Abstract
Memory performance can be enhanced by expectations regarding the appearance of ensuing stimuli. Here, we investigated the influence of stimulus-category expectation on memory performance in aging, and used fMRI to explore age-related alterations in associated neural mechanisms. Unlike younger adults, who demonstrated both working memory (WM) and long-term memory (LTM) performance benefits for face stimuli when this stimulus category was expected, older adults did not exhibit these memory benefits. Concordantly, older adults did not exhibit expectation-period activity modulation in visual association cortex (i.e., fusiform face area (FFA)). However, within the older population, individuals who demonstrated face-expectation memory benefits also exhibited expectation-period FFA activity modulation equivalent to younger adults. The older cohort also displayed diminished expectation-related functional connectivity between regions of the prefrontal cortex and the FFA, relative to younger adults, suggesting that network alterations underlie the absence of expectation-mediated cortical modulation and memory benefits. This deficit may have broader consequences for the effective utilization of predictive cues to guide attention and engender optimal cognitive performance in older individuals.
Keywords: Memory, Attention, Expectation, Aging, fMRI, Functional Connectivity
1. Introduction
Expectations of future events allow us to dynamically optimize allocation of our limited cognitive resources. It is well established that attention-directing cues regarding the spatial location, features or object category of ensuing stimuli enable more effective processing of sensory information (Bollinger, Rubens, Zanto, & Gazzaley, 2010; Bressler, Tang, Sylvester, Shulman, & Corbetta, 2008; Capotosto, Babiloni, Romani, & Corbetta, 2009; Chawla, Rees, & Friston, 1999; Corbetta & Shulman, 2002; Desimone & Duncan, 1995; Giesbrecht, Weissman, Woldorff, & Mangun, 2006; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Posner, Snyder, & Davidson, 1980; Ress, Backus, & Heeger, 2000; Schmidt, Vogel, Woodman, & Luck, 2002; Sakai & Passingham, 2003; Serences, Yantis, Culberson, & Awh, 2004). Accordingly, predictive category cueing has been shown to enhance the speed and accuracy by which stimuli are detected and discriminated (Esterman & Yantis, 2009; Puri & Wojciulik, 2008; Puri et al., 2009). Extending these findings into the memory domain, we have recently demonstrated that predictive category cueing can also result in improved working memory (WM) and long-term memory (LTM) performance (Bollinger et al., 2010). Thus, extensive evidence suggests that expectations act as an attentional filter to facilitate the extraction of goal-directed information, resulting in performance benefits across multiple domains.
Expectation-mediated cognitive benefits are associated with neural activity modulation within sensory cortical areas prior to stimulus presentation, a phenomenon known as activity “baseline shifts” (Kastner et al., 1999; Chelazzi, Duncan, Miller, & Desimone, 1998; Luck, Chelazzi, Hillyard, & Desimone, 1997). This process biases selective activation, such that attended stimuli are afforded a processing advantage (Chelazzi et al., 1998; Kastner, et al., 1999; Luck et al., 1997; Stokes, Thompson, Nobre & Duncan, 2009). The prevailing view is that pre-stimulus activity modulation is mediated via top-down control by a fronto-parietal attention network, which includes the intraparietal sulcus (IPS), superior parietal lobule (SPL), dorsal supramarginal gyrus (SMG), frontal eye fields (FEF), middle frontal gyrus (MFG), and inferior frontal junction (IFJ) (Bollinger et al., 2010; Desimone & Duncan, 1995; Corbetta & Shulman, 2002; Kastner et al., 1999; Bressler et al., 2008; Esterman & Yantis, 2009; Ungerleider, Courtney, & Haxby, 1998).
In the current study, we explored the hypothesis that memory deficits in healthy older adults are associated with less effective use of predictive cues to guide optimal cognitive performance. Normal aging has been associated with cognitive decline across a wide variety of domains, including those abilities aided by expectation in younger adults, such as perception, WM, and LTM (Bennett, Golob, & Starr, 2004; Curran, Hills, Patterson, & Strauss, 2001; Friedman; Nielsen-Bohlman & Knight, 1995; Pelosi & Blumhardt, 1999). Moreover, studies directed at exploring the underlying neural basis of cognitive aging have revealed age-associated deficits in top-down modulation of visual cortical activity when stimuli are present, which have been shown to contribute to memory impairment (Gazzaley, Cooney, McEvoy, Knight, & D’Esposito, 2005; Gazzaley et al., 2008). Additionally, alterations in the fronto-parietal attention network have been documented in older adults (O’Sullivan et al., 2001; Andrews-Hanna et al., 2007; Madden et al., 2010; Grady et al., 2009). Thus, an age-related loss in the benefits that expectations have on subsequent memory may result from an inability of older adults to modulate pre-stimulus activity in response to predictive cues, perhaps as a consequence of deficient top-down control networks.
In the current study, we collected fMRI and memory performance data while healthy older participants performed delayed-recognition tasks that differed only in the instructions informing them of the category of stimuli to be remembered (Predictive tasks - participants knew the to-be-remembered stimulus would be a face (“Stimulus-known faces,” SKf trials) or a scene (“Stimulus-known scenes,” SKs trials); Neutral tasks - participants did not know whether the stimulus would be a face or a scene (“Stimulus-unknown,” SUf and SUs trials); Passive baseline tasks - participants passively viewed face and scene stimuli (PVf and PVs trials) (Fig. 1). In a manner analogous to the Posner spatial cueing paradigm (Posner et al., 1980), SKf and SKs conditions served as the valid or ‘predictive’ conditions, while SUf and SUs conditions were considered ‘neutral’, such that participants were expecting a memory task on the forthcoming stimuli, but were not biased towards either of the two categories. No invalid trials were included. This paradigm was recently used to evaluate neural mechanisms of expectation-related influences on memory in a cohort of younger adults (Bollinger et al., 2010). Data from older adults obtained in the current study were contrasted with data from the prior study to assess age-related changes in the impact of predictive cueing on WM and LTM performance, expectation-period activity modulation in visual association cortex, and fronto-parietal control networks assessed via functional connectivity analysis (Rissman, Gazzaley, & D’Esposito, 2004; Gazzaley, Rissman, & D’Esposito, 2004; Clapp, Rubens, & Gazzaley, 2010; Zanto, Rubens, Bollinger, & Gazzaley, 2010; Bollinger et al., 2010).
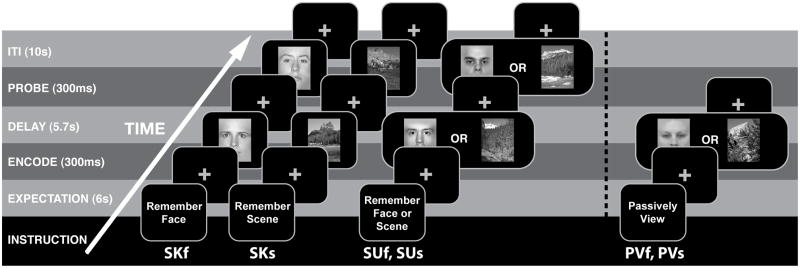
Figure 1. Experimental Paradigm.
All participants performed 4 tasks (Stimulus-Known Faces (SKf trials), Stimulus-Known Scenes (SKs trials), Stimulus Unknown (SUf and SUs trials), and Passive View (PVf and PVs trials)), which were blocked and counterbalanced and stimuli were randomized. For Passive View trials, delay and probe periods were removed (see methods). Note that fixation crosses were green, red, and grey for expectation, delay, and inter-trial interval (ITI) periods, respectively (not shown in figure). SKf, stimulus-known face trials; SKs, stimulus-known scene trials; SUf, stimulus-unknown face trials; SUs, stimulus-unknown scene trials; PVf, passive-view face trials; PVs, passive-view scene trials.
2. Materials and Methods
2.1. Participants
Seventeen healthy older adults (mean age 72.2±1.81 years, range 60–86 years, 7 males) gave written informed consent to participate in this study, which was approved by the University of California, San Francisco Committee for Human Research. All participants had normal or corrected-to-normal vision and were screened to ensure they had no history of neurological, psychiatric or vascular disease, were not depressed, and were not taking any psychotropic medications. All participants had a minimum of 12 years education. Data from a cohort of 18 younger participants (mean age 22.1±3.41 years, range 18–28 years, 8 males) who previously engaged in the same experiment was utilized for age-group comparisons (Bollinger, et al., 2010).
2.2. Neuropsychological Testing
Prior to the experiment, older adults were administered a battery of thirteen neuropsychological tests. Participants were required to score within two standard deviations of published age-matched normative values on these tests to be included in the study. The neuropsychological evaluation consisted of tests designed to assess general intellectual function (Folstein, Folstein, & McHugh, 1975), verbal learning (CVLT-II), geriatric depression (GDS), visual-spatial function (modified Rey-Osterrieth figure), visual-episodic memory (memory for details of a modified Rey-Osterrieth Complex Figure (ROCF) (Rey, 1941; Osterrieth, 1944)), visual-motor sequencing (trail making tests A and B), phonemic fluency (words beginning with the letter ‘D’), semantic fluency (animals), calculation ability (arithmetic), executive functioning (Wechsler, 1981; Kanwisher, McDermott, & Chun, 1997), working memory and incidental recall (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Epstein & Kanwisher, 1998), backward digit span and digit symbol, and WAIS-R (Kanwisher et al., 1997). Group scores for these tests are presented in Table 1.
Table 1. Participant Demographics.
Values represent group mean values. Standard deviations are in parentheses.
| Younger (SD) | Older (SD) | |
|---|---|---|
| N | 18 | 17 |
| Mean Age (year) | 22.1 (3.4) | 72.2 (1.8) |
| Age Range | 18–28 | 60–86 |
| Percent Male | 44.4 | 41.2 |
| Education (years) | 12+ | 12+ |
| MMSE | n/a | 29.5 (0.2) |
| GDS | n/a | 2.5 (0.4) |
| Executive Composite | ||
| WAIS-R Digit Span (backward) | 5.5 (0.3) | |
| Trailmaking Test A (s) | 34.5 (11.4) | |
| Semantic Fluency Test | 22.6 (1.7) | |
| Phonemic Fluency Test | 15.7 (1.6) | |
| Calculation Ability (out of 5) | 4.6 (0.1) | |
| Stroop: color-word naming | 57.2 (4.3) | |
| Memory Composite | ||
| CVLT: Trial 5 Recall | 12.9 (0.6) | |
| CVLT: short delay free recall | 11.1 (0.7) | |
| CVLT: short delay cued recall | 13.2 (0.4) | |
| CVLT: long delay free recall | 12.3 (0.7) | |
| CVLT: long delay cued recall | 12.8 (0.6) | |
| Memory for Modified Rey | 13.0 (0.6)* | |
| Processing Speed Component | ||
| Trail Making Test B (s) | 68.4 (19.1) | |
| WAIS Digit Symbol Test | 55.1 (3.1) | |
| Stroop: color naming | 87.1 (3.6) | |
MMSE = Mini-Mental State Examination (Folstein et al, 1975)
GDS = Geriatric Depression Scale
WAIS = Wechsler Adult Intelligence Scale
Information from 16 participants
2.3. Task Design
Stimuli consisted of grayscale images of faces and natural scenes presented on a black background (Fig. 1). The face stimuli consisted of a variety of neutral-expression male and female faces from a wide age range. Hair and ears were digitally removed from the images and a blur was applied along the contours of the face in order to remove any non-face specific features. Each stimulus was used in only one trial per experimental session. Individual face and scene stimuli were randomized to different conditions across participants to ensure that potentially distinctive stimuli did not confound a particular condition. Images were 225 pixels wide and 300 pixels tall (14 X 18 cm), subtended 3 degrees of visual angle from fixation, and were presented foveally.
The experiment utilized three delayed-recognition conditions, Stimulus-Known Faces (SKf trials, predictive), Stimulus-Known Scenes (SKs trials, predictive), and Stimulus-Unknown (SUf and SUs trials, neutral). In addition, a Passive View (PVf and PVs trials, passive) task was used as a baseline. Participants were given detailed instructions and underwent several practice trials immediately prior to the scanning session.
At the initiation of each WM block, participants were presented with the predictive instruction of “Remember the Face” or “Remember the Scene” (SKf or SKs) (i.e., faces or scenes appear as the target stimulus 100% of the time for each task, respectively) or the neutral instruction of “Remember the Face or the Scene” (SU) (i.e., faces or scenes were equally likely to appear as the target stimulus). Thus, for the predictive conditions, the relevant stimulus appeared in 100% of trials, while for the neutral conditions each stimulus category appeared in 50% of trials. A 6-second expectation-period was signaled by a grey-to-green color change of a fixation cross on each trial. This was followed by a brief 300ms target stimulus and a subsequent 5.7-second delay period during which a red fixation cross was presented. Target stimuli were brief to encourage expectation of the stimulus by the participant. The trial concluded with a probe stimulus that was always consistent in stimulus category with the target stimulus. Participants were instructed to indicate whether or not the probe was exactly the same stimulus as the target by responding with a button press (right for match, left for non-match) as quickly as possible without sacrificing accuracy. Equal numbers of match and non-match trials were presented. The probe stimulus was followed by a 9.7-second inter-trial interval during which a grey fixation cross was present. Instructions only appeared for the first trial of each block, after which a new trial onset was cued by grey-to-green color change of the fixation cross, as described. Switching is often challenging for older adults and when it is not controlled in the experimental design it results in ambiguity when interpreting age-related changes as being due the manipulation being tested or the result of task-switching deficits. To minimize this confound, we used a mixed, event-related, block design, such that participants are instructed at the start of the block and are not cued as to the task goals on each trial.
WM performance data statistically matched a unimodal Gaussian distribution for younger (p = 0.04, Jarque-Bera test) but not older adults (p = 0.19, Jarque-Bera test). LTM performance data did not statistically match a unimodal Gaussian distribution for younger (p = 0.5, Jarque-Bera test) or older adults (p = 0.23, Jarque-Bera test). Performance data appeared linear and not bimodal for both younger and older groups.
Trials with shorter expectation-periods were inserted at random in the WM blocks to encourage stimulus expectation throughout the period (not in the Passive view blocks). Four of these trials were included in each WM block, two trials with 2-second expectation periods and two trials with 4-second expectation periods, all of which were excluded from the final analysis in order to hold constant the temporal separation of expectation and encode regressors in the general linear model (GLM, see Data Acquisition and Analysis). Note that although trials with shorter expectation-periods were modeled, they were removed from further analysis, yielding 15 trials per WM block, which is balanced with the number of trials in the Passive View condition. Thus, for each object category (faces and scenes), equal numbers of predictive, neutral, and passive view trials with full-length expectation periods were presented in the experiment. For the passive view trials, delay and probe periods were removed in order to ensure that all target stimuli (predictive, neutral, and passive view) were preceded by an equivalent period of fixation. Trials were presented in a pseudo-randomized block design (10 blocks total; 2 SKf, 2 SKs, 2 PV (PVf and PVs) and 4 SU (2 SUf and 2 SUs)) with 19 trials per WM block (15 of which were included in the final analysis) and 15 trials per passive-view block. During all delayed-recognition task delay periods, participants were explicitly instructed to “maintain a mental image” of the memoranda, and to avoid mnemonic strategies. In post-experiment questionnaires, all participants reported using mental imagery during delay periods as well as being awake and alert during the experiment, and that experimental instructions were clear and remembered.
A surprise post-experiment recognition test was given approximately 30 minutes after the main experiment to assess incidental LTM. Participants were presented images of faces and scenes from each condition in the experiment. Stimuli were presented at a self-paced rate. 60% of tested stimuli were the memoranda from non-match WM trials and passive-view trials and thus were viewed only once prior to the post-experiment test. As lures, the remaining 40% of stimuli were novel face or scene images. Participants were instructed to respond with a confidence score using a four-point Likert scale for each stimulus (4 = confident the stimulus did appear in the experiment, 3 = less confident the stimulus did appear in the experiment, 2 = less confident the stimulus did not appear in the experiment, 1 = confident the stimulus did not appear in the experiment). In order to normalize confidence scores to each participant’s response bias, indices for each condition were calculated as the confidence score for stimuli of a particular condition minus the confidence score for novel images. Older adults performed significantly better than chance for stimuli from all conditions (SKf: p < 0.0005; SUf: p < 0.0005; PVf: p < 0.0005; SKs: p < 0.0005; SUs: p < 0.0005; PVs: p < 0.01).
2.4. Region-of-Interest Localization
An independent functional localizer task was used to identify the face-selective fusiform face area (FFA) (Allison et al., 1994; Puce, Allison, Gore & McCarthy, 1995; Kanwisher et al., 1997) and the scene-selective parahippocampal place area (PPA) (Epstein & Kanwisher, 1998) in the visual association cortex of each participant. Participants performed 10 blocks of a 1-back task. Each block was 16-seconds in length and included face stimuli, scene stimuli, or fixation (rest). Blocked face and scene stimuli regressors were used to generate SPM[T] images, from which regions-of-interest (ROIs) were identified. For the contrast of faces > scenes, a face-selective ROI, the right FFA, was identified as the cluster of 35 contiguous voxels with the highest t value within the right fusiform gyrus of each participant (MNI-coordinate range for normalized right FFA ROIs: 37 to 47mm, −76 to −38mm, −26 to −4mm; mean t value (SD): 4.37 ±1.86). The right FFA has been shown to be more strongly activated by faces (Cox, 1996; Gazzaley, Cooney, Rissman, & D’Esposito, 2005). For the contrast of scenes > faces, a scene-selective ROI, the left PPA, was also identified as the cluster of 35 contiguous voxels with the highest t value within the left parahippocampal gyrus of each participant (MNI-coordinate range for normalized left PPA ROIs: −37 to −23mm, −65 to −27mm, −20 to −4mm; mean t value (SD): 5.64 ±1.22). The left PPA has been shown to be more selective for scenes (Kanwisher et al., 1997) and the strongest region of attentional modulation for scenes (Gazzaley et al., 2005). The ROI voxel extent was based on methodology from similar studies (Rissman et al., 2004; Gazzaley et al., 2004; Clapp et al., 2010) and was used in order to achieve a reasonable balance between regional specificity (diminished by the use of a larger cluster) and susceptibility to noise (a problem with smaller clusters). Fronto-parietal ROIs were identified with the contrast of 1-back > rest as regions that survived a single-voxel statistical threshold of p < 0.001 with a 75-voxel cluster-extent threshold to correct for multiple comparisons at p < 0.05 (Table 3).
Table 3. Univariate Contrasts.
Cluster Table: Univariate Contrasts for the older group. SKf > SUf, SUf > SKf, 1-back > rest (functional localizer, see methods). SKf, stimulus-known face trials; SUf, stimulus-unknown face trials.
| Brain region | BA | # voxels | Mean t val | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| OLDER: SKf > SUf | ||||||
| L Middle Frontal Gyrus | 9/46 | 107 | 3.363 | −29 | 25 | 43 |
| R Middle Frontal Gyrus | 9 | 38 | 2.989 | 24 | 36 | 48 |
| L Supramarginal Gyrus | 40 | 66 | 3.050 | −58 | −40 | 48 |
| R Supramarginal Gyrus | 40 | 67 | 2.959 | 57 | −25 | 45 |
| L Intraparietal Sulcus | 40 | 71 | 2.992 | −49 | −40 | 57 |
| R Intraparietal Sulcus | 40 | 41 | 2.967 | 44 | −52 | 46 |
| R Precentral Gyrus | 6 | 37 | 2.971 | 20 | −13 | 66 |
| L Precentral Gyrus | 19 | 205 | 3.045 | −31 | −51 | 7 |
| Anterior Cingulate Cortex | 8 | 62 | 3.096 | −2 | 45 | 50 |
| R Hippocampus | -- | 43 | 3.587 | 27 | −7 | −21 |
| R Thalamus | -- | 43 | 3.024 | 14 | −22 | 18 |
| L Thalamus | -- | 75 | 3.328 | −15 | −16 | 19 |
| OLDER: SUf > SKf | ||||||
| L Lateral Occipital Cortex | 18/19 | 541 | 3.350 | −28 | −93 | 3 |
| L Precentral Gyrus | 6 | 39 | 3.067 | −48 | −2 | 54 |
| R Precentral Gyrus | 18/19 | 625 | 3.328 | 30 | −87 | 1 |
| R Fusiform Gyrus | 37 | 58 | 3.071 | 39 | −55 | −13 |
| R Middle Temporal Gyrus | 37 | 41 | 3.052 | 42 | −52 | 10 |
| OLDER: 1-back > rest | ||||||
| L Inferior Frontal Junction | 6 | 124 | 4.762 | −46 | 5 | 34 |
| R Inferior Frontal Junction | 6 | 312 | 4.884 | 46 | 9 | 32 |
| L Intraparietal Sulcus | 7 | 445 | 4.916 | −58 | −40 | 48 |
| R Intraparietal Sulcus | 7 | 175 | 4.950 | 26 | −54 | 55 |
| L Precentral Gyrus | 6 | 269 | 4.729 | −35 | −4 | 55 |
| R Precentral Gyrus | 6 | 154 | 4.731 | 29 | −1 | 56 |
| L Lingual Gyrus | 19 | 247 | 4.676 | 19 | −58 | 7 |
| Anterior Cingulate Cortex | 32 | 494 | 4.683 | −1 | 13 | 53 |
| R Insula | 47 | 173 | 4.560 | 32 | 26 | 0 |
| Fusiform/Calcarine | 18/19/20 | 7086 | 5.479 | 0 | −76 | 5 |
L. Left: R. Right
2.5. Data Acquisition and Analysis
All images were acquired on a Siemens 3T Magnetom Trio with stimuli presented on an LCD monitor positioned behind the head of participants and viewed using a mirror rigidly attached to a 12-channel receive-only head coil. Echo planar imaging (EPI) data were acquired (FA = 80°, TE = 30 ms, TR = 2000 ms) as twenty-nine interleaved 3.0 mm axial T2*-weighted slices (0.5 mm inter-slice gap) with 1.8 × 1.8 × 3.0 mm sized voxels (FOV = 230 mm; 128 × 128 matrix). In addition, high-resolution (T1-MPRAGE) anatomical volumes were acquired (1 × 1 × 1 mm voxel size; FOV = 160 × 240 × 256 mm, TR = 2300 ms, TE = 2.98 ms, FA = 9°). Raw blood oxygen level dependant (BOLD) images were corrected offline for slice-timing acquisition and motion artifacts. A 5 mm isotropic Gaussian smoothing kernel was applied prior to modeling the data. All trial stages were modeled as events convolved with the canonical synthetic hemodynamic response function HRF (SPM5, Wellcome Department of Imaging Neuroscience, London, England) and inserted in the GLM. The onset of the expectation regressor was time-locked with the grey-to-green fixation-cross color change, the onset of the encode regressor was time-locked with target-stimulus onset, and the onset of the probe regressor was time-locked with probe-stimulus onset. In addition, three translational (X, Y, Z) and three rotational (pitch, roll, yaw) motion parameters were included in the GLM. The resulting parameter estimates yielded scalar beta weights corresponding to the relative changes in signal strength associated with each trial stage. Incorrect trials were modeled with a separate regressor and excluded from the final analysis. Group whole-brain maps were calculated from Montreal Neurological Institute (MNI) normalized data and all figures appear in neurological convention. For all analyses, a single-voxel statistical threshold of p < 0.01 was used. Where applicable, a Monte Carlo simulation was employed utilizing the AlphaSim function in the AFNI toolbox (Cox, 1996) which prescribed a cluster extent of 35 nearest-neighbor voxels, in addition to the single-voxel threshold of p < 0.01, to achieve a statistic corrected for multiple comparisons of p < 0.05. All values are presented as the mean ± SEM.
A primary aim of this study was to examine age-related alterations in top-down control networks and associated expectation-driven baseline shifts, which are largest in magnitude under predictive conditions in younger adults (Bollinger et al., 2010). For this reason, we used SU, the neutral condition, as the main baseline and contrasted data from these trials against SKf, the predictive condition. This analysis duplicated the approach previously utilized in younger adults (Bollinger et al., 2010).
2.6. Functional Connectivity
Functional connectivity network maps were created for each participant as described previously using a beta series connectivity analysis approach (Rissman et al., 2004; Gazzaley et al., 2004) For this analysis, a new GLM design matrix was constructed to model each trial stage (expectation, encode, probe) with a unique covariate, resulting in 516 covariates of interest ((19 trials X 8 WM blocks X 3 covariates per WM trial) + (15 trials X 2 PV blocks X 2 covariates per PV trial)). Note that although trials with shorter expectation-periods were modeled, they were removed from further analysis, yielding 15 trials per WM block. Beta values averaged across each ROI (FFA and PPA) were then correlated across trials with every brain voxel resulting in condition-specific correlation maps. Although multiple trial stages were modeled, only the expectation-period was subject to analysis. Single-participant maps were subsequently normalized to the MNI template (2 × 2 × 2 mm voxel size) and Gaussian smoothed (5 mm FWHM) for group analysis. Group-beta series connectivity main effect analysis derived t-maps for each condition. Regions that survived Bonferroni correction for all non-cerebellar brain voxels (~1.6 X 105; t > 7.69; Table 3), in a manner identical to our recent report (Bollinger et al., 2010), were reported. Nonparametric permutation tests were used to calculate whole-brain contrast maps between conditions (Nichols & Holmes, 2002) (Fig. 5). Functional-connectivity maps were corrected for multiple comparisons in a manner identical to univariate maps.
To examine age-related changes in expectation networks via a contrast of age-group data, an established method (Buckner et al., 2004) was used to derive a hybrid template from 43 younger and 43 older participants’ anatomical data collected by our group. Older and younger adult data (Bollinger et al., 2010) were normalized to this template and then contrasted using a non-parametric analysis permutation method identical to that used for the within-group comparisons (Nichols & Holmes, 2002). This approach minimizes the bias resulting from fitting older adult data, which reflects functional and anatomical changes that occur during normal aging, to a canonical template derived from the anatomy of younger adults. Instead, this method controls for age-related anatomical changes and allows for a valid, direct evaluation of functional changes across disparate aged cohorts.
2.7. Regression Analyses
To further explore the potential sources of expectation-period FFA activity modulation, functional connectivity measures were extracted from significant fronto-parietal ROIs derived from the SKf main effects analysis, as well as ROIs obtained from the independent functional localizer. Connectivity values from each ROI were regressed against expectation-period FFA activity modulation measures for each condition across-participants, as well as indices for differences between conditions. Resultant Pearson’s r coefficients and corresponding p values for regions that survived FDR correction for multiple comparisons (i.e., correction for the number of fronto-parietal ROIs for which Pearson’s r coefficients were calculated) are reported. In addition, across-participant regression analyses were performed between connectivity indices (SKf-SUf) from each ROI and memory performance indices (SKf-SUf) for both WM recognition accuracy and LTM recognition scores. Resultant Pearson’s r coefficients and corresponding p values for regions that survived FDR correction for multiple comparisons (i.e., correction for the number of fronto-parietal ROIs for which Pearson’s r coefficients were calculated) are reported.
3. Results
3.1. Working Memory and Long-Term Memory Performance – Older vs. Younger Adults
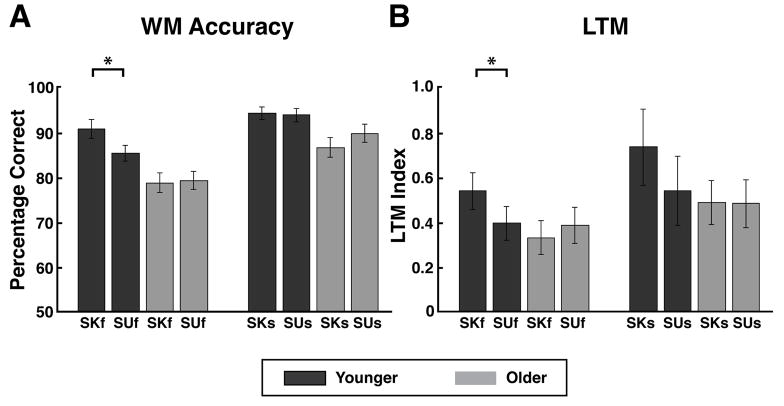
Previous results revealed that younger adults who performed the identical experiment exhibited significant WM and LTM performance benefits of predictive cuing for face stimuli, but not scene stimuli (Bollinger, et al., 2010) (Fig. 2A, B; Table 2A, B). To examine age-related changes in memory performance benefits engendered by predictive cueing, 2 × 2 × 2 ANOVAs with factors of age (older, younger), cue (predictive, neutral), and stimulus category (face, scene) were conducted for WM accuracy and LTM recognition scores (calculated using the 4-point Likert confidence score for stimuli of a particular condition minus the confidence score for novel images).
Figure 2. Behavioral Performance. Younger vs. Older Adults.
(A) WM Accuracy. Compared to neutrally cued stimuli (SUf & SUs), younger adults were significantly more accurate for predictively cued faces (SKf) (*p < 0.05) but not scenes (SKs) (p > 0.05). Older adults performed equivalently for predictively and neutrally cued faces (p > 0.05), as well as predictively and neutrally cued scene stimuli (p > 0.05).
(B) LTM Performance. Compared to neutrally cued stimuli, younger adults remembered predictively cued faces better (*p < 0.05) but not scenes (p > 0.05). Older adults equivalently remembered predictively and neutrally cued faces (p > 0.05) and scene stimuli (p > 0.05).
SKf, stimulus-known face trials; SKs, stimulus-known scene trials; SUf, stimulus-unknown face trials; SUs, stimulus-unknown scene trials; WM, working memory; LTM, long-term memory.
Table 2A. Behavioral Results.
(A) Behavioral results: Older and younger group mean behavioral performance measures for WM accuracy and LTM performance in each task. Standard deviations are in parentheses.
| Younger | Older | |||
|---|---|---|---|---|
| WM | LTM | WM | LTM | |
| Total | 91.1% (1.4) | 0.36 (0.055) | 83.6% (1.6) | 0.41 (0.066) |
| SKf | 90.8% (2.1) | 0.54 (0.083) | 78.8% (2.3) | 0.33 (0.076) |
| SUf | 85.4% (1.7) | 0.40 (0.075) | 79.3% (2.1) | 0.39 (0.080) |
| PVf | -- | 0.40 (0.076) | -- | 0.36 (0.080) |
| Faces | 88.1% (1.7) | 0.47 (0.074) | 79.0% (1.9) | 0.36 (0.070) |
| PCIFaces | 5.4% (1.8) | 0.14 (0.057) | −0.5% (2.2) | −0.056 (0.068) |
| SKs | 94.3% (1.4) | 0.74 (0.160) | 86.7% (2.1) | 0.49 (0.097) |
| SUs | 93.9% (1.5) | 0.54 (0.083) | 89.8% (2.1) | 0.49 (0.107) |
| PVs | -- | −0.092 (0.105) | -- | 0.41 (0.131) |
| Scenes | 94.1% (1.3) | 0.64 (0.124) | 88.2% (1.9) | 0.49 (0.097) |
| PCIScenes | 0.4% (1.3) | 0.20 (0.09) | −3.1% (1.7) | 0.0033 (0.063) |
Table 2B. Neural Results: Expectation period.
(B) Neural results: Expectation period. Older and younger group mean expectation-related FFA and PPA activity measures. Standard deviations are in parentheses.
| FFA | PPA | ||||
|---|---|---|---|---|---|
| Younger | Older | Younger | Older | ||
| SKf | 3.36 (0.69) | 1.17(0.82) | SKs | 0.93 (0.57) | 1.31 (0.49) |
| SUf | 1.87 (0.71) | 2.11 (0.65) | SUs | 1.39 (0.55) | 2.22 (0.57) |
| PVf | 1.24 (0.45) | 2.46(1.13) | PVs | 1.33 (0.36) | 2.08 (0.42) |
| SKs | 1.07 (0.66) | 0.45 (0.97) | SKf | 0.83 (0.55) | 1.89 (0.53) |
| PCI | 1.49 (0.68) | −0.94 (0.82) | PCI | −0.44 (0.54) | −0.91 (0.62) |
For WM accuracy, the ANOVA revealed main effects of age (F(1,33) = 12.58, p < 0.005) and stimulus category (F(1,33) = 39.73, p < 0.0001). While a three-way interaction was not observed (F(1,33) = 0.42, p > 0.4), two-way interactions were significant for cue x stimulus category (F(1,33) = 4.67, p < 0.05) and age x cue (F(1, 33) = 7.22, p < 0.05). For LTM recognition, the ANOVA revealed main effects of age (F(1,33) = 5.92, p < 0.05) and cue (F(1,33) = 4.23, p < 0.05). While a three-way interaction was not observed (F(1,33) = 0.001, p > 0.9), a two-way interaction was significant for age x cue (F(1, 33) = 7.25, p < 0.05). To evaluate the age x cue interactions for both WM and LTM, post-hoc t-tests were performed and revealed that for faces, while younger adults performed significantly better for SKf compared to SUf in terms of both WM and LTM (WM: t(17) = 3.01, p < 0.01; LTM: t(17) = 2.50, p < 0.05), older adults did not perform differently on these two conditions using either memory measure (WM: t(16) = 0.200, p > 0.8; LTM: t(16) = 0.817, p > 0.4) (Fig. 2A & B; Table 2A). This was not observed for scenes, as SKs was not significantly different than SUs for either age group for either WM or LTM (Younger –WM: t(17) = 0.329, p > 0.7, LTM: t(17) = 2.10, p > 0.05; Older – WM: t(16) = 0.200, p > 0.8, LTM: t(16) = 0.052, p > 0.9).
In summary, the current results revealed that while younger adults experienced WM and LTM benefits by predictive expectations of face stimuli (Bollinger et al., 2010), older adults displayed neither a WM nor LTM benefit from predictive cueing, and a significant age-related decrease in expectation benefits.
3.2. fMRI data
3.2.1. Expectation-Period Univariate Activity in Visual Cortical Areas – Older vs. Younger Adults
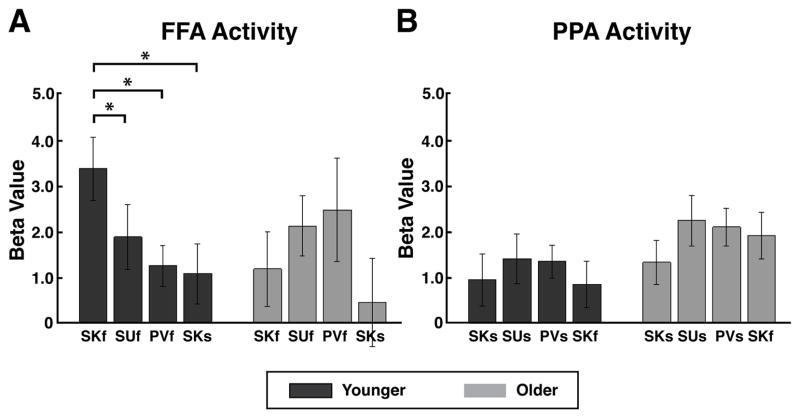
Expectation-driven neural activity modulation in stimulus-selective visual regions has recently been observed in response to category cueing in perceptual and WM tasks in younger adults (Puri et al., 2009; Bollinger et al., 2010). We hypothesized that age-related deficits in this neural biasing might underlie the lack of predictive cueing benefits on memory performance in older adults. To evaluate this, we examined expectation-period univariate activity in stimulus-selective visual cortical regions (i.e., fusiform face area (FFA) and parahippocampal place area (PPA)) for each older participant. Older and younger group mean beta values for FFA and PPA during expectation-periods for each task are presented in Table 2B.
For expectation-period FFA activity, a 2 × 3 × 2 ANOVA with factors of age (younger, older), cue (predictive, neutral, passive) and stimulus category (faces, scenes) did not reveal an effect of age (F(1, 33) = 0.04, p > 0.8), cue (F(2,66) = 1.70, p > 0.18), or stimulus category (F(1, 33) = 1.78, p > 0.19). Two-way interactions were significant for age x cue (F(2, 66) = 3.78, p < 0.05) and cue x stimulus category (F(2, 66) = 4.13, p < 0.05). To evaluate the age x cue interaction, post-hoc within-group t-tests revealed that while younger adults displayed significantly increased expectation-period FFA activity for the SKf condition compared to SUf (t(17) = 2.49, p < 0.05), PVf (t(17) = 3.13, p < 0.001), and SKs (t(17) = 2.73, p < 0.05), older adults displayed expectation-period FFA activity for SKf that was equivalent to SUf (t(16) = 1.14, p > 0.2), PVf (t(16) = 1.17, p > 0.2), and SKs (t(16) = 0.61, p > 0. 5) (Fig. 3A, Table 2B). Across-group analysis focused on difference scores, to avoid direct comparisons of BOLD signal across age-groups, which minimizes confounds due to age-related vascular changes (Gazzaley et al., 2005; D’Esposito, Deouell, & Gazzaley, 2003). Expectation-period FFA activity modulation driven by predictive cues (SKf-SUf) was significantly decreased in older adults compared to younger adults (t(33) = 2.29, p < 0.05). For expectation-period PPA activity, a 2 × 3 × 2 ANOVA with factors of age (younger, older), cue (predictive, neutral, passive) and stimulus category (faces, scenes) revealed no significant main effects or interactions (p values > 0.05) (Fig. 3B, Table 2B).
Figure 3. Expectation-period FFA/PPA Activity: Younger vs. Older Adults.
(A) FFA Activity. In younger adults, expectation-related FFA activity (i.e., baseline shift) was greater for predictively (SKf) than for neutrally cued face trials (SUf) (*p < 0.05), while in older adults expectation-related FFA activity was equivalent for both predictively and neutrally cued face trials (p > 0.05).
(B) PPA Activity. In younger and older adults, PPA activity was equivalent for both predictively (SKs) and neutrally (SUs) cued scene stimuli (p values > 0.05).
FFA, fusiform face area; PPA, parahippocampal place area; SKf, stimulus-known face trials; SKs, stimulus-known scene trials; SUf, stimulus-unknown face trials; SUs, stimulus-unknown scene trials.
In order to evaluate if age-related differences in representational specificity of visual cortical areas (Park, Polk, Park, Minear, Savage & Smith, 2004) contribute to the current findings, univariate data from the FFA during the encoding period was collapsed across conditions for each stimulus category and compared between groups. A 2 × 2 ANOVA with factors of age (younger, older), and stimulus category (faces, scenes) revealed an effect of stimulus category (F(1, 33) = 107.26, p < 0.00001), but not age (F(1, 33) = 0.03, p > 0.8) or an interaction (F(1, 33) = 1.36, p > 0.25). Post-hoc analysis revealed increased FFA responsivity for faces compared with scenes for each group (Younger: t(17) = 8.27, p < 0.00001; Older: t(16) = 6.35, p < 0.00001), but no differences between groups for face (t(33) = 0.29, p > 0.7) or scene (t(33) = 0.99, p > 0.3) stimuli (Table 2C). Thus, no age-related differences in FFA representational specificity were observed.
Table 2C. Neural Results: Encode period.
(C) Neural results: Encode period. Older and younger group mean FFA and PPA activity measures from the encode period. Standard deviations are in parentheses. WM, working memory; LTM, long term memory; FFA, fusiform face area; PPA, parahippocampal place area.
| FFA | PPA | ||||
|---|---|---|---|---|---|
| Younger | Older | Younger | Older | ||
| Faces | 9.63 (1.24) | 9.11(1.37) | Faces | 0.01 (0.57) | 1.37 (0.50) |
| Scenes | 2.23 (0.67) | 3.21(0.71) | Scenes | 6.05(0.46) | 5.54 (0.71) |
In summary, the current results revealed that while younger adults display face-expectation associated FFA activity increases (Bollinger et al., 2010), older adults did not display FFA modulation from predictive cueing for face stimuli. In addition, older adults displayed a significant age-related decrease in face-expectation associated FFA activity modulation, revealing a deficit in expectation-mediated neural biasing. The absence of significant expectation-period activity modulation in older adults in the FFA is consistent with the lack of benefits from predictive information on face WM and LTM performance, both of which occur in younger adults (i.e., baseline shifts and memory benefits).
3.2.2. Subgroups of Older Adults vs. Younger Adults
As a population, the older age group exhibited significant decreases in the benefit of predictive cues on WM and LTM performance for faces (Table 2A), as well as on FFA expectation-period baseline shifts (Table 2B), compared to young adults. To assess if a relationship existed between these behavioral and neural expectation-related effects, across-participant regression analyses were performed, but these did not reveal significant correlations (p values > 0.2). In another evaluation of neuro-behavioral relationships, the older-adult group was split into performance subgroups in a similar manner as a previous report (Gazzaley et al., 2005). A predictive cue index (PCI) assessed as the difference between the predictive-cue condition and the neutral-cue condition (i.e., SK-SU) indexes the memory-performance benefits obtained by object-category foreknowledge. For both PCIWM and PCILTM, the subgroup of six older participants showing the smallest memory benefits of predictive cueing demonstrated a significantly reduced PCIFFA compared to the younger cohort (WM: t(22) = 2.40, p < 0.05; LTM: t(22) = 3.53, p < 0.005), whereas the subgroup of six older participants with the greatest benefits (and preserved predictive-cue associated memory benefits relative to younger adults (Z > −1)) did not show a reduced PCIFFA (WM: t(22) = 0.30, p > 0.7; LTM: t(22) = 0.79, p > 0.4). These results indicate that neural differences in pre-stimulus modulation observed at the population level were driven by older adults that did not experience memory benefits of cueing, thus establishing a relationship between age-related deficits in expectation-period activity modulation and memory performance.
3.2.3. Whole-brain Univariate Analysis
A central aim of the current study was to examine age-related alterations in the neural mechanisms of top-down control mediating expectation-driven baseline shifts and subsequent memory benefits. Therefore the remainder of the neural analyses focused on face-present trials only, where these effects were observed in younger adults (Bollinger et al., 2010). Furthermore, given our goal of exploring the processes that bias sensory processing during specific expectation (i.e., an ensuing stimulus category is predicted with 100% certainty: SKf condition), SUf serves as a “non-specific” (neutral) expectation condition in the comparison. Examination of whole-brain univariate data using the main contrast of interest, SKf > SUf, revealed significant activation in fronto-parietal regions in older adults, comparable to those previously reported in younger adults during expectation-periods for perceptual tasks (Corbetta & Shulman, 2002; Esterman & Yantis, 2009; Puri et al., 2009), and in the WM tasks used in the current study (Bollinger et al., 2010). This included the right IPS, bilateral MFG, right precentral gyrus, and right dorsal SMG (Table 3). These results revealed that frontal and parietal regions were active during the expectation-period in both younger and older adults.
3.2.4. Functional Connectivity Results: Expectation Networks
It has been proposed that frontal and parietal regions comprise a fronto-parietal network that generate top-down signals to bias processing of expected stimuli in sensory cortices (Kastner, et al., 1999; Shulman, et al., 1999; Hopfinger, Buonocore, & Mangun, 2000; Corbetta & Shulman, 2002; Serences, et al., 2004; Bressler, et al., 2008; Esterman & Yantis, 2009; Summerfield & Egner, 2009; Sakai & Passingham, 2003). Univariate findings, including those presented here, report coincident expectation-driven fronto-parietal activity and visual cortical activity modulation to support this claim, although this evidence is indirect. The beta-series correlation method is a functional connectivity analysis approach that utilizes trial-by-trial variability to measure covariance in activity between spatially disparate regions, and thus offers a more powerful tool for assessing network interactions (Rissman et al., 2004; Gazzaley et al., 2004; Gazzaley et al., 2007; Bollinger et al., 2010; Clapp et al., 2010; Wais, Rubens, Boccanfuso & Gazzaley, 2010; Zanto et al., 2010). To evaluate functional connectivity in older adults, connectivity maps using the FFA as a seed-region were calculated for the expectation-period of the SKf condition. This revealed a set of fronto-parietal network regions: bilateral MFG, right IFJ, bilateral IPS, and precuneus. In order to examine predictive-cue specificity of FFA-functional connectivity maps, a non-parametric analysis (Nichols & Holmes, 2002) was used to contrast FFA-connectivity maps during the expectation-periods, SKf > SUf. This contrast revealed significant connectivity with bilateral occipital cortices and left hippocampus, but not with fronto-parietal regions (Table 4).
Table 4. Functional Connectivity Contrasts (FFA).
Cluster Table: FFA-connectivity Contrasts.
SKf > SUf (older group), SUf > SKf (older group), SKf > SUf (younger>older group), SKf > SUf (older>younger group). SKf, stimulus-known face trials; SUf, stimulus-unknown face trials.
| Brain region | BA | # voxels | Mean p val | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| OLDER: SKf >SUf | ||||||
| L Occipital Cortex | 17 | 65 | 0.005 | −11 | −100 | −3 |
| R Occipital Cortex | 17 | 134 | 0.004 | 14 | −100 | 6 |
| L Middle Temporal Gyrus | 21 | 41 | 0.005 | −52 | −33 | −2 |
| L Hippocampus | -- | 95 | 0.006 | −34 | −32 | 8 |
| OLDER: SUf > SKf | ||||||
| L Nucleus Accumbens | -- | 41 | 0.007 | −14 | 12 | −2 |
| YOUNGER > OLDER: SKf > SUf | ||||||
| L Middle Frontal Gyrus | 46 | 73 | 0.007 | −32 | 30 | 27 |
| R Middle Frontal Gyrus | 46 | 430 | 0.006 | 31 | 14 | 20 |
| R Inferior Frontal Junction | 6 | 321 | 0.004 | 29 | 6 | 42 |
| L Inferior Frontal Gyrus | 11 | 67 | 0.007 | −32 | 55 | −4 |
| R Supramarginal Gyrus | 40 | 40 | 0.005 | 42 | −45 | 29 |
| R Precentral Gyrus | 4 | 42 | 0.006 | 46 | −10 | 28 |
| R Middle Temporal Gyrus | 21 | 55 | 0.004 | 58 | −40 | 1 |
| R Superior Temporal Gyrus | 38 | 147 | 0.005 | 50 | 4 | −19 |
| R Insula | -- | 49 | 0.004 | 36 | 6 | −13 |
| R Basal Ganglia | -- | 241 | 0.005 | 12 | 2 | −2 |
| L Basal Ganglia | -- | 472 | 0.004 | −16 | 12 | 3 |
| OLDER > YOUNGER: SKf > SUf | ||||||
| L Intracalcarine Cortex | 17 | 222 | 0.006 | −24 | −68 | 4 |
| Retrosplenial Cortex | 29 | 59 | 0.005 | 5 | −45 | 3 |
| Posterior Cingulate Cortex | 23 | 77 | 0.005 | −21 | −29 | 43 |
| L Planum Temporale | 41 | 122 | 0.005 | −43 | −35 | 18 |
L, Left; R, Right
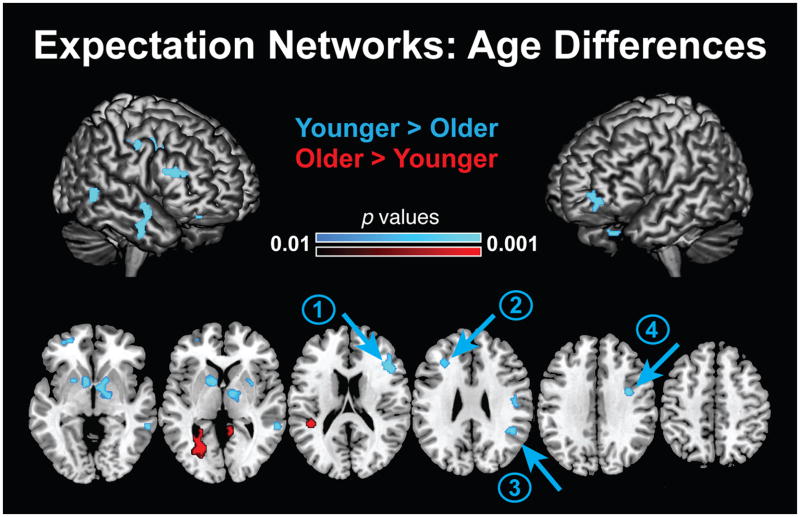
These functional connectivity results for older adults are in striking contrast to those previously reported in younger adults using the identical paradigm and analytical approach (Bollinger et al. 2010). In younger adults, the contrast SKf > SUf revealed increased FFA connectivity within multiple fronto-parietal cortical regions, including bilateral MFG, right inferior frontal gyrus (IFG), and right IFJ, right IPS, right SPL, and right precentral gyrus (Bollinger et al. 2010). To directly explore age-related differences in predictive-cue related FFA connectivity, we performed a between-group contrast of expectation networks (SKf > SUf) using a common normalization template generated using the structural MR data from a large sample of young and elderly participants limited to the same age ranges as in the current study. This analysis revealed greater FFA-connectivity in the younger vs. older age group in multiple frontal regions, including bilateral MFG (Fig. 4, arrows 1 & 2; Table 4), right dorsal SMG (Fig. 4, arrow 3; Table 4), right IFJ, (Fig. 4, arrow 4; Table 4), right precentral gyrus, and left IFG and basal ganglia -left nucleus accumbens, caudate, putamen and right pallidum, putamen (Table 4). Four regions showed predictive-cue related connectivity increases in older adults: left intracalcarine cortex, left planum temporale, posterior cingulate cortex (PCC), and retrosplenial cortex (Fig. 4; Table 4).
Figure 4. FFA Functional Connectivity: SKf > SUf.
Older- versus younger-group expectation-related FFA connectivity contrast SKf > SUf. Axial slices and surface renderings illustrate FFA-seed functional connectivity group contrasts. This analysis revealed greater FFA-connectivity in the younger group for multiple frontal regions, including bilateral MFG (arrows 1 & 2), right dorsal SMG (arrow 3), right IFJ (arrow 4), and right inferior frontal gyrus, as well basal ganglia - left nucleus accumbens, caudate, putamen and right pallidum, putamen. Four regions showed predictive-cue related connectivity increases in older adults: left intracalcarine cortex, left planum temporale, PCC, and retrosplenial cortex. FFA, fusiform face area; MFG, middle frontal gyrus; SMG, supramarginal gyrus; IFJ, inferior frontal junction; PCC, posterior cingulate cortex; SKf, stimulus-known face condition; SUf, stimulus-unknown face condition; PVf, passive-view face condition.
Data from younger adults revealed a subset of regions within the fronto-parietal network (i.e., right IFJ, MFG, IFG, and IPS) whose expectation-related functional connectivity with the FFA correlated across participants with the level of FFA activity modulation during face-predictive expectation (i.e., SKf) (Bollinger et al., 2010). This suggested that the communication between these regions may be driving the modulation. To examine the possibility of age-related alterations in the relationship between network connectivity and FFA activity baseline shifts, we conducted an across-participant regression analysis of connectivity between the FFA and fronto-parietal regions and FFA expectation-period activity modulation. Three different sets of regions-of-interest (ROIs) were used: 1) fronto-parietal ROIs identified in the younger > older expectation network contrast (Figure 4), 2) ROIs identified in the older group SKf connectivity main effects and, 3) ROIs selected from the independent localizer contrast of 1-back > rest in older adults (this included bilateral IFJ, IPS, precentral gyrus, right insula, and ACC (Table 3). While FFA-connectivity of IFJ, MFG, IFG, and IPS regions correlated with expectation-period FFA activity modulation in younger adults (Bollinger et al., 2010), similar regions identified in older adults failed to display the same relationship using any of the ROI selection methods (all p values > 0.2). Although not causal, the current results suggest that functional connectivity between fronto-parietal networks and sensory target regions, proposed to mediate top-down activity modulation prior to stimulus presentation during predictive expectation in younger adults, is disrupted in older adults.
3.3. Neurobehavioral Correlations
In contrast to younger adults, predictive instructions failed to result in benefits in WM or LTM performance for face stimuli in older adults. In order to investigate if age-related alterations in expectation-driven networks were associated with this deficit, we conducted across-participant, neural-behavioral regression analyses using FFA-connectivity with fronto-parietal ROIs (described in the preceding section) and memory performance measures. While in younger adults, expectation-mediated changes (SKf-SUf) of FFA-connectivity with the right IFJ and right precuneus predicted improvements in WM recognition accuracy (SKf-SUf), and FFA-connectivity (SKf-SUf) with the left MFG predicted LTM recognition (Bollinger et al., 2010), regression analyses in older adults failed to reveal significant positive neurobehavioral correlations (all p values > 0.25).
4. Discussion
The current study generated converging behavioral and neural evidence that normal aging is associated with decreased utilization of predictive cues to guide attention and result in WM and LTM performance benefits. This conclusion is supported by six results: 1) While younger adults displayed improved WM and LTM performance for predictively cued face stimuli compared to neutrally cued face stimuli (Bollinger et al., 2010), older adults did not display these memory benefits (significant age x cue interaction), 2) While younger adults displayed FFA-activity modulation during the expectation period for predictively-cued face stimuli (Bollinger et al., 2010), older adults did not display this modulation (significant age x cue interaction), 3) A subgroup analysis of the older population revealed that older adults who exhibited memory benefits by predictive cueing also displayed expectation-period modulation, the same as younger adults, while those older adults who did not display cue associated benefits in WM and LTM demonstrated a significant deficit in expectation-period FFA modulation compared to younger adults, 4) Relative to younger adults, older adults displayed decreased expectation-period functional connectivity between the FFA and a fronto-parietal network of regions thought to mediate sensory cortical neural biasing, 5) While in younger adults the magnitude of FFA-connectivity measures with fronto-parietal regions correlated with expectation-period activity modulation in the FFA (Bollinger et al., 2010), older adults did not display this relationship, and 6) While analysis in younger adults revealed that predictive-cue associated increases in FFA-connectivity with a fronto-parietal regions predicted improvements in WM recognition accuracy and LTM recognition, similar regression analyses in older adults failed to show significant correlations. In summary, the current findings reveal that older adults display a deficit in the utilization of predictive cues to guide attentional resources that optimize WM and LTM performance, and the absence of these memory performance benefits in older adults is associated with deficient expectation-mediated neural biasing by the fronto-parietal attention network. Thus, while younger adults show that a sensory cortical node (i.e., FFA) can be dynamically linked in a network with fronto-parietal brain regions based upon expectations (Bollinger et al., 2010), these mechanisms are functionally impaired in older adults.
Our behavioral results are consistent with previous studies that showed older individuals benefit less than younger adults from predictive knowledge on cued RT tasks (Rabbitt, 1979). Of note, several studies did not show that older adults benefit less from predictive cueing compared to younger adults, however all of these studies utilized experimental paradigms in which a percentage of “valid” cues were actually invalid (Curran et al., 2001; Nissen & Corkin,1985; Hartley, Kieley & Slabach, 1990). This presents a potential confound in interpreting across-group comparisons because the finding may have been generated by strategic differences across age groups. For example, younger adults, unlike older adults, may be more restrained in their use of predictive information when they are aware that it will result in diminished performance on a subset of the trials (i.e., invalid trials). This is supported by results of increased benefit (valid vs. neutral), but also increased cost (invalid vs. neutral) in the older participants (Nissen & Corkin, 1985). Furthermore, a previous study that did not contain invalid cues (similar to the current study) showed that older participants did not benefit to the same degree as the younger adults (Hoyer & Familant, 1987).
Recent reports have demonstrated age-related deficits in top-down modulation of activity in category-specific, visual cortical regions when stimuli are present, and that these reductions in modulation correlate with WM performance deficits (Gazzaley et al., 2005). The current results complement these findings by revealing diminished expectation-period activity modulation in older adults when stimuli are absent. A subgroup analysis revealed that this neural effect is associated with age-related deficits in predictive-cue based WM and LTM performance benefits. Thus, this evidence converges to suggest that age-related deficits in top-down modulation may be a generalizable phenomenon of impaired activity modulation during both stimulus-present and absent time periods. Of note, although this is true at the population level, significant within-group heterogeneity in the older population raises the interesting potential of an undetermined protective factor.
The current experiments revealed widespread age-related reductions in FFA-functional connectivity with fronto-parietal network regions (e.g., bilateral MFG and right IFJ). Moreover, the strong correlations between fronto-parietal regions - FFA connectivity and FFA activity modulation present in younger adults, which was interpreted as a basis for the baseline shifts (Bollinger et al., 2010), was completely absent in the older cohort. Several theories propose that age-associated WM and LTM declines emerge from changes in the functional integration between brain systems, in addition to general dysfunction of specific grey matter areas (Giorgio et al., 2010) or white matter (O’Sullivan et al., 2001; Madden, Bennett, & Song, 2009). This “disconnection” account of cognitive aging states that a disruption of distributed neural systems is a fundamental mechanism of aging-related variability in cognitive performance (Madden et al., 2009). To our knowledge, the current results provide the first evidence that this functional disconnection occurs prior to stimulus onset during periods of expectation.
In a search for unifying theories, researchers have posited that perceptual, WM, and LTM deficits are hallmarks of cognitive aging that are due to an inability to ignore irrelevant information (Gazzaley et al., 2005; Hasher et al., 1999), a decline in processing speed (Salthouse, 1996), deficits in contextual processing (West & Schwarb, 2006; Braver et al., 2001), changes in white matter (Madden et al., 2009; Thomas et al., 2008), and decreased neural selectivity (Goh et al., 2010). These theories are not mutually exclusive and many are in fact complementary. In light of the current results, and the established role of predictive mechanisms in facilitating a broad range of behavior, we propose the expectation deficit hypothesis of cognitive aging, which posits that age-related impairments in engaging attentional neural networks during periods of expectation result in widespread costs in cognitive performance in older individuals. This hypothesis will be further evaluated to determine its generalizability across expectation-driven behaviors.
Acknowledgments
This work was supported by the National Institute of Health Grant 5R01AG030395(AG). We would like to thank Ezequiel Morsella, Peter Wais, Joaquin Anguera, and Chips McSteeley Jr. II for reading this manuscript and their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacob Bollinger, Email: jbolling@phy.ucsf.edu.
Michael T. Rubens, Email: michael.rubens@ucsf.edu.
Edrick Masangkay, Email: edrick.gazzlab@gmail.com.
Jonathan Kalkstein, Email: jkalkstein@gmail.com.
Adam Gazzaley, Email: adam.gazzaley@ucsf.edu.
References
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD. Face recognition in human extrastriate cortex. J Neurophysiol. 1994;71:821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Golob EJ, Starr A. Age-related differences in auditory event-related potentials during a cued attention task. Clin Neurophysiol. 2004;115:2602–2615. doi: 10.1016/j.clinph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J, Rubens M, Zanto T, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working memory and long-term memory performance. J Neurosci. 2010;30:14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowsky JS, Taylor SF, Yesavage JA, Mumenthaler MS, Jagust WJ, Reed BR. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol Gen. 2001;130:746–763. [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos A, Marcus D, Morris J, Snyder A. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2010;20:859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell L, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Esterman M, Yantis S. Perceptual Expectation Evokes Category-Selective Cortical Activity. Cereb Cortex. 2009;20:1245–1253. doi: 10.1093/cercor/bhp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D. Event-related brain potential investigations of memory and aging. Biol Psychol. 2000;54:175–206. doi: 10.1016/s0301-0511(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Nat Acad Sci USA. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp WC, D’Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney J, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney J, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, D’Esposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Weissman DH, Woldorff MG, Mangun GR. Pre-target activity in visual cortex predicts behavioral performance on spatial and feature attention tasks. Brain Res. 2006;1080:63–72. doi: 10.1016/j.brainres.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JAE, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2009;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AA, Kieley JM, Slabach EH. Age differences and similarities in the effects of cues and prompts. J Experimental Psychol Hum Percept Perform. 1990;16:523–537. doi: 10.1037//0096-1523.16.3.523. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory Control, Circadian Arousal, and Age. Attention and Performance. 1999;XVII:653–675. [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hoyer W, Familant M. Adult age differences in the rate of processing expectancy information. Cognitive Development. 1987;2:59–70. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennet IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415, 435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Saniol J, Bucur B, Cabeza R. Adult age differences in connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cereb Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Nissen M, Corkin S. Effectiveness of attentional cueing in older and younger adults. Journal of Gerontology. 1985;40:185–191. doi: 10.1093/geronj/40.2.185. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une gure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi L, Blumhardt LD. Effects of age on working memory: an event-related potential study. Brain research Cognitive brain research. 1999;7:321–334. doi: 10.1016/s0926-6410(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensetive regions in the extrastriate cortex studied by funtional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Puri AM, Wojciulik E, Ranganath C. Category expectation modulates baseline and stimulus-evoked activity in human inferotemporal cortex. Brain Res. 2009;1301:89–99. doi: 10.1016/j.brainres.2009.08.085. [DOI] [PubMed] [Google Scholar]
- Puri AM, Wojciulik E. Expectation both helps and hinders object perception. Vis Res. 2008;48:589–597. doi: 10.1016/j.visres.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Some experiments and a model for attentional selectivity with old age. In: Baumeister F, editor. Biological Effects of Aging. Springer-Verlag; Hamberg: 1979. [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal innteractions reflect future task operations. Nat Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Vogel E, Woodman G, Luck S. Voluntary and automatic attentional control of visual working memory. Perception & psychophysics. 2002;64:754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, Awh E. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol. 2004;92:3538–3545. doi: 10.1152/jn.00435.2004. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes M, Thompson R, Nobre AC, Duncan J. Shape-specific preparatory activity mediates attention to targets in human visual cortex. Proc Natl Acad Sci USA. 2009;106:19569–19574. doi: 10.1073/pnas.0905306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Egner T. Expectation (and attention) in visual cognition. Trends Cogn Sci. 2009;13:403–409. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung K, Peterson MS, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Nat Acad Sci USA. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Rubens MT, Boccanfuso J, Gazzaley A. Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. J Neurosci. 2010;30(8541):50. doi: 10.1523/JNEUROSCI.1478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. The Psychological Corporation; New York: 1981. [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: The role of the inferior frontal junction. Neuroimage. 2010;53:736–745. doi: 10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]