Abstract
Background
There is a need to better understand the safety of TNF inhibitors in patients with psoriatic disease in whom TNF inhibitors are frequently used as monotherapy.
Objective
Examine the risks of infection and malignancy with the use of TNF antagonists in adult patients with psoriatic disease.
Methods
Systematic search for trials of TNF antagonists for adults with plaque psoriasis (PsO) and psoriatic arthritis (PsA). We included randomized, placebo-controlled trials of etanercept, infliximab, adalimumab, golimumab, and certolizumab for the treatment of PsO and PsA. 20 out of 820 identified studies with a total of 6,810 patients were included. Results were calculated using fixed effects models and reported as pooled odds ratios (OR).
Results
ORs for overall infection and serious infection over a mean of 17.8 weeks were 1.18 (95% CI: 1.05, 1.33) and 0.70 (95% CI: 0.40, 1.21), respectively. When adjusting for patient-years, the incidence rate ratio for overall infection was 1.01 (95% CI: 0.92, 1.11). The OR for malignancy was 1.48 (95% CI: 0.71, 3.09), and 1.26 (95% CI: 0.39, 4.15) when non-melanoma skin cancer was excluded.
Limitations
Short duration of follow-up and rarity of malignancies and serious infections.
Conclusions
There is a small increased risk of overall infection with the short-term use of TNF antagonists for psoriasis that may be attributable to differences in follow-up time between treatment and placebo groups. There was no evidence of an increased risk of serious infection and a statistically significant increased risk in cancer was not observed with short-term use of TNF inhibitors.
Keywords: psoriasis, psoriatic arthritis, meta-analysis, malignancy, cancer, infection, biologics, safety, TNF-alpha
Classifications: biologics (Rx), biostatistics, clinical trials, controlled trials, epidemiology, infections, immunobiologics, immunosuppression, psoriasis, psoriatic arthritis
INTRODUCTION
Psoriasis is a common, chronic, inflammatory disease that is associated with impairment in health-related quality of life even when objectively mild, and an increased risk of death from cardiovascular disease, cancer, and infection in patients with severe disease.1–7 The treatment of psoriasis has undergone a revolution with the advent of TNF-α antagonists that suppress inflammatory pathways. These agents are generally safe and well tolerated; however, due to their immunosuppressive properties, the risk of infection and malignancy associated with these agents has been of concern.
Most studies evaluating the risks of malignancy and infection with TNF inhibitors have evaluated these agents in patients with RA or inflammatory bowel disease (IBD). Observational studies and meta-analyses of randomized controlled trials (RCTs) have indicated an increased risk of non-serious and serious infections8–14 in these patient populations with the use of anti-TNF agents.15–17 Some meta-analyses and observational studies in the RA population have found an increased risk of malignancy,8, 9, 18, 19 although there is conflicting evidence.20–27 It is unclear, however, if safety data from RA patients generalizes to patients with psoriatic disease. In particular, patients with psoriasis are typically treated with monotherapy, whereas concomitant use of systemic immunosuppressants is common in the RA and IBD patient populations. 8, 9 Importantly, there may be a synergistic effect with the use of TNF antagonists and concomitant immunosuppressants on the risk of malignancy and serious infection.28
To date, the safety profile of these agents in patients with psoriatic disease has not been extensively evaluated. Individual RCTs lack the sample size and trial duration to detect rare adverse events such as cancer and serious infections. Additionally, open-label extension trials and post-marketing surveillance databases often lack adequate control groups and spontaneous reports are generally not reliable for assessing malignancy and infection risk due to severe underreporting.29 In this study, we sought to evaluate the risk of malignancy, serious infection, and non-serious infection associated with the use of anti-TNF-α agents in adult patients with plaque psoriasis and psoriatic arthritis by conducting a meta-analysis of RCTs.
METHODS
Based on the Cochrane Handbook for Systemic Reviews of Interventions guidelines,30 we used a predefined, peer-reviewed protocol to perform the study selection, assessment of eligibility criteria, data extraction, and statistical analysis of RCTs of patients with plaque psoriasis (PsO) and psoriatic arthritis (PsA). This article was prepared in accordance with the PRISMA statement.31 This study was granted an Institutional Review Board exemption by the University of Pennsylvania.
Data Sources and Search Strategy
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from inception to July 30th, 2009 using the terms psoriasis, psoriatic arthritis combined with controlled trial, clinical trial phase II, clinical trial phase III, clinical trial phase IV, and randomized trial, combined with biological, biologics, TNF, tumor necrosis factor, or with terms unique to each biologic agent including etanercept, Enbrel, infliximab, Remicade, adalimumab, Humira, golimumab, CNT0 148, certolizumab, and CDP870. To obtain data from unpublished or unidentified clinical studies, we searched clinicalstudyresults.org and contacted industry sponsors of the anti-TNF agents and corresponding authors of published studies (Centocor, Horsham, PA; Schering-Plough, Kenilworth, NJ; Abbott Laboratories, Abbott Park, IL; Amgen, Thousand Oaks, CA; and UCB, Inc., Smyrna, GA).
Selection and Outcomes
We included RCTs of the 4 currently licensed anti-TNF agents (etanercept, infliximab, adalimumab, golimumab), and 1 anti-TNF agent currently under investigation (certolizumab) for the treatment of adult patients with moderate to severe PsO and/or PsA, limited to the English language. Study participants must have been adult patients with a diagnosis of PsO or PsA randomized to receive treatment with an anti-TNF agent or placebo for at least 12 weeks.
Studies were evaluated by two independent reviewers (K.A. and J.N.) using the Jadad scale32, which scores the quality of studies on a scale of 0 to 5. A Jadad score of 3 or greater was required for inclusion; this primarily indicates blinding, randomization, and report of withdrawals and dropouts.
Data Abstraction
Data were independently abstracted by two authors (K.A. and E.D.) for our two primary outcomes of malignancy and infection, with disagreement resolved by consensus. We additionally classified infections as serious or non-serious. Serious infection was defined as an infection that was considered a serious adverse event (SAE), and non-serious infection as an infection that was not recorded as an SAE by study investigators. We classified reported malignancies as non-melanoma skin cancers (NMSC) and a composite group of other cancers. We obtained the time point of diagnosis for each malignancy and person-years of follow-up for each treatment arm from published reports and/or industry sponsors. All industry sponsors as well as corresponding authors were contacted to verify and/or obtain (if not reported in the original publication) the number of infections and malignancies. We were able to obtain requested unpublished data from all of the above sponsors except UCB.
Data on the following measures were also abstracted: study design, sample size, intention-to-treat analysis, trial duration, blinding period, outcome measures, treatment regimen, and withdrawals and dropouts.
Statistical Analysis
We determined the number of patients with at least 1 infection or malignancy during the randomized, placebo-controlled period. In instances where the number of events instead of the number of subjects experiencing an event was reported, an assumption of one event per subject was made. All patients from eligible trials who received at least one dose of study drug were included in the denominator of our outcome measures (intention-to-treat method). We calculated an odds ratio (OR) based on the number of subjects experiencing the events (malignancy, infection) and the number of subjects receiving treatment in each group. Homogeneity testing was performed using the I2 test.33 We produced a pooled estimate of risk for each outcome, with results expressed as overall ORs with associated 95% confidence intervals (CIs). A fixed effects model with Mantel-Haenszel methods34 was used, as it is considered to be superior to a random effects model when pooling trials with few or no events and typically produces narrower confidence intervals.30 We calculated ORs across all included studies, and additionally performed subanalyses by indication and drug. We calculated a number needed to harm (NNH) based on the Mantel-Haenszel fixed effects model estimate if the OR was statistically significant.
We additionally calculated rate-adjusted estimates of risks for malignancy and infection using incidence rate ratios (IRRs). The rate ratio was calculated for each study based on the number of events and person-years of follow-up in each treatment group. The IRR was calculated by pooling the rate ratios across studies using Mantel-Haenszel weights.35
All treatment regimens (e.g. low dose, high dose) were combined for comparison. We observed zero events in some groups for some of the outcome measures, particularly malignancy. For both the OR and IRR calculations, when no events were observed in one arm of the RCT, we used a continuity correction of 0.5.36 If no events occurred in either study arm, the study was effectively excluded from the analysis.
Sensitivity analyses included calculating ORs of the pooled estimates of risk using the random effects model, and additionally calculating the ORs and IRRs using multiple different continuity corrections. We also performed an analysis omitting all NMSC. The influence of individual studies on the pooled effect-size estimate was analyzed by performing an influence analysis, in which the pooled estimates were recalculated omitting 1 study at a time. We used funnel plots to evaluate the potential for publication bias with respect to our primary endpoints (malignancy and infection).30, 37–39 We also used the Egger test to evaluate the risk of publication bias, with a 2-tailed P value of less than 0.05 considered to be statistically significant.30
All analyses were performed using Stata version 10.0 (StataCorp, College Station, Texas) and Review Manager version 5.0.21 (Nordic Cochrane Center, Copenhagen, Denmark).
RESULTS
Search Results and Trial Characteristics
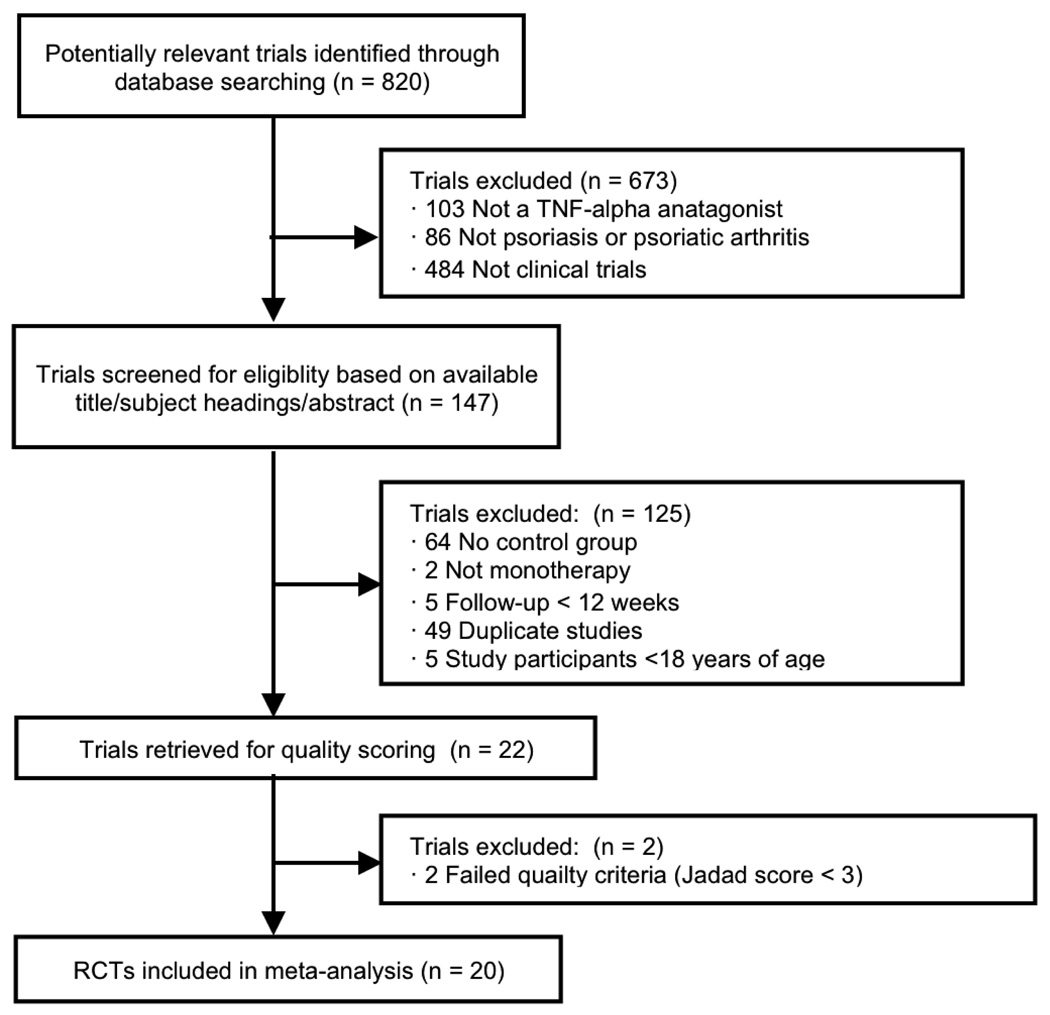
Of 820 potentially relevant publications identified through database searching, 20 clinical trials including 6,810 adult patients (5,427 patients in PsO and 1,383 patients in PsA studies) qualified for inclusion (Figure 1). Seven trials specifically included patients with active PsA unresponsive to DMARDs and/or non-steroidal anti-inflammatory drugs (NSAIDS), although five of these trials additionally required that patients have active psoriatic skin lesions and/or a documented history of PsO. The remaining 13 trials specifically included those with moderate to severe PsO. All PsA trials allowed for the use of at least one concomitant DMARD, whereas PsO trials excluded those on concomitant immunosuppressant therapy.
Figure 1.
Selection of Studies for Meta-analysis
All trials compared one of the following treatments with a placebo: 6 trials with adalimumab, 7 with etanercept, 5 with infliximab, 1 with certolizumab, and 1 with golimumab. Two separate 24-week trials were designed with early escape at week 16, which allowed patients to enter either the treatment group from placebo or begin a higher dose of study drug if there were 2 treatment groups in the trial. For these trials, any patient who received at least one dose of anti-TNF treatment was included in the treatment group.40 This resulted in 4,598 patients included in the treatment group and 2,313 patients included in the placebo group for the meta-analysis (see Table 1 for trial characteristics).
Table 1.
Trial Characteristics
| Treatment Group (n = 4598) | Placebo Group (n = 2313) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Trial Name/Registry Number |
Disease Indicationa |
Permitted Concomitant Systemic Therapyb |
Duration of Placebo- Controlled Trial, wks |
Treatment (Dose)c | No. of Patients |
Patient-years follow-up |
No. of Patients |
Patient-years follow-up |
| Mease et al, 200547 |
Study M02-518 / NCT00646386 |
PsA | MTX (≤30 mg/wk) or prednisone (≤10 mg/day) at stable dose; rescue therapy after 12 wks with DMARDS or corticosteroids |
24 | Adalimumab (40 mg eow) | 151 | 66.8 | 162 | 71.1 |
| Gordon et al, 200646 |
Study M02-528 / NCT00645814 |
PsO | None | 12 | Adalimumab (40 mg eow) Adalimumab (40 mg weekly) |
45 50 |
10.2 11.2 |
52 | 11.8 |
| Genovese et al, 200757 |
Study M02-570 / NCT00646178 |
PsA | MTX (≤30 mg/wk) or prednisone (≤10 mg/day), or other DMARD at stable dose |
12 | Adalimumab (40 mg eow) | 51 | 11.8 | 49 | 10.8 |
| Menter et al, 200850 |
Study M03-656 / NCT00237887 |
PsO | None | 16 | Adalimumab (80 mg at wk 0, then 40 mg eow starting at wk 1) |
814 | 250.4 | 398 | 120.7 |
| Saurat et al, 200851 |
CHAMPION / Study M04-716 / NCT00235820 |
PsO | None | 16 | Adalimumab (80 mg at wk 0, then 40 mg eow starting at wk 1) MTX (7.5 mg, increased as needed and as tolerated to 25 mg weekly)d |
108 110 |
34.7 34.8 |
52 | 16.3 |
| Akihiko et al, 201078 |
Study M04-688 / NCT00338754 |
PsO | None | 24 | Adalimumab (80 mg at wk 0, then 40 mg eow starting at wk 1) Adaliumumab (40 mg eow) Adalimumab (80 mg eow) |
38 43 42 |
16.3 17.2 17.9 |
46 | 19.1 |
| Unpublished41 | Study C87040 / NCT00245765 |
PsO | None | 12 | Certolizumab pegol (400 mg at wk 0, then 200 mg every 2 wks) Certolizumab pegol (400 mg every 2 wks) |
59 58 |
Unknown | 59 | Unknown |
| Mease et al, 200048 |
Study 20021630 | PsA and PsO |
MTX (≤25 mg/wk) or prednisone (≤10 mg/day) at stable dose |
12 | Etanercept (25 mg twice weekly) | 30 | 6.5 | 30 | 6.1 |
| Gottlieb et al, 200352 |
Study 20021632 | PsO | None | 24 | Etanercept (25 mg twice weekly) | 57 | 23.6 | 55 | 14.5 |
| Leonardi et al, 200342 |
Study 20021639 | PsO | None | 12 | Etanercept (25 mg weekly) Etanercept (25 mg twice weekly) Etanercept (50 mg twice weekly) |
160 162 164 |
34.3 34.6 35.8 |
166 | 34.9 |
| Mease et al, 200449 |
NCT00317499 | PsA | MTX (≤25 mg/wk) or prednisone (≤10 mg/day) at stable dose |
24 | Etanercept (25 mg twice weekly) | 101 | 81.0 | 104 | 59.2 |
| Papp et al, 200544 |
Study 20021642 | PsO | None | 12 | Etanercept (25 mg twice weekly) Etanercept (50 mg twice weekly) |
196 194 |
42.9 42.6 |
193 | 41.0 |
| Tyring et al, 200755 |
NCT00111449 | PsO | None | 12 | Etanercept (50 mg twice weekly) | 312 | 68.8 | 306 | 65.9 |
| van de Kerkhof et al, 200843 |
NCT00333034 | PsO | None | 12 | Etanercept (50 mg weekly) | 96 | Unknown | 46 | Unknown |
| Kavanaugh et al, 200945 |
NCT00265096 | PsA | Stable dose of MTX, prednisone, or NSAIDS |
24 with early escape at week |
Golimumab (50 mg every 4 wks) Golimumab (100 mg every 4 wks) All golimumabe |
146 146 343 |
62 67 142 |
113 | 42 |
| Gottlieb et al, 200458 |
SPIRIT / NCT00230529 |
PsO | NSAIDS | 30 | Infliximab (3 mg/kg at wks 0, 2, 6) Infliximab (5 mg/kg at wks 0, 2, 6) |
98 99 |
56 58 |
51 | 20.5 |
| Antoni et al, 200556 |
IMPACT | PsA | MTX or other DMARD at stable dose |
16 | Infliximab (5 mg/kg at wks 0, 2, 6, 14) | 52 | 16 | 51 | 16 |
| Antoni et al, 200540 |
IMPACT 2 / NCT00051623 |
PsA | MTX (≤25 mg/wk) or prednisone (≤10 mg/day) at stable dose |
24 with early escape at wk 16 |
Infliximab (5 mg/kg at wks 0, 2, 6, 14, 22) All infliximabf |
100 150 |
45 53 |
97 | 37 |
| Reich et al, 200554 |
EXPRESS I / NCT00106834 |
PsO | NSAIDS | 24 | Infliximab (5 mg/kg at wks 0, 2, 6, 14, 22) | 298 | 135 | 76 | 33 |
| Menter et al, 200753 |
EXPRESS II / NCT00106847 |
PsO | NSAIDS | 14 | Infliximab (3 mg/kg at wks 0, 2, 6) Infliximab (5 mg/kg at wks 0, 2, 6) |
313 314 |
85 85 |
207 | 54 |
PsA = psoriatic arthritis; PsO = plaque psoriasis
MTX = methotrexate; DMARDS = disease-modifying antirheumatic drugs; NSAIDS = non-steroidal anti-inflammatory drugs
eow = every other week
Not included in meta-analysis.
All golimumab group includes all patients who received at least one dose of study drug.
All infliximab group includes all those that received at least one dose of study drug.
One clinical trial was not published in a peer-reviewed journal, and data on this study were obtained through a poster abstract.41 We found no trials meeting our inclusion-criteria that were published in a language other than English. Mean duration of the placebo-controlled phases across trials was 17.8 weeks (range 12–30 weeks). Overall, the percent of withdrawals during the course of the study was significantly greater in the placebo group than in the treatment group (16.1 vs. 6.8 percent, p = 0.005). According to the published manuscripts, the greater withdrawal rate in the placebo group was largely due to lack of efficacy. One study did not report dropouts specifically for each treatment group, but did report overall dropouts and an adequate description of appropriate double-blinding, and thus qualified for inclusion according to the Jadad criteria.32, 42 Similarly, there was a significant difference in patient-years (PY) of follow-up between treatment and placebo groups (total of 1516.4 and 673.9 PY, respectively, p=0.0004), partially due to differential drop out between the placebo and treatment groups, but additionally because many trials included multiple treatment groups for different doses of study drug (see Table 1). We were unable to obtain information on PY of follow-up for two studies.41, 43
Malignancies
A total of 21 malignancies were reported in published data in patients who received at least one dose of an anti-TNF agent and 4 malignancies in patients who received placebo across the 20 included clinical trials. An additional 7 malignancies (4 basal cell carcinomas, 1 squamous cell carcinoma, 1 prostate cancer, and 1 breast cancer) in the treatment group and 2 malignancies (2 squamous cell carcinomas) in the placebo group were identified after contacting the industry sponsors.42, 44 A total of 28 malignancies in the treatment group and 6 malignancies in the placebo group were used in the analysis (Table 2).
Table 2.
Malignancies and Infectious Events Occurring in Randomized Controlled Trialsa
| Treatment Group (n = 4598) | Placebo Group (n = 2313) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Treatment (Dose)b | No. of Pts |
Pts with ≥ 1 Serious Infection |
Pts with ≥ 1 Infectious Event |
No. of Maligc |
Type of Malignancyd |
Time of Malig, wk |
No. of Pts |
Pts with ≥ 1 Serious Infection |
Pts with ≥ 1 Infectious Event |
No. of Malig |
Type of Malignancy |
Time of Malig, wk |
| Mease et al, 200547 |
Adalimumab (40 mg eow) | 151 | 1 | 68 | 0 | 162 | 1 | 64 | 0 | ||||
| Gordon et al, 200646 |
Adalimumab (40 mg eow) Adalimumab (40 mg weekly) |
45 50 |
0 1 |
5 13 |
1 1 |
Metastatic SCCe Breast Cancer |
2.1 5.1 |
52 | 0 | 8 | 0 | ||
| Genovese et al, 200757 |
Adalimumab (40 mg eow) | 51 | 0 | 9 | 0 | 49 | 1 | 16 | 0 | ||||
| Menter et al, 200850 |
Adalimumab (80 mg at wk 0, then 40 mg eow starting at wk 1) |
814 | 5 | 235 | 6 | NMSC × 4 Breast cancer Melanoma in situ |
8.1, 8.1, 13, 15.6 1.6 4.1 |
398 | 4 | 89 | 2 | NMSC Uterine- carcinoma |
13.9 4.1 |
| Saurat et al, 200851 |
Adalimumab (80 mg at wk 0, then 40 mg eow starting at wk 1) |
108 | 0 | 51 | 0 | 52 | 0 | 23 | 0 | ||||
| Akihiko et al, 201078 |
Adalimumab (80 mg at wk 0, then 40 mg eow starting at wk 1) Adaliumumab (40 mg eow) Adalimumab (80 mg eow) |
38 43 42 |
0 0 0 |
21 18 21 |
0 | 46 | 0 | 23 | 0 | ||||
| Unpublished41 | Certolizumab pegol (400 mg at wk 0, then 200 mg every 2 wks) Certolizumab pegol (400 mg every 2 wks) |
59 58 |
1 2 |
16 27 |
0 | 59 | 0 | 24 | 0 | ||||
| Mease et al, 200048 |
Etanercept (25 mg twice weekly) | 30 | 0 | 17f | 0 | 30 | 0 | 17f | 0 | ||||
| Gottlieb et al, 200352 |
Etanercept (25 mg twice weekly) | 57 | 0 | 28g | 0 | 55 | 1 | 14g | 0 | ||||
| Leonardi et al, 200342 |
Etanercept (25 mg weekly) Etanercept (25 mg twice weekly) Etanercept (50 mg twice weekly) |
160 162 164 |
2 0 1 |
18g 15 10 |
1 0 2 |
BCC BCC Prostate Cancer |
11.6 9.1 7.3 |
166 | 2 | 22g | 2 | SCC × 2 | 0.7, 1.9 |
| Mease et al, 200449 |
Etanercept (25 mg twice weekly) | 101 | 0 | 33 | 0 | 104 | 1 | 39 | 0 | ||||
| Papp et al, 200544 |
Etanercept (25 mg twice weekly) Etanercept (50 mg twice weekly) |
196 194 |
1 0 |
36h 35 |
0 4 |
Breast Cancer SCC BCC × 2 |
1.9 7.9 8.0, 12.0 |
193 | 1 | 29h | 0 | ||
| Tyring et al, 200755 |
Etanercept (50 mg twice weekly) | 312 | 1 | 88 | 3 | SCC BCC Pancreatic- Carcinoma |
0.6 Unknown 10.7 |
306 | 1 | 71 | 1 | Bladder- carcinoma |
11.0 |
| van de Kerkhof et al, 200843 |
Etanercept (50 mg weekly) | 96 | 0 | 27 | 0 | 46 | 0 | 12 | 0 | ||||
| Kavanaugh et al, 200945 |
Golimumab (50 mg every 4 wks) Golimumab (100 mg every 4 wks) All Golimumabi |
146 146 343 |
1 1 2 |
48 60 118 |
0 3 3 |
BCC × 2 Prostate cancer As above |
19.3,19.8 9.9 As above |
113 | 4 | 27 | 0 | ||
| Gottlieb et al, 58 |
Infliximab (3 mg/kg at wks 0, 2, 6) Infliximab (5 mg/kg at wks 0, 2, 6) |
98 99 |
0 1 |
31 37 |
2 1 |
SCC × 2 BCC |
8.9, 7.8 31.8 |
51 | 0 | 11 | 0 | ||
| Antoni et al, 200556 |
Infliximab (5 mg/kg at wks 0, 2, 6, 14) | 52 | 1 | 6 | 0 | 51 | 0 | 9 | 0 | ||||
| Antoni et al, 200540 |
Infliximab (5 mg/kg wks 0, 2, 6, 14, 22) All Infliximabc |
100 150 |
3 3 |
34 47 |
0 0 |
97 | 2 | 29 | 1 | BCC | 9 9 |
||
| Reich et al, 54 |
Infliximab (5 mg/kg wks 0, 2, 6, 14, 22) | 298 | 3 | 125 | 2 | SCC BCC |
5.7 5.2 |
76 | 0 | 30 | 0 | ||
| Menter et al, 200753 |
Infliximab (3 mg/kg at wks 0, 2, 6) Infliximab (5 mg/kg at wks 0, 2, 6) |
313 314 |
0 1 |
106 97 |
1 1 |
BCC BCC |
9.9 9.4 |
207 | 1 | 62 | 0 | ||
A total of 9 malignancies (in bold) were unpublished and obtained from industry sponsors.
Eow = every other week
Malig = malignancy
SCC = Squamous cell carcinoma; BCC = Basal cell carcinoma; NMSC = non-melanoma skin cancer (unspecified)
Non-cutaneous metastatic SCC on the left side of the neck. Swollen lymph node on the left side of the neck was present at screening.
Only upper respiratory tract events reported.
Only upper respiratory tract infections (URIs) and sinusitis reported.
Only URIs and flu syndrome reported.
Early escape at week 16. All malignancies occurred in patients receiving only the 100 mg dose
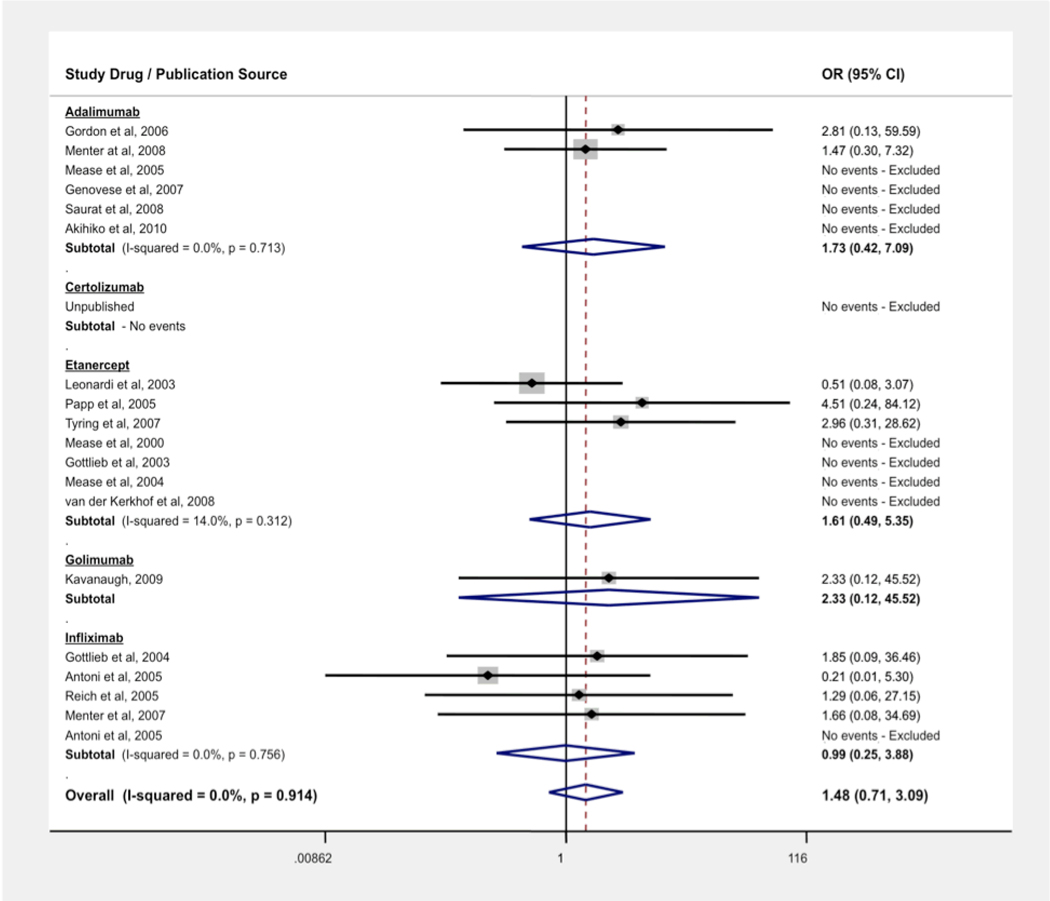
The pooled OR for malignancies in patients with PsO and PsA using anti-TNF agents was 1.48 (95% CI: 0.71, 3.09). We found no evidence of statistical heterogeneity, the measure of inconsistency between trials (I2 = 0.0%, p = 0.91). We also conducted subanalyses by drug (see Figure 2). Using the rate-adjusted analysis, we found an IRR of 0.99 (95% CI: 0.51, 1.90).
Figure 2.
Odds Ratio (OR) of Malignancy Associated with Anti-TNF Treatment vs Control
Two 24-week trials were designed with early escape at week 16. In the study by Kavanaugh et al.,45 all malignancies occurred in patients receiving only the 100 mg dose of golimumab throughout the 24-weeks. In another trial by Antoni et al. of infliximab,40 only one malignancy occurred in the trial in a patient receiving placebo.
Overall, 70.6% of malignancies included in our analysis were non-melanoma skin cancers (NMSC). The OR for NMSC in patients using anti-TNF agents across all trials was 1.33 (95% CI: 0.58, 3.04). The IRR for NMSC was 0.72 (95% CI: 0.42, 1.24).
The OR for all malignancies excluding NMSC was 1.28 (95% CI: 0.39, 4.15). Subanalysis by disease indication resulted in an OR of 0.83 (95% CI: 0.14, 4.96) for PsA trials (n = 7) and an OR of 1.64 (95% CI: 0.73, 3.70) for PsO trials (n = 13). Similar results were obtained when using a Mantel-Haenszel random-effects model, with ORs of 1.38 (95% CI: 0.64, 3.01) and 1.23 (95% CI: 0.37, 4.06) for all malignancies and malignancies excluding NMSC, respectively. The rate-adjusted analysis for all malignancies excluding NMSC yielded an IRR of 0.56 (95% CI: 0.31, 1.01).
Infections
A total of 1358 patients in the treatment group and 619 patients in the placebo group experienced an infectious event (serious or non-serious). Several studies (n = 7)43, 46–51 did not specify whether reported non-serious infections were by number of events or by number of patients experiencing at least 1 non-serious infection. For non-serious infections, four studies42, 44, 48, 52 only reported patients experiencing the most common infections that occurred during the trial, which included upper respiratory tract infections, flu syndrome, and sinusitis (Table 2).
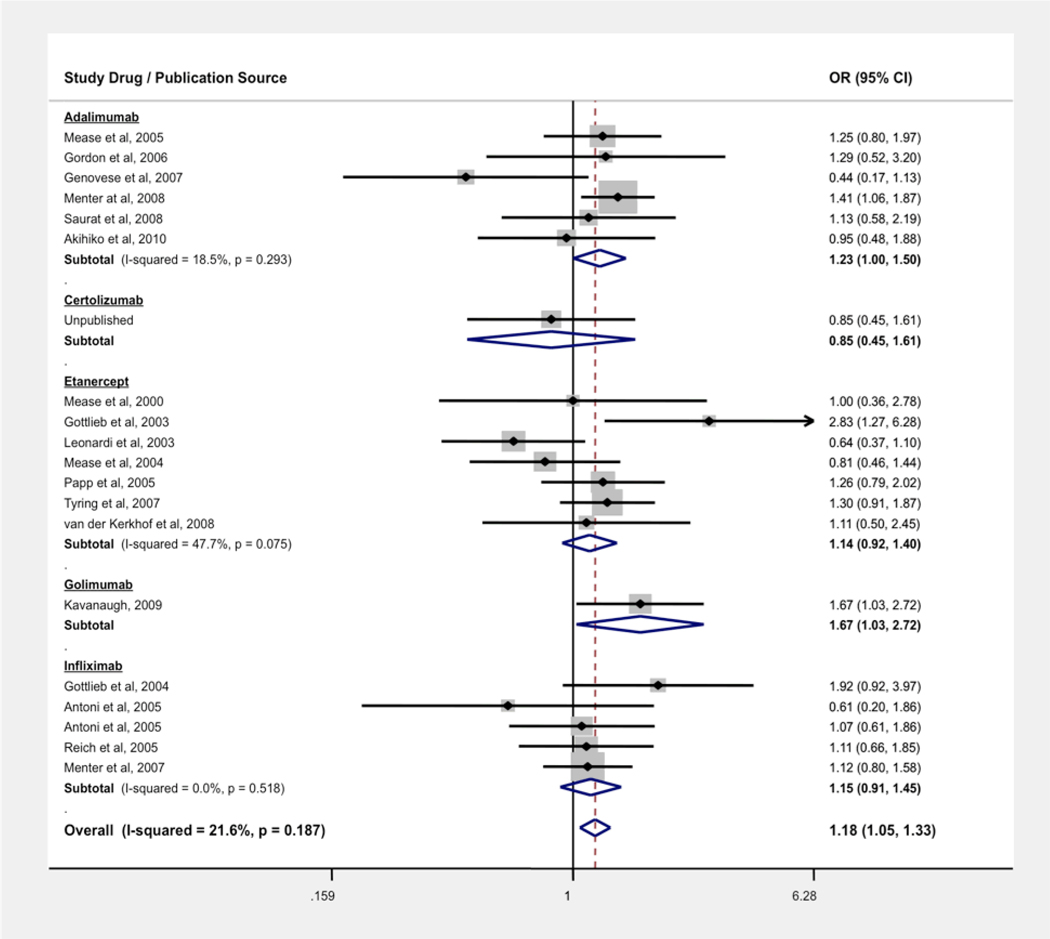
The OR for any infectious event in patients with PsA or PsO treated with an anti-TNF agent was 1.18 (95% CI: 1.05, 1.33), with 97.6% of infections being non-serious, i.e., not recorded as an SAE. The ORs were 1.22 (1.06, 1.40) for PsO trials and 1.09 (0.87, 1.37) for PsA trials when separating by indication. We also stratified the risk of infection by drug (see Figure 3). The NNH for treatment with all anti-TNF agents was 29. There was no evidence of statistically significant heterogeneity (I2 = 21.6%, p = 0.187). The estimated OR for non-serious infection only was 1.20 (95% CI: 1.07, 1.35).
Figure 3.
Odds Ratio (OR) of Overall Infection Associated with Anti-TNF Treatment vs Control
Serious infections were reported in 28 (0.61%) patients in the treatment group and 19 (0.82%) patients in the placebo group, resulting in a pooled OR of 0.70 (95% CI: 0.40, 1.21). Stratification by disease indication resulted in an OR of 0.78 (95% CI: 0.38, 1.58) for PsO and 0.60 (95% CI: 0.25, 1.44) for PsA. Similar results were found when using a random-effects model.
When adjusting for patient-years, the IRR for overall infection was 1.01 (95% CI: 0.92, 1.11) and 0.59 (95% CI: 0.35, 0.99) for serious infection. The estimated IRR for non-serious infection was 1.02 (95% CI: 0.93, 1.13).
Publication Bias
We found no evidence of publication bias with Egger tests for malignancy (P = 0.54), NMSC (p = 0.29), malignancies excluding NMSC (p = 0.48), overall infection (p = 0.18), non-serious infection (p = 0.16), or serious infection (p = 0.14). Funnel plots were also created for the above outcomes, all of which were found to be symmetrical.
DISCUSSION
Our systematic review and meta-analysis combined data from 20 RCTs of adult patients with plaque psoriasis and psoriatic arthritis treated with anti-TNF-α agents. To our knowledge, this is the largest review to date of RCTs examining the risk of infection and malignancy with the use of anti-TNF-α agents in patients with psoriatic disease.
Our study suggests that there may be a small increased risk of overall infection with the short-term use of TNF-alpha antagonists for psoriatic disease. However, 97.6% of reported infections were non-serious, and the large majority of these were upper respiratory tract infections. Thus, although our finding for an increased risk of overall infection may be statistically significant, it may have limited clinical implications, and it appears that the short-term risk-to-benefit profile with respect to overall infection is favorable. Moreover, models that adjust for differences in follow-up time indicated no statistically significant increased risk of infection, suggesting that the observed infection risk may be an effect of differential follow-up as opposed to an effect of the TNF inhibitor.
There was no evidence of an increased risk of serious infection and a statistically significant increased risk of cancer was not observed. When adjusting for patient-years of follow-up, we found a marginally statistically significant decreased risk of serious infection. .41, 42, 44, 50, 53–55 From the 9 trials with detailed information,40, 45–47, 49, 52, 56–58 cellulitis was the most common serious infection occurring in the placebo group (n = 3) compared to only one reported case in the treatment group. Thus, an improvement in skin disease and decreased scratching with anti-TNF therapy may be a plausible explanation for this unexpected finding. It must be emphasized, however, that serious infections including atypical infections such as tuberculosis have been reported in a variety of TNF inhibitor treated patient populations including patients with psoriatic disease. Therefore, clinicians should ensure that patients are up to date with vaccinations, and have appropriate screening for tuberculosis and invasive fungal infections prior to initiation of TNF inhibitors, and are closely monitored for infection during the course of treatment.59
Our study results differ from a similar meta-analysis performed in the RA population by Bongartz et al, which found pooled ORs of 3.3 (95% CI: 1.2–9.1) for malignancy and 2.0 (95% CI, 1.3–3.1) for serious infection with the use of anti-TNF antibodies (infliximab and adalimumab).8 This meta-analysis included 9 randomized controlled trials, limited to the placebo-controlled phase. Despite our inclusion of a greater number of trials with more patients, more malignancies and serious infections occurred in the meta-analysis by Bongartz et al. then in our analysis of patients with psoriatic disease. This difference in safety profile is consistent with results from open-label studies of etanercept in psoriasis and RA, which found the exposure-adjusted rates of serious infectious events in the psoriasis population to be lower than that in the RA population (1.2 vs. 4.2 events per 100 patient-years, respectively).60, 61
Several factors could explain the differing results between our meta-analysis and that of the meta-analysis in the RA population. On average, the duration of the placebo-controlled phases of the included trials for the Bongartz analysis was longer than those included in our study (mean of 32.7, range 12–54 weeks compared to mean of 17.8, range 12–30 weeks for our study), which could have led to increased detection of adverse events, especially if the risk increases over time. However, others have found in the RA population that serious infections appear to peak in the first 90 days of treatment with an anti-TNF agent.62 Additionally, the large majority of malignancies reported in the study by Bongartz et al. occurred within the first 24 weeks (25 vs. 8 occurring at >24 weeks).18
The most notable difference between the trials included in our meta-analysis and previous meta-analyses done within the RA population was the use of concomitant immunosuppressive therapy. In the trials included in the meta-analysis by Bongartz et al. (n = 5005),8 approximately 77.1% of the patients were on MTX, 6.5% were on other DMARDS, and 54.9% were on corticosteroids concomitantly at baseline.63–71 In the 13 PsO trials (n = 5434) included in our meta-analysis, none allowed for simultaneous immunosuppressive therapy, and in the 7 included PsA trials (n = 1485), approximately 44.6% were on MTX, 5.5% were on other DMARDS, and 10.5% were on corticosteroids at baseline. There is some evidence that there may be a synergistic effect when combining other systemic immunosuppressants with TNF-alpha inhibitors on the risk of serious infection and malignancy.72–76 This suggests that the risk profile of these agents may be significantly altered when using combination therapy, and that this should be taken into account when interpreting the existing literature.
Limitations
There are several limitations to our study that should be noted. Conducting meta-analyses with rare event data is inherently difficult. Small changes in the numerator or denominator can significantly affect the estimated risk. Due to the rarity of events and short duration of follow-up, the confidence intervals we calculated for malignancy and serious infection were wide and do not rule out potential associations that could be clinically significant. Moreover, safety endpoints were grouped (e.g. serious infections, malignancy) and therefore, this analysis could not determine the risk of specific individual outcomes such as tuberculosis or lymphoma. Additionally, we were unable to assess the risk of cancer and serious infection associated with chronic use of TNF inhibitors. This limitation is of special concern for malignancy, which may take years of exposure in order to accurately define risk. In general, side effects which are delayed in onset or occur at a rate of <1 in 1000 patients per year are often only recognized after a medication is in widespread use, with over half of medications entering the market having serious adverse events discovered only after FDA approval. Thus, it has been suggested that a novel drug should have at least 20,000 patients exposed with direct observation (i.e., active surveillance for adverse events as opposed to relying on spontaneous reports) before being widely marketed to the general population.77
On average, the clinical trials included in our meta-analysis often had shorter durations of follow-up in placebo groups compared with treatment groups because of higher rate of treatment failure in the former. Since our pooled ORs were based on event data, longer follow-up time in the treatment groups could have biased our results to an overestimation of the true risk, as there is more time in the treatment group to detect an adverse event. To adjust for unequal follow-up times, we performed a meta-analysis of rates. However, the statistical methods are not as well developed for this type of analysis, and it requires an assumption of a constant, underlying risk which may not be appropriate.30 Thus, while the rate-adjusted estimates are informative, they should be interpreted with caution.
Most of the malignancies found during the placebo-controlled portions of the trials were NMSC (70.6%). We performed an analysis omitting all NMSC from the analysis, as there is a potential for unmasking bias for trials including patients with psoriatic disease as skin cancers may be easier to detect as the psoriasis clears from effective treatment. However, excluding NMSC did not change our results.
The trials included in this meta-analysis were clinically heterogeneous with respect to study drug, trial design, disease indication, previous and concomitant immunosuppressant treatment, and disease duration. However, we found no evidence of statistical heterogeneity for any of our measured outcomes, suggesting that outcomes for the included trials were statistically similar enough to validly pool results across these studies.
The estimated ORs for our outcomes were based on the number of subjects experiencing the events (malignancy, infection) and the number of subjects receiving treatment in each group. As one patient can experience more than one non-serious infection, the rate of non-serious infection and number of patients experiencing at least 1 non-serious infection may not be equal. In trials where it was not specified whether the number of events instead of the number of subjects experiencing an event was reported,43, 46–51 an assumption of one event per subject was made. For the overall infection analysis, this could have led to an overestimation of effect.
Conclusions
There have been limited studies examining the risk profile of TNF inhibitors in the psoriatic population. The existing literature on risk of infection and malignancy with the use of the TNF-alpha inhibitors from the RA population may not generalize to psoriatic patients. Of special importance, RA is typically treated with concomitant immunosuppressive therapy while psoriasis is not. There is some evidence that there may be a synergistic effect with the use of anti-TNF agents and other immunosuppressants on the risk of infection and malignancy. Thus, compared to the existing literature, our study may provide a more accurate picture of the risks associated with TNF inhibitors when used as monotherapy.
Our results suggest that the short-term risk-to-benefit profile of the TNF-alpha inhibitors in adult patients with psoriatic disease is favorable. However, larger, long term studies with appropriate control groups will be necessary to fully assess the risk of cancer and serious infection associated with chronic use of TNF inhibitors in the psoriatic population.
Acknowledgments
Funding Sources: Supported in part by grant K23AR051125 from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (JMG) and a National Research Service Award from the National Institute of Health (EDD).
Abbreviations and Acronyms
- PsO
plaque psoriasis
- PsA
psoriatic arthritis
- RCT
randomized, controlled trial
- RA
rheumatoid arthritis
- IBD
irritable bowel disease
- TNF
tumor necrosis factor
- SAE
serious adverse event
- NMSC
non-melanoma skin cancer
- DMARD
disease-modifying antirheumatic drug
- OR
odds ratio
- IRR
incidence rate ratio
- NNH
number needed to harm
- NSAIDS
non-steroidal anti-inflammatory drugs
- EOW
every other week
Footnotes
Prior Presentation: This study was previously presented at the 2010 Society for Investigative Dermatology Annual Meeting in Atlanta, GA, and the abstract from this meeting published in the Journal for Investigative Dermatology.
Financial Disclosures: Dr. Gelfand receives grant support and is an investigator for Amgen and Pfizer. He is a consultant for Pfizer, Genentech, Celgene, Amgen, Centocor, and Luitpold. Dr. Dommasch, Dr. Abuabara, Mr. Shin, Dr. Nguyen, and Dr. Troxel have no relevant financial relationships to declare.
REFERENCES
- 1.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: A study from the US population. Journal of the American Academy of Dermatology. 2004;51:704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The Risk of Lymphoma in Patients with Psoriasis. J Invest Dermatol. 2006;126:2194–2201. doi: 10.1038/sj.jid.5700410. [DOI] [PubMed] [Google Scholar]
- 3.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of Myocardial Infarction in Patients With Psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 4.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, Margolis DJ, Strom BL. The Risk of Mortality in Patients With Psoriasis: Results From a Population-Based Study. Arch Dermatol. 2007;143:1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 5.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: Results from NHANES 2003–2004. Journal of the American Academy of Dermatology. 2009;60:218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. European Heart Journal. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abuabara K, Azfar R, Shin D, Neimann A, Troxel A, Gelfand J. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the United Kingdom. British Journal of Dermatology:no-no. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and Meta-analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 9.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-Tumor Necrosis Factor treatments in rheumatoid arthritis: meta and exposure adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.091025. ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 10.Schiff MH, Burmester GR, Kent JD, Pangan AL, Kupper H, Fitzpatrick SB, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:889–894. doi: 10.1136/ard.2005.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domm S, Cinatl J, Mrowietz U. The impact of treatment with tumour necrosis factor-alpha; antagonists on the course of chronic viral infections: a review of the literature. British Journal of Dermatology. 2008;159:1217–1228. doi: 10.1111/j.1365-2133.2008.08851.x. [DOI] [PubMed] [Google Scholar]
- 12.Furst DE. The Risk of Infection with Biologic Therapies for Rheumatoid Arthritis. Semin Arthritis Rheum. 2008 doi: 10.1016/j.semarthrit.2008.10.002. Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 13.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, Gromnica-Ihle E, Antoni C, Herzer P, Kekow J, Schneider M, Zink A. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis & Rheumatism. 2005;52:3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 14.Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-{alpha} therapy. Rheumatology. 2003;42:617–621. doi: 10.1093/rheumatology/keg263. [DOI] [PubMed] [Google Scholar]
- 15.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, Zink A. Risk of Herpes Zoster in Patients With Rheumatoid Arthritis Treated With Anti-TNF-{alpha} Agents. JAMA. 2009;301:737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 16.Favalli EG, Desiati F, Atzeni F, Sarzi-Puttini P, Caporali R, Pallavicini FB, Gorla R, Filippini M, Marchesoni A. Serious infections during anti-TNFalpha treatment in rheumatoid arthritis patients. Autoimmun Rev. 2008;8:266–273. doi: 10.1016/j.autrev.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.BIOBADASER Group; Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: A multicenter active-surveillance report. Arthritis & Rheumatism. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 18.Bongartz T, Warren FC, Mines D, Matteson EL, Abrams KR, Sutton AJ. Etanercept therapy in rheumatoid arthritis and the risk of malignancies. A systematic review and individual patient data meta-analysis of Randomized Controlled Trials. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.094904. ard.2008.094904. [DOI] [PubMed] [Google Scholar]
- 19.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and Safety of Tumor Necrosis Factor Antagonists in Crohn's Disease: Meta-Analysis of Placebo-Controlled Trials. Clinical Gastroenterology and Hepatology. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, Sundstrom C, Bertilsson L, Coster L, Geborek P, Jacobsson LT, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414–1420. doi: 10.1136/ard.2004.033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askling J, Baecklund E, Granath F, Geborek P, Fored M, Backlin C, et al. Anti-TNF therapy in RA and risk of malignant lymphomas Relative risks and time-trends in the Swedish Biologics Register. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.085852. ard.2007.085852. [DOI] [PubMed] [Google Scholar]
- 22.Geborek P, Bladstrom A, Turesson C, Gulfe A, Petersson IF, Saxne T, et al. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis. 2005;64:699–703. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonardi CL, Toth D, Cather JC, Langley RG, Werther W, Compton P, Kwon P, Wetherill G, Curtin F, Menter A. A Review of Malignancies Observed during Efalizumab (Raptiva®) Clinical Trials for Plaque Psoriasis. Dermatology. 2006;213:204–214. doi: 10.1159/000095037. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis & Rheumatism. 2004;50:1740–1751. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: Analyses from a large US observational study. Arthritis & Rheumatism. 2007;56:2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 PERSON-YEARS of observation. Arthritis & Rheumatism. 2007;56:1433–1439. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 27.Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatologic Therapy. 2009;22:418–430. doi: 10.1111/j.1529-8019.2009.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MR, Korman BD, Korman NJ. Combination Immunosuppressive Therapies: The Promise and the Peril. Arch Dermatol. 2007;143:1053–1057. doi: 10.1001/archderm.143.8.1053. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand JM. Pharmacovigilance: verifying that drugs remain safe. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy. 2nd Edition. Philadelphia, PA: Saunders Elsevier; 2007. [Google Scholar]
- 30.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 32.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 35.Guevara J, Berlin J, Wolf F. Meta-analytic methods for pooling rates when follow-up duration varies: a case study. BMC Medical Research Methodology. 2004;4:17. doi: 10.1186/1471-2288-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 37.Light RPD. Summing Up: The Science of Reviewing Research Cambridge. MA and London: Harvard University Press; 1984. [Google Scholar]
- 38.Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. International Journal of Epidemiology. 2002;31:88–95. doi: 10.1093/ije/31.1.88. [DOI] [PubMed] [Google Scholar]
- 39.Stern JAC EM, Davey SG. Investigating and dealing with publication and other biases. In: Egger AD, DSG M, editors. Systematic Review in Health Care. Meta-Analysis in Context London: BMJ Publishing Group; 2001. pp. 189–208. [Google Scholar]
- 40.Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Annals of the rheumatic diseases. 2005 doi: 10.1136/ard.2004.032268. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00051623?term=NCT00051623&rank=1. [DOI] [PMC free article] [PubMed]
- 41.Ortonnne JP, Tasset C, Reich K, Sterry W. Safety and efficacy of subcutaneous certolizumab pegol, a new anti-TNFa monoclonal antibody, in patients with moderate-to-severe chronic plaque psoriasis: Preliminary results from a double-blind, placebo-controlled trial Abstract P21. American Academy of Dermatology 65th Annual Meeting February 2–6, 2007. Journal of the American Academy of Dermatology. 2007 Available at: http://www.mrw.interscience.wiley.com/cochrane/clcentral/articles/995/CN-00615995/frame.html.
- 42.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 43.van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159:1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x. [DOI] [PubMed] [Google Scholar]
- 44.Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 45.Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–986. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 46.Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–3289. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 48.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 49.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 50.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139:1627–1632. doi: 10.1001/archderm.139.12.1627. discussion 32. [DOI] [PubMed] [Google Scholar]
- 53.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31 e1–31 e15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 55.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 56.Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–1236. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- 57.Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol. 2007;34:1040–1050. [PubMed] [Google Scholar]
- 58.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Menter A, Reich K, Gottlieb AB, Bala M, Li S, Hsu MC, et al. Adverse drug events in infliximab-treated patients compared with the general and psoriasis populations. J Drugs Dermatol. 2008;7:1137–1146. [PubMed] [Google Scholar]
- 60.Tyring S, Gordon KB, Poulin Y, Langley RG, Gottlieb AB, Dunn M, et al. Long-term Safety and Efficacy of 50 mg of Etanercept Twice Weekly in Patients With Psoriasis. Arch Dermatol. 2007;143:719–726. doi: 10.1001/archderm.143.6.719. [DOI] [PubMed] [Google Scholar]
- 61.Moreland LW, Cohen SB, Baumgartner SW, Tindall EA, Bulpitt K, Martin R, et al. Long-term safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol. 2001;28:1238–1244. [PubMed] [Google Scholar]
- 62.Dixon WG, Symmons DPM, Lunt M, Watson KD, Hyrich KL, Consortium BSfRBRCC, et al. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: Lessons from interpreting data from observational studies. Arthritis & Rheumatism. 2007;56:2896–2904. doi: 10.1002/art.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clair EWS, Heijde DMFMvd, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: A randomized, controlled trial. Arthritis & Rheumatism. 2004;50:3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 64.Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) The Journal of Rheumatology. 2003;30:2563–2571. [PubMed] [Google Scholar]
- 65.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: A randomized, placebo-controlled, 52-week trial. Arthritis & Rheumatism. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 66.Lipsky PE, van der Heijde DMFM, St. Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and Methotrexate in the Treatment of Rheumatoid Arthritis. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 67.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, MacFarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis & Rheumatism. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 68.van de Putte LBA, Atkins C, Malaise M, Sany J, Russell AS, van Riel PLCM, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Annals of the Rheumatic Diseases. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van de Putte LBA, Rau R, Breedveld FC, Kalden JR, Malaise MG, van Riel PLCM, et al. Efficacy and safety of the fully human anti-tumour necrosis factor Œ± monoclonal antibody adalimumab (D2E7) in DMARD refractory patients with rheumatoid arthritis: a 12 week, phase II study. Annals of the Rheumatic Diseases. 2003;62:1168–1177. doi: 10.1136/ard.2003.009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis & Rheumatism. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 71.Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: A large, randomized, placebo-controlled trial. Arthritis & Rheumatism. 2006;54:1075–1086. doi: 10.1002/art.21734. [DOI] [PubMed] [Google Scholar]
- 72.Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis & Rheumatism. 2004;50:1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- 73.Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Annals of the rheumatic diseases. 2007;66:228–234. doi: 10.1136/ard.2006.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: A one-year randomized, placebo-controlled study. Arthritis & Rheumatism. 2006;54:2807–2816. doi: 10.1002/art.22070. [DOI] [PubMed] [Google Scholar]
- 75.The Wegener's Granulomatosis Etanercept Trial Research Group. Etanercept plus Standard Therapy for Wegener's Granulomatosis. N Engl J Med. 2005;352:351–361. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 76.Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T cell lymphoma in inflammatory bowel disease. Gut. 2008;57:1639–1641. doi: 10.1136/gut.2008.163279. [DOI] [PubMed] [Google Scholar]
- 77.Okie S. Safety in Numbers - Monitoring Risk in Approved Drugs. New England Journal of Medicine. 2005;352:1173–1176. doi: 10.1056/NEJMp058029. [DOI] [PubMed] [Google Scholar]
- 78.Akihiko A, Hidemi N, Takafumi E, Mamitaro O. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: Efficacy and safety results from a Phase II/III randomized controlled study. The Journal of Dermatology. 2010;37:299–310. doi: 10.1111/j.1346-8138.2009.00748.x. [DOI] [PubMed] [Google Scholar]