Abstract
We identified a conserved pattern of residues L-X(3–4)-R-X(2)-L-X(4)-G, in which -X(n)- represents n residues of any amino acids, in two enzymes acting on polyunsaturated fatty acids, diacylglycerol kinase epsilon (DGKε) and phosphatidylinositol-4-phosphate-5-kinase Iα (PIP5K Iα). DGKε is the only one of the 10 mammalian isoforms of DGK that exhibits arachidonoyl specificity and is the only isoform with the aforementioned motif. Mutations of the essential residues in this motif result in loss of arachidonoyl specificity. Furthermore, DGKα can be converted to an enzyme having this motif by substituting only one residue. When DGKα was mutated so that it gained the motif, the enzyme also gained some specificity for arachidonoyl-containing diacylglycerol. This motif is also present in an isoform of phosphatidylinositol-4-phosphate-5-kinase that we demonstrated had arachidonoyl-specificity for its substrate. Single residue mutations within the identified motif of this isoform result in loss of activity against an arachidonoyl substrate. The importance of acyl chain specificity for the phosphatidic acid activation of phosphatidylinositol-4-phosphate-5-kinase is also shown. We also demonstrate that the acyl chain dependence of this phosphatidic acid activation is dependent on the substrate. This is the first demonstration of a motif that endows specificity for an acyl chain in two studied enzymes, DGKε and PIP5K Iα.
Keywords: diacylglycerol kinase, acyl chain specificity, phosphatidylinositol-4-phosphate-5-kinase, arachidonic acid, PI-cycling
INTRODUCTION
Polyunsaturated fatty acids (PUFA) are essential nutrients for humans and are major components of cell membrane phospholipids. PUFA from the diet play an important role in the regulation of prostaglandin and proinflammatory cytokine synthesis.1
One of the most abundant PUFAs in mammalian cells isarachidonic acid (AA), and its derivatives are key mediators of a wide variety of physiological and pathophysiological processes, such as atherosclerosis,2 arthritis, asthma and tumorigenesis.3, 4 AA is the precursor of a large family of bioactive compounds called eicosanoids, produced by cyclooxygenases and lipoxygenases.5, 6 Because of the potent biological actions of eicosanoids and of free AA itself, this fatty acid is maintained atvery low levels in the cells, by being converted into cellular lipids by the enzymes arachidonoyl-CoA synthetase and lysophospholipid acyltransferases.7 Therefore, under physiological conditions, AA is generally found esterified at the sn-2 position of glycerophospholipids, such as choline and ethanolamine glycerophospholipids, phosphatidic acid and phosphatidylinositol.
To utilize the arachidonic acid pathway, the enzymes are required to distinguish the acyl chain length and saturation of the substrate. It is common that only one of the isoforms of a particular enzyme has specificity towards substrates with an arachidonate moiety. Thus, only the epsilon isoform of diacylglycerol kinase (DGKε) shows substrate specificity in vitro for diacylglycerols with an arachidonoyl acyl chain at the sn-2 position.8–10 Phosphorylation of 1 -stearoyl-2-arachidonoyl-glycerol (SAG) (for all lipid abbreviations, used in this study, refer to Table 1) is the first step in the resynthesis of phosphatidylinositols (PIs), and therefore, DGKε contributes to the enrichment of 1 -stearoyl-2-arachidonoyl species of PIs.11, 12 It is intriguing, that the domain responsible for the substrate recognition and specificity of DGKε and other enzymes with arachidonate specificity has still not been identified. In the present study we propose a region located in the accessory domain of DGKε that can recognize an arachidonoyl group. We identified a motif L-X(3–4)-R-X(2)-L-X(4)-G, in which -X(n)- represents n residues of any amino acids in this domain, that is present in DGKε, as well as in phosphatidylinositol-4-phosphate-5-kinase type I. This motif is similar to a PUFA-recognizing domain recently identified in lipoxygenases (LOX)on the basis of a 1.85 Å resolution structure of an 8R -lipoxygenase from Plexaura homomalla, which reveals a U -shaped channel, defined by invariant amino acids that would allow substrate access to the catalytic iron. 13 We showed that several residues in this motif are involved in the substrate specificity of two enzymes, acting on PUFA-containing substrates, DGKε and PIP5K Iα.
Table 1.
Lipids used and/or referred to in this study
| Abbreviation | Full name | Alternative notation (sn-1/sn-2) |
|---|---|---|
| DAG | ||
| AAG | 1-Arachidoyl-2-arachidonoyl-sn-glycerol | 20:0/20:4 DAG |
| DOG | 1,2-Dioleoyl-sn-glycerol | 18:1/18:1 DAG |
| PAG | 1-Palmitoyl-2-arachidonoyl-sn-glycerol | 16:0/20:4 DAG |
| SAG | 1-Stearoyl-2-arachidonoyl-sn-glycerol | 18:0/20:4 DAG |
| SLG | 1-Stearoyl-2-linoleoyl-sn-glycerol | 18:0/18:2 DAG |
|
| ||
| PA | ||
| AAPA | 1-Arachidoyl-2-arachidonoyl phosphatidic acid 1,2- | 20:0/20:4 PA |
| DAPA | Diarachidonoyl phosphatidic acid | 20:4/20:4 PA |
| PAPA | 1-Palmitoyl-2-arachidonoyl phosphatidic acid | 16:0/20:4 PA |
| POPA | 1-Palmitoyl-2-oleoyl phosphatidic acid | 16:0/18:1 PA |
| SAPA | 1-Stearoyl-2-arachidonoyl phosphatidic acid | 18:0/20:4 PA |
| SLPA | 1-Stearoyl-2-linoleoyl phosphatidic acid | 18:0/18:2 PA |
| SOPA | 1-Stearoyl-2-oleoyl phosphatidic acid | 18:0/18:1 PA |
|
| ||
| PC | ||
| DOPC | 1,2-Dioleoyl-sn-glycero-3-phosphocholine | 18:1/18:1 PC |
|
| ||
| PS | ||
| DOPS | 1,2-Dioleoyl-sn-glycero-3-phospho-L-serine | 18:1/18:1 PS |
|
| ||
| PI4P | ||
| SA-PI4P | 1-Stearoyl-2-arachidonoyl phosphatidylinositol-4- phosphate | 18:0/20:4 PI4P |
| DP-PI4P | 1,2-Dipalmitoyl phosphatidylinositol-4-phosphate | 16:0/16:0 PI4P |
RESULTS
Mutations in the LOX-like motif of DGKε greatly affect the activity of the enzyme
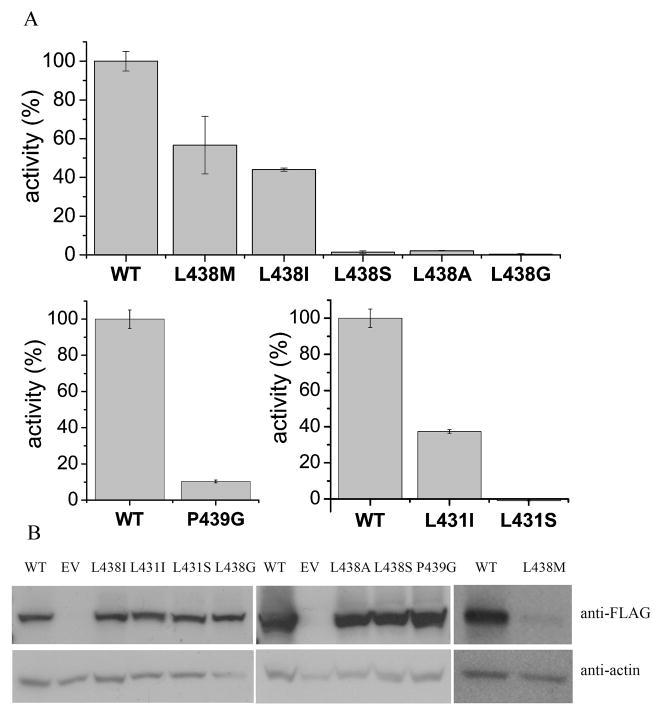
We identified a motif L-X(3–4)-R-X(2)-L-X(4)-G in the accessory domain of DGKε (Table 2), similar to that in lipoxygenases, but different in having an L, rather than an I as the first residue (Table 3). This motif is highly conserved in DGKε among different species. To determine if the identified motif plays a role in the DGKε specificity towards arachidonate-containing substrates, we mutated the residues in this region of the protein and measured the activities of FLAG-DGKε WT and mutant proteins using the micelle-based assay with SAG as a substrate. The results showed that the mutations in this LOX-like region of DGKε greatly affect the activity of the enzyme (Fig. 1). Notably, this effect is strongly correlated with the size and/or shape of a side chain of the mutated amino acids. From these results at a single substrate concentration, it is clear that all of the mutations result in a significant loss of enzymatic activity, even substitution of a single amino acid with a similar residue, thus demonstrating the importance of this region of DGKε for enzymatic activity.
Table 2.
A partial sequence alignment of verterbrate DGKε. The conserved residues, similar to those in LOX, are colored in red. Note the high degree of sequence conservation in this region of the protein from avian to mammalian species.
| NP_003638 [Homo sapiens] | 421 | KDLNKKVELELDGERVALPSLEGIIVLNIGYWG | 453 |
| NP_062378 [Mus musculus] | 418 | KDLNKKIELELDGERVELPNLEGIIVLNIGYWG | 450 |
| XP_618342 [Bos taurus] | 418 | KDLNKKVELELDGERVELPNLEGIIVLNIGYWG | 450 |
| XP_001503369 [Equus caballus] | 418 | KDLNKKVELELDGERVELPNLEGIIVLNIGYWG | 450 |
| XP_548222 [Canis familiaris] | 418 | KDLNKKIELELDGERVELPNLEGIIVLNIGYWG | 450 |
| XP_001521727 [Ornithorhynchus anatinus] | 414 | KDLNKKVELELDGERVELPNLEGIIVLNIGYWG | 446 |
| XP_523803 [Pan troglodytes] | 421 | KDLNKKVELELDGERVALPSLEGIIVLNIGYWG | 453 |
| XP_001234226 [Gallus gallus] | 421 | KDLNKKVELELDGERIELPNLEGIIVLNIGYWG | 453 |
| XP_002192399 [Taeniopygia guttata] | 407 | KDLNKKVELELDGERIELPNLEGIIVLNIGYWG | 439 |
Table 3.
A partial sequence alignment of LOX. The conserved residues, located in U-shaped channel, that would allow substrate access to the catalytic iron, 13 are colored in red.
| LOX15 [Oryctolagus cuniculus] | 389 | LIVPHLRYTLEINVRARNGLVSDFGIFDQIM | 419 |
| LOX15 [Homo sapiens] | 388 | LIIPHLRYTLEINVRARTGLVSDMGIFDQIM | 418 |
| LOX12 [Homo sapiens] | 388 | FLIPHIRYTMEINTRARTQLISDGGIFDKAV | 418 |
| LOX12 [Rattus norvegicus] | 389 | LLVPHLLYTMEINVRARSDLISERGFFDKAM | 419 |
| LOX5 [Rattus norvegicus] | 395 | LLVAHVRFTIAINTKAREQLNCEYGLFDKAN | 425 |
| LOX1 [Arabidopsis thaliana] | 547 | LLEPHFRDTMNINALARQILINGGGIFEITV | 577 |
Figure 1.
A. Comparison of the enzyme activities for FLAG-DGKε WT and mutants L438M, L438I, L438S, L438A, L438G, L431I, L431S, P439G. Enzyme activity is expressed as a percentage of the activity of FLAG-DGKε WT protein with SAG as a substrate. B. Western blots showing the expression levels of DGKε WT and mutants (anti-FLAG panel), and the expression levels of actin (anti-actin panel) in the transfected cells. Enzyme activity was normalized for the amount of protein in the lysates. The activity measured with mock-transfected cells was normalized for the amount of actin and subtracted from the values obtained using cells overexpressing one of the DGKε constructs. The activity measured with mock-transfected cells was on average 5–10% of the activity measured with DGKε WT.
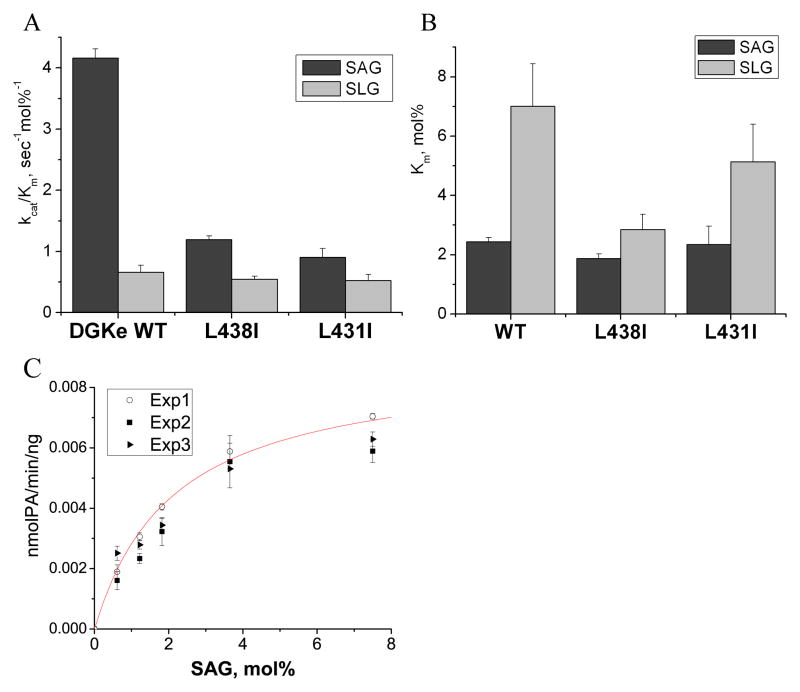
Affecting the substrate binding site would be expected to lower the activity of the enzyme against all substrates. However, if the binding site is specific for arachidonoyl groups, then the loss of activity should be greater for an arachidonoyl-containing substrate. In order to test this question more critically, we performed a kinetic analysis and calculated the Michaelis-Menten parameters for FLAG-DGKε WT and its single residue mutants, L431I and L438I, using SAG and SLG as substrates (Table 4). The arachidonoyl-containing substrate, SAG, is the major species of diacylglycerol in the PI-cycle. It was compared with a structurally similar diacylglycerol not containing arachidonic acid, SLG, as a critical test of the extent of arachidonoyl-specificity. Other more structurally different diacylglycerols, such as dioleoylglycerol or dipalmitoylglycerol, have very low activity with wild type DGKε12, 14 and also show very weak activity with mutant forms of this enzyme (data not shown). They therefore do not provide a critical comparison to test relative acyl chain specificity.
Table 4.
Summary of the kinetic parameters for FLAG-DGKε WT and its L438I and L431I mutants. Results are presented as the mean ±S.D. Although the measured activity at one substrate concentration did not show differences in substrate specificity (Fig.1), a more complete analysis of the kinetics does clearly illustrate that both kcat, that represents the catalytic rate constant, as well as kcat/Km, that represents the pseudo first order rate constant at low substrate concentrations, are affected by the mutations, particularly for the SAG substrate (see Fig. 7 for the graphic representation of Table 4).
| Km, mol% | kcat, sec−1 | kcat/Km, sec−1mol%−1 | |
|---|---|---|---|
| FLAG-DGKε WT | |||
| SAG | 2.44 ± 0.14 | 10.2 ± 0.3 | 4.16 ± 0.14 |
| SLG | 7.0 ± 1.4 | 4.6 ± 0.6 | 0.66 ± 0.11 |
|
| |||
| FLAG-DGKε L438I | |||
| SAG | 1.88 ± 0.15 | 2.24 ± 0.07 | 1.19 ± 0.05 |
| SLG | 2.9 ± 0.5 | 1.56 ± 0.09 | 0.54 ± 0.04 |
|
| |||
| FLAG-DGKε L431I | |||
| SAG | 2.4 ± 0.6 | 2.1 ± 0.2 | 0.91 ± 0.14 |
| SLG | 5.1 ± 1.2 | 2.7 ± 0.4 | 0.53 ± 0.09 |
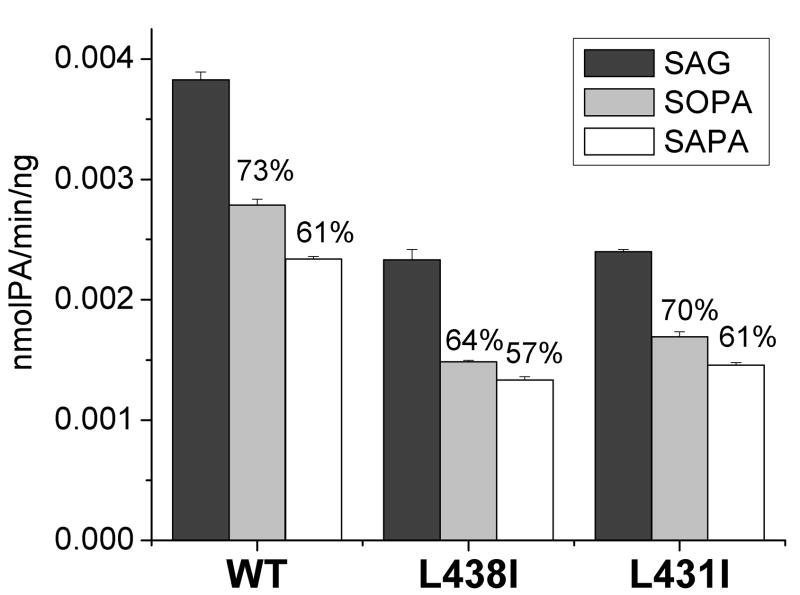
Almost all other mutants, L431S, L438S, L438A, L438G, and P439G, had very low activity (less than 2% of WT), which did not allow us to perform the kinetic analysis. Our results showed that kcat/Km was reduced for both L431I and L438I mutations of DGKε. However the reduction of kcat/Km was much greater for SAG as substrate (four-fold decrease) compared with SLG (18% decrease). The number of substrate molecules turned over per enzyme molecule per second (k cat), was also reduced, particularly with the substrate SAG. There was less affect on the affinity of the enzyme for these substrates as measured by Km.
To test if the L431I and L438I mutations of FLAG-DGKε affect the mode of inhibition by PA, we compared the inhibition of FLAG-DGKε WT and mutant proteins by SOPA and SAPA (Fig. 2). Our results showed that L431I and L438I mutations of DGKε do not affect the extent of PA inhibition.
Figure 2.
Comparison of inhibition of DGKε by PA. DGK enzymatic activity was measured with 15 mM Triton X-100, 0.1 mM [γ-32P]-ATP, and 2 mol % SAG (shown as dark grey bars) or with the addition of either 2 mol % SAPA (light grey bars) or SOPA (white bars). The numbers above the bars show the enzymatic activity of proteins in presence of PA as a percentage of the enzymatic activity in the absence of PA.
Arachidonate preference can be introduced into DGKα by V656L mutation
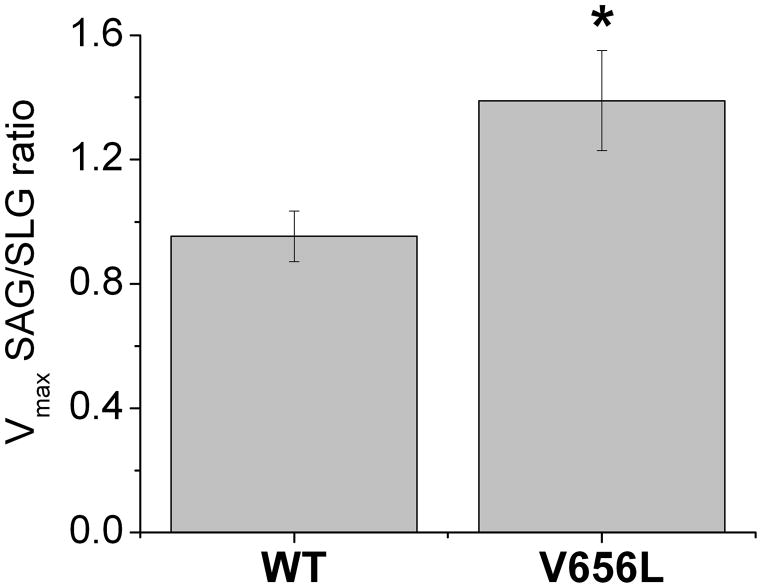
We found that only one other isoform of DGK, DGKα, has a motif with some similarity to the LOX-like motif in DGKε, but with a V656 residue instead of the Leu in DGKε (Table 5). These motifs are found in the accessory domain of both DGKα as well as DGKε. It was shown that DGKα does not have a preference for DAG with an arachidonate moiety.15 Therefore, to test if the arachidonate specificity could be introduced, at least to some extent, into DGKα, we mutated the V656 residue in human DGKα to L to obtain a protein with the same motif as in DGKε (LxxxRxxLxxxxG) and compared the Michaelis-Menten parameters for 3×HA-DGKα WT and the V656L mutant (Table 6). Our results showed that there is no statistically significant difference in the Kmor in the overall efficiency, V max/Km, of DGKα WT and V656L mutant, although it should be noted that there is an intrinsically large error in the determination of Km. However, there is a difference in the Vmax for these proteins. There was a significant 22% increase in Vmax for SAG with the introduction of the mutation in contrast to a 16% decrease for SLG. When taken as a ratio of Vmax for SAG to Vmax for SLG, it shows a significant difference between DGKα WT and the V656L mutant proteins (Fig. 3).
Table 5.
A partial sequence alignment of mammalian DGKα. The conserved residues, similar to those in LOX, are colored in red.
| NP_963848 [Homo sapiens] | 639 | PDILKTCVPDLSDKRLEVVGLEGAIEMGQIYTK | 671 |
| NP_058091 [Mus musculus] | 634 | PDILKTCVPDMSDKRLEVVGIEGAIEMGQIYTR | 666 |
| NP_001071328 [Bos taurus] | 638 | PDILKTCVPDLSDKRLEVVGLEGAIEIGQIYTK | 670 |
| NP_542965 [Ruttus Norvegicus] | 631 | PDILKTCVPDMSDKRLEVVGIEGVIEMGQIYTR | 663 |
| XP_855720 [Canis familiaris] | 477 | PDILKTCVPDLTDKRLEVVGLEGAIEMGQIYTK | 509 |
| XP_001112067 [Macaca mulatta] | 527 | PDILKTCVPDLSDKRLEVVGLEGAIEMGQIYTK | 559 |
| NP_999197 [Sus scrofa] | 638 | PDILKTCVPDLSDKRLEVVGLEGAIEMGQIYTK | 670 |
| XP_001169813 [Pan troglodytes] | 527 | PDILKTCVPDLSDKRLEVVGLEGAIEMGQIYTK | 559 |
Table 6.
Summary of the kinetic parameters for 3×HA-DGKα WT and V656L mutant. Kinetic analysis shows that the V656L mutation of DGKα increases the Vmax of the enzyme for SAG and decreases it for SLG, thus introducing the arachidonoyl preference (see also Fig. 3B). Values of Vmaxare relative values since the absolute amount of enzyme in the cell preparations is not known. Vmax of 3×HA-DGKα V656L is normalized for the amount of protein relatively to WT. Results are presented as the mean ±S.D.
| Km, mol% | Vmax, nmol PA min−1 | Vmax/Km, mol%−1sec−1 | |
|---|---|---|---|
| 3×HA-DGKα WT | |||
| SAG | 2.1 ± 0.4 | 1.44 ± 0.09 | 0.70 ± 0.13 |
| SLG | 2.8 ± 0.3 | 1.51 ± 0.08 | 0.55 ± 0.07 |
|
| |||
| 3×HA-DGKα V656L | |||
| SAG | 1.8 ± 0.3 | 1.75 ± 0.11 | 1.00 ± 0.17 |
| SLG | 1.5 ± 0.4 | 1.26 ± 0.12 | 0.87 ± 0.25 |
Figure 3.
Comparison of SAG to SLG ratios for Vmax parameters for 3×HA-DGKα WT and V656L mutant proteins. V656L mutation of DGKα increases the arachidonoyl preference of the enzyme. *The difference between Vmaxratios of SAG to SLG for DGKα WT and V656L mutant is statistically significant with P < 0.05.
PIP5K type I exhibits preference for arachidonoyl-PI(4)P
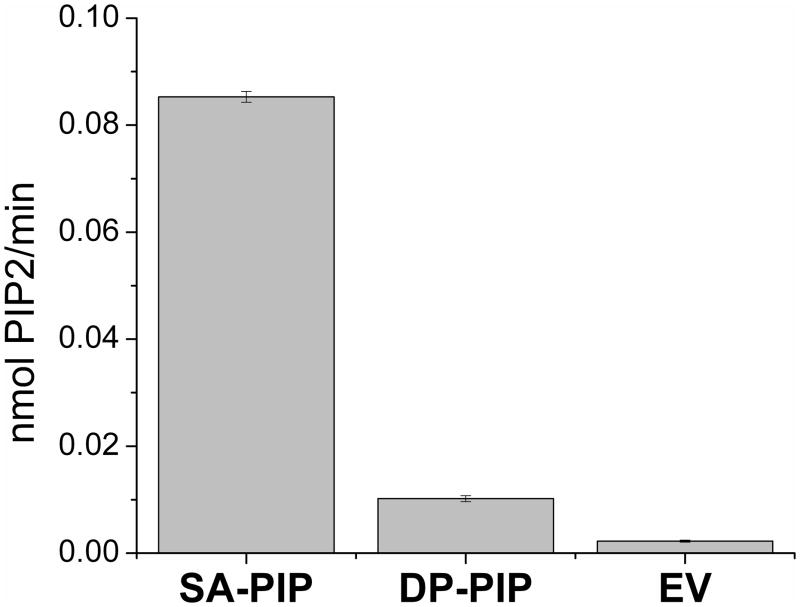
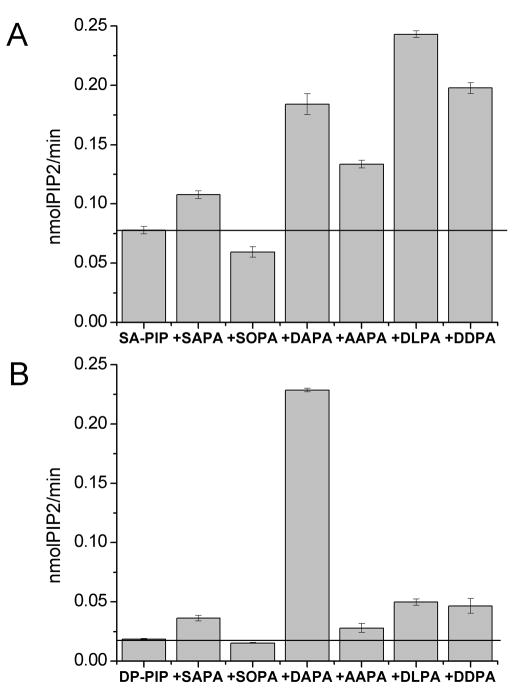
We found that phosphatidylinositol 4-phosphate 5-kinases (PIP5K) type I, but not type II, have a motif LxxxxRxxLxxxxG. There are three active isoforms of PIP5K type I, namely α, β and γ that are encoded by distinct genes. All three isoforms of the type I PIP5K have the same L-X(3–4)-R-X(2)-L-X(4)-G motif as DGKε mentioned above. In the present work we have used human PIP5K Iα which has been suggested to fulfill a “housekeeping” function.16 To test if PIP5K type I forms have specificity towards an arachidonate -containing substrate, we compared the activity of human PIP5K type Iα with SA-PI(4)P vs. DP-PI(4)P as substrates. There are few molecular forms of the substrate PI(4)P that have been synthesized. These two were chosen because of their availability. Our results showed that PIP5K type Iα phosphorylates SA-PI(4)P about 8 times faster than DP-PI(4)P (Fig. 4). This is the first demonstration that this enzyme exhibits substrate acyl chain preference, although by itself it is not a critical test of arachidonoyl specificity.
Figure 4.
Comparison of the enzyme activities for c-myc-PIP5K Iα with SA-PI(4)P and DP-PI(4)P as a substrate. As source of SA-PI(4)P, brain PI(4)P (Avanti Polar Lipids) was used. Only SA-PI(4)P and DP-PI(4)P were compared, since other PI(4)P are not commercially available. Negativ e control (EV) is performedwith SA -PI(4)P as a substratewith the addition of beads immunoprecipitated from mock -transfected COS-7 cells.
To test the role of the identified motif in the activity of PIP5K type Iα, we made L202I and L210I mutations in this region of the protein and performed a kinetic analysis of these mutant proteins in comparison with the WT PIP5K type Iα. Our results showed that both L202I and L210I mutations decrease the substrate affinity and the enzyme efficiency of PIP5K type Iα for arachidonate-containing PI(4)P (Table 7).
Table 7.
Summary of the kinetic parameters for c-myc-PIP5K Iα WT and L202I and L210I mutants. Our results show that L202I and L210I mutations of PIP5K Iα significantly decrease the substrate affinity of the enzyme (12-fold increase in Km for L202I mutant and 2-fold for L210I). Consequently, the mutations also affect the enzyme efficiency (about 6-fold decrease in Vmax/Km for L202I and 2-fold for L210I). Values of Vmaxare relative values since the absolute amount of enzyme in the cell preparations is not known. Vmax for PIP5K Iα L202I and L210I mutants is normalized for the amount of proteins relatively to WT. Kinetic parameters are calculated using the effective concentration of PI4P at the surface of the micelle. The effective surface concentration of PI(4)P was determined by multiplying the mole fraction of PI(4)P at the surface of the micelle by the total concentration of PI(4)P to be comparable to previously published work.45Results are presented as the mean ± S.D.
| Km, μM | Vmax, nmol/min | Vmax/Km, 1/μM/min |
|---|---|---|
| PIP5K Iα WT | ||
| 0.3 ± 0.1 | 0.125 ± 0.008 | 0.35 ± 0.10 |
|
| ||
| PIP5K Iα L202I | ||
| 3.6 ± 0.7 | 0.200 ± 0.027 | 0.06 ± 0.01 |
|
| ||
| PIP5K Iα L210I | ||
| 0.7 ± 0.2 | 0.097 ± 0.012 | 0.14 ± 0.05 |
We also determined the activation of PIP5K Iα with different PAs for both SA-PI(4)P (Fig. 5A) and DP-PI(4)P substrates (Fig. 5B). Our results using SA-PI(4)P as substrate showed that PIP5K is activated most by polyunsaturated PAs with the same fatty acids at sn-1 and sn-2 positions (di-PUFA-PA), such as DAPA, DLPA and DDPA. For the DP-PI(4)P substrate, PIP5K Iα is activated 12 -fold by DAPA (Fig. 5B), showing that PIP5K can exhibit a very specific arachidonate preference for PA activation when DP-PI(4)P is used as substrate.
Fig. 5.
Activation of PIP5K by PA with A) SA-PI(4)P and B) DP-PI(4)P as substrate. As source of SA-PI(4)P, brain PI(4)P (Avanti Polar Lipids) was used. PIP5K enzymatic activity was measured with 20 μM PI(4)P and 50 μM PA.
Discussion
Acyl chain specificity of DGKε
We previously showed12 that the inhibition and substrate specificity of DGKε are both determined by selectivity for a combination of the sn-1 and sn-2 acyl chains of PA or DAG, respectively, preferring the most prevalent acyl chain composition of lipids involved specifically in the PI cycle, 1-stearoyl-2-arachidonoyl.12 The inhibition of DGKε by PA is competitive. Thus, the active site of DGKε not only recognizes the lipid headgroup but also a combination of the two acyl chains in PA or DAG. Taken together, these findings suggest that the substrate-binding pocket of DGKε should have a specific size and length, which is best suited for SAG. This isoform of DGK also contains a motif L-X(3–4)-R-X(2)-L-X(4)-G. Mutations of several residues in this motif in DGKε result in a marked loss of activity (Fig. 1). It should be pointed out that this marked sensitivity of the enzymatic activity of DGKε to these single residue substitutions contrasts sharply with the very small changes in the kinetics of this enzyme that were observed when 58 residues were removed from the amino terminus of the enzyme.12 In general, the less bulky the amino acid side chain in the mutated forms of the LOX-like motif, the lower the enzymatic activity.
The greater loss of activity by introducing mutations with less bulky amino acid side chains can be explained by the location of residues L438 and L431 at the bottom of the substrate -binding pocket, in analogy with the LOX enzyme,13 although the shape of this binding site may be different in DGKε. In the case of DGKε, the mutation of L438 or L431 residues to another amino acid with a smaller side chain would increase the volume of the substrate-binding pocket. The less fixed position of the substrate would then decrease the interaction between the hydroxyl group of the substrate and the phosphate group of the ATP that is bound to the ATP-binding site of the catalytic domain, which is outside of the acyl chain binding pocket. This would slow the rate of catalysis and the effect would depend on the size of the side chain of the mutated amino acid.
We also compared the mode of inhibition of FLAG-DGKε WT and mutants L438I and L431I by PA (Fig. 2). These mutations have greatly affected kcat/Km(about 4 -fold decrease) for SAG, but not for SLG (Fig. 6). In contrast with this large decrease in the rate of phosphorylation of arachidonoyl substrates by mutations of the LOX-like motif, relatively little change in acyl chain specificity of PA inhibition is exhibited in these mutant forms of DGKε compared with the wild type protein (Fig. 2). This can be explained by the fact that PA competes with the substrate for the substrate-binding site, but in the PA the hydroxyl group is already phosphorylated, and therefore, the distance of the PA from the ATP-binding site would not affect the extent of inhibition.
Figure 6.
Graphic presentation of the kinetic parameters for FLAG-DGKε WT and its L438I and L431I mutants. Results are presented as the mean ± S.D. A. Results show that kcat/Km is greatly affected (about 4-fold decrease) by L438I and L431I mutations of DGKε for SAG, but not SLG substrate. B. Comparison of Km parameters shows that L438I and L431I mutations of DGKε slightly affect the substrate affinity of the enzyme, decreasing the preference for SAG over SLG. C. An example of kinetic data of 3 independent experiments (Exp1, Exp2 and Exp3) is shown for FLAG-DGKε WT with SAG as a substrate. Every experiment is performed in triplicate. A nonlinear regression curve fitting Exp1 data is shown in red.
Furthermore, DGK shares the catalytic domain with other lipid kinases, such as sphingosine kinase and ceramide kinase,17, 18 but at the same time it is highly specific for DAG as substrate and does not catalyze the phosphorylation of sphingosine or ceramide. This suggests that the accessory domain is responsible for substrate recognition.19 The LOX-like motif, which we identified in DGKε, is located in the accessory domain of DGKε, further suggesting that it is involved in the substrate recognition and binding.
Acyl chain specificity of DGKα
Notably, DGKε is the only DGK isoform that has an identified LOX-like motif, and it is the only isoform that has specificity for substrates with an arachidonate moiety. Although, one other isoform, DGKα, has a region with some similarity to that in DGKε, but with a V656 residue instead of the Leu in DGKε, and with the first Leu649 not being conserved among the mammalian species (Table 5).
Our results showed that the V656L mutation of DGKα that introduces a LOX-like motif in this isoform results in SAG having a higher Vmax than SLG (Fig. 3). Therefore, this mutation increases the substrate preference of DGKα towards arachidonoyl substrates, making its substrate preference more similar to the DGKε isoform. This supports our fin dings that the essential residues in the LOX-like motif play an important role in arachidonate-containing substrate specificity and recognition. The V656L mutant of DGKα is not as specific for arachidonoyl groups as is DGKε, but perhaps one should not expect a newly introduced property in a protein by a single residue substitution to result in optimized function.
It is interesting, that DGKα also preferentially acts on substrates containing an arachidonoyl group when this group is incorporated into alkylacylglycerols.15 Although diacylglycerols are better substrates for DGKα than the alkylacylglycerols, no specificity is exhibited for arachidonoyl -containing diacylglycerols. This data may be explained by our observation that DGKα has a region similar to the LOX-like motif in DGKε, but since it is not completely identical, DGKα does not exhibit specificity for arachidonoyl-containing DAG, unless this region is mutated so that the conserved residues are identical to those of DGKε, as is shown in our study.
Acyl chain specificity of PIP5K Iα
We identified a motif L-X(3–4)-R-X(2)-L-X(4)-G in PIP5K type I, which converts PI(4)P to PI(4,5)P2, and we confirmed that this enzyme exhibits preference for arachidonoyl-PI(4)P, and that the mutations in the identified region decrease the catalytic efficiency and substrate affinity for this enzyme (Table 7). Moreover, we also determined the activation of PIP5K Iα with different PAs for both SA-PI(4)P and DP-PI(4)P substrates (Fig. 5). For DP-PI(4)P as substrate, the best activator of PIP5K Iα is DAPA (Fig. 5B), showing that PIP5K exhibits arachidonate preference not only for the substrate, but also for its activator PA. The relationship between the arachidonoyl-requirement for the substrate of PIP5K Iα and the acyl chain requirements for PA as an activator is not yet known. However, the role of PA for PIP5K as an activator clearly has to be different from the competitive inhibition that PA exhibits with DGKε.12 A previous study identified dilinoleoyl-PA as a potent activator of the phosphorylation of SA-PI(4)P by this enzyme.20 Our results extend these findings and demonstrate that PIP5K stimulation by PA is sensitive to acyl chain composition and it depends on the substrate as well. We show that the PI-cycle intermediate, SAPA, is not the best activator for the phosphorylation of SA-PI(4)P, but rather it is di-PUFA-PA. The levels of di-PUFA-PA would be low in most tissues, but high in brain, where it would stimulate PI-cycling.
Conclusions
Enzymes and other proteins that interact with lipid exhibit specificity for certain lipid structures. In some cases, this is primarily a consequence of interacting with a lipid headgroup. An example is the PH domain that interacts with phosphatidylinositol phosphates with little specificity for the acyl chain. However, other proteins and enzymes can be specific for the nature of the acyl chain. Acyl chain specificity can have important physiological consequences, such as the segregation of arachidonoyl groups for signal transduction and perhaps other functions.
There has been current interest in the molecular basis of PUFA specificity. A recent proposal, based on the crystallographic structure of a coral lipoxygenase, has identified a pattern of amino acid residues that are important for recognizing arachidonic acid.13 In the present study we demonstrate that a similar amino acid pattern, L-X(3–4)-R-X(2)-L-X(4)-G, is found in two other proteins that exhibit relative specificity for PUFA groups. While this motif does not predict a specific conformation of a PUFA binding site and this motif is found in other proteins that do not interact with PUFA groups, it has allowed us to identify the region in DGKε that is involved in its unique arachidonoyl specificity among the 10 isoforms of mammalian DGK. We present further supporting evidence for this hypothesis by demonstrating the arachidonoyl specificity of DGKα can be increased by a site-specific mutation that results in the introduction of this proposed motif. In addition, we show that the enzyme PIP5K Iα that also contains this motif exhibits specificity for PUFA moieties in both the substrate and in PA activators. Among the enzymes, lipoxygenases, DGK and PIP5K there are gross differences in the structure of the substrate and in the nature of the reaction that is catalyzed. One would therefore not expect a priori for the amino acid pattern or extent of acyl chain specificity to be identical in all three cases. One of the differences is that in lipoxygenases the first residue of this motif is an I, rather than L. Nevertheless, we demonstrate that there is a strong relationship between the amino acid pattern that we have identified and the property of PUFA specificity in two enzymes, DGKε and PIP5K Iα. It is also possible that a similar amino acid pattern can play a role in the substrate specificity and recognition of other enzymes with specificity towards PUFA-containing substrates, but further studies are needed to address this issue.
Amino acid patterns in forming structures that recognize particular features of substrates or ligands have been discovered in a number of proteins. There is also the so-called “CRAC” motif that has been proposed to be responsible for cholesterol recognition.21, 22 This amino acid pattern is also quite flexible in definition, it does not define a specific structure and the molecular basis of its relationship to cholesterol binding is not known. Nevertheless, there are an increasing number of examples of this motif being responsible for cholesterol interactions in proteins.23–27 Other examples include a phosphorylation site for Aurora B kinase, the mitosis-specific serine/threonine protein kinase, (R/K)1–3-X-(S/T) or (R/K)-(R/K)-X0–2-(S/T) where X denotes any amino acid;28, 29 the Phox homology domain for binding PI, (R/K)(R/K)(Y/F)xxFxxLxxxL or R(R/K)xxLxx(Y/F);30 the lysosomal targeting sequences, Tyr -X-X-Hyd and LL (where Hyd is any hydrophobic amino acid).31 In all of these cases, as in the motif described in this paper, the motif is part of a structurally specific interaction site. However, the structure of this site is not determined solely by the motif with its large degree of variation and limited number of constraints. Nevertheless, identification of such motifs has been found to be a valuable tool in cell biology. Thus our manuscript extends this concept to PUFA recognition in two studied enzymes. This is of particular importance because of the roles of arachidonic acid in prostanoid metabolism and as an sn-2 acyl chain of lipids in the PI-cycle.
Materials and methods
DGKε constructs
The FLAG epitope-tagged DGKε, 3×HA-DGKα and c -Myc-PIP5K type Iα expression vectors were prepared as previously described.32–34 All correspond to human forms of the respective enzymes. The mutants of FLAG-DGKε, 3×HA-DGKα and c -Myc-PIP5K type Iα were designed using the QuikChange Lightning Kit (Stratagene, La Jolla, CA) according to the instructions of the manufacturer. The presence of the desired mutations was verified by sequencing analysis.
Cell culture
COS -7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO/Invitrogen) containing 10% fetal bovine serum (GIBCO/Invitrogen) at 37 °C in an atmosphere of 5% CO2. The cells were grown to about 70%–80% confluency and transiently transfected with the expression vectors using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The cells were harvested 48 hours after transfection by scraping them into 1X PBS containing 1:100 protease inhibitor cocktail for use with mammalian cells and tissue (Sigma-Aldrich). The cells were pelleted at 5000g at 4 °C and kept at −90 °C until further use.
Immunoblot Analysis
Amounts of protein in the lysates of transfected COS -7 cells were quantified by immunoblotting. Protein samples for immunoblot analysis were prepared by incubation with 2% SDS buffer at 95 °C for 5 min. The resultant proteins were separated by 7.5% Tris-glycine SDS-PAGE and electroblotted onto an Immobilon-P polyvinylidenedifluoride membrane (Millipore). The membrane was then incubated with either a 1:2000 dilution of mouse anti-FLAGM2 (Sigma), 0.5 μg/ml concentration of mouse THE™ anti-HA tag IgG1(GenScript, A01244), 1:800 dilution of mouse anti-c-Myc (Santa Cruz, sc-40), or 1:800 dilution of goat anti-actin (Santa Cruz, sc-1616) as the primary antibody and either a 1:2000 dilution of horseradish peroxidase-conjugated goat anti-mouse (Santa Cruz, sc-2005) or donkey anti-goat antibody (Santa Cruz, sc-2020) as the secondary antibody. The antibody complexes were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences) and X-Omat LS film (Eastman Kodak Co.) according to the manufacturer’s instructions. A 3XFLAG-tagged bacterial alkaline phosphatase (3XFLAG-BAP) (Sigma-Aldrich) with a molecular mass of 49.9 kDa was used for DGKε and its mutants as a standard in different lanes of the same blots.
Enzyme Preparations for Enzymatic Activity Assay
Prior to assay, cell pellets of COS-7 cells overexpressing human 3×FLAG-DGKε WT, 3×HA -DGKα WT or mutants were resuspended in ice-cold cell lysis buffer (1% (v/v) (octylphenoxy) polyethoxyethanol (Nonidet P-40), 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM activated sodium orthovanadate, and 1:100 protease inhibitor cocktail for use with mammalian cells and tissue (Sigma-Aldrich)), allowed to lyse for 10 minutes on ice, sonicated for 5 minutes and then centrifuged at 100,000 g, 30 min at 4 °C. The supernatants were used in the assay of DGK activity. For PIP5K enzyme, cellpellets of COS-7 cells overexpressing human c-Myc-PIP5K type Iα were resuspended in ice-cold cell lysis buffer (2% (v/v) (octylphenoxy)polyethoxyethanol (Nonidet P-40), 20 mM Tris/HCl pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 μg/mL aprotinin and leupeptin, 1 mM PMSF, 5 mM NaF, 100 μg/mL soybean trypsin inhibitor, and 1:100 protease inhibitor cocktail for use with mammalian cells and tissue (Sigma-Aldrich)), allowed to lyse for 10 minutes on ice, sonicated for 10 minutes and then incubated with agarose beads conjugated with anti-c-Myc antibodies (Santa Cruz, sc-40 AC) at 4 C overnight. After that the beads were centrifuged and washed 1 time with IP kinase buffer (25 mM Tris, pH 7.5, 100 mM NaCl, 0.1% Triton X-100); 1 time with PBS pH 6.0, 0.5% Triton X-100; 1 time with 25 mM Tris, pH 8, 100 mM NaCl, 0.1% Triton X-100; 1 time with 25 mM Tris, pH 7.5, 500 mM NaCl, 0.1% Triton X-100; and 1 time with IP kinase buffer 20. After the final wash the beads were briefly centrifuged and resuspended in 1× assay buffer.
Quantification of Phosphatidic Acid and PI(4)P
The concentration of all PA and PI(4)P stocks used in this study was determined experimentally based on an assay for inorganic phosphate as described previously.35 Briefly, 30 μl of 10% (w/v) Mg(NO3)2in 95% ethanol was added to an aliquot of PA (up to 80 nmol) in an acid-washed Pyrex tube, which was subsequently flamed until the organic phosphate was completely ashed. 350 μl of 0.5 N HCl was then added, and the mixture was subsequently incubated at 100 °C for 15 min. Afterward, 750 μL of a 1:6 mixture of 10% (w/v) L-ascorbic acid, 0.42% (w/v) ammonium molybdate tetrahydrate in 1 N H2SO4was added. The mixture was then incubated at 60 °C for 10 min and allowed to cool to room temperature where its absorbance was read at 820 nm.
Detergent-Phospholipid-Mixed Micelle-based DGK Enzymatic Activity Assay
DGK was assayed for enzymatic activity using a detergent-phospholipid-mixed micelle-based protocoldescribed by Walsh et al. 9 as previously employed in our laboratory.32 Lipid films composed of the substrate (DAG) along with any phospholipid component required in the assay (PA and/or 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) (for DGKε) and/or 1,2-dioleoyl-sn-glycero-3-[phospho-L-serine] (DOPS) (for DGKα)) were made at a constant total lipid concentration of 5.75 mM (see Table 1 for the list of the lipids). Mixed micelles were formed by hydrating these lipid films with 50 μL of 4×Assay Buffer (200 mM Tris -HCl (pH 7.5), 400 mM NaCl, 20 mM MgCl2, 4 mM EGTA, 1 mM dithiothreitol) containing 60 mM Triton X-100 and subsequently vortexing the hydrated lipid film for 2 min. Lysates from COS-7 cells expressing DGK were added to the mixed micelles along with double distilled H2O to a final volume of 180 μL. The reaction was initiated by adding 20 μL of 1 mM [γ-32P]-ATP (50 μCi/ml) (PerkinElmer Life Sciences), allowed to proceed for 10 min at 25 °C, and terminated with 2 ml of stop solution (1:1 CHCl3/CH3OH, 0.25 mg/mL dihexadecyl phosphate). The organic layer was washed three times with 2 ml of wash solution (7:1 H2O/CH3OH, 1% HClO4, 0.1% H3PO4). An aliquot of the organic layer was used to quantify the incorporation of 32P into PA using Cerenkov counting. All enzymatic activity data presented in this study should be considered as data obtained from initial rate experiments, because the formation of the product PA was linear over the 10-min reaction period. Negative controls were run with the addition of lysates from mock-transfected COS-7 cells and were confirmed to have activity levels significantly below lysates from cells overexpressing DGK. The measured activities of DGK enzymes could only be detected if exogenous lipid substrate was added, further indicating that endogenous lipids do not provide a sufficient concentration of substrate for their phosphorylation to be detected. In addition, the DGK activity of cells transfected with DGKε is specific for SAG, while cells expressing DGKα can phosphorylate either SAG or SLG equally well. This finding is in accord with the known substrate specificities of these isoforms and is additional evidence that we are measuring the properties of the over-expressed enzyme. The activity measured with mock-transfected cells was subtracted from the values obtained using cells overexpressing one of the DGKε or DGKα constructs. The assays were performed in triplicate and presented as the mean ± S.D. Each experiment was independently repeated at least two times. The day to day variations using the same enzyme preparation and the same lipids were not much greater than those for an individual experiment. The concentrations of the individual lipid components of the mixed micelles are listed as their mole percentage of the detergent-phospholipid-mixed micelle, because DGKs are interfacial enzymes, and therefore, the concentrations of the individual lipid components at the surface of the mixed micelle are important in affecting DGK enzymatic activity rather than the bulk concentrations of the lipid components.
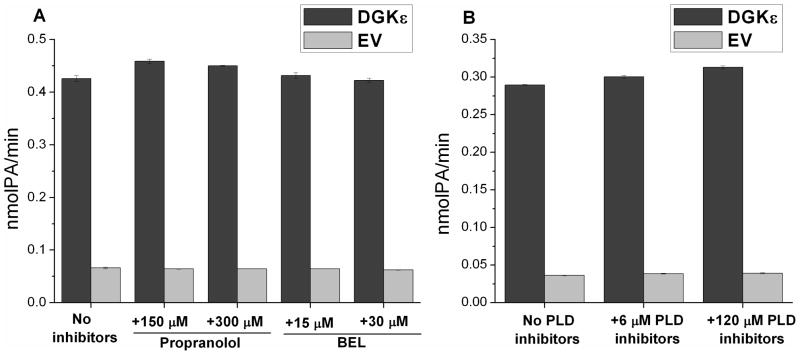
We also confirmed that the measured activity of DGK is not substantially affected by product degradation as a result of the hydrolysis of PA by endogenous PA phosphatases (PAP), or as a result of the upregulation by endogenous protein kinase C (PKC) and phospholipases D (PLD). Our results showed that the DGKε activity measured in the absence and presence of either propranolol (PAP36 and PKC37 inhibitor, from Sigma- Aldrich), bromoenol lactone (BEL, PAP inhibitor,38 from Sigma-Aldrich) (Fig. 7A), or PLD inhibitors (Avanti Polar Lipids, equimolar mix of VU0359595,39 VU015505640 and VU0285655–141 inhibitors) (Fig. 7B) are not significantly different.
Figure 7.
Comparison of the FLAG-DGKε enzyme activities in the absence and presence of PA phosphatase (PAP) inhibitors, protein kinase C (PKC) and phospholipase D (PLD) inhibitors. A. Propranolol (PAP and PKC inhibitor) and bromoenol lactone (BEL, PAP inhibitor) do not affect the measured DGKε activity. B. 6 μM or 120 μM PLD inhibitors (Avanti Polar Lipids, equimolar mix of VU0359595, VU0155056 and VU0285655-1 inhibitors) do not significantly affect the measured DGKε activity. Negative control (EV) is performed with the lysates from COS-7 cells transfected with empty vector (p3XFLAG-CMV-7.1, Sigma-Aldrich).
Detergent-Phospholipid-Mixed Micelle-based PIP5K Enzymatic Activity Assay
PIP5 kinase activity assay was performed as described by Parker et al.42 with the following modifications. Reactions were performed in a 100 μL reaction volume in a standard buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 100 mM NaCl, 1 mM EGTA, 0.1% Triton X-100 and 50 μM [γ-32P]ATP (2μCi/reaction). The reaction was stopped after 10 min by the addition of 500 μL of 1 N HCl and 2 mL of chloroform:methanol (1:1) simultaneously. The assay was washed twice with 1 mL of methanol:1N HCl. An aliquot of the organic layer was used to quantify the incorporation of 32P into PI(4,5)P2 using Cerenkov counting. Negative controls were run with the addition of beads immunoprecipitated from mock-transfected COS-7 cells and were confirmed to have activity levels significantly below immunoprecipitates from cells overexpressing PIP5K. Kinetic parameters were calculated using the effective concentration of PI4P at the surface of the micelle. To be consistent with previously presented data on this enzyme,42 the effective surface concentra tion of PI(4)P was calculated by multiplying themole fraction of PI(4)P at the surface of the micelle by the total concentration of PI(4)P.
Kinetic Analysis of the Micelle-Based Assay of DGK and PIP5K Activity
The Michaelis-Menten constants, Vmax and Km, were evaluated by a nonlinear regression analysis(initial velocity (v 0) versus substrate concentration ([S])), as well as by using Hanes plots ([S]/v0 versus [S]). The content of DGK and PIP5K was determined using immunoblot analysis as described above. Microcal Origin software was used to determine kcat, Vmaxand K m parameters.
Acknowledgments
We are grateful to Ms Jessica Ngui-Yen for assistance in preparing one of the mutant proteins. This work was supported in part by a grant from the Natural Sciences and Engineering Research Council of Canada, grant 9848 (to R.M.E.) and from the National Institutes of Health GrantR01CA095463 (to M.K.T.).
Abbreviations
- AA
arachidonic acid
- BEL
PAP inhibitor
- DAG
diacylglycerol
- DGK
diacylglycerol kinase
- LOX
lipoxygenase
- PA
phosphatidic acid
- PAP
PA phosphatases
- PI
phosphatidylinositide
- PI(4
5)P2, phosphatidylinositol-4,5-bisphosphate
- PI(4)P
phosphatidylinositol-4-phosphate
- PIP5K
phosphatidylinositol 4-phosphate 5-kinase
- PKC
protein kinase C
- PLD
phospholipases D
- di -PUFA-PA
phosphatidic acid with two polyunsaturated acyl chains
- PUFA
polyunsaturated fatty acid; see also Table 1 for lipid nomenclature used.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laye S. Polyunsaturated fatty acids, neuroinflammation and wellbeing. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4–6):295–303. doi: 10.1016/j.plefa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Back M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009;23:41–48. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Wang S, Wu N, Yang CS. Leukotriene A4 hydrolase as a target for cancer prevention and therapy. Curr Cancer Drug Targets. 2004;4:267–283. doi: 10.2174/1568009043333041. [DOI] [PubMed] [Google Scholar]
- 4.Rådmark O, Samuelsson B. 5-lipoxygenase: mechanisms of regulation. J Lipid Res. 2009;50:S40–S45. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 6.Buczynski MW, Dumlao DS, Dennis EA. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Chacón G, Astudillo AM, Balgoma D, Balboa MA, Balsinde J. Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim Biophys Acta. 2009;1791:1103–1113. doi: 10.1016/j.bbalip.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott SM, Bazan NG. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoylinositol lipid signaling. Proc Natl Acad Sci U S A. 2001;98:4740–4745. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh JP, Suen R, Lemaitre RN, Glomset JA. Arachidonoyl-diacylglycerol kinase from bovine testis. Purification and properties. J Biol Chem. 1994;269:21155–21164. [PubMed] [Google Scholar]
- 10.Thirugnanam S, Topham M, Epand R. Physiological implications of the contrasting modulation of the activities of the epsilon- and zeta-isoforms of diacylglycerol kinase. Biochemistry. 2001;40(35):10607–10613. doi: 10.1021/bi010609s. [DOI] [PubMed] [Google Scholar]
- 11.Milne SB, Ivanova PT, Armstrong MD, Myers DS, Lubarda J, Shulga YV, Topham MK, Brown HA, Epand RM. Dramatic differences in the roles in lipid metabolism of two isoforms of diacylglycerol kinase. Biochemistry. 2008;47(36):9372–9379. doi: 10.1021/bi800492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lung M, Shulga YV, Ivanova PT, Myers DS, Milne SB, Brown HA, Topham MK, Epand RM. Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J Biol Chem. 2009;284:31062–31073. doi: 10.1074/jbc.M109.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neau DB, Gilbert NC, Bartlett SG, Boeglin W, Brash AR, Newcomer ME. The 1.85 A structure of an 8R-lipoxygenase suggests a general model for lipoxygenase product specificity. Biochemistry. 2009;48(33):7906–7915. doi: 10.1021/bi900084m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Biol Chem. 1996;271:10237–10241. [PubMed] [Google Scholar]
- 15.Epand RM, Kam A, Bridgelal N, Saiga A, Topham MK. The alpha isoform of diacylglycerol kinase exhibits arachidonoyl specificity with alkylacylglycerol. Biochemistry. 2004;43:14778–14783. doi: 10.1021/bi0484724. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli-Daley LA, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, Abrams CS, Kanaho Y, De Camilli P. Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. J Biol Chem. 2010;285(37):28708–28714. doi: 10.1074/jbc.M110.132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273(37):23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 18.Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J Biol Chem. 2002;277(26):23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 19.Mérida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409(1):1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 20.Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, Davis JN. Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate. J Cell Biochem. 2007;100(1):112–128. doi: 10.1002/jcb.21027. [DOI] [PubMed] [Google Scholar]
- 21.Epand RM. Cholesterol and the interaction of proteins with membrane domains. Prog Lipid Res. 2006;45(4):279–294. doi: 10.1016/j.plipres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139(12):4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- 23.Boesze-Battaglia K, Brown A, Walker L, Besack D, Zekavat A, Wrenn S, Krummenacher C, Shenker BJ. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J Biol Chem. 2009;284(16):10650–10658. doi: 10.1074/jbc.M809094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saher G, Quintes S, Mobius W, Wehr MC, Kramer-Albers EM, Brugger B, Nave KA. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci. 2009;29:6094–6104. doi: 10.1523/JNEUROSCI.0686-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell Biochem. 2010;51:77–108. doi: 10.1007/978-90-481-8622-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scolari S, Müller K, Bittman R, Herrmann A, Müller P. Interaction of Mammalian Seminal Plasma Protein PDC-109 with Cholesterol: Implications for a Putative CRAC Domain. Biochemistry. 2010;49(42):9027–9031. doi: 10.1021/bi101257c. [DOI] [PubMed] [Google Scholar]
- 27.Jafurulla M, Tiwari S, Chattopadhyay A. Identification of cholesterol recognition amino acid consensus (CRAC) motif in G-protein coupled receptors. Biochem Biophys Res Commun. 2011;404:569–573. doi: 10.1016/j.bbrc.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Kwon HR, Bae CD, Park J, Hong KU. Specific primary sequence requirements for Aurora B kinase-mediated phosphorylation and subcellular localization of TMAP during mitosis. Cell Cycle. 2010;9(10):2027–2036. doi: 10.4161/cc.9.10.11753. [DOI] [PubMed] [Google Scholar]
- 29.Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15:778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 30.Sato TK, Overduin M, Emr SD. Location, location, location: membrane targeting directed by PX domains. Science. 2001;294(5548):1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- 31.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3(12):919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 32.Dicu AO, Topham MK, Ottaway L, Epand RM. Role of the hydrophobic segment of diacylglycerol kinase epsilon. Biochemistry. 2007;46:6109–6117. doi: 10.1021/bi6024726. [DOI] [PubMed] [Google Scholar]
- 33.Topham MK, Prescott SM. Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism. J Cell Biol. 2001;152(6):191135–43. doi: 10.1083/jcb.152.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo B, Regier DS, Prescott SM, Topham MK. Diacylglycerol kinases. Cell Signal. 2004;16(9):983–989. doi: 10.1016/j.cellsig.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 36.Pappu AS, Hauser G. Propranolol-induced inhibition of rat brain cytoplasmic phosphatidate phosphohydrolase. Neurochem Res. 1983;8(12):1565–1575. doi: 10.1007/BF00964158. [DOI] [PubMed] [Google Scholar]
- 37.Sozzani S, Agwu DE, McCall CE, O’Flaherty JT, Schmitt JD, Kent JD, McPhail LC. Propranolol, a phosphatidate phosphohydrolase inhibitor, also inhibits protein kinase C. J Biol Chem. 1992;267(28):20481–20488. [PubMed] [Google Scholar]
- 38.Balsinde J, Dennis EA. Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J Biol Chem. 1996;271(50):31937–31941. doi: 10.1074/jbc.271.50.31937. [DOI] [PubMed] [Google Scholar]
- 39.Lewis JA, Scott SA, Lavieri R, Buck JR, Selvy PE, Stoops SL, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I. Impact of alternative halogenated privileged structures for PLD1 specificity. Bioorg Med Chem Lett. 2009;19:1916–1920. doi: 10.1016/j.bmcl.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nature Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavieri R, Scott SA, Lewis JA, Selvy PE, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part II. Identification of the 1,3,8-triazaspiro[4,5]decan-4-one privileged structure that engenders PLD2 selectivity. Bioorg Med Chem Lett. 2009;19:2240–2243. doi: 10.1016/j.bmcl.2009.02.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker GJ, Loijens JC, Anderson RA. Detection of phosphatidylinositol-4-phosphate 5-kinase activity using thin-layer chromatography. Methods Mol Biol. 1998;105:127–139.41. doi: 10.1385/0-89603-491-7:127. [DOI] [PubMed] [Google Scholar]