Abstract
Aims
A common variant, rs9939609, in the FTO (fat mass and obesity) gene is associated with adiposity in Europeans, explaining its relationship with diabetes. However, data are inconsistent in South Asians. Our aim was to investigate the association of the FTO rs9939609 variant with obesity, obesity-related traits and Type 2 diabetes in South Asian individuals, and to use meta-analyses to attempt to clarify to what extent BMI influences the association of FTO variants with diabetes in South Asians.
Methods
We analysed rs9939609 in two studies of Pakistani individuals: 1666 adults aged ≥ 40 years from the Karachi population-based Control of Blood Pressure and Risk Attenuation (COBRA) study and 2745 individuals of Punjabi ancestry who were part of a Type 2 diabetes case–control study (UK Asian Diabetes Study/Diabetes Genetics in Pakistan; UKADS/DGP). The main outcomes were BMI, waist circumference and diabetes. Regression analyses were performed to determine associations between FTO alleles and outcomes. Summary estimates were combined in a meta-analysis of 8091 South Asian individuals (3919 patients with Type 2 diabetes and 4172 control subjects), including those from two previous studies.
Results
In the 4411 Pakistani individuals from this study, the age-, sex- and diabetes-adjusted association of FTO variant rs9939609 with BMI was 0.45 (95% CI 0.24–0.67) kg/m2 per A-allele (P = 3.0× 10−5) and with waist circumference was 0.88 (95% CI 0.36–1.41) cm per A-allele (P = 0.001). The A-allele (30% frequency) was also significantly associated with Type 2 diabetes [per A-allele odds ratio (95% CI) 1.18 (1.07–1.30); P = 0.0009]. A meta-analysis of four South Asian studies with 8091 subjects showed that the FTO A-allele predisposes to Type 2 diabetes [1.22 (95% CI 1.14–1.31); P = 1.07× 10−8] even after adjusting for BMI [1.18 (95% CI 1.10–1.27); P = 1.02× 10−5] or waist circumference [1.18 (95% CI 1.10–1.27); P = 3.97× 10−5].
Conclusions
The strong association between FTO genotype and BMI and waist circumference in South Asians is similar to that observed in Europeans. In contrast, the strong association of FTO genotype with diabetes is only partly accounted for by BMI.
Introduction
Cardiometabolic diseases associated with overweight and obesity have become a global public health problem [1]. The South Asian population has been shown to have one of the highest risks for these conditions [2,3]. Approximately one in four persons aged 15 years or above is already overweight or obese in Pakistan and 20% of those aged 40 years or above suffer from diabetes [4].
Large-scale studies in Europeans have highlighted the significance of common variants in the FTO (fat mass- and obesity-associated) gene for susceptibility to adiposity. The adiposity-associated FTO variants are also associated with Type 2 diabetes, although recent studies of different ethnic groups have demonstrated apparent differences in the way that FTO contributes to disease risk. In populations of European origin, the strong association first observed between FTO single nucleotide polymorphisms and Type 2 diabetes was completely removed by adjusting for BMI [5]. Several studies of Europeans support these original findings [6–8], although others report BMI-independent associations between FTO variants and diabetes [9,10]. Studies in East Asian individuals show that, whilst the FTO risk allele is rarer compared with Europeans, it has a similar effect on body mass index [11–15]. In contrast, there are few studies of FTO in South Asians. One study consisted of a relatively small sample size—532 patients with Type 2 diabetes and 386 control subjects from the Sikh community in Northern India—and the other study showed a very weak association between FTO and BMI [16,17]. Both studies suggested the FTO association with Type 2 diabetes remained after adjusting for BMI.
To provide more definitive evidence for the role of FTO variation in BMI and Type 2 diabetes in South Asians, we used data from 1666 individuals recruited to the Karachi population-based Control of Blood Pressure and Risk Attenuation (COBRA) study and 2745 individuals of Punjabi ancestry ascertained as part of a Type 2 diabetes case–control study, the UK Asian Diabetes Study/Diabetes Genetics in Pakistan (UKADS/DGP). We assessed the relationship of the FTO rs9939609 variant with measures of adiposity (BMI and waist circumference) and with fasting glucose and Type 2 diabetes. We meta-analysed our results with those from two previously published papers describing the effect of FTO variants in South Asians.
Research design and methods
COBRA study
Study design
A population-based sample representative of adults in Karachi was recruited in a cluster randomized trial of strategies to control hypertension. Ethical approval was obtained from the Ethics Review Committee at the Aga Khan University, Pakistan. The sampling details have been described previously [2]. Briefly, a multistage cluster random sampling design was used to randomly select 12 geographical clusters in Karachi, the largest metropolitan city in Pakistan. A census was carried out and a listing of all individuals from all households in the selected areas was made. All subjects aged 40 years or above and able to consent were invited to participate in the study by trained community health workers. All subjects were evaluated after informed consent was obtained. A range of anthropometric and biochemical data were collected for all subjects. Information on use of anti-diabetes medication was recorded. Diabetes was defined as fasting plasma glucose > 7.0 mmol/l (126 mg/dl) or taking anti-diabetes medication [18]
UKADS/DGP study
Study design
Participants in the study were from two genetically homogeneous Punjabi populations, one resident in the UK and one indigenous to the Mirpur region of Azad Kashmir, Pakistan. Informed consent was obtained from all study participants and the study was approved by the Birmingham East, North and Solihull Research Ethics Committee and the Baqai Institute of Diabetology and Endocrinology Institutional Review Board. All subjects were of Punjabi ancestry, confirmed over three generations, and originated predominantly from the Mirpur District, Pakistan. A range of anthropometric and biochemical data were collected for all subjects.
UKADS subjects
UK-resident subjects with Type 2 diabetes (n = 892) were recruited to the UKADS [19] from Birmingham and Coventry. Ethnically matched control subjects (n = 287) were recruited from the same geographical areas through community screening. As control subjects were recruited from community venues and places of worship, fasting samples or oral glucose tolerance tests were not feasible. Normal glucose tolerance was therefore defined as random blood glucose < 7 mmol/l. Genomic DNA was extracted from venous blood using the Nucleon® protocol (Nucleon Biosciences, Coatbridge, UK).
DGP subjects
Indigenous Pakistani subjects were recruited in collaboration with the Baqai Institute of Diabetology and Endocrinology, Karachi, Pakistan, as part of the DGP study. Subjects with Type 2 diabetes (n = 717) were recruited either from hospitals within Mirpur District or from specifically organized Diabetes Awareness camps. Control subjects (n = 912) were recruited from community screening camps set up throughout Mirpur District. Normal glucose tolerance was defined as fasting whole blood glucose ≤ 5.6 mmol/l. Genomic DNA was extracted from saliva using the Oragene® DNA sample collection kit and extraction protocol (DNA Genotek Inc., XXXXX, ON, Canada).
Genotyping
All subjects (UKADS/DGP, n = 2808; COBRA, n = 1718) were genotyped for the FTO rs9939609 single nucleotide polymorphism using either TaqMan genotyping assays (Applied Biosystems, Warrington, UK) or the KASPar® method (KBiosciences, Hoddesdon, UK). Genotype success rates were 98% (UKADS/DGP) and 97% (COBRA), resulting in 4411 subjects being successfully genotyped (UKADS/DGP, n = 2745; COBRA, n = 1666). Blind duplicates were genotyped (UKADS/DGP, 10.4%; COBRA, 7.6%) resulting in error rates of 0% (0/286) and 2.1% (3/141), respectively.
Statistical analyses
Deviation from Hardy–Weinberg equilibrium in the groups without diabetes was tested using a χ2 goodness-of-fit test (UKADS: χ2 = 0.06, d.f. = 1, P = 0.80; DGP: χ2 = 0.18, d.f. = 1, P = 0.67; COBRA: χ2 = 0.07, d.f. = 1, P = 0.79). Association between the single nucleotide polymorphism and categorical variables such as Type 2 diabetes and obesity category was tested using logistic regression. The association between rs9939609 and Type 2 diabetes was tested before and after adjusting for BMI and waist circumference. Obesity categories were defined in line with recently presented guidelines for Indian Asian populations [20]. A BMI of < 23 kg/m2 was classified as normal weight or underweight, 23 to <25 kg/m2 as overweight and ≥ 25 kg/m2 as obese. Linear regression was used to test association between rs9939609 and continuous variables. All regression analyses were adjusted for age, sex and diabetes as appropriate. All analyses in the COBRA study accounted for clustering at the census level. For the UKADS/DGP study, analyses were adjusted for country of residence. Heterogeneity of effect sizes between the UKADS and DGP cohorts was assessed using Cochran’s Q statistics; no significant heterogeneity was observed for either the BMI (P = 0.747), waist circumference (P = 0.909), overweight/obesity (P = 0.546) or Type 2 diabetes (P = 0.463) analyses. Genotype distributions were compared between subjects without diabetes from the studied groups using a χ2-test of independence (see also Supporting Information, Table S1). Summary statistics from the COBRA and UKADS/DGP studies were combined using an inverse variance weighted meta-analysis, implemented in the Stata module Metan. Within the COBRA study, a sensitivity analysis was performed on those belonging to the single largest ethnic subgroup (Muhajirs); the per FTO A-allele increase in BMI was calculated for both the Muhajir group and the whole COBRA group. Heterogeneity of these effect sizes was tested using Cochran’s Q statistics (see also Supporting Information, Table S2). All statistical analyses were performed using Stata/IC version 10 (2007) (StataCorp, College Station, TX, USA) or SAS 9.1.3 (SAS Institute, Cary, NC, USA).
The clinical details of individuals from the two studies are shown in Table 1. Allele frequencies of rs9939609 were similar to those seen in previously studied Indian Asian populations [16,17] (see also Supporting Information, Table S1).
Table 1.
Demographic and health characteristics of study participants in the two studies
| UKADS/DGP study | COBRA study | |||
|---|---|---|---|---|
| Control subjects | Subjects with Type 2 diabetes | Population control subjects | Subjects with Type 2 diabetes | |
| n | 1177 | 1568 | 1281 | 385 |
| Age (years) | 56.4 (10.7) | 55.8 (11.9) | 51.1 (10.7) | 53.5 (10.7) |
| Per cent male | 50.0 | 51.6 | 46.3 | 40.0 |
| Fasting plasma glucose (mmol/l) | 5.4 (0.6)* | N/A | 5.3 (0.6) | 10.6 (4.0) |
| Systolic blood pressure (mmHg) | 132.0 (20.3) | 137.9 (21.1) | 135.6 (22.8) | 143.4 (24.2) |
| Diastolic blood pressure (mmHg) | 83.7 (11.6) | 84.5 (11.3) | 85.9 (12.8) | 88.3 (12.6) |
| Total cholesterol (mmol/l) | 4.8 (1.2) | 4.8 (1.1) | 4.8 (1.0) | 5.0 (1.2) |
| LDL cholesterol (mmol/l) | 2.9 (1.1) | 2.7 (1.1) | 3.0 (0.8) | 3.1 (0.9) |
| HDL cholesterol (mmol/l) | 1.2 (0.3) | 1.2 (0.4) | 1.1 (0.3) | 1.0 (0.3) |
| Random blood glucose (mmol/l) | 5.4 (0.9)† | N/A | N/A | N/A |
| HbA1c (%) | N/A | 9.0 (2.7)‡ | N/A | N/A |
| Triglycerides (mmol/l) | 1.8 (1.0) | 2.5 (1.8) | 1.7 (0.9) | 2.2 (1.3) |
| BMI (kg/m2) | 25.7 (5.2) | 27.5 (4.8) | 25.2 (5.2) | 26.7 (5.6) |
| Waist circumference (cm) | 93.8 (13.0) | 99.8 (11.4) | 88.1 (12.0) | 93.2 (11.7) |
| Height (m) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) |
| Weight (kg) | 68.7 (14.8) | 73.9 (13.6) | 63.4 (13.8) | 66.4 (15.0) |
Except where specified, values are means (SD).
In the DGP control subjects, fasting plasma glucose is estimated from fasting whole blood glucose measurement, using a conversion factor of 1.15.
Random blood glucose recorded for UKADS control subjects only.
HbA1c recorded for UKADS/DGP subjects with diabetes only.
COBRA, Control of Blood Pressure and Risk Attenuation; DGP, Diabetes Genetics in Pakistan; N/A, not available; UKADS, UK Asian Diabetes Study.
Meta-analysis
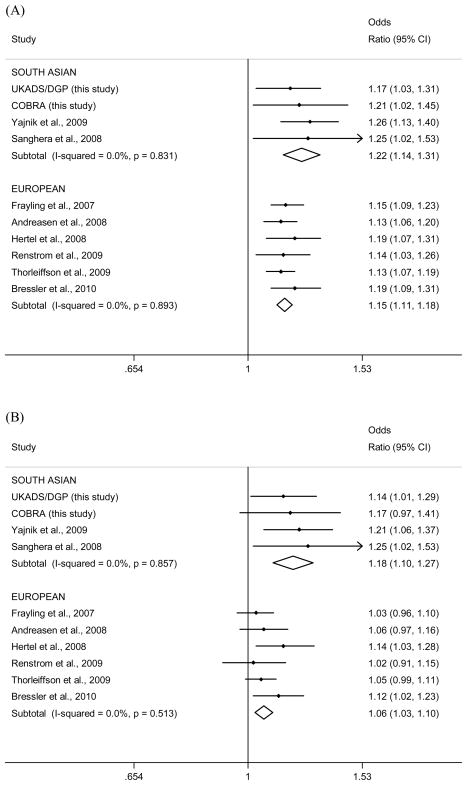
To assess more comprehensively the role of FTO variation in South Asians, we performed a meta-analysis of our data with those from two previous publications [16,17]. These publications represented those identified by a PubMed search of the term ’Asian and FTO‘ that included FTO genotyping in patients with Type 2 diabetes and control subjects in individuals from the Indian subcontinent (South Asians). Results of the South Asian meta-analysis were compared with a similar meta-analysis of previously published studies of European populations [5–8,10,21], identified by a PubMed search of the term ’FTO and diabetes‘. Publications were selected for inclusion if they investigated European populations and reported per FTO risk-allele odds ratios for Type 2 diabetes before and after adjustment for BMI. Two of the selected studies investigated single nucleotide polymorphisms other than rs9939609: rs1121980 and rs8050136. These studies were included as both single nucleotide polymorphisms were good proxies for rs9939609 [r2≥ 0.85 in the CEPH (CEU) HapMap samples] (Fig. 1). We performed an inverse variance weighted meta-analysis of the per-allele effect of rs9939609 (or its proxies) on Type 2 diabetes, before and after correcting for BMI, using the Metan command in Stata/IC (version 10).
Figure 1.
Meta-analysis plots for the association between the FTO rs9939609 single nucleotide polymorphism and Type 2 diabetes before (a) and after (b) adjustment for BMI. In the pooled South Asian populations, rs9939609 was significantly associated with diabetes before (P = 1.07× 10−8) and after (P = 1.02× 10−5) BMI adjustment. Significant heterogeneity was detected between the South Asian studies and the European studies after BMI adjustment (P = 0.009). The single nucleotide polymorphisms included in the meta-analysis from Renstrom et al. (7) and Thorleiffsson et al. (8) were rs1121980 and rs8050136, respectively, both of which are good proxies for rs9939609 [D’/r2 values of 1/1 and 0.96/0.85, respectively, estimated using the CEPH (CEU) HapMap samples]. Data from Sanghera et al [17] (using subjects from the Sikh Diabetes Study) were reanalysed to give allelic odds ratios before inclusion in the meta-analysis. COBRA, Control of Blood Pressure and Risk Attenuation; DGP, Diabetes Genetics in Pakistan; UKADS, UK Asian Diabetes Study.
Results
Association of FTO rs9939609 with BMI and obesity in Pakistani individuals
We observed a significant increase in BMI with increasing numbers of A-alleles in both the COBRA study [0.52 kg/m2 (95% CI 0.15–0.89); P = 0.006] and the UKADS/DGP study [0.42 kg/m2 (95% CI 0.16–0.68); P = 0.002] and when combined in a meta-analysis of the two studies [0.45 kg/m2 (95% CI 0.24–0.67); P = 3.0× 10−5]. A similar pattern was observed for waist circumference, with a per-allele effect of 1.20 cm (95% CI 0.33–2.07; P = 0.007) in the COBRA study, 0.70 cm (95% CI 0.04–1.37; P = 0.038) in the UKADS/DGP study and 0.88 cm (95% CI 0.36–1.41; P = 0.001) in a meta-analysis of the two studies. When BMI was split into a dichotomous trait of overweight/obesity vs. normal weight (defined as BMI ≥ 23 vs. < 23 kg/m2) we again saw a similar effect, with a per-allele effect of odds ratio of 1.21 (95% CI 1.03–1.41; P = 0.02) in the COBRA study, 1.13 (95% CI 0.97–1.30; P = 0.11) in the UKADS/DGP study and 1.17 (95% CI 1.05–1.30; P = 0.005) in the meta-analysis (Table 2).
Table 2.
Association of FTO variant rs9939609 with adiposity in Pakistani individuals
| COBRA study (n = 1666) | UKADS/DGP study (n = 2745) | Combined studies‡ (n = 4411) | ||||
|---|---|---|---|---|---|---|
| Phenotype | Per risk allele effect size (95% CI)* | P | Per risk allele effect size (95% CI)* | P | Per risk allele effect size (95% CI)* | P |
| BMI (kg/m2) | 0.52 (0.15–0.89) | 0.006 | 0.42 (0.16–0.68) | 0.002 | 0.45 (0.24–0.67) | 3.0 × 10−5 |
| Waist circumference (cm) | 1.20 (0.33–2.07) | 0.007 | 0.70 (0.04–1.37) | 0.038 | 0.88 (0.36–1.41) | 0.001 |
| Overweight/obesity† | 1.21 (1.03–1.41) | 0.02 | 1.13 (0.97–1.30) | 0.11 | 1.17 (1.05–1.30) | 0.005 |
β-coefficient (BMI and waist circumference analysis) or odds ratio (overweight/obesity analysis).
Subjects classed as overweight if BMI ≥ 23 kg/m2. The reference category was those subjects with BMI < 23 kg/m2.
Estimated using an inverse variance weighted meta-analysis. β-coefficients refer to the per-A-allele, one degree of freedom test.
COBRA, Control of Blood Pressure and Risk Attenuation; DGP, Diabetes Genetics in Pakistan; UKADS, UK Asian Diabetes Study.
Association of FTO rs9939609 with Type 2 diabetes in Pakistani individuals
We observed an association of FTO genotype with Type 2 diabetes in both studies, each copy of the A-allele increasing the risk of diabetes with an odds ratio of 1.21 (95% CI 1.02–1.45; P = 0.03, age- and sex-adjusted) in the COBRA study, 1.17 (95% CI 1.03–1.31; P = 0.01, age-, sex- and cohort-adjusted) in the UKADS/DGP study and 1.18 (95% CI 1.07–1.30; P = 0.0009) in a meta-analysis of the two studies. These associations remained very similar when adjusting for BMI, with odds ratios of 1.17 (95% CI 0.97–1.41; P = 0.09) in the COBRA study, 1.14 (95% CI 1.01–1.29; P = 0.03) in the UKADS/DGP study and 1.15 (95% CI 1.04–1.27; P = 0.008) in a meta-analysis of the two studies; or waist circumference, with odds ratios of 1.15 (95% CI 0.94–1.41; P = 0.17) in the COBRA study, 1.15 (95% CI 1.01–1.30; P = 0.03) in the UKADS/DGP study and 1.15 (95% CI 1.03–1.28; P = 0.01) in a meta-analysis of the two studies. We also observed an association between FTO genotype and fasting glucose across all individuals in the COBRA cohort, and this also remained similar even after adjusting for BMI or waist circumference (Table 3).
Table 3.
Association of FTO variant rs9939609 with hyperglycaemia in Pakistani individuals
| Phenotype | Model | COBRA study n = 1666 | P | UKADS/DGP study n = 2745 | P | Combined studies n = 4411 | P |
|---|---|---|---|---|---|---|---|
| Fasting glucose (mmol/l) (β-coefficient) | I | 0.24 (0.02–0.46) | 0.03 | N/A | N/A | N/A | N/A |
| II | 0.21 (−0.01 to 0.43) | 0.06 | |||||
| III | 0.19 (−0.03 to 0.41) | 0.09 | |||||
| Diabetes* (odds ratio) | I | 1.21 (1.02–1.45) | 0.03 | 1.17 (1.03 –1.31) | 0.01 | 1.18 (1.07–1.30) | 0.0009 |
| II | 1.17 (0.97–1.41) | 0.09 | 1.14 (1.01–1.29) | 0.03 | 1.15 (1.04–1.27) | 0.008 | |
| III | 1.15 (0.94–1.41) | 0.17 | 1.15 (1.01–1.30) | 0.03 | 1.15 (1.03–1.28) | 0.011 | |
Model I adjusted for age and gender; model II adjusted for age, gender and BMI; model III adjusted for age, gender and waist circumference.
In the COBRA study, diabetes was defined as patients taking anti-diabetes medications or having fasting plasma glucose ≥ 7.0 mmol/l (126 mg/dl). Fasting glucose analysis in the COBRA study includes patients with diabetes.
COBRA, Control of Blood Pressure and Risk Attenuation; DGP, Diabetes Genetics in Pakistan; N/A, not available; UKADS, UK Asian Diabetes Study.
Meta-analysis of association of FTO variant with Type 2 diabetes in South Asian individuals
A meta-analysis of our two studies and two other South Asian studies showed that the association between FTO genotype and Type 2 diabetes is only partly accounted for by BMI, with a combined odds ratio for Type 2 diabetes of 1.22 (95% CI 1.14–1.31; P = 1.07× 10−8) before adjusting for BMI and 1.18 (95% CI 1.10–1.27; P = 1.02× 10−5) after adjusting for BMI (Fig. 1). The association between rs9939609 and diabetes also remained significant after adjustment for waist circumference in the South Asians [see also Supporting Information, Fig. S1; odds ratio 1.18 (95% CI 1.10–1.27); P = 3.97× 10−5). This meta-analysis includes data from both a population-based cohort and case–control studies, but, despite differences in study design, effect sizes for all studies of people of South Asian origin were very similar, both before (P for heterogeneity = 0.83) and after (P for heterogeneity = 0.86) adjustment for BMI. In a meta-analysis of six published studies investigating European populations [5–8,10,21], we found that the association between FTO and Type 2 diabetes was greatly reduced by adjusting for BMI [unadjusted: odds ratio 1.15 (95% CI 1.11–1.18), P = 2.07× 10−20; BMI-adjusted: odds ratio 1.06 (95% CI 1.03–1.10), P = 4.72× 10−4], although it did retain significance (Fig. 1). The BMI-adjusted effect size, however, was significantly larger in the South Asians compared with the Europeans (heterogeneity P = 0.009).
Discussion
Our study is the first from Pakistan to investigate the association of the FTO variant rs9939609 with obesity-related traits and diabetes and the first meta-analysis of several studies from South Asian individuals. We found that the FTO variant is strongly associated with body mass index and waist circumference in the COBRA and UKADS/DGP studies of South Asians, in keeping with multiple reports in European individuals. The estimate of the per-allele effect of each FTO rs9939609 A-allele is 0.45 (95% CI 0.24–0.67) kg/m2, which is very similar to the estimate of 0.39 kg/m2 in Europeans reported by the Genomewide Investigation of Anthropometric Measures (GIANT) consortium [22]. The most important finding from our study is that we have shown a robust association between an FTO variant and Type 2 diabetes in South Asian individuals, and that this association is only partially attenuated by accounting for BMI or waist circumference. In contrast, a meta-analysis of the association between FTO variants and Type 2 diabetes in European individuals showed a much reduced effect when accounting for BMI.
While the association of FTO with diabetes has been reported previously by two case–control studies from the Indian subcontinent, this relationship could not be explained by BMI or waist circumference [16,17]. However, these two studies were either relatively small in sample size [17] or showed inconclusive effects of the FTO variant on BMI [16]. Genetic association studies have repeatedly shown that meta-analyses of several studies provide a more accurate estimate of the real effect—mainly because they increase power. Our meta-analysis, including a total of 3919 South Asian patients with Type 2 diabetes and 4172 control subjects, provides strong evidence that, in South Asians, BMI and central obesity can only partly account for the association between the FTO A-allele and diabetes. We have also shown, through meta-analysis of six studies of Europeans, that adjusting for BMI partially accounts for the association between FTO and Type 2 diabetes in Europeans as well as South Asians. However, the effect of BMI adjustment is much stronger in Europeans compared with South Asians, with most of the Type 2 diabetes association accounted for. We suggest that BMI may be a poorer surrogate measure of adiposity in South Asians compared with Europeans. It is also possible that a single cross-sectional measure of BMI does not adequately reflect the lifelong effects of the FTO polymorphism, and this contributes to residual association between FTO and diabetes in both ethnic groups. In our study of South Asians, adjustment for waist circumference does not appear to explain much more of the association between the FTO variant and diabetes compared with BMI adjustment. This is surprising as central obesity is a particularly well-established correlate of diabetes and cardiovascular risk in South Asians [23].
Our study has limitations. We did not use an oral glucose tolerance-based test to characterize control subjects and it is possible that the control populations contain a small proportion of subjects with undiagnosed diabetes, but this issue is unlikely to result in false positive associations and population-based controls have been successfully used in many genetic studies of Type 2 diabetes. The differences in study designs (cross-sectional and case–control) add to the complexity of interpreting the pooled results, such as those related to the clinical expression and diagnosis of disease. However, the consistency of our main results across several studies means we believe our results are robust and generalizable to the vast majority of native and migrant populations of the Indian subcontinent. Clearly, these findings are of importance as approximately half the population has at least one FTO adiposity-increasing allele. Moreover, the prevalence of overweight and obesity is rapidly escalating in the youth and adults of Pakistan, further enhancing the risk of diabetes [24]. Our findings suggest that this risk is especially elevated in those with the A-allele at FTO variant rs9939609.
In conclusion, we observed a significant association of FTO rs9939609 A-alleles with Type 2 diabetes in South Asians, but this relationship was only partly accounted for by BMI or waist circumference. Overall, our results indicate that the FTO variant has similar effects on BMI and waist circumference in Pakistani individuals compared with Europeans. In contrast to Europeans, where most of the Type 2 diabetes association is explained by BMI adjustment, the effect of the FTO variant on diabetes in Pakistani individuals appears to be only partly accounted for by the effects of FTO on BMI and waist circumference. This could be explained by a poorer correlation between surrogate measures of adiposity and true levels and distributions of adiposity in South Asians compared with Europeans.
Supplementary Material
Additional Supporting Information may be found in the online version of this article:
Figure S1. Meta-analysis plot for the association between the FTO rs9939609 single nucleotide polymorphism and Type 2 diabetes after adjustment for waist circumference.
Table S1. FTO rs9939609 genotype frequencies stratified by cohort and ethnic subgroup.
Table S2. Sensitivity analysis for the Muhajir subgroup.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
Acknowledgments
Funding
The COBRA study was supported by a project grant from the Wellcome Trust, UK. The DGP collection was supported by a project grant from Diabetes UK. Genotyping in both the UKADS and DGP cohorts was funded by Diabetes UK and Eli Lilly.
COBRA study
We would like to thank the members of the study group, including Dr Muhammaed Murtaza, Ms Aisha Zohara, Tayyaba Abbasi, Farina Hanif, Madiha Yameen and Mr Nadir Khan, Azam Khan and Iftikhar Haji, and Dr Philippe Frossard from Aga Khan University (AKU) for DNA extraction of all samples and pretesting of genotyping procedures, Dr Zahra Hasan (AKU) for facilitating provision of NanoDrop 1000; the data management team (including Mr Rasool Bux) and more than 40 field workers and support staff and the laboratory team at Peninsula Medical School, University of Exeter for genotyping of all samples. Finally, we would to thank all study participants for their cooperation. The work for this grant was supported by Wellcome Trust grant 080747/Z/06/Z.
UKADS/DGP study
The authors would like to thank all the patients with diabetes and control subjects for agreeing to participate in this study. We are grateful to Shanaz Mughal, Kam Johal, Dr Anthony Dixon, Dr Sri Bellary and Tahera Mehrali within the UK and Dr Asher Fawwad, Dr Waheed Iqbal and Dr Abdul Razzaq in Pakistan for recruiting the study subjects and coordinating data collection. The UK Asian Diabetes Study was conceived and managed by Professor Anthony Barnett, Professor Sudhesh Kumar and Dr Paul O’Hare and was supported by Pfizer, Sanofi-Aventis, Servier Laboratories UK, Merck Sharp and Dohme/Schering-Plough, Takeda UK, Roche, Merck Pharma, Daiichi-Sankyo UK, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Bristol-Myers Squibb, Solvay Health Care and Assurance Medical Society UK. Funding for the collection of DNA and clinical data from the Pakistan resident population was provided by Diabetes UK (project number 07/0003512). Funding for genetic analyses was provided by Diabetes UK (project number 09/0003926) and Eli Lilly.
Sikh Diabetes Study
Part of this study was supported by the National Institute of Health grant number KO1 TW006087, funded by the Fogarty International Center; R01 DK082766, funded by National Institute of Diabetes and Digestive and Kidney Diseases, USA.
Abbreviations
- COBRA
Control of Blood Pressure and Risk Attenuation
- DGP
Diabetes Genetics in Pakistan
- UKADS
UK Asian Diabetes Study
Footnotes
(Clinical Trials Registry No; NCT 00327574)
Competing interests
Nothing to declare.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Jafar TH, Qadri Z, Chaturvedi N. Coronary artery disease epidemic in Pakistan: more electrocardiographic evidence of ischaemia in women than in men. Heart. 2008;94:408–413. doi: 10.1136/hrt.2007.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350:2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 4.Jafar TH, Levey AS, Jafary FH, White F, Gul A, Rahbar MH, et al. Ethnic subgroup differences in hypertension in Pakistan. J Hypertens. 2003;21:905–912. doi: 10.1097/00004872-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 7.Renstrom F, Payne F, Nordstrom A, Brito EC, Rolandsson O, Hallmans G, et al. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18:1489–1496. doi: 10.1093/hmg/ddp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 9.Legry V, Cottel D, Ferrieres J, Arveiler D, Andrieux N, Bingham A, et al. Effect of an FTO polymorphism on fat mass, obesity, and type 2 diabetes mellitus in the French MONICA Study. Metabolism. 2009;58:971–975. doi: 10.1016/j.metabol.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Hertel JK, Johansson S, Raeder H, Midthjell K, Lyssenko V, Groop L, et al. Genetic analysis of recently identified type 2 diabetes loci in 1638 unselected patients with type 2 diabetes and 1858 control participants from a Norwegian population-based cohort (the HUNT study) Diabetologia. 2008;51:971–977. doi: 10.1007/s00125-008-0982-3. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Wu Y, Loos RJ, Hu FB, Liu Y, Wang J, et al. Variants in the fat mass- and obesity-associated (FTO) gene are not associated with obesity in a Chinese Han population. Diabetes. 2008;57:264–268. doi: 10.2337/db07-1130. [DOI] [PubMed] [Google Scholar]
- 12.Tan JT, Dorajoo R, Seielstad M, Sim XL, Ong RT, Chia KS, et al. FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes. 2008;57:2851–2857. doi: 10.2337/db08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Li H, Loos RJ, Yu Z, Ye X, Chen L, et al. Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes. 2008;57:2834–2842. doi: 10.2337/db08-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta K, Nakata Y, Matsuo T, Kamohara S, Kotani K, Komatsu R, et al. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet. 2008;53:546–553. doi: 10.1007/s10038-008-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabara Y, Osawa H, Guo H, Kawamoto R, Onuma H, Shimizu I, et al. Prognostic significance of FTO genotype in the development of obesity in Japanese: the J-SHIPP study. Int J Obes (Lond) 2009;33:1243–1248. doi: 10.1038/ijo.2009.161. [DOI] [PubMed] [Google Scholar]
- 16.Yajnik CS, Janipalli CS, Bhaskar S, Kulkarni SR, Freathy RM, Prakash S, et al. FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia. 2009;52:247–252. doi: 10.1007/s00125-008-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanghera DK, Ortega L, Han S, Singh J, Ralhan SK, Wander GS, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet. 2008;9:59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Bellary S, O’Hare JP, Raymond NT, Gumber A, Mughal S, Szczepura A, et al. Enhanced diabetes care to patients of south Asian ethnic origin (the UK Asian Diabetes Study): a cluster randomised controlled trial. Lancet. 2008;371:1769–1776. doi: 10.1016/S0140-6736(08)60764-3. [DOI] [PubMed] [Google Scholar]
- 20.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170. [PubMed] [Google Scholar]
- 21.Bressler J, Kao WH, Pankow JS, Boerwinkle E. Risk of type 2 diabetes and obesity is differentially associated with variation in FTO in whites and African-Americans in the ARIC study. PLoS One. 2010;5:e10521. doi: 10.1371/journal.pone.0010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249–796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhawan J, Bray CL, Warburton R, Ghambhir DS, Morris J. Insulin resistance, high prevalence of diabetes, and cardiovascular risk in immigrant Asians. Genetic or environmental effect? Br Heart J. 1994;72:413–421. doi: 10.1136/hrt.72.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jafar TH, Chaturvedi N, Pappas G. Prevalence of overweight and obesity and their association with hypertension and diabetes mellitus in an Indo-Asian population. CMAJ. 2006;175:1071–1077. doi: 10.1503/cmaj.060464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article:
Figure S1. Meta-analysis plot for the association between the FTO rs9939609 single nucleotide polymorphism and Type 2 diabetes after adjustment for waist circumference.
Table S1. FTO rs9939609 genotype frequencies stratified by cohort and ethnic subgroup.
Table S2. Sensitivity analysis for the Muhajir subgroup.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.