ± SD: 2.73 ± 0.88) evaluated by using Spearman's correlation (P < 0.02). B: Relation between plasma zinc concentrations and Sequential Organ Failure Assessment (SOFA) scores (5.77 ± 3.78) evaluated by Spearman's correlation (P = 0.05). C: Relation between SLC39A8 mRNA expression in blood monocytes [expressed as ΔCt (Delta Ct); mean ± SD: 6.1 ± 1.4] and SOFA scores evaluated by Spearman's correlation (P < 0.03). Lower ΔCt values equated to increased mRNA expression levels; thus, mRNA expression increased as the SOFA score increased. ΔCt = Ct (cycle threshold) target − Ct reference.
TABLE 4.
Relation between plasma zinc concentrations, vasopressor use, and severity of illness in septic patients (n = 22)1
| n | Plasma zinc concentration2 | P | |
| μg/dL | |||
| Required vasopressors | 0.0083 | ||
| No | 9 | 57.2 ± 20.2 (54.5) | — |
| Yes | 13 | 35.6 ± 8.03 (36) | — |
| SOFA cardiovascular category4 | 0.0395 | ||
| 0 | 9 | 58.9 ± 20.1 (57.3) | — |
| 1 | 3 | 38 ± 9.3 (36.2) | — |
| 2 | 0 | — | — |
| 3 | 6 | 37.5 ± 8.1 (35.5) | — |
| 4 | 4 | 32.6 ± 10.4 (34.3) | — |
SOFA, Sequential Organ Failure Assessment.
All values are means ± SDs; medians in parentheses.
Two-sample Wilcoxon rank sum test
0, no vasopressors; 1, mean arterial pressure <70 mm Hg; 2, dopamine ≤5 μg ⋅ kg−1 ⋅ min−1 or dobutamine (any dose); 3, dopamine >5 μg ⋅ kg−1 ⋅ min−1 or epinephrine/norepinephrine ≤0.1 μg/min; 4, dopamine >15 μg ⋅ kg−1 ⋅ min−1 or epinephrine/norepinephrine > 0.1 μg/min.
Kruskal-Wallis rank test.
Cytokine measurements
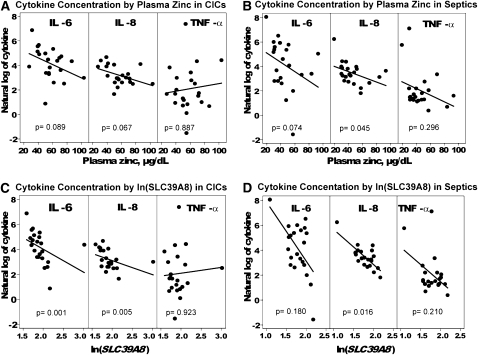
Cytokine concentrations for the 8 cytokines evaluated [interleukin (IL)-10, -6, -8, and -1β, interferon-γ, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and tumor necrosis factor-α] are presented in Table 5 for all 3 groups. All cytokine concentrations increased as plasma zinc concentrations decreased (P = 0.05) when all groups were evaluated collectively, but IL-6 and -8 concentrations had the strongest correlation to plasma zinc concentrations (P < 0.001 for each cytokine). We evaluated the relation between cytokine and plasma zinc concentration in the CIC or septic group. In the CIC group, there tended to be an inverse relation between IL-6 and -8 concentrations and plasma zinc concentration (Figure 3, A and B), but this relation was not significant. In the septic group, the trend was the same for IL-6, but IL-8 showed a significant inverse correlation (P = 0.045).
TABLE 5.
Cytokine and chemokine concentrations (in pg/mL) for each patient cohort1
| Cytokine/chemokine | Septic (n = 22) | CIC (n = 22) | Healthy (n = 12) |
| IL-10 | 80.4 ± 261.3 | 142.7 ± 594.6 | 10.0 ± 20.3 |
| IL-6 | 287.0 ± 665.9 | 131.8 ± 202.9 | 21.9 ± 30.8 |
| IL-8 | 55.8 ± 104.6 | 32.7 ± 27.0 | 33.2 ± 69.1 |
| Interferon-γ | 215.5 ± 622.7 | 61.0 ± 166.6 | 85.7 ± 138.6 |
| IL-1β | 10.1 ± 31.6 | 9.6 ± 33.1 | 4.1 ± 9.8 |
| MCP-1 | 214.8 ± 265.5 | 148.7 ± 125.8 | 154.8 ± 148.8 |
| MIP-1α | 141.2 ± 162.6 | 116.3 ± 48.0 | 130.3 ± 103.1 |
| TNF-α | 76.4 ± 267.1 | 88.3 ± 340.2 | 11.6 ± 19.1 |
All values are means ± SDs. CIC, critically ill control; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; TNF-α, tumor necrosis factor-α. No significant differences were shown between cohorts for each individual cytokine by ANOVA.
FIGURE 3.
Correlations between plasma cytokine and zinc concentrations in critically ill control (CIC) (A) or septic (B) patients and between plasma cytokine concentrations and SLC39A8 mRNA expression in CIC (C) and septic (D) patients (n = 22 septic and 22 CIC patients). ΔCt and cytokine values were log transformed to obtain a normal distribution and analyzed by Spearman's rank correlation. Lower ΔCt values equated to increased mRNA expression levels; thus, mRNA expression increased as cytokine concentrations increased. P value adjustment was made by using the Holm's procedure for multiple comparisons. ΔCt = Ct (cycle threshold) target − Ct reference.
Zinc transporters
The total amount of RNA obtained from peripheral blood monocytes was initially screened to quantitatively determine the expression of each of the 24 known human zinc transporters for each study subject. We observed that SLC39A8 was the only zinc transporter with a higher expression in CIC and septic patients than in healthy control subjects (Table 6). In addition, we observed that SLC39A8 expression was consistently higher in the sepsis cohort than in CIC subjects. We determined whether there was a correlation between plasma zinc concentrations and the expression of SLC39A8 and 3 other related zinc transporters (SLC39A1, SLC39A4, SLC39A14). Only 2 transporters had significant correlations between gene expression levels and plasma zinc concentrations when the combined groups, including healthy control subjects, were evaluated. Strikingly, there was a strong inverse correlation between a low plasma zinc concentration and elevated SLC39A8 expression (P < 0.01) (Table 7). In contrast, the opposite effect was observed between the plasma zinc concentration and expression of SLC39A14, which is the human zinc transporter that is most closely related to SLC39A8 (P = 0.02). However, compared with the expression of SLC39A8, the expression of SLC39A14 was substantially lower in monocytes across all study subjects, which suggested that SLC39A14 had a negligible influence on the zinc metabolism within this cell type.
TABLE 6.
RNA expression by real-time polymerase chain reaction for each of the 4 zinc transporters analyzed in septic (n = 22), critically ill control (CIC) (n = 22), and healthy (n = 12) subjects1
| Transporter | Septic ΔCt | Septic RCN | CIC ΔCt | CIC RCN | Healthy ΔCt | Healthy RCN |
| SLC39A1 | 8.2 ± 3.72 | 0.76 | 6.9 ± 0.9 | 0.95 | 7.0 ± 0.9 | 0.90 |
| SLC39A4 | 20.3 ± 2.2 | 0.00 | 20.4 ± 2.0 | 0.00 | 20.3 ± 1.9 | 0.00 |
| SLC39A8 | 6.1 ± 1.4 | 2.23 | 7.4 ± 3.1 | 1.22 | 8.1 ± 1.3 | 0.61 |
| SLC39A14 | 12.8 ± 1.5 | 0.02 | 12.6 ± 2.6 | 0.03 | 11.8 ± 0.4 | 0.03 |
ΔCt, Ct (cycle threshold) target − Ct reference; RCN, relative copy number.
Mean ± SD (all such values).
TABLE 7.
Correlation between plasma zinc concentration and the 4 importers of interest for each patient group1
| All groups |
Septic (n = 22) |
CIC (n = 22) |
Healthy (n = 12) |
|||||
| Transporters | ρ2 | P | ρ2 | P | ρ2 | P | ρ2 | P |
| SLC39A1 | < −0.01 | >0.99 | 0.09 | 0.68 | 0.10 | 0.66 | <0.01 | 0.99 |
| SLC39A4 | −0.14 | 0.29 | −0.24 | 0.27 | 0.06 | 0.79 | −0.82 | <0.01 |
| SLC39A8 | 0.45 | <0.01 | 0.29 | 0.20 | 0.43 | 0.05 | −0.04 | 0.90 |
| SLC39A14 | −0.32 | 0.02 | −0.13 | 0.58 | −0.22 | 0.33 | 0.16 | 0.62 |
CIC, critically ill control.
Plasma zinc values were compared with ΔCt [Ct (cycle threshold) target − Ct reference] values by using Spearman's correlation: negative ρ (indirect) and positive ρ (direct).
On the basis of the observation that SLC39A8 expression was increased in conjunction with a declining plasma zinc concentration, we evaluated SLC39A8 expression in the context of severity-of-illness scores in sepsis and CIC patients. The relation between SLC39A8 expression and SAPS II was not significant for the sepsis or CIC cohorts (P = 0.674 and P = 0.613, respectively). However, an increased SLC39A8 expression was directly correlated with SOFA scores in the sepsis cohort and achieved statistical significance (P = 0.027) (Figure 2C). An additional evaluation was conducted to analyze the association, if any, between SLC39A8 expression and SOFA variables [vasopressor use, mean arterial pressure, acute lung injury ratio (PaO2/FiO2), platelet count, or creatinine] (Table 8). In the septic cohort, a direct correlation was shown between SLC39A8 expression and the platelet count (P < 0.01). Finally, SLC39A8 transcript levels were evaluated in relation to IL-6 and -8 and tumor necrosis factor-α plasma concentrations and compared between CIC and sepsis cohorts. IL-6 and -8 concentrations were selected because they had been previously identified as the only factors that achieved statistical significance in relation to declining plasma zinc concentrations. In both groups, transcript levels were shown to have been directly correlated with IL-8 plasma concentrations (Figure 3, C and D).
TABLE 8.
Association between SLC39A8 expression and 5 sequential organ failure assessment categories in the septic cohort (n =22)1
| P | Spearman's rank correlation | |
| Vasopressors2 | 0.6 | NA |
| Mean arterial pressure3 | 0.49 | NA |
| PaO2/FiO24 | 0.67 | −0.12 |
| Platelets5 | <0.01 | 0.60 |
| Creatinine6 | 0.85 | −0.04 |
NA, not applicable; PaO2/FiO2, ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen.
Wilcoxon rank sum test.
Kruskal-Wallis rank test.
Mean ± SD: 230 ± 10.
Mean ± SD: 181 ± 89 K (thousand)/μL.
Mean ± SD: 1.9 ± 1.9 mg/dL.
DISCUSSION
The decrease in plasma zinc concentrations observed in the study was consistent with observations in previous reports and likely occurred independent of body loss, which supported the hypothesis that zinc is mobilized from the vasculature into vital organs to support zinc-dependent metabolic functions (20, 21). This hypothesis was further supported by the observed increase in the expression of SLC39A8, which is a zinc importer, in peripheral monocytes. Unique to this study, we observed that plasma zinc concentrations declined more in the sepsis cohort than in the CIC group, despite similar severity-of-illness scores. This dichotomy between groups persisted even when variables that could have potentially affected zinc concentrations were accounted for, including age, propofol use, insulin use, and body mass index (22–25). The post hoc analysis revealed that these factors did not significantly alter plasma zinc concentrations, which indicated that the changes observed represented authentic changes in zinc metabolism as a consequence of critical illness. All things considered, these findings showed that perturbations in zinc metabolism were enhanced in the setting of severe infection. In addition, zinc concentrations predictably declined with increased severity-of-illness scores, which was consistent with a study that was recently conducted in a critically ill pediatric cohort (26). Cvijanovic et al (26) and Wong et al (27) reported that the extent of the decline in plasma zinc concentrations was associated with increased organ failure and mortality. Whether zinc redistribution is a beneficial compensatory mechanism or an indication of declining function and worse outcomes remains to be determined.
Plasma zinc concentrations significantly decreased with an increasing severity of illness in the sepsis cohort on the basis of both SOFA scores and systemic inflammatory response syndrome criteria. Cardiovascular variables (vasopressor use and mean arterial pressure) had the most significant effect on the association between low plasma zinc concentrations and increased SOFA scores. To our knowledge, this was a novel finding in the setting of human sepsis. Because circulatory collapse that leads to shock is one of the most severe manifestations of sepsis, it deserves further exploration. In support of this observation, animal studies have shown that zinc deficiency is associated with a lower systolic arterial blood pressure and the reduced activities of serum angiotensin-converting enzyme (28, 29) and carbonic anhydrase (29). In addition, zinc has been shown to activate the endothelin-converting enzyme that is an enzyme that is responsible for endothelin-1 synthesis, which is a central vasoconstricting factor produced by the endothelium (29). Taken together, we predicted that a dysregulation in zinc metabolism (eg, lower plasma zinc concentrations) is associated with the severity of illness and may contribute to cardiovascular instability in the setting of severe infection.
Zinc transporters comprised by SLC30 (also known as ZnT) and SLC39 (also known as Zip) family members exhibit tissue-specific expression and respond differently to physiologic stimuli including cytokines and hormones (30, 31). Our group recently reported that SLC39A8 is unique relative to other SLC39 family members in that it is induced at the onset of inflammatory stress and is required for zinc-dependent cytoprotection (13). This is consistent with animal studies that demonstrated the induction of SLC39A14, which is a mouse ortholog to human SLC39A8, during the acute phase response that led to the mobilization of zinc from the vasculature into the liver (32). We observed that mRNA expression of most zinc transporters, if expressed at all, was essentially unaltered in monocytes during the early stages of critical illness or sepsis. In sharp contrast, SLC39A8 expression was increased in critically ill and septic subjects and particularly in those with lower plasma zinc concentrations. The increase in SLC39A8 expression correlated with an increasing disease severity (SOFA score) irrespective of the infecting organism. We believe that the alteration of SLC39A8 gene expression in all critically ill subjects was remarkable and warrants further investigation to understand whether this transporter is intricately linked to immune function and serves as a marker for disease severity. A significant correlation between increased SLC39A8 expression and IL-6 and -8 concentrations was observed in CIC subjects, whereas only IL-8 achieved significance within the sepsis cohort, despite the fact that sepsis subjects had lower plasma zinc concentrations, higher cytokine and chemokine concentrations, and higher SLC39A8 expression. Taken together, these findings suggested that critically ill sepsis adults who were systemically infected were prone to larger variations in zinc metabolism and immune activation.
Zinc is vital to many cellular functions, including protein synthesis, signal transduction, and gene transcription (33) and is necessary to maintain proper immune function (1, 5). In this investigation, we showed that the activation of the innate immune response, as determined by elevated cytokine and chemokine production, inversely correlated with a low plasma zinc concentration and was most significant in the septic cohort. This result was consistent with results of previous animal studies that demonstrated that zinc deficiency resulted in an exaggerated inflammatory response after endotoxin administration or cecal ligation and puncture, which is a method used to induce polymicrobial sepsis. In particular, our group recently reported that nutritional zinc depletion resulted in an increase in the activation of the innate immune response and occurred in conjunction with a significant increase in vital organ injury and mortality in a murine model (12, 34). In addition, zinc deficiency was associated with increased oxidative stress in elderly subjects, and zinc supplementation decreased nuclear transcription factor κB activity (34–36). These observations were supported in this investigation by the demonstration that lower plasma zinc concentrations were associated with higher illness scores and increased cytokine and chemokine production. Because of this information, zinc supplementation may play an important role as an antiinflammatory agent with the ability to modulate the proinflammatory response and result in less organ dysfunction and death; however, the determination of the optimal dose of zinc supplementation in septic patients will be crucial to prevent innate immune suppression and risk of secondary infection.
The evaluation of plasma zinc status, immune activation, and zinc transporter expression was conducted in the first 24 h of critical illness before initiation of nutrition supplementation and before subjects could achieve a fasting state. An accurate determination of zinc nutritional status in study subjects before the onset of critical illness was not possible. Therefore, a major limitation of our findings is that we were not able to determine whether decreased plasma zinc concentrations were a consequence of a nutritional deficiency before illness, which possibly contributed to illness, because of changes in metabolism in response to a critical illness and infection or both. This limitation underscores the need for a reliable biomarker that can be used to determine the zinc metabolic status, particularly in vulnerable populations including the elderly and individuals with chronic disease. The evaluation of a causal relation between zinc concentrations and survival was not possible because of the low mortality encountered in our study population.
The development of accurate methods to determine the zinc nutritional status in the setting of comorbidity has largely been unsuccessful. Past strategies have included biomarkers that were unreliable in the inflammatory setting (eg, metallothionein) or analytic measures that were cumbersome in a critical care setting (eg, nutrition monitoring with excreted zinc measurements). In our experience, the analysis of the plasma zinc content was predictive of the severity of illness. Whether, this type of analysis was reliable in terms of determining the patient outcome, and possibly the need for zinc supplementation, in the setting of sepsis remains to be determined.
To our knowledge, this study is the first to compare zinc metabolism in conjunction with innate immune activation and zinc transporter expression in critically ill adults with and without sepsis within the early stages of intensive care unit admission. Findings from this study, although they were based on a relatively small sample size, provided evidence in support of the hypothesis that zinc is mobilized into the intracellular compartment during the early stages of sepsis and showed, for the first time to our knowledge, that larger changes in zinc metabolism correlated with an increased severity of illness in humans as determined by objective physiologic variables, particularly cardiovascular dysfunction, and the activation of the innate immune response. In addition, to our knowledge, this is the first human study to identify SLC39A8 as a unique zinc transporter in the setting of critical illness. SLC39A8 has the potential to be a determinant of disease severity or a biomarker for zinc nutritional status and warrants further investigation.
Supplementary Material
Acknowledgments
We thank Elizabeth Joseph for technical assistance.
The authors’ responsibilities were as follows—BYB and MCE: designed the research; BYB, MCE, JH, and RAD: conducted the research; MDW and DLK: provided essential reagents; BYB and GP: analyzed data; BYB and DLK: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr 2004;24:277–98 [DOI] [PubMed] [Google Scholar]
- 2.Prasad AS. Clinical and biochemical manifestations of zinc deficiency in human subjects. J Am Coll Nutr 1985;4:65–72 [DOI] [PubMed] [Google Scholar]
- 3.Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition 1993;9:218–24 [PubMed] [Google Scholar]
- 4.Prasad AS. Zinc and immunity. Mol Cell Biochem 1998;188:63–9 [PubMed] [Google Scholar]
- 5.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68:447S–63S [DOI] [PubMed] [Google Scholar]
- 6.Prasad AS. Zinc: mechanisms of host defense. J Nutr 2007;137:1345–9 [DOI] [PubMed] [Google Scholar]
- 7.Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 2007;85:837–44 [DOI] [PubMed] [Google Scholar]
- 8.Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 2003;133:1485S–9S [DOI] [PubMed] [Google Scholar]
- 9.Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr 1985;5:341–63 [DOI] [PubMed] [Google Scholar]
- 10.Hambidge M. Human zinc deficiency. J Nutr 2000;130:1344S–9S [DOI] [PubMed] [Google Scholar]
- 11.Braunschweig CL, Sowers M, Kovacevich DS, Hill GM, August DA. Parenteral zinc supplementation in adult humans during the acute phase response increases the febrile response. J Nutr 1997;127:70–4 [DOI] [PubMed] [Google Scholar]
- 12.Knoell DL, Julian MW, Bao S, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 2009;37:1380–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 2008;294:L1127–36 [DOI] [PubMed] [Google Scholar]
- 14.Srinivas U, Braconier JH, Jeppsson B, Abdulla M, Akesson B, Ockerman PA. Trace element alterations in infectious diseases. Scand J Clin Lab Invest 1988;48:495–500 [DOI] [PubMed] [Google Scholar]
- 15.Shanbhogue LK, Paterson N. Effect of sepsis and surgery on trace minerals. JPEN J Parenter Enteral Nutr 1990;14:287–9 [DOI] [PubMed] [Google Scholar]
- 16.Gavrilin MA, Bouakl IJ, Knatz NL, et al. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci USA 2006;103:141–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuhammer AM, Cherian MG. Quantification of metallothioneins by a silver-saturation method. Toxicol Appl Pharmacol 1986;82:417–25 [DOI] [PubMed] [Google Scholar]
- 18.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr 2003;78:756–64 [DOI] [PubMed] [Google Scholar]
- 19.Armenti JIBaVT Handbook of drug-nutrient interaction. 2nd ed. Totowa, NJ: Humana Press, 2010 [Google Scholar]
- 20.Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci 1982;389:39–48 [DOI] [PubMed] [Google Scholar]
- 21.Brown KH. Effect of infections on plasma zinc concentration and implications for zinc status assessment in low-income countries. Am J Clin Nutr 1998;68:425S–9S [DOI] [PubMed] [Google Scholar]
- 22.Miyata S. [Zinc deficiency in the elderly.] Nippon Ronen Igakkai Zasshi 2007;44:677–89 (in Japanese) [PubMed] [Google Scholar]
- 23.Niskanen LK, Salonen JT, Nyyssonen K, Uusitupa MI. Plasma lipid peroxidation and hyperglycaemia: a connection through hyperinsulinaemia? Diabet Med 1995;12:802–8 [DOI] [PubMed] [Google Scholar]
- 24.Chen MD, Lin PY, Lin WH, Cheng V. Zinc in hair and serum of obese individuals in Taiwan. Am J Clin Nutr 1988;48:1307–9 [DOI] [PubMed] [Google Scholar]
- 25.Kang TM. Propofol infusion syndrome in critically ill patients. Ann Pharmacother 2002;36:1453–6 [DOI] [PubMed] [Google Scholar]
- 26.Cvijanovich NZ, King JC, Flori HR, Gildengorin G, Wong HR. Zinc homeostasis in pediatric critical illness. Pediatr Crit Care Med 2009;10:29–34 [DOI] [PubMed] [Google Scholar]
- 27.Wong HR, Shanley TP, Sakthivel B, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 2007;30:146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahlheim H, White CL, Rothemund J, von Lutterotti N, Jacob IC, Rosenthal J. Effect of zinc depletion on angiotensin I-converting enzyme in arterial walls and plasma of the rat. Miner Electrolyte Metab 1989;15:125–9 [PubMed] [Google Scholar]
- 29.Tubek S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol Trace Elem Res 2007;117:39–51 [DOI] [PubMed] [Google Scholar]
- 30.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr 2004;24:151–72 [DOI] [PubMed] [Google Scholar]
- 31.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci 2004;61:49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 2005;102:6843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol 2008;97:149–76 [DOI] [PubMed] [Google Scholar]
- 34.Bao S, Liu MJ, Lee B, et al. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2010;298:L744–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao B, Prasad AS, Beck FW, et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr 2010;91:1634–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med 2004;37:1182–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.