Abstract
Preclinical and clinical evidence suggest an association between alcoholism and the primary regulator of extracellular dopamine concentrations, the dopamine transporter (DAT). However, the nature of this association is unclear. We determined if ten days of voluntary alcohol self-administration followed by withdrawal could directly alter DAT function, or if genetically-mediated changes in DAT function and/or availability could influence vulnerability to alcohol abuse. Heterozygous (DAT+/-) and homozygous mutant (DAT-/-) and wildtype (DAT+/+) mice were allowed to consume 5% alcohol in a schedule-induced polydipsia (SIP) task. In vivo fixed potential amperometry in anesthetized mice was used to (1) identify functional characteristics of mesoaccumbens dopamine neurons related to genotype, including dopamine autoreceptor (DAR) sensitivity, DAT efficiency, and DAT capacity, (2) determine if any of these characteristics correlated with alcohol drinking observed in DAT+/+ and DAT+/- animals, and (3) determine if SIP-alcohol self-administration altered DAR sensitivity, DAT efficiency, and DAT capacity by comparing these characteristics in wildtype (DAT+/+) mice that were SIP-alcohol naïve, with those that had undergone SIP-alcohol testing. DAT-/- mice consumed significantly less alcohol during testing and this behavioral difference was related to significant differences in DAR sensitivity, DAT efficiency, and DAT capacity. These functional characteristics were correlated to varying degrees with g/kg alcohol consumption in DAT+/+ and DAT+/- mice. DAR sensitivity was consistently reduced and DAT efficiency was enhanced in SIP-alcohol experienced DAT+/+ mice in comparison to naïve animals. These results indicate that DAR sensitivity is reduced by SIP-alcohol consumption and that DAT efficiency is modified by genotype as well as SIP-alcohol exposure. DAT capacity appeared to be strictly associated with SIP-alcohol consumption.
Keywords: dopamine transporter, dopamine autoreceptor, schedule-induced polydipsia, fixed potential amperometry, alcohol self-administration
Introduction
It is well known that mesolimbic dopamine (DA) neurons mediate many of the reinforcing properties of addictive drugs including alcohol (Koob and Bloom, 1988; Wise, 2005; Blaha and Phillips, 1996). Acute or repeated administration of addictive drugs or alcohol stimulates DA release in both the shell and core of the nucleus accumbens (NAc) of animals and humans (Di Chiara et al., 1996; Yim et al., 1998; Yoshimoto et al., 1998; Boileau et al., 2003; Tang et al., 2003), but with a preferential action and lack of habituation in the shell region (Bassareo et al., 2003; Di Chiara, 2002; Di Chiara et al., 2004).
Evidence suggests an association between the primary regulator of forebrain DA extracellular concentrations, the DA transporter (DAT), and alcoholism, although the direction of this association is unclear: Genotypic differences in the DAT may influence susceptibility to alcoholism, or alcohol consumption may modify DAT functionality. Several imaging studies (Tiihonen et al., 1995; Gilman et al., 1998; Little et al., 1998) have reported a reduction in striatal DAT densities in withdrawn late onset alcoholics, while others failed to replicate this result (Volkow et al., 1996; Heinz et al., 1998). Linkage and association studies indicate that polymorphisms in the human DAT gene (Dat1) can be significantly associated with alcoholism (Heinz et al., 2004; Köhnke et al., 2005; Ueno et al., 1999), and that DAT density is increased in human late onset alcoholics (Repo et al., 1999). In knockout (KO) mouse studies, depending on alcohol concentration in 2-bottle choice tests, deletions of the DAT are reported to increase alcohol consumption in male mice, while female DAT KO mice have been reported to show increased as well as decreased preference for alcohol, in comparison to wild-type mice (Hall et al., 2003; Savelleva et al., 2002).
The main goal of this study was to determine differences in susceptibility to voluntary alcohol self-administration in homozygous DAT KO (DAT-/-), heterozygous DAT KO (DAT+/-) and wildtype control mice (DAT+/+). Alcohol consumption (5% v/v) of mice in these groups was tested over 10 days in a schedule-induced polydipsia task. When rodents are food but not water deprived and given intermittent presentations of small amounts of food, many develop excessive fluid consumption, called schedule-induced polydipsia (SIP). The SIP task was selected because it has a strong dopaminergic basis (Mittleman et al., 1990; Robbins and Koob, 1980), and is sensitive to genotypic differences in alcohol consumption (Mittleman et al., 2003). A second goal was to identify several functional characteristics of mesoaccumbens DA neurons related to genotype, including DA autoreceptor (DAR) sensitivity, DAT efficiency, and DAT capacity and to determine if any of these characteristics correlated with differences in alcohol drinking observed in heterozygous (DAT+/-) animals. The final goal was to determine if DA dynamics were influenced by chronic alcohol consumption in SIP. Thus, DAR sensitivity, DAT efficiency, and DAT capacity in SIP-naïve, wildtype (DAT+/+) mice were compared with those that had undergone SIP-alcohol testing.
Materials and Methods
Animals
Homozygote (DAT-/-), heterozygote (DAT+/-) and wildtype (DAT+/+) littermate mice were bred by crossing DAT-/- males with DAT+/- females, or by crossing DAT+/- mice of both sexes (Giros et al., 1996). All breeders were on an inbred C57BL6 background. After weaning animals were separated by sex and housed 2-5 per cage and allowed to mature. They were kept on a 12 hr light/dark cycle (lights on at 0800 hr) with food and water ad libitum. Mice were singly housed four-six days before an experiment began. Mice randomly assigned to the SIP task were weighed and then food-restricted in order to decrease their body weight to 90% of ad libitum weight. As DAT-/- mice gain weight at a slower rate than wildtype mice (Hall et al., 2003; Giros et al., 1996) a minimum baseline weight of 17 g was set so that no animal had a target weight less than 15 g. Restricted feeding occurred at the beginning and end of the 12 hr light phase to ensure gradual weight loss; each animal's weight was taken immediately before the second (late afternoon) feeding. Restricted feeding occurred throughout SIP testing. DAT+/+ Mice not assigned to the SIP task (SIP naïve) were treated identically to those tested in SIP: They were singly housed, and food deprived for the same length of time and to the same extent as mice tested in SIP. Then both groups were allowed to recover to their pre-food-restriction weights. All procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee.
Schedule-induced polydipsia
Within 1-3 days after the target weights were reached mice were given a daily (2-5 pm) 40 minute SIP session (2 acclimatization days then 10 testing days) in 10 identical operant chambers (Med Associates, St. Albans, VT) in which a 20 mg grain-based food pellet (Research Diets, New Brunswick, NJ: Formula A/I) was dispensed every 60 sec. Pellet retrieval was detected as head entries into the food magazine by an infrared photocell beam mounted 3 mm above the floor of the magazine. A drinking tube positioned to the right of the food magazine was attached to a graduated cylinder containing a 5% alcohol solution. Fluid consumption (ml) was determined at the end of each session by subtracting the amount of fluid in the cylinder reservoir from the amount at the beginning of the session. The amount of alcohol consumed was converted to g/kg intake. Locomotor activity was monitored by the number of times the animals crossed an additional, infrared beam located 1 cm above the cage floor and 13 cm from the wall housing the food magazine and drinking tube. Each chamber was equipped with a house light and exhaust fan which were turned on at the start of each session. A microprocessor (CeNeS, Cambridge, UK) controlled food pellet delivery and recorded the number of head entries and locomotor activity.
Blood alcohol concentration
Immediately after the final SIP session 15-30 μl of blood was collected into a heparinized capillary tube via a scalpel nick of the tip of the tail. Tubes were centrifuged to separate the serum. Blood alcohol concentration (BAC) was calculated using an Analox GL-5 (Analox Instruments, London, UK). For each determination, 5 μl of plasma was injected into the Analox instrument and BAC was expressed as milligrams per deciliter (mg/dl). After blood was collected all mice were immediately returned to free feeding.
Fixed potential amperometry (FPA)
Mice tested in SIP as well as naïve animals were allowed to recover to their pre-food-restricted weights and then tested with FPA. At least one week elapsed between SIP and FPA testing. Mice were anesthetized with urethane (1.5 g/kg, i.p.), mounted in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the skull flat, and maintained at 36 ± 0.5°C. A concentric bipolar stimulating electrode (SNE-100; Rhodes Medical Co., CA) was implanted into the medial forebrain bundle (MFB coordinates in mm: -2.1AP from bregma; +1.1ML from midline -4.8DV from dura) and carbon fiber electrode (250 μm length × 10 μm o.d.; Thornel Type P, Union Carbide, Pittsburgh, PA) into the ipsilateral NAc core (coord. in mm: +1.5AP from bregma; +1.0ML from midline; -4.3DV from dura) (Franklin and Paxinos, 1997). An Ag/AgCl reference/stainless-steel auxiliary electrode was placed on contralateral cortical tissue -3.0 mm from bregma. Amperometric recordings in a Faraday cage consisted of applying a fixed potential (+0.8V) to the recording electrode and monitoring oxidation current continuously (10K samples/sec) with an electrometer (e-corder 401 and Picostat, eDAQ Inc., Colorado Springs, CO, USA) filtered at 50 Hz (Dugast et al., 1994; Forster and Blaha, 2003). A delay of 10-20 minutes allowed the recording electrode to stabilize before the start of the experiment.
DAR sensitivity
Variance in DAR sensitivity was determined by applying a pair of test stimuli (T1 and T2; with ten 0.5 msec duration 800 μA pulses at 50 Hz separated by six sec) to the MFB six times every 60 sec to evoke DA efflux. Five sets of conditioning pulses (1, 5, 10, 20, and 40) (0.5-ms pulse duration at 15 Hz) were given immediately prior (0.3 sec at the end of the conditioning pulse train) to T2. T1 was recorded in the absence of conditioning pulses and served as an ‘on-going’ control (100%) response for each set of applied conditioning pulses effect on DAR for T2. DAR-mediated inhibition of evoked DA efflux, in terms of the change in amplitude of T2 with respect to T1, was expressed as a mean percentage change (T1/T2*100) by each set of conditioning pulses (Benoit-Marand et al., 2000, 2001). Thus, low to high DAR sensitivity corresponded to low to high percent inhibition of evoked DA efflux.
DAT efficiency
DAT uptake efficiency was assessed by applying six sets of stimulations of 0.5 msec duration pulses, 30 sec apart at 50 Hz to the MFB at a fixed intensity (800 μA). DAT efficiency was determined by the mean half-life decay (i.e., the time for 50% decrease from the maximum evoked increase to the pre-stimulus baseline level in the amperometric signal) for each set of stimulation frequencies applied to each mouse strain (Suaud-Chagny et al., 1995; Benoit-Marand et al., 2000). Thus, low to high DAT efficiency corresponded to long to short half-life decay.
DAT capacity
Baseline levels of MFB stimulation-evoked DA (fifteen 0.5 msec duration pulses at 50 Hz applied at 30 sec intervals) was recorded for three minutes preceding the administration of the selective DAT inhibitor nomifensine at a dose (10 mg/kg, i.p.) half-maximal for complete inhibition of DAT (Lee et al., 2006). Recording continued for at least 30-60 minutes. Higher doses of nomifensine increase half-life values proportionately to the extent that a maximal dose (e.g. 20 mg/kg i.p.) reduces clearance rates to a level that approximate those observed in DAT KO mice that completely lack DAT proteins. Under conditions of a submaximal dose of nomifensine, a relatively short half-life decay in DA clearance following submaximal inhibition of available DAT sites corresponds to a relatively higher DAT capacity (density). In contrast, a relatively long half-life decay in DA clearance following submaximal inhibition of available DAT sites corresponds to a relatively lower DAT capacity (density). Inhibition of DAT by the drug-induced change in the half-life decay at 30 minutes post- versus pre-nomifensine half-life values were thus used to determine differences in DAT capacity among the genotypes and conditions.
Effect of other monoamine transporters
Administration of the selective serotonin or norepinephrine transporter inhibitor fluoxetine (20 mg/kg, i.p.) or desipramine (20 mg/kg, i.p.), respectively, assessed the effect on DA uptake by other transporter systems in the absence of DAT (Mateo et al., 2004). Inhibition of DA uptake by the drug-induced change in the half-life decay at 30 minutes post-drug with respect to pre-drug half-life values determined any contributions of additional DA uptake by other transporter systems among the different genotypes. Recording continued for at least 30 minutes and at most 60 minutes.
Histology and PCR determination
At the end of each experiment direct anodic current (100 μA for 10 sec) was applied to the stimulating electrode and the recording electrode to produce a lesion. Mice were then euthanized (intracardial urethane 0.345 g/ml) and their brains removed and prepared in 30%/10% sucrose/formalin plus 0.1% potassium ferricyanide for cryostat sectioning. Placements of electrodes were determined under a light microscope and recorded on representative coronal diagrams (Franklin and Paxinos, 1997). Three to five millimeters of tail were collected from each animal after euthanasia to extract DNA for confirmation of the genotype of the animals via PCR and gel electrophoresis (Bauer et al., 1994). DNA was extracted from mouse tail using a modified salt method after Proteinase K digestion O/N. DNA was re-suspended in 100 μl Tris-EDTA buffer and 1 μl was used in a 10 μl PCR reaction containing known primers (Giros et al, 1991). Known controls were run with each litter to ensure accurate genotypic analysis
Data analyses and statistics
The influence of SIP-alcohol exposure was determined with repeated measures analysis of variance (ANOVA) using g/kg alcohol intake, activity and entries into the food magazine as the dependent variables. Genotype (DAT-/-, DAT+/-, DAT+/+) was the between subjects factors, with Day (1-10) as the within subjects factor. ANOVA with Genotype as the between subjects factor and Number of conditioning pulses as the within subjects factor was used to determine DAR sensitivity differences. ANOVA with Genotype as the between subjects factor was used to analyze DAT efficiency and capacity. These 2 conditions were compared using an ANOVA with Genotype and Condition (pre and post-nomifensine) as, respectively, the between and within factors. It should be noted that a separate set of ANOVAs was then conducted that were similar to those mentioned above. For these ANOVAs Sex (M, F) was additionally included, along with Genotype as the between subject factors. These ANOVAs were used to compare the DAT+/- and DAT+/+ groups. The DAT-/- group was excluded from these analyses because of the unbalanced number of males and females within this genotype.
Similar ANOVAs with Group (SIP, SIP-naïve) and Sex as the between subjects factor were used to determine neurochemical differences between SIP-alcohol and SIP-naïve DAT+/+ mice. Significant interactions were followed with simple main effects tests or Dunnett's t-tests, when appropriate.
Pearson product moment correlations were used to explore relationships between neurochemical characteristics (DAR sensitivity and DAT efficiency and capacity) and behavioral variables (g/kg alcohol intake, rear chamber activity, and food magazine entries). In addition to showing relationships between average g/kg alcohol consumption and the various neurochemical measures, these correlations revealed that relationships among alcohol consumption, food entries and DAT capacity were significant. Given the potential overlap in the relationship of g/kg alcohol intake and food entries in their association with DAT capacity, hierarchical regression models were then conducted. The same model was used, respectively, to predict DAR sensitivity (at 40 conditioning pulses), DAT efficiency and DAT capacity (post-nomifensine). For each model, average food entries was entered in the first step. Average g/kg consumption was entered in the second step in order to determine if alcohol consumption would continue to predict variance in the neurochemical measures once food entries were taken into account.
Results
PCR confirmation of genotype
PCR indicated that the distribution of genotypes included 17 DAT-/-, 49 DAT+/- and 35 DAT+/+ controls, or a total of 101 mice (male, n=45; female, n=56). The baseline, non-food deprived weight of these groups averaged, respectively, 17.5±0.39g (DAT-/-), 23.32±0.51g (DAT+/-) and 23.91±0.95g (DAT+/+). With the exception of 9 DAT+/+ mice that remained naïve, all of these mice underwent SIP testing. Once SIP testing had concluded and animals were no longer food deprived, 11 DAT-/- (male, n=3; female, n=8), 35 DAT+/- (male, n=18; female, n=17) and 16 DAT+/+ (male n=8; female, n=8) mice were randomly assigned to FPA testing. Additionally, 9 DAT+/+ SIP-naïve mice (male, n=4; female, n=5) were also tested with FPA. Mice that did not undergo FPA testing were used in other studies.
Behavioral differences related to genotype
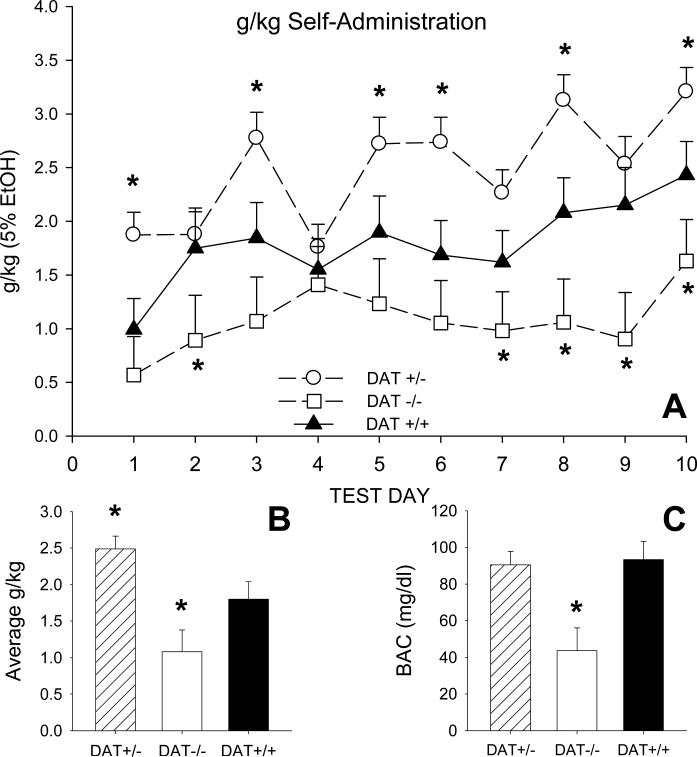
As shown in Figure 1 A, there were consistent genotype-related differences in the consumption of 5% alcohol solution across the 10 SIP test days (Genotype X Day F=2.55, df=18,576, p=.002). Thus, DAT-/- mice consumed significantly less alcohol than DAT+/+ controls on test day 2, and during the final 4 test days. In contrast, DAT+/- mice had significantly higher levels of alcohol consumption on days 1, 3, 5, 6, 8 and 10, in comparison to controls (Dunnett's t-test). These differences in daily levels of consumption accounted for the observed group difference in mean levels of alcohol drinking (Genotype F=19.65, df=2,64, p<.001; Figure 1 B). When averaged across the 10 test days, g/kg consumption in both DAT-/- (1.08±0.17 g/kg) and DAT+/- (2.49±0.21 g/kg) mice differed significantly from that of DAT+/+ controls (1.80±0.19 g/kg; Dunnett's t-tests). There were sex differences in alcohol consumption (Genotype X Sex F=4.07, df=1,71, p<.05). Simple main effects tests indicated female DAT+/- mice consumed an average 2.85±0.27 g/kg while DAT+/+ females averaged 1.55±0.33 g/kg (Genotype F=9.185, df=1,38, p<.005).
Fig. 1.
(A) Average daily consumption (g/kg) of 5% alcohol solution in DAT homozygote (-/-), heterozygote (+/-) and wildtype (+/+) littermate mice. All mice were tested in the schedule-induced polydipsia paradigm for 40 minutes each day. Results showed that g/kg alcohol consumption in DAT-/- and DAT+/- mice was, respectively, significantly less than or greater then that of DAT+/+ mice. (B) Alcohol consumption (g/kg) averaged over the 10 test days. (C) Blood alcohol concentration (BAC) determined immediately following removal from the test chambers on test day 10. The vertical bar in each figure shows one standard error of the difference (SED) in group means. * significantly different from DAT+/+ (Dunnett's t-test).
Blood alcohol concentrations (BACs) partly mirrored these differences in g/kg alcohol consumption (Genotype F=6.16, df=2,89, p=.003). As shown in Figure 1 C, BACs were significantly lower in DAT-/- (43.68±7.43 mg/dl) mice in comparison to DAT+/+ controls (93.28±11.29 mg/dl) while DAT+/+ and DAT+/- (90.51±7.43 mg/dl) mice did not differ significantly (Dunnett's t-tests). There were no sex differences in BAC between these latter two groups.
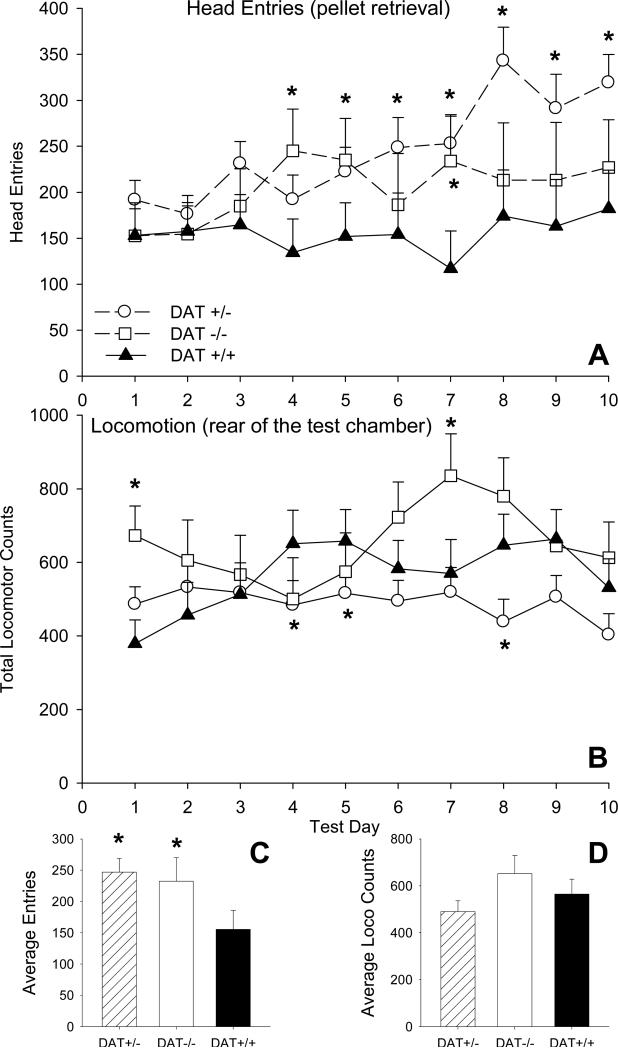
Other behaviors that occurred during SIP also differed as a function of mouse genotype. Figure 2 A shows the total number of daily head entries to retrieve the food pellet delivered at 1 min intervals. The pattern of head entries among the groups differed over test days (Genotype X Day F=3.48, df=18,576 p<.001). In comparison to DAT+/+ controls, DAT-/- mice entered the food magazine significantly more frequently on test days 4, 5 and 7 while entries were consistently elevated in DAT+/- mice throughout the final 5 days of testing (Dunnett's t-tests). When considered over the 10 SIP test days both DAT-/- and DAT+/- mice showed significantly higher numbers of head entries than control DAT+/+ mice (Genotype F=5.48, df=2,64, p=.006; Fig 2C). Head entries did not differ significantly between male and female DAT+/+ and DAT+/- mice.
Fig. 2.
(A) Average daily entries into the food magazine to retrieve the 20 mg food pellet that was delivered every 60 sec during the SIP tests. [right] Head entries averaged over the 10 SIP tests. Results indicated that both the DAT-/- and DAT+/- genotypes had significantly increased numbers of entries into the food magazine in comparison to DAT+/+ mice. (B) Average daily activity in the SIP test chambers as indicated by photocell beam breaks. (C and D) Food entries and locomotor activity averaged over the 10 SIP tests. The vertical bars show one standard error of the difference (SED) in group means. * significantly different from DAT+/+ (Dunnett's t-test).
In SIP, nonspecific locomotion was measured at the rear of the test chamber, away from the front of the chamber where the animal retrieved reward pellets and drank. During SIP testing there were less pronounced genotype-related differences in locomotion in the test chambers (Figure 2 B; Genotype X Day F=6.05, df=18,576, p<.001). In comparison to DAT+/+ controls, DAT+/- mice were less active in the test chambers on test days 4 and 8. Differences between DAT+/+ control and DAT-/- mice were inconsistent. Activity in DAT-/- mice was higher than DAT+/+ controls on days 1 and 7, but significantly lower than control levels on days 4 and 5. When considered over the 10 SIP test days there were no group differences in activity at the rear of the test chamber (Fig. 2 D). There were no significant sex differences in nonspecific locomotion between DAT+/+ and DAT+/- mice.
Differences in dopaminergic dynamics related to genotype following SIP-alcohol exposure
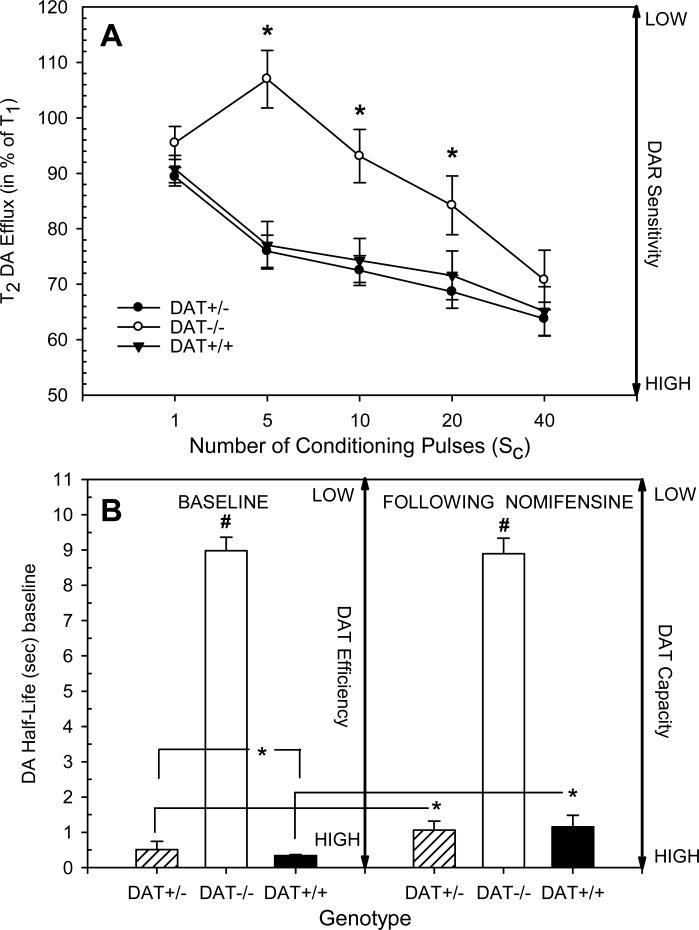
Figure 3 A shows DAR sensitivity reflected as a mean percentage change in accumbens DA efflux evoked by the second test stimulus (T2) with respect to the peak increase in DA efflux evoked by the first test stimulus (T1) following application of 1 to 40 conditioning pulses 0.3 sec prior to T2. There were profound differences in DAR sensitivity between DAT-/- mice and those of the other two genotypes (Genotype X Number of conditioning pulses F= 5.89, df=8,236, p<.001). Specifically, in DAT-/- mice the peak increase in accumbens DA efflux in response to the second test stimulus (T2) was significantly less attenuated by 5, 10 and 20 conditioning pulses indicating overall lower DAR sensitivity compared to DAT+/- or DAT+/+ mice (Dunnett's t-tests). In contrast, overall DAR sensitivity did not differ significantly between DAT+/- and DAT+/+ mice, and there were no significant sex differences between these two genotypes.
Fig. 3.
(A) DA autoreceptor (DAR) sensitivity expressed as mean percentage change in accumbens DA efflux. DA efflux evoked by a test stimulus (T1) was compared to DA efflux when a second test stimulus (T2) was preceded by 1 to 40 conditioning pulses 0.3 sec prior to T2. DAT-/- mice showed much smaller reductions in DA efflux following 5, 10 and 20 conditioning pulses than the other genotypes indicating a reduced sensitivity in DARs. The vertical bars indicate the standard error of the group means. * significantly different from DAT+/+ (Dunnett's t-test). (B, left) DAT uptake efficiency at baseline (pre-nomifensine treatment) was measured as the half-life decay duration (i.e., the time for 50% decrease from the maximum evoked increase to the pre-stimulus baseline level in the amperometric signal) of the DA signal to pre-stimulation levels following 15 pulses at 50 Hz. (B, right) DAT capacity was measured by the half-life decay duration of the DA signal to pre-stimulation levels following similar stimulation in the presence of submaximal DAT blockade with nomifensine (10 mg/kg i.p.). Results indicated that DAT-/- mice differed significantly from the other genotypes in DAT efficiency and capacity, and DAT+/- mice had significantly lower DAT efficiency then DAT+/+ mice. Additionally, nomifensine significantly reduced DAT capacity in DAT+/- and DAT+/+ mice but had no-effect on DAT-/- animals. The vertical bars indicate the standard error of the group means. * groups differed significantly (simple main effects tests). # significantly different from the other genotypes (Dunnett's t-tests).
Half-life decay durations of the DA signal in response to 15 pulses of stimulation at 50 Hz before (baseline) and 30 min after nomifensine (10 mg/kg) administration served as measures of DAT efficiency and capacity, respectively. As shown in Figure 3 B, overall analysis indicated that regardless of condition, pre (baseline) and post (nomifensine) measures of DA half-life were significantly longer in duration in DAT-/- mice in comparison to the other two genotypes (Genotype F= 166.92, df=2, 49, p<.001). Additional analysis of the DAT+/+ and DAT+/- genotypes indicated that DAT+/- mice, regardless of sex, had significantly lower DAT efficiency (mean half-life decay = 0.52±0.04 sec) then DAT+/+ mice (mean half-life decay = 0.35±0.07 sec; Genotype F=4.43, df=1,42, p<.05).
Analysis additionally revealed that there were significant differences in how these genotypes responded to nomifensine (Genotype X Condition F=4.33, df=2, 49, p=.019). Simple main effects tests showed that nomifensine significantly increased DA half-life decay durations in DAT+/- (Condition F= 19.58 df=1,29, p<.001) and DAT+/+ mice (Condition F=6.86, df=1, 11, p=.025), but had no significant effect on DAT-/- mice (Condition F=1.28, df=1,9, p=ns). DAT capacity did not differ significantly between DAT+/- and DAT+/+ mice. There were however sex differences in the effects of nomifensine within these two groups (Sex F=5.46, df=1,38, p<.03). Regardless of genotype, female mice exhibited lower DAT capacity (mean half-life decay = 1.51±0.22 sec) than males (mean half-life decay = 0.84±0.19 sec).
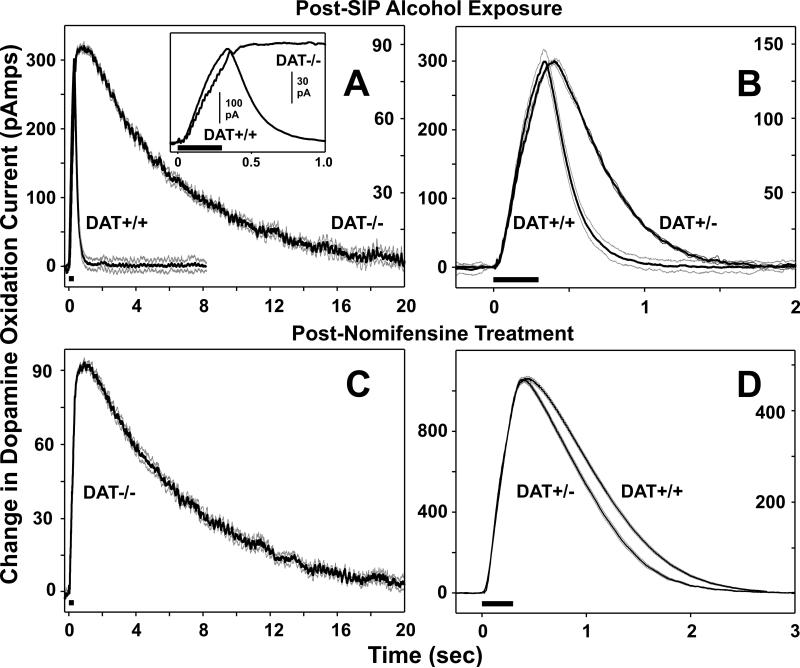
Figure 4 illustrates the marked differences in DA efficiency and capacity as measured in individual mice from all three genotypes. As expected, deletion of the DAT resulted in a markedly longer duration in the half-life decay of the stimulation evoked DA signal and was approximately one-third and two-thirds the amplitude in evoked DA compared to DAT+/+ and DAT+/- mice, respectively (Figure 4 A and B). As shown in figure 4 B, DA half-life decay measured in an individual DAT+/- mouse was longer in duration (i.e. less efficient DA reuptake) compared to DA half-life recorded in a DAT+/+ mouse. As might be expected, administration of nomifensine failed to alter DA half-life decay in DAT-/- (Figure 4 C), whereas non-maximal blockade of the DAT with this drug enhanced DA half-life decay to a similar degree in DAT+/+ and DAT+/- mice (Figure 4 D).
Fig. 4.
Representative examples from individual DAT+/+, DAT+/- and DAT-/- mice depicting the time course for accumbens DA efflux evoked by 15 pulses of medial forebrain bundle stimulation at 50 Hz (black bars). (A) Time course of DA recovery to pre-stimulation levels was significantly prolonged for DAT-/- mice compared to DAT+/+ mice. Left and right y-axis scales correspond to DAT+/+ and DAT-/- responses, respectively. Inset: Expanded time frame for DA responses recorded in DAT-/- and DAT+/+ mice. (B) Time course of DA recovery to pre-stimulation levels for DAT+/- and DAT+/+ mice. As noted in the Results, these two groups differed significantly in this measure. Left and right y-axis scales correspond to DAT+/+ and DAT+/- responses, respectively. (C) Time course of DA recovery to pre-stimulation levels was unaffected following blockade of DATs by nomifensine. In contrast, (D) nomifensine treatment markedly delayed DA recovery in both DAT+/+ and DAT+/- mice. Bold dark lines represent the means and light gray lines the SEMs for six evoked responses recorded in individual mice 5 min before and 30 min after nomifensine administration (10 mg/kg i.p.). All recordings were made at least 1weeks following 10 days of SIP-alcohol. Left and right y-axis scales correspond to DAT+/+ and DAT+/- responses, respectively.
No significant enhancement in stimulation evoked DA half-life decay duration of the DA signal to pre-stimulation levels following 15 pulses at 50 Hz was observed in DAT+/+ or DAT+/- mice following the administration of selective serotonin transporter inhibitor fluoxetine (10 mg/kg, i.p.) or selective norepinephrine transporter inhibitor desipramine (10 mg/kg, i.p.). Most significantly, neither of these transporter inhibitors altered the DA half-life decay in DAT-/- mice (results not shown).
Relationships between behavior and dopaminergic dynamics
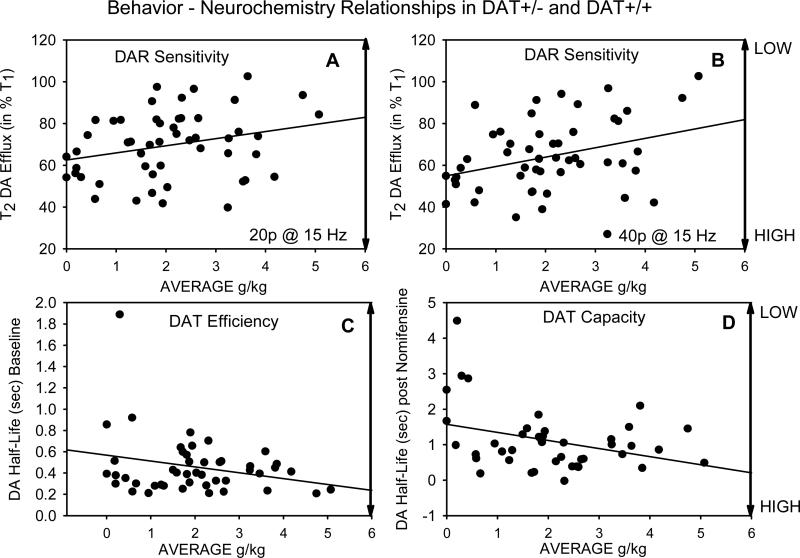
Relationships among behavioral variables and those indexing DA dynamics were investigated in DAT+/- and DAT+/+ mice. Relationships in these mice between behaviors occurring during SIP-alcohol (g/kg consumption, head entries, and nonspecific locomotion) and stimulation-evoked DA responses recorded by FPA (DAR sensitivity, DAT efficiency and capacity) were initially determined using Pearson Product Moment correlations. As shown in Table 1, average g/kg consumption of alcohol over the 10 test days was inversely related to DAR sensitivity. Thus, higher levels of alcohol consumption corresponded to lower degrees of attenuation of accumbens DA efflux following 20 or 40 conditioning pulses (i.e., lower DAR sensitivity). As an example of these significant associations, Figure 5 A and B show the inverse relationship between these variables following 20 and 40 conditioning pulses, respectively. As shown in Figure 5 C and D, respectively, variability in average g/kg consumption had a trend level association with DAT efficiency at pre-nomifensine baseline and was significantly associated with DAT capacity determined 30 min following nomifensine. In the latter case higher levels of alcohol consumption during SIP were associated with shorter durations of DA half-life decay (i.e., higher DAT capacity). As shown in Table 1, food magazine entries showed a similar relationship in that this variable was also significantly associated with shorter durations of DA half-life decay following nomifensine. DAT efficiency at pre-nomifensine baseline and DAT capacity determined 30 min following nomifensine were themselves highly inter-correlated, as was DAR sensitivity at 5 to 40 pulses.
Table 1.
* Bivariate correlations in DAT +/+ and DAT+/- mice
| Behavioral Variables | Neurochemical Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AVG g/kg | AVG Entries | AVG Loco | 5 pulses | 10 pulses | 20 pulses | 40 pulses | DAT Half-life Baseline | ||
| AVG Entries (pellet retrieval) | r p= N |
0.181 0.203 51 |
|||||||
| AVG Locomotion | r p= N |
-0.128 0.370 51 |
-0.490 0.000 51 |
||||||
| 5 pulses | r p= N |
-0.128 0.37 51 |
-0.016 0.91 51 |
-0.047 0.74 51 |
|||||
| 10 pulses | r p= N |
0.270 0.055 51 |
0.039 0.784 51 |
-0.017 0.908 51 |
0.847 0.000 51 |
||||
| 20 pulses | r p= N |
0.274 0.050 51 |
0.069 0.630 51 |
-0.033 0.816 51 |
0.758 0.000 51 |
0.892 0.000 51 |
|||
| 40 pulses | r p= N |
0.327 0.019 51 |
0.150 0.293 51 |
-0.114 0.428 51 |
0.689 0.000 51 |
0.845 0.000 51 |
0.862 0.000 51 |
||
| DA Half-Life Baseline | r p= N |
-0.256 0.086 46 |
0.109 0.471 46 |
-0.040 0.791 46 |
-0.347 0.018 46 |
-0.393 0.007 46 |
-0.417 0.004 46 |
-0.387 0.008 46 |
|
| DA Half-Life nomifensine | r p= N |
-0.344 0.026 42 |
-0.325 0.036 42 |
0.225 0.151 42 |
-0.120 0.447 42 |
-0.076 0.632 42 |
-0.176 0.266 42 |
-0.270 0.084 42 |
0.354 0.021 42 |
Relationships between behavioral variables and indices of dopaminergic function.
r= Pearson product moment correlations, P= Significance levels, N=number of mice in each correlation. Bold P values indicate significant correlations. Note that 5 mice were eliminated prior to determining DA half-life at baseline due to electrode failure and 4 animals were eliminated from the pre/post nomifensine analysis of DA half-life due to failure to respond to the nomifensine injection.
Fig. 5.
(A and B) The relationship between DAR sensitivity expressed as mean percentage change in accumbens DA efflux and g/kg alcohol consumption in individual DAT+/- and DAT+/+ mice. The results of 20 and 40 conditioning pulses are shown. In both cases low DAR sensitivity was significantly associated with the largest amount of g/kg alcohol consumption. (C) DAT uptake efficiency at baseline and g/kg alcohol consumption in individual DAT+/- and DAT+/+ mice. (D) DAT capacity following non-maximal maximal DAT blockade with nomifensine (10 mg/kg i.p.) and g/kg alcohol consumption in individual DAT+/- and DAT+/+ mice. High DAT capacity was significantly associated with high levels of g/kg alcohol consumption. The relationship between DAT efficiency and alcohol consumption approached significance. See Figure 3 and the Methods for additional details.
Hierarchical regression models were used to determine the separate influence of average food magazine entries (entered in Step 1) and average g/kg alcohol consumption (entered in step 2) on DAR sensitivity (40 pulses), baseline DAT efficiency, and DAT capacity following nomifensine, respectively. Results from these models are shown in Table 2. When entered in step 1, average entries failed to predict DAR sensitivity following 40 pulses. When g/kg consumption was added in step 2, it emerged as the only significant predictor of DAR sensitivity. The model predicting baseline DAT efficiency showed no significant effects although g/kg alcohol consumption was marginally (p=.06) and negatively associated with DAT efficiency. For the analysis of DAT capacity, average entries emerged as a significant negative predictor in step 1. However, when g/kg consumption was added in step 2, the influence of food entries became non-significant and alcohol consumption became a significant negative predictor of DAT capacity.
Table 2.
* Hierarchical regression models predicting neurochemical characteristics using behavioral variables including food entries (Step 1) and average alcohol consumption (Step 2)
| Beta | t | Partial r | R2 Change | F changeA | Adj. R2 | |
|---|---|---|---|---|---|---|
| Predicting 40 Pulse response with behavioral measures | ||||||
| Step 1 | 0.02 | 1.13 (1,49) | 0.003 | |||
| AVG Entries | 0.15 | 1.06 | 0.15 | |||
| Step 2 | 0.09 | 5.05* (1,48) | 0.079 | |||
| AVG Entries | 0.09 | 0.68 | 0.10 | |||
| AVG G/KG | 0.31 | 2.25 * | 0.31 | |||
| Predicting DAT half-life at baseline with behavioral measures | ||||||
| Step 1 | 0.01 | .53 (1,44) | -0.01 | |||
| AVG Entries | 0.11 | 0.07 | 0.11 | |||
| Step 2 | 0.08 | 3.63 (1,43) | 0.05 | |||
| AVG Entries | 0.16 | 1.05 | 0.16 | |||
| AVG G/KG | -0.28 | -1.91 + | -0.28 | |||
| Predicting DAT half-life after nomifensine with behavioral measures | ||||||
| Step 1 | 0.11 | 4.72* (1,40) | 0.08 | |||
| AVG Entries | -0.33 | -2.17 * | -0.33 | |||
| Step 2 | 0.09 | 4.35* (1,39) | 0.15 | |||
| AVG Entries | -0.28 | -1.93 | -0.30 | |||
| AVG G/KG | -0.30 | -2.09 * | -0.32 | |||
Degrees of freedom in parentheses
Degrees of freedom in parentheses
p<.05
p=.06
SIP-alcohol vs. SIP-alcohol naïve comparison
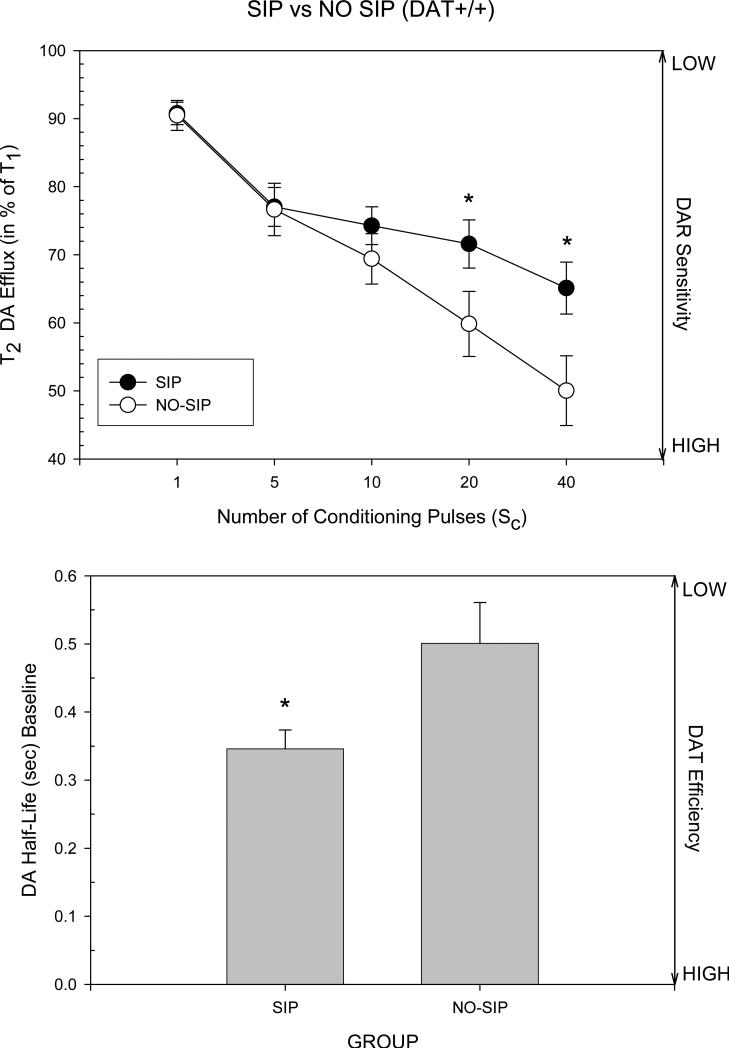
In order to determine if the SIP-alcohol experience influenced the observed relationships between g/kg alcohol consumption and dopaminergic dynamics, FPA was performed in DAT+/+ mice that were either SIP-naïve (n=9) or had been tested in SIP for 10 days (n=16). Figure 6 (Top) shows that these two groups differed in DAR sensitivity as expressed by differences in attenuation of stimulation-evoked accumbens DA efflux by conditioning pulses (Group X Pulse F=5.55, df=3,69, p=.002). Specifically both male and female DAT+/+ mice that had undergone SIP showed significantly less attenuation following 20 and 40 conditioning pulses in comparison to SIP-naïve mice (Dunnett's t-tests), suggesting that DAR sensitivity had been reduced in these mice following their SIP-alcohol experience.
Fig. 6.
(Top) Comparison of DAR sensitivity in SIP-naïve and SIP-alcohol experienced DAT+/+ mice. Mice experienced with alcohol consumption in SIP showed lower levels of DAR sensitivity than SIP-naïve mice. The vertical bars indicate the standard error of the group means. * significantly different from the SIP- naïve control group (Dunnett's t-test). (Bottom) DAT uptake efficiency at baseline in SIP-naïve and SIP-alcohol experienced DAT+/+ mice. Mice experienced with alcohol consumption in SIP had higher DAT efficiency than SIP-naïve mice. The vertical bars indicate the standard error of the group means. * significant group difference as determined by ANOVA. See Figure 3b and the Methods for additional details.
SIP experience also altered DAT efficiency at the pre-nomifensine baseline. As shown in Figure 6 (Bottom), the mean duration of DA half-life decay in SIP experienced mice was significantly shorter (greater DAT efficiency) in comparison to SIP-naïve animals (Group F=7.44, df=1, 19, p<.02), and both sexes were equivalently affected.
SIP experience altered DAT capacity in a Sex dependent manner (Group X Sex F=5.91, df=1,15, p<.05). Simple main effects tests revealed that the mean DA half-life was significantly shorter (higher DAT capacity) in SIP-exposed males (0.78±0.17 sec) then in SIP naïve males (1.69±0.23 sec; Group F=10.25, df=1,9 p<.02). Females were non-significantly affected (results not shown).
Stereotaxic placements
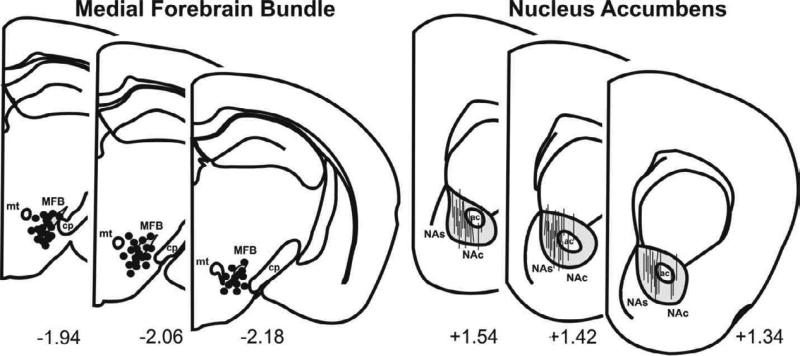
As shown in Figure 7, histological analysis revealed that the tips of the stimulating electrodes (n=101) were confined within the anatomical boundaries of the MFB for all groups of mice, ranging from 1.94 to 2.18 mm posterior to bregma, 0.6 to 1.3 mm lateral to midline, and 4.1 to 5.2 mm ventral to the cortical surface of the brain. The carbon fibers of the electrochemical recording electrodes were confined within the core region of the NAc for all groups of mice, ranging from 1.3 to 1.6 mm posterior to bregma, 0.8 to 1.3 mm lateral to midline, and (shank to tip) 3.0 to 4.0 mm ventral to the cortical surface of the brain.
Fig. 7.
Representative coronal sections illustrating the site of implantation for (Left) medial forebrain bundle (MFB) stimulating electrodes (●) and (Right) striatal carbon fiber recording electrodes ( | ). Only electrode sites for one half of the mice tested (n=50 per site) are shown as the remainder (n=51) closely overlapped with those depicted here. NAc and NAs, correspond to nucleus accumbens core and shell, respectively; ac, anterior commissure; mt, mammillothalamic tract; cp, cerebral peduncle. Numbers correspond to mm from bregma in accordance with the mouse atlas of Franklin and Paxinos (1997).
Discussion
The main behavioral results of this experiment indicated that in the SIP-alcohol paradigm, mice lacking the DAT (homozygous DAT-/-) consumed significantly less g/kg of 5% alcohol, and had significantly lower BACs (mg/dl) than control mice (wildtype DAT+/+). As a group heterozygous DAT+/- mice consumed more alcohol than controls, although this did not correspond to an increase in BAC (Figure 1). Female DAT+/- mice consumed significantly more alcohol than DAT+/+ females. Behaviorally, both DAT-/- and DAT+/- mice exhibited increased numbers of head entries into the food magazine during the SIP sessions in comparison to DAT+/+ (Figure 2).
When tested with FPA following SIP-alcohol, overall DAR sensitivity (T1/T2*100) in DAT-/- mice was significantly less compared to both DAT+/- and DAT+/+ mice, while these two latter groups exhibited comparable DAR sensitivity over the entire series of conditioning pulses tested (Figure 3). DAT+/- mice had significantly lower DAT efficiency than DAT+/+ animals at baseline, and nomifensine significantly increased DA half-life decay (sec) in both of these genotypes. Compared to DAT+/- and DAT+/+ mice, both DAT efficiency and capacity were completely absent in DAT-/- mice, as reflected respectively by a nearly 18-fold higher duration in baseline DA half-life decay (Benoit-Marand et al., 2000; Jones et al.,1998) and failure of nomifensine to significantly alter this duration (Figure 3 B). Consistent with previous voltammetric results from brain slices showing a lack of effect of desipramine or fluoxetine on the clearance of DA in DAT+/+ and DAT-/- mice (Mateo et al., 2004), blockade of serotonin and norepinephrine transporters in all three genotypes failed to alter the duration of stimulation evoked DA half-life decay. Together, these data provide confirmatory evidence of the specificity of stimulation-evoked DA efflux in the NAc in vivo and are highly consistent with the notion of a lack of compensatory uptake of DA by alternative transporters in the NAc of DAT-/- mice.
Measures of g/kg alcohol consumption were related to dopaminergic dynamics in DAT+/- and DAT+/+ mice following 10 days of SIP-alcohol. In these mice average alcohol consumption was inversely correlated with DAR sensitivity, with high levels of drinking corresponding to low levels in DAR sensitivity. Thus, high levels of drinking corresponded to a smaller decrease in evoked DA efflux following a series of conditioning pulses (Table 1; Figure 5 A and B). Additionally, the degree of alcohol consumption in these mice was weakly correlated with DAT efficiency, but strongly related to DAT capacity. In both cases high levels of drinking corresponded to low DA half-life decay times (i.e., relatively faster rates in DA clearance in the absence and presence of incomplete inhibition of DA uptake by nomifensine, Figure 5 C and D). Although average food entries initially showed a zero order correlation with DAT capacity this association became non-significant when considered along with average alcohol consumption (Table 2).
In order to determine if these relationships between dopaminergic dynamics and SIP-alcohol consumption were due to pre-existing individual differences in the functional characteristics of the mesoaccumbens DA system, or occurred as a function of 10 days of SIP-alcohol drinking, DAR sensitivity and DAT efficiency and capacity were assessed in SIP-naïve and compared to SIP-alcohol exposed DAT+/+ mice. DAR sensitivity was significantly lower in SIP-alcohol exposed animals in comparison to SIP-alcohol naïve mice (Figure 6, Top). Additionally, in DAT+/+ mice, the SIP-alcohol experience also significantly increased DAT efficiency in comparison to SIP-naïve animals (Figure 6, Bottom). DAT capacity in SIP-alcohol exposed males was significantly increased compared to SIP-naïve males, while DAT capacity in females did not change significantly. These results are suggestive that in DAT+/+ mice, reduced DAR sensitivity, increased DAT efficiency and possibly increased DAT capacity (in males) likely resulted from the SIP-alcohol experience.
Coupled with the correlational and hierarchical regression analyses, these results are suggestive that SIP-alcohol experience has varied levels of influence on measures of DA dynamics. As all three analyses (Pearson correlations, hierarchical regression models, and SIP-alcohol vs. SIP naïve) indicated that DAR sensitivity was significantly associated with g/kg alcohol consumption it is reasonable to conclude that this is a very consistent effect of the SIP-alcohol experience. Changes in DAT efficiency and capacity that could be associated with alcohol consumption were less consistently indicated by the three analyses. Thus, an association between DAT efficiency and g/kg alcohol consumption was only weakly confirmed by the Pearson correlations and hierarchical regression analyses. These same two analyses significantly associated average alcohol consumption with changes in DAT capacity but this was only confirmed in male DAT+/+ mice by the SIP-alcohol vs. SIP naïve analysis. It therefore seems most parsimonious to suggest that alcohol drinking in SIP may have less consistent effects on DAT efficiency and capacity.
Behaviors related to genotype
The reduced consumption of 5% alcohol observed in DAT-/- mice may initially seem at odds with reported enhancements in 2-bottle choice consumption of high concentrations (24 to 32%) of alcohol in DAT-/- mice (Hall et al., 2003). However, it should be noted that in the current study 8 of 11 mice in the DAT-/- mice group were female. These results are thus more consistent with other findings showing that female DAT-/- mice have either significantly reduced alcohol drinking (Savelieva et al., 2002), or a trend toward decreased consumption of alcohol (Hall et al., 2003) in 2-bottle choice.
The reduced consumption of 5% alcohol observed in these DAT-/- mice appears to be a behaviorally specific manifestation of DAT deletion. Although these mice also showed significant elevations in head entries into the food magazine, which could have indirectly reduced the opportunity to drink from the drinking tube, this possibility is unlikely for a number of reasons. First, elevations in average daily head entries were comparable in both DAT-/- and DAT+/- animals although they displayed significant differences in alcohol consumption. Secondly, in comparison to controls, DAT-/- mice showed significant increases in entries on test days 4, 5, and 7, but significantly reduced alcohol consumption on test days 2, 7, 8, 9, and 10. Thus, the lack of correspondence between drinking and head entries, as well as the observed similarities in locomotor activity across groups, argues against a relationship between these behaviors and reduced alcohol consumption in DAT-/- mice and supports the notion that reduced SIP-alcohol consumption is directly related to DAT deletion.
It should be remembered however, that DAT deletion is also accompanied by developmental compensatory changes in dopaminergic dynamics such as a marked reduction in DAR activity (~90%), reduced expression of the DA synthesizing rate-limiting enzyme tyrosine hydroxylase (~90%), and reduced DA D1 and D2 receptor expression (~50%) (Giros et al., 1996; Jones et al., 1999; Jaber et al., 1999; Sora et al., 2001). Thus, the reduced sensitivity of DARs observed in DAT-/- mice is likely a developmental consequence of complete DAT deletion and not related to SIP-alcohol exposure. It is therefore possible that the developmental reduction in postsynaptic D2 receptors that is a prominent characteristic of these mice, and strongly implicated in the reinforcing effects of alcohol (Cohen et al., 1997; Vanover and Woolverton, 1994), may directly or additionally account for their relatively low levels of alcohol consumption.
We also found that female DAT+/- mice consumed significantly more alcohol than DAT+/+ females. This result is consistent with previous observations showing that these mice consume more alcohol in 2-bottle choice (Savelieva et al., 2002) and show increased preference for low concentrations of ethanol (Hall et al., 2003).
Dopaminergic dynamics related to genotype following SIP-alcohol exposure
DAR sensitivity in DAT+/+ and DAT+/- mice
DAR sensitivity following SIP-alcohol was similar in DAT+/+ and DAT+/- mice and significantly higher than in DAT-/- mice, suggesting that this dopaminergic characteristic was unaffected by partial deletion of DAT in heterozygous mice. Although DAR sensitivity was not examined pre and post SIP-alcohol in DAT-/+ or DAT-/- mice, in SIP-exposed DAT+/+ mice DAR sensitivity was significantly lower than in SIP-naïve DAT+/+ mice, suggesting that repeated SIP-alcohol exposure reduced DAR sensitivity in these animals.
The relationship between DAR sensitivity and alcohol exposure is not straightforward. Unlike the present study, Budygin et al. (2007) failed to observe a change in DAR sensitivity as assessed by inhibition of electrically evoked DA efflux by bath applied quinpirole in rat striatal/accumbens slices measured 30 min following 5 or 10 days of alcohol vapor exposure. However, in a study using a primate model of long-term voluntary excessive alcohol drinking Budygin et al. (2003) found that 18 months of alcohol exposure was associated with an increase in striatal DAR sensitivity when assessed within 2 hours of alcohol exposure. Additional studies using autoradiography to assess D2 receptor density during alcohol consumption and post-withdrawal have also yielded conflicting results (Tajuddin and Druse, 1996; Djouma and Lawrence, 2002).
DAT efficiency and capacity in DAT+/+ mice
SIP-alcohol exposure resulted in a significant increase in DAT efficiency and respectively a significant elevation and no change in DAT capacity in DAT+/+ male and female mice, compared to SIP-naïve mice. Consistent with previous findings comparing wildtype and heterozygous DAT knockout mice (Jones et al., 1998), evoked DA was approximately 2-fold greater in magnitude and DAT uptake twice as efficient in DAT+/+ mice, compared to DAT+/- mice (Figure 4 B). The enhancement in DAT efficiency, corresponding to an increase in Vmax of the DA transporter, but not an increase in DAT density (capacity), is consistent with a number of in vitro studies assessing these dopaminergic characteristics in rats under a variety of chronic alcohol treatment conditions. For example, Carrolla et al. (2006) examined the rate of [3H]DA accumulation in striatal and NAc homogenates taken from High-Alcohol-Drinking replicate line 1 (HAD-1) rats 2 hours after 24 hour access to 15% alcohol for 8 weeks. They found a significant increase in the Vmax of the DA transporter in the NAc of female HAD-1 rats. However, based on other molecular and autoradiographic studies these investigators argued that this increased efficiency following chronic alcohol exposure was due to an increase in DAT capacity (e.g. Jiao et al., 2006; Mash et al., 1996; Rothblat et al., 2001). Additionally, using fast-scan cyclic voltammetry, Budygin et al. (2007) found enhanced DA uptake rates (efficiency) in rat NAc and striatum brain slices prepared within 30 min after removal from alcohol vapor chambers following 5 and 10 day alcohol exposure. Most significantly, these investigators attributed the change to an increase in Vmax and not to an increase in DAT capacity, as there was no apparent change in Km (DA affinity) and both control and alcohol exposed rats showed identical sensitivity to nomifensine-mediated uptake inhibition.
The present findings are consistent with the possibility that SIP-alcohol exposure decreased DAR sensitivity while increasing DAT efficiency, with an increase in DAT capacity, at least in male DAT+/+ mice. Discrepancies between the present voltammetry investigation with other studies, especially in regard to DAR sensitivity may be due to a number of methodological differences including (1) species variations (rats, primates, and mice), (2) BACs achieved by vapor inhalation, voluntary drinking and SIP, and the related possibility that alcohol dependent (vapor inhalation) and non-dependent (SIP-alcohol) animals are being compared, (3) the duration of alcohol exposure (5 days to 18 months), (4) in vitro versus in vivo analyses (brain slices vs. anesthetized), (5) the methodology of DAR assessment (quinpirole vs. conditioning pulse inhibition), or (6) the latency between alcohol consumption and testing.
It is also important to note one additional caveat to the conclusion that SIP-alcohol exposure decreased DAR sensitivity while increasing DAT efficiency. SIP-alcohol drinking consists of both exposure to the SIP task as well as the consumption of alcohol during the task. It is well known that the acquisition of SIP drinking is specifically dependent on DA neurotransmission in the mesoaccumbens DA system (Mittleman et al., 1990, 1994; Robbins and Koob, 1980; Wallace et al., 1983). The possibility that exposure to the SIP task with water as the available fluid could result in the relatively permanent changes in dopaminergic dynamics observed in the current study has not been investigated.
Relationships between behavior and dopaminergic dynamics
An additional goal of this study was to determine whether DAR sensitivity, DAT efficiency, and/or DAT capacity correlated with differences in the degree of alcohol consumption observed in DAT+/- and DAT+/+ mice. As noted above, low-high levels of alcohol consumption inversely corresponded to high-low levels of DAR sensitivity in these mice (Figure 5, A & B). It has been demonstrated that acute administration of alcohol can elevate dialysate DA levels by ~80% above baseline in both DAT+/+ and DAT-/- mice (Mathews et al., 2006), and it seems reasonable to assume that the effects of acute alcohol administration in our DAT+/+ and DAT+/- mice would be similar. Keeping this possibility in mind, the currently observed relationship between DAR sensitivity and alcohol consumption could occur via two different mechanisms. Reductions in DAR sensitivity could result from a direct action of repeated exposure to high brain alcohol concentrations that in turn lead to enhanced extracellular concentrations of DA. Alternatively, high brain alcohol–induced increases in extracellular concentrations of DA may lead to a downregulation of DAR sensitivity in high alcohol DAT+/+ and DAT+/- drinkers. Regardless of the mechanism, as these results were obtained after a period of at least 1 week of alcohol abstinence they indicate that previous exposure to relatively high levels of alcohol consumption in the SIP-alcohol paradigm leads to relatively low levels of DAR sensitivity in the NAc, and this change is alcohol driven.
In contrast to the inverse relationship between alcohol consumption and DAR sensitivity, low-high levels of alcohol consumption in DAT+/+ and DAT+/- mice varied strongly with low-high levels of DAT capacity, while low-high levels of drinking in these mice varied less consistently with low-high levels of DAT efficiency (Figure 5 C and D, Table 1). Coupled with data obtained in SIP-alcohol experienced and SIP-naïve DAT+/+ mice, it is possible that similar to these mice, high levels of alcohol consumption led to increased levels of DAT capacity, while differences in DAT efficiency are an intrinsic manifestation of genetic variability of DAT deletion in DAT+/- mice that are less influenced by alcohol exposure. Relatively high levels of DAT efficiency and capacity would be expected to reduce extracellular concentrations of DA in the NAc.
The mechanisms accounting for alcohol-induced changes in DAT efficiency or capacity are unknown. It has been previously shown that acute alcohol administration does not alter DA uptake in mouse, rat, or monkey striatum (Budygin et al., 2001, 2003, 2005; Jones et al., 2006; Mathews et al., 2006; Yavich and Tiihonen, 2000; Yim and Gonzales, 2000). However, in vitro and in vivo evidence suggest that increased extracellular DA levels that accompany reductions in DAR activity, may participate in the presynaptic regulation of DAT (Dickinson et al., 1999; Zahniser et al., 1999; Mayfield et al., 2001; Wu et al., 2002). Ligand binding studies suggest that chronic alcohol exposure followed by withdrawal leads to increases in DAT capacity within terminal target sites of central dopaminergic systems (e.g. Jiao et al., 2006; Mash et al., 1996; Rothblat et al., 2001). This could include changes in DAT efficiency as well, that could be secondary to elevated DA concentrations and not a direct response to alcohol (Budygin et al., 2007). Thus, it is conceivable that, coupled with neuroadaptive reductions in DAR sensitivity, alcohol-induced elevations in extracellular DA concentrations in the NAc of DAT+/+ and DAT+/- mice served to increase DAT capacity, and to a lesser extent, efficiency, in a compensatory fashion.
Conclusions
Overall, these results provide an indication of DA dynamics that are associated with alcohol consumption, along with those characteristics that are altered by alcohol consumption. It should be noted that the present results were obtained in mice tested in a SIP-alcohol paradigm which involves both mild food restriction and the scheduled delivery of food. We have previously shown that strain differences in SIP-alcohol drinking between C57BL/6J and DBA/2J mice are the same as those obtained using more traditional methods of alcohol self-administration (Crabbe et al., 1999; Mittleman et al., 2003; Risinger et al., 1998). These similarities in results are therefore suggestive that data obtained using a SIP-alcohol paradigm can be generalized to other methods of alcohol self-administration, such as 2-bottle choice or 24 hr access.
Acknowledgements
Supported by 1U01AA13506 to GM, 1U01AA13503 to DG, and 1U01AA13509 to DBM. The authors wish to thank Marc G. Caron for his generous gift of DAT breeders and J. Gayle Beck for her critical discussion of the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassareo V, De Luca MA, Aresu M, Aste A, Ariu T, Di Chiara G. Differential adaptive properties of accumbens shell dopamine responses to ethanol as a drug and as a motivational stimulus. Eur. J. Neurosci. 2003;17:1465–1472. doi: 10.1046/j.1460-9568.2003.02556.x. [DOI] [PubMed] [Google Scholar]
- Bauer D, Warthoe P, Rohde M, Strauss M. Detection and differential display of expressed genes by DDRT-PCR. PCR Methods Appl. 1994;4:97–108. doi: 10.1101/gr.4.2.s97. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: Time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Jaber M, Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur. J. Neurosci. 2000;12:2985–2992. doi: 10.1046/j.1460-9568.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Phillips AG. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviours. Behav. Pharmac. 1996;7:675–708. [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: A preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Mathews TA, Lapa GB, Jones SR. Local effects of acute ethanol on dopamine neurotransmission in the ventral striatum in C57BL/6 mice. Eur. J. Pharmacol. 2005;523:40–45. doi: 10.1016/j.ejphar.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Läck AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology. 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J. Pharmacol. Exp. Ther. 2001;297:27–34. [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Sanger DG. Evidence for the involvement of dopamine receptors in ethanol-induced hyperactivity in mice. Neuropharmacology. 1997;36:1099–1108. doi: 10.1016/s0028-3908(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain. Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G. Ethanol as a neurochemical surrogate of conventional reinforcers: the dopamine-opioid link. Alcohol. 1996;13:13–17. doi: 10.1016/0741-8329(95)02034-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J. Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Djouma E, Lawrence AJ. The effect of chronic ethanol consumption and withdrawal on mu-opioid and dopamine D(1) and D(2) receptor density in Fawn-Hooded rat brain. J. Pharmacol. Exp. Ther. 2002;302:551–559. doi: 10.1124/jpet.102.035915. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur. J. Neurosci. 2003;17:1–11. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Gilman S, Koeppe RA, Adams KM, Junck L, Kluin KJ, Johnson-Greene D, Martorello S, Heumann M, Bandekar R. Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (11C)dihydrotetrabenazine and positron emission tomography. Ann. Neurol. 1998;44:326–336. doi: 10.1002/ana.410440307. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Giros B, el Mestikawy S, Bertrand L, Caron MG. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Sex-dependent modulation of ethanol consumption in vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) knockout mice. Neuropsychopharmacology. 2003;28:620–628. doi: 10.1038/sj.npp.1300070. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Gallinat J, Schumann G, Puls I. Pharmacogenetic insights to monoaminergic dysfunction in alcohol dependence. Psychopharmacology (Berl) 2004;174:561–570. doi: 10.1007/s00213-004-1903-x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Higley JD, Jones DW, Gorey JG, Saunders R, Zajicek K, Suomi SJ, Lesch KP, Weinberger DR, Linnoila M. In vivo observation of an association between serotonin transporters and sensitivity to alcohol intoxication. Am. J. Psychiatry. 1998;155:1023–1028. doi: 10.1176/ajp.155.8.1023. [DOI] [PubMed] [Google Scholar]
- Jaber M, Dumartin B, Sagne C, Haycock JW, Roubert C, Giros B, Bloch B, Caron MG. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur. J. Neurosci. 1999;11:3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006;1073-1074:175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Köhnke MD, Batra A, Kolb W, Köhnke AM, Lutz U, Schick S, Gaertner I. Association of the dopamine transporter gene with alcoholism. Alcohol. 2005;40:339–342. doi: 10.1093/alcalc/agh179. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, Roberts DW, Kim U. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur. J. Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, McPhinton PR, Dalack GW, Cook EH, Cassin BJ, Watson SJ. Brain dopamine transporter messenger RNA and binding sites in cocaine users. Arch. Gen. Psychiatry. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- Mash DC, Staley JK, Doepel FM, Young SN, Ervin FR, Palmour RM. Altered dopamine transporter sites in alcohol-preferring vervet monkeys. Neuroreport. 1996;7:457–462. doi: 10.1097/00001756-199601310-00020. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, John CE, Banks ML, Jones SR. Voltammetric assessment of dopamine clearance in the absence of the dopamine transporter: no contribution of other transporters in core or shell of nucleus accumbens. J. Neurosci. Meth. 2004;140:183–187. doi: 10.1016/j.jneumeth.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Maiya R, Keller D, Zahniser NR. Ethanol potentiates the function of the human dopamine transporter expressed in Xenopus oocytes. J. Neurochem. 2001;79:1070–1079. doi: 10.1046/j.1471-4159.2001.00656.x. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Rosner AL, Schaub CL. Polydipsia and dopamine: behavioral effects of dopamine D1 and D2 receptor agonists and antagonists. J. Pharmacol. Exp. Ther. 1994;271:638–50. [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin. Exp. Res. 2003;27:918–925. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Whishaw IQ, Jones GH, Koch M, Robbins TW. Cortical, hippocampal and striatal mediation of schedule-induced behaviors. Behav. Neurosci. 1990;104:399–409. doi: 10.1037//0735-7044.104.3.399. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin. Exp. Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Repo E, Kuikka JT, Bergström KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology (Berl) 1999;147:314–318. doi: 10.1007/s002130051173. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Koob GF. Selective disruption of displacement behavior by lesions of the mesolimbic dopamine system. Nature. 1980;285:409–412. doi: 10.1038/285409a0. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Rubin E, Schneider JS. Effects of chronic alcohol ingestion on the mesostriatal dopamine system in the rat. Neurosci. Lett. 2001;300:63–66. doi: 10.1016/s0304-3940(01)01548-8. [DOI] [PubMed] [Google Scholar]
- Savelieva KV, Caudle WM, Findlay GS, Caron MG, Miller GW. Decreased ethanol preference and consumption in dopamine transporter female knock-out mice. Alcohol Clin. Exp. Res. 2002;26:758–764. [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc. Natl. Acad. Sci. USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Dugast C, Chergui K, Msghina M, Gonon F. Uptake of dopamine released by impulse flow in the rat mesolimbic and striatal systems in vivo. J. Neurochem. 1995;65:2603–2611. doi: 10.1046/j.1471-4159.1995.65062603.x. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. Effects of chronic alcohol consumption and aging on dopamine D2 receptors in Fischer 344 rats. Alcohol Clin. Exp. Res. 1996;20:144–151. doi: 10.1111/j.1530-0277.1996.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Tang A, George MA, Randall JA, Gonzales RA. Ethanol increases extracellular dopamine concentration in the ventral striatum in C57BL/6 mice. Alcohol Clin. Exp. Res. 2003;27:1083–1089. doi: 10.1097/01.ALC.0000075825.14331.65. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Bergström K, Hakola P, Karhu J, Ryynänen OP, Föhr J. Altered striatal dopamine re-uptake sites in habitually violent and nonviolent alcoholics. Nature Med. 1995;1:654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T, Komiyama T, Mitsushio H, Sano A, Tanabe H. Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism. Mol. Psychiatry. 1999;4:552–557. doi: 10.1038/sj.mp.4000562. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Woolverton WL. Behavioral effects of the dopamine autoreceptor agonist PD 128483 alone and in combination with cocaine. J. Pharmacol. Exp. Ther. 1994;270:1049–1056. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin. Exp. Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Wallace M, Singer G, Finlay J, Gibson S. The effects of 6-OHDA lesions of the nucleus accumbens septum on schedule-induced drinking, wheel running and corticosterone levels in the rat. Pharmacol. Biochem. Behav. 1983;18:129–136. doi: 10.1016/0091-3057(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J. Comp. Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J. Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Tiihonen J. Ethanol modulates evoked dopamine release in mouse nucleus accumbens: Dependence on social stress and dose. Eur. J. Pharmacol. 2000;401:365–373. doi: 10.1016/s0014-2999(00)00456-8. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin. Exp. Res. 1998;22:367–374. [PubMed] [Google Scholar]
- Yoshimoto K, Yoshida T, Sorimachi Y, Hirano A, Takeuchi Y, Ueda S. Effects of age and ethanol on dopamine and serotonin release in the rat nucleus accumbens. Physiol. and Behav. 1998;64:347–351. doi: 10.1016/s0031-9384(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J. Pharm. Exp. Ther. 1999;289:266–277. [PubMed] [Google Scholar]