Abstract
The fragile X premutation is a tandem CGG trinucleotide repeat expansion on the FMR1 gene between 55 and 200 repeats in length. A CGG knock-in (CGG KI) mouse with CGG trinucleotide repeat lengths between 70 and 350 has been developed and used to model the histopathology and cognitive deficits reported in carriers of the fragile X premutation. Previous studies have shown that CGG KI mice show progressive deficits in processing spatial and temporal information. To characterize the motor deficits associated with the fragile X premutation, male and female CGG KI mice ranging from 2–16 months of age with trinucleotide repeats ranging from 72–240 CGG in length were tested for their ability to perform a skilled ladder rung walking test. The results demonstrate that both male and female CGG KI mice showed a greater number of foot slips as a function of increased CGG repeat length, independent of the age of the animal or general activity level.
Keywords: Fragile X Premutation, Transgenic Mice, Motor Function, Endophenotype, Neurodevelopmental Disorder, Neurodegenerative Disorder, FXTAS
Introduction
The FMR1 gene is polymorphic for the length of a CGG trinucleotide repeat in the 5′ untranslated region. In the general population there are <45 CGG repeats on the FMR1 gene, while in the full mutation underlying fragile X syndrome (FXS) there are >200 CGG repeats and the FMR1 gene is transcriptionally silenced. In the fragile X premutation there are between 55–200 CGG repeats and increased transcription of FMR1 mRNA [29]. Additionally, the fragile X premutation has now been associated with a number of neurocognitive sequelae, such as working memory deficits and impaired spatial information processing [1, 3, 9, 15, 17, 30, 31, 41–44]. The fragile X premutation can also result in the late onset neurodegenerative disorder called fragile X-associated tremor/ataxia (FXTAS), which occurs in upwards of 40% in males and 8–16% of female carriers of the fragile X premutation identified from known fragile X probands [14, 38, 46, 52]. FXTAS sequelae include cerebellar gait ataxia and intention tremor that may be targetable symptoms for pharmacological intervention [33, 52].
To further investigate the consequences of the fragile X premutation, a CGG knock-in (KI) mouse has been developed [6, 11–13, 65]. Behavioral characterizations of these mice have demonstrated subtle motor deficits on an accelerating rotarod as well as impaired spatial memory in the water maze, but only when mice were tested at greater than 12 months of age [53, 62]. Subsequent studies have identified abnormal embryonic development as well as spatial memory deficits evident as early as 12 weeks of age in CGG KI mice [16, 22, 36, 37]. Whether there are early motor deficits in CGG KI mice has yet to be determined.
To determine whether the CGG KI mouse model shows specific motor performance deficits at earlier ages than 12 months of age, more sensitive motor tasks are needed. Therefore, in the present study male and female mice ranging from 2 to 16 months of age were tested on a ladder rung task adapted from procedures reported by Soblosky et al. [58, 59] and refined by Whishaw and colleagues [24, 25, 48, 49, 57–59], among others [4, 21, 56]. This task was chosen because it has been shown to be sensitive to subtle sensorimotor deficits in both mice and rats [24, 58]. In the ladder rung task, mice were allowed to walk along a narrow walkway on a floor made of parallel thin rounded rods at a constant separation. Successful performance in this task requires the animal to precisely determine where to place a paw on a narrow rung, followed by a skillful limb advance and paw placement. In this task, foot slips, defined as the number of times the mouse’s paw fell through the rung floor, were used as an index of skilled motor performance during walking. We found that both male and female CGG KI mice show a greater number of foot slips in this test than wildtype littermates. Furthermore, a CGG dosage effect was evident because within the CGG KI mice with expanded CGG trinucleotide repeats on the Fmr1 gene, the number of foot slips showed a positive association with CGG repeat length, such that mice with long CGG repeat lengths had a greater number of foot slips than mice with more intermediate length CGG repeats.
Methods and Materials
Animals
Forty-two male and 30 female CGG KI mice from 2 to 16 months of age as well as 41 male and 20 female wildtype mice of the same ages were used as subjects for this task. All wildtype mice were littermates with CGG KI mice included in the study. All CGG KI mice were bred onto a congenic C57BL/6J background as verified by microsattelite analysis from founder mice on a mixed FVB/N × C57BL/6J background [37, 64, 65]. Mice were housed in same sex, mixed genotype groups with between one and four mice per cage in a temperature and humidity controlled vivarium. A 12 h light-dark cycle was used with ad libitum access to water and food. All experiments were conducted during the light phase of the cycle and conformed to UC Davis IACUC approved protocols.
Genotyping
DNA was extracted from mouse tails by incubating with 10 mg/mL Proteinase K (Roche Diagnostics; Mannheim, Germany) in 300 μL lysis buffer containing 50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 150 mM NaCl, 1% SDS overnight at 55 °C. One hundred μL saturated NaCl was then added and the suspension was centrifuged. One volume of 100% ethanol was added, gently mixed, and the DNA was pelleted by centrifugation and the supernatant discarded. The DNA was washed and centrifuged in 500 μL 70% ethanol. The DNA was then dissolved in 100 μL milliQ-H2O. CGG repeat lengths were determined by PCR using the Expanded High Fidelity Plus PCR System (Roche Diagnostics). Briefly, approximately 500–700 ng of DNA was added to 50 μL of PCR mixture containing 2.0 μM/L of each primer, 250 μM/L of each dNTP (Invitrogen; Tigard, OR), 2% dimethyl sulfoxide (Sigma-Aldrich; St. Louis, MO), 2.5 M Betaine (Sigma-Aldrich), 5 U Expand HF buffer with mg (7.5 μM/L). The forward primer was 5′-GCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ and the reverse primer was 5′-AGCCCCGCACTTCCACCACCAGCTCCTCCA-3′. PCR steps were 10 min denaturation at 95 °C, followed by 34 cycles of 1 min denaturation at 95 °C, annealing for 1 min at 65 °C, and elongation for 5 min at 75 °C to end each cycle. PCR ends with a final elongation step of 10 min at 75 °C. DNA CGG band sizes were determined by running DNA samples on a 2.5% agarose gel and staining DNA with ethidium bromide [11, 36, 64, 65]. Genotyping was performed twice on each animal, once using tail snips taken at weaning and again on tail snips collected at sacrifice. In all cases the genotypes matched.
Apparatus
The ladder rung apparatus was modeled on previously described ladder rung walk apparatus [24, 48–50, 57–59]. The apparatus consisted of two, 28 cm tall × 65 cm long black walls separated by 10 cm. The floor was elevated 10 cm from the bottom of the walls and was made from 43 parallel 1 mm diameter bars separated by 1.5 cm.
Ladder Rung Testing
All performance on the ladder rung test was recorded with a digital video camera (Sony Handycam; Sony, Inc., Tokyo, Japan) connected by a firewire connector to a PC laptop. The video camera was positioned at one end of the apparatus to record the full length of the beam floor. This allowed the experimenter to score whether the mouse’s limbs extended below the beam floor as well as allowed the experimenter to observe the general posture of the mouse above the beam floor.
For testing, mice were gently placed in the apparatus and allowed to freely explore the apparatus and walk back and forth along the apparatus for 2 min. Mice typically explored the apparatus by walking the length of the apparatus, looking over the edge, and returning to the start position. During testing, the experimenter recorded how many times the mouse moved between the two ends of the apparatus as a general activity measure. All experiments were performed by the same experimenter. The digital recordings were later independently scored from the recordings by two experimenters blinded to the genotype of the animals (intraclass correlation coefficient = 0.94, p<0.001).
Dependent Measures and Statistical Analysis
Along with recording the number of times each mouse traversed the apparatus, the number of times the animals’ fore or hind paws slipped below the ladder rungs was recorded as the dependent variable. A foot slip was recorded whenever the limb of the mouse passed below a rung sufficiently to clearly see the wrist of the animal. In this way, slipping was defined as the mouse completely missing or falling off a rung while walking across the apparatus.
To determine whether parametric analyses of variance (ANOVA) were appropriate for the data, tests of normality and homoscedasticity were performed. Once it was determined that parametric statistics were appropriate for the data, the data were plotted and placed into CGG repeat length groups as follows: the mice in the wildtype group all had between 8–12 CGG repeats (mean 10 +/− 0.25 SEM; n=61), mice included in the Low CGG repeat group ranged between 72–116 CGG repeats (mean 86 +/− 3.1; n=20), and the mice included in the High CGG repeat group ranged between 140–240 CGG repeats (mean 170 +/− 9.2; n=52). These groupings were used to categorize group by CGG repeat length because it was determined that the 24 CGG gap in the data between the Low (e.g., 116) and the High (e.g., 140) CGG repeat groups as well as the large gap between Wildtype (8–12 CGG repeats) and Low (e.g., 72) CGG repeat groups invalidated any using CGG repeat length as a continuous variable for statistical analysis. Similar groupings were also used in previous studies of the CGG KI mice [36]. To determine if age significantly contributed to ladder rung test performance, mice were further separated into two groups based upon a median split of age, with one group ≤ 6 months of age (mean 4.5 +/− .99 mo; range 2–6 mo) and the other ≥ 7 months of age (mean 10.75 +/− 2.1 mo; range 7–16 mo). The data were analyzed as follows. A 3 (Group) × 2 (Sex) × 2 (Age) analysis of covariance (ANCOVA) with locomotor activity as a potential covariate was used to determine which factors contribute to task performance. Subsequent analyses were performed to further characterize all main effects. All analyses were considered significant at p < 0.05. Statistical analyses were performed in R 2.7.1 language and environment (The R Foundation for Statistical Computing; Aukland University, Aukland, New Zealand; http://www.r-project.org/) and statistical power was calculated using both R and the statistical program G*Power 3 [26, 27].
Results
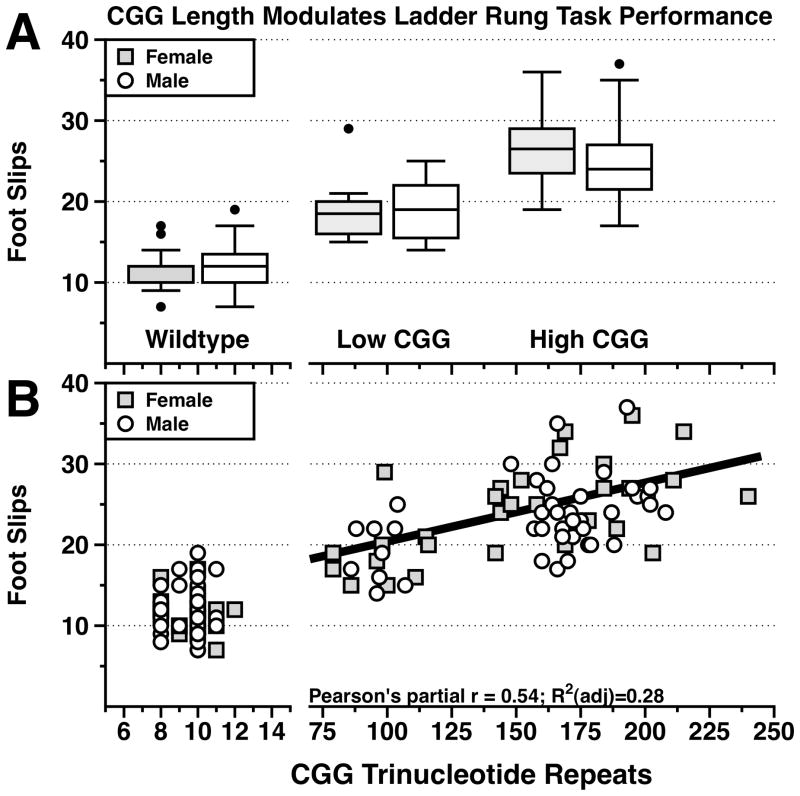
For all mice, data were grouped by CGG repeat length (wildtype, low CGG, high CGG), Sex (male, female), and Age (≤ 6 mo, ≥ 7 mo) and analyzed using three way ANCOVA with number of foot slips as the dependent variable and locomotor activity as a covariate. There was a main effect of CGG repeat length group (F(2,120)=5.4373, p=.005), but no main effect of Age (F(1,120)=0.54, p=0.47) or Sex (F(1,120)=0.06, p=0.94), nor were there any significant interactions among variables (lowest p value p=0.45). There was also no significant contribution of locomotor behavior for performance on the ladder rung task (F(1,120)=0.23, p=0.63). These results suggest that Age, Sex, and activity level did not contribute to task performance and only the CGG repeat length group factor significantly contributed to ladder rung task performance (Figure 1A).
Figure 1. CGG repeat length modulates ladder rung task performance.
A. Boxplots stratified by Sex and CGG repeat length group. Note that the High CGG repeat group showed a greater number of foot slips than mice in the Low CGG repeat group (p<0.005). Both the High and Low CGG repeat groups had a greater number of foot slips than wildtype mice (p<0.0001, p<0.005 respectively). Wildtype male n=41, female n=20; Low CGG male n=10, female n=10; High CGG male n=32, female n=20.
B. Scatterplot of CGG repeat length and the number of foot slips for each animal included the present study. A partial correlation performed comparing the number of foot slips to CGG repeat length (adjusted for influence of Sex) within CGG KI mice with expanded CGG trinucleotide repeats demonstrated a positive association between CGG repeats and the number of foot slips (Pearson’s partial r=0.54; R2(adj)=0.28).
To further characterize the significant main effect of CGG repeat length group, a Tukey-Kramer post hoc pairwise comparisons test demonstrated that the wildtype mice showed significantly fewer foot slips (mean 12.8 +/− 0.55) than the Low (mean 19.1 +/− 0.88) or High (mean 25.3 +/− 0.65) CGG repeat groups (p<0.005, p<0.0001 respectively), and that the Low CGG repeat groups had significantly fewer foot slips than the High CGG repeat group (p<0.005) (Figure 1A).
To characterize any possible relationship between CGG repeat length and performance on the ladder rung task in CGG animals with expanded CGG trinucleotide repeats, a partial correlation coefficient adjusted for Sex was calculated. A positive association was observed between the CGG trinucleotide repeat length and the number of foot slips during ladder rung task performance (Figure 1B; Pearson’s partial r=0.54; R2(adj)=0.28).
Discussion
The current experimental results reveal that both male and female CGG KI mice are impaired in performance of a skilled ladder rung walking task compared to wildtype littermates. Specifically, CGG KI mice showed a greater number of foot slips with increasing CGG repeat length (Figure 1B). These findings suggest that the length of an expanded CGG trinucleotide repeat on the Fmr1 gene is related to impaired locomotor performance in CGG KI mice as observed in the ladder rung task. The present data also provide the first demonstration of motor deficits in CGG KI mice under 12 months of age. Interestingly, mice as young as two months of age appeared to show motor deficits similar to the mice over 12 months of age. Contrary to our initial hypotheses, age did not contribute to task performance, nor were there differences between sexes for skilled ladder rung performance. Male and female mice showed similar decrements in performance with increasing CGG repeat length (Figure 1A, B). These data suggest that the ladder rung task is likely revealing an early motor deficit as opposed to directly modeling the late onset cerebellar gait ataxia reported in human cases of FXTAS.
The early appearance of motor deficits was not entirely unexpected, considering recent reports that embryonic cortical development is abnormal in CGG KI mice [22] and that dendritic complexity is reduced and synaptic structure is altered in cultured hippocampal neurons [16]. Furthermore, it has been found that CGG repeat length and age modulate performance on a spatial processing task in 23–43 year old human female fragile X premutation carriers [30].
The lack of differential performance between sexes was unexpected as it has been reported that fragile X syndrome and FXTAS are more prevalent in males than females, presumably due to a protective influence of a second non mutated FMR1 gene on the second X chromosome [7, 38]. Despite the reduced prevalence of FXTAS in female carriers of the fragile X premutation relative to males, females with FXTAS do not show reduced FXTAS symptoms once diagnosed [7, 34]. Furthermore, it is possible that the ladder rung task is sensitive enough to probe the underlying motor networks that may be similarly disrupted in male and female CGG KI mice.
Although not quantified, CGG KI mice also showed a hunched posture all ages and a discernible shaking while walking along the ladder rung apparatus. Similar behaviors were not observed in wildtype mice. This tremoring during the performance of a motor task is of interest because no gross motor abnormalities or tremoring are apparent when CGG KI mice are observed in an open field. These results suggest that motor abnormalities in CGG KI mice may not be apparent until the mice are challenged by a difficult motor task, such as the ladder rung task, which may unmask a previously unidentified motor tremor. These postural tremor-like behaviors need to be further investigated and carefully described in CGG KI mice.
These results also indicate that the ladder rung task is a sensitive and robust assay that allows for a high throughput analysis of motor function in CGG KI mice. As each mouse was only exposed to the apparatus for approximately 2 min in the present experiment and there was no adaptation period preceding data collection, performance of the task served as a rapid assay of motor function without the potential confounds of motor learning that may mask between group effects, as suggested in earlier studies [24, 58]. This point is important as the rotarod task used to test motor function in mice requires the mouse be placed on a rotating drum and the time to fall is typically used as the outcome measure. For the rotarod, there are often early training trials given to mice on the rotarod apparatus that may potentially mask any differences that are present in baseline motor function as mice are trained to set performance criteria before administration of accelerating rotarod testing [58, 59].
The present experiment did not explicitly employ the subtle gait measurements described by Whishaw and colleagues [8, 24, 50]. This is because the sides of the apparatus used in this study were opaque and prevented the requisite recording the mouse’s foot placement from the side for more precise analysis of limb movements. Such measurements could provide additional evidence for subtle motor deficits, as well as the precise nature of the observed missteps. For example, more sophisticated analyses of gait would reveal whether animals make predictable errors such as consistently under or overestimating the location of subsequent beams. Such errors could indicate a possible dysfunction in frontal-parietal network-dependent vector calculations underlying action in peripersonal space [23, 54, 63]. Alternately, if animals showed a trend toward a general clumsiness or lack of precision in motor performance that could indicate a more purely motor deficit [4, 5]. The first possibility is intriguing in for the CGG KI mice as frontal-parietal network dysfunction is hypothesized to underlie spatiotemporal, arithmetic, and attentional deficits in FXS as well as the fragile X premutation [3, 10, 18–20, 28, 30, 32, 35, 39, 40, 43, 47, 51, 55, 60, 61], as well as being involved in skilled walking behaviors [2, 4, 5, 23, 45]. The latter possibility, based more directly on motor function is also important for the extension of the CGG KI mouse as a murine model of motor deficits present in FXTAS [62]. Such follow-up studies are currently underway to explore these possibilities in CGG KI mice as well as to correlate any performance deficits to neuropathological features, which are present throughout the neocortex and cerebellum of CGG KI mice [37, 64, 65].
The primary benefit of this modification of the ladder rung task is that extensive pre-training is not required and that testing times and trials are significantly shortened compared to the versions of the task reported previously [24,50]. Furthermore, spontaneous exploration is encouraged, and the effect of this behavior on potential error production can be considered. This results in a high throughput screen that is sufficiently sensitive to detect subtle motor impairments in transgenic mouse models.
In summary, the present experiment identified age-independent motor deficits in CGG KI mice. The detection of motor deficits in young CGG KI mice is important as performance on the ladder rung task may be used as outcome measures for behavioral or pharmacological therapeutic intervention in this mouse model as it pertains to FXTAS as well as other late onset neurodegenerative disorders. This modification might make the test more attractive to other groups.. Because mice as young as 2 months of age show deficits, the need to limit testing to mice greater than 12 months of age to identify potential endpoints and outcome measures is mitigated.
Acknowledgments
Funding
This work was supported by National Institute of Health (NIH) grants, NINDS RL1 NS062411 and TL1 DA024854. This work was also made possible by a Roadmap Initiative grant (UL1 DE019583) from the National Institute of Dental and Craniofacial Research (NIDCR) in support of the NeuroTherapeutics Research Institute (NTRI) consortium; and by a grant (UL1 RR024146) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations
- FXS
Fragile X Syndrome
- FXTAS
Fragile X-Associated Tremor/Ataxia Syndrome
- FMR1
Fragile X Mental Retardation 1 gene
- KI
Knock-in
- WT
Wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams, Nguyen DV, Hessl, Brunberg JA, Tassone F, Zhang, et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B. 2010;153B:775–785. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andujar JE, Lajoie K, Drew T. A contribution of area 5 of the posterior parietal cortex to the planning of visually guided locomotion: limb-specific and limb-independent effects. J Neurophysiol. 2010;103:986–1006. doi: 10.1152/jn.00912.2009. [DOI] [PubMed] [Google Scholar]
- 3.Aziz, Stathopulu, Callias, Taylor, Turk, Oostra, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B. 2003;121:119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- 4.Beloozerova IN, Sirota MG. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J Physiol. 1993;461:1–25. doi: 10.1113/jphysiol.1993.sp019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beloozerova IN, Sirota MG. Integration of motor and visual information in the parietal area 5 during locomotion. J Neurophysiol. 2003;90:961–971. doi: 10.1152/jn.01147.2002. [DOI] [PubMed] [Google Scholar]
- 6.Berman RF, Willemsen R. Mouse Models of Fragile X-Associated Tremor Ataxia. J Inv Med. 2009;57:837–841. doi: 10.231/JIM.0b013e3181af59d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry K, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- 8.Blume SR, Cass DK, Tseng KY. Stepping test in mice: a reliable approach in determining forelimb akinesia in MPTP-induced Parkinsonism. Exp Neurol. 2009;219:208–211. doi: 10.1016/j.expneurol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois JA, Seritan A, Casillas EM, Hessl D, Schneider A, Yang Y, et al. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72:175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bregman JD, Dykens E, Watson M, Ort SI, Leckman JF. Fragile-X syndrome: variability of phenotypic expression. J Am Acad Child Adolesc Psychiatry. 1987;26:463–471. doi: 10.1097/00004583-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer, Huizer, Severijnen, Hukema, Berman RF, Oostra, et al. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107:1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwer, Severijnen, de J, Hessl, Hagerman RJ, Oostra, et al. Altered hypothalamus Äìpituitary Äìadrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwer, Willemsen R, Oostra The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am J Med Genet B. 2009;31:71–83. doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunberg J, Jacquemont, Hagerman RJ, Berry K, Grigsby, Leehey, et al. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- 15.Cellini E, Forleo P, Ginestroni A, Nacmias B, Tedde A, Bagnoli S, et al. Fragile X premutation with atypical symptoms at onset. Arch Neurol. 2006;63:1135–1138. doi: 10.1001/archneur.63.8.1135. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Tassone F, Berman RF, Hagerman P, Hagerman RJ, Willemsen R, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chonchaiya W, Utari A, Pereira GM, Tassone F, Hessl D, Hagerman RJ. Broad clinical involvement in a family affected by the fragile X premutation. J Dev Behav Pediatr. 2009;30:544–551. doi: 10.1097/DBP.0b013e3181c35f25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10:11–16. doi: 10.1002/mrdd.20003. [DOI] [PubMed] [Google Scholar]
- 19.Cornish K, Swainson R, Cunnington R, Wilding J, Morris P, Jackson G. Do women with fragile X syndrome have problems in switching attention: preliminary findings from ERP and fMRI. Brain Cogn. 2004;54:235–239. doi: 10.1016/j.bandc.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, et al. Annotation: Deconstructing the attention deficit in fragile X syndrome: a developmental neuropsychological approach. J Child Psychol Psychiatry. 2004;45:1042–1053. doi: 10.1111/j.1469-7610.2004.t01-1-00297.x. [DOI] [PubMed] [Google Scholar]
- 21.Cummings BJ, Engesser-Cesar C, Cadena G, Anderson AJ. Adaptation of a ladder beam walking task to assess locomotor recovery in mice following spinal cord injury. Behav Brain Res. 2007;177:232–241. doi: 10.1016/j.bbr.2006.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham CL, Martinez Cerdeno V, Navarro Porras E, Prakash AN, Angelastro JM, Willemsen R, et al. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Mol Genet. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drew T, Andujar JE, Lajoie K, Yakovenko S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev. 2008;57:199–211. doi: 10.1016/j.brainresrev.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Farr TD, Liu, Colwell, Whishaw IQ, Metz GA. Bilateral alteration in stepping pattern after unilateral motor cortex injury: a new test strategy for analysis of skilled limb movements in neurological mouse models. J Neurosci Methods. 2006;153:104–113. doi: 10.1016/j.jneumeth.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 27.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 28.Franke P, Leboyer M, Gansicke M, Weiffenbach O, Biancalana V, Cornillet-Lefebre P, et al. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psych Res. 1998;80:113–127. doi: 10.1016/s0165-1781(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 29.GarciaArocena D, Hagerman P. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19R1:R83–89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivistava S, Tassone F, Harvey D, et al. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn. 2011;75:255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigsby, Brega AG, Engle K, Leehey, Hagerman R, Tassone F, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 32.Grigsby JP, Kemper MB, Hagerman RJ, Myers CS. Neuropsychological dysfunction among affected heterozygous fragile X females. Am J Med Genet B. 1990;35:28–35. doi: 10.1002/ajmg.1320350107. [DOI] [PubMed] [Google Scholar]
- 33.Hagerman RJ, Hall, Coffey, Leehey, Bourgeois JA, Gould, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Int Ageing. 2008;3:251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagerman RJ, Leavitt, Farzin, Jacquemont, Greco CM, Brunberg J, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodapp, Dykens, Ort, Zelinsky, Leckman Changing patterns of intellectual strengths and weaknesses in males with fragile X syndrome. J Autism Dev Disord. 1991;21:503–516. doi: 10.1007/BF02206873. [DOI] [PubMed] [Google Scholar]
- 36.Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal Ordering Deficits in Female CGG KI Mice Heterozygous for the Fragile X Premutation. Behav Brain Res. 201(213):263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123:1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacquemont, Hagerman RJ, Leehey, Hall, Levine, Brunberg J, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am J Med Genet B. 1999;83:286–295. [PubMed] [Google Scholar]
- 40.Kemper, Hagerman RJ, Altshul S. Cognitive profiles of boys with the fragile X syndrome. Am J Med Genet B. 1988;30:191–200. doi: 10.1002/ajmg.1320300118. [DOI] [PubMed] [Google Scholar]
- 41.Keri S, Benedek G. Visual pathway deficit in female fragile X premutation carriers: a potential endophenotype. Brain Cogn. 2009;69:291–295. doi: 10.1016/j.bandc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Keri S, Benedek G. The perception of biological and mechanical motion in female fragile X premutation carriers. Brain Cogn. 2010;72:197–201. doi: 10.1016/j.bandc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Kogan CS, Boutet I, Cornish KM, Zangenehpour S, Mullen KT, Holden JJ, et al. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004;127:591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- 44.Kogan CS, Cornish KM. Mapping self-reports of working memory deficits to executive dysfunction in Fragile X Mental Retardation 1 (FMR1) gene premutation carriers asymptomatic for FXTAS. Brain Cogn. 2010;73:236–243. doi: 10.1016/j.bandc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Lajoie K, Andujar JE, Pearson K, Drew T. Neurons in area 5 of the posterior parietal cortex in the cat contribute to interlimb coordination during visually guided locomotion: a role in working memory. J Neurophysiol. 2010;103:2234–2254. doi: 10.1152/jn.01100.2009. [DOI] [PubMed] [Google Scholar]
- 46.Leehey, Berry K, Min, Hall, Rice, Zhang, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22:203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 47.Loat, Craig, Plomin Investigating the relationship between FMR1 allele length and cognitive ability in children: a subtle effect of the normal allele range on the normal ability range? Ann Hum Genet. 2006;70:555–565. doi: 10.1111/j.1469-1809.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 48.Metz G. Skilled Limb Use in Rat Models of Human Neurological Disease. In: Spink AJ, Ballintijn MR, Bogers ND, Grieco F, Loijens LWS, Noldus LPJJ, Smit G, Zimmerman PH, editors. Proceedings of Measureing Behavior. Maastricht; The Netherlands: 2008. [Google Scholar]
- 49.Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- 50.Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 51.Miezejeski CM, Jenkins EC, Hill AL, Wisniewski, French JH, Brown WT. A profile of cognitive deficit in females from fragile X families. Neuropsychologia. 1986;24:405–409. doi: 10.1016/0028-3932(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Ortigas MC, Bourgeois JA, Schneider A, Olichney, Nguyen DV, Cogswell J, et al. Improving Fragile X-Associated Tremor/Ataxia Syndrome Symptoms With Memantine and Venlafaxine. J Clin Psychopharmacol. 2010;30:642–644. doi: 10.1097/JCP.0b013e3181f1d10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, et al. A mouse model of the fragile X premutation: Effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42:85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redish AD, Touretzky DS. The reaching task: evidence for vector arithmetic in the motor system? Biol Cybern. 1994;71:307–317. doi: 10.1007/BF00239618. [DOI] [PubMed] [Google Scholar]
- 55.Rivera, Menon, White CD, Glaser B, Reiss Functional brain activation during arithmetic processing in females with fragile X Syndrome is related to FMR1 protein expression. Hum Brain Map. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt RA, Zuckerman J, Martin HA, Wolfe KF., Jr A novel discrete gross motor learning task: modifications of the Bachman ladder. Res Q. 1971;42:78–82. [PubMed] [Google Scholar]
- 57.Shriner AM, Drever FR, Metz GA. The development of skilled walking in the rat. Behav Brain Res. 2009;205:426–435. doi: 10.1016/j.bbr.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soblosky JS, Colgin LL, Chorney-Lane D, Davidson JF, Carey ME. Ladder beam and camera video recording system for evaluating forelimb and hindlimb deficits after sensorimotor cortex injury in rats. J Neurosci Methods. 1997;78:75–83. doi: 10.1016/s0165-0270(97)00131-3. [DOI] [PubMed] [Google Scholar]
- 59.Soblosky JS, Colgin LL, Chorney-Lane D, Davidson JF, Carey ME. Some functional recovery and behavioral sparing occurs independent of task-specific practice after injury to the rat’s sensorimotor cortex. Behav Brain Res. 1997;89:51–59. doi: 10.1016/s0166-4328(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 60.Steyaert J, Legius E, Borghgraef M, Fryns JP. A distinct neurocognitive phenotype in female fragile-X premutation carriers assessed with visual attention tasks. Am J Med Genet A. 2003;116A:44–51. doi: 10.1002/ajmg.a.10821. [DOI] [PubMed] [Google Scholar]
- 61.Tassone F, Hagerman RJ, Taylor, Mills, Harris, Gane, et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet B. 2000;91:144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 62.Van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, et al. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behav Brain Res. 2005;162:233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Ward, Brown Covert orienting of attention in the rat and the role of striatal dopamine. J Neurosci. 1996;16:3082–3088. doi: 10.1523/JNEUROSCI.16-09-03082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenzel HJ, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 2010 doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willemsen R, Hoogeveen W, Reis, Holstege, Severijnen, Nieuwenhuizen, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]