Abstract
Objective
The purpose of this study was to determine the presence and relative composition of neutral lipids in human saliva.
Design
Whole unstimulated saliva was collected from 12 subjects ranging from 21 to 29 years old. Samples were lyophilized, and lipids were extracted using chloroform-methanol. Lipids were analyzed by thin-layer chromatography.
Results
Human saliva contains cholesterol, fatty acids, triglycerides, wax esters, cholesterol esters and squalene. The mean total neutral lipid content was 12.1 +/− 6.3 µg/ml.
Conclusions
This lipids in human saliva closely resemble the lipids found on the skin surface. These salivary lipids are most likely produced by the sebaceous follicles in the oral mucosa and sebaceous glands associated with major salivary glands.
1.0 Introduction
Cholesterol and other neutral lipids, phospholipids and glycolipids have all been reported as lipid components of human saliva;1 however, the most recently published analysis of human salivary lipids indicated that neutral lipids dominated, with polar components like phospholipids and glycolipids comprising only 1 – 5% of total lipid mass.2 The major lipids identified were cholesterol esters, triglycerides, fatty acids and cholesterol.
Sebaceous glands in the oral mucosa and the vermillion border of the lip have often been referred to as ectopic sebaceous glands and considered benign.3,4 In fact, the vermillion border of the lip and all regions of the oral mucosa contain sebaceous follicles.5–8 These are sebaceous glands without associated terminal hairs. Although some sebaceous follicles are found in all regions of skin excluding the palmar and plantar regions, these secretory units uniquely surround all orifices of the body, including the vermillion border of the lip. This anatomic distribution suggests a protective function.9 The sebaceous follicles produce sebaceous lipids with the same composition as the pilosebaceous units of the skin.10 In addition, sebaceous glands are associated with normal major salivary glands.11–13 Thus, the secretions of the major salivary glands can be a source of sebaceous lipids. In Fact, some of the previous salivary lipid analyses were done using saliva collected from cannulated parotid and submandibular glands.2 Enlarged sebaceous glands called Fordyce spots, if present on the lips, are sometimes treated for cosmetic reasons.14
These observations on sebaceous follicles in the oral cavity suggest that the lipids found in saliva should contain, in addition to the previously noted neutral lipids, wax monoesters and squalene. These two lipids are unique biochemical markers of human sebum. Wax esters are not found anywhere else in the human body. Very minor amounts of squalene is present in most human tissues since it is an intermediate in the biosynthesis of cholesterol.15 In this context, small amounts of squalene have been detected in mouse salivary glands.16 Other than in human sebum, significant proportions of squalene have been reported in the skin surface lipids of the otter, kinkajou, beaver and the semiaquatic mole, Scalopus aquaticus.17,18 Squalene is a very effective water proofing agent, and these other mammals that produce squalene all live in wet environments. This suggests that our ancestors may have evolved in or near water. The purpose of the present study was to test the hypothesis that wax monoesters and squalene are present in human saliva.
2.0 Materials and methods
2.1 Collection of saliva
The study population consisted of twelve subjects ages 21–29 years, five females and seven males. Once informed consent was obtained, subjects were submitted to basic oral exams conducted by Dr. Chris Barwacz, University of Iowa. These exams included hard and soft tissue screening as well as periodontal probing to evaluate oral health. Upon ensuring that subjects were in good oral health, samples of salivary lipids were taken. Subjects were asked to drool into a 50 ml centrifuge tube providing approximately 10 ml of saliva. Saliva samples were placed in a freezer until all samples had been obtained.
2.2 Extraction of lipids
Subsequently, the samples were thawed in a water bath and transferred into 50 ml Erlenmeyer flasks. These flasks were again frozen and then lyophilized. Once lyophilization was complete lipid was extracted from all 24 samples using 5 ml of chloroform:methanol, 2:1 (v/v).19 The extracts were then filtered into test tubes and dried under nitrogen. These dried extracts were then dissolved in 100 µl of toluene in preparation for thin-layer chromatographic (TLC) analysis.
2.3 Thin-layer chromatography20
Twenty × twenty cm glass plates coated with 0.25-mm-thick silica gel G (Adsorbosil-plus-1; Alltech Associates; Deerfield IL) were washed with chloroform:methanol, 2:1, activated in a 110° C oven, and the adsorbent was scored into 6-mm-wide lanes. Calibrated glass capillaries were used to apply 5 µl samples 2 cm from the bottom edge of the plate. The chromatogram was developed to 20 cm with hexane, followed by toluene to 20 cm, followed by hexane:ethyl ether:acetic acid, 70:30:1, to 12 cm.
After development, chromatograms were air dried, sprayed with 50% sulphuric acid, and slowly heated to 220° C on an aluminum slab on a hot plate. After 2 hrs, charring was complete and a digital image of the chromatograms was captured using a UMAX flatbed scanner with MagicScan software. The TIF images were analyzed using TNIMAGE (Thomas Nelson, Bethesda MD).
2.4 Identification of squalene and wax monoesters
The lipid that matched the squalene standard and a fraction containing a mixture of cholesterol esters and wax esters were isolated by preparative TLC. Lipid was applied as a streak to an undivided silica gel TLC plate. After development as described above, the plate was sprayed with 0.02 % 2’,7’-dichlorofluorescene in 95% ethanol. After drying, the plate was viewed under UV light. The silica gel containing the bands of interest were scraped from the plate and placed in small glass columns. Lipids were eluted with chloroform:methanol, 2:1. The samples were dried under nitrogen.
The isolated squalene was dissolved in n-hexane prior to analysis by gas-liquid chromatography. This was done with a Shimadzu GC-14A equipped with a flame ionization detector. A 30 m EC-WAX quartz capillary column was operated isothermally at 175° C.
The chain length distributions within the cholesterol ester and wax esters is such that the individual lipid types cannot be individually isolated. The mixed cholesterol ester/wax ester fraction was treated with 2 ml of 1 M NaOH in methanol at 50° for 1 hr. After cooling to room temperature, 2 ml of 1 N HCl (aq) and 10 ml of chloroform were added and mixed. The chloroform layer was transferred to a clean tube and dried under nitrogen. The residue was dissolved in 100 µl of chloroform:methanol, 2:1, and 10 µl of this was applied to a lane on a thin-layer plate about 2 cm from the bottom edge. The chromatogram was developed to 20 cm with a mobile phase of hexane:ethyl ether:acetic acid, 70:30:1. After air drying, the plate was sprayed with 50% sulfuric acid (aq) and slowly heated to 220° to induce charring. Stearic acid, stearyl alcohol and cholesterol standards were included on separate lanes.
3.0 Results
The total neutral lipid content of the whole saliva analyzed in the current study ranged from 3–23 µg/ml. The average was 12.1 +/− 6.3 µg/ml (mean +/− standard deviation).
A carbon density profile of the chromatographically separated lipids is shown in Figure 1. Bands corresponded to the standards for squalene (SQ), cholesterol esters (CE), wax esters (WE), triglycerides (TG), fatty acids (FA) and cholesterol (CH). The area of each peak was converted to µg of lipid through use of standard curves.
Figure 1.
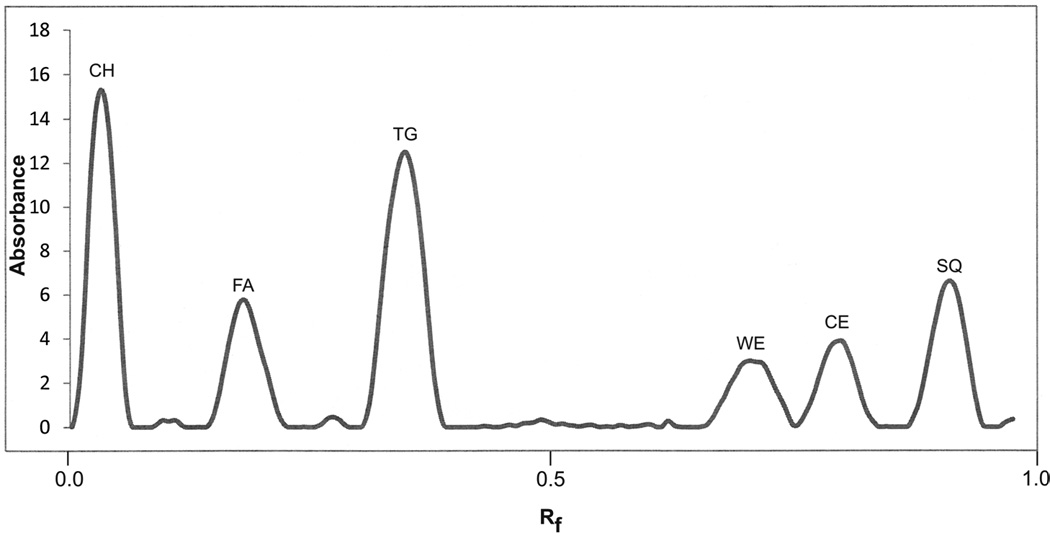
Carbon density profile of charred lipids after separation by TLC. CH, cholesterol; FA, fatty acids; TG, triglyserides; WE, wax esters; CE, cholesterol esters; SQ, squalene
Table 1 summarizes the individual lipid contents of the saliva from each of the subjects. There is high variation in the salivary content of each of the neutral lipids; however, all of the neutral lipids were present in all saliva samples with the exception of cholesterol in subjects # 6 and # 8. Cholesterol was generally the least abundant of the lipids, and it seems likely that it would have been detected in these samples if a larger portion of the samples had been applied to the TLC plate.
Table 1.
For each of 12 subjects the µg/ml for each lipid component is given.
| Subject # | CH | FA | TG | WE | CE | SQ |
|---|---|---|---|---|---|---|
| 1 | 1.72 | 2.01 | 7.28 | 0.28 | 1.01 | 0.23 |
| 2 | 0.03 | 1.87 | 2.29 | 0.44 | 0.58 | 0.35 |
| 3 | 0.06 | 4.10 | 8.19 | 4.41 | 2.38 | 3.78 |
| 4 | 0.02 | 7.79 | 6.38 | 0.63 | 1.98 | 0.46 |
| 5 | 0.03 | 7.52 | 7.29 | 0.09 | 4.05 | 0.42 |
| 6 | 0.00 | 2.53 | 2.60 | 0.15 | 1.72 | 0.24 |
| 7 | 0.01 | 2.44 | 4.85 | 0.92 | 2.83 | 1.33 |
| 8 | 0.00 | 2.99 | 5.47 | 0.50 | 1.40 | 0.44 |
| 9 | 0.04 | 4.85 | 4.11 | 0.33 | 8.47 | 0.98 |
| 10 | 0.46 | 0.21 | 1.57 | 0.04 | 0.69 | 0.04 |
| 11 | 0.04 | 2.37 | 2.20 | 0.35 | 0.81 | 0.43 |
| 12 | 0.04 | 1.42 | 2.95 | 1.03 | 2.21 | 1.89 |
Figure 2 demonstrates that the salivary component tentatively identified as squalene based on its mobility on TLC comigrates with authentic squalene on gas-liquid chromatography.
Figure 2.
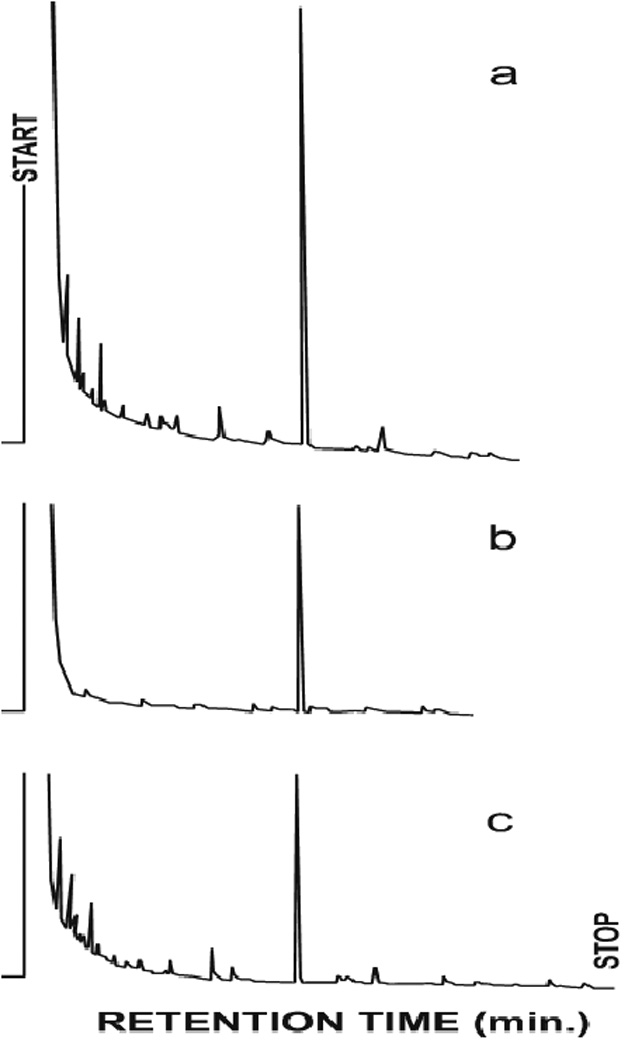
Gas chromatography of squalene isolated from human saliva. (a) mixture of authentic squalene and isolated material (b) authentic squalene (c) squalene isolated from saliva. In each case the retention time of the single peak was 19.1 min.
The hydrolysis products from the cholesterol ester/wax ester fraction consisted of fatty acids (Rf = 0.35), cholesterol (Rf = 0.09) and fatty alcohol (Rf = 0.14).
4.0 Discussion
The average total neutral lipid of whole saliva found in the present study of 12.1 µg/ml is in good agreement with the value of 13.1 µg/ml reported by Larsson et al.2 Some of the earlier measurements of salivary lipid content were considerably higher21,22; however, these studies included phospholipids and glycolipids which would have contributed to the discrepancy between the earlier and present findings.
The composition of the salivary neutral lipids found in the present study differs from previous reports in that it includes squalene and wax esters. It seems likely that in the previous TLC analysis the squalene would have migrated to the solvent front and that the wax esters would not have separated from the cholesterol esters.2 The TLC system used in the present study was developed for the analysis of skin surface lipids, which includes squalene and wax monoesters.20 The identity of squalene was confirmed by comparison of the isolated material with authentic squalene by gas-liquid chromatography. The identity of wax monoesters is supported by the presence of fatty alcohols and fatty acids upon hydrolysis of a wax ester-enriched fraction. Squalene and wax esters are biochemical markers of human sebum. In fact, the profile of neutral lipids found in this study is remarkably similar to the profile of skin surface lipids, which is dominated by sebum. This points to the origin of salivary lipids in the sebaceous follicles in the mucosa and salivary glands associated with the major salivary glands.
It should be pointed out that oral epithelial cells do contain some free fatty acids and cholesterol.23 Given the very low concentration of cholesterol found in the saliva, this does not seem a likely source of significant contamination. Also, bacteria do not contain any of these neutral lipids. The possibility that the triglycerides, fatty acids or cholesterol from food may have contributed to the present lipid profiles cannot be entirely ruled out. This, too, is unlikely as all twelve subjects likely had different diets.
Skin surface lipids have long been known to have antimicrobial properties attributable to the free fatty acid component. The free fatty acids are produced by hydrolysis of the sebaceous triglycerides. More recently, it has been shown that lauric acid (C12:0) and sapienic acid (C16:1Δ6) are by far the most potent antimicrobial fatty acids from sebum.24 This raises the possibility that the salivary lipids may be a part of innate immunity in the oral cavity. In this regard, the sebaceous glands of fetal skin, and probably those in fetal oral mucosa, become active during the third trimester of gestation. The fetal sebum contributes to a coating on the skin surface known as vernix caseosa. Through interaction with lung surfactant near the end of gestation some of the vernix caseosa is dislodged from the skin surface and is ingested shortly before birth. It is thought that the vernix caseosa remaining on the skin provides benefits including both antimicrobial and antioxidant activities.25 It may be that the ingested vernix caseosa provides some similar benefits.
Future studies will be directed at determining if salivary lipid content or composition, especially the extent of triglyceride hydrolysis, varies as a function of age or disease.
Acknowledgments
Funding
Supported in part by RO1 DEO18032 (NIH/NIDCR). AB was recipient of a Dows Research Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None
Ethical approval
This study was approved by the biomedical institutional review board (IRB-01) at the University of Iowa.
References
- 1.Slomiany BL, Murty VL, Slomiany A. Salivary lipids in health and disease. Progress in Lipid Research. 1985;24(4):311–324. doi: 10.1016/0163-7827(85)90009-8. [DOI] [PubMed] [Google Scholar]
- 2.Larsson B, Olivecrona G, Ericson T. Lipids in human saliva. Archives of oral Biology. 1996;41(1):105–110. doi: 10.1016/0003-9969(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 3.Meleti M, Vescovi P, Mooi WJ, van der Waal I. Pigmented lesions of the oral mucosa and perioral tissues: a flow-chart for the diagnosis and some recommendations for the management. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2008;105(5):606–616. doi: 10.1016/j.tripleo.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Gulec AT, Haberal M. Lip and oral mucosal lesions in 100 renal transplant recipients. Journal of the American Acadamy of Dermatology. 2010;62(1):96–101. doi: 10.1016/j.jaad.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Gorsky M, Buchner A, Fundoianu-Dayan D, Cohen C. Fordyce’s granules in the oral mucosa of adult Israeli Jews. Community Dentistry and Oral Epidemiology. 1986;14(4):231–232. doi: 10.1111/j.1600-0528.1986.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 6.Batsakis JG, el-Naggar AK. Sebaceous lesions of salivary glands and oral cavity. Annals of Otology, Rhinology and Laryngology. 1990;99(5 Pt1):416–418. doi: 10.1177/000348949009900517. [DOI] [PubMed] [Google Scholar]
- 7.Daley T. Pathology of intraoral sebaceous glands. Journal of Oral Pathology and Medicine. 1993;22(6):241–245. doi: 10.1111/j.1600-0714.1993.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 8.Olivier JH. Fordyce granules on the prolabial and oral mucous membranes of a selected population. South African Dental Journal. 2006;61(2):72–74. [PubMed] [Google Scholar]
- 9.Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186(4158):19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- 10.Nordstrom KM, McGinley KJ, Lessin SR, Leyden JJ. Neutral lipid composition of Fordyce’s granules. British Journal of Dermatology. 1989;121(5):669–670. doi: 10.1111/j.1365-2133.1989.tb08205.x. [DOI] [PubMed] [Google Scholar]
- 11.Meza-Chavez L. Sebaceous glands in normal and neoplastic parotid glands: possible significance of sebaceous glands in respect to the origin of tumors of the salivary glands. American Journal of Pathology. 1949;25(4):627–645. [PMC free article] [PubMed] [Google Scholar]
- 12.Linhartova A. Sebaceous glands in salivary gland tissue. Archives of Pathology. 1974;98(5):320–324. [PubMed] [Google Scholar]
- 13.Martinez-Madrigal F, Micheau C. Histology of the major salivary glands. American Journal of Surgical Pathology. 1989;13(10):879–899. doi: 10.1097/00000478-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Kang HY, Lee E-S, Kim YC. Treatment for Fordyce spots with 5-aminolaevulinic acid-photodynamic therapy. British Journal of Dermatology. 2007;156(2):399–400. doi: 10.1111/j.1365-2133.2006.07653.x. [DOI] [PubMed] [Google Scholar]
- 15.Do R, Kiss RS, Gaudet D, Engert JC. Squalene synthase: a critical enzyme in the cholesterol biosynthesis pathway. Clinical Genetics. 2009;75(1):19–29. doi: 10.1111/j.1399-0004.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 16.Hindy AM, Yoshiga K, Takada K, Okuda K. Sterol composition and biosynthesis in mouse salivary glands. Archives of oral Biology. 1986;31(2):87–93. doi: 10.1016/0003-9969(86)90031-2. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm JS, McCormick JM, Colton SW, 6th, Downing DT. Variation of skin surface lipid composition among mammals. Comparative Biochemistry and Physiology. 1981;69B(1):75–78. doi: 10.1016/0305-0491(83)90353-x. [DOI] [PubMed] [Google Scholar]
- 18.Downing DT, Stewart ME. Skin surface lipids of the mole Scalopus aquatecus. Comparative Biochemistry and Physiology. 1987;86B(4):667–670. doi: 10.1016/0305-0491(87)90207-0. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloan Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 20.Downing DT, Strauss JS, Pochi PE. Variability in the chemical composition of human skin surface lipids. Journal of Investigative Dermatology. 1969;53(5):322–327. doi: 10.1038/jid.1969.157. [DOI] [PubMed] [Google Scholar]
- 21.Slomiany BL, Slomiany A, Mandel ID. Lipid composition of human submandibular gland secretion from light and heavy calculus formers. Archives of oral Biology. 1980;31(11–12):699–702. doi: 10.1016/0003-9969(80)90129-6. [DOI] [PubMed] [Google Scholar]
- 22.Slomiany BL, Murty VL, Aono M, Slomiany A, Mandel ID. Lipid composition of parotid and submandibular saliva from caries-resistant and caries-susceptible adults. Archives of oral Biology. 1982;27(10):803–808. doi: 10.1016/0003-9969(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 23.Law S, Wertz PW, Swartzendruber DC, Squier CA. Regional variation in content, composition and organization of porcine epithelial barrier lipids revealed by thin-layer chromatography and transmission electron microscopy. Archives of oral Biology. 1995;40(12):1085–1091. doi: 10.1016/0003-9969(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 24.Drake DR, Brogden KA, Dawson DV, Wertz PW. Antimicrobial lipids at the skin surface. Journal of Lipid Research. 2008;49(1):4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Hoath SB, Pickens WL, Visscher MO. The biology of vernix caseosa. International Journal of Cosmetic Science. 2006;28(5):319–333. doi: 10.1111/j.1467-2494.2006.00338.x. [DOI] [PubMed] [Google Scholar]


