Abstract
We report pure-tone hearing threshold findings in 56 college students. All subjects reported normal hearing during telephone interviews, yet not all subjects had normal sensitivity as defined by well-accepted criteria. At one or more test frequencies (0.25–8 kHz), 7% of ears had thresholds ≥25 dB HL and 12% had thresholds ≥20 dB HL. The proportion of ears with abnormal findings decreased when three-frequency pure-tone-averages were used. Low-frequency PTA hearing loss was detected in 2.7% of ears and high-frequency PTA hearing loss was detected in 7.1% of ears; however, there was little evidence for “notched” audiograms. There was a statistically reliable relationship in which personal music player use was correlated with decreased hearing status in male subjects. Routine screening and education regarding hearing loss risk factors are critical as college students do not always self-identify early changes in hearing. Large-scale systematic investigations of college students’ hearing status appear to be warranted; the current sample size was not adequate to precisely measure potential contributions of different sound sources to the elevated thresholds measured in some subjects.
Keywords: college student population, hearing loss, high frequency, noise
Introduction
Estimating the prevalence of hearing loss among children and adolescents has been a longstanding goal given the need to allocate resources appropriately to support hearing-impaired school-based populations (for reviews, see Lundeen, 1991; Holmes et al., 2004; Meinke & Dice, 2007; Ross et al., 2010). Normative data from pediatric and older adult populations are readily available (for recent review, see Pascolini & Smith, 2009). A smaller number of studies focus on the 18–25 year old population; however, there is increasing evidence indicating that median thresholds within this population may be closer to +5 dB HL rather than the expected 0 dB HL norms (for review, see Borchgrevink, 2003). Outcomes such as this have prompted questions as to whether the prevalence of hearing loss in adolescents is increasing, and if so, to what extent can this exposure be attributed to acoustic insult (for examples, see Niskar et al., 1998; 2001; Shargorodsky et al., 2010). Historical data are described first, followed by discussion of recent data and the current data set. The most often cited epidemiological studies, in chronological order, include the Pittsburgh Study (1958–1960), the Health Examination Survey (1963–1965), the National Speech and Hearing Survey (1968–1969), and, the National Academy of Sciences/Institute of Medicine study in D.C. area children (1971). The most recent data come from the Third National Health and Nutrition Examination Survey (NHANES III; 1988–1994) and NHANES 2005–2006.
In “the Pittsburgh Study,” the initially reported prevalence of hearing loss was less than 2% in a sample of 4,078 Pittsburgh children ages 5 to 14 years old, tested at 0.25-, 0.5-, 1-, 2-, 4-, 6- and 8-kHz (Eagles et al., 1963). However, after applying the conventional conversion across standards (from American Standards Association, 1951; to International Organization for Standardization, 1964; and American National Standards Institute, 1969), the prevalence of significant hearing impairment in one or both ears was estimated at approximately 5% (Silverman & Lane, 1970; see Lundeen, 1991 for additional discussion). Outcomes were generally similar in the Health Examination Survey. In this study, a nationally representative sample of 6,768 children, 6–11 years of age, were tested at 0.25-, 0.5-, 1-, 2-, 3-, 4-, 6- and 8-kHz (Roberts & Ahuja, 1975). The prevalence of hearing loss, using a criterion of 15 dB or more above audiometric zero (ASA-1951) within the critical speech range of 0.5–2 kHz, was estimated to be less than 1.5%, although self-report and parental report of hearing problems was 5% or higher (Roberts & Ahuja, 1975; for review, see Leske, 1981).
The National Speech and Hearing Survey is the largest study to date, and estimates of the prevalence of hearing loss in children were well within the 2–5% range described in the earlier studies. In this study, a nationally representative sample of 38,568 students in grades 1 through 12 were tested at 0.5-, 1-, 2-, 3- and 4-kHz (Hull et al., 1971). Within individual frequencies, thresholds >25 dB HL were observed at a rate of approximately 2% within 0.5-, 1-, and 2-kHz frequencies, and at a rate of 3–4% within 3- and 4-kHz frequencies. At 4-kHz, thresholds >25 dB were detected at a rate that was almost twice as great in boys as in girls (Hull et al., 1971). When a pure-tone-average (PTA) threshold >25 dB HL for frequencies of 0.5-, 1-, and 2-kHz was used as a criterion, 2.63% of the subjects did not meet the hearing criterion (Lundeen, 1991).
More recent studies have suggested a somewhat higher prevalence of hearing loss in children, although the referral criterion used in those studies was more conservative and higher referral rates would thus be expected. One such study was the Institute of Medicine study. In this study, 1,639 children, 4–11 years of age, in the Washington D.C. area were screened at 0.25-, 0.5-, 1-, 2-, 4-, and 8-kHz. Results showed 6.7% of the subjects had hearing loss in one or both ears using a threshold criterion of 15 dB HL or greater from 0.5 to 2-kHz (4.5% unilateral and 2.2% bilateral; Kessner et al., 1974; see also Chapter 1 in Northern & Downs, 1984). A second study with a higher reported prevalence for hearing loss in adolescents was the Third National Health and Nutrition Examination Survey (NHANES III). In NHANES III, a sample of 6,166 children ages 6 to 19 yrs old were tested from 1988–1994, and 7.1% were reported to have hearing loss in one or both ears using a criterion of low-frequency pure-tone-average (LFPTA) threshold ≥ 16 dB HL at 0.5-, 1-, and 2-kHz (Niskar et al., 1998). That number increased to 12.7% using a criterion of high-frequency pure-tone-average (HFPTA) threshold ≥ 16 dB HL at 3-, 4-, and 6-kHz, and 14.9% when either LFPTA or HFPTAs were considered (Niskar et al., 1998). When the NHANES III data were recently compared to the NHANES 2005–2006 data (collected from 1,771 participants aged 12–19), a 31% increase in prevalence of any hearing loss (defined as unilateral or bilateral LFPTA or HFPTA >15 dB) was reported. Specifically, there was an increase from 14.9% with measured hearing loss ≥ 16 dB in NHANES III to 19.5% with measured hearing loss > 15 dB in NHANES 2005–2006 (Shargorodsky et al., 2010). The NHANES is now an ongoing cross-sectional health survey with ~5,000 individuals randomly selected for participation each year.
In this report, we present data on the prevalence of hearing loss in a population of 56 college students who had self-reported “normal hearing” during initial telephone screenings. The data were collected in the course of screening individuals for potential participation in a study that required thresholds ≤ 25 dB HL at 0.25-, 0.5-, 1-, 2-, 3-, 4-, 6- and 8-kHz. We anticipated that all or most subjects would pass the hearing screening given the self-report of normal hearing, and we were surprised to find that a significant subset of the subjects did not meet the screening criteria. The prevalence of hearing loss in this self-reported “normal hearing population” is described here, along with descriptive associations between hearing thresholds and self-reported hearing history
Methods
Subjects
All protocols and procedures were approved by Investigational Review Boards at the University of Florida (IRB-01) and the University of Michigan (IRBMED), and all data were collected under the supervision of an NIH-selected Data Safety Monitoring Board. Participants included 57 college students (20 male, 37 female) who responded to advertisements posted at multiple locations on the University of Florida campus (mean age = 21.1 years; S.D. = 3.1; range = 18–31 years old); students were not required to be enrolled in a specific Department or program and were not systematically polled on their specific degree program. Advertisements invited normally hearing subjects to participate in a study of temporary changes in hearing after use of a personal music player (PMP). Participants passed the initial telephone screening by self-reporting normal hearing sensitivity, and were invited to schedule appointments for further information. After providing informed consent, all participants completed questionnaires regarding their health history, and their individual exposure to various sound sources.
Questionnaires
The Hearing Survey used for screening the current subjects (Appendix A) was modeled after an unpublished hearing survey used at the Karolinska Institutet. Three questions were used to establish pigmentation (eye color, hair color, effects of sun exposure). The Tinnitus Survey used for screening the current subjects (Appendix B) was modeled after the Tinnitus Ototoxicity Monitoring Interview (TOMI) developed by Fausti et al. (2007).
Facilities
All data collection was completed at Shands Hospital at the University of Florida, in the Speech and Hearing Clinic. All audiometric tests were conducted in a double-walled sound-treated test booth meeting American National Standards Institute ANSI/ASA S3.1-1999 (R2008) specifications.
Otoscopy
Visual examination of the ear canal and tympanic membrane was conducted to ensure normal anatomy and no presence of debris. One subject did not pass the otoscopic examination, and was excluded from all subsequent tests.
Tympanometry
Tympanometric measures were collected using a GSI 38 immittance measurement device that was in compliance with ANSI S3.39 and IEC 601-1 criteria. Normal middle ear function was defined by Type A 226 Hz tympanograms bilaterally with a pressure peak within the region of −140 to +40 daPa based on the 90% range for adults (see Margolis & Hunter, 2000) All of the subjects had normal middle ear function.
Pure-Tone Audiometry
All pure-tone threshold measurements were conducted using a GSI 61 diagnostic audiometer with 3A insert earphones in a double-walled sound-treated test booth meeting ANSI/ASA S3.1-1999 (R2008) specifications for audiometric test rooms. The GSI 61 clinical audiometer was professionally calibrated annually according to ANSI 3.6 1996, and checked biologically each day. Pure-tone air conduction thresholds were obtained using a modified Hughson-Westlake procedure for test frequencies of 0.25-, 0.5-, 1-, 2-, 3-, 4-, 6-, and 8-kHz (Hughson & Westlake, 1944). Initial descent towards threshold was accomplished in 10-dB steps. Beginning with the first non-response, levels were increased by 2 dB for each non-response, and decreased by 4 dB after each correct detection response. Using a 2-dB step size instead of the 5-dB step size more commonly used in the clinic has two main effects: 1) thresholds measured using a 2-dB step size are 1–2 dB lower (better) than those measured using 5-dB steps, and 2) the standard deviation for test-retest differences is decreased when a 2-dB step is used (i.e., reliability is improved) (Jerlvall & Arlinger, 1986; Marshall & Jesteadt, 1986; Marshall et al., 1996). Threshold was defined as the lowest level at which two responses were obtained out of three presentations on an ascending run. Responses were evaluated for reliability by using repeat testing at 2- and 8-kHz in each ear. Responses were considered reliable if the absolute difference between test and retest thresholds was ≤ 5 dB, a criterion previously used by Fausti et al. (1999). As noted by Campbell et al. (2003), this criterion is more stringent than frequently recommended; however, it assures good subject reliability.
If air-conduction thresholds were >25 dB HL, subjects were not eligible to participate in the later PMP study and no additional testing was conducted. If air-conduction thresholds were ≥ 15 dB HL and ≤ 25 dB HL, then bone-conduction pure-tone audiometry was conducted for test frequencies of 0.25, 0.5, 1, 2, 3, and 4 kHz. If air-bone gaps were ≤10 dB, and right-left thresholds were symmetric within 15 dB, subjects were allowed to enroll in the PMP study. Normal otoscopy and tympanometry findings provide additional evidence of normal middle ear status for these subjects.
We describe pure-tone air conduction thresholds and pass/refer rates based on conventional criterion including 10, 15, 20, or 25 dB HL. The 20 and 25 dB HL levels were chosen as other studies have used 20 dB HL (Sarafraz & Ahmadi, 2009) or 25 dB HL (Holmes et al., 1996; Holmes et al., 1997; Shah et al., 2009) criterion. In addition, the American Academy of Audiology (AAA) and ASHA have established a 20 dB HL level at 1-, 2-, and 4-kHz as the standard screening criterion. The 15 dB HL level was chosen as the NHANES III studies defined hearing loss as thresholds of 16 dB HL or greater. Finally, we selected the 10 dB HL criterion because this level has been suggested to provide an early warning for subtle hearing loss (Couzad & Rousey, 1966). Unless otherwise specified, referral data are based on number of ears that failed to meet the hearing criterion. Monaural and binaural failure rates are distinguished only when the percent of subjects failing in one or both ears is specifically stated.
Evidence of Notched Audiogram Configuration
Hearing loss is typically attributed to noise exposure if the configuration of the patient’s audiogram is “notched.” We therefore assessed the potential for audiometric notches in the current subjects’ audiograms using the definitions of Niskar et al. (2001) and Coles et al. (2000). Niskar et al. (2001) defined a noise notch as 1) thresholds ≤ 15 dB HL at 0.5- and 1.0-kHz, 2) 3-, 4-, or 6-kHz threshold at least 15 dB worse than thresholds at 0.5- and 1-kHz, and 3) 3-, 4-, or 6-kHz threshold at least 10 dB worse than 8-kHz threshold. Coles et al. (2000) defined a noise notch as a hearing threshold at 3-, 4-, or 6-kHz that is at least 10 dB greater than 1- or 2- and 8-kHz. The 10-dB notch depth used by Coles et al. (2000) is less restrictive than the 15-dB notch depth used by Niskar et al. (2001). Other audiometric notch definitions are described in the literature, and it is clear that the definition of a notch affects the measured prevalence of audiometric notches (Nondahl et al., 2009). Importantly, not all individuals identified as having an audiometric notch report a positive history of noise exposure, and not all individuals reporting a positive history of noise have an audiometric notch (Hong, 2005; Nondahl et al., 2009; Osei-Lah & Yeoh, 2010). Although there is not a precise relationship between presence of an audiometric notch and positive history of noise exposure (see Nondahl et al., 2009), the “notched” audiogram in combination with the noise history is the most-used clinical metric for assessing potential noise-induced hearing loss (NIHL).
Statistical Analyses
Tabular and graphical presentations of hearing thresholds are presented as a function of audiometric test frequency, gender, ear (left vs. right), reported PMP use (user vs. nonuser), and impulse noise exposure (exposed vs. not exposed). Inferential analyses concerning differences associated with the independent variables listed were obtained using repeated measures analyses of variance (ANOVA). Specifically, tests of main effects from these analyses and post hoc comparisons of least squares means are presented to establish the statistical significance of differences which are apparent in the tables and graphs. Chi-square and Fisher exact tests were used to test for male/female differences in various risk factors associated with ear related medical history reported by respondents (Table 1) and hearing loss (Table 2). All analyses were carried out using PROC MIXED and PROC FREQ in version 9.1 of SAS. In our data tables, confidence intervals accompany the point estimates of percentages. These intervals convey information concerning the variability of our estimates taking into account our sample sizes. These confidence intervals were computed using the normal approximation to the binomial distributions. For percentages near zero, the lower bound of the intervals can be less than zero. In these cases we report the lower bound as zero.
Table 1.
Percentages (with Confidence Intervals) of Self-Reported Hearing Issues
| Total Percent Reporting Mean (CI) | Male N=20 Mean (CI) | Female N=36 Mean (CI) | p-value (male vs. female) | |
|---|---|---|---|---|
| Percent Reporting Parents/Siblings with Hearing Loss | 12.5 (3.8,21.2) | 10.0 (0,23.1) | 13.9 (2.6,25.2) | 0.67 |
| Percent Reporting Previous Ear Infection | 55.4 (42.4,68.4) | 50.0 (28.1,71.9) | 58.3 (42.2,74.4) | 0.55 |
| Percent Reporting Previous Ear Disease | 0.0 | 0.0 | 0.0 | N/A |
| Percent Reporting Previous Hearing Loss | 5.4 (0,11.3) | 5.0 (0,14.5) | 5.6 (0,13.1) | 1.0 |
| Percent Reporting Hypersensitivity to Loud Sound | 7.1 (0.4,13.8) | 10.0 (0,23.1) | 5.6 (0,13.1) | 0.61 |
| Percent Reporting Ever Having Tinnitus | 50.0 (37.9,63.1) | 40.0 (18.5,61.5) | 55.6 (39.4,71.8) | 0.40 |
| Percent Reporting Previous Tinnitus | 0.77 | |||
| Always (percent of those ever having tinnitus) | 0.0 | 0.0 | 0.0 | |
| Often (percent of those ever having tinnitus) | 3.6 (0,8.5) | 0.0 | 5.0 (0,12.1) | |
| Occasionally (percent of those ever having tinnitus) | 46.4 (33.3,59.5) | 37.5 (16.3,58.7) | 50.0 (33.6,66.3) | |
| Rarely (percent of those ever having tinnitus) | 50.0 (36.9,63.1) | 62.5 (41.3,83.7) | 45.0 (28.7,61.3) | |
| Percent Reporting Current Tinnitus | 0.0 | 0.0 | 0.0 | N/A |
Note: P-values are derived from Fisher exact tests for the 2 × 2 tables. The p-value for the single 2 × 5 table is derived from an exact version of the Pearson Chi-square test as implemented by SAS. The normal approximation was used for the confidence intervals. When the lower bound was below zero, a value of 0 was used.
Table 2.
Percentages (with Confidence Intervals) of Self-Reported Exposure to Loud Sound.
| Total Percent Reporting Mean (CI) | Male N=20 Mean (CI) | Female N=36 Mean (CI) | p-value (male vs. female) | |
|---|---|---|---|---|
| Most Recent Exposure to Loud Sound | 0.49 | |||
| Past 24 hours | 8.9 (1.4,16.4) | 5.0 (0,14.5) | 11.1 (0.8,21.4) | |
| Past 48 hours | 5.4 (0,11.3) | 0.0 | 8.3 (0,17.3) | |
| Past week | 46.4 (33.3,59.5) | 50.0 (28.1,71.9) | 44.4 (28.2,60.6) | |
| Past month | 21.4 (10.7,32.1) | 30.0 (9.9,50.1) | 16.7 (4.5,28.9) | |
| Past year | 17.9 (7.9,27.9) | 15.0 (0,30.6) | 19.4 (6.5,32.3) | |
| Percent Reporting Exposure to Loud Sound | 39.3 (26.5,52.1) | 45.0 (23.2,66.8) | 36.1 (20.4,51.8) | 0.51 |
| Bars, Clubs | 30.4 (18.3,42.4) | 25.0 (6.0,44.0) | 33.3 (17.9,48.7) | 0.56 |
| Hunting, Shooting | 1.8 (0,5.3) | 5.0 (0,14.6) | 0.0 | 0.35 |
| Sports | 16.1 (6.5,25.7) | 30.0 (15.0,45.0) | 8.3 (0,20.4) | 0.06 |
| Percent Reporting Exposure to Loud Music | 62.5 (49.8,75.1) | 55.0 (33.2,76.8) | 66.7 (51.3,82.1) | 0.41 |
| Musical Instrument | 9.3 (1.7,16.9) | 25.0 (0,44) | 0.0 | 0.005 |
| Concerts, Discos | 22.2 (11.3,33.2) | 15.0 (0,30.6) | 26.5 (12.1,40.9) | 0.50 |
| Headphones (walkman/iPod®) | 46.4 (33.4,59.5) | 35.0 (14.1,56.0) | 52.8 (36.5,69.1) | 0.26 |
| Loud Music in Car | 21.8 (11.0,32.6) | 5.0 (0,14.6) | 31.4 (16.3,46.6) | 0.04 |
| Percent Reporting Exposure to Loud Impulse Noise | 44.5 (31.5,57.5) | 65.0 (44.1,85.9) | 33.3 (17.9,48.7) | 0.02 |
| Percent Reporting Exposure to One-Two Sources of Loud Sound | 39.3 (26.5,52.1) | 45.0 (23.2,66.8) | 36.1 (20.4,51.8) | 0.57 |
| Percent Reporting Exposure to Three or More Sources of Loud Sound | 28.6 (16.8,40.4) | 25.0 (6.0,44.9) | 30.6 (15.5,45.7) | 0.76 |
Note: P-values are derived from Pearson Chi-Square tests or Fisher exact tests of the null hypothesis of no difference between males and females. P-values are derived from Fisher exact tests for 2×2 tables. The p-value for the 2 by 5 table is derived from an exact version the Pearson Chi-square tests as implemented by SAS.
Results
Pure-Tone Audiometry
Average pure-tone air-conduction thresholds are shown in Figure 1A; there were no differences between right and left ear thresholds. When we compared average thresholds for male and female subjects (see Figure 1B), there was a statistically significant main effect of gender (F=12.76, df=1,54; p=0.0008), and a statistically significant interaction for frequency x gender (F=2.20, df=7,378; p=0.0332). Adolescent and young adult males frequently have worse hearing thresholds than females (Hull et al., 1971; Niskar et al., 1998; Serra et al., 2005; Kim et al., 2009; Shah et al., 2009; Shargorodsky et al., 2010), and these gender-based differences extend into adulthood (Agrawal et al., 2008; Ciletti & Flamme, 2008). The frequencies at which male and female subjects had statistically significant differences in hearing sensitivity in this study included 0.25-, 0.5-, 4-, 6-, and 8-kHz, with males having worse thresholds than females (p’s<0.05).
Figure 1.
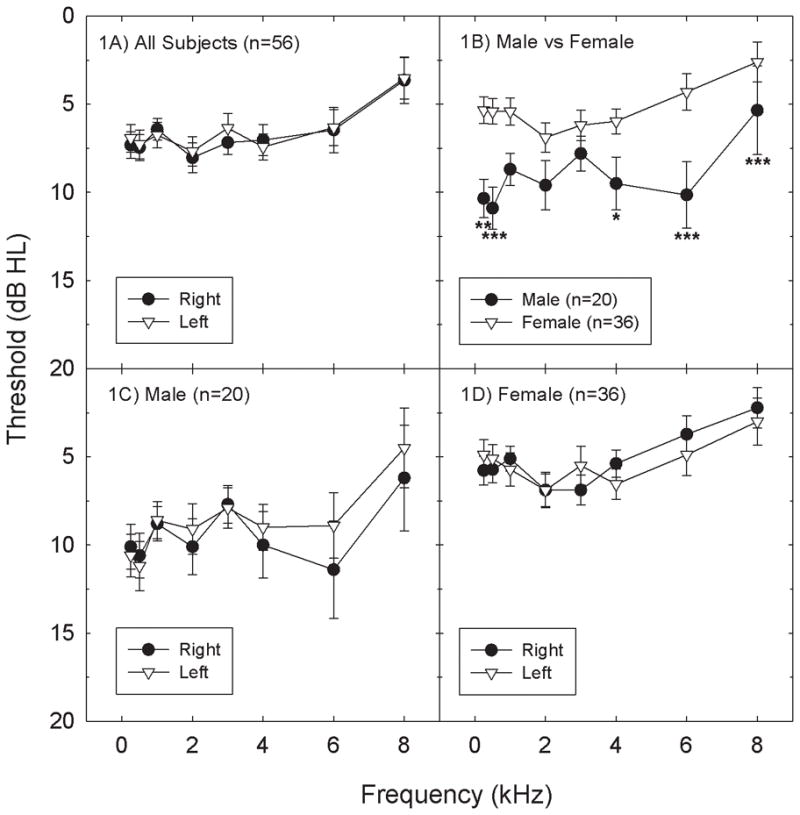
Threshold sensitivity (mean +/− S.E.) did not significantly differ as a function of ear (right versus left) in the total subject sample (1A), or in subsets that were limited to just male (1C) or female (1D) subjects. When average thresholds (right and left ear combined) for male and female subjects were compared (1B), male subjects had significantly (p<0.05) worse threshold sensitivity than female subjects at all test frequencies (0.25, 0.5, 4, 6, and 8 kHz). One, two or three asterisks indicates statistically significant difference at the 0.05, 0.01, or 0.001 level respectively.
Figure 1 also illustrates threshold sensitivity (mean +/− S.E.) for right and left ears within the male subject subset (see Figure 1C) and the female subject subset (see Figure 1D). Within both the male and female subject cohorts, thresholds varied with frequency (male: F=2.98, df=7,133; p=0.0061; female: F=6.33, df=7,245; p<0.0001), but there were no statistically reliable differences between right and left ear thresholds within ear cohort (p’s>0.05). Given that there were no reliable differences between right and left ears (Figures 1A, 1C, and 1D), thresholds were averaged across ears for all subsequent analyses.
Surveys
Hearing and health history reported by subjects via questionnaires revealed a small percentage with previous hearing loss (5.4%, n = 3), and a family history of hearing loss (12.5%, n = 7) (see Table 1). Differences in measured thresholds as a function of these factors were explored using a Chi-Square analysis. However, no statistically significant relationships were found between these variables (p>0.05). A history of ear disease and current tinnitus were also considered, but no subjects reported either of these conditions. Finally, we considered the possibility that tobacco use (cigarette, cigar, or pipe smoking, or use of chewing tobacco) influenced hearing outcomes (as shown by Agrawal et al., 2008; 2009), but too few subjects reported tobacco use to conduct this analysis.
When survey responses regarding pigmentation were evaluated, there was no relationship between any measure of pigmentation and measured threshold sensitivity (p > 0.05). These results contrast with other data from humans and animals suggesting vulnerability to noise insult might vary with race (Agrawal et al., 2008) as well as eye color, skin color, hair color, and/or sensitivity to sun exposure (Conlee et al., 1986; Jerger et al., 1986; Conlee et al., 1988; Barrenas & Lindgren, 1990; Barrenas & Lindgren, 1991; Barrenas & Hellstrom, 1996; Ishii & Talbott, 1998). Given the lack of statistically significant outcomes, these data are not discussed further. It remains possible that group differences as a function of pigmentation would emerge with an increase in sample size.
Surveys on self-reported sound exposure were more informative (see Table 2). Almost half of the subjects reported recreational use of a PMP (46.4%, 7 male, 19 female) and/or accidental, unprotected impulse noise exposure (i.e., gunshot, firecracker) (44.5%, 13 male, 11 female). Of these, 16 of 56 subjects (28.6%) reported both use of a PMP and accidental impulse noise exposure (5 male, 11 female), and 10 subjects (17.9%) reported neither PMP use nor accidental impulse noise (5 male, 5 female). There were smaller numbers of subjects reporting exposure to other sound sources including bar/nightclub sounds (30.4%; 5 male, 12 female), loud music in personal vehicles (21.8%, 1 male, 11 female), music at concerts or discos (22.2%; 3 male, 9 female), and/or musical instrument use (9.3%, 5 male, 0 female). Only 1.8% of the subjects (1 male, 0 female) reported use of firearms during hunting and/or shooting activities. Detailed self-report sound exposure survey responses are presented in Table 2.
Audiometric Thresholds as a Function of Reported Sound Exposure
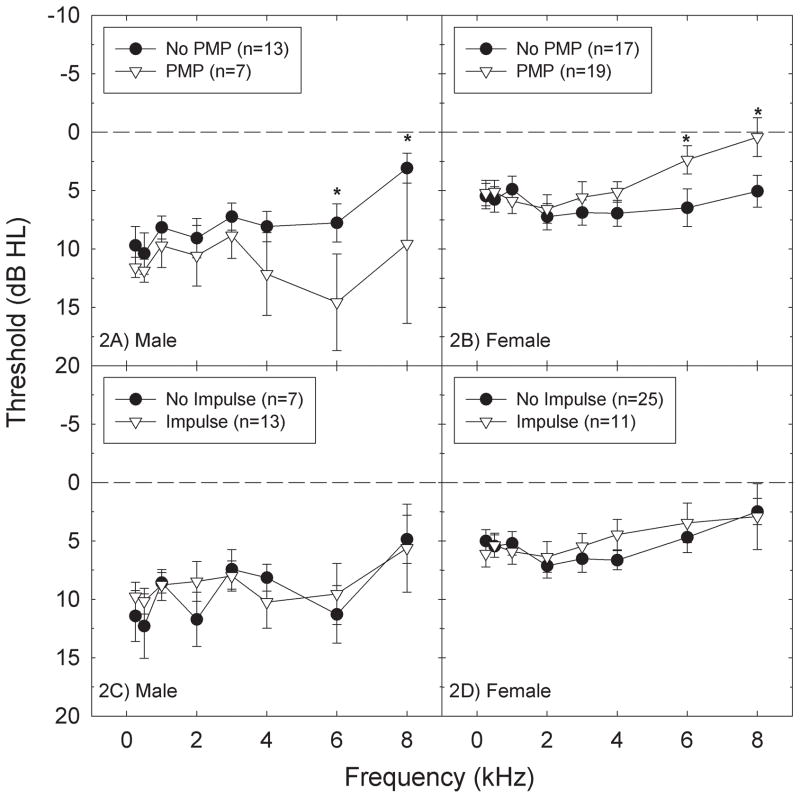
Threshold sensitivity as a function of self-reported sound exposure was evaluated for the two types of exposures that were most commonly reported: PMP use and impulse noise exposure. When thresholds of subjects that use PMPs were compared to those not reporting PMP use, there was an apparent elevation in threshold sensitivity in male subjects that reported use of these devices, particularly at higher test frequencies (Figure 2A). A mixed model ANOVA used to evaluate group differences within frequencies revealed statistically significant differences at 6-kHz (F=4.69, df=1,126; p=0.0321) and 8-kHz (F=4.28, df=1,126; p=0.0406). Group differences in female subjects were smaller and were in the opposite direction, with better high-frequency threshold sensitivity measured in the subset of female subjects that reported use of PMPs (Figure 2B; 6 kHz: F=4.37, df=1,238; p=0.0377; 8 kHz: F=5.87, df=1,238; p=0.0162).
Figure 2.
Threshold sensitivity (mean +/− S.E.) significantly differed at only 6 kHz and 8 kHz as a function of reported PMP use in both male (2A) and female (2B) subjects. Male subjects that reported use of PMPs had significantly worse threshold sensitivity than male subjects that did not report use of PMPs; the opposite relationship was observed in female subjects. Threshold sensitivity (mean +/− S.E.) did not differ as a function of reported impulse noise exposure in either male (2C) or female (2D) subjects. One asterisk indicates statistically significant difference at the 0.05 level.
In contrast to the group differences observed as a function of PMP use, there were no statistically reliable differences in threshold sensitivity as a function of self-reported accidental, unprotected exposure to impulse noise in either males (Figure 2C) or females (Figure 2D) (all p’s > 0.05). The failure to detect any effect of accidental, unprotected impulse noise exposure on threshold sensitivity may suggest that reported exposures were in fact minimal and thus were not likely to result in auditory trauma.
Notched Audiometric Configuration
Using the 15-dB audiometric notch defined by Niskar et al. (2001), none of the audiograms from any of the current subjects had a notched audiometric configuration. Using the alternative medicolegal definition of an audiometric notch offered by Coles et al. (2000), 4 subjects (7%) had an audiometric notch (1 in the left ear and 3 in the right ear). Rabinowicz et al. (2006) similarly reported that the Coles et al. (2000) criteria detected higher percentages of audiometric notches than the Niskar et al. (2001) criteria (79% vs 55%, and 72% vs 45%, in two separate samples of audiograms). Taken together, even using the relatively less restrictive definition of a notched audiometric configuration from Coles et al. (2000), the 7% prevalence observed in our study is less than the 15.5% prevalence of audiometric notches reported within the NHANES III data set for the younger (12–19 year old) population (Niskar et al., 2001), and is also less than the 16.4% prevalence rate for any high-frequency hearing loss in the most recent 2005–2006 NHANES data set (Shargorodsky et al., 2010). Given that noise insult is generally expected to result in a notched audiometric configuration if the noise is sufficient to induce hearing loss, and that audiometric notches were generally not observed in the current sample, NIHL may not be the best explanation for the elevated thresholds detected during screening of this self-reported normally hearing population.
Screening “Referrals” Detected Using Different Criteria
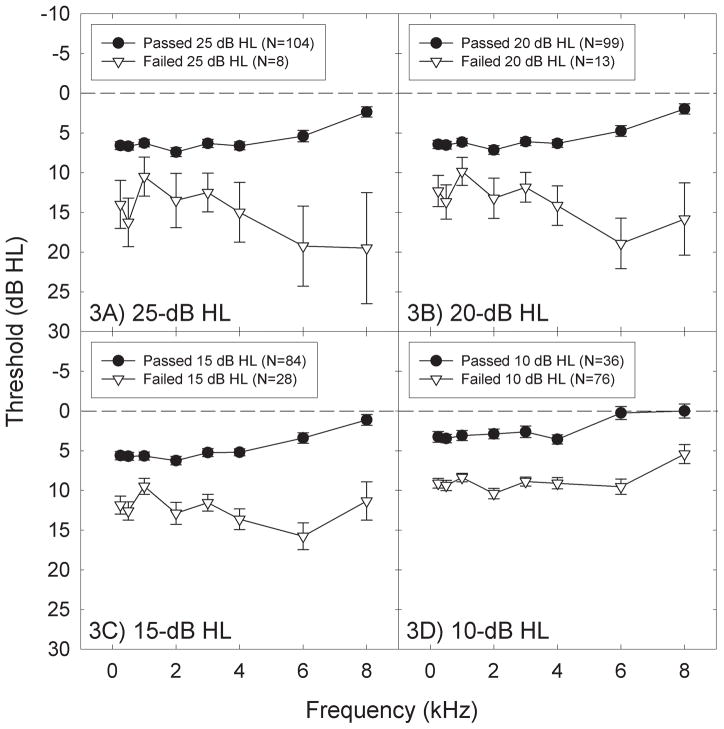
We first examined threshold findings at 0.5-, 1-, 2-, and 4-kHz, as per the ASHA frequency criteria. An ear was scored as a “refer” when thresholds were worse than the criteria at one or more of the test frequencies. Thresholds in 4 ears were > 25 dB HL; thresholds in 6 ears were > 20 dB HL; thresholds in 21 ears were > 15 dB HL, and thresholds in 59 ears were > 10 dB HL. We then rescored all individual ear data as pass or refer using an expanded frequency list, with all test frequencies from 0.25- to 8-kHz. The prevalence of screening referrals increased when additional frequencies were considered (Table 3). There were 8 ears with thresholds > 25 dB HL; 13 ears with thresholds > 20 dB HL; 27 ears with thresholds > 15 dB HL, and 74 ears with thresholds > 10 dB HL. At the 20 and 25 dB HL screening levels, more than twice as many ears referred the screening test using the expanded frequency list. Average thresholds within the passing and referring subsets of ears are shown in Figure 3.
Table 3.
Number and Percent of Ears that Referred Hearing Screening Tests at Individual Frequencies at 10, 15, 20, and 25 dB HL (with Confidence Intervals). Data are presented for failures at each frequency, failures within subsets of the frequencies, and failures at any of the 8 test frequencies.
| > 10 dB HL | > 15 dB HL | > 20 dB HL | > 25 dB HL | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency | n | % (CI) | n | % (CI) | n | % (CI) | n | % (CI) |
| 0.25 kHz | 29 | 25.9 (14.4,37.4) | 8 | 7.1 (0.4,13,9) | 3 | 2.7 (0,6.9) | 0 | 0 |
| 0.50 kHz | 35 | 31.3 (19.1,43.4) | 6 | 5.34 (0,11.3) | 2 | 1.78 (0,5.2) | 0 | 0 |
| 1 kHz | 20 | 17.9 (7.8,27.9) | 4 | 3.56 (0,8.4) | 0 | 0 | 0 | 0 |
| 2 kHz | 36 | 32.1 (19.9,44.4) | 11 | 9.8 (2,17.6) | 3 | 2.7 (0,6.9) | 2 | 1.78 (0,5.2) |
| 3 kHz | 23 | 20.5 (0,105.4) | 8 | 7.14 (0.4,13.9) | 1 | 0.90 (0,3.4) | 1 | 0.89 (0,3.3) |
| 4 kHz | 25 | 22.3 (11.4,33.2) | 9 | 8.0 (0.9,15.2) | 2 | 1.8 (0,5.2) | 1 | 0.89 (0.3.3) |
| 6 kHz | 31 | 27.7 (16,39.4) | 11 | 9.8 (2,17.6) | 5 | 4.5 (0,9.9) | 3 | 2.68 (0,6.9) |
| 8 kHz | 11 | 9.8 (2.0,17.6) | 7 | 6.3 (0.12.6) | 5 | 4.46 (0,9.9) | 3 | 2.68 (0,6.9) |
| 0.5, 1, 2, or 4 kHz | 59 | 52.68 (39.6,65.8) | 22 | 19.64 (9.2,30) | 6 | 5.36 (0,11.3) | 4 | 3.57 (0.8.4) |
| 3, 4, or 6 kHz | 48 | 42.85 (29.9,55.8) | 18 | 16.07 (6.5,25.7) | 7 | 6.25 (0,12.6) | 4 | 3.57 (0,8.4) |
| 0.25, 0.5, 1, 2, 3, 4, 6, or 8 kHz | 76 | 67.86 (55.6,80.1) | 28 | 25 (13.7,36.3) | 13 | 11.61 (3.2,20) | 8 | 7.14 (0,13.9) |
Figure 3.
All ears were individually scored as “Pass” or “Refer” with an ear scored as a “Refer” when thresholds were worse than the “Pass” criterion at any frequency from 0.25 to 8 kHz. Thresholds within Pass and Refer categories (mean +/− S.E.) are shown for pass criteria of ≤25-dB HL (3A), ≤20-dB HL (3B), ≤15-dB HL (3C), and ≤10-dB HL (3D). As the pass criterion was lowered, the number of ears classified as referrals increased.
The subset of ears with thresholds > 25 dB HL at any frequency from 0.25- to 8-kHz were best characterized by a sloping high-frequency hearing loss (Figure 3A). The most robust threshold elevations were observed at 6- and 8-kHz, although thresholds were clearly elevated at all of the test frequencies. As the pass criterion shifted from 25 dB HL (3A) to more restrictive pass criteria (3B, 3C, 3D), an increasing subset of the ears that had passed the 25 dB HL criteria were classified as referrals, decreasing the number of ears classified as “pass” and increasing the number of ears classified as “refer”.
For this sample, decreasing the pass-refer test criterion from 25 dB HL (Figure 3A) changed the shape of the average audiogram for the referring ears. The worst thresholds for the >20 dB HL (Figure 3B) and >15 dB HL (Figure 3C) criterion were measured at 6 kHz instead of at 8 kHz. Averaging thresholds across all ears that had thresholds > 10 dB HL (Figure 3D) flattened the audiometric configuration, and a general shift that did not vary with frequency emerged (Figure 3D). These data highlight the potential impact of choosing different screening criteria for detecting atypical hearing in populations of subjects.
Hearing Loss Defined Using Pure-Tone-Average (PTA) Thresholds
Hearing screening refer outcomes are typically based on pure-tone-average (PTA) thresholds at a discrete set of frequencies. We determined the percent of ears that did not meet a 15-dB HL hearing screening criterion based on LFPTA and HFPTA thresholds (defined using the Niskar et al., 1998 criteria). LFPTA was defined as the average threshold at 0.5-, 1-, and 2-kHz, and HFPTA was defined as the average threshold at 3-, 4-, and 6-kHz. In this sample, there were 3 ears that had LFPTAs ≥ 16 dB HL and 8 ears that had HFPTAs ≥ 16 dB HL. Thus, there were more than twice as many ears with HFPTA losses (7.17%) as with LFPTA losses (2.77%). The apparently disproportionate contribution of high-frequency hearing loss to the hearing screening referrals using the >16 dB HL criteria is consistent with the NHANES data sets (Niskar et al., 2001; Shargorodsky et al., 2010), however, there was not a statistically significant difference in the rate of hearing loss at high and low frequencies in this study (Fisher exact test, p>0.05).
Importantly, there were relatively equal number of ears referred during the hearing screening tests if the PTA threshold was fixed at a criterion higher than that used by Niskar and colleagues (>20 dB, >25 dB HL), or at a criterion lower than that used by Niskar and colleagues (>10 dB HL) (see table 4).
Table 4.
Number and Percent of Ears that Referred Pure-Tone-Average (PTA) Hearing Screening Tests at 10, 15, 20, and 25 dB HL (with Confidence Intervals).
| Number of Ears That Refer at LFPTA 0.5, 1, and 2 kHz | Number of Ears That Refer at HFPTA 3, 4, and 6 kHz | |||
|---|---|---|---|---|
| PTA Threshold | n | % (CI) | n | % (CI) |
| >25 dB HL | 0 | 0 | 1 | 0.89 (0,2.6) |
| >20 dB HL | 2 | 1.79 (0,4.2) | 2 | 1.79 (0,4.2) |
| >15 dB HL | 4 | 3.57 (0.1,7.0) | 9 | 8.04 (0,13.1) |
| >10 dB HL | 29 | 25.9 (17.8,34.0) | 28 | 25.00 (0,33.0) |
When comparing findings across studies, it is absolutely critical that the criteria used to define hearing loss are consistent. Ross and colleagues recently reevaluated the NHANES III data using three different definitions of hearing loss (Ross et al., 2010). When hearing loss was defined as PTA ≥15 dB HL at 0.5-, 1-, and 2-kHz in the affected ear (case definition 1), there was a 6.3% refer rate. When hearing loss was defined as PTA ≥15 dB HL at 0.5-, 1-, 2-, and 4-kHz in the affected ear (case definition 2), there was a 5.8% refer rate. Finally, when hearing loss was defined as PTA ≥20 dB HL at 0.5-, 1-, and 2-kHz or PTA >25 dB HL at two or more frequencies above 2-kHz (3-, 4-, 6-, and 8-kHz) in the affected ear (case definition 3), the refer rate was 3.0%.
Data from the current study were analyzed using each of the different criteria for the most informative comparisons across studies (see Table 5). We note here that small differences across studies should be interpreted with caution as our protocol for measurement of hearing thresholds utilized 2-dB intensity increments, which could produce thresholds 1–2 dB lower than protocols which use 5-dB steps in intensity during threshold assessment. In other words, thresholds would have likely been measured as 1–2 dB higher (worse) if a conventional 5-dB step size had been used.
Table 5.
Percent Screening Referrals Across Studies as a Function of the Hearing Loss Criterion. Current Study Data are Percent (with Confidence Intervals).
| NHANES Data | NSHS | HES | D.C. Study | FL adolescents | FL juvenile detainees | College under-graduates | Current Data % (CI) | |
|---|---|---|---|---|---|---|---|---|
| LFPTA≥ 25 dB HL at 0.5, 1, 2, and 4 kHz | a3.1% |
j0.9% 1/112 (0,2.6) |
||||||
| LFPTA≥ 25 dB HL at 0.5, 1 and 2 kHz | d2.63% |
j0% 0/112 |
||||||
| LFPTA≥15 dB HL at 0.5, 1 and 2 kHz | b5.6% |
j2.7% 3/112 (0,5.7) |
||||||
| LFPTA≥15 dB HL at 0.5, 1, and 2 kHz | c6.3% | e1.5% | f6.7% |
j3.6% 4/112 (0.13,7.1) |
||||
| LFPTA≥15 dB HL at 0.5, 1, 2, and 4 kHz | c5.8% |
j2.7% 3/112 (0,5.7) |
||||||
| LFPTA≥20 dB HL at 0.5, 1, and 2 kHz or HFPTA> 25 dB HL at ≥2 frequencies above 2 kHz | c3.0% |
j1.8% 2/112 (0,4.3) |
||||||
| HFPTA≥25 dB HL at 3, 4, and 6 kHz | a8.5% |
j0.9% 1/112 (0,2.6) |
||||||
| HFPTA≥16 dB HL at 3, 4, and 6 kHz | b12.7% |
j7.2% 8/112 (2.4,12) |
||||||
| 1, 2, or 4 kHz; ≥25 dB HL | g7% | h12% |
j1.8% 2/112 (0,4.3) |
|||||
| 1, 2, 4, or 6 kHz; ≥25 dB HL | g17% | h26% |
j3.6% 4/112 (0.15,7.1) |
|||||
| 0.5, 1, 2, 4, or 6 kHz; ≥20 dB HL | i26% |
j11.6% 13/112 (0,17.5) |
NHANES (1999–2004, N=1,458, age 20–29 years): Percent of subjects that referred (Agrawal et al., 2008)
NHANES III (1988–1994, N=6,768, ages 6–19 years): Percent of subjects that referred (Niskar et al., 1998)
NHANES III: Percent of subjects that referred (Ross et al., 2010)
NSHS: National Speech and Hearing Survey (N=38,568, grades 1–12): Percent of subjects that referred (the report by Hull et al., 1971 is limited to single-frequency outcomes; for PTA data, see Lundeen, 1991)
HES: Health Examination Survey (N=6,768, ages 6–11 years old): Percent of subjects that referred (Roberts & Ahuja, 1975)
D.C. Study (N=1,639, ages 4–11 years old): Percent of subjects that referred (Kessner et al., 1974)
FL adolescents (N=342, ages 10–20 years old): Percent of subjects that referred (Holmes et al., 1997)
FL juvenile detainees (N=226, ages 9–18 years old): Percent of ears that referred (Holmes et al., 1996)
University of Pennsylvania, Undergraduate Education Majors (N=258, ages 17–21 years old): Percent of subjects that referred (Widen et al., 2009)
Current Data: Percent of ears that referred; followed by number of ears that referred, out of 112 ears tested, (with confidence intervals)
Discussion
Based on review of the literature, Borchgrevink (2003) suggested that median hearing thresholds within the 18 to 25 year old population may be closer to +5 dB HL rather than the expected 0 dB HL norms. The data from our subject population are consistent with that conclusion. Average thresholds across all subjects in Figure 1A were approximately 7 dB HL, and these data were collected with a 2-dB step size protocol that may produce 1–2 dB “improvements” in hearing when compared to clinically conventional (5-dB step) tests.
When we evaluated hearing loss in this self-reported normal hearing population, the proportion of subjects with hearing loss was highly dependent on the specific criteria used to define normal hearing. Using the ASHA-recommended PTA at 0.5-, 1-, 2-, and 4-kHz, and the pass criterion of 25 dB HL, as used in the National Speech and Hearing Survey with a reported 2.63% refer rate, none of the subjects exceeded the criterion. Given that potential subjects were asked if they had any hearing difficulties as part of an initial telephone screening, it is not that surprising that we did not have any subjects that met the ASHA hearing loss referral criteria. If we considered single-frequency thresholds, however, 3 subjects referred at one or more frequencies in one or both ears (5.36% of the sample; 1 subject referred bilaterally and 2 subjects referred unilaterally).
A 15 dB HL low-fence threshold may be justified when testing adolescents, given that 15 dB HL hearing losses can impact communication and language learning (see Table 3.6 in Northern & Downs, 1984; Bess et al., 1998; Tharpe & Bess, 1999; see also Ross et al., 2010). Among the major studies that have used a 15 dB HL screening criteria at 0.5-, 1-, and 2-kHz, reported low-frequency refer rates range from approximately 2% to 7% (Roberts & Ahuja, 1970; Kessner et al., 1974; Roberts & Ahuja, 1975; Roberts & Ahuja, 1975). Using the 15 dB HL LFPTA criteria, three subjects referred the 15 dB HL hearing screening (5.36% refer rate); two subjects referred unilaterally and the third referred bilaterally. When we compared the current 5.35% refer rate based on our LFPTA data to those extracted from the younger subjects of the NHANES III study, in which, according to the Niskar (1998) analysis, 5.6% of the subjects had LFPTA thresholds >16 dB HL at 0.5-, 1- and 2-kHz, we had an almost identical rate of LFPTA referrals. Statistical comparison of the outcomes for the current smaller sample with outcomes from a larger nationally representative sample is challenging, but the confidence intervals shown in Table 5 do allow comparisons between the current data and other published data sets. In virtually every case, the prevalence of screening referrals for low frequency hearing loss in other earlier studies falls within the confidence intervals shown for our data in Table 5. This observation lends confidence to the conclusion that the low-frequency screening referral data reported here are generally equivalent to those reported in earlier, nationally representative studies.
While the reported refer rates are not obviously increased relative to the existing data from large, unscreened samples, there is one important difference between the larger national samples and the current smaller sample. In contrast to the earlier studies, all subjects in our study self-reported normal hearing. This suggests a subset of the current subjects had hearing loss they were not aware of. This is not surprising, however. Widén et al (2009) reported a 26% referral rate from a college-aged student sample (258 American undergraduates) using a 20 dB HL pass criterion at 0.5-, 1-, 2-, 4- and 6-kHz; they found that only 4% of that sample reported hearing loss prior to testing.
In this population which had self-reported normal hearing, reported prevalence of tinnitus was relatively comparable to that in other studies. None of our subjects reported permanent tinnitus, but 50% subjects reported they had had tinnitus at some point previously (of those reporting previous tinnitus, ~4% reported they have tinnitus always or frequently, 46% reported they have tinnitus occasionally, and 50% reported they rarely have tinnitus). In another survey study of American college students, 85% reported they have had tinnitus, with ~2% reporting they have tinnitus always or frequently, 23% reporting they have tinnitus sometimes, and 59% reporting they have tinnitus rarely (Danhauer et al., 2009). Other studies report a generally similar incidence of tinnitus, with 66% of college students reporting tinnitus in a population that had reported sources of noise exposure including loud music (up to 50% of students), and loud workplace noise (up to 29% of students) (Rawool & Colligon-Wayne, 2008). Reports are variable across the literature, however, with Widén et al (2009) reporting temporary tinnitus in only 15% of their college student subjects. In Swedish high-school students aged 13 to 19 years (N=1285), the prevalence of tinnitus was similarly only 9% (Widen & Erlandsson, 2004).
Together, these outcomes highlight the need for routine hearing screening of young adult populations such that patients with “sub-clinical” deficits can be counseled to avoid loud sound exposure, and recommendations for additional health monitoring can be provided as needed. The similar referral rates observed in the unscreened NHANES III sample and the screened sample described here are striking, and suggest that subjects may not have appreciable deficits with 15 dB HL thresholds.
Although most reported sources of noise exposure did not provide any predictive power for explaining higher thresholds measured in some subjects, we found one potential gender-specific interaction among sources of sound exposure and threshold sensitivity. Male subjects that reported use of PMPs had significantly worse high-frequency thresholds compared to within-gender controls reporting no PMP use (~7 dB at 8-kHz, Figure 2A). Some studies report no threshold differences between those that use PMPs and those that do not (Wong et al., 1990; Mostafapour et al., 1998; Kumar et al., 2009; Shah et al., 2009). Others report small differences in conventional pure-tone audiometric thresholds (Meyer-Bisch, 1996; Kim et al., 2009). Mean differences of 2–3 dB at 4–6 kHz have been reported by others as well, although the group differences were not statistically reliable (West & Evans, 1990). One of the most detailed descriptions of PMP use among college-student populations was recently provided by Danhauer et al. (2009). They report more than 50% of subjects use a PMP 5–7 days per week, with 85% reporting at least 30 min use per day (1/2 to 1 hour: 30%; 1–2 hours: 39%; 3–4 hours: 15%). Use of PMPs has an even higher prevalence in other studies, with Vogel et al. (2009) reporting 90% of Dutch youth (ages 12–19) use these devices. An earlier study with 14-year old high school students from Argentina had lower reported PMP use (less than 20% of subjects reported use) (Serra et al., 2005); however, it is difficult to be certain whether population differences were influenced by age, geographical location/socio-economic status, or, timing of the data collection, as PMP use has almost certainly increased between 1998 (when the Argentina study started) and 2006 (when the Danhauer surveys were distributed). In the current sample, 46% of our subjects reported PMP use (see Table 2).
The Chi-square distribution tests did not reveal any other factors predictive of threshold sensitivity. We specifically considered the potential effects of impulse noise exposure because high-frequency deficits have been tightly linked to impulse noise exposure, and, specifically, to firearm use (with deficits often worse in the left ear than the right, see Prosser et al., 1988; Cox & Ford, 1995; Holmes et al., 1997; Nondahl et al., 2000). However, in contrast to the differences between PMP users and those that did not report use of these devices, there was no predictive relationship between accidental, unprotected impulse noise exposure and threshold sensitivity in male or female subjects (Figures 2C, 2D). Even when the analysis was limited to the left ear (not shown), there was no evidence that hearing was worse in subjects reporting exposure to impulse noise. Only 3.5% of the subjects reported use of firearms (hunting/shooting, see Table 2), which did not allow systematic exploration of that more specific impulse noise variable.
It is not necessarily surprising that effects of PMPs on hearing were greater in males than in females in this limited sample. Male listeners often choose higher listening levels than female listeners (Rice et al., 1987; Williams, 2005; Torre, 2008; Vogel et al., 2009). Gender-based differences in hearing loss in young adult population are also consistent with a study on American and Swedish college student attitudes. Specifically, Widén et al. (2006) found that males had more pro-noise attitudes than females, and may prefer louder volume levels than females. NIHL reportedly affects males at a 3:1 greater rate than females in the general population (Nelson et al., 2005). Greater hearing loss in males than in females could be secondary to greater exposures in males than in females; alternatively, a greater vulnerability of males compared to females is also possible. Data suggesting gender differences in hearing loss vulnerability are mixed.
While male versus female differences were generally expected, it was surprising that there was a statistically reliable relationship between PMP use and lower thresholds in female subjects. While speculative, one potential explanation for this outcome is protection of the female auditory system via sound conditioning, if female PMP users were listening at levels and durations that induce long-term protection against a decline in auditory sensitivity. Sound conditioning was initially described by Canlon and colleagues (Canlon et al., 1988; Canlon & Fransson, 1995) as a phenomena in which long-term exposure to continuous low-level sound (1 kHz, 81 dB SPL, 24 days) reduces hearing deficits typically associated with exposure to a subsequent sound that otherwise induces significant NIHL. Typical delays between conditioning and trauma exposure are 5–6 days, although protection extends for at least two months post-conditioning (McFadden et al., 1997). Interrupted sounds, in which the background conditioning sounds are on for only 6 hours/day, are also protective (Subramaniam et al., 1993; Skellett et al., 1998). Taken together, any interpretation of gender-based differences, and any potential role for conditioning, will remain highly speculative until additional data on the levels at which both male and female subjects use PMPs become available. PMP use by itself is likely to prove inadequate for measuring total risk of hearing loss, and future studies with larger samples in a broader population are critical.
Conclusions
Approximately half (46.4%) of the subjects in our study reported recreational use of a PMP, and most had previous exposure to other recreational sound sources such as loud music at concerts, in nightclubs, in their cars, and at other settings. Despite the exposures acknowledged in their histories, all of the students self-reported normal hearing sensitivity. With additional hearing testing using a variety of common clinical procedures, we found that hearing loss in this sample of “normal-hearing” college-age students occurred at about the same rate as reported in previous studies using unscreened populations of adolescents and young adults.
Data collected in this study provided evidence of a gender-related association between PMP use and elevated hearing thresholds. There was no additional relationship between other specific sources of sound or total sources of reported sound exposure and the measured thresholds, but it is possible that we did not have adequate power to detect effects of some sound sources given the small samples reporting any single specific source of exposure, and we may not have included all possible relevant noise sources. Although the current sample size is considerably smaller than the national normative studies, our data are consistent with the possibility that PMP use may be related to hearing loss in an individual, particularly if the music player is used for long durations at high volumes, or if it is used by an individual exposed to other sound sources which have a cumulative detrimental effect.
One might speculate that subjects with the highest measured thresholds engaged in the riskiest listening behaviors. However, the current survey did not provide data that adequately address this issue. It remains possible that some unidentified factor (race, history of smoking, undiagnosed hypertension) and/or other source of sound exposure co-varied with reported PMP use and influenced the current outcome. Additional data from a larger, randomly-selected, population is essential given remaining gaps in information about hearing in college age students.
Acknowledgments
Portions of this research have been presented in abstract form (Le Prell et al., 2009; 2010). We thank Robert Dobie, David Dolan, Joseph Hall, Alice Holmes, and Josef Miller for comments on an earlier version of this manuscript, and Patrick Antonelli, Glenn Green, Sharon Kujawa, and Josef Miller for help developing the screening questionnaire. We thank Robert Dobie, Joseph Hall, Rick Mowery, Catherine Ross, and Darby Thompson (members of the NIH-selected Data Safety Monitoring Board), as well as Gordon Hughes at the NIH, for helpful feedback and suggestions. Kari Morgenstein, Marissa Rosa, Jason Schmitt, and Lindsey Willis provided technical assistance, and Susan DeRemer provided assistance with IRB applications.
Abbreviations
- NIHL
Noise-induced hearing loss
- PMP
Personal music player
- PTA
Pure-tone average
Appendix A
The Survey used for screening the current subjects was modeled after a hearing survey used at the Karolinska Institutet (Ann-Catherine Lindblad, unpublished)
Q-1) Do you have relatives (parents/siblings) with hearing loss?
__________YES (1) __________NO (2)
Q-2) When is the last time you were exposed to loud noise?
__________ (1) past 24 hours
__________ (2) past 48 hours
__________ (3) past week
__________ (4) past month
__________ (5) past year
Q-3) Have you ever experienced tinnitus, defined as a ringing, humming, buzzing, or other noise in your ears or in your head, even if only for a brief period, shortly after exposure to loud noise?
-
__________YES (1) __________NO (2)
CF-13)if yes, does this happen:
__________ (1) Always
__________ (2) Often
__________(3) Occasionally
-
__________ (4) Rarely
CF-14)if yes, does your tinnitus also occur in the absence of exposure to loud noise?
__________YES (1) __________NO (2)
Q-4) Have you ever experienced hearing loss?
-
__________YES (1) __________NO (2)
CF-15)if yes, check all that apply FOR THE RIGHT EAR:
__________ (1) Always
__________ (2) Often
__________ (3) Occasionally
-
__________ (4) Only after loud sounds
CF-16)if yes, check all that apply FOR THE LEFT EAR:
__________ (1) Always
__________ (2) Often
__________ (3) Occasionally
__________ (4) Only after loud sounds
Q-5) Have you ever had an ear infection?
-
__________YES (1) __________NO (2)
CF-17)if yes, have you had an ear infection within the past 3 months?
__________YES (1) __________NO (2)
Q-6) Have you ever had any other ear disease?
-
__________YES (1) __________NO (2)
CF-18)If yes, please specify: __________
Q-7) Do you consider yourself to be overly sensitive to loud sounds?
-
__________YES (1) __________NO (2)
CF-19)if yes, check all that apply FOR THE RIGHT EAR:
__________ (1) Always
__________ (2) Often
__________ (3) Occasionally
-
__________ (4) Only after loud sounds
CF-20) if yes, check all that apply FOR THE LEFT EAR:
__________ (1) Always
__________ (2) Often
__________ (3) Occasionally
__________ (4) Only after loud sounds
Q-8) Are you exposed to loud sound in your leisure time?
-
__________YES(1) __________NO (2)
CF-21) if yes, please check all that apply:
__________ (1) Bars, Clubs
__________ (2) Hunting, Shooting Range
__________ (3) Sports Events
__________ (4) Other (please specify: __________)
Q-9) Are you exposed to loud music in your leisure time?
-
__________YES(1) __________NO (2)
CF-22) if yes, please check all that apply:
-
__________ (1) I play a musical instrument
(Please specify: __________)
__________ (2) I go to concerts/discos
__________ (3) I use headphones to listen to walkman/iPod
__________ (4) I listen to loud music in my car
Q-10) Have you ever had an accidental exposure to loud impulse noise (i.e., gunshot, firecracker), while you were not wearing hearing protection?
__________YES(1) __________NO (2)
Q-11) What is/was your natural hair color?
__________ (1) Red
__________ (2) Blond
__________ (3) Light Brown/Auburn
__________ (4) Dark Brown
__________ (5) Black
Q-12) What is/was your natural eye color?
__________ (1) Blue
__________ (2) Brown
__________ (3) Green
__________ (4) Hazel
__________ (5) Grey
Q-13) How is your skin affected by sunbathing?
__________ (1) Turns red/burns easily
__________ (2) Tans slowly or not at all
__________ (3) Slightly red sometimes
__________ (4) Tans normally
__________ (5) Never burns/turns red
__________ (6) Darker tan than most people
Appendix B
The Tinnitus Survey used for screening the current subjects was modeled after the Tinnitus Ototoxicity Monitoring Interview (TOMI) developed by Fausti et al. (2007) as a tool for tracking drug-induced tinnitus in patients. The tinnitus visual rating scales were developed based on the sensory scaling work by Bartoshuk and colleagues (Snyder et al., 2004; Bartoshuk et al., 2005; Bartoshuk et al., 2006; Snyder et al., 2006)
Instructions to subjects: Tinnitus is ringing, humming, buzzing or other noises in your ears or head. Almost everyone hears noises in the ears or head that are brief and fade away-these sounds are normal. I am going to ask you about persistent tinnitus that lasts at least 5 minutes, and occurs at least twice a week.
Q-1) “Do you have persistent tinnitus, meaning that it lasts at least 5 minutes, and occurs at least twice a week?”
-
__________YES(1) __________NO (2)
If yes, How long have you had tinnitus?
__________ (1) Less than 1 year
__________ (2) 1–2 years
__________ (3) 3–5 years
__________ (4) 6–10 years
__________ (5) 11–20 years
__________ (6) More than 20 years
__________ (7) Not Sure
Q-2) “Do you have tinnitus currently?”
-
__________YES(1) __________NO (2)
IF YES: Continue to the next question.
IF NO: The tinnitus interview is complete.
Q-3) “What does your tinnitus sound like? (mark all that apply)?”
__________ (1) Ringing
__________ (2) Hissing
__________ (3) Buzzing
__________ (4) Sizzling
__________ (5) Crickets
__________ (6) Whistle
__________ (7) Hum
__________ (8) Other: __________
Q-4) “Does your tinnitus have a pulsing quality to it?”
__________YES(1) __________NO (2)
Q-5) “Where is your tinnitus located?”
__________ (1) Left ear only
__________ (2) Right ear only
__________ (3) Both ears
__________ (4) Inside head
__________ (5) Other: __________
Q-6) “Is your tinnitus louder on one side than the other?”
__________ (1) Right louder than left
__________ (2) Left louder than right
__________ (3) Equal
Q-7) “How loud is your tinnitus on average?”
__________ (1) Not loud at all
__________ (2) Slightly loud
__________ (3) Moderately loud
__________ (4) Very loud
__________ (5) Extremely loud
Q-8) “Please rate the loudness of this tinnitus, using a scale from 1 to 10.”

Mark the number line above using an X, and enter your rating here, in numeric form._____ _____. _____
Q-9) “Please rate how objectionable/bothersome this tinnitus is, using a scale from 1 to 10.”

Mark the number line above using an X, and enter your rating here, in numeric form. _____ _____. _____
Q-10) “How much of the time do you think your tinnitus is present?”
__________ (1) Occasionally
__________ (2) Some of the time
__________ (3) Most of the time
__________ (4) Always
Q-11) “On average, how much of a problem is your tinnitus?”
__________ (1) Not a problem
__________ (2) Slight problem
__________ (3) Big problem
__________ (4) Very big problem
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Support for this research was provided by an inter-institutional subcontract to the University of Florida, with funds from the National Institutes of Health via NIH/NIDCD U01 DC 008423 awarded to Josef Miller.
References
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30:139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute. Specifications for audiometers, S3.6–1969. American National Standards Institute; New York: 1969. [Google Scholar]
- American Standards Association. American standard specification for audiometers for general diagnostic purposes, Z24.5–1951. New York: ANSI; 1951. [Google Scholar]
- Barrenas ML, Hellstrom PA. The effect of low level acoustic stimulation on susceptibility to noise in blue- and brown-eyed young human subjects. Ear Hear. 1996;17:63–68. doi: 10.1097/00003446-199602000-00008. [DOI] [PubMed] [Google Scholar]
- Barrenas ML, Lindgren F. The influence of inner ear melanin on susceptibility to TTS in humans. Scand Audiol. 1990;19:97–102. doi: 10.3109/01050399009070759. [DOI] [PubMed] [Google Scholar]
- Barrenas ML, Lindgren F. The influence of eye colour on susceptibility to TTS in humans. Br J Audiol. 1991;25:303–307. doi: 10.3109/03005369109076602. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Fast K, Snyder DJ. Differences in our sensory worlds: invalid comparisons with labeled scales. Curr Dir Psychol Sci. 2005;14:122–125. [Google Scholar]
- Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Borchgrevink HM. Does health promotion work in relation to noise? Noise Health. 2003;5:25–30. [PubMed] [Google Scholar]
- Campbell KCM, Kelly E, Targovnik N, Hughes LH, Van Saders C, et al. Audiologic monitoring for potential ototoxicity in a Phase I clinical trial of a new glycopeptide antibiotic. J Am Acad Audiol. 2003;14:157–168. [PubMed] [Google Scholar]
- Canlon B, Borg E, Flock A. Protection against noise trauma by pre-exposure to a low level acoustic stimulus. Hear Res. 1988;34:197–200. doi: 10.1016/0378-5955(88)90107-4. [DOI] [PubMed] [Google Scholar]
- Canlon B, Fransson A. Morphological and functional preservation of the outer hair cells from noise trauma by sound conditioning. Hearing Research. 1995;84:112–124. doi: 10.1016/0378-5955(95)00020-5. [DOI] [PubMed] [Google Scholar]
- Ciletti L, Flamme GA. Prevalence of hearing impairment by gender and audiometric configuration: results from the National Health and Nutrition Examination Survey (1999–2004) and the Keokuk County Rural Health Study (1994–1998) J Am Acad Audiol. 2008;19:672–685. doi: 10.3766/jaaa.19.9.3. [DOI] [PubMed] [Google Scholar]
- Coles RR, Lutman ME, Buffin JT. Guidelines on the diagnosis of noise-induced hearing loss for medicolegal purposes. Clin Otolaryngol Allied Sci. 2000;25:264–273. doi: 10.1046/j.1365-2273.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Abdul-Baqi KJ, McCandless GA, Creel DJ. Differential susceptibility to noise-induced permanent threshold shift between albino and pigmented guinea pigs. Hear Res. 1986;23:81–91. doi: 10.1016/0378-5955(86)90177-2. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Abdul-Baqi KJ, McCandless GA, Creel DJ. Effects of aging on normal hearing loss and noise-induced threshold shift in albino and pigmented guinea pigs. Acta Otolaryngol (Stockh) 1988;106:64–70. doi: 10.3109/00016488809107372. [DOI] [PubMed] [Google Scholar]
- Couzad R, Rousey CL. Hearing and speech disorders among delinquent children. Corr Psych J Social Ther. 1966;35:173–181. [Google Scholar]
- Cox HJ, Ford GR. Hearing loss associated with weapons noise exposure: when to investigate an asymmetrical loss. J Laryngol Otol. 1995;109:291–295. doi: 10.1017/s0022215100129950. [DOI] [PubMed] [Google Scholar]
- Danhauer JL, Johnson CE, Byrd A, DeGood L, Meuel C, et al. Survey of college students on iPod use and hearing health. J Am Acad Audiol. 2009;20:5–27. doi: 10.3766/jaaa.20.1.2. quiz 83–24. [DOI] [PubMed] [Google Scholar]
- Eagles EL, Wishik SM, Doerffer LG, Melnick W, Levine HS. Hearing sensitivity and related factors in children. Laryngoscope. 1963 Supplement. [Google Scholar]
- Fausti SA, Helt WJ, Gordon JS, Reavis KM, Phillips DS, et al. Audiologic monitoring for ototoxicity and patient management. In: Campbell KCM, editor. Pharmacology and Ototoxicity for Audiologists. Clifton Park: Thomson Delmar Learning; 2007. [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, Phillips DS, Frey RH, et al. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Holmes AE, Kaplan HS, Nichols SW, Griffiths SK, Weber FT, et al. Screening for hearing loss in juvenile detention centers. J Am Acad Audiol. 1996;7:332–338. [PubMed] [Google Scholar]
- Holmes AE, Kaplan HS, Phillips RM, Kemker FJ, Weber FT, et al. Screening for hearing loss in adolescents. Speech-Language-Hearing in the Schools. 1997;28:70–75. [Google Scholar]
- Holmes AE, Niskar AS, Kieszak SM, Rubin C, Brody DJ. Mean and median hearing thresholds among children 6 to 19 years of age: the Third National Health And Nutrition Examination Survey, 1988 to 1994, United States. Ear Hear. 2004;25:397–402. doi: 10.1097/01.aud.0000134553.60120.3a. [DOI] [PubMed] [Google Scholar]
- Hong O. Hearing loss among operating engineers in American construction industry. Int Arch Occup Environ Health. 2005;78:565–574. doi: 10.1007/s00420-005-0623-9. [DOI] [PubMed] [Google Scholar]
- Hughson W, Westlake HD. Manual for program outline for rehabilitation of aural casualties both military and civilian. Transactions of the American Academy of Ophthalmology and Otolaryngology. 1944;48(Suppl):1–15. [Google Scholar]
- Hull FM, Mielke PW, Jr, Timmons RJ, Willeford JA. The National Speech and Hearing Survey: preliminary results. ASHA. 1971;13:501–509. [PubMed] [Google Scholar]
- International Organization for Standardization. ISO Recommendation R389. New York: ANSI; 1964. Standard reference zero for the calibration of pure-tone audiometers. [Google Scholar]
- Ishii EK, Talbott EO. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. J Occup Environ Med. 1998;40:661–666. doi: 10.1097/00043764-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Jerger J, Jerger S, Pepe P, Miller R. Race difference in susceptibility to noise-induced hearing loss. Am J Otol. 1986;7:425–429. [PubMed] [Google Scholar]
- Jerlvall L, Arlinger S. A comparison of 2-dB and 5-dB step size in pure-tone audiometry. Scand Audiol. 1986;15:51–56. doi: 10.3109/01050398609045954. [DOI] [PubMed] [Google Scholar]
- Kessner DM, Snow C, Singer J. Assessment of medical care in children, Contrasts in Health Status. Vol. 3. Washington, D. C: Institute of Medicine, National Academy of Sciences; 1974. [Google Scholar]
- Kim MG, Hong SM, Shim HJ, Kim YD, Cha CI, et al. Hearing threshold of Korean adolescents associated with the use of personal music players. Yonsei Med J. 2009;50:771–776. doi: 10.3349/ymj.2009.50.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Mathew K, Alexander SA, Kiran C. Output sound pressure levels of personal music systems and their effect on hearing. Noise Health. 2009;11:132–140. doi: 10.4103/1463-1741.53357. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Guire K, Hall JWI, Holmes AE. Prevalence of 6 kHz “notch” in populations of adolescents and young adults. Presented at IX European Federation of Audiology Societies (EFAS) Congress in Tenerife; Spain. 2009. [Google Scholar]
- Le Prell CG, Hall JWI, Sakowicz B, Campbell KCM, Kujawa SG, et al. Temporary threshold shift subsequent to music player use: comparison with hearing screenings in populations of adolescents and young adults. Paper presented at the National Hearing Conservation Association; Orlando, FL. February, 2010.2010. [Google Scholar]
- Leske MC. Prevalence estimates of communicative disorders in the U.S. Language, hearing and vestibular disorders. ASHA. 1981;23:229–237. [PubMed] [Google Scholar]
- Lundeen C. Prevalence of hearing impairment among school children. Lang Speech Hear Serv Sch. 1991;22:269–271. [Google Scholar]
- Margolis RH, Hunter LL. Acoustic immittance measurements. In: Roeser RJ, Valente M, Hosford-Dunn H, editors. Audiology Diagnosis. New York: Thieme; 2000. pp. 381–342. [Google Scholar]
- Marshall L, Hanna TE, Wilson RH. Effect of step size on clinical and adaptive 2IFC procedures in quiet and in a noise background. J Speech Hear Res. 1996;39:687–696. doi: 10.1044/jshr.3904.687. [DOI] [PubMed] [Google Scholar]
- Marshall L, Jesteadt W. Comparison of pure-tone audibility thresholds obtained with audiological and two-interval forced-choice procedures. J Speech Hear Res. 1986;29:82–91. doi: 10.1044/jshr.2901.82. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Henderson D, Shen YH. Low-frequency ‘conditioning’ provides long-term protection from noise-induced threshold shifts in chinchillas. Hear Res. 1997;103:142–150. doi: 10.1016/s0378-5955(96)00170-0. [DOI] [PubMed] [Google Scholar]
- Meinke DK, Dice N. Comparison of audiometric screening criteria for the identification of noise-induced hearing loss in adolescents. Am J Audiol. 2007;16:S190–202. doi: 10.1044/1059-0889(2007/023). [DOI] [PubMed] [Google Scholar]
- Meyer-Bisch C. Epidemiological evaluation of hearing damage related to strongly amplified music (personal cassette players, discotheques, rock concerts)--high-definition audiometric survey on 1364 subjects. Audiology. 1996;35:121–142. doi: 10.3109/00206099609071936. [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Lahargoue K, Gates GA. Noise-induced hearing loss in young adults: the role of personal listening devices and other sources of leisure noise. Laryngoscope. 1998;108:1832–1839. doi: 10.1097/00005537-199812000-00013. [DOI] [PubMed] [Google Scholar]
- Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M. The global burden of occupational noise-induced hearing loss. Am J Ind Med. 2005;48:446–458. doi: 10.1002/ajim.20223. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes A, Esteban E, Rubin C, et al. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA. 1998;279:1071–1075. doi: 10.1001/jama.279.14.1071. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, et al. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics. 2001;108:40–43. doi: 10.1542/peds.108.1.40. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, et al. Recreational firearm use and hearing loss. Arch Fam Med. 2000;9:352–357. doi: 10.1001/archfami.9.4.352. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Shi X, Cruickshanks KJ, Dalton DS, Tweed TS, et al. Notched audiograms and noise exposure history in older adults. Ear Hear. 2009;30:696–703. doi: 10.1097/AUD.0b013e3181b1d418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northern JL, Downs MP. Hearing in Children. Baltimore: Williams & Wilkins; 1984. [Google Scholar]
- Osei-Lah V, Yeoh LH. High frequency audiometric notch: an outpatient clinic survey. Int J Audiol. 2010;49:95–98. doi: 10.3109/14992020903300423. [DOI] [PubMed] [Google Scholar]
- Pascolini D, Smith A. Hearing Impairment in 2008: a compilation of available epidemiological studies. Int J Audiol. 2009;48:473–485. doi: 10.1080/14992020902803120. [DOI] [PubMed] [Google Scholar]
- Prosser S, Tartari MC, Arslan E. Hearing loss in sports hunters exposed to occupational noise. Br J Audiol. 1988;22:85–91. doi: 10.3109/03005368809077802. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Galusha D, Slade MD, Dixon-Ernst C, Sircar KD, et al. Audiogram notches in noise-exposed workers. Ear Hear. 2006;27:742–750. doi: 10.1097/01.aud.0000240544.79254.bc. [DOI] [PubMed] [Google Scholar]
- Rawool VW, Colligon-Wayne LA. Auditory lifestyles and beliefs related to hearing loss among college students in the USA. Noise Health. 2008;10:1–10. doi: 10.4103/1463-1741.39002. [DOI] [PubMed] [Google Scholar]
- Rice CG, Rossi G, Olina M. Damage risk from personal cassette players. Br J Audiol. 1987;21:279–288. doi: 10.3109/03005368709076420. [DOI] [PubMed] [Google Scholar]
- Roberts J, Ahuja EM. Hearing levels of children by age and sex, United States. Vital Health Stat. 1970;11(102):1–56. [PubMed] [Google Scholar]
- Roberts J, Ahuja EM. Hearing levels of U.S. youths 12–17 years. Vital Health Stat. 1975;11(145):1–84. doi: 10.1037/e458822004-001. [DOI] [PubMed] [Google Scholar]
- Roberts J, Ahuja EM. Hearing sensitivity and related medical findings among youths 12–17 years, United States. Vital Health Stat. 1975;11(154):1–112. [PubMed] [Google Scholar]
- Ross DS, Visser SN, Holstrum WJ, Qin T, Kenneson A. Highly variable population-based prevalence rates of unilateral hearing loss after the application of common case definitions. Ear Hear. 2010;31:126–133. doi: 10.1097/AUD.0b013e3181bb69db. [DOI] [PubMed] [Google Scholar]
- Sarafraz M, Ahmadi K. A practical screening model for hearing loss in Iranian school-aged children. World J Pediatr. 2009;5:46–50. doi: 10.1007/s12519-009-0008-3. [DOI] [PubMed] [Google Scholar]
- Serra MR, Biassoni EC, Richter U, Minoldo G, Franco G, et al. Recreational noise exposure and its effects on the hearing of adolescents. Part I: an interdisciplinary long-term study. Int J Audiol. 2005;44:65–73. doi: 10.1080/14992020400030010. [DOI] [PubMed] [Google Scholar]
- Shah S, Gopal B, Reis J, Novak M. Hear today, gone tomorrow: an assessment of portable entertainment player use and hearing acuity in a community sample. J Am Board Fam Med. 2009;22:17–23. doi: 10.3122/jabfm.2009.01.080033. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772–778. doi: 10.1001/jama.2010.1124. [DOI] [PubMed] [Google Scholar]
- Silverman SR, Lane HS. Deaf Children. In: Davis H, Silverman SR, editors. Hearing and Deafness. 3. New York: Holt, Rinehart, & Winston; 1970. [Google Scholar]
- Skellett RA, Cullen JK, Jr, Fallon M, Bobbin RP. Conditioning the auditory system with continuous vs. interrupted noise of equal acoustic energy: is either exposure more protective? Hear Res. 1998;116:21–32. doi: 10.1016/s0378-5955(97)00199-8. [DOI] [PubMed] [Google Scholar]
- Snyder DJ, Fast K, Bartoshuk LM. Valid comparisons of suprathreshold sensations. Journal of Consciousness Studies. 2004;11:96–112. [Google Scholar]
- Snyder DJ, Prescott J, Bartoshuk LM. Modern psychophysics and the assessment of human oral sensation. Adv Otorhinolaryngol. 2006;63:221–241. doi: 10.1159/000093762. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Henderson D, Spongr VP. Protection from noise induced hearing loss: is prolonged ‘conditioning’ necessary? Hear Res. 1993;65:234–239. doi: 10.1016/0378-5955(93)90216-n. [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Bess FH. Minimal, progressive, and fluctuating hearing losses in children. Characteristics, identification, and management. Pediatr Clin North Am. 1999;46:65–78. doi: 10.1016/s0031-3955(05)70081-x. [DOI] [PubMed] [Google Scholar]
- Torre P., 3rd Young adults’ use and output level settings of personal music systems. Ear Hear. 2008;29:791–799. doi: 10.1097/AUD.0b013e31817e7409. [DOI] [PubMed] [Google Scholar]
- Vogel I, Verschuure H, van der Ploeg CP, Brug J, Raat H. Adolescents and MP3 players: too many risks, too few precautions. Pediatrics. 2009;123:e953–958. doi: 10.1542/peds.2008-3179. [DOI] [PubMed] [Google Scholar]
- West PD, Evans EF. Early detection of hearing damage in young listeners resulting from exposure to amplified music. Br J Audiol. 1990;24:89–103. doi: 10.3109/03005369009077849. [DOI] [PubMed] [Google Scholar]
- Widen SE, Erlandsson SI. Self-reported tinnitus and noise sensitivity among adolescents in Sweden. Noise Health. 2004;7:29–40. [PubMed] [Google Scholar]
- Widen SE, Holmes AE, Erlandsson SI. Reported hearing protection use in young adults from Sweden and the USA: effects of attitude and gender. Int J Audiol. 2006;45:273–280. doi: 10.1080/14992020500485676. [DOI] [PubMed] [Google Scholar]
- Widen SE, Holmes AE, Johnson T, Bohlin M, Erlandsson SI. Hearing, use of hearing protection, and attitudes towards noise among young American adults. Int J Audiol. 2009;48:537–545. doi: 10.1080/14992020902894541. [DOI] [PubMed] [Google Scholar]
- Williams W. Noise exposure levels from personal stereo use. Int J Audiol. 2005;44:231–236. doi: 10.1080/14992020500057673. [DOI] [PubMed] [Google Scholar]
- Wong TW, Van Hasselt CA, Tang LS, Yiu PC. The use of personal cassette players among youths and its effects on hearing. Public Health. 1990;104:327–330. doi: 10.1016/s0033-3506(05)80524-4. [DOI] [PubMed] [Google Scholar]