Abstract
The accessory protein negative factor (Nef) from human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) is required for optimal viral infectivity and the progression to acquired immunodeficiency syndrome (AIDS). Nef interacts with the endocytic machinery, resulting in the down-regulation of cluster of differentiation antigen 4 (CD4) and major histocompatibility complex class I (MHCI) molecules on the surface of infected cells. Mutations in the C-terminal flexible loop of Nef result in a lower rate of internalization by this viral protein. However, no loop-dependent binding of Nef to adaptor protein-2 (AP-2), which is the adaptor protein complex that is required for the internalization of proteins from the plasma membrane, could be demonstrated. In this study we investigated the relevance of different motifs in Nef from SIVmac239 for its internalization, CD4 down-regulation, binding to components of the trafficking machinery, and viral infectivity. Our data suggest that the binding of Nef to the catalytic subunit H of the vacuolar membrane ATPase (V-ATPase) facilitates its internalization. This binding depends on the integrity of the whole flexible loop. Subsequent studies on Nef mutant viruses revealed that the flexible loop is essential for optimal viral infectivity. Therefore, our data demonstrate how Nef contacts the endocytic machinery in the absence of its direct binding to AP-2 and suggest an important role for subunit H of the V-ATPase in viral infectivity.
INTRODUCTION
Negative factor (Nef) from human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) was initially described as a “negative factor” for viral replication (Fisher et al., 1986). However, subsequent studies revealed Nef to be essential for high viral loads and the development of acquired immunodeficiency syndrome (AIDS) (Kestler et al., 1991; Daniel et al., 1992; Deacon et al., 1995; Kirchhoff et al., 1995). Via endocytic motifs, Nef also interacts with intracellular trafficking pathways, resulting in its internalization from the plasma membrane (Foti et al., 1997; Mangasarian et al., 1997; Craig et al., 1998; Greenberg et al., 1998a). These tyrosine- or dileucine-based motifs are implicated in the binding to adaptor protein (AP) complexes whose function is to connect proteins to clathrin (Pearse and Robinson, 1990).
The tyrosine-based motif conforms to the amino acid sequence “Yxxθ ” and the dileucine-based motif corresponds to the sequence “D/ExxxLθ ” (Y, tyrosine; D, aspartic acid; E, glutamic acid; x, any amino acid; θ, amino acid with bulky hydrophobic side chain, i.e., leucine, isoleucine, phenylalanine, methionine, or valine). AP complexes are heterotetramers consisting of two large subunits, a medium chain, and a small chain. Four different AP complexes are known to mediate protein transport between different subcellular compartments. AP-1, AP-3, and presumably also AP-4 are involved in vesicle-mediated protein transport from the trans-Golgi network (TGN) to endosomes and lysosomes (Pearse and Robinson, 1990; Dell'Angelica et al., 1997, 1999; Hirst et al., 1999). AP-2 is involved in the internalization of proteins from the plasma membrane and is found in clathrin-coated pits and vesicles, which mediate protein transport from the plasma membrane to early endosomes (Pearse and Robinson, 1990).
Known functions of Nef include cellular activation pathways, increased virion infectivity as well as internalization of cluster of differentiation antigen 4 (CD4) and major histocompatibility complex I (MHC I) determinants (Fisher et al., 1986; Kestler et al., 1991; Aiken et al., 1994; Baur et al., 1994; Sawai et al., 1994, 1996; Schwartz et al., 1996; Fackler et al., 1999, 2000). However, because motifs in Nef that are required for the internalization of CD4 (Aiken et al., 1994; Bresnahan et al., 1998; Craig et al., 1998; Greenberg et al., 1998a; Lu et al., 1998; Piguet et al., 1998) do not affect the sequestration of MHC I determinants, they are mechanistically different. Nef-mediated down-regulation of MHC I molecules depends on the ability of Nef to misroute their trafficking, resulting in their retrieval to the TGN (Schwartz et al., 1996; Greenberg et al., 1998b; Le Gall et al., 1998). Whereas the removal of MHC I determinants is thought to protect infected cells from lysis by cytotoxic T lymphocytes (Collins et al., 1998a), the internalization of CD4 might protect infected cells from superinfection (Benson et al., 1993) and prevent receptor interference during virus production (Lama et al., 1999; Ross et al., 1999).
A Nef-binding protein (Nef-binding protein-1 [NBP-1]) was identified as the catalytic subunit H (V1H) of the vacuolar membrane ATPase (V-ATPase) (Lu et al., 1998). Two acidic amino acid residues in the C-terminal flexible loop of Nef from HIV-1SF2 were found to be essential for its binding to V1H. This mutant Nef protein was not only highly defective for its internalization but also lost its ability to down-regulate CD4 (Lu et al., 1998). These data suggested that the tyrosine- or dileucine-based motifs are not the only signals important for the internalization of Nef and CD4. Except for the tyrosine-based motifs that were found near the N terminus of Nef from SIV and HIV-2, the endocytic ability of all Nef proteins depends on the C-terminal flexible loop. However, although a weak interaction between Nef from HIV-1 and AP-2 had been suggested, most reports could not demonstrate its direct binding to AP-2, the AP complex that is required for the internalization of proteins from the plasma membrane (Greenberg et al., 1998a; Lock et al., 1999). Because AP-1 is not involved in this trafficking, the loop-dependent interaction between Nef and the β-subunit of AP-1 (Greenberg et al., 1998a) also could not account for the internalization of Nef. Thus, this study investigated how sequences in the flexible loop of Nef affect its internalization and whether mutations of these motifs contribute to viral infectivity.
MATERIALS AND METHODS
Generation of Constructs
CD8-Nef hybrid proteins consisting of the extracellular and transmembrane portion of human CD8, a cytosolic portion representing the whole open reading frame of SIVmac239 Nef, and a C-terminal c-myc sequence were created as previously described (Baur et al., 1994; Sawai et al., 1996). Similarly, the CD8-V1H construct was generated by polymerase chain reaction-cloning, by using the full-length V1H (previously named NBP-1) clone (Lu et al., 1998) as a template. Mutations in the nef gene were generated by using the QuikChange site-directed mutagenesis kit (Stratagene, San Diego, CA) or the Transformer site-directed mutagenesis kit (Clonetech, Palo Alto, CA) according to the manufacturers' instructions. A construct expressing the α chain of the interleukin-2 receptor was kindly provided by Dr. Warner Greene (University of California, San Francisco, CA). The proviral constructs pVP-1 and pVP-2 were kindly provided by Dr. Paul Luciw (University of California, Davis, CA). To generate proviruses with mutations in the nef gene, the pEF BOS-CD8-Nef constructs were digested with BglII and NdeI. The resulting fragment spanned most of the open reading frame of Nef and included all different mutations. This fragment was then subcloned into the pVP-2 proviral vector, which was previously cut with the same enzymes. pVP-1 proviral construct (10 μg) containing the 5′ coding region of SIVmac239 was digested with SphI and ApaI, and 10 μg of the pVP-2 proviral construct containing the 3′ coding region of SIVmac239 was digested with SphI and PvuI. The larger fragments were gel purified and ligated overnight at 16°C by using T4-DNA-Ligase (New England Biolabs, Boston, MA). Glutathione S-transferase (GST)-Nef fusion proteins were generated by polymerase chain reaction-cloning of the respective SIVmac239 Nef, HIV-1NL4-3, or HIV-1SF2 Nef cDNA into the BamHI/EcoRI site of the pGEX-2TK (Amersham-Pharmacia Biotech, Piscataway, NJ) vector. The μ2 (AP-2) construct was kindly provided by M. Robinson, University of Cambridge, Cambridge, England.
Cell Culture and Transfections
293-T, Jurkat, and CEMx174 cells were obtained from American Type Culture Collection (Manassas, VA). The following reagent was obtained through the AIDS Research and Reference Reagent Program, AIDS Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health: CMMT-CD4-LTR-β-Gal (sMAGI) from Dr. Julie Overbaugh (Chackerian et al., 1995). 293-T and sMAGI cells were grown in DMEM media containing 10% fetal calf serum, penicillin, and streptomycin in the presence of 0.5 mg/ml Geneticin (Life Technologies, Grand Island, NY) or 0.2 mg/ml Geneticin (Life Technologies) and 0.1 mg/ml Hygromycin B (Roche Molecular Biochemicals, Indianapolis, IN), respectively. Jurkat and CEMx174 cells were grown in RPMI 1640 media containing 10% fetal calf serum, penicillin, and streptomycin. Transfections of 293-T cells were performed with Lipofectamine (Life Technologies) according to the manufacturer's instructions by using 1–5 μg (10–20 μg for kinetic assay) of total DNA. Transfections of Jurkat and CEMx174 cells were performed by electroporation by using 10 × 106 cells and 15–30 μg of total DNA at a setting of 200 V, 950 μF.
Steady-State Internalization Assays
293-T and Jurkat cells were harvested 24 h after transfection and washed in cold phosphate-buffered saline (PBS). Half of the cells was saved for Western blotting and half was resuspended in 200 μl of PBS, 3% bovine serum albumin. To each tube 10 μl of anti-CD25-PE and 10 μl of anti-CD8-fluorescein isothiocyanate antibodies were added and the tubes were incubated on ice for 45 min. The cells were washed three times in PBS, 3% bovine serum albumin and resuspended in 500 μl of PBS. One microliter of propidium iodide (40 μg/ml final concentration) was added to each tube to stain dead cells. Fluorescence-activated cell sorter analysis (FACS) was performed with the “FACSCalibur” (Becton Dickinson, San Jose, CA) machine, by using the CELLQuest software (Becton Dickinson). The cells were gated for viability and CD25 (interleukin-2 receptor α chain), representing all transfected cells. The level of surface expression of the different hybrid CD8-Nef proteins was calculated as the percentage of FITC signal obtained with the truncated CD8 protein (=100%). Recycling assays were performed as described previously (Piguet et al., 1998).
Kinetic Internalization Assays
Forty-eight hours after transfection, 293-T cells were harvested in Tris-EDTA buffer (1 mM EDTA, 50 mM Tris, pH 7.2) and washed once in PBS, 2% fetal calf serum. Twenty-five percent of the cells were saved for Western blotting and the remaining cells were stained for 45 min with 20 μl of anti-CD8-PE antibody. The cells were washed in PBS, 2% fetal calf serum and resuspended in 200 μl of the same. Eppendorf tubes containing 500 μl of DMEM media, 5% fetal calf serum were prewarmed to 37°C in a water bath. Cell suspension (150 μl) was added to the prewarmed media. Aliquots (100 μl) were removed at different time points (0, 5, 10, and 15 min) and added to 2 ml of cold PBS, pH 7.5. After 2 min, 10 ml of PBS, pH 8 was added for neutralization. The cells were pelleted and resuspended in 500 μl of PBS. FACS analysis was performed and the geometric mean fluorescence of the cells at the different time points was measured. The geometric mean fluorescence at time point 0 min was subtracted and the resulting value was divided through the value at time point 0 min. This value represents the percentage of internalization at a given time point (Chambers et al., 1993; Mangasarian et al., 1997).
CD4 Down-Regulation
293-T cells were cotransfected with 5 μg of a CD4-expressing plasmid and 5 μg of the respective Nef wild-type or mutant construct as described above. Twenty-four hours after transfection the cells were stained with an anti-CD4-PE antibody and analyzed by FACS. The CD4 level of the positive control was set to 100% and all other CD4 levels were calculated relative to this value.
Yeast Two-Hybrid Binding Assay
Yeast two-hybrid binding assays were performed with the Matchmaker Two-Hybrid System 2 (Clontech) according to the manufacturer's instructions. The liquid culture β-galactosidase assay was performed with CPRG and ONPG reagents by using the Y187 cell line according to the protocol from Clontech (Yeast Protocols Handbook). All SIVmac239 Nef sequences were subcloned into the EcoRI/BamHI site of the pAS2-1 vector that has the GAL4 DNA-binding domain upstream of the cloning site. The open reading frames of V1H and AP-2 (μ2) were subcloned into the pACT2 vector that has the GAL4 activation domain upstream of the cloning site.
In Vitro Binding Assay
In vitro binding experiments were performed with GST-Nef proteins, purified from Escherichia coli and in vitro translated μ2 (AP-2). Proteins were incubated with 10 mM PMSF, 150 mM NaCl, 50 mM Tris, pH 7.4) for 3 h, 4°C and washed three times in the same buffer. Proteins were separated by SDS-PAGE. The protein gels were subsequently exposed to film to visualize bound μ2 (AP-2) or stained with Coomassie to visualize the GST input. In vitro translation of μ2 (AP-2) was performed by using the TNT T7-coupled reticulocyte lysate system (Promega, Madison, WI) according to the manufacturer's instructions.
Antibodies and Immunoblotting
The fluorescence-labeled antibodies against CD8, CD4, and CD25 were obtained from Becton Dickinson. The c-myc (A14) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and the anti-actin antibody was obtained from Roche Molecular Biochemicals. The following reagents were obtained through the AIDS Research and Reference Reagent Program, AIDS Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health: SIVmac p27Gag monoclonal antibody (55–2F12) from Dr. Niels Pedersen (Higgins et al., 1993) and CD4 antiserum (T4-4) from Dr. R. Sweet (Willey et al., 1992). The anti-SIV Nef antibody was kindly provided by Earl T. Sawai (University of California, Davis). Protein immunoblots were prepared as described (Mandic and Lowe, 1999) by using antibodies against c-myc, SIV Nef, p27Gag, CD4, and actin.
Viral Infectivity Assay
Proviral constructs were generated as described above and transfected into CEMx174 cells. After the appearance of a cytopathic effect the cell media was harvested and assayed for reverse transcriptase (RT) activity. 1 × 104 sMAGI cells were seeded into each well of a 96-well plate. The following morning 100 μl of viral supernatant was added to each well and the cells were incubated at 37°C for 5 h. The viral supernatant was then replaced with fresh media and the cells were incubated for additional 24 h. Infected cells were visualized by β-galactosidase staining and counted under a microscope. The relative infectivity was calculated as the ratio of infected (blue) cells and RT activity.
RESULTS
Effects of Mutations in Nef from SIVmac239 on Its Internalization
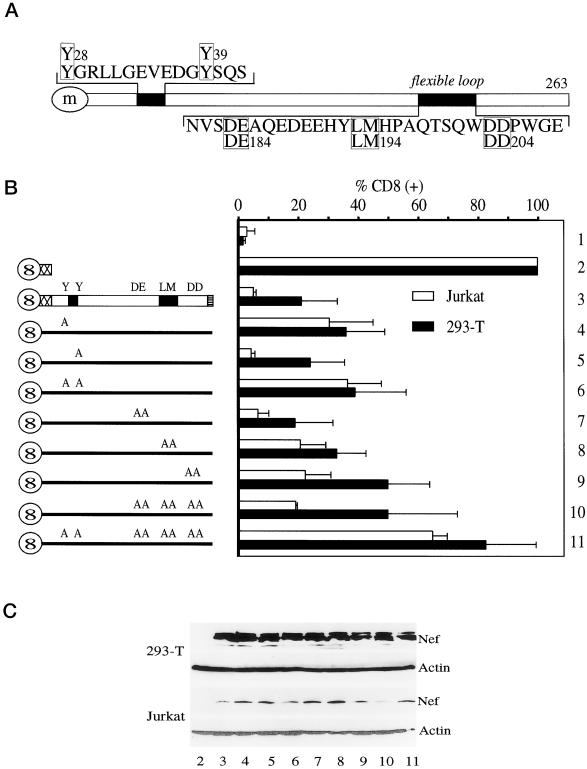
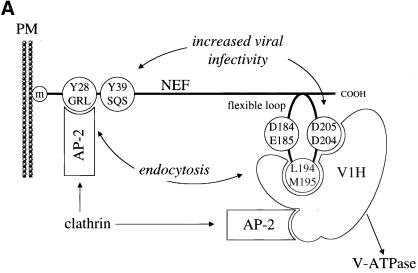
As shown in Figure 1A, Y28GRL and Y39SQS are tyrosine-based motifs that were implicated previously in the binding to AP-2 (Piguet et al., 1998). They are located in the N-terminal flexible anchor domain of Nef (Geyer et al., 1999) and are present only in Nef from SIV and HIV-2 but not HIV-1. Three additional motifs are located in the C-terminal flexible loop of Nef that is conserved between different alleles of Nef. D184E185 corresponds to a recently described motif in Nef from HIV-1 that is required for the trafficking from the early-to-late endosomes and lysosomes and that was reported to bind to β-COP (Piguet et al., 1999). The L194M195 motif represents a dileucine-based motif and is required for the internalization of Nef from the plasma membrane (Bresnahan et al., 1999). Finally, the D204D205 motif corresponds to the binding site of V1H in Nef from HIV-1SF2 that is also required for the internalization of Nef and CD4 (Lu et al., 1998).
Figure 1.
Effect of mutations in Nef from SIVmac239 on its internalization. (A) Schematic representation of Nef from SIVmac239 and location of different endocytic motifs. Whereas the two tyrosine-based endocytic motifs (Y28GRL and Y39SQS), which were implicated in the binding to AP-2, are found near the N terminus, the three motifs implicated in the binding to β-COP (D184E185), AP complexes (L194M195), and V1H (D204D205) are located in the C-terminal flexible loop of the protein. (m) is myristate. (B) Effects of mutations in Nef from SIVmac239 on its internalization at steady state. Hybrid CD8-Nef proteins were expressed in Jurkat or 293-T cells and their surface levels [% CD8(+)] were evaluated by FACS. The surface expression of CD8 alone (lane 2) was set to 100% and the amount of internalization of the chimeras (lanes 3–11) was calculated relative to this value. The empty pEF-BOS vector was used as a negative control (lane 1). Error bars indicate SEM from at least three independent experiments. (C) Levels of expression of Nef chimeras in 293-T and Jurkat cells. Levels of different Nef proteins were determined by Western blotting (lanes 2–11). They were compared with the endogenous actin.
To investigate the importance of these different motifs for the internalization of Nef, hybrid mutant CD8-Nef proteins were expressed in 293-T or Jurkat cells (Figure 1B). Because previous studies used mainly 293-T cells, we also tested Jurkat cells, which can be infected by HIV. A functional identity between the hybrid CD8-Nef and wild-type Nef proteins had been established (Lu et al., 1998). Whereas levels of expression of these proteins were similar in 293-T cells, a slightly greater variability was observed in Jurkat cells (Figure 1C). First, the steady-state surface expression of the hybrid CD8-Nef proteins was examined [Figure 1B, CD8(+)]. The Y28A (Figure 1B, lane 4) but not the Y39A (Figure 1B, lane 5) mutant proteins exhibited a higher surface expression than the wild-type protein (Figure 1B, lane 3). When both mutations were combined (Y28A/Y39A; Figure 1B, lane 6), the surface expression of this protein was similar to the Y28A mutant protein (Figure 1B, lane 4). Moreover, the DE184AA mutant protein (Figure 1B, lane 7) had a similar surface expression to the wild-type protein (Figure 1B, lane 3). This result is different from Nef from HIV-1, where the respective mutation in the β-COP-binding site resulted in a higher surface expression of Nef due to its increased recycling to the plasma membrane (Piguet et al., 1999). The LM194AA and the DD204AA mutant proteins (Figure 1B, lanes 8 and 9) both showed higher surface levels than were observed with the wild-type protein (Figure 1B, lane 3), which manifested the importance of these motifs for the internalization of Nef. Surprisingly, the DE184AA/LM194AA/DD204AA triple mutant protein (Figure 1B, lane 10) did not exhibit a higher surface expression than the most severe single loop mutant protein alone (DD204AA; Figure 1B, lane 9). Finally, a Nef protein with a combination of all investigated mutations (Y28A/Y39A/DE184AA/LM194AA/DD204AA) (Figure 1B, lane 11) exhibited surface expression levels comparable to the truncated CD8 protein (Figure 1B, lane 2). This observation suggests that the L194M195 and D204D205 motifs are part of a common binding surface that likely interacts with the same protein. Despite variations in levels of surface expression of these different proteins between 293-T and Jurkat cells, the pattern of different mutations in Nef on their steady-state surface expression was similar (Figure 1B). Thus, in the absence of tyrosine-based motifs, the internalization of Nef depends entirely on its flexible loop. Because the triple loop mutant protein (Figure 1B, lane10; Figure 2) exhibited a similar impairment for the internalization as the LM194AA and DD204AA mutant proteins, an intact flexible loop rather than a specific short motif in the loop is required for the internalization of the protein.
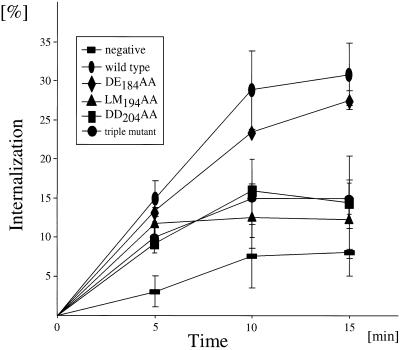
Figure 2.
Internalization rates of Nef loop mutant proteins from SIVmac239. Shown is a kinetic internalization assay in 293-T cells. Internalization rates of the Nef DE184AA, LM194AA, DD204AA, and DE184AA/LM194AA/DD204AA mutant proteins were compared with the wild-type Nef protein and the truncated CD8 protein (=negative). Error bars indicate SEs of the mean from three independent experiments.
Internalization Rates of the LM194AA and DD204AA Loop Mutant Nef Proteins from SIVmac239 Are Similar
To verify that increased steady-state surface levels of the LM194AA and DD204AA mutant proteins were indeed due to a reduction in rates of internalization from the plasma membrane, they were also tested in a kinetic internalization assay (Figure 2). After 15 min, 31% of the wild-type hybrid CD8-Nef protein and 8% of the truncated CD8 protein were internalized. The latter represents the nonspecific internalization of plasma membrane proteins. Internalization levels were reduced to 12 and 14% for the LM194AA and the DD204AA mutant proteins, respectively (Figure 2). These two mutant proteins did not lose fully their ability for internalization to levels of the truncated CD8 protein, which revealed the contribution of the tyrosine-based motif near the N terminus of Nef from SIVmac239. These results also confirm a similarly reduced internalization rate for the LM194AA and DD204AA mutant proteins as observed at steady state and again suggest that these two motifs are not independent from each other but rather behave as a single functional unit (Figure 1B, lanes 8 and 9). The DE184AA mutant protein exhibited a slightly reduced ability for internalization compared with the wild-type protein, which was not obvious at steady state (Figure 1B, lane 7). Finally, the triple loop mutant protein exhibited a similarly reduced internalization rate to that observed for the LM194AA and DD204AA mutant proteins (Figure 2). No recycling of these proteins could be detected (our unpublished results). Overall, these data confirm results from the steady-state surface expression (Figure 1B).
Requirement of the Flexible Loop in Nef from SIVmac239 for CD4 Down-Regulation
To assess whether the loss of internalization that was observed for the mutant Nef proteins also affected their ability to down-regulate CD4, wild-type and hybrid mutant CD8-Nef proteins were coexpressed with CD4 in 293-T cells and surface levels of CD4 were quantified. As expected, the wild-type hybrid CD8-Nef protein (Figure 3, lane 3) down-regulated CD4 to 32% of control levels (100%; Figure 3, lane 2). The Y39A mutant protein also down-regulated CD4 efficiently (23%; Figure 3, lane 5), whereas the Y28A (55%; Figure 3, lane 4) and the Y28A/Y39A (46%; Figure 3, lane 6) mutant proteins lost some of their ability for CD4 down-regulation. Interestingly, the LM194AA (72%; Figure 3, lane 8) and DD204AA (81%; Figure 3, lane 9) mutant proteins both lost most of their ability for CD4 down-regulation and the DE184AA/LM194AA/DD204AA and Y28A/Y39A/DE184AA/LM194AA/DD204AA triple mutant proteins (104 and 134%; Figure 3, lanes 10 and 11) even induced slightly higher surface levels of CD4 than the truncated CD8 protein. The DE184AA mutant protein was also slightly impaired for CD4 down-regulation (52%, Figure 3, lane 7). Therefore, effects of the different hybrid CD8-Nef proteins on CD4 down-regulation correlated with their ability for internalization.
Figure 3.
Effects of mutations in Nef from SIVmac239 on the internalization of CD4. (A) Internalization of CD4. Shown is the effect of mutations in Nef on CD4 down-regulation at steady state. Different Nef proteins and CD4 were coexpressed in 293-T cells. Levels of surface expression of CD4 [% CD4(+)] were evaluated by FACS. The surface expression of CD4 in the presence of CD8 alone (negative) was set to 100% and levels of CD4 in the presence of other hybrid mutant Nef proteins were calculated relative to this value. Error bars indicate SEMs of the mean from at least three independent experiments. (B) Levels of expression of Nef chimeras in 293-T cells. Levels of different Nef proteins were determined by Western blotting (lanes 2–11). They were compared with the endogenous actin.
V1H binds to the Flexible Loop of Nef from SIVmac239
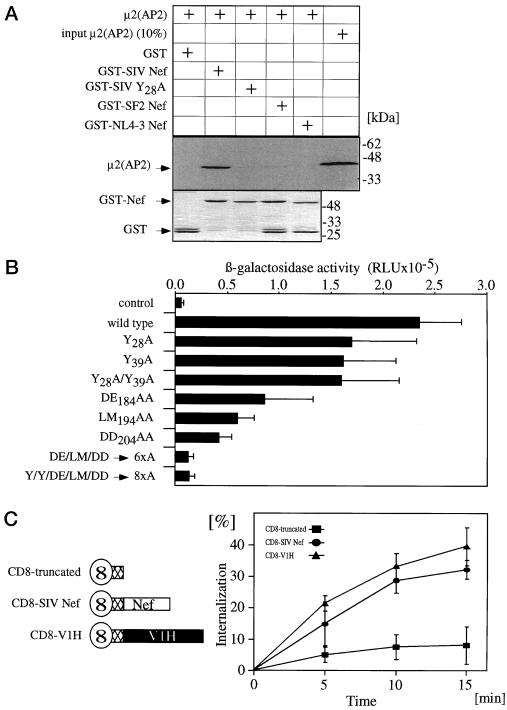
Using in vitro binding assays, previous studies showed that the N-terminal tyrosine-based motifs in SIVmac239 Nef bind to the medium chain (μ2) of AP-2. Whereas Nef from SIVmac239 bound strongly to μ2, the mutant Nef protein, where both of the N-terminal tyrosine-based endocytic motifs were mutated, failed to bind to AP-2 (Piguet et al., 1998; Bresnahan et al., 1999). After performing an in vitro binding assay, we also found that this Nef binds directly to μ2 (Figure 4A). However, because the Y28A mutant protein lost all its binding to μ2, this binding was dependent on the tyrosine 28 alone. Additionally, no binding of μ2 to Nef from HIV-1NL4-3 and HIV-1SF2 was observed, which argues against a direct binding of Nef to AP-2 in the absence of a functional tyrosine-based motif (Figure 4A). We also used the yeast two-hybrid assay to examine the binding of μ2 to all other mutant Nef proteins from SIVmac239 and lost the binding to μ2 only when the tyrosine 28 was mutated to alanine. The loop as well as the tyrosine 39 mutant proteins could still bind to μ2 (AP-2) (our unpublished results).
Figure 4.
In vitro binding assays. (A) μ2 binds to the N-terminal tyrosine-based motif in Nef from SIVmac239. Shown is an autoradiogram of the binding of hybrid GST-Nef proteins from SIVmac239 (GST-SIV Nef and GST-SIV Y28A) and HIV-1 (GST-SF2 and GST-NL4–3 Nef) to the in vitro translated μ2 chain from AP-2 (top). Input of the GST proteins is shown in the lower panel. (B) V1H binds to the flexible loop of Nef from SIVmac239. Shown is a liquid yeast two-hybrid assay testing the interaction of wild-type and mutant Nef proteins from SIVmac239 with V1H. The β-galactosidase activity is expressed in relative light units (RLU). (C) Similar internalization rate of V1H and Nef. Kinetic internalization assay of CD8, CD8-SIV Nef, and CD8-V1H chimeras in 293-T cells.
Our previous studies also demonstrated binding of Nef from SIVmac239 to V1H in vivo. However, the exact binding site for V1H in this Nef remained unknown (Lu et al., 1998). Because previous studies mapped interactions with medium chains μ1 and μ2 by using yeast two-hybrid approaches (Aguilar et al., 1997), we chose the same assay to examine the binding to V1H. As seen in Figure 4B, the LM194AA and DD204AA mutant proteins lost most of their binding to V1H and the DE184AA mutant protein exhibited a weak binding. As expected, the triple loop mutant proteins lost all their binding to V1H. In sharp contrast, the Y28A and Y39A mutant Nef proteins exhibited strong binding to V1H (Figure 4B). Because all these mutations affected the binding to V1H, we conclude that V1H not only binds to Nef but also that this binding depends on a larger part of the flexible loop of Nef rather than on a specific single short motif.
V1H Is Internalized Similarly to Nef from SIVmac239
To test whether V1H itself can be internalized from the plasma membrane, we performed an internalization assay by using the hybrid CD8-V1H and CD8-SIV Nef proteins. Interestingly, in our kinetic assays, CD8-V1H chimera internalized as efficiently as the CD8-SIV Nef fusion protein (Figure 4C). This observation demonstrates that V1H has the intrinsic ability to be internalized from the plasma membrane. Thus, its interaction with Nef could be sufficient for the observed internalization of the viral protein (Figure 4B).
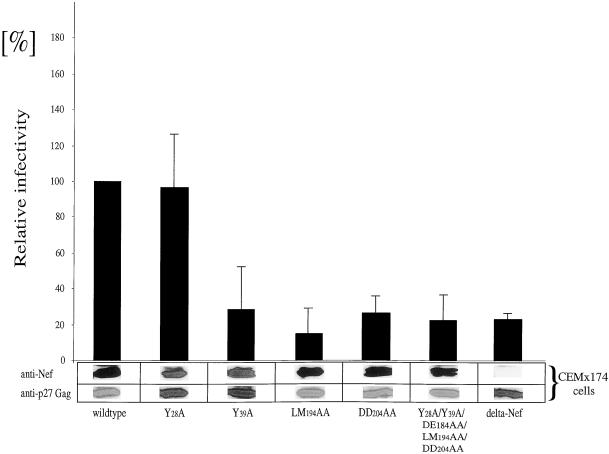
Flexible Loop of Nef from SIVmac239 Is Required for High Viral Infectivity
To investigate the relevance of mutations in the nef gene for viral infectivity, proviruses with corresponding mutations were created and a single round of replication assay was performed (Figure 5). Western blotting demonstrated equal levels of expression of Nef and p27Gag proteins in CEMx174 producer cells (Figure 5). Surprisingly, whereas the Y28A mutation did not affect viral infectivity (Figure 5, lane 2), both the LM194AA and the DD204AA mutant proteins (Figure 5, lanes 4 and 5) showed a dramatic reduction in viral infectivity to levels of the delta Nef virus (Figure 5, lane 7), Interestingly, the Y39A mutation also decreased greatly viral infectivity (Figure 5, lane 3). As expected from the observations for the single motif mutant proteins, a virus with all investigated mutations combined (Y28A/Y39A/DE184AA/LM194AA/DD204AA) resulted in a loss of infectivity to levels of the delta Nef virus (Figure 5, lane 6). These results suggest an important role of the flexible loop of Nef in the enhancement of virion infectivity.
Figure 5.
The flexible loop in SIVmac239 Nef is required for high viral infectivity. Proviral constructs with mutations in the nef gene were transfected into CEMx174 cells and new virions were tested in a single-round of replication assay. The relative infectivity of the wild-type virus was arbitrarily set to 100%. Comparable ratios of protein expression of Nef and p27Gag were determined by Western blotting of CEMx174 producer cells. Error bars indicate SEMs from at least three independent experiments.
DISCUSSION
In this study we demonstrated that the first (Y28GRL) but not the second (Y39SQS) tyrosine-based motif in Nef from SIVmac239 binds to the medium chain of AP-2. The L194M195, D204D205 and to a lesser extent D184E185 motifs were also required for the internalization of Nef and CD4. These motifs mediated the binding to V1H and did not bind to μ2 (AP-2). Viruses with mutations of the dileucine (L194M195) or the diacidic (D204D205) motifs as well as the second (Y39SQS) but not the first (Y28GRL) tyrosine-based motifs lost their infectivity to levels of the delta Nef virus. We conclude that Nef from SIVmac239 interacts via its C-terminal flexible loop with V1H, the catalytic subunit of the V-ATPase, and that this interaction enables Nef to be internalized from the plasma membrane in the absence of its direct binding to AP-2. The observed reduction in the infectivity of the loop mutant viruses also suggests an important role of the flexible loop and V1H in viral infectivity (Figure 6A).
Figure 6.
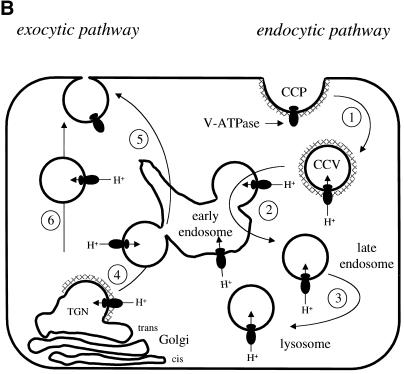
Model: Requirement of motifs in SIVmac239 Nef for its internalization and viral infectivity. (A) Shown is the interaction of the C-terminal flexible loop of Nef with V1H. Both the L194M195 and D204D205 and to a lesser degree the D184E185 motifs are required for the binding to V1H, which then binds to AP-2. AP-2 also binds directly to the first tyrosine-based motif (Y28GRL). AP-2 binds to clathrin and V1H binds to and is part of the V-ATPase. Both loop motifs (L194M195 and D204D205) and the second tyrosine-based motif (Y39SQS) are required for optimal viral infectivity. (B) Shown is the trafficking route of the V-ATPase in the cell (modified from Stevens and Forgac, 1997). It is similar to trafficking routes of Nef. The exocytic (left side) and endocytic (right side) pathways are highlighted for clarity. On the right side is the route of the V-ATPase from the plasma membrane to early endosomes (1), late endosomes (2), and lysosomes (3). The V-ATPase also trafficks in the exocytic route from the TGN directly (6) or indirectly via the endosomal compartment (4 and 5) to the plasma membrane (CCP, clathrin-coated pit; CCV, clathrin coated vesicle; m, myristate; PM, plasma membrane; TGN, trans-Golgi network).
Because the second tyrosine-based motif (Y39SQS) lacks a bulky hydrophobic residue at position 4, which is a hallmark of tyrosine-based endocytic motifs (Ohno et al., 1995; Aguilar et al., 1997), it is not surprising that this sequence in Nef from SIVmac239 did not contribute to the internalization of CD4 or the binding to μ2 (AP-2). Interestingly, the tyrosine 28 can be phosphorylated in cells (our unpublished results). This observation agrees with our previous finding with Nef from SIVpbj14 (Luo and Peterlin, 1997). Because a phosphorylated tyrosine 28 does not bind to μ2, it cannot act as a tyrosine-based endocytic motif (Owen and Evans, 1998). This finding could explain the cell-dependent differences for the internalization of Nef, which were observed between the Jurkat and 293-T cells (Figure 1B).
However, we were surprised that the flexible loop of Nef behaved like a functional unit. Mutations of the dileucine or diacidic motifs were indistinguishable in our functional assays. Both motifs were also required for the binding to V1H but did not bind to μ2. Our data from the yeast two-hybrid binding studies are consistent with previously demonstrated interactions between Nef from SIVmac239 and V1H in cells (Lu et al., 1998). AP-2 and the V-ATPase are both recruited into clathrin-coated pits at the plasma membrane (Mellman et al., 1986; Pearse and Robinson, 1990; Dell'Angelica et al., 1997, 1999; Hirst et al., 1999). Moreover, the reported binding to subunits of AP-1 required only the dileucine motif in Nef (Bresnahan et al., 1998, 1999; Craig et al., 1998; Greenberg et al., 1998a; Rapoport et al., 1998). Because our functional studies demonstrated clearly that the dileucine and diacidic motifs act as a unit, we were looking for a protein that requires both sequences for its binding to Nef. V1H fulfilled these criteria. Moreover, V1H binds directly to μ2 (Geyer and Peterlin, unpublished data), which is consistent with previous reports of AP-2 binding to the V-ATPase (Myers and Forgac, 1993; Liu et al., 1994), and internalizes efficiently from the plasma membrane when expressed as a CD8-V1H chimera (Figure 4C). This interaction between V1H and AP-2 might be critical for the trafficking of the V-ATPase, and therefore also of Nef and CD4. Given the dynamic balance of assembly and disassembly of multiprotein complexes, it remains to be established whether V1H acts as an adaptor molecule when dissociated from the V-ATPase or whether Nef recruits the entire proton pump (Kane, 2000).
Interestingly, the direct interaction between Nef and AP-2 via the first tyrosine-based endocytic motif (Y28GRL) was irrelevant for its enhancement of virion infectivity. Rather, the mutation of the second motif (Y39SQS → A39SQS), which was not required for its internalization, had a profound effect, suggesting that tyrosine-mediated internalization does not play a major role for the infectivity of SIVmac239. The significance of this tyrosine 39 is also highlighted in chimeric viruses between HIV and SIV (SHIV) (Mandell et al., 1999). When SHIV starts to replicate efficiently, it is this tyrosine that appears in Nef from HIV. In sharp contrast, the appearance of the first tyrosine-based motif has not been observed. Because the flexible loop is highly conserved between different Nef alleles it is not surprising that no mutations appeared in this region of Nef. Unlike the tyrosine-based motif (Y28GRL), mutations in the flexible loop also contributed to the infectivity of SIVmac239. Interestingly, recent in vivo studies reported the reversion of a D204 → R204 mutation in Nef from a mutant SIVmac239 (Kirchhoff et al., 1999) as well as the reduction in infectivity of a mutant HIV-1 bearing mutations in the dileucine motif in Nef (Craig et al., 1998), again emphasizing the importance of the dileucine and diacidic motifs for viral infectivity.
How could the interaction between Nef and V1H affect viral infectivity? It could help in the formation of new virions. As part of the V-ATPase, V1H is known to control the pH of different compartments and to move along the endocytic and secretory pathways (Stevens and Forgac, 1997) (Figure 6B). Interestingly, the influenza virus encodes a protein that controls the acidification of cellular organelles to prevent the activation of its envelope and fusion with these compartments before the virus is released (Steinhauer et al., 1991). The interaction between Nef and V1H could play a similar role and prevent the activation of the fusogenic activity of gp41 by acidic pH in certain strains of HIV-1 (Fackler and Peterlin, 2000). Equally intriguing, the human T-cell leukemia virus-I (HTLV-I) encodes a membrane-associated protein, p12(I), which also binds to the V-ATPase (Franchini et al., 1993; Collins et al., 1998b). The p12(I) gene is not only found in the same location in the genome of HTLV-I as the nef gene in that of HIV and SIV but also its gene product is important for virion infectivity. Thus, these latter effects together with the internalization and proteolysis of cellular and viral structures could be equally important for effects of Nef in infected cells. These and other aspects of Nef and V1H are currently under investigation and could reveal the underlying mechanism of Nef-mediated enhancement of infectivity in primate lentiviruses that leads to the development of AIDS.
ACKNOWLEDGMENTS
We thank Michael Armanini and Paula Zupanc-Ecimovic for administrative support, Paul Luciw and colleagues for reagents and suggestions on generation of the SIVmac239 mutant viruses, and Pat Bresnahan and Wes Yonemoto for supplying the protocol for the kinetic assay and valuable suggestions. O.T.F. and M.G. were supported by fellowships from the Deutsche Forschungsgemeinschaft and the European Molecular Biology Organization, respectively. This work was funded by grants from the National Institutes of Health (1RO1AI38532-01) and the Howard Hughes Medical Institute.
Abbreviations used:
- AP
adaptor proteins
- β-Cop
β-coatomer protein
- CD4
cluster of differentiation antigen 4
- HIV
human immunodeficiency virus
- HTLV-I
human T cell leukemia virus-I
- MHC I
major histocompatibility complex class I
- NBP-1
Nef binding proein 1
- Nef
negative factor
- RT
reverse transcriptase
- SIV
simian immunodeficiency virus
- V1H
subunit H of the V-ATPase
- V-ATPase
vacuolar membrane ATPase or universal proton pump
REFERENCES
- Aguilar RC, Ohno H, Roche KW, Bonifacino JS. Functional domain mapping of the clathrin-associated adaptor medium chains μ1 and μ2. J Biol Chem. 1997;272:27160–27166. doi: 10.1074/jbc.272.43.27160. [DOI] [PubMed] [Google Scholar]
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C, Peterlin BM. HIV-1 Nef leads to inhibition or activation of T-cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Benson RE, Sanfridson A, Ottinger JS, Doyle C, Cullen BR. Down-regulation of cell-surface CD4 expression by simian immunodeficiency virus prevents viral super infection. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan PA, Yonemoto W, Ferrell S, Williams-Herman D, Gelezunias R, Greene W. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 down-regulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- Bresnahan PA, Yonemoto W, Greene WC. Cutting edge: SIV Nef protein utilizes both leucine- and tyrosine-based protein sorting pathways for down-regulation of CD4. J Immunol. 1999;163:2977–2981. [PubMed] [Google Scholar]
- Chackerian B, Haigwood NL, Overbaugh J. Characterization of a CD4-expressing macaque cell-line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology. 1995;213:386–394. doi: 10.1006/viro.1995.0011. [DOI] [PubMed] [Google Scholar]
- Chambers JD, Simon SI, Berger EM, Sklar LA, Arfors K-I. Endocytosis of β2 integrins by stimulated human neutrophils analyzed by flow cytometry. J Leukocyte Biol. 1993;53:462–469. doi: 10.1002/jlb.53.4.462. [DOI] [PubMed] [Google Scholar]
- Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998a;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12(I) reduces viral infectivity in vivo. Blood. 1998b;91:4701–4707. [PubMed] [Google Scholar]
- Craig HM, Pandori MW, Guatelli JC. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Ellett A, Hooker DJ, McPhee DA, Greenway AL, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of H.IV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Bonifacino JS. AP-4, a novel protein complex related to clathrin adaptors J. Biol Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Lu XB, Frost JA, Geyer M, Jiang B, Luo W, Abo A, Alberts AS, Peterlin BM. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol Cell Biol. 2000;20:2619–2627. doi: 10.1128/mcb.20.7.2619-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Luo W, Geyer M, Alberts AS, Peterlin BM. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- Fisher AG, Ratner L, Mitsuya H, Marselle LM, Harper ME, Broder S, Gallo RC, Wong-Staal F. Infectious mutants of HTLV-III with changes in the 3′ region and markedly reduced cytopathic effects. Science. 1986;233:655–659. doi: 10.1126/science.3014663. [DOI] [PubMed] [Google Scholar]
- Foti M, Mangasarian VP, Lew DP, Krause K-H, Trono D, Carpentier J-L. Nef-mediated clathrin-coated pit formation. J Cell Biol. 1997;139:37–47. doi: 10.1083/jcb.139.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G, Mulloy JC, Koralnik IJ, Lomonico A, Sparkowski JJ, Andresson T, Goldstein DJ, Schlegel R. The human T-cell lymphotrophic virus type-I P12(I) protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+-A.T.Pase. J Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M, Munte CE, Schorr J, Kellner R, Kalbitzer HR. Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein. J Mol Biol. 1999;289:123–138. doi: 10.1006/jmbi.1999.2740. [DOI] [PubMed] [Google Scholar]
- Greenberg M, De Tulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for down-regulation of CD4. Curr Biol. 1998a;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Iafrate AJ, Skowronski J. The SH3 domain-binding and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998b;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JR, Sutjipto S, Marx PA, Pedersen NC. Shared antigenic epitopes of the major core protein of human and simian immunodeficiency virus isolates. J Med Primatol. 1993;21:265–269. [PubMed] [Google Scholar]
- Hirst J, Bright NA, Rous B, Robinson MS. Characterization of a fourth adaptor-related protein complex. Mol Biol Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane PM. Regulation of V-ATPases by reversible disassembly. FEBS Lett. 2000;469:137–141. doi: 10.1016/s0014-5793(00)01265-5. [DOI] [PubMed] [Google Scholar]
- Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Carl S, Sopper S, Sauermann U, Mätz-Rensing, Stahl-Hennig C. Selection of the R17Y substitution in SIVmac239 Nef coincided with a dramatic increase in plasma viremia and rapid progression to death. Virology. 1999;254:61–70. doi: 10.1006/viro.1998.9522. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Greenough TC, Brettler DB, Sulivan JL, Desrosier RC. Absence of intact Nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J-M, Schwartz O. Nef interacts with the μ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC class I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Feng Y, Forgac M. Activity and in vitro reassembly of the coated vesicle (H+)-ATPase requires the 50-kDa subunit of the clathrin assembly complex AP-2. J Biol Chem. 1994;269:31592–31597. [PubMed] [Google Scholar]
- Lock M, Greenberg ME, Iafrate AJ, Swigut T, Muench J, Kirchhoff F, Shohdy N, Skowronski J. Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of C.D4 endocytosis. EMBOJ. 1999;18:2722–2733. doi: 10.1093/emboj/18.10.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Yu H, Liu S-H, Brodsky FM, Peterlin BM. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- Luo W, Peterlin BM. Activation of the T-cell receptor signaling pathway by Nef from an aggressive strain of simian immunodeficiency virus. J Virol. 1997;71:9531–9537. doi: 10.1128/jvi.71.12.9531-9537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell CP, Reyes RA, Cho K, Sawai ET, Fang AL, Schmidt KA, Luciw PA. SIV/HIV nef recombinant virus (SHIVnef) produces simian AIDS in rhesus macaques. Virology. 1999;265:235–251. doi: 10.1006/viro.1999.0051. [DOI] [PubMed] [Google Scholar]
- Mandic R, Lowe AW. Characterization of an alternatively spliced isoform of rat vesicle associated membrane protein-2 (VAMP-2) FEBS Lett. 1999;451:209–213. doi: 10.1016/s0014-5793(99)00551-7. [DOI] [PubMed] [Google Scholar]
- Mangasarian VP, Foti M, Aiken C, Chin D, Carpentier J-L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Myers M, Forgac M. The coated vesicle vacuolar (H+)-ATPase associates with and is phosphorylated by the 50-kDa polypeptide of the clathrin assembly protein (AP-2) J Biol Chem. 1993;268:9184–9186. [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF, Robinson MS. Clathrin adapters, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Piguet V, Chen YL, Mangasarian A, Foti M, Carpentier JL, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier JL, Trono D. Nef-induced C.D4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TM, Oran AE, Cullen BR. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- Sawai ET, Baur A, Struble H, Peterlin BM, Levy JA, Cheng-Mayer C. Human-immunodeficiency-virus type-1 nef associates with a cellular serine kinase in T-lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai ET, Khan IH, Montbriand PM, Peterlin BM, Cheng-Mayer C, Luciw PA. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr Biol. 1996;6:1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J-M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Steinhauer DA, Wharton SA, Skehel JJ, Wiley DC, Hay AJ. Amantadine selection of a mutant influenza-virus containing an acid-stable hemagglutinin glycoprotein – evidence for virus-specific regulation of the pH of glycoprotein transport vesicles. Proc Natl Acad Sci USA. 1991;88:11525–11529. doi: 10.1073/pnas.88.24.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TH, Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol. 1997;13:779–808. doi: 10.1146/annurev.cellbio.13.1.779. [DOI] [PubMed] [Google Scholar]
- Willey RL, Maldarelli F, Martin MA, Strebel K. Human-immunodeficiency-virus type-1 vpu protein regulates the formation of intracellular gp 160-CD4. J Virol. 1992;66:226–234. doi: 10.1128/jvi.66.1.226-234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]