Abstract
Drug and alcohol use have been associated with a worse prognosis in short-term and cross-sectional analyses of HIV-infected populations, but longitudinal effects on adherence to antiretroviral therapy (ART) and clinical outcomes in advanced AIDS are less well characterized. We assessed self-reported drug and alcohol use in AIDS patients, and examined their association with non-adherence and death or disease progression in a multicenter observational study. We defined non-adherence as reporting missed ART doses in the 48 hours before study visits. The association between drug use and ART non-adherence was evaluated using repeated measures generalized estimating equation (GEE) models. The association between drug and alcohol use and time to new AIDS diagnosis or death was evaluated via Cox regression models, controlling for covariates including ART adherence. Of 643 participants enrolled between 1997–1999 and followed through 2007, at entry 39% reported ever using cocaine, 24% amphetamines, and 10% heroin. Ongoing drug use during study follow-up was reported by 9% using cocaine, 4% amphetamines, and 1% heroin. Hard drug (cocaine, amphetamines, or heroin) users had 2.1 times higher odds (p=0.001) of ART non-adherence in GEE models and 2.5 times higher risk (p=0.04) of AIDS progression or death in Cox models. Use of hard drugs was attenuated as a risk factor for AIDS progression or death after controlling for non-adherence during follow-up (HR=2.11, p=0.08), but was still suggestive of a possible adherence-independent mechanism of harm. This study highlights the need to continuously screen and treat patients for drug use as a part of ongoing HIV care.
Keywords: Substance use, drug use, alcohol use, HIV/AIDS, Outcomes, Adherence, Antiretroviral Therapy, Mortality
INTRODUCTION
Adherence to antiretroviral therapy (ART) is crucial to optimize treatment efficacy and prolong survival in patients with HIV disease [1–3]. The longitudinal effect of alcohol and illicit substance use (hereafter, “drug use”) on adherence to highly active antiretroviral therapy (HAART) and on clinical outcomes in patients with advanced AIDS has been less well characterized.
Drug and alcohol use are highly prevalent among HIV-infected populations in the United States [4]; but the long-term impact of these substances among patients on HAART is unclear [5–9]. Drug and alcohol use have been associated with decreased use of HAART [10–15], non-adherence to HAART [14–20], decreased viral suppression [14,21–24], and HIV disease progression in cross-sectional and short-term analyses of HIV-infected populations [6, 15, 16, 19, 22, 23, 25, 26]. Active drug use was linked to disease progression in a university-based HIV-infected population, followed for up to 21/2 years from a single clinic [24]. Even intermittent use of intravenous drugs was associated with diminished virologic response to HAART in a population with lower HIV severity [14]. Recent heroin or cocaine use and homelessness were associated with increased short-term mortality in HIV-infected patients with alcohol problems [27]. Little is known about the prevalence of drug and alcohol use and its impact on morbidity and mortality over longer periods or after prolonged immune reconstitution.
The objective of this study is to describe the prevalence and impact of self-reported alcohol and drug use in patients enrolled in a decade-long clinical trial of AIDS patients who experienced immune reconstitution on therapy. We assessed the impact of reported drug and alcohol use on self-reported adherence to antiretroviral medications and on HIV disease progression and death within the context of an observational extension of a multicenter, randomized clinical study.
METHODS
Study Population
In the clinical trial, we enrolled 643 HIV-infected subjects without prior Mycobacterium avium complex (MAC) and with documented immune reconstitution (CD4 cell counts <50 followed by CD4 >100 cells/mm3 on two separate occasions before study entry) from October 1997 through April 1999. AIDS Clinical Trials Group (ACTG) 362, a prospective, placebo-controlled multicenter trial of discontinuing MAC prophylaxis, was stopped in October 1999 after failing to show a treatment difference for azithromycin versus placebo. We enrolled 433 of the participants still on study in an observational cohort to evaluate cardiovascular, metabolic, and neurological outcomes of HAART therapy [28]. Self-administered questionnaires were used to assess alcohol and drug use at entry and during follow-up visits among participants [26]. The protocol and its successive amendments were approved by institutional review boards at 29 participating U.S. ACTG sites; all participants provided written informed consent.
Data Collection and Event Definitions
Subjects were assessed at baseline, weeks 4 and 8, and then at 8–32 week intervals through the end of the study at week 512. They were evaluated for alcohol and drug use, medication adherence, new opportunistic infections or cardiovascular events, interval medical histories, CD4 counts, plasma HIV-1 RNA measurements, and any changes in ART or other medications. HIV-1 RNA measurements were obtained using the Roche Amplicor standard assay (Roche Diagnostics, Branchburg, NJ), with a lower limit of quantification of 500 copies/mL.
Data was collected from October 1997 to April 2007 on ART use before and at study entry and throughout the follow-up period. Individual clinical events and specific diagnoses were reviewed and corroborated using the Adult ACTG Criteria for Clinical Events by study clinicians (SEC, SLK, JSC) [29].
Adherence Assessment
At each follow-up visit, subjects were asked whether they were prescribed and adhering to ART. Non-adherence to ART was defined as missing any doses during the prior 48 hours. In addition, we defined “ever non-adherent” to ART if the subject reported ever missing ART in the past week at baseline or in the prior 48 hours at any follow-up visit throughout the study.
At baseline, participants completed a self-report questionnaire to assess potential factors affecting adherence [30]. Baseline adherence was assessed based on this question: “When was the last time you skipped any of your medications?” with the options “within the past week,” ”within the past 2 weeks,” “2–4 weeks ago,” “1–3 months ago,” “more than 3 months ago,” and “never skip medications or not applicable.” The responses to this question were grouped as “within the past week” versus “more than a week ago” (i.e., any of last 5 options) to be most similar to the 2-day adherence assessment.
Assessment of Drug and Alcohol Use
At each visit, subjects completed a self-reported questionnaire describing their current use of alcohol, cocaine (or crack), heroin, and amphetamines, and whether they were in methadone treatment. Patients reported their quantity and frequency of their alcohol consumption during the past 30 days. Drinking was dichotomized in 2 ways, as heavy drinkers (regularly drinking >4 drinks per day on average when they drank over the past 30 days) or not; and as binge drinkers (5 or more drinks within a couple of hours at least once in the last 30 days) or not [16,31]. Ongoing use of cocaine, heroin, and/or amphetamines within 30 days prior to a study visit was referred to as “ongoing hard drug use.” Participants were also asked whether or not they injected cocaine, amphetamines or heroin in the past 30 days, and frequency of use per day. They reported whether they had ever used cocaine, amphetamines and heroin at baseline and throughout the study, and alcohol use in the 30 days prior to each visit.
Statistical Analysis
Non-adherence to ART over the prior two days and recent substance use over the prior 30 days were assigned to the nearest scheduled study visit, at weeks 0 and 4, at 8 week intervals from weeks 8 to 224,, and at 16–32 week intervals from week 224 to 512. The association between drug use and non-adherence to ART was evaluated using repeated measures generalized estimating equation (GEE) models, with an assumption of equal correlation between any pair of visits within each participant. The repeated measures models considered the indicator of recent hard drug use and non-adherence for each study visit. Other possible predictors of non-adherence included demographic factors (e.g. age, sex, race/ethnicity), HIV disease severity (baseline viral load, baseline CD4 count, Karnofsky score<80, prior AIDS defining condition), and treatment indicators (years of combination ART at entry, randomization to azithromycin or placebo). We performed backward selection of candidate predictors for multivariable models, retaining covariates with p<0.10.
The association between drug and alcohol use and time to new AIDS diagnosis or death was evaluated via Cox regression models, controlling for the potential confounders described above along with ART non-adherence. Two approaches for controlling for non-adherence were considered: one which controlled only for recent non-adherence to ART in the week prior to study entry (i.e., baseline non-adherence), and a second approach using time-updated indicators of ART non-adherence during study follow-up (time-dependent non-adherence). The association between ART non-adherence and virologic treatment failure (HIV-1 RNA ≥ 500 copies/mL) was evaluated in two ways. First, non-adherence was evaluated as a time-dependent covariate in a Cox model for time to first viral load failure. Second, non-adherence immediately prior to each visit was evaluated as a correlate of virologic failure at that visit in a GEE repeated measures analysis. All analyses were performed using Statistical Analysis System 9.1 (SAS Institute, Cary, NC). P-values less than 0.05 were considered statistically significant.
RESULTS
Subject characteristics
The demographic and clinical characteristics of the 643 participants are presented in Table 1. All subjects were on ART at baseline. Subjects had an average of 11 visits (IQR=5–16) at which adherence and drug and alcohol use were assessed. Maximum time on study was 9.4 years and median time on study was 6.0 years (IQR=1.9–8.6). The rate of loss to follow up was approximately 6% per year during the first two years of the clinical trial and less than 3% per year during the last 7 years of the observational study. Of the 643 subjects, 434 completed the clinical trial, 53 died during study follow-up, 17 were at sites that lost funding to continue the study, and 144 were lost to follow-up (81 refused contact, 51 were unable to be contacted, 4 moved, 1 was incarcerated, and 7 discontinued due to other reasons). The 144 lost to follow-up were more likely to be younger and black or Hispanic rather than white non-Hispanic, but were similar in other baseline characteristics (gender, CD4 count, HIV-1 RNA, adherence, ART use, and lifetime drug use or alcohol use reported in the 30 days prior to entry).
Table 1.
Baseline and on Study Health Characteristics of AIDS Patients in ACTG 362, by Self-Reported Hard Drug Use During Study Follow-up
| Characteristic | Hard Drug User During Study1 | ||||
|---|---|---|---|---|---|
| Total (N=643) | No (N=566) | Yes (N=77) | P-Value2 | ||
| Age at entry (years) | Median | 40 | 40 | 40 | 0.95 |
| < 35 years | 135 (21%) | 120 (21%) | 15 (19%) | 0.96 | |
| 35-39 years | 184 (29%) | 162 (29%) | 22 (29%) | ||
| 40-44 years | 129 (20%) | 112 (20%) | 17 (22%) | ||
| 45+ years | 195 (30%) | 172 (30%) | 23 (30%) | ||
| Gender | Male | 559 (87%) | 487 (86%) | 72 (94%) | 0.07 |
| Female | 84 (13%) | 79 (14%) | 5 (6%) | ||
| Race/Ethnicity | White Non-Hispanic | 369 (57%) | 320 (57%) | 49 (64%) | 0.22 |
| Black Non-Hispanic | 131 (20%) | 115 (20%) | 16 (21%) | ||
| Hispanic | 117 (18%) | 105 (19%) | 12 (16%) | ||
| Other | 26 (4%) | 26 (5%) | 0 (0%) | ||
| IV Drug Use (IVDU) | Never | 544 (85%) | 490 (87%) | 54 (70%) | <0.001 |
| Current/Previous | 99 (15%) | 76 (13%) | 23 (30%) | ||
| Risk Behavior Category | MSM | 354 (55%) | 310 (55%) | 44 (57%) | <0.001 |
| MSM and IVDU | 38 (6%) | 26 (5%) | 12 (16%) | ||
| IVDU | 61 (9%) | 50 (9%) | 11 (14%) | ||
| High risk Heterosexual Contact | 117 (18%) | 114 (20%) | 3 (4%) | ||
| Others or Missing | 73 (11%) | 66 (12%) | 7 (9%) | ||
| Baseline CD4 | Median | 226 | 227 | 222 | 0.60 |
| <200 | 241 (37%) | 209 (37%) | 32 (42%) | ||
| 200-299 | 208 (32%) | 186 (33%) | 22 (29%) | ||
| 300+ | 194 (30%) | 171 (30%) | 23 (30%) | ||
| Baseline Viral Load | 500 or less | 417 (65%) | 371 (66%) | 46 (60%) | 0.54 |
| 500-20,000 copies | 112 (17%) | 92 (16%) | 20 (26%) | ||
| 20,000 copies or more | 100 (16%) | 89 (16%) | 11 (14%) | ||
| Missing | 14 (2%) | 14 (2%) | 0 (0%) | ||
| Antiretroviral Therapy at Entry | NRTI + PI based regimen | 470 (73%) | 417 (74%) | 53 (69%) | 0.91 |
| NRTI + NNRTI + PI based regimen | 105 (16%) | 91 (16%) | 14 (18%) | ||
| NNRTI + PI based regimen | 30 (5%) | 26 (5%) | 4 (5%) | ||
| NRTI + NNRTI based regimen | 14 (2%) | 12 (2%) | 2 (3%) | ||
| Other regimen | 24 (4%) | 20 (4%) | 4 (5%) | ||
| Self-reported Drinking: | |||||
| Baseline Binge Drinking | 119 (19%) | 97 (17%) | 22 (29%) | 0.02 | |
| Binge Drinking During Study | 276 (43%) | 227 (40%) | 49 (64%) | <0.001 | |
| Baseline Heavy Drinking | 31 (5%) | 23 (4%) | 8 (10%) | 0.02 | |
| Heavy Drinking During Study | 102 (16%) | 81 (14%) | 21 (27%) | 0.01 | |
| Self-reported Baseline Drug Use3 | |||||
| Baseline Cocaine Use | 15 (2%) | --- | 15 (19%) | ||
| Baseline Amphetamine Use | 3 (0%) | --- | 3 (4%) | ||
| Baseline Heroin Use | 2 (0%) | --- | 2 (3%) | ||
| Any Baseline Hard Drug Use | 20 (3%) | --- | 20 (26%) | ||
| Self-reported Drug Use During Study4: | |||||
| Cocaine Use During Study | 57 (9%) | --- | 57 (74%) | ||
| Amphetamine Use During Study | 27 (4%) | --- | 27 (35%) | ||
| Heroin Use During Study | 7 (1%) | --- | 7 (9%) | ||
| Baseline Non-adherence5 | No | 516 (80%) | 459 (81%) | 57 (74%) | 0.31 |
| Yes | 100 (16%) | 85 (15%) | 15 (19%) | ||
| Missing | 27 (4%) | 22 (4%) | 5 (6%) | ||
| Non-adherence During Study6 | 316 (49%) | 266 (47%) | 50 (65%) | 0.003 | |
MSM= men who have sex with men; IVDU=intravenous drug use; NRTI=nucleoside reverse transcriptase inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor.
Hard drug user: defined as reporting drug use (cocaine, heroin and/or amphetamine use) within 30 days prior to entry or prior to any study visit.
P-value calculated by Fisher’s Exact Test for binary characteristics, by Chi-Square test for categorical characteristics, and by Wilcoxon Rank Sum Test for continuous characteristics.
Baseline drug use defined as use during the 30 days prior to entry for each individual drug, or for any hard drug (cocaine, heroin, amphetamines)
Drug use during study defined as use during the 30 day prior to entry or any study visit
Baseline non-adherence defined as self-reported non-adherence over 7 days prior to entry
Non-adherence during study defined as self-reported baseline non-adherence as above, or in 2 days prior to any study visit
Of the 53 deaths during the study, 28 were determined to be non-HIV associated, 10 were HIV-associated, 4 were other disease processes, and 11 were of unknown causes. Seventy AIDS-related events occurred over the course of the study, half of which were microscopically or histologically confirmed. The vast majority were opportunistic infections including 15 with esophageal candidiasis, 14 with Pneumocystis jiroveci pneumonia, 7 with cytomegalovirus disease and 6 with cryptosporidiosis. There were 4 confirmed cases of MAC disease and 7 with HIV wasting syndrome. Only 5 developed HIV-associated malignancies, 3 with Kaposi’s sarcoma and 2 with lymphoma.
Incidence and prevalence of drug and alcohol use
At baseline, 253 (39%) reported ever having used cocaine, 154 (24%) amphetamines and 65 (10%) heroin. Only 28 (2%) reported ever having used methadone treatment at baseline. Ninety-nine patients (15%) reported ever having injected drugs at baseline. While on study, 6 patients (0.9%) reported having injected cocaine, 4 (0.6%) amphetamines, and 3 (0.5%) heroin, with 1 (0.2%) having recently injected more than one drug within 30 days of a study visit. Baseline drug use in the participants who enrolled in the observational study was similar to those who did not continue (data not shown).
During the study, 9% reported having used cocaine, 4% amphetamines, 1% heroin, and 4% methadone treatment in the past 30 days prior to study entry or any subsequent study visit (See Table 1). Of the 77 patients who used hard drugs in the 30 days prior to study entry or any study visit, only 8 (11%) were new users who had not reported using hard drugs prior to entry.
At baseline, of the 373 patients who reported use of alcohol in the prior 30 days (58%), 31 (8%) were heavy drinkers and 119 (32%) were binge drinkers. During the study, 102 (16%) patients were classified as heavy drinkers and 276 (43%) were classified as binge drinkers. Most of the patients classified as heavy drinkers were also binge drinkers (97%) but only a third of binge drinkers were also classified as heavy drinkers (36%). Of those who reported being heavy or binge drinkers during the study, 69 (68%) had reported not being heavy drinkers, and 151 (55%) were not binge drinkers in the 30 days prior to their baseline visit (See Table 1).
Current or prior IV drug use, heavy or binge drinking, and non-adherence to ART were significantly associated with hard drug use during the study (Table 1). At any visit, the maximum prevalence of reporting binge drinking was 19% and hard drug use was<5% (See Figure 1). Figure 1 depicts the percentage of heavy and binge alcohol use and drug use through the study with reported non-adherence to ART by week.
Figure 1. Percent of AIDS Patients in ACTG 362 Self-Reporting Drug Use1 and Non-Adherence2 During Study Follow-up.
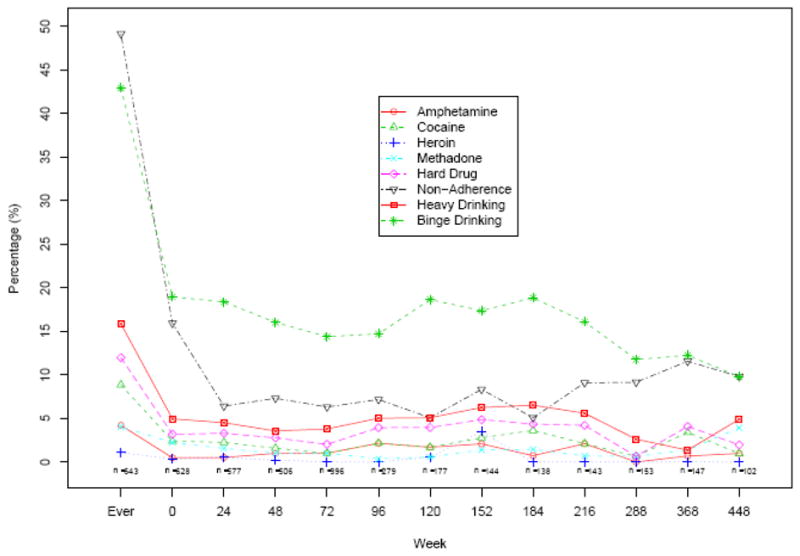
1 Hard drug use: defined as reporting drug use within 30 days prior to entry or prior to any study visit.
2 Non-adherence: defined as missing any antiretroviral therapy within the past week prior to entry or in the past 2 days at any study visit.
ART non-adherence
At baseline, 15% of participants reported ART non-adherence during the prior week (Figure 1). While on study, reported ART non-adherence at any one visit ranged from 5 to 12%. Overall, the percentage of patients who reported ART non-adherence at least once between baseline and the end of study follow-up, e.g., “ever non-adherent,” was 49% (316). We found each additional decade of age was associated with a 25% decrease in odds of non-adherence (adjusted OR=0.75, p<0.001), using repeated measures GEE models. The odds of non-adherence increased slightly over the study follow-up (OR=1.02 per 24 weeks, p=0.03).
Association of drug and alcohol use with ART non-adherence
In multivariable GEE analyses adjusting for age, sex, and correlation among visits for each subject, hard drugs users had 2.14 times higher odds (95% CI: 1.36, 3.38, p<0.001) of ART non-adherence compared to non-users (see Table 2). In addition, those who recently used cocaine had 2.60 times higher odds (95% CI: 1.63, 4.13, p<0.001) and those who recently used methadone had 2.33 times higher odds than non-users (95% CI: 1.16, 4.69, p=0.02). Heavy drinking was not associated with non-adherence, but binge drinking was associated with a 1.53 times higher odds (95% CI: 1.21, 1.95, p<0.01) of ART non-adherence.
Table 2.
Association between Hard Drug Use and Non-Adherence based on GEE Repeated Measures Models Adjusting for Within-Participant Correlation among Multiple Study Visits
| Substance Use1 | Adjusted Odds Ratio2 | 95% Confidence Interval | P-value |
|---|---|---|---|
| Recent Cocaine Use | 2.60 | (1.63, 4.13) | <0.001 |
| Recent Heroin Use | 1.94 | (0.39, 9.58) | 0.42 |
| Recent Amphetamine Use | 1.79 | (0.73, 4.39) | 0.20 |
| Recent Methadone Treatment | 2.33 | (1.16, 4.69) | 0.02 |
| Recent Hard Drug Use (Cocaine, Amphetamine, or Heroin) | 2.14 | (1.36, 3.38) | 0.001 |
| Heavy Drinking | 1.19 | (0.72, 1.98) | 0.50 |
| Binge Drinking | 1.53 | (1.21, 1.95) | <0.001 |
Defined as reported use in the 30 days prior to a visit.
Each row represents a separate model for the specified substance use, adjusted for visit week, age, sex, and correlation among visits for a given participant.
Association of non-adherence with AIDS progression or death
Of the 55 subjects with new AIDS diagnoses during the study, 15 subsequently died. In multivariate Cox proportional hazards models adjusting for potential confounders, there was no association of baseline ART non-adherence with risk of AIDS or death (adjusted HR=0.78, 95% CI=0.50,1.21, p=0.26) (See Table 3). However, time-updated measures of non-adherence during the study were associated with an almost two-fold increase in the odds of developing an AIDS condition or death (adjusted HR=1.84, 95% CI=1.15,2.94, p=0.01).
Table 3.
Association between Hard Drug Use and Risk of AIDS Progression or Death based on Cox Models for Time to Event
| Recent Substance Use at Entry1 | Adjusted Hazard Ratio2 | 95% Confidence Interval | P-value |
|---|---|---|---|
| Without adjusting for non-adherence: | |||
| Recent Hard Drug Use (Cocaine, Heroin, or Amphetamine) | 2.46 | (1.06, 5.69) | 0.04 |
| Baseline Viral Load > 500 copies/mL | 2.12 | (1.38, 3.26) | <0.001 |
| Karnofsky Score ≤ 80 | 2.01 | (1.23, 3.28) | 0.005 |
| Adjusting for non-adherence reported at study entry: | |||
| Recent Hard Drug Use (Cocaine, Heroin, or Amphetamine) | 2.58 | (1.11, 5.97) | 0.03 |
| Baseline Viral Load > 500 copies/mL | 2.14 | (1.39, 3.30) | <0.001 |
| Karnofsky Score ≤ 80 | 2.07 | (1.27, 3.39) | 0.004 |
| Non-adherence reported at entry | 0.78 | (0.50, 1.21) | 0.26 |
| Adjusting for non-adherence reported throughout study follow-up as time-dependent covariate: | |||
| Recent Hard Drug Use (Cocaine, Heroin, or Amphetamine) | 2.11 | (0.90, 4.92) | 0.08 |
| Baseline Viral Load > 500 copies/mL | 1.92 | (1.24, 2.97) | 0.003 |
| Karnofsky Score ≤ 80 | 1.93 | (1.18, 3.15) | 0.01 |
| Time-updated Non-Adherence | 1.84 | (1.15, 2.94) | 0.01 |
Defined as reported use in the 30 days prior to study entry.
We adjusted for baseline HIV RNA>500 copies/ml and Karnofsky score only since all other covariates (age, sex, baseline CD4, non-white status, randomized treatment status [azithromycin versus placebo], years on antiretroviral therapy at entry, and prior AIDS defining event) had p>0.20 or higher.
Association of drug and alcohol use with AIDS progression or death
The association between drug and alcohol use and a new AIDS diagnosis or death was evaluated via Cox regression models, controlling for covariates with and without adjustment for non-adherence (See Table 3). After controlling for baseline VL and Karnofsky Score, hard drug users had 2.46 times higher risk (p=0.04) of a new AIDS outcome or death. When we controlled for baseline adherence in addition, use of hard drugs was associated with a significant increase in risk of an AIDS outcome or death (adjusted HR=2.58, p=0.03). However, when we controlled for adherence as a time-dependent covariate throughout study follow-up, risk from hard drug use was attenuated (adjusted HR=2.11, p=0.08). Neither binge drinking nor heavy drinking was found to increase the risk of an AIDS outcome or death when controlling for baseline VL and Karnofsky score.
In Cox regression models for time to death, the effect of hard drug use was associated with a 3-fold higher risk after adjusting for age, baseline RNA>500 copies/mL, and Karnofsky score ≤80 (adjusted HR=3.09, p=0.03), but no association was found for alcohol use.
Association of non-adherence with virologic treatment failure (VTF)
Non-adherence was associated with VTF (e.g. HIV-1 RNA ≥500 copies/ml) when evaluated as time-dependent non-adherence in a Cox regression model controlled for baseline hard drug use (adjusted HR=1.42, p=0.004). Likewise, non-adherence at each visit correlated with VTF at that visit in a GEE repeated measured analysis adjusted for recent hard drug use, visit week, age, sex, and correlation among visits for a given participant (adjusted OR=1.41, p<0.001). In this GEE model, we found that recent hard drug use was also significantly associated with VTF (adjusted OR=1.63, p=0.002).
DISCUSSION
Our study patients with advanced AIDS who had responded to HAART had a high prevalence of drug and alcohol use. Almost 40% of participants reported a history of hard drug use and 12% reported some ongoing use during the study. Similarly, over half reported recent use of alcohol, with 43% reporting binge drinking during the study. These data are in keeping with statistics on alcohol and drug use in HIV-infected persons in care for HIV in the United States [10,16], and are significantly higher than those found in the general population [32].
Our findings are consistent with previous studies that have found that active drug use is associated with non-adherence to HAART and incomplete viral suppression when actively using drugs [14,16, 17]. Adherence to ART is essential to decrease the incidence of opportunistic infections and improve the overall outcomes of patients with HIV. Our study used a self-reported measure of adherence that has been used successfully throughout the ACTG and many other HIV clinical trials. This adherence measure predicted AIDS progression and death in the first year of follow-up of this study [26]. In the current analysis, hard drug users had higher odds of developing a new AIDS-related condition or dying, even after controlling for adherence. Similar to findings from a four year observational study of illicit drug users in Baltimore by Lucas et al., we found that drug users, had a two-fold higher risk of developing new opportunistic conditions or death after adjusting for HAART use and time-dependent adherence [17].
Although drug users may have been excluded from clinical trials in the past, more recent ACTG studies exclude subjects only if, in the opinion of the investigator, active drug or alcohol use would interfere with adherence to study requirements. Thus, compared with AIDS patients in care, patients on this trial were probably less likely to use drugs and alcohol. Those who used alcohol may also have been more likely to admit to drinking >4 drinks on one occasional despite drinking more than that, leading to underreporting of “heavy drinking” in this study. Reporting binge drinking has previously been shown to be associated with ART nonadherence [20,33], although its impact on clinical outcomes warrants further investigation.
Drug and alcohol use may increase the risk of AIDS progression and death through direct biological effects and/or indirect behavioral effects [17]. In vitro, opioids impair lymphocyte function [34] and increase HIV-1 replication in mononuclear cells [35]. Drug use may also increase HIV replication mediated through effects on immune activation [36]. Cocaine appears to promote HIV infection in vivo, interacting with host immunity, cell susceptibility and activating sigma-1 receptors [37]. Drug use may also affect the immune system and other host defenses that may predispose to OI-related morbidity and mortality. For example, smoking tobacco, which is frequently associated with drug use, is a risk factor for developing cryptococcal infections in AIDS [38]. Alcohol, in addition to smoking, may paralyze respiratory cilia function and predispose users to recurrent bacterial pneumonias [39,40]. Carrico et al. found that compared to non-users, weekly stimulant use (cocaine and methamphetamines) was associated with higher HIV viral loads, higher neopterin levels, a measure of immune activation, and lower tryptophan levels [41].
Cohorts of injection drug users in the pre-HAART era had similar rates of CD4 lymphocyte decline [42] and progression of AIDS compared with men who have sex with men [43]. Moore et al. found that HIV-infected drug users had similar risk of disease progression in the pre-HAART era but had higher rates of disease progression in the HAART era compared to non-drug users [17,44], suggesting that non-adherence may account for much of the difference
Our study had several limitations. Patients who achieve immunorestoration and choose to participate in a decade long observational study are likely atypical of other AIDS patients. Research volunteers have been shown to be less impulsive, more satisfied, and more positive than the general population [45]. As a result of this self-selection, our participants may under-represent the true prevalence of drug and alcohol abuse among patients with HIV in clinical practice and consequently, may have a lower likelihood of using illicit substances and alcohol than the average patient with HIV/AIDS on HAART in clinical practice. Patients who use drugs and alcohol may be more likely to underreport their drug use, miss study visits or prematurely discontinue the study compared to non-users; drug users tend to minimize or deny drug use and are more likely to discontinue HAART or the clinical trial if they were using drugs. Misclassification from this underreporting would reduce the power of our study.
We did not assess lifetime use of alcohol or tobacco, or other potential covariates such as homelessness, depression, health literacy, hepatitis status, insurance status, and recruitment and retention strategies used. Although self-reported measures of drug use are useful, we probably underestimated the use of drugs by not performing more other objective measures of drug use such as urine drug screening. We also did not ask about other drugs of abuse including amyl nitrates, marijuana, recreational use of prescription drugs or other drugs considered to be “party drugs.”
This study of patients with advanced AIDS who had responded to HAART and were followed for close to ten years found a high prevalence of alcohol and drug use. Binge drinking and drug use was associated with increased risk of non-adherence that only partially explained poorer virologic control and increased risk of progression of AIDS or death. Our study highlights the need to routinely and continuously screen all patients with HIV for current drug and alcohol use, and to offer alcohol and drug treatment services and other targeted interventions where appropriate to optimize HIV management and outcomes.
Acknowledgments
National Institute of Allergy and Infectious Diseases, National Institutes of Health (AI068636 to AIDS Clinical Trials Group and AI068634 to Statistical and Data Analysis Center, Harvard School of Public Health)
We thank the study participants and the following institutions and individuals for their participation in ACTG 362:
Bev Putnam, MSN and Graham Ray, MSN-University of Colorado Health Sciences Center (A6101) CTU Grant # AI69450, AI054907 and GCRC Grant # RR025780;
Harold Kessler, MD and Elke Narkiewicz, RN-Rush Presbyterian/St. Luke’s (A2702) CTU Grant # AI25915;
Baiba Berzins, MPH -Northwestern University (Site A2701) CTU Grant # AI06947;
Oluwatoyin Adeyemi, MD -Cook County CORE Center (A2705) CTU Grant # AI25915;
Susan Cahill, RN and Julie Hoffman, RN- University of California, San Diego Antiviral Res (A0701) CTU Grant # AI69432;
Robert A. Salata, M.D. and Patricia Walton BSN RN-Case Western University (A2501) CTU Grant # AI69501; Akron City Hospital, Summa Health Systems (A2506);
Jody Lawrence, MD and Mary Payne, BS, BA, RN- UCSF, San Francisco General Hospital (A0801) CTU Grant # 5UO1 AI069502-03;
Jane Reid, RNc, MS, ANP and Carol Greisberger, RN- University of Rochester Medical Center (A1101) CTU Grant # AI27658, GCRC Grant # RR00044;
Tammy O’Hara, RN and Gene Morse, PharmD- University of Buffalo (A1102);
Jane Norris, PA-C and Sandra Valle, PA-c-Stanford University (A0501) CTU Grant # AI 69556;
Connie A. Funk, RN, MPH & Frances Canchola, RN-University of Southern California (A1201) CTU Grant # AI27673;
Susan L. Koletar, MD and Kathy Watson, RN- Ohio State University (A2301) CTU Grant #1U01 AI 69474;
Charlene Gaca, RN and Timothy Cooley, MD - Boston Medical Center (A0104) CTU Grant # AI069472;
Mallory Witt, M.D. and Sadia Shaik - Harbor UCLA Medical Center (A0603) CTU Grant # AI069424;
Janet Forcht RN and Judith Aberg, MD- New York University/NYC HHC at Bellevue Hospital Center (A0401) CTU Grant # AI27665, AI069532; GCRC Grant # RR00096;
Marshall Glesby and Valery Hughes – Cornell University (A2201) Weill Medical College GCRC Grant #RR00047; Memorial Sloan- Kettering (A2202);
Sheryl Storey, PA-C and Jeff Schouten, MD – University of Washington, Seattle (A1401) Grant #AI27664;
Henry H. Balfour, Jr., M.D. and Christine Fietzer, R.N., B.S.N. - University of Minnesota (A1501) Grant # AI27661
University of Alabama at Birmingham (A5801);
Clifford Gunthel, MD and Ericka R. Patrick, MSN- Emory University HIV/AIDS Clinical Trials Unit (A5802) CTU Grant # A1069418;
Richard B. Pollard, MD and Gerianne Casey, RN-University of Texas, Galveston (A6301) CTU Grant # AI032782;
Mark Rodriguez, RN and Lisa Kessels, RN, ACRN, CCRC- Washington University (A2101) CTU Grant # AI25903;
Judith Feinberg MD and Tammy Mansfield RN ACRN-University of Cincinnati College of Medicine (A2401) CTU Grant # AI069513;
Mitchell Goldman, MD and Beth Zwickl, RN, MSN-Indiana University (A2601) CTU Grant # AI025859; Wishard Memorial Hospital (A2603);
Carol Dukes Hamilton, MD and Joan Riddle, RN-Duke University Medical Center (A1601) CTU Grant # 5U01 AI069 484-02;
Charles van der Horst, MD and David Ragan, RN- University of North Carolina University of North Carolina (A3201) CTU Grant # 5-U01 AI069423-03; GCRC M01 RR000046-48; cfar P30 AI050410(-11);
Nancy Hanks, RN, University of Hawaii and Debra Ogata-Arakaki, RN, University of Hawaii (A5201) CTU Grant #AI34853;
Ellen Chusid, PhD and Walter Weiss, PA – Mount Sinai Medical Center (A1801);
Mary Waldron, MD and Donna Mildvan, MD-Beth Israel Medical Center (A2851) CTU Grant # AI46370;
John McNeil, MD – Howard University (A5301) Grant #AI34835;
Rob Roy MacGregor, MD and Kathryn Maffei, RN-University of Pennsylvania, Philadelphia (A6201) CTU Grant # AI069467;
Ilene Wiggins, RN and Andrea Weiss, RPh- Johns Hopkins University (A0201) CTU Grant # AI27668, GCRC Grant #RR00052;
John Mellors, MD and Barbara Rutecki, MSN, MPH, CRNP-Pitts CRS (A1001) CTU Grant # AI069494;
Susan Swindells MBBS and Frances Van Meter APRN-University of Nebraska Medical Center (A1505) CTU Grant # AI27661;
Rebecca A. Clark, MD, PhD – Charity Hospital/Tulane University (A1702);
James Paul Steinberg, MD – Emory University Comprehensive Hemophilia Program (A9413).
Sylvia Stoudt, RN and Dennis Israelski, MD -Willow Clinic Menlo Park (A0507) CTU Grant #: AI69556;
Neel French, MD-Louis A Weiss Memorial Hospital (A2708) CTU Grant # AI25915;
Debbie Slamowitz, RN and Patricia Cain, RN-Santa Clara Valley Medical Center, San Jose (A0506) CTU Grant #: AI69556;
Sylvia Stoudt, RN and Dennis Israelski, MD-San Mateo County AIDS Program (A0505) CTU Grant # AI69556;
Ardis Moe, M.D. and Suzette A. Chafey, RN, BSN, MPH-UCLA Medical Center (A0601) CTU Grant # 5 U01 AI 069424-03;
Michael Conklin RN NP and Ge-Youl Kim RN BSN - Connect Care (A2102) CTU Grant # AI25903;
Mary Albrecht, MD and Carol Silver, RN; Clyde Crumpacker, MD and Carol Delaney, RN (Site A0102)-Beth Israel Deaconess (A0103) CTU Grant #AI069472; Harvard Massachusetts General (A0101);
Craig Lindquist, MD, PhD and Deborah Mullaney-Fricke-Marin Country Department of Health, San Rafael, CA (A0809) CTU Grant # AI27663.
Footnotes
This paper was presented in part as an oral presentation at the 4th International Conference on HIV Treatment Adherence, April 7, 2009, Miami.
This study was conducted under the Clinicaltrails.gov number NCT00000883.
References
- 1.Haubrich RH, Little SJ, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS. 1999;13(9):1099–107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 2.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Epidemiology of HIV/AIDS-United States 1981–2005. MMWR. 2006 June 2;55(21):589–592. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5521a2.htm. [PubMed]
- 6.Cofrancesco J, Jr, Scherzer R, Tien PC, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008;22(3):357–65. doi: 10.1097/QAD.0b013e3282f3cc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia F, Cook JA, Cohen MH, et al. The relationship between non-injection drug use behaviors on progression to AIDS and death in a cohort of HIV seropositive women in the era of highly active antiretroviral therapy use. Addiction. 2005;100(7):990–1002. doi: 10.1111/j.1360-0443.2005.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohli R, Lo Y, Howard AA, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41(6):864–72. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 10.Turner BJ, Fleishman JA, Wenger N, et al. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16(9):625–33. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 12.Bassetti S, Battegay M, Furrer H, et al. Why is highly active antiretroviral therapy (HAART) not prescribed or discontinued? Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 1999;21(2):114–9. [PubMed] [Google Scholar]
- 13.Mocroft A, Madge S, Johnson AM, et al. A comparison of exposure groups in the EuroSIDA study: starting highly active antiretroviral therapy (HAART), response to HAART, and survival. J Acquir Immune Defic Syndr. 1999;22(4):369–78. doi: 10.1097/00126334-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Weber R, Huber M, Rickenbach M, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV Cohort Study. HIV Medicine. 2009;10:407–416. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 15.Conen A, Fehr J, Glass TR, et al. Self-reported alcohol consumption and its association with adherence and outcome of antiretroviral therapy in the Swiss HIV Cohort Study. Antivir Ther. 2009;14(3):349–57. [PubMed] [Google Scholar]
- 16.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114(7):573–80. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 17.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163(5):412–20. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 18.Cohn SE, Umbleja T, Mrus J, Bardequez AD, Andersen J, Chesney MA. Prior Illicit Drug Use and Missed Prenatal Vitamins Predict Non-adherence to Antiretroviral Therapy in Pregnancy. AIDS Patient Care and STDs. 2008;22:29–40. doi: 10.1089/apc.2007.0053. [DOI] [PubMed] [Google Scholar]
- 19.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17(10):756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood E, Montaner JS, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169(7):656–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Palepu A, Tyndall M, Yip B, et al. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. J Acquir Immune Defic Syndr. 2003;32(5):522–6. doi: 10.1097/00126334-200304150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27(3):251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 25.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 26.Cohn SE, Kammann E, Williams P, Currier JS, Chesney MA. Association of adherence to Mycobacterium avium complex prophylaxis and antiretroviral therapy with clinical outcomes in Acquired Immunodeficiency Syndrome. Clin Infect Dis. 2002;34(8):1129–36. doi: 10.1086/339542. [DOI] [PubMed] [Google Scholar]
- 27.Walley AY, Cheng DM, Libman H, et al. Recent drug use, homelessness and increased short-term mortality in HIV-infected persons with alcohol problems. AIDS. 2008;22(3):415–420. doi: 10.1097/QAD.0b013e3282f423f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currier JS, Williams PL, Koletar SL, et al. Discontinuation of Mycobacterium avium complex prophylaxis in patients with antiretroviral therapy-induced increases in CD4+ cell count. Ann Intern Med. 2000;133:493–503. doi: 10.7326/0003-4819-133-7-200010030-00008. [DOI] [PubMed] [Google Scholar]
- 29.Koletar SL, Williams PL, Wu J, et al. Long-term follow-up of HIV-infected individuals who have significant increases in CD4+ cell counts during antiretroviral therapy. Clin Infect Dis. 2004;39(10):1500–6. doi: 10.1086/424882. [DOI] [PubMed] [Google Scholar]
- 30.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 31.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much. A Clinician’s Guide. 2005. Washington, DC: National Institutes of Health, U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 32.Wright D, Sathe N, Spagnola K. State Estimates of Substance Use from the 2004–2005 National Surveys on Drug Use and Health (DHHS Publication No. SMA 07-4235, NSDUH Series H-31) Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. State Estimates of Substance Use from the 2004–2005 National Surveys on Drug Use and Health. OAS Series #H-31, DHHS Publication No. (SMA) 07-4235, Rockville, MD; 2007. [Google Scholar]
- 33.Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005 Jul;29(7):1190–7. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 34.Mientjes GH, Miedema F, van Ameijden EJ, et al. Frequent injecting impairs lymphocyte reactivity in HIV-positive and HIV-negative drug users. AIDS. 1991;5(1):35–41. doi: 10.1097/00002030-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Peterson PK, Sharp BM, Gekker G, et al. Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4(9):869–73. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Dhillon NK, Williams R, Peng F, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13(6):483–95. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 37.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78(6):1198–203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 38.Hajjeh RA, Conn LA, Stephens DS, et al. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. Cryptococcal Active Surveillance Group. J Infect Dis. 1999;179(2):449–54. doi: 10.1086/314606. [DOI] [PubMed] [Google Scholar]
- 39.Elliott MK, Sisson JH, Wyatt TA. Effects of Cigarette Smoke and Alcohol on Ciliated Tracheal Epithelium and Inflammatory Cell Recruitment. Am J Respir Cell Mol Biol. 2007;36(4):452–9. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leitch GJ, Frid LH, Phoenix D. The effects of ethanol on mucociliary clearance. Alcohol Clin Exp Res. 1985;9:277–280. doi: 10.1111/j.1530-0277.1985.tb05749.x. [DOI] [PubMed] [Google Scholar]
- 41.Carrico AW, Johnson MO, Morin SF, et al. Stimulant use is associated with immune activation and depleted tryptophan among HIV-positive persons on anti-retroviral therapy. Brain Behav Immun. 2008;22:1257–1262. doi: 10.1016/j.bbi.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyles CM, Margolick JB, Astemborski J, et al. The influence of drug use patterns on the rate of CD4+ lymphocyte decline among HIV-1-infected injecting drug users. AIDS. 1997;11(10):1255–62. doi: 10.1097/00002030-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Spijkerman IJ, Langendam MW, Veugelers PJ, et al. Differences in progression to AIDS between injection drug users and homosexual men with documented dates of seroconversion. Epidemiology. 1996;7(6):571–7. doi: 10.1097/00001648-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 45.Tishler CL, Bartholomae S, Rhodes AR. Personality profiles of normal healthy research volunteers: a potential concern for clinical drug trial investigators? Med Hypotheses. 2005;65(1):1–7. doi: 10.1016/j.mehy.2005.01.018. [DOI] [PubMed] [Google Scholar]


