Abstract
The melanocortin MC1 receptor is a G -protein coupled receptor expressed in melanocytes of the skin and hair and is known for its key role in regulation of human pigmentation. Melanocortin MC1 receptor activation after ultraviolet radiation exposure results in a switch from the red/yellow pheomelanin to the brown/black eumelanin pigment synthesis within cutaneous melanocytes; this pigment is then transferred to the surrounding keratinocytes of the skin. The increase in melanin maturation and uptake results in tanning of the skin, providing a physical protection of skin cells from ultraviolet radiation induced DNA damage. Melanocortin MC 1 receptor polymorphism is widespread within the Caucasian population and some variant alleles are associated with red hair colour, fair skin, poor tanning and increased risk of skin cancer. Here we will discuss the use of mouse coat colour models, human genetic association studies, and in vitro cell culture studies to determine the complex functions of the melanocortin MC1 receptor and the molecular mechanisms underlying the association between melanocortin MC1 receptor variant alleles and the red hair colour phenotype. Recent research indicates that melanocortin MC1 receptor has many non-pigmentary functions, and that the increased risk of skin cancer conferred by melanocortin MC1 receptor variant alleles is to some extent independent of pigmentation phenotypes. The use of new transgenic mouse models, the study of novel melanocortin MC1 receptor response genes and the use of more advanced human skin models such as 3D skin reconstruction may provide key elements in understanding the pharmacogenetics of human melanocortin MC1 receptor polymorphism .
Keywords: melanocortin 1 receptor, melanoma, red hair colour, melanocyte, tanning, pigmentation
1. Introduction
The melanocortin MC1 receptor is a seven transmembrane G-protein coupled receptor expressed in the melanocytes of the skin and hair follicles, but absent from melanocytes in the uveal tract of the eye. Activation of melanocortin MC1 receptor by ultraviolet radiation increases synthesi s of the dark eumelanin pigment, resulting in the visible darkening of the skin known as the tanning response. Melanocortin MC1 receptor stimulation also results in increased melanocyte dendricity, proliferation, cell survival and DNA repair [for a detailed review of melanocortin MC1 receptor function see (Abdel-Malek et al., 2010; Abdel-Malek et al., 2008; Beaumont et al., 2009; Garcia-Borron et al., 2005)]. Loss of melanocortin MC1 receptor function results in synthesis of the red/yellow pheomelanin pigment by melanocytes, giving rise to red hair colour in humans or yellow coat colour in mice.
Melanocortin MC1 receptor was one of the first genes to be associated with normal human pigmentation variation, and is the most important predictor of skin and hair colour within the Caucasian population. Genetic association studies have identified melanocortin MC1 receptor polymorphisms that are associated with red hair, fair skin, poor tanning, freckling and increased skin cancer risk; this is termed the red hair colour phenotype. Here we describe the use of genetic association studies to determine the penetrance and interaction of several common variant melanocortin MC1 receptor alleles found in the Brisbane twin nevus study, the use of melanoma and non-melanocytic transfected cell lines to analyse melanocortin MC1 receptor wild type and variant allele function, as well as the use of genotyped melanocyte strains in monoculture and co-culture, a more physiologically intact system. We will also discuss other more complex systems for further elucidation of the role of melanocortin MC1 receptor in the regulation of pigmentation and the response to ultraviolet radiation, such as the use of 3D skin reconstruction and transgenic mouse models.
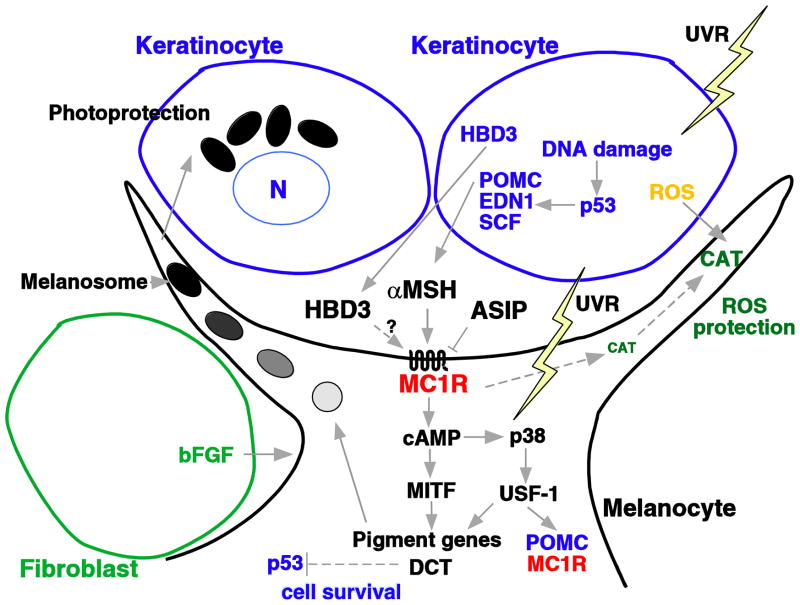
Intense research into melanocortin MC1 receptor function using human and mouse genetics and in vitro model systems has revealed a network of interactions involved in the upstream regulation and downstream induced signalling pathways. However, recent research has posed new questions about key components involved in the melanocortin MC1 receptor response. Figure 1 summarises the different ligands, signalling pathways and responses involved in melanocortin MC1 receptor activation. The canonical pathway after melanocortin MC1 receptor binding by the α-melanocyte stimulating hormone agonist, produced in response to ultraviolet radiation-induced DNA damage within the surrounding keratinocytes, involves a cAMP signalling cascade. This ultimately results in upregulation of the Microphthalmia transcription factor, a master regulator of melanocyte cell differentiation. Microphthalmia transcription factor controls the transcription of many important pigment genes such as tyrosinase, the main enzyme responsible for melanin synthesis.
Figure 1.
The melanocyte and the tanning response. Adapted from (Beaumont et al., 2009). Exposure of human skin to ultraviolet radiation results in the release of various factors by keratinocytes and melanocytes. Proopiomelanocortin (POMC) is converted to α-melanocyte stimulating hormone ( MSH), which stimulates melanocortin MC1 receptor (α-MC1R) cAMP signaling in the melanocyte causing the upregulation of genes involved in melanogenesis, ultimately resulting in the tanning response. Agouti signaling protein (ASIP) anatgonises the action of α-MSH. Dotted lines and ? indicate interactions that are in need of further research. ROS, reactive oxygen species; UVR = ultraviolet radiation, HBD3 = human β-defensin 3, END1 = endothelin 1, SCF, stem cell factor; CAT, catalase; bFGF, basic fibroblast growth factor; MITF, microphthalmia transcription factor; DCT, dopachrome tautomerase; USF-1, upstream transcription factor 1.
Recently, a novel melanocortin MC1 receptor ligand has been found. Black coat colour of dogs was found to be due to an activating mutation in an antibacterial β-defensin gene, CBD103 (Candille et al., 2007). This gene also induced a black coat colour in transgenic mice, and can bind to mouse melanocortin MC1 receptor, but does not activate cAMP. The human equivalent, human β-defensin 3, was also found to bind to melanocortin MC1 receptor. Human-defensin 3 is also produced by keratinocytes in response to ultraviolet radiation (Glaser et al., 2009). Further research is needed to determine if human β-defensin 3 acts as a melanocortin MC1 receptor agonist by binding and stimulating an alternative, non-cAMP dependent pathway.
After ultraviolet radiation exposure of the skin, keratinocytes can produce reactive oxygen species that are detected and transferred to melanocytes by passive diffusion (Pelle et al., 2005) and absorbed by the melanin pigments they produce. Moreover, α-melanocyte stimulating hormone treatment has been found to protect melanocytes from reactive oxygen species by induction of catalase enzyme activity (Song et al., 2009) or stimulating intracellular trafficking of the catalase protein to the cell periphery (Maresca et al., 2010).
Other non-pigmentary actions of melanocortin MC1 receptor may be mediated through early response genes such as the nuclear hormone receptor NR4A family, which are involved in DNA repair (Smith et al., 2008). Dopachrome tautomerase, previously thought to be primarily involved in melanin synthesis, may also play a role in protection from DNA damage (Michard et al., 2008) and cell survival (Sendoel et al., 2010).
2. Melanocortin MC1 receptor genetic association studies with pigmentation phenotypes
In the late 20th century the role of melanocortin MC1 receptor in melanogenesis was discovered largely due to investigations into the genetics of mouse coat colour, as well as studies on cultured melanocytes and melanoma cells, and the notable effects of the melanocortin MC1 receptor agonist α-melanocyte stimulating hormone on skin colour in humans. The association between melanocortin MC1 receptor variant alleles and red hair in humans was first described by Valverde and co-workers (Valverde et al., 1995), where they employed direct sequencing of the melanocortin MC1 receptor gene in a group of unrelated Caucasian volunteers, some of whom were chosen for extremes of hair colour. More detailed studies utilising a large adolescent twin collection in Australia (Brisbane twin nevus study), revealed the relatively common high penetrance melanocortin MC 1 receptor variant R alleles responsible for the red hair colour phenotype in Caucasians. These variant alleles include D84E, R151C, R160W and D294H (Box et al., 1997; Duffy et al., 2004). Low penetrance variant r alleles included V60L, V92M and R163Q. Two other melanocortin MC1 receptor alleles, R142H and I155T, did not show a statistically significant association with red hair, probably due to their relatively low frequencies in the population (Duffy et al., 2004), although I155T was significantly associated with blonde hair.
R142H and I155T have both been found to show association with red hair colour in familial studies (Box et al., 1997; Flanagan et al., 2000). The association of R142H with red hair colour was confirmed by a recent meta-analysis, and given the odds ratios were similar to the other high penetrance R variants (Raimondi et al., 2008), and in vitro studies have found a functional impairment similar to the other R variant receptors (Beaumont et al., 2007), we thus classify R142H as a high penetrance R variant allele. Although I155T was initially classified as a familial R allele (Beaumont et al., 2005), given the lack of significant association with red hair colour in the meta-analysis (Raimondi et al., 2008), and the relatively small functional impairment of the I155T receptor compared to the other R variant receptors [(Beaumont et al., 2009) and see discussion below], here we classify I155T as a potential r allele (Table 1). Although interestingly, the meta-analysis did find that the I155T allele was significantly associated with melanoma (Raimondi et al., 2008). An increasing number of studies have found that the association of melanocortin MC1 receptor variant alle les with skin cancer risk is independent of pigmentation phenotype, demonstrating melanocortin MC 1receptor has non -pigmentary functions (Bastiaens et al., 2001; Box et al., 2001; Kanetsky et al., 2010; Landi et al., 2005; Liboutet et al., 2006). However, given that gaps exist in our understanding of the melanogenic intermediates and the process of melanin polymerization, there remains the possibility of unknown melanocortin MC1 receptor mediated pigmentary responses being involved.
Table 1.
MC1R variant alleles in hair and skin colour.
| MC1R Allele | Red hair % | Blonde hair % | Light brown hair % | Dark brown hair % | Black hair % | Fair skin % | |
|---|---|---|---|---|---|---|---|
| Wild Type | +/+ | 0 | 10.4 | 34.7 | 48.6 | 6.3 | 21 |
| +/V60L | +/r | 0.4 | 14.6 | 34.8 | 44.7 | 5.5 | 24 |
| V60L/V60L | r/r | 2.5 | 17.5 | 32.5 | 47.5 | 0 | 50 |
| +/D84E | +/ R | 0 | 21.7 | 34.8 | 43.5 | 0 | 64 |
| D84E/D84E | R/R | - | - | - | - | - | - |
| +/V92M | +/r | 1.4 | 15.9 | 35.9 | 42.3 | 4.6 | 39 |
| V92M/V92M | r/r | 0 | 0 | 20 | 70 | 10 | 43 |
| +/R142H | +/R | 0 | 15.4 | 15.4 | 61.5 | 7.7 | 10 |
| R142H/R142H | R/R | - | - | - | - | - | - |
| +/R151C | +/R | 0.9 | 23.5 | 42.6 | 30.9 | 2.2 | 45 |
| R151C/R151C | R/R | 74.1 | 14.8 | 3.7 | 7.4 | 0 | 94 |
| +/I155T | +/r | 0 | 24 | 36 | 40 | 0 | 31 |
| I155T/I155T* | r/r | 0 | 0 | 0 | 100 | 0 | 0 |
| +/R160W | +/R | 4 | 14.6 | 45.5 | 34.8 | 1.1 | 44 |
| R160W/R160W | R/R | 57.1 | 14.3 | 14.3 | 14.3 | 0 | 50 |
| +/R163Q | +/r | 0 | 16 | 40 | 42 | 2 | 24 |
| R163Q/R163Q | r/r | 0 | 0 | 50 | 25 | 25 | 50 |
| +/D294H | +/R | 0 | 14 | 34 | 48 | 4 | 40 |
| D294H/D294H* | R/R | 100 | 0 | 0 | 0 | 0 | 100 |
Adapted from data in (Beaumont et al., 2007).
For these genotypes data was obtained from only one individual. R = high penetrance allele, r = low penetrance allele, r= familial association with R/functionally classified asequivalent to r.
In order to look more specifically at individual variant alleles, we have separated out effects of melanocortin MC1 receptor variants in the+/R or +/r heterozygous and R/R, or r/rhomozygous states as shown in Table 1 [adapted from (Beaumont et al., 2007)], this allows consideration of melanocortin MC1 receptor alleles without potential transcomplementation effects. We have also removed the one red haired individual who was initially genotyped as wild type, as we later sequenced the melanocortin MC1 receptor gene to find this individual was compound heterozygous for two rare frame shift mutations (Beaumont et al., 2008).
Although red hair colour is generally inherited in a recessive manner, the+/R heterozygote effect of the common melanocortin MC1 receptor alleles c an be seen by increases in the percentage fair skinned individuals, as well as increases in the percentage of red and particularly blonde hair. This correlates with data obtained from our in vitrostudies on dominant negative effects of melanocortin MC1 receptor variant receptors on the wild type melanocortin MC1 receptor (Beaumont et al., 2007). The only variant that did not show a lightening effect on skin and hair colour in the heterozygous state was the R142H allele. This was the only variant that did not show a dominant negative effect on the wild type receptor in vitro (Beaumont et al., 2007).
Unfortunately, due to their rarity we were unable to obtain a large number of some R/R homozygous genotypes such as R142H, I155T and D294H, however the strong effect of the R160W and particularly the R151C variants on hair and skin colour in the homozygous state was confirmed, while the V60L had the largest effect of the r/rvariants, which again agrees with our in vitro studies.
3. Melanocortin MC1 receptor in vitro studies in melanoma and non-melanocytic cell lines
Given their association with a loss of eumelanin pigment, it was assumed that melanocortin MC1 receptor variant alleles would be loss of function receptors. However, it was clear from the genetic studies that there were some differences in the strength of penetrance between different alleles. Due to difficulties culturing primary melanocytes and obtaining the different melanocortin MC1 receptor variant strains, many in vitro studies on melanocortin MC1 receptor function have been performed in melanoma or non-melanocytic cell lines, transfected with melanocortin MC1 receptor wild type or variant alleles. The use of expression vector transfected cell lines also removes the influence of different genetic backgrounds and levels of melanocortin MC1 receptor protein expression found between melanocyte strains.
The assay of cAMP after stimulation with melanocortin MC 1 receptor agonists such as the super-potent α-melanocyte stimulating hormone analogue [Nle4, D-Phe7] α-melanocyte stimulating hormone (NDP-MSH) has routinely been used as a measure of melanocortin MC1 receptor function. We have systematically studied the cellular localisation and function of melanocortin MC1 receptor variant alleles in transiently transfected melanoma cells, as well as stably transfected HEK293 cells. Notably, we discovered that a number of melanocortin MC1 receptor variant receptors were intracellularly retained, thus resulting in a corresponding reduction in cAMP signalling (Beaumont et al., 2009; Beaumont et al., 2005; Beaumont et al., 2007).
We have also determined that the in vitro functional ability of variant receptors is similar to the strength of the reported genetic associations. The relative cAMP signalling abilities of the common melanocortin MC1 receptor variant alleles in our assays were as follows: V92M ≥ wild type > R163Q > I155T > V60L ≈ R160W > R151C≈D84E≈R142H > D294H [(Beaumont et al., 2009; Beaumont et al., 2007) and see Table 1].
All variant receptors showed some decrease in cAMP signalling compared to the wild type receptor, with the exception of the V92M variant low penetrance r allele. It remains to be seen whether the V92M variant displays altered function in some other way such as internalisation, desensitisation or non-cAMP mediated signalling. Some reports have noted a reduced affinity of the V92M variant for α-melanocyte stimulating hormone (Xu et al., 1996), however we did not see altered affinity in our studies.
Although the α-melanocyte stimulating hormone induced signalling pathways have been well studied, the functional effects of the novel melanocortin MC1 receptor ligand human β-defensin 3 [(also known as HBD3, defensin, beta 103A (DEFB103A)] are as yet unknown. For the purpose of characterising the signalling pathways involved, we have used stably transfected HEK293 cell lines and B16 cells. B16 mouse melanoma is another commonly used cell line in the study of melanocortin MC1 receptor. Unlike many human melanoma cell lines, B16 endogenously express mouse melanocortin MC1 receptor and retain the ability to respond to α-melanocyte stimulating hormone.
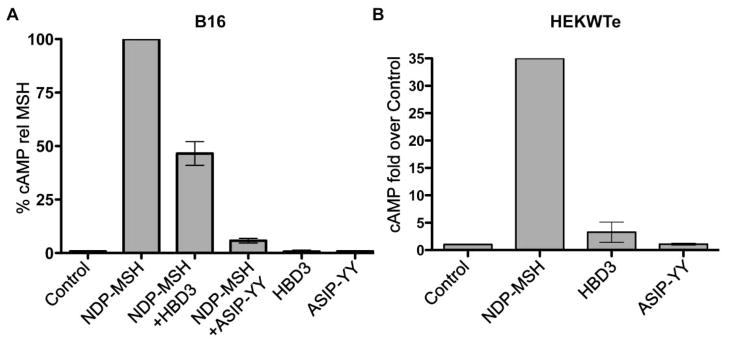
Our preliminary data confirms that human β-defensin 3 does not activate cAMP signalling in B16 (Figure 2A) or melanocortin MC 1 receptor expressing HEK293 cells (Figure 2B), and that human β-defensin 3 can compete with NDP-MSH to inhibit melanocortin MC1 receptor-induced cAMP signalling, although not to the same extent as the known melanocortin MC1 receptor antagonist agouti signaling protein (Figure 2B). This is probably due to the lower affinity of the human β-defensin 3 ligand for melanocortin MC1 receptor compared to NDP-MSH or agouti signaling protein. Human β-defensin 3 binding competition studies with α-melanocyte stimulating hormone have been published previously (Candille et al., 2007), although binding of human β-defensin 3 to mouse melanocortin MC1 receptor was not determined. Note that HEK293 cells transfected with the empty vector pcDNA3.1 did not respond to any of the melanocortin MC1 receptor ligands (data not shown), confirming the specificity of the ligands for melanocortin MC1 receptor.
Figure 2.
cAMP accumulation in B16 or HEK293 cells stably transfected with melanocortin MC1 receptor (HEKWTe). Cells were incubated in serum-free media for at least 2 hours. Cells were pre-incubated with 0.1mM IBMX for 15min, then stimulated with the indicated ligands for 10min. cAMP levels were quantified with the cAMP EIA system (Amersham Biosciences). The bars indicate the range from two independent experiments
A) 0.5nM or 1nM NDP-MSH alone or in combination with 100nM agouti signaling protein peptide (ASIP-YY) (McNulty et al., 2005) or HBD3, or 100nM ASIP-YY or HBD3 alone. Data was normalised to NDP-MSH, which was set at 100%. Results are from two independent experiments.
B) 1nM NDP-MSH, 100nM HBD3 or 100nM ASIP-YY. Data was expressed as fold over the control. Results are from 2 independent experiments, except for NDP-MSH, which is from only one experiment.
We have also looked at melanocortin MC1 receptor induced mitogen-activated protein kinase (MAPK) activation, as an alternative to the traditional cAMP pathway. Interestingly, although melanocortin MC1 receptor-induced MAPK signalling is thought to be activated via crosstalk with cAMP (Busca et al., 2000), our preliminary data indicates that despite being unable to activate cAMP (Figure 2) human β-defensin 3 may activate ERK1/2 in HEK293 cells transfected with melanocortin MC1 receptor (Figure 3, lane 6). MAPK may be responsible for the proliferative effect of melanocortin MC1 receptor activation (Dumaz and Marais, 2005), ERK also phosphorylates microphthalmia transcription factor (Hemesath et al., 1998). The way in which the human β-defensin 3 melanocortin MC1 receptor ligand is able to induce melanogenesis - at least in dogs and mouse models - is still in need of further research.
Figure 3.
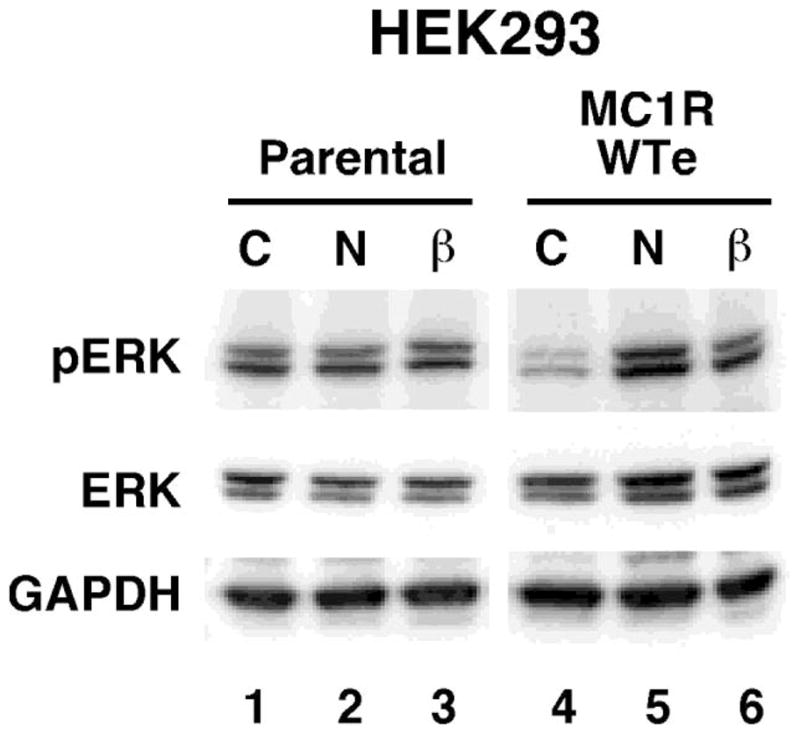
ERK phosphorylation in response to α-melanocyte stimulating hormone and human β-Defensin 3. Lanes are as indicated C=Control, N=10nM NDP-MSH, β=10nM HBD3. HEK293 untransfected cells (parental) or HEK293 stably expressing melanocortin MC 1 receptor (WTe) were pre-incubated in serum-free media for at least 2 hours, then stimulated with the indicated ligands for 5min. Western immuno-blotting with the indicated antibodies was performed using total cell extracts. pERK (Cell Signalling) is specific to the phosphorylated Thr202/Tyr204 sites of ERK1/2, ERK (Cell Signalling) recognises all forms of the ERK protein. Anti-GAPDH (R&D Systems) was used as a loading control. This blot is representative of three independent experiments.
4. Genotyped primary melanocytes in monoculture and co-culture
Although initially there was some difficulty demonstrating melanocortin MC1 receptor-induced melanogensis using in vitro cultured melanocytes, by changing culture conditions, the use of human melanocytes of defined melanocortin MC1 receptor genotype has provided a major experimental approach by our laboratory and others to define the functional consequences for each melanocortin MC1 receptor allele as, under the appropriate growth conditions and short-term passage, melanocytes can continue to express the pigmentary phenotype characteristic of the skin type from which they were derived (Leonard et al., 2003). Clonal melanocyte cultures allow large amounts of a single cell type to be prepared for biochemical, cellular, molecular and genetic analysis and have facilitated investigation of melanocyte differentiation, and responses to growth factors, hormones and ultraviolet radiation (Kadekaro et al., 2006). However, despite maintaining their ex-vivo skin pigmentation phenotype, melanocytes in culture do display different morphological and phenotypic characteristics to that of cells seen in situ. They grow in vitro with a bipolar morphology and acquire expression of several melanoma-associated antigens (Hsu et al., 2002). This suggests an important role of the microenvironment in controlling the phenotype of melanocytes. In normal skin, melanocytes are intimately associated with neighbouring keratinocytes forming the epidermal-melanin unit, which allows not only the transfer of melanin-containing melanosomes into keratinocytes, but also extensive interactions. Upon co-culture with keratinocytes, melanocytes regain a multidendritic differentiated phenotype resembling that seen in vivo (Roberts et al., 2008). Moreover, the defined serum-free keratinocyte medium used in co-culture permits the study of melanocortin MC1 receptor signalling in medium lacking exogenous cAMP elevating agents normally required for melanocyte monoculture (Halaban, 2000).
Melanocytes in this environment also have a repertoire of signalling pathways activated by keratinocyte-derived factors that may better resemble the situation in vivo (Cook et al., 2003). This is an important aspect for studies on melanocortin MC1 receptor function in light of the considerable cross-talk between different melanocyte signal transduction cascades (Figure 1). Such pathway interactions may underlie the important role of keratinocytes in the regulation of dendricity, the proportions of pheomelanin and eumelanin, degree of ultraviolet-B stimulated eumelanogenesis, and require consideration.
We have employed the melanocyte:keratinocyte co-culture system to study the effects of ultraviolet radiation and NDP-MSH on the signalling proteins p38 and p53 in vitro. There has been increasing evidence for the involvement of these two proteins in pigmentary and ultraviolet radiation responses. p38 has been shown to be activated in response to α-melanocyte stimulating hormone in human melanocytes, and there was a synergistic interaction between ultraviolet radiation and melanocortin MC1 receptor stimulation on p38 signalling (Newton et al., 2007). This may be important for the tanning response as ultraviolet radiation induced expression of proopiomelanocortin, tyrosinase and melanocortin MC1 receptor in melanocytes has been reported to be dependent on p38 mediated phosphorylation of the transcription factor USF-1 (Corre et al., 2004; Galibert et al., 2001). However, a recent report has also linked p38 with negative regulation of pigmentation via degradation of tyrosinase (Bellei et al., 2010). Ultraviolet radiation-induced DNA damage activates p53 in keratinocytes, which is able to induce the expression of the melanocortin precursor proopiomelanocortin (Cui et al., 2007). In melanocytes, p53 is an early response gene induced by ultraviolet radiation, thought to be involved in DNA repair and cell cycle arrest (Yang et al., 2006). p53 may also induce expression of the tyrosinase related genes (Nylander et al., 2000).
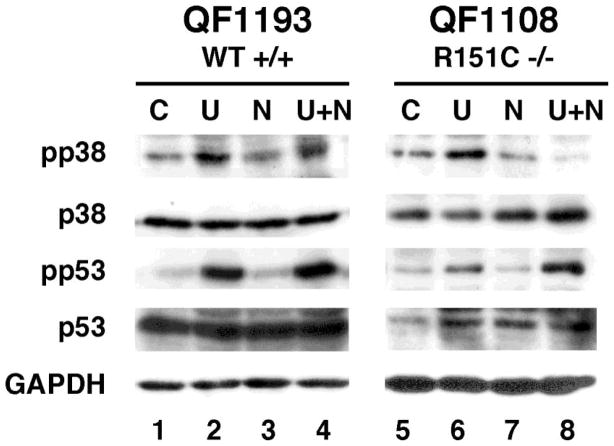
We wished to investigate the relative contributions of p38 and p53 signalling in response to ultraviolet radiation and NDP-MSH in co-cultures. All of our melanocyte strains have been genotyped for melanocortin MC1 receptor so we can also look at the effect of melanocortin MC1 receptor variant alleles. In these preliminary results, p38 was activated in melanocortin MC1 receptor wild type co-cultures by ultraviolet radiation, with a synergistic effect of NDP-MSH (Figure 4, lane 4). Melanocortin MC1 receptor R151C co-cultures still activated p38 activation in response to ultraviolet radiation, however NDP-MSH no longer had a synergistic effect (Figure 4, lane 8). In fact, the ultraviolet radiation p38 response seemed to be repressed in the presence of NDP-MSH in the R151C co-cultures. When compared with the results for monocultures (data not shown), it was concluded that the p53 activation was mainly due to the keratinocytes in the co-cultures. In contrast, the diminished responses of p38 in variant co-cultures might be attributed to the reduced function of the melanocortin MC1 receptor variant receptor in the melanocytes.
Figure 4.
Melanocyte co-culture p38 and p53 responses to NDP-MSH and ultraviolet radiation. Co-cultures of melanocortin MC1 receptor wild type (WT–QF1193) or homozygous R151C melanocytes (QF1108) and keratinocytes were treated with 20 mJ/cm2 ultraviolet radiation(U), 20 nM NDP -MSH (N) or both (UN) and incubated for 1 hr. Total cell extracts were used for western immuno-blotting. Activated forms of p38 and p53 were detected using antibodies specific for the Thr180/Tyr182 (pp38) and Ser15 (pp53) phosphorylated sites (Cell Signaling). These blots are representative of 2-3 independent experiments.
These results confirm previous melanocyte monoculture studies in which phosphorylation of p38 was reduced in the red hair colour variants compared to the wild type melanocortin MC1 receptor strains with NDP-MSH and ultraviolet irradiation (Newton et al., 2007). Using the co-culture model in combination with monoculture studies, we are able to delineate the contributions of the melanocortin MC1 receptor signalling pathway to ultraviolet radiation and α-melanocyte stimulating hormone responses.
Dopachrome tautomerase is a member of the tyrosinase related protein family, and is also known as TYRP2. Dopachrome tautomerase has been recognized for its role in the formation of eumelanin by catalysing the conversion of dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) (Ito and Wakamatsu, 2008). Expression of dopachrome tautomerase is strongly induced by α-melanocyte stimulating hormone in melanocyte co-cultures (Roberts et al., 2008). The importance of dopachrome tautomerase beyond melanin synthesis has recently been realized with the finding that dopachrome tautomerase is able to promote cell survival and inhibit apoptosis in melanoma cells, possibly via inhibition of p53 (Sendoel et al., 2010). The biochemical mechanism of this inhibition is as yet unknown, but the authors speculated it might be related to the dopachrome tautomerase activity this protein. Dopachrome tautomerase was also linked to DNA damage protection and reduced sensitivity to oxidative stress in amelanotic melanoma cells (Michard et al., 2008).
We have previously studied the induction of dopachrome tautomerase in melanocyte strains homozygous for melanocortin MC1 receptor variants (Roberts et al., 2008). Here we have preliminary data on the induction of dopachrome tautomerase in heterozygote melanocortin MC1 receptor strains in co-culture (Figure 4). The wild type strain shows a good induction of dopachrome tautomerase by both NDP-MSH and forskolin (Figure 4, lanes 2 and 3), whereas the variant strains, particularly the R160W heterozygote, show diminished responses to NDP-MSH (Figure 4, lanes 5, 6, 8 and 9). These results are in line with our in vitro transfection studies in melanoma cells on the dominant negative effect of variant receptors on wild type melanocortin MC1 receptor (Beaumont et al., 2007), and may also help explain the heterozygote effect on pigmentation phenotypes and skin cancer risk in genetic association studies.
5. Further models for studying melanocortin MC1 receptor function
Although cell co-culture models allow interactions between melanocytes and keratinocytes, melanocytes integrated into the basal layer of a 3D reconstructed epidermis is closer to mimicking the in vivo situation in human skin, and has been used by a number of groups, particularly in the study of melanocyte responses to ultraviolet radiation (Duval et al., 2001). Immediate pigmentation can be seen in response to ultraviolet radiation, and it can be assumed that pigmentation could also be induced by melanocortin MC1 receptor agonists, although the melanocortin MC1 receptor has yet to be studied in the skin reconstruction model. One disadvantage of the 3D skin reconstruction is that cell and biochemical manipulations such as siRNA knockdown or overexpression studies are not easily performed. Imaging of cells also becomes more difficult with the move from a 2D to a 3D cell culture system.
Mouse models have been important for the identification of many different pigmentation genes via the study of coat colour mutants. Murine melanocortin MC1 receptor maps to the mouse extension locus, which was known to influence pigmentation due to a number of naturally occurring dominant and recessive coat colour phenotypes. The extension locus alleles include wild type (E+), recessive yellow (e), tobacco darkening (Etob), sombre (Eso), and sombre 3J (Es-o3J) (Searle, 1968). The e allele encodes a non-functional mouse melanocortin MC1 receptor, while the other dominant darkening alleles encode constitutively active or hyperactive mouse melanocortin MC1 receptor (Robbins et al., 1993). Mutations in the melanocortin MC1 receptor antagonist agouti signal protein in mice also give rise to a number of coat colour phenotypes (Bultman et al., 1992). Temporal expression of agouti is responsible for the black/yellow banding pattern in wild type agouti mice, loss of agouti expression results in a black coat colour, whereas overexpression results in a yellow coat colour.
While we have discovered many important functions of the melanocortin system in mice, melanocortin MC1 receptor regulated pigmentation in humans has some major differences. Agouti signaling protein does not produce the same obvious pheomelanin banding effect in human hair, indeed agouti signaling protein expression is yet to be detected in human skin, and the human melanocortin MC1 receptor expressed in transgenic mice appeared resistant to the effects of agouti signaling protein(Healy et al., 2001) . However, agouti signaling protein antagonism of the human melanocortin MC1 receptor has been demonstrated in vitro (Suzuki et al., 1997). Moreover genome wide association studies have found associations between agouti signaling protein polymorphism and skin colour as well as skin cancer risk(Duffy et al., 2010) , with the agouti signaling protein tagged rs4911442 risk allele showing epistatic effects roughly equivalent to amelanocortin MC 1 receptor r allele. Human melanocortin MC1 receptor also has a higher sensitivity to ligands and a lower expression level relative to murine melanocortin MC1 receptor. To address some of these issues, Jackson and co-workers developed mice transgenic for human melanocortin MC1 receptor , which was under control of the human melanocortin MC1 receptor regulatory sequences (Jackson et al., 2007). In this mouse model, unlike the previous model where melanocortin MC1 receptor was expressed under the control of the mouse regulatory sequences, agouti antagonism and a normal agouti banding pattern could be seen(Jackson et al., 2007) .
Another major difference between mouse and human pigmentation is that mature mice do not have skin pigmentation, except for the ears and tail; this is due to a lack of melanocytes in the epidermis of the skin.D’Orazio and co -workers created a “humanized” mouse model, which mimics the human skin with the presence of melanocytes in the basal layer of the epidermis (D'Orazio et al., 2006). In this model, tanning in the trunk area of the transgenic melanocortin MC1 receptore/e mouse could be achieved by topical application of the cAMP activating agent forskolin (D'Orazio et al., 2006).
Robinson and co-workershave created an albino and pigmented hairless melanocortin MC1 receptor model in the mouse (Robinson et al., 2010). This allowed the authors to study the effects of melanocortin MC1 receptor on not only pigmentation, but non-pigmentary effects as well. The question of whether melanocortin MC1 receptor really exerts an effect on skin cancer risk independent of pigmentation status seems to have been answered in this model. P53 positive keratinocyte clones were used as a readout for inadequate repair of DNA damage and clonal proliferation. Significantly higher numbers of p53-positive clones were found in albino mice null for melanocortin MC1 receptor, compared to albino mice expressing wild type melanocortin MC1 receptor. This indicates that melanocortin MC1 receptor, at least in this model, does have non-pigmentary protective effects on skin cancer development.
Another important tool used in the study of melanocortin MC1 receptor function is microarray analysis. It is becoming increasingly clear the melanocortin MC1 receptor signalling activates a large number of target genes involved in many processes such as melanin synthesis, melanosome granule maturation, melanosome trafficking and transfer, cell morphology, DNA repair, cell survival and others. A recent study identified 255 genes that were altered by α-melanocyte stimulating hormone in murine melanocytes (Le Pape et al., 2009). Unsurprisingly, α-melanocyte stimulating hormone treatment upregulated known genes important for pigmentation, while murine agouti signal protein, as an inverse agonist (Siegrist et al., 1997), downregulated the same genes. Surprisingly, murine agouti signal protein also upregulated a number of additional genes involved in differentiation and developmental processes, as well as cell adhesion, motility and extra cellular matrix-receptor interactions (Le Pape et al., 2009). The authors suggested that agouti signal protein activates expression of genes that are typically expressed during morphogenesis, and are reactivated in cancer cells to promote migration and invasion. Supporting this, melan-a melanocytes and B16 melanoma cells treated with murine agouti signal protein showed increased migration.
6. Conclusions
The use of naturally occurring mouse coat colour mutants, human genetic association studies, in vitro studies using transfected cells or genetically defined melanocyte monocultures and co-cultures have all contributed to our knowledge of melanocortin MC1 receptor function in human pigmentation and skin cancer risk. It is becoming increasingly apparent that the function of melanocortin MC1 receptor is much more complex than just regulating the switch from the red/yellow pheomelanin to the brown/black eumelanin that was originally described. Future melanocortin MC1 receptor pharmocogenetic studies may utilise new transgenic mouse models, new information about novel melanocortin MC1 receptor response genes obtained in micro array studies, or more physiologically relevant models of the human skin such as in vitro 3D reconstructions.
Figure 5.
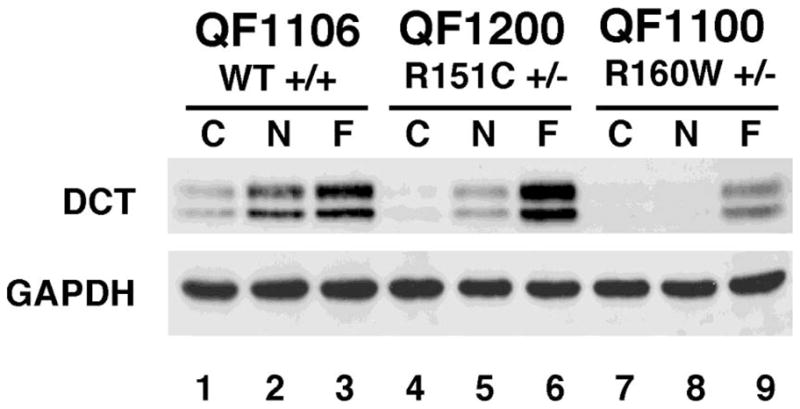
Dopachrome tautomeraseinduction in melanocyte co -cultures. Melanocytes were either melanocortin MC1 receptor wild type (WT–QF1106 strain), heterozygous for R151C (QF1200 strain) or heterozygous for R160W (QF1100 strain). Co-cultures of melanocytes with keratinocytes were treated with the following compounds: C = Control, N = 20nM NDP-MSH or F = 10uM forskolin, for four days, then total cell extracts were used for western immuno-blotting with dopachrome tautomerase(Santa Cruz) and GAPDH antibodies. This blot is representative of responses seen in three different wild type and variant strains.
Acknowledgments
We thank AmjadYousuf for his contribution to the cAMP assays. This work was funded in part by grants from the National Health and Medical Research Council of Australia (NHMRC-511191, NHMRC-552485) and the Australian Research Council (ARC-DP0771169) to RAS,and from the National Institutes of Health(NIH -R01DK064265) to GM. RAS is an NHMRC Senior Research Fellow (NHMRC-511029).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186. doi: 10.1111/j.1755-148X.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Knittel J, Kadekaro AL, Swope VB, Starner R. The melanocortin 1 receptor and the UV response of human melanocytes--a shift in paradigm. Photochem Photobiol. 2008;84:501–508. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- Bastiaens MT, ter Huurne JAC, Kielich C, Gruis NA, Westendorp RGJ, Vermeer BJ, Bavinck NJB. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. American Journal of Human Genetics. 2001;68:884–894. doi: 10.1086/319500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont KA, Liu YY, Sturm RA. Chapter 4 The Melanocortin-1 Receptor Gene Polymorphism and Association with Human Skin Cancer. Prog Mol Biol Transl Sci. 2009;88C:85–153. doi: 10.1016/S1877-1173(09)88004-6. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SL, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA. Red hair is the null phenotype of MC1R. Hum Mutat. 2008;29:E88–E94. doi: 10.1002/humu.20788. [DOI] [PubMed] [Google Scholar]
- Bellei B, Maresca V, Flori E, Pitisci A, Larue L, Picardo M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem. 2010;285:7288–7299. doi: 10.1074/jbc.M109.070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Irving RE, Russell A, Chen W, Griffiths LR, Parsons PG, Green AC, Sturm RA. Melanocortin-1 receptor genotype is a risk factor for basal and squamous cell carcinoma. Journal of Investigative Dermatology. 2001;116:224. doi: 10.1046/j.1523-1747.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Human Molecular Genetics. 1997;6:1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- Busca R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne JP, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases(ERKs) in melanocytes. Embo J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, Kerns JA, Schmutz SM, Millhauser GL, Barsh GS. A β-defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AL, Donatien PD, Smith AG, Murphy M, Jones MK, Herlyn M, Bennett DC, Leonard JH, Sturm RA. Human melanoblasts in culture: expression of BRN2 and synergistic regulation by fibroblast growth factor-2, stem cell factor, and endothelin-3. J Invest Dermatol. 2003;121:1150–1159. doi: 10.1046/j.1523-1747.2003.12562.x. [DOI] [PubMed] [Google Scholar]
- Corre S, Primot A, Sviderskaya E, Bennett DC, Vaulont S, Goding CR, Galibert MD. UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1) J Biol Chem. 2004;279:51226–51233. doi: 10.1074/jbc.M409768200. [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D'Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13:447–461. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol. 2010;130:520–528. doi: 10.1038/jid.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N, Marais R. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. Febs J. 2005;272:3491–3504. doi: 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- Duval C, Regnier M, Schmidt R. Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure. Pigment Cell Res. 2001;14:348–355. doi: 10.1034/j.1600-0749.2001.140506.x. [DOI] [PubMed] [Google Scholar]
- Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet. 2000;9:2531–2537. doi: 10.1093/hmg/9.17.2531. [DOI] [PubMed] [Google Scholar]
- Galibert MD, Carreira S, Goding CR. The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. EMBO J. 2001;20:5022–5031. doi: 10.1093/emboj/20.17.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, Schwarz A, Schwarz T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–1123. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Halaban R. The regulation of normal melanocyte proliferation. Pigment Cell Res. 2000;13:4–14. doi: 10.1034/j.1600-0749.2000.130103.x. [DOI] [PubMed] [Google Scholar]
- Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ. Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet. 2001;10:2397–2402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–536. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K. Chemistry of mixed melanogenesis--pivotal roles of dopaquinone. Photochem Photobiol. 2008;84:582–592. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- Jackson IJ, Budd PS, Keighren M, McKie L. Humanized MC1R transgenic mice reveal human specific receptor function. Hum Mol Genet. 2007;16:2341–2348. doi: 10.1093/hmg/ddm191. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Wakamatsu K, Ito S, Abdel-Malek ZA. Cutaneous photoprotection and melanoma susceptibility: reaching beyond melanin content to the frontiers of DNA repair. Front Biosci. 2006;11:2157–2173. doi: 10.2741/1958. [DOI] [PubMed] [Google Scholar]
- Kanetsky PA, Panossian S, Elder DE, Guerry D, Ming ME, Schuchter L, Rebbeck TR. Does MC1R genotype convey information about melanoma risk beyond risk phenotypes? Cancer. 2010;116:2416–2428. doi: 10.1002/cncr.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi MT, Kanetsky PA, Tsang S, Gold B, Munroe D, Rebbeck T, Swoyer J, Ter-Minassian M, Hedayati M, Grossman L, Goldstein AM, Calista D, Pfeiffer RM. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. J Natl Cancer Inst. 2005;97:998–1007. doi: 10.1093/jnci/dji176. [DOI] [PubMed] [Google Scholar]
- Le Pape E, Passeron T, Giubellino A, Valencia JC, Wolber R, Hearing VJ. Microarray analysis sheds light on the de-differentiating role of agouti signal protein in murine melanocytes via the Mc1r. PNAS. 2009 doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JH, Marks LH, Chen W, Cook AL, Boyle GM, Smit DJ, Brown DL, Stow JL, Parsons PG, Sturm RA. Screening of human primary melanocytes of defined melanocortin-1 receptor genotype: Pigmentation marker, ultrastructural andUV -survival studies. Pigment Cell Res. 2003;16:198–207. doi: 10.1034/j.1600-0749.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- Liboutet M, Portela M, Delestaing G, Vilmer C, Dupin N, Gorin I, Saiag P, Lebbe C, Kerob D, Dubertret L, Grandchamp B, Basset-Seguin N, Soufir N. MC1R and PTCH gene polymorphism in French patients with basal cell carcinomas. J Invest Dermatol. 2006;126:1510–1517. doi: 10.1038/sj.jid.5700263. [DOI] [PubMed] [Google Scholar]
- Maresca V, Flori E, Bellei B, Aspite N, Kovacs D, Picardo M. MC1R stimulation by alpha-MSH induces catalase and promotes its re-distribution to the cell periphery and dendrites. Pigment Cell Melanoma Res. 2010;23:263–275. doi: 10.1111/j.1755-148X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- McNulty JC, Jackson PJ, Thompson DA, Chai B, Gantz I, Barsh GS, Dawson PE, Millhauser GL. Structures of the agouti signaling protein. J Mol Biol. 2005;346:1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Michard Q, Commo S, Belaidi JP, Alleaume AM, Michelet JF, Daronnat E, Eilstein J, Duche D, Marrot L, Bernard BA. TRP-2 specifically decreases WM35 cell sensitivity to oxidative stress. Free Radic Biol Med. 2008;44:1023–1031. doi: 10.1016/j.freeradbiomed.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Newton RA, Roberts DW, Leonard JH, Sturm RA. Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides. 2007;28:2387–2396. doi: 10.1016/j.peptides.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Nylander K, Bourdon JC, Bray SE, Gibbs NK, Kay R, Hart I, Hall PA. Transcriptional activation of tyrosinase and TRP-1 by p53 links UV irradiation to the protective tanning response. J Pathol. 2000;190:39–46. doi: 10.1002/(SICI)1096-9896(200001)190:1<39::AID-PATH492>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Pelle E, Mammone T, Maes D, Frenkel K. Keratinocytes act as a source of reactive oxygen species by transferring hydrogen peroxide to melanocytes. J Invest Dermatol. 2005;124:793–797. doi: 10.1111/j.0022-202X.2005.23661.x. [DOI] [PubMed] [Google Scholar]
- Raimondi S, Sera F, Gandini S, Iodice S, Caini S, Maisonneuve P, Fargnoli MC. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer. 2008;122:2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Newton RA, Leonard JH, Sturm RA. Melanocytes expressing MC1R polymorphisms associated with red hair color have altered MSH-ligand activated pigmentary responses in coculture with keratinocytes. J Cell Physiol. 2008;215:344–355. doi: 10.1002/jcp.21318. [DOI] [PubMed] [Google Scholar]
- Robinson S, Dixon S, August S, Diffey B, Wakamatsu K, Ito S, Friedmann PS, Healy E. Protection against UVR involves MC1R-mediated non-pigmentary and pigmentary mechanisms in vivo. J Invest Dermatol. 2010;130:1904–1913. doi: 10.1038/jid.2010.48. [DOI] [PubMed] [Google Scholar]
- Searle AG. An extension series in the mouse. J Hered. 1968;59:341–342. doi: 10.1093/oxfordjournals.jhered.a107739. [DOI] [PubMed] [Google Scholar]
- Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465:577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist W, Drozdz R, Cotti R, Willard DH, Wilkison WO, Eberle AN. Interactions of alpha-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. J Recept Signal Transduct Res. 1997;17:75–98. doi: 10.3109/10799899709036595. [DOI] [PubMed] [Google Scholar]
- Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, Muscat GE. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J Biol Chem. 2008;283:12564–12570. doi: 10.1074/jbc.M800480200. [DOI] [PubMed] [Google Scholar]
- Song X, Mosby N, Yang J, Xu A, Abdel-Malek Z, Kadekaro AL. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009;22:809–818. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Tada A, Ollmann MM, Barsh GS, Im S, Lamoreux ML, Hearing VJ, Nordlund JJ, AbdelMalek ZA. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. Journal of Investigative Dermatology. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Xu X, Thornwall M, Lundin LG, Chhajlani V. Val92Met variant of the melanocyte stimulating hormone receptor gene. Nat Genet. 1996;14:384. doi: 10.1038/ng1296-384. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhang G, Pittelkow MR, Ramoni M, Tsao H. Expression profiling of UVB response in melanocytes identifies a set of p53-target genes. J Invest Dermatol. 2006;126:2490–2506. doi: 10.1038/sj.jid.5700470. [DOI] [PubMed] [Google Scholar]