Abstract
Objective
To determine potential factors regulating gluconeogenesis in Extremely Low Birth Weight (ELBW) infants receiving total parenteral nutrition (TPN).
Study design
Seven infants (0.824±0.068 kg; 25.4±0.5 wks; 3.3±0.2 d) were studied for 11 hours, with parenteral lipid and amino acids continued at pre-study rates. Glucose was supplied at pre-study rates for the first 5 h (period 1), was then reduced to 6 mg/kg.min for 1 h and further to ~3mg/kg.min for 5 h (period 2); 2.5 mg/kg.min of the glucose was replaced by [U-13C]glucose throughout the study for measurements of glucose production and gluconeogenesis. Concentrations of glucose, insulin, glucagon and cortisol were determined. Data obtained during periods 1 and 2 were compared using paired t-test.
Results
Gluconeogenesis and glucose production remained unchanged (2.12±0.23 vs. 1.84±0.25 mg/kg.min (NS) and 2.44±0.27 vs. 2.51±0.31 mg/kg.min (NS), respectively), despite a 60% reduction of the glucose infusion rate and subsequent 30% (124.7±10.8 to 82.6±8.9 mg/dL (p=0.009) and 70% (26.9±4.7 to 6.6±0.4 μU/mL (p=0.002)) decreases in glucose and insulin concentrations, respectively. Cortisol and glucagon concentrations remained unchanged.
Conclusion
In ELBW infants receiving TPN, gluconeogenesis is a continuous process that is not affected by infusion rates of glucose or concentrations of glucose or insulin.
The fine regulation of glucose metabolism in adults is primarily exerted by insulin and secondarily by its counter regulatory hormones glucagon, epinephrine, cortisol and growth hormone (1). In contrast, infants born prematurely are at high risk of disturbed glucose homeostasis resulting from limited substrate availability and potentially immature regulation of glucose metabolism. To prevent hypoglycemia and promote growth and development, infants born prematurely routinely receive total parenteral nutrition with a glucose supply exceeding the normal glucose turnover rates for infants [2-8] from their first days of life. However, their tolerance for parenteral glucose is low resulting in a frequent occurrence of hyperglycemia [2-3, 9]. There is a paucity of data on which substrates and hormones regulate glucose production from its two sources, gluconeogenesis and glycogenolysis, during total parenteral nutrition (TPN) in preterm infants. This information is crucial to optimize nutritional strategies in this population.
We and others have demonstrated that preterm infants initiate gluconeogenesis within the first days of life both from endogenous body fuel stores and exogenous lipid and amino acid substrate [10-13]. Further, a variable suppression of glucose production was demonstrated in preterm infants receiving parenteral glucose [4, 14-16]. In these studies, the infants received only glucose (i.e. no lipid and amino acids) and the partition of glucose production into its components gluconeogenesis and glycogenolysis were not measured.
Cowett et. al addressed the impact of insulin and its counter-regulatory hormones on glucose production in a systematic fashion in newborn lambs [17]. They conclude that insulin has a greater effect on glucose uptake than on glucose production and, glucagon, cortisol and growth hormone have no major effects on glucose production even during hyperinsulinemic hypoglycemia.
In a recent study, we demonstrated that in very low birth weight infants receiving routine TPN, glucose production was not completely suppressed and that gluconeogenesis constituted the major part of residual glucose production [18]. Building on these results, the present study was conducted during routine TPN providing glucose at high rates and in response to reducing the glucose infusion (as part of TPN) to half normal newborn glucose turnover rate. This approach enabled us to investigate potential factors regulating gluconeogenesis in extremely low birth weight (ELBW) infants. We hypothesized that gluconeogenesis would remain unchanged in response to reduction in the glucose supply and subsequent decreases in concentrations of glucose and insulin in ELBW infants receiving TPN due to imprecise hormonal regulation of glucose metabolism. In addition because normoglycemia had to be maintained in all infants, glucagon and cortisol, which have their effects under hypoglycemic conditions [19-21], were not expected to change. Therefore, these hormones would not affect gluconeogenesis during administration of TPN providing gluconeogenic substrates.
Methods
The protocol was reviewed and approved by the Institutional Review Board for Human Research at Baylor College of Medicine and the Advisory Board of the General Clinical Research Center at Texas Children's Hospital, Houston TX. The infants were recruited from the Neonatal Intensive Care Unit at Texas Children's Hospital and they were enrolled with written parental consent.
Seven premature infants (5 boys and 2 girls) fulfilling our inclusion criteria i.e. gestational age ≤ 29 wks; AGA (appropriate for gestational age); and absence of syndromes, anomalies and sepsis were studied. The mothers did not have diabetes or substance abuse. All mothers except one had received antenatal steroids. Four infants were delivered vaginally and three by cesarean section. The average Apgar score at 5 min was 7 with no infant having less than 5. Subject characteristics are shown in Table I. Further, the infants must be clinically stable on IPPV (Intermittent Positive Pressure Ventilation) or CPAP (Continuous Positive Airway Pressure) and not have a mother with diabetes or substance abuse.
Table 1.
Subject Characteristics
| Subject # | Sex | Gestational Age wks | Birth Weight kg | Postnatal Age days |
|---|---|---|---|---|
| 1 | m | 27 | 0.930 | 3 |
| 2 | m | 27 | 1.085 | 3 |
| 3 | m | 25 | 0.962 | 3 |
| 4 | m | 24 | 0.572 | 4 |
| 5 | m | 26 | 0.808 | 4 |
| 6 | f | 25 | 0.747 | 3 |
| 7 | f | 24 | 0.605 | 3 |
| Mean ± SE | 25.4 ± 0.5 | 0.824 ± 0.068 | 3.3 ± 0.2 |
At the time of the study, 6/7 infants were receiving caffeine and 2/7 dopamine (7.5 μg/kg.min and 10.0 μg/kg.min, respectively). The infants had received antibiotics (Ampicillin + Gentamicin) prior to study, but in all of them antibiotics had been discontinued before start of the study. No infant had positive cultures or clinical signs of sepsis. In addition, concentrations of C-reactive protein (CRP) were measured to confirm the absence of severe illness. None of the infants had received insulin. The infants were clinically stable with oxygen saturation between 85 and 95% either on Intermittent Positive Pressure Ventilation (IPPV) (n=2) (20/5 cm H2O and FiO2 0.28 ± 0.03) or Continuous Positive Airway Pressure (CPAP) (n=5) (8 ± 1 cm H2O and FiO2 0.27 ± 0.04).
Parenteral nutrition
Amino acids (TrophAmine, Braun Medical Inc. Bethlehem, PA) were administered at the pre-study rates as ordered by the attending physician throughout the 11 hour study (2.04 ± 0.10 mg/kg.min; range 1.67-2.36 mg/kg.min corresponding to 2.4-3.4 g/kg.d).
Lipid (20% Intralipid, Kabivitrum, Stockholm, Sweden) was also administered at the pre-study rates throughout the 11 h study (1.12 ± 0.21 mg/kg.min; range 0.50-2.08 mg/kg.min = 0.72-3.00 g/kg.d).
To test the effect of a change in the glucose infusion rate on gluconeogenesis, we used two different glucose infusion rates. During the first 5 h of the study, glucose was given at the pre-study rates (8.4 ± 0.5 mg/kg.min; range 6.9-10.0 mg/kg.min corresponding to 10.0-14.4 g/kg.d) (denoted period 1). At study hour 5 (directly after a 5 hour blood sample), the glucose infusion rate was reduced step wise (to minimize counter regulatory responses) first to 6 mg/kg.min (i.e. corresponding to the normal glucose turn over rate of newborn infants [2-8]) for one hour and then further to 3.4 ± 0.2 mg/kg.min (range 2.9-4.1 mg/kg.min) for the remaining 5 h of the 11 h study period (denoted period 2).
Compounds labeled with stable isotopes
During the entire 11 hour study period, 2.5 ± 0.1 mg/kg.min of the above glucose infusion rates was replaced by [U-13C]glucose (metabolically equivalent to unlabelled natural glucose and used to measure glucose appearance rate and glucose production from gluconeogenesis and glycogenolysis). In addition, throughout the entire 11 hour study, [1-13C]leucine was given at a constant rate of 0.01 ± 0.0 mg/kg.min (0.6 ± 0.0 mg/kg.hr) to measure leucine turn over. The [U-13C]glucose (99 atom % 13C) and [1-13C]leucine (99 atom % 13C) were purchased sterile and pyrogen free from Cambridge Isotope Laboratories (Andover, MA) and were mixed and dissolved in normal saline at 12.5 and 0.5 mg/ml, respectively, by the Investigation Pharmacy at Texas Children's Hospital, Houston, TX. The tracer solutions were again tested for sterility and pyrogenicity and placed in sealed vials under sterile conditions by the investigational pharmacy. The TPN solution and the compounds labeled with stable isotopes were infused via umbilical venous catheters.
Blood sampling
Blood samples (a total of 3 ml/kg) were obtained at start of the study and at study hours 4.5, 5 (representing period 1), and 10.5 and 11 (representing period 2). The blood samples were obtained via umbilical artery catheters already in place for clinical care purposes.
Analyses
The isotopic enrichments and the mass isotopomer distribution of glucose and lactate during the [U-13C]glucose infusion were determined by gas chromatography – mass spectrometry (GCMS) (6890/5973 Agilent Technologies, Wilmington, DE). The pentaacetate and acetyl-pentafluorobenzyl derivative of glucose and lactate, respectively, were prepared as previously described (10). The blood samples were analyzed for blood glucose concentration using a glucose analyzer (YSI 2300 Stat Plus, YSI Inc. Yellow Springs, OH, USA). The 13C isotopic enrichments of α-ketoisocaproic acid (KIC), the intracellular transamination product of leucine, was measured by GCMS using the oxime-terbutyldimethylsilyl derivative [10]
Insulin and Glucagon concentrations were determined by radioimmunoassays (Millipore, Billerica, MA). Other measurements were Cortisol, Adiponectin and C-reactive protein concentrations with non radioactive human ELISA kits; Cortisol kit from IBL Transatlantic, Toronto, ON and, Adiponectin and CRP from Millipore Corporation, Billerica, MA.
All kinetic measurements were performed under steady state conditions. Rates of total glucose appearance in plasma, glucose production and gluconeogenesis were measured at study hours 4.5 and 5 (period 1) and 10.5 and 11 (period 2). Total plasma glucose appearance rate (glucose Ra) was calculated from the M+6 enrichment of [U-13C]glucose in plasma using established isotope dilution equations [6,10].
Rate of glucose production (mg/kg.min) (GPR) = glucose Ra – exogenous glucose (labeled and unlabeled).
Fractional gluconeogenesis (i.e. gluconeogenesis as a fraction of glucose Ra) was calculated using [U-13C]glucose mass isotopomers distribution analyses (MIDA) [10-12, 22]. Rates of gluconeogenesis were calculated as the product of total glucose appearance rate (glucose Ra) [10] and fractional gluconeogenesis
Rate of Gluconeogenesis (mg/kg.min) (GNG rate) = gluc Ra × GNG % Ra
Glycogenolysis was calculated by subtracting the rate of gluconeogenesis from the glucose production rate.
Rate of glycogenolysis (mg/kg.min) = GPR - GNG rate
Total leucine rate of appearance was calculated from the [13C]KIC enrichment using the “reciprocal pool” model [23]. Rate of appearance of endogenous leucine was calculated by subtracting the rate of infusion of exogenous leucine (TPN) from total leucine Ra and was considered an indicator of proteolysis. Leucine turnover values were converted to protein turnover assuming the content of leucine in body proteins is ~8% [24].
Statistical analyses
Absolute rates of gluconeogenesis, concentration of glucose, insulin, adiponectin, cortisol and glucagon obtained during the first 5 h period (period 1 representing high glucose infusion rate) were compared with those obtained during the last 5 h period (period 2 representing low glucose infusion rate) using paired t-tests. To account for multiple testing a p value <0.01 was used to define significance. Linear regression analysis was used to analyze relationships between measured variables i.e. gluconeogenesis, glucose appearance rate, glucose production rate, glucose concentration, and the various hormone concentrations. All results are provided as mean ± SE.
Results
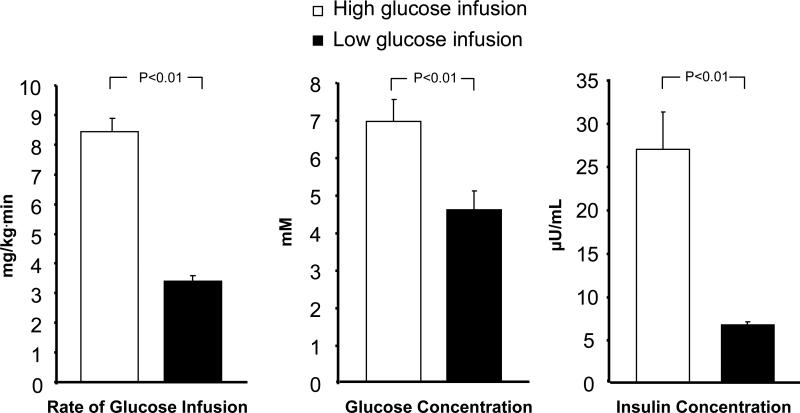
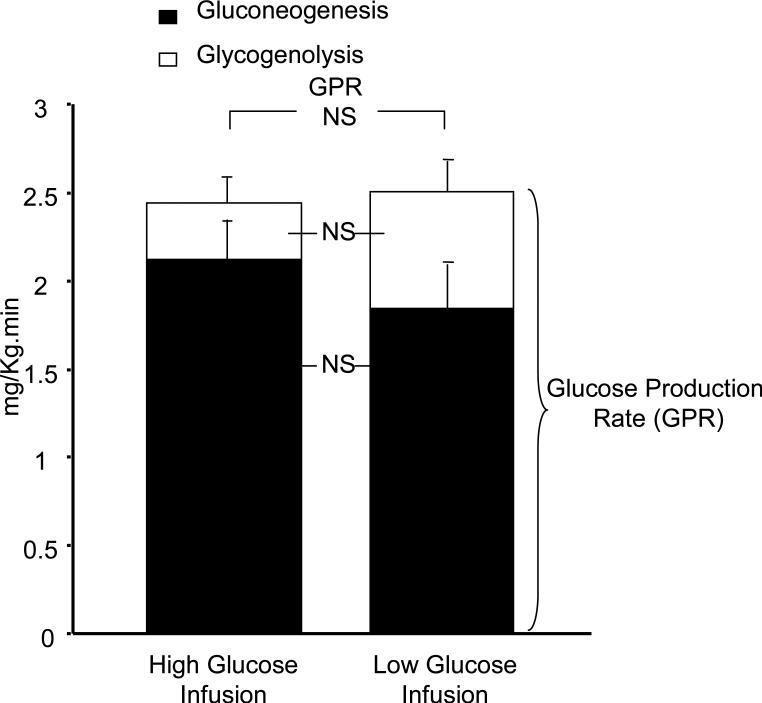
Concentrations of glucose and insulin are depicted in Figure 1, and glucagon, cortisol, adiponectin and CRP in Table II. Glucose Appearance Rate averaged 10.88 ± 0.61 mg/kg.min (60.39 ± 3.39 µmol/kg.min) during period 1 and 5.92 ± 0.24 mg/kg.min (32.86 ± 1.33 µmol/kg.min) during period 2 (p=<0.001). Glucose Production Rates did not differ significantly between periods 1 and 2, 2.4 ± 0.3 mg/kg.min (13.5 ± 1.5 μmol/kg.min) vs. 2.5 ± 0.3 mg/kg.min (13.9 ± 1.7 μmol/kg.min) (NS). This shows that the difference in glucose appearance rate between periods 1 and 2 was solely a result of the difference in the glucose infusion rates. Rates of Gluconeogenesis and Glycogenolysis are shown in Figure 2 demonstrating that there was no difference in the rates between the two infusion rate periods (NS). Gluconeogenesis accounted for 89 ± 5% and 75 ± 5% of glucose production during periods 1 and 2, respectively (NS).
Figure. 1.
Rates of glucose infusion and concentrations of insulin and glucose during the high and low glucose infusion rate periods.
Table 2.
Concentrations of Glucagon, Cortisol, Adiponectin and CRP during high and low glucose infusion
| Period 1 High glucose infusion period | Period 2 Low glucose infusion period | p Value | |
|---|---|---|---|
| Glucagon (pg/mL) | 85.8 ± 8.2 | 94.8 ± 11.5 | 0.29 |
| Cortisol (ng/mL) | 151 ± 29 | 183 ± 39 | 0.09 |
| Adiponectin (ng/mL) | 9576 ± 1794 | 9686 ± 1383 | 0.92 |
| CRP (μg/mL) | 1.26 ± 0.46 | 1.37 ± 0.55 | 0.45 |
Figure. 2.
Rates of glucose production, gluconeogenesis and glycogenolysis during the high and low glucose infusion rate periods. Error bars refer to gluconeogenesis and glycogenolysis, respectively.
Total leucine rate of appearance was 0.73 ± 0.04 mg/kg.min (5.59 ± 0.33 µmol/kg.min) during period 1 and 0.71 ± 0.03 mg/kg.min (5.38 ± 0.22 µmol/kg.min) for period 2 (NS). Thus, the total rate of proteolysis was 13.2 ± 0.8 g/kg.day during period 1 and 12.7 ± 1.4 g/kg.day during period 2 (NS). Endogenous leucine Ra from proteolysis averaged 0.45 ± 0.04 mg/kg.min (3.40 ± 0.27 µmol/kg.min) during period 1 and 0.42 ± 0.02 mg/kg.min (3.20 ± 0.16 µmol/kg.min) for period 2, which corresponds to a total rate of endogenous proteolysis of 8.04 ± 0.65 g/kg.day during period 1 and 7.55 ± 0.37 g/kg.day during period 2 (NS).
Linear Regression Analyses did not show any effect of glucose infusion rate or glucose, insulin, glucagon, cortisol, adiponectin, and CRP concentrations on gluconeogenesis during either of the glucose infusion rate periods. During period 1, linear regression analysis demonstrated that glucose infusion rate explained 54% of the variation in glucose concentration, (R2=0.54; p=0.04). Adding glucose production to the regression analysis, increased the R2 value to 0.83 (p=0.013). During period 2, the glucose infusion rate was the same in all subjects by design (~3 mg/ kg.min). Thus, as expected there was no relationship between glucose infusion rate and glucose concentration. However, blood glucose concentration was significantly related to the glucose production rate, R2=0.60; p=0.026. The plasma glucose concentration was also not affected by glucagon, cortisol, adiponectin or CRP concentrations during either period 1 or 2.
Leucine rate of appearance from proteolysis and thus, total protein turnover was not affected by glucose infusion rate, plasma glucose, insulin or cortisol concentrations during the two glucose infusion rate periods.
Discussion
We recently reported that gluconeogenesis continues in preterm infants receiving routine TPN providing glucose at rates exceeding normal infant glucose turnover rates [18]. However, there are no reports on the regulation of gluconeogenesis in preterm infants. Our primary purpose was to determine whether insulin or glucose concentration regulates gluconeogenesis in ELBW infants receiving TPN. We demonstrated that gluconeogenesis remained unchanged and accounted for the major contribution to glucose production whether the infants received TPN with a glucose supply exceeding normal infant glucose turnover rate or the glucose infusion rate was reduced by 60%. In response to the reduction of the glucose infusion rate, glucose and insulin concentrations decreased by 30% and 70%, respectively (Figure 1). This clearly demonstrates that gluconeogenesis is not acutely affected by either insulin or glucose concentrations in ELBW infants receiving TPN. There was a strong relationship between the decreases in glucose and insulin concentrations between periods 1 and 2 showing that these immature infants were able to adjust insulin in response to the lower glucose concentrations.
The secondary objective was to investigate potential effects of the insulin counter regulatory hormones, glucagon and cortisol (limitations in the blood volumes that can be safely withdrawn in ELBW infants precluded measurement of epinephrine and growth hormone) on gluconeogenesis. We did not observe any changes in these hormones in response to the reduction in the glucose infusion rate and subsequent decreases in glucose and insulin concentrations. Glucagon increased plasma free fatty acid and ketone body concentrations in humans even under conditions of elevated plasma insulin concentrations and stimulated synthesis of phosphoenolpyruvate, a key step of the gluconeogenic pathway [20, 25]. Cortisol is known to be a counter regulatory hormone during prolonged hypoglycemia and stimulates gluconeogenesis by increasing the delivery of gluconeogenic substrates via lipolysis and proteolysis, and by enhancing the activity of key gluconeogenic enzymes [26-31]. Because our infants received lipid and amino acid substrate and remained normoglycemic, the lack of changes in glucagon and cortisol concentrations is not surprising. Under these conditions, the importance of these hormones in the regulation of gluconeogenesis is limited. In neonatal lambs, there was an imprecise response in the concentrations of glucagon and cortisol to hyperinsulinemic hypoglycemia and these hormones did not affect glucose production [17].
Incomplete suppression of glucose production in preterm infants was reported previously [4, 14-15]. However, in these earlier studies the infants received only glucose i.e. no lipid and amino acids. This is an important difference because TPN is given soon after birth according to current nutritional guidelines. Further, the contribution from gluconeogenesis and glycogenolysis were not measured. Kalhan et al [13] did not observe any differences in gluconeogenesis between preterm infants receiving glucose alone or glucose plus lipid and amino acids using a method that included only the gluconeogenic contribution from pyruvate (i.e. glycerol is not included). In contrast, the method used in the present study provides an estimate of total gluconeogenesis i.e. new glucose generated from all non-carbohydrate sources including glycerol, which accounts for the major part of gluconeogenesis in preterm infants receiving TPN [10-11]. Thus, as expected, estimates of gluconeogenesis were lower in their study [13].
Gluconeogenesis remained unchanged in the infants despite the substantial reduction in glucose infusion rate. However, the infants were able to maintain normoglycemia by producing glucose primarily via the gluconeogenic pathway in addition to the low glucose supply confirming our previous reports [10-12]. This indicates that the rates of gluconeogenesis were already appropriately high during the low glucose infusion period but was not suppressed during the high glucose infusion rate period, despite exogenous glucose was supplied at rates exceeding the estimated needs of the infants.
Studies in human adults have shown fine regulation of glucose metabolism in response to intravenous glucose administration with complete or nearly complete suppression of glucose production [14, 32-33]. Similarly, Cowett et. al reported that in adult sheep, glucose production was suppressed at relatively low glucose infusion rates (~6 mg/kg.min), while it was sustained in newborn lambs until the glucose infusion reached very high rates (~22 mg/kg.min) (34). In addition, there is evidence for the continuation of gluconeogenesis from lactate during a wide range of glucose and insulin infusion rates in newborn lambs [35]. Further, Farrag et. al showed that suppression of glucose production in preterm infants (32-33 wks) was only minimally affected by the insulin concentration during infusion of insulin at various rates [36]. In contrast, they observed a near complete suppression of glucose production in adults (~89%) during insulin infusion [36]. In the present study, gluconeogenesis accounted for ~80% of glucose production regardless of glucose infusion rate and resulting glucose concentration, suggesting persistent glucose production in extremely low birth weight infants is primarily a result of ongoing gluconeogenesis.
Hepatic insulin resistance could be a potential reason for the lack of suppression of gluconeogenesis in extremely low birth weight infants. However, insulin sensitivity is difficult to measure in these infants. Some studies have demonstrated correlation between adiponectin and insulin sensitivity [37-38]. Therefore, we measured adiponectin as a potential indicator of insulin sensitivity. We found that adiponectin concentrations were similar during the two glucose infusion periods and were not related to rates of gluconeogenesis. The adiponectin concentrations found in our infants were of the same magnitude as those of the lean insulin sensitive adolescents we have previously reported [39]. This might indicate that sustained gluconeogenesis in these infants is not due to hepatic insulin resistance.
Our data also demonstrate that despite substantially higher insulin concentrations during infusion of glucose at high rates, blood glucose concentrations were elevated. We speculate that further increase in insulin would not have any major effects on the blood glucose concentration, which would support the reports of minimal clinical benefits and negative outcome of early insulin therapy in extremely low birth weight infants [40-41]. Additionally, similar protein turnover data during the two periods demonstrates that reduced supply of glucose as a part of TPN does not increase proteolysis in extremely low birth weight infants. This might imply that ongoing gluconeogenesis during TPN providing gluconeogenic substrate prevented a potential need of increased proteolysis to sustain gluconeogenesis to meet glucose demands when the glucose supply is low. In the absence of TPN, Hertz et al [16] showed that glucose supplied at or above normal infant glucose turnover rates did not affect proteolysis in extremely premature infants.
Because gluconeogenesis is an ongoing process enabling preterm infants receiving TPN to remain normoglycemic even during a glucose supply corresponding to half normal turnover rate, the glucose infusion rate is the primary factor that can be optimized to reduce the risk of hyperglycemia. Maintaining a glucose infusion rate corresponding to normal infant glucose turnover rates as part of total parenteral nutrition might be a feasible approach to prevent both hypo- and hyperglycemia and yet provide sufficient energy for growth in ELBW infants during their first days of life.
Acknowledgments
We would like to thank Drs Dennis M. Bier and Morey W. Haymond at the Children's Nutrition Research Center, Houston, TX for invaluable help and advice; Cindy Bryant, Pamela Gordon, Geneva Shores and Susan Sharma for excellent assistance; the pharmacist at Texas Children's Hospital for preparing isotope solutions; and the staff of the neonatal intensive care unit for professional collaboration.
Funded by NIH (RO1 HD 37857), USDA (Cooperative Agreement #58-6250-6-001), and the General Clinical Research Center, National Center for Research Resources, (NIH MO1-RR-001888). The contents of this publication do not necessarily reflect the views of policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Cryer PE. Glucose counterregulation: prevention and correction of hypoglycemia in humans. Am J Physiol Endocrinol Metab. 1993;264(27):E149–E155. doi: 10.1152/ajpendo.1993.264.2.E149. [DOI] [PubMed] [Google Scholar]
- 2.Cowett AA, Farrag HM, Gelardi NL, Cowett RM. Hyperglycemia in the micropremie: Evaluation of the metabolic disequilibrium during the neonatal period. Prenat Neonat Med. 1997;2:360–365. [Google Scholar]
- 3.Cowett RM, Oh W, Pollak A, Schwartz R, Stonestreet BS. Glucose disposal of low birth weight infants: steady state hyperglycemia produced by constant intravenous glucose infusion. Pediatrics. 1979;63:389–396. [PubMed] [Google Scholar]
- 4.Kalhan SC, Oliven A, King KC, Lucero C. Role of glucose in the regulation of endogenous glucose production in the human newborn. Pediatr Res. 1986;20:49–52. doi: 10.1203/00006450-198601000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Adam PAJ, King K, Schwartz R. Model for the investigation of intractable hypoglycemia: Insulin-Glucose interrelationships during steady state infusions. Pediatrics. 1968;41:91–105. [PubMed] [Google Scholar]
- 6.Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA. Measurement of “true” glucose production rates with 6,6-dideuteroglucose. Diabetes. 1977;26:1016–23. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 7.Sunehag AL, Ewald U, Larsson A, Gustafsson J. Glucose production rate in extremely Immature Neonates (<28 weeks) studied by use of deuterated glucose. Pediatr Res. 1993;33:97–100. doi: 10.1203/00006450-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Tyrala EE, Chen X, Boden G. Glucose metabolism in the infant weighing less than 1100 grams. J Pediatr. 1994;125:283–287. doi: 10.1016/s0022-3476(94)70212-8. [DOI] [PubMed] [Google Scholar]
- 9.Hays SP, O'Brian Smith E, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in Extremely Low Birth Weight Infants. Pediatrics. 2006;118:1811–1818. doi: 10.1542/peds.2006-0628. [DOI] [PubMed] [Google Scholar]
- 10.Sunehag AL, Haymond MW, Schanler RJ, Reeds PJ, Bier DM. Gluconeogenesis in very low birth weight infants receiving total parenteral nutrition. Diabetes. 1999;48:791–800. doi: 10.2337/diabetes.48.4.791. [DOI] [PubMed] [Google Scholar]
- 11.Sunehag AL. Parenteral glycerol enhances gluconeogenesis in very premature infants. Pediatr Res. 2003;53:635–641. doi: 10.1203/01.PDR.0000054774.90893.0F. [DOI] [PubMed] [Google Scholar]
- 12.Sunehag AL. The role of parenteral lipids in supporting gluconeogenesis in very premature infants. Pediatr Res. 2003;54:480–486. doi: 10.1203/01.PDR.0000081298.06751.76. [DOI] [PubMed] [Google Scholar]
- 13.Kalhan SC, Parimi P, Beek RV, Gilfillan C, Saker F, Gruca L, Sauer PJJ. Estimation of gluconeogenesis in newborn infants. Am J Physiol Endocrinol Metab. 2001;281:E991–E997. doi: 10.1152/ajpendo.2001.281.5.E991. [DOI] [PubMed] [Google Scholar]
- 14.Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983;71:467–475. doi: 10.1172/JCI110791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunehag AL, Gustafsson J, Ewald U. Very Immature infants (≤ 30 Wk) respond to glucose infusion with incomplete suppression of glucose production. Pediatr Res. 1994;36:550–555. doi: 10.1203/00006450-199410000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Hertz DE, Karn CA, Liu YM, Liechty EA, Denne SC. Intravenous glucose suppresses glucose production but not proteolysis in extremely premature newborns. J Clin Invest. 1993;92:1752–1758. doi: 10.1172/JCI116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowett RM, Rapoza RE, Gelardi NL. Insulin counter regulatory hormones are ineffective in neonatal hyperinsulinemic hypoglycemia. Metabolism. 1999;48(5):568–574. doi: 10.1016/s0026-0495(99)90052-5. [DOI] [PubMed] [Google Scholar]
- 18.Chacko SK, Sunehag AL. Gluconeogenesis continues in premature infants receiving TPN. Arch Dis Child Fetal Neonatal Ed. 2010;95:F413–418. doi: 10.1136/adc.2009.178020. [DOI] [PubMed] [Google Scholar]
- 19.Gerich JE, Schneider V, Dippe SE, Langlois M, Noacco C, Karam JH, Forsham PH. Characterization of the glucagon response to hypoglycemia in man. J Clin Endocrinol Metab. 1974;38:77–82. doi: 10.1210/jcem-38-1-77. [DOI] [PubMed] [Google Scholar]
- 20.Gerich JE, Cryer PE. Contrainsulin hormones: Biochemical and Physiological aspects. In: Cowett RM, editor. Principles of Perinatal-Neonatal Metabolism. Springer-Verlag; New York: 1991. pp. 103–127. [Google Scholar]
- 21.Jackson L, Williams FLR, Burchell A, Coughtrie MWH, Hume R. Plasma Catecholamines and the counterregulatory responses to hypoglycemia in infants: A critical role for epinephrine and cortisol. J Clin Endocrinol Metab. 2004;89(12):6251–6256. doi: 10.1210/jc.2004-0550. [DOI] [PubMed] [Google Scholar]
- 22.Tayek JA, Katz J. Glucose production, recycling, Cori cycle, and gluconeogenesis in humans: relation to serum cortisol. Am J Physiol Endocrinol Metab. 1997;272:E476–E484. doi: 10.1152/ajpendo.1997.272.3.E476. [DOI] [PubMed] [Google Scholar]
- 23.Mathews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-13C] Leucine. Am J Physiol Endocrinol Metab. 1980;238:E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- 24.Sunehag AL, Haymond MW. Maternal protein homeostasis and milk protein synthesis during feeding and fasting in humans. Am J Physiol Endocrinol Metab. 2003;285:E420–E426. doi: 10.1152/ajpendo.00080.2003. [DOI] [PubMed] [Google Scholar]
- 25.Schade D, Eaton R. Modulation of fatty acid metabolism by glucagon in man. I. Effects in normal subjects. Diabetes. 1975;24:502–509. doi: 10.2337/diab.24.5.502. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein RE, Wasserman DH, McGuinness OP, Brooks LD, Cherrington AD, Abumrad NN. Effects of chronic elevation in plasma cortisol on hepatic carbohydrate metabolism. Am J Physiol Endocrinol Metab. 1993;264(27):E119–E127. doi: 10.1152/ajpendo.1993.264.1.E119. [DOI] [PubMed] [Google Scholar]
- 27.Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40(10):1228–32. doi: 10.2337/diab.40.10.1228. [DOI] [PubMed] [Google Scholar]
- 28.Darmaun D, Matthews DE, Bier DM. Physiological hypercortisolemia increases proteolysis, glutamine, and alanine production. Am J Physiol Endocrinol Metab. 1988;255(18):E366–373. doi: 10.1152/ajpendo.1988.255.3.E366. [DOI] [PubMed] [Google Scholar]
- 29.Simmons PS, Miles JM, Gerich JE, Haymond MW. Increased proteolysis; an effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984;(73):412–420. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon M, Gerich J, Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988;4:17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- 31.Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci. 2001;101:739–747. doi: 10.1042/cs1010739. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe RR, Allsop JR, Burke JF. Glucose metabolism in man: Responses to intravenous glucose infusion. Metabolism. 1979;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- 33.Long CL, Spencer JL, Kinney JM, Geiger JW. Carbohydrate metabolism in normal man and effect of glucose infusion. J Appl Physiol. 1971;31:102–109. doi: 10.1152/jappl.1971.31.1.102. [DOI] [PubMed] [Google Scholar]
- 34.Cowett RM, Susa JB, Oh W, Schwartz R. Endogenous glucose production during constant glucose infusion in the newborn lamb. Pediatr Res. 1978;12:853–857. doi: 10.1203/00006450-197808000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Susa JB, Cowett RM, Oh W, Schwartz R. Suppression of gluconeogenesis and endogenous glucose production by exogenous insulin administration in the newborn lamb. Pediatr Res. 1979;13:594–598. doi: 10.1203/00006450-197905000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Farrag HM, Nawrath LM, Healey JE, Dorcus EJ, Rapoza RE, Oh W, Cowett RM. Persistent glucose production and greater peripheral sensitivity to insulin in the neonate vs. the adult. Am J Physiol Endocrinol Metab. 1997;272(35):E86–E93. doi: 10.1152/ajpendo.1997.272.1.E86. [DOI] [PubMed] [Google Scholar]
- 37.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE, Shulman G, Caprio S. Low adiponectin levels in adolescent obesity: A marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab. 2003;88(5):2014–2018. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 38.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of adipocyte protein adiponectin are decreased in parallel with decreased insulin sensitivity during the progression to type-2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 39.Sunehag AL, Toffolo G, Campioni M, Bier DM, Haymond MW. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy, obese adolescents. J Clin Endocrinol Metab. 2005;90(8):4496–4502. doi: 10.1210/jc.2005-0626. [DOI] [PubMed] [Google Scholar]
- 40.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, et al. Early insulin therapy in Very-Low-Birth-Weight Infants. N Eng J Med. 2008;359(18):1873–1884. doi: 10.1056/NEJMoa0803725. [DOI] [PubMed] [Google Scholar]
- 41.Poindexter BB, Karn CA, Denne SC. Exogenous insulin reduces proteolysis and protein synthesis in extremely low birth weight infants. J Pediatr. 1998;132:948–53. doi: 10.1016/s0022-3476(98)70389-0. [DOI] [PubMed] [Google Scholar]