Abstract
Melanocortin receptors belong to the seven-transmembrane (TM) domain proteins that are coupled to G-proteins and signaled through intracellular cyclic adenosine monophosphate. Many structural features conserved in other G-protein coupled receptors (GPCRs) are found in the melanocortin receptors. There are five melanocortin receptor subtypes and each of the melanocortin receptor subtypes has a different pattern of tissue expression and has its own profile regarding the relative potency of different melanocortin peptides. α-, β-, and γ-MSH and ACTH are known endogenous agonist ligands for the melanocortin receptors. Agouti and AgRP are the only known naturally occurring antagonists of the melanocortin receptors. We have examined the molecular basis of all five human melanocortin receptors for different ligand binding affinity and potency using chimeric and mutated receptors. Our studies indicate that human melanocortin MC1 receptor, human melanocortin MC3 receptor, human melanocortin MC4 receptor and human melanocortin MC5 receptor utilize orthosteric sites for non selective agonists, α-MSH and NDP- β-MSH, high affinity binding and utilize allosteric sites for selective agonist or antagonist binding. Furthermore, our results indicate that molecular determinants of human melanocortin MC2 receptor for ACTH binding and signaling are different from that of other melanocortin receptors. Many studies also indicate that agonists can induce different conformation changes of melanocortin receptors, which then lead to the activation of different signaling pathways, even when the expression level of receptor and the strength of stimulus-response coupling are the same. This finding may provide new information for the design of drugs for targeting melanocortin receptors.
Index words: orthosteric binding, allosteric binding, melanocortin receptors, GPCR
1. Introduction
THE MELANOCORTIN SYSTEM consists of 1) the melanocortin peptides α–, β–, and γ–melanocyte-stimulating hormone (α–, β–, γ–MSH) and adrenocorticotropic hormone (ACTH), 2) a family of five seven-transmembrane G protein-coupled melanocortin receptors, and 3) the endogenous melanocortin antagonists agouti and agouti-related protein (AgRP) (Cone et al., 1996; Gantz and Fong, 2003; Holder and Haskell-Luevano, 2004; Yang and Harmon, 2003). The melanocortins are involved in a diverse number of physiological functions, including pigmentation, steroidogenesis, energy homeostasis, exocrine secretion, sexual function, analgesia and inflammation. Melanocortin MC1 receptor is the melanocyte α-MSH receptor, expressed on cutaneous melanocytes, where it has a key role in determining skin and hair pigmentation. Melanocortin MC1 receptor is also expressed at leukocytes, where it may mediate the anti-inflammatory property. Melanocortin MC2 receptor is the adrenocortical ACTH receptor, expressed in the adrenal cortex zona reticularis and zona fasiculata, where it mediates the effects of ACTH on steroid secretion. Melanocortin MC3 receptor is identified in many areas of the central nervous system and peripheral tissues and involved in energy homeostasis. Melanocortin MC4 receptor is expressed predominantly in the central nervous system and regulates both food intake and sexual function. Melanocortin MC5 receptor is expressed in numerous human peripheral tissues and is mainly involved in exocrine function, particularly sebaceous gland secretion discovered by targeted deletion of that receptor (Adan et al., 1999; Cone, 1999; 2005; Gantz and Fong, 2003). The physiological significance of the melanocortin receptor family has promoted considerable research activity over past decade (Adan et al., 1997; Adan and Vink, 2001; Chen et al., 2000a; Chen et al., 2000b; Chen et al., 2007a; Huszar et al., 1997; Hwa et al., 2001). The ultimate goal for the development of any new therapeutic agent for melanocortin receptors is to identify a drug that produces the desired effect with minimal side effects. To this end, the concept of directed signaling and functional selectivity has generated significant interest as a means to develop compounds that can selectively activate or block receptor-signaling pathways that lead only to the desired therapeutic effect. This is of particular importance for the melanocortin receptors as a potential target for the treatment of skin cancer, food intake, and exocrine disorders because many existing melanocortin receptor agonists for melanocortin receptors are not receptor subtype specific and have unwanted side effects. Several approaches have been undertaken to develop melanocortin receptor agonists and antagonists. In this review, current understanding of the molecular basis of human melanocortin receptors responsible for ligand binding and signaling will be discussed.
2. Structural features of the melanocortin receptors
Melanocortin receptors belong to the seven-transmembrane (TM) domain receptor proteins that are coupled to proteins G [G-protein-coupled receptors (GPCRs)] and signaled mainly through intracellular cyclic adenosine monophosphate. The cloning of the human melanocortin receptor genes has led to a tremendous progress in understanding the biological effects of the melanocortin peptides and the melanocortin receptors. So far, five human melanocortin receptor genes, including human melanocortin MC1 receptor, human melanocortin MC2 receptor, human melanocortin MC3 receptor, human melanocortin MC4 receptor, and human melanocortin MC5 receptor, have been cloned (Gantz et al., 1996; Gantz et al., 1993; Gantz et al., 1997; Gantz et al., 1994a; Gantz et al., 1994b; Mountjoy et al., 1992). Melanocortin receptors consist of a single polypeptide featuring seven α-helical TM domains, an extracellular N-terminus, and an intracellular C-terminus. Melanocortin receptors share many structural features conserved in other GPCRs: the consensus N-linked glycosylation sites near the amino terminus, a palmitoylation site in the COOH-terminal tail, and sites for phosphorylation in the first and third intracellular domains and in the COOH-terminal tail. All human melanocortin receptors contain the conserved amino acids, Aspartic acid–arginine–tyrosine (DRY) motif at the junction of the TM3 domain and contain cysteine at C terminus. However, melanocortin receptors have several features which are different from other GPCR. Melanocortin receptors are the smallest G protein-coupled receptors known, with short amino- and carboxyl-terminal ends and a very small second extracellular loop. Human melanocortin receptors also lack several features found in most GPCRs; one, or two, cysteine residues in the first and second extracellular loops and prolines found in the fourth and fifth TM domains. The melanocortin receptors contain conserved cysteine residues at different region of the receptor, including cysteine residues within extracellular loops (ELs) and cysteine residues within TMs, and cysteine residue in C terminus. Functional studies indicate that cysteines in EL3 may form a disulfide bond which is crucial for receptor function while cysteine residues in TMs of the human melanocortin receptors may have different roles in receptor expression, ligand binding and receptor activation (Yang et al., 2007).
3. The orthosteric binding pocket in the melanocortin receptors
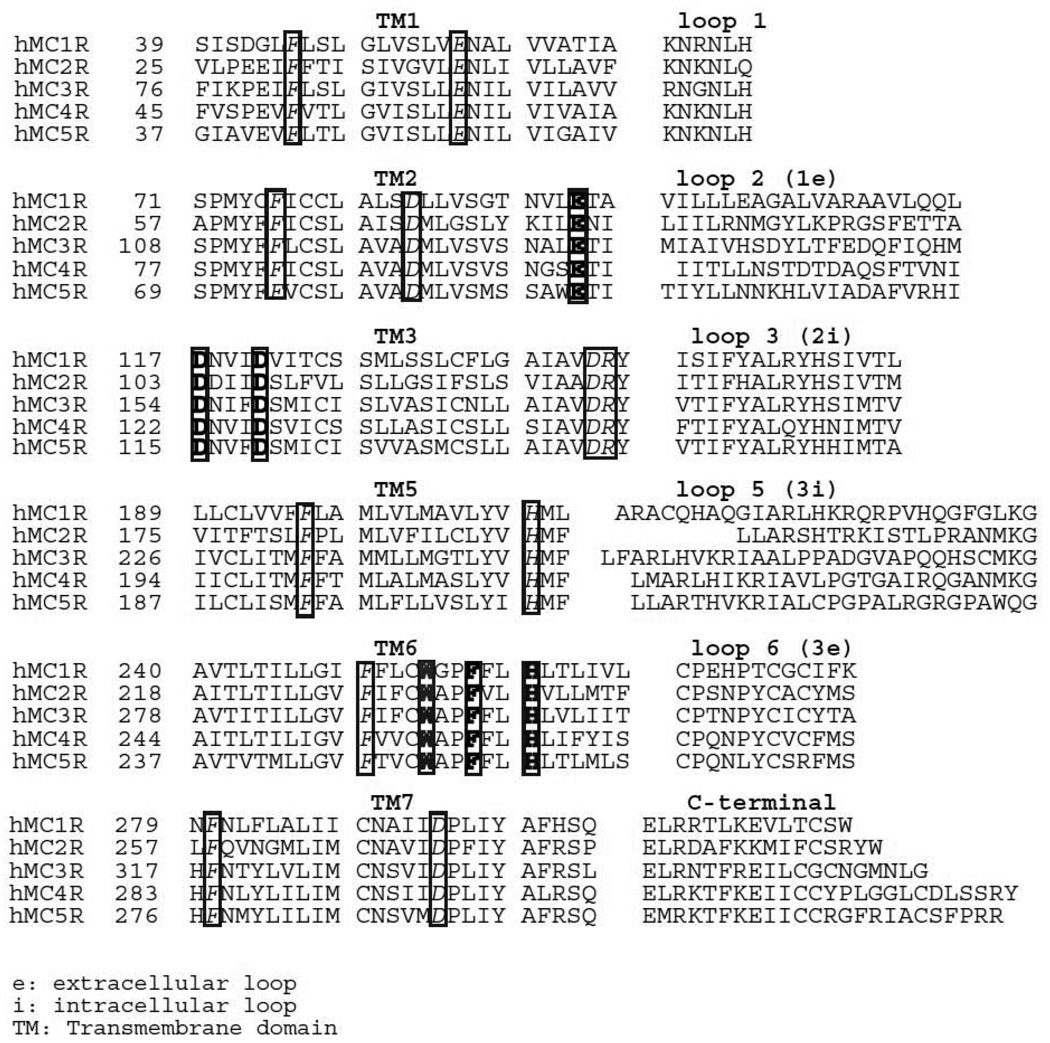
The orthosteric site is defined as the receptor area where the endogenous agonist binds. Melanocortin receptors share same endogenous ligands and therefore should have orthosteric binding sites for endogenous melanocortins. Sequence comparison of human melanocortin receptors show high sequence homologies, ranging from 60% identity between human melanocortin MC4 receptor and melanocortin MC5 receptor, 45% identity between human melanocortin MC3 receptor and melanocortin MC1 receptor and between human melanocortin MC3 receptor and melanocortin MC2 receptor to 38% identity between human melanocortin MC2 receptor and melanocortin MC4 receptor (Gantz et al., 1993; Yang and Harmon, 2003). The alignment of the human melanocortin receptor subtype primary sequences also highlights the highly conserved residues among these receptors (Figure 1). To determine the molecular basis of human melanocortin receptors for the endogenous ligand binding, site-directed mutagenesis is used to examine the role of the conserved amino acid residues in human melanocortin receptors for ligand orthosteric binding and chimeric receptors are used to examine the role of non-conserved amino acid residues for ligand allosteric binding. We have examined all five human melanocortin receptor binding sites by using above two approaches. Based on the assumption that receptor residues conserved among the human melanocortin receptors might participate in ligand orthosteric binding, conserved basic, negative TM residues and aromatic, hydroxyl and sulfhydryl containing residues of human melanocortin receptors have been mutated and tested. Glutamine acids 2.4 and aspartic acid 3.2 and 3.6 in TM3 of the melanocortin receptors are conserved among all five melanocortin receptor subtypes. Mutation of this glutamic acid or aspartic acid to alanine resulted in significantly decrease in endogenous agonist binding and receptor signaling, suggesting that these residues are crucial for the melanocortin orthosteric binding. Phenylalanine F6.51 and histidine 6.54 are also conserved among all five melanocortin receptors. Mutation of these residues to alanine resulted in significantly decrease in endogenous agonist binding and receptor signaling, suggesting that these residues are also involved in melanocortin orthosteric binding (Fleck et al., 2005; Haskell-Luevano et al., 2001; Nickolls et al., 2003; Pogozheva et al., 2005; Yang et al., 2000). Table 1 enumerates the human melanocortin receptor residues involved in ligand orthosteric binding. For human melanocortin MC1 receptor, glutamic acid 94 in TM2, aspartic acid 117 and 121 in TM3, tryptophan 254, phenylalanine 257 and histidine 260 in TM6 were identified to be involved in MSH orthosteric binding (Yang et al., 1997). For human melanocortin MC3 receptor, glutamic acid 131 in TM2, aspartic acid 154 and 158 in TM3, tryptophan 292, phenylalanine 295 and histidine 298 in TM6 are identified to be involved in MSH orthosteric binding (Chen et al., 2006). For human melanocortin MC4 receptor, glutamic acid 100 in TM2, aspartic acid 122 and 126 in TM3, tryptophan 258, phenylalanine 261 and histidine 264 in TM6 are important for MSH orthosteric binding (Chen et al., 2007c; Yang et al., 2000). For human melanocortin MC5 receptor, glutamic acid 92 in TM2, aspartic acid 115 and 119 in TM3, tryptophan 251, phenylalanine 254 and histidine 257 in TM6 were identified to be involved in MSH orthosteric binding (unpublished data).
Figure 1.
Primary sequences of human melanocortin receptors. The conserved TM residues in these receptors are denoted by italic. The residues involved in ligand orthosteric binding were highlighted by bold.
Table 1.
Receptor mutation on NDP-α-MSH binding affinity and potency
| Receptor binding affinity and potency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Residue mutation | hMC1R | hMC2R | hMC3R | hMC4R | hMC5R | |||||
| TM2 (2.6) | E94 | ↓ | E80 | ↓ | E131 | ↓ | E100 | ↓ | E92 | ↓ |
| TM3 (3.25) | D117 | ↓ | D103 | ↓ | D154 | ↓ | D122 | ↓ | D115 | ↓ |
| TM3 (3.29) | D121 | ↓ | D107 | ↓ | D158 | ↓ | D126 | ↓ | D119 | ↓ |
| TM6 (6.48) | W254 | ↓ | W232 | ↓ | W292 | ↓ | W258 | ↓ | W251 | ↓ |
| TM6 (6.51) | F257 | ↓ | F235 | ↓ | F295 | ↓ | F261 | ↓ | F254 | ↓ |
| TM6 (6.54) | H260 | ↓ | H238 | ↓ | H298 | ↓ | H264 | ↓ | H257 | ↓ |
binding affinity and potency decrease.
Melanocortin MC2 receptor is unique among melanocortin receptors. Although melanocortin MC2 receptor share some conserved amino acid residues in TM region with other melanocortin receptors, it shows low sequence homology with other melanocortin receptors, only 38% identity between melanocortin MC2 receptor and melanocortin MC4 receptor. Pharmacological characterization also indicates that melanocortin MC2 receptor has different pharmacological profile compared with other melanocortin receptors. First, ACTH is the only endogenous ligand for melanocortin MC2 receptor. α-MSH, β-MSH and γ-MSH are unable to bind to melanocortin MC2 receptor and activate receptor. Second, substitution of Phe7 with DPhe at ACTH decreases ligand binding affinity and potency at melanocortin MC2 receptor whereas substitution of phenylalanine (Phe)7 at MSH with DPhe results in increase ligand binding affinity and potency at melanocortin MC1 receptor, melanocortin MC3 receptor, melanocortin MC4 receptor and melanocortin MC5 receptor. Furthermore, Phe7-ACTH1-17 is a potent melanocortin MC2 receptor agonist but DPhe7-ACTH1-17 losses its binding ability at melanocortin MC2 receptor. Thirdly, DNal (2’)-ACTH1-17 is a potent agonist at melanocortin MC2 receptor but substitution of Phe7 at MSH with D-Naphthylalanine (2’) [DNal (2’)] switches ligand from agonist to antagonist at melanocortin MC3 and MC4 receptors. All these results suggest that melanocortin MC2 receptor has different ligand binding pocket compared with other melanocortin receptors. Mutagenesis study indicates that human melanocortin MC2 receptor utilize some conserved amino acid residues for ACTH binding. However, study also indicates that melanocortin MC2 receptor has a broad binding pocket in which both conserved and unique amino acid residues are involved and the residues in TM2, TM3 and TM6 of. Mutations of the conserved amino acid residues, glutamic acid 80 in TM2, aspartic acid 107 in TM3, phenylalanine 178 in TM4, tryptophan 232, phenylalanine 235 and histidine 238 in TM6 and phenylalanine 258 in TM7, significantly reduced ACTH binding affinity and signaling that is similar to other melanocortin receptors. Mutations of unique amino acids aspartic acid 104, phenylalanine 108 in TM3, phenylalanine 168 in TM4 and phenylalanine 178 in TM4 significantly decreased ACTH binding and signaling, implying that melanocortin MC2 receptor orthosteric binding sites are much broader than that of other melanocortin receptors (Chen et al., 2007b).
4. An allosteric binding pocket in the melanocortin receptors
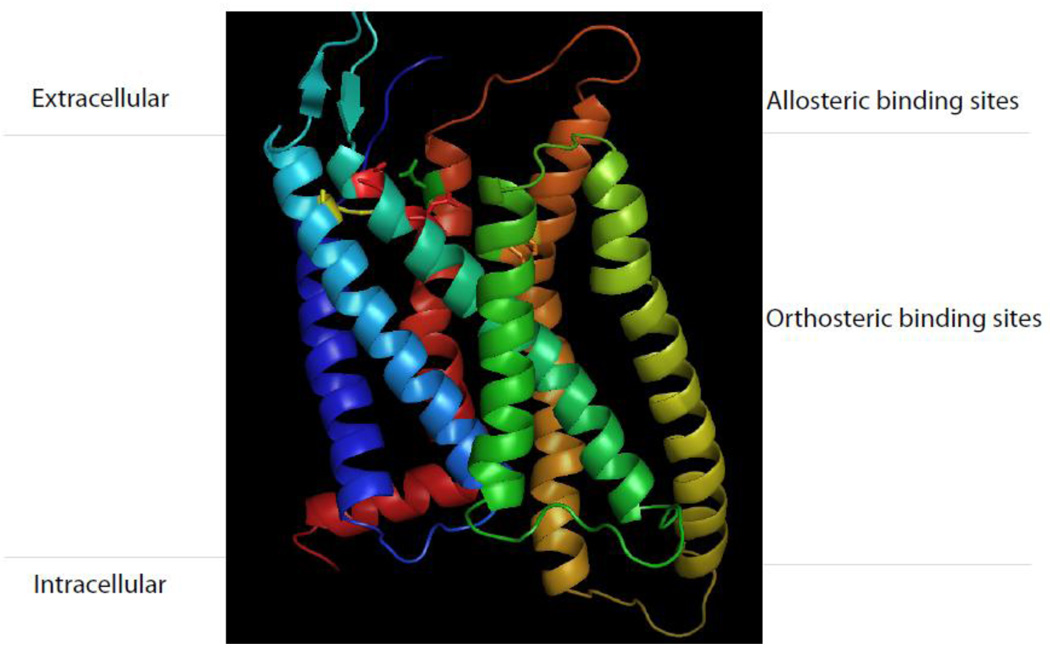
The allosteric site is defined as the receptor area that is distinct from the binding site of the endogenous ligands. Allosteric ligands alter receptor conformation through binding at sites distinct from the orthosteric ligand binding site. Melanocortin receptor family is a member of G-protein-coupled receptor families that share same endogenous ligand. While ligand selectivity for melanocortin receptor family is usually achievable, it is often difficult to obtain selectivity among subtypes of the family. Thus selective orthosteric ligands, which presumably interact with the same amino acid sequences, would tend to be non-selective within a family. Allosteric ligands may imprint specific conformations on the receptor protein that are not induced by orthosteric agonists. Therefore, targeting allosteric domains allows to achieving binding site selectivity to an extent that is not achieved by traditional orthosteric agonists. Furthermore, selectivity at the allosteric site of the melanocortin receptors allowed for the pharmacological separation of receptors that previously could not be distinguished using orthosteric ligands. Increasing evidence demonstrates that melanocortin receptors allow for ligand interactions outside the domain where the endogenous ligand binds. Agouti signaling protein (ASIP) and AgRP are endogenous melanocortin receptor antagonists. ASIP is selective for melanocortin MC1 receptor and melanocortin MC4 receptor but AgRP is selective antagonist for melanocortin MC3 receptor and melanocortin MC4 receptor. Study indicates that AgRP selectively binds to melanocortin MC3 receptor and melanocortin MC4 receptor using orthosteric and allosteric binding (Yang et al., 1999). The allosteric site is located extracellular loop and the orthosteric site is located at transmembrane region (Oosterom et al., 2001; Yang et al., 2003; Yang et al., 1999) (Figure 2). This selective allosteric binding is achieved from less well conserved amino acids among the extracellular regions of the melanocortin receptors while orthosteric binding is shared by MSH and AgRP.
Figure 2.
Schematic representation of antagonist AgRP binding pocket at melanocortin receptor. AgRP utilizes both orthosteric and allosteric sites for selective binding at melanocortin receptor.
Many small synthetic melanocortin MC4 receptor selective agonists have been developed and characterized. These synthetic compounds have different chemical structure from that of MSH but they are selective for different melanocortin receptor subtype. For example, synthetic nonpeptide compound N- (3R)-1 4-tetrahydroisoquinolinium-3-ylcarbonyl - (1R)-1-(4-chlorobenzyl)-2- 4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl) piperidin-1-yl -2-oxoethylamine (THIQ) is a synthetic small compound which is a selective melanocortin MC4 receptor agonist. Many studies indicate that both conserved amino acid residues and non-conserved residues are involved in THIQ binding (Pogozheva et al., 2005; Yang et al., 2009). Mutations of the conserved residues, aspartic acids 122 and 126 in the TM3 of the melanocortin MC4 receptor, reduce the binding affinity and potency of both NDP-MSH and THIQ. However, mutation of non-conserved residues, isoleucine 129 and leucine 125 in the TM3 of the melanocortin MC4 receptor, significantly reduces the potency of THIQ but not on binding affinity or potency of the classic orthosteric agonists, suggesting that these residues are involved in allosteric binding (Haskell-Luevano et al., 2001; Pogozheva et al., 2005; Yang et al., 2000).
5. Agonist-selective signaling of melanocortin receptors: Mechanism and implication
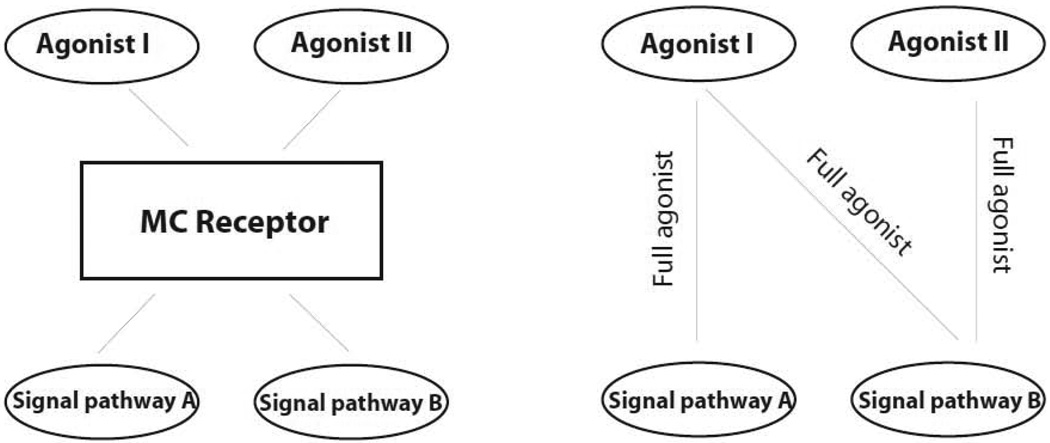
Melanocortin agonists bind to melanocortin receptors with high affinity and shift the receptor to its active conformation and induce a physiological effect. Recent studies indicate that agonists possess different efficacies on different signaling pathways of particular melanocortin receptors (1, 2). A new theory may accommodate these phenomena that melanocortin agonist can induce different conformation changes of one particular melanocortin receptor, which then lead to the activation of different signaling pathways. Activation of different signaling pathways may lead to the different cellular responses in vitro or physiological responses in vivo which were shown in a schematic diagram (Figure 3). Agonist I and agonist II activate the receptor, which transduce the signal to two pathways, pathway A and pathway B. Agonist I is a full agonist on pathway A and pathway B. However, agonist II is a full agonist on pathway B but has no effect on pathway A.
Figure 3.
Schematic representation of melanocortin receptor agonist-selective signaling. Agonist I and II exhibit different efficacies in pathway A and B. Agonist I is a full agonist for pathway A and B. Agonist II is a full agonist for only pathway B.
Many studies indicate that melanocortin MC4 receptor agonist can induce different conformation changes of melanocortin MC4 receptor, which then lead to the activation of different signaling pathways, even when the expression level of receptor and the strength of stimulus-response coupling are the same. For example, the classical signaling pathway for the melanocortin MC4 receptor is to couple to the heterotrimeric stimulatory G protein (Gs) and receptor activation leads to increased camp production, and consequently protein kinase a (PKA) activation. Recently, many studies indicate melanocortin MC4 receptor can couple to all three major classes of G proteins, Gs, Gi/o, and Gq, changing second messengers such as camp and calcium and activating MAPK including ERK1/2 and JNK (Chai et al., 2006a; Chai et al., 2006b; Chai et al., 2009. Functional studies indicate that synthetic melanocortin MC4 receptor agonists differ in their ability to couple the same receptor to different G proteins. The peptide agonists exhibited high intrinsic activity in camp, calcium and receptor internalization, whereas nonpeptide agonists only exhibited high intrinsic activity in the camp signal pathway and impaired ability to mobilize calcium or internalize the receptor, suggesting that these agonists induce different receptor conformational stats. NDP-MSH activates receptor and induces receptor internalization while nonpeptide THIQ activates melanocortin receptor but failed to induce receptor internalization. Further analysis indicates that different region of the melanocortin MC4 receptor is involved in receptor activation and internalization. Deletion of the partial C terminal of the melanocortin MC4 receptor completely abolished agonist induced receptor. Internalization but still maintained agonist mediated receptor activation (Yang et al., 2005).
Molecular analysis of the melanocortin receptors has indicated that different amino acid residues at melanocortin receptors are crucial for agonist or antagonist mediated receptor activation or inhibition. SHU 9119 is a cyclic melanocortin analogue that contains D-Naphthylalanine instead of D-Phenylalanine in the core melanocortin sequence which loss agonist activity at human melanocortin MC3 receptor and human melanocortin MC4 receptor (Yang et al., 2002). Molecular analysis of the melanocortin MC3 receptor and MC4 receptor indicate that leucine 165 at melanocortin MC3 receptor and leucine 133 at melanocortin MC4 receptor are crucial for SHU9119 antagonist activity. Replacement of leucine 165 to methionine 165 and leucine 133 to methionine 133 switch SHU9119 from antagonist to agonist at melanocortin MC3 receptor and melanocortin MC4 receptor (Yang et al., 2002). Evidence indicates that different amino acid residues of the melanocortin receptors are involved in different G protein binding. Mutation of aspartic acid 90 to asparagines 90 at melanocortin MC4 receptor abolishes Gs pathway but remains Gq pathway.
6. Melanocortin receptor modeling using computational methods
To obtain accurate information on the three-dimensional surrounding the binding pocket of melanocortin receptors is a big challenge and the knowledge of the melanocortin receptor’s three-dimensional structure is critical to an understanding of how melanocortin receptors carry out their functions. For GPCR, the three dimensional structure of a receptor is usually obtained from X-ray crystallography studies, but for most GPCRs crystallization is still an unresolved problem. An alternative approach to building a molecular model of a protein is the homology modeling procedure where the target protein is built starting from the experimental known 3-d structure of a related protein. Four GPCR crystal structures have been identified. Their structures reveal two important features. One is structural convergence (the similarities in structure). Another is structural divergence (the difference in structure). It is evident from superimposition of the TM domains that these are very similar. The docked ligands are also similar in occupying much the same space. Thus GPCRs tend to accommodate these small molecules with similar spatial arrangement, but interactions with amino acid chains of ligands are quite different. Many groups have developed 3-dimensional models for human melanocortin MC1 receptor and human melanocortin MC4 receptor (Fleck et al., 2007; Haskell-Luevano et al., 1996; Hogan et al., 2006; Pogozheva et al., 2005; Prusis et al., 1997; Sun and Fry, 2007). We utilized automatic comparison of the melanocortin receptor with already known four GPCRs and developed 3 dimensional models for human melanocortin MC2 receptor, human melanocortin MC3 receptor and human melanocortin MC5 receptor. Our results indicate that these melanocortin receptors are more similar to adenosine receptor than other three GPCR (rhodopsin, β1-adrenergic and β2-adrenergic receptors). We utilized adenosine receptor as template since crystal structure of this receptor has been identified. A three-dimensional molecular model of NDP-MSH interacting with the human melanocortin MC3 receptor and human melanocortin MC5 receptor suggests that NDP-MSH binding sites at human melanocortin MC3 receptor and human melanocortin MC5 receptor are similar to that of human melanocortin MC1 receptor and human melanocortin MC4 receptor while ACTH binding sites at human melanocortin MC2 receptor is different from all other melanocortin receptors. Melanocortin MC2 receptor binding sites includes a series of amino acids in TM domains 2, 3, 5 and 7 which form a broad binding pocket for ACTH.
7. Conclusion
Melanocortin receptor family is of the most important class of GPCR in the genome because of its tremendous molecular diversity and potential targets for therapeutic application. The majority of current ligands affect melanocortin receptor activity by binding to orthosteric site as the endogenous cognate ligand for the receptor. Over the past one decade, novel opportunities for drug discovery have risen from a greater understanding of the complexity of melanocortin receptor signaling. A striking example of this is the appreciation that melanocortin receptors possess functional allosteric binding sites. Allosteric modulator ligands bind receptor domains topographically distinct from the orthosteric site, altering the biological activity of the orthosteric ligand by changing its binding affinity, functional efficacy, or both. This additional allosteric ligands offer the way for not only receptor-selective but also signaling pathway-selective therapies. While still a relatively new concept in melanocortin receptor pharmacology, allosteric ligands provide a remarkable precision in the targeting of drugs to closely related melanocortin receptor subtypes and engendering stimulus-bias in orthosteric ligand signaling, opening up new avenues for not only receptor-selective but also signaling-pathway-selective therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan RA, Oosterom J, Toonen RF, Kraan MV, Burbach JP, Gispen WH. Molecular pharmacology of neural melanocortin receptors. Receptors Channels. 1997;5:215–223. [PubMed] [Google Scholar]
- Adan RA, Szklarczyk AW, Oosterom J, Brakkee JH, Nijenhuis WA, Schaaper WM, Meloen RH, Gispen WH. Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. Eur J Pharmacol. 1999;378:249–258. doi: 10.1016/s0014-2999(99)00465-3. [DOI] [PubMed] [Google Scholar]
- Adan RA, Vink T. Drug target discovery by pharmacogenetics: mutations in the melanocortin system and eating disorders. Eur Neuropsychopharmacol. 2001;11:483–490. doi: 10.1016/s0924-977x(01)00125-0. [DOI] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Ammori JB, Mulholland MW. Melanocortin-3 receptor activates MAP kinase via PI3 kinase. Regul Pept. 2006a doi: 10.1016/j.regpep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006b;27:2846–2857. doi: 10.1016/j.peptides.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides. 2009;30:1098–1104. doi: 10.1016/j.peptides.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000a;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000b;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang W, Tucci F, Tran JA, Fleck BA, Hoare SR, Joppa M, Markison S, Wen J, Sai Y, Johns M, Madan A, Chen T, Chen CW, Marinkovic D, Arellano M, Saunders J, Foster AC. Discovery of 1-[2-[(1S)-(3- dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl] -4-[(2R)-methyl-3-(4- chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J Med Chem. 2007a;50:5249–5252. doi: 10.1021/jm070806a. [DOI] [PubMed] [Google Scholar]
- Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- Chen M, Aprahamian CJ, Kesterson RA, Harmon CM, Yang Y. Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry. 2007b;46:11389–11397. doi: 10.1021/bi700125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in [corrected] [Nle4,D-Phe7]-alpha-melanocyte-stimulating [corrected] hormone binding and signaling. J Biol Chem. 2007c;282:21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317. discussion 318. [PubMed] [Google Scholar]
- Fleck BA, Chen C, Yang W, Huntley R, Markison S, Nickolls SA, Foster AC, Hoare SR. Molecular interactions of nonpeptide agonists and antagonists with the melanocortin-4 receptor. Biochemistry. 2005;44:14494–14508. doi: 10.1021/bi051316s. [DOI] [PubMed] [Google Scholar]
- Fleck BA, Ling N, Chen C. Substituted NDP-MSH peptides paired with mutant melanocortin-4 receptors demonstrate the role of transmembrane 6 in receptor activation. Biochemistry. 2007;46:10473–10483. doi: 10.1021/bi700406k. [DOI] [PubMed] [Google Scholar]
- Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- Gantz I, Konda Y, Yang YK, Miller DE, Dierick HA, Yamada T. Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet Cell Genet. 1996;74:286–290. doi: 10.1159/000134436. [DOI] [PubMed] [Google Scholar]
- Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- Gantz I, Muraoka A, Yang YK, Samuelson LC, Zimmerman EM, Cook H, Yamada T. Cloning and chromosomal localization of a gene (GPR18) encoding a novel seven transmembrane receptor highly expressed in spleen and testis. Genomics. 1997;42:462–466. doi: 10.1006/geno.1997.4752. [DOI] [PubMed] [Google Scholar]
- Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994a;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- Gantz I, Yamada T, Tashiro T, Konda Y, Shimoto Y, Miwa H, Trent JM. Mapping of the gene encoding the melanocortin-1 (alpha-melanocyte stimulating hormone) receptor (MELANOCORIN MC1R) to human chromosome 16q24.3 by Fluorescence in situ hybridization. Genomics. 1994b;19:394–395. doi: 10.1006/geno.1994.1080. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Sawyer TK, Trumpp-Kallmeyer S, Bikker JA, Humblet C, Gantz I, Hruby VJ. Three-dimensional molecular models of the hMELANOCORIN MC1R melanocortin receptor: complexes with melanotropin peptide agonists. Drug Des Discov. 1996;14:197–211. [PubMed] [Google Scholar]
- Hogan K, Peluso S, Gould S, Parsons I, Ryan D, Wu L, Visiers I. Mapping the binding site of melanocortin 4 receptor agonists: a hydrophobic pocket formed by I3.28(125), I3.32(129), and I7.42(291) is critical for receptor activation. J Med Chem. 2006;49:911–922. doi: 10.1021/jm050780s. [DOI] [PubMed] [Google Scholar]
- Holder JR, Haskell-Luevano C. Melanocortin ligands: 30 years of structure-activity relationship (SAR) studies. Med Res Rev. 2004;24:325–356. doi: 10.1002/med.10064. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R444–R451. doi: 10.1152/ajpregu.2001.281.2.R444. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Cismowski MI, Wang X, Wolff M, Conlon PJ, Maki RA. Molecular determinants of melanocortin 4 receptor ligand binding and MELANOCORIN MC4/MELANOCORIN MC3 receptor selectivity. J Pharmacol Exp Ther. 2003;304:1217–1227. doi: 10.1124/jpet.102.044974. [DOI] [PubMed] [Google Scholar]
- Oosterom J, Garner KM, den Dekker WK, Nijenhuis WA, Gispen WH, Burbach JP, Barsh GS, Adan RA. Common requirements for melanocortin-4 receptor selectivity of structurally unrelated melanocortin agonist and endogenous antagonist, Agouti protein. J Biol Chem. 2001;276:931–936. doi: 10.1074/jbc.M007261200. [DOI] [PubMed] [Google Scholar]
- Pogozheva ID, Chai BX, Lomize AL, Fong TM, Weinberg DH, Nargund RP, Mulholland MW, Gantz I, Mosberg HI. Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry. 2005;44:11329–11341. doi: 10.1021/bi0501840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusis P, Schioth HB, Muceniece R, Herzyk P, Afshar M, Hubbard RE, Wikberg JE. Modeling of the three-dimensional structure of the human melanocortin 1 receptor, using an automated method and docking of a rigid cyclic melanocyte-stimulating hormone core peptide. J Mol Graph Model. 1997;15:307–317. doi: 10.1016/s1093-3263(98)00004-7. 334. [DOI] [PubMed] [Google Scholar]
- Sun H, Fry D. Molecular modeling of melanocortin receptors. Curr Top Med Chem. 2007;7:1042–1051. doi: 10.2174/156802607780906573. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cai M, Chen M, Qu H, Melanocorin MCPherson D, Hruby V, Harmon CM. Key amino acid residues in the Melanocortin-4 Receptor for nonpeptide THIQ Specific Binding and Signaling. Regul Pept. 2009 doi: 10.1016/j.regpep.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chen M, Kesterson RA, Jr, Harmon CM. Structural insights into the role of the ACTH receptor cysteine residues on receptor function. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1120–R1126. doi: 10.1152/ajpregu.00240.2007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Lai Y, Gantz I, Georgeson KE, Harmon CM. Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity. J Biol Chem. 2002;277:20328–20335. doi: 10.1074/jbc.M201343200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Lai Y, Gantz I, Yagmurlu A, Georgeson KE, Harmon CM. Molecular determination of agouti-related protein binding to human melanocortin-4 receptor. Mol Pharmacol. 2003;64:94–103. doi: 10.1124/mol.64.1.94. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Loux TJ, Georgeson KE, Harmon CM. Molecular mechanism of the intracellular segments of the melanocortin-4 receptor for NDP-MSH signaling. Biochemistry. 2005;44:6971–6979. doi: 10.1021/bi047521+. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4,D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–23010. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]
- Yang YK, Dickinson CJ, Zeng Q, Li JY, Thompson DA, Gantz I. Contribution of melanocortin receptor exoloops to Agouti-related protein binding. J Biol Chem. 1999;274:14100–14106. doi: 10.1074/jbc.274.20.14100. [DOI] [PubMed] [Google Scholar]
- Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- Yang YK, Harmon CM. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obes Rev. 2003;4:239–248. doi: 10.1046/j.1467-789x.2003.00104.x. [DOI] [PubMed] [Google Scholar]