Abstract
Previous reports using dual x-ray absorptiometry (DXA) suggest that up to 70% of adults with thalassemia major (Thal) have low bone mass. However, few studies have controlled for body size and pubertal delay, variables known to affect bone mass in this population. In this study, bone mineral content and areal density (BMC, aBMD) of the spine and whole body were assessed by DXA, and volumetric BMD and cortical geometries of the distal tibia by peripheral quantitative computed tomography (pQCT) in subjects with Thal (n=25, 11 male, 10 to 30 yrs) and local controls (n=34, 15 male, 7 to 30 yrs). Z-scores for bone outcomes were calculated from reference data from a large sample of healthy children and young adults. Fasting blood and urine were collected, pubertal status determined by self-assessment and dietary intake and physical activity assessed by written questionnaires. Subjects with Thal were similar in age, but had lower height, weight and lean mass index Z-scores (all p<0.001) compared to controls. DXA aBMD was significantly lower in Thal compared to controls at all sites. Adult Thal subjects (>18 yrs, n=11) had lower tibial trabecular vBMD (p=0.03), cortical area, cortical BMC, cortical thickness, periosteal circumference and section modulus Z-scores (all p<0.01) compared to controls. Cortical area, cortical BMC, cortical thickness, and periosteal circumference Z-scores (p=0.02) were significantly lower in young Thal (≤18 yrs, n=14) compared to controls. In separate multivariate models, tibial cortical area, BMC, and thickness and spine aBMD and whole body BMC Z-scores remained lower in Thal compared to controls after adjustment for gender, lean mass and/or growth deficits (all p<0.01). Tanner stage was not predictive in these models. Osteocalcin, a marker of bone formation, was significantly reduced in Thal compared to controls after adjusting for age, puberty and whole body BMC (p=0.029). In summary, we have found evidence of skeletal deficits that cannot be dismissed as an artifact of small bone size or delayed maturity alone. Given that reduced bone density and strength are associated with increased risk of fracture, therapies focused on increasing bone formation and bone size in younger patients are worthy of further evaluation.
1.0 INTRODUCTION
The identification of reduced bone mass in patients with thalassemia (Thal) is not a new finding. Despite improvements in treatment strategies over the last few decades, it has been estimated that 60 to 90% of adult patients with thalassemia present with osteopenia or osteoporosis [1,2]. Incidence of low bone mass is dependent upon the underlying type of thalassemia [3,4], and increases with age [1]; patients with thalassemia appear to gain bone at a slower rate and lose bone faster compared to healthy populations [5]. Many factors contribute to the etiology of low bone mass including: ineffective erythropoiesis which leads to bone marrow hyperplasia [6], growth hormone and sex steroid deficiencies [1,2], hypothyroidism [7], vitamin D deficiency [8], and severe anemia [6,9] with presumed, but poorly characterized reduced physical activity. Even well transfused patients with normal gonadal function who are supplemented with calcium have been shown to have low bone mass by dual x-ray absorptiometry (DXA) [1,10], suggesting other factors are also involved.
One of the main challenges to the assessment of bone health in patients with Thal is accurate interpretation of densitometry results. Many patients with Thal have height deficits, and delayed puberty and/or bone age. Bone mineral density (BMD) assessed by DXA is derived from a 2-dimensional image and is often referred to as an “areal” (g/cm2) BMD (aBMD); it is not a true volumetric density [11], and DXA underestimates BMD in small patients compared to those who are of normal size for chronological age [12]. Additionally, excessive hepatic and cardiac iron stores are frequently observed in patients with thalassemia. In very high concentrations, iron is radiographically dense and may lead to potential errors in accurate aBMD assessment [13,14]. Peripheral quantitative computed tomography (pQCT) is an alternative bone densitometry technique that is able to assess volumetric density (vBMD) at peripheral sites as well as estimate geometric properties of bone that are directly proportional to bone strength. pQCT has the additional advantage that peripheral sites are assessed, where iron is not accumulated. The radiation dosage to patients is slightly lower than DXA, making it appropriate for use in pediatric populations.
Though many studies have identified bone mass deficits in patients with thal [1-6], few have examined potential confounding by size deficits or assessed other measures of bone strength. The purpose of this study was to characterize deficits in aBMD by DXA, vBMD and bone geometry by pQCT and markers of bone turnover controlling for size and puberty in a contemporary sample of young patients with Thal (10-30 yr) who were previously identified as having low bone mass by DXA, compared to a healthy active control group. Dietary and physical activity influences on bone mass and strength were also explored.
2.0 MATERIALS AND METHODS
2.1 Subjects
Subjects with Thal were recruited from three hematology clinics in the U.S.: the Children's Hospital & Research Center, Oakland (CHRCO), the Children's Hospital of Philadelphia (CHOP) and the University of California at San Francisco (UCSF) as part of a longitudinal interventional study. Results included herein were limited to baseline assessments among subjects with complete pQCT and DXA data. Subjects with Thal were considered eligible if they were between 6 and 30 years of age, with a DXA aBMD Z-score less than −1.0 at the spine, hip or whole body. Children were excluded if they had other chronic medical conditions known to affect bone health, had a history of bone marrow transplant (BMT), or had taken a bisphosphonate in the previous year. Potentially eligible subjects based on age and diagnosis were identified and approached. A total of 37 subjects were excluded due to: previous BMT (n=16), conflicting medical conditions (n=8), aBMD Z-scores > −1.0 (n=5), current bisphosphonate therapy (n=3), current pregnancy (n=2), or death prior to contacting (n=3).
Healthy control subjects of similar age, gender and ethnicity were also recruited from the local community at CHRCO. Informed written consent was obtained from all subjects or legal guardians and assent from the subjects <18 yrs. The protocol was approved by the Committee for the Protection of Human Subjects of the Institutional Review Board at CHRCO, CHOP and UCSF.
2.2 Methods
Subjects were measured at two centers, CHRCO (n=18 Thal, all controls) and CHOP (n=7 Thal). All subjects had a fasting morning blood draw, urine collection, anthropometry, pubertal assessment, DXA and pQCT examinations, and completed a brief medical history and physical activity questionnaire. In addition, Thal subjects < 20 yrs had a bone age examination. Height and weight were assessed by trained research anthropometrists in duplicate and the mean used in the analysis. Sexual maturation [15] was assessed in subjects <21 years of age using a validated self-assessment pictorial questionnaire [16]. Physical activity was estimated using a self-assessment questionnaire of weekly hours of inactivity as well as hours spent per week in weight bearing plus cardiovascular type activities (e.g. running, soccer, basketball, dance), weight bearing, non-cardiovascular type activities (e.g. weight lifting, yoga, baseball, walking) and non-weight bearing, cardiovascular type activities (e.g. swimming, biking). Self reported fracture history included age and location of fracture, as well as circumstances surrounding fracture (e.g. fall, motor vehicle accident). Fractures were not adjudicated through individual x-ray.
Review of medical charts for the Thal subjects included retrieval of information regarding endocrine diagnoses and relevant ongoing hormone therapy. Based on these data, the following definitions were used: Growth Failure: Height Z-score of less than −2.5 and/or ongoing growth hormone therapy. Diabetes Mellitus: Fasting glucose > 126 mg/dL, and/or non-fasting glucose > 200 mg/dL and/or exogenous insulin administration and/or use of oral hypoglycemic medications. Hypothyroidism: Ongoing thyroid hormone replacement therapy. Hypogonadism: Females: >13 yrs, not yet Tanner B2 (i.e., prepubertal breast development) or >14 yrs requiring estrogen replacement therapy or >16 yrs with primary amenorrhea. Males: >14 yrs, not yet Tanner G2 (i.e., prepubertal genital development) OR on androgen replacement therapy OR >17 yrs, not yet Tanner G4 (i.e. mid-pubertal genital development). For the analysis of hypogonadism only females >13 yrs and males >14 yrs were included.
Following blood sample collection, serum and plasma were separated from erythrocytes and stored at −70°C until analyzed. For transfused patients, blood samples were collected at least 2 weeks from the previous transfusion and they were instructed to refrain from taking their chelator medication for 24 hrs prior to blood sampling. A 2nd morning void spot urine sample was also collected. Complete blood cell counts and serum ferritin were assessed by usual methods in the clinical laboratory. Urinary N-telopeptide of type 1 collagen, serum osteocalcin, 25-OH vitamin D, and intact parathyroid hormone (PTH-CAP) were assessed by a commercial laboratory (ARUP laboratories, Salt Lake City, UT).
2.3 Bone Assessments
BMC and aBMD of the lumbar spine (L1-L4, fast array spine) and whole body were measured according to manufacturer guidelines on Hologic QDR 4500 scanners with Discovery software upgrades. The in vivo precision of spine BMD measurements was determined by duplicate measurement of 30 healthy subjects of similar age. The root mean square error (RMSE) of spine BMD was 0.025 g/cm2 (coefficient of variation [CV] = 0.7 %). The in vitro CV of the CHRCO and CHOP DXA instruments was less than 1% for standard phantoms. All DXA scans were analyzed by a single operator (EBF) using Hologic software version 12.6.
pQCT measurements were performed using a Stratec XCT2000 imager (pQCT, Stratec, Pforzheim, Germany, software v5.5). In vivo reproducibility of trabecular vBMD at the 3% site was 0.8% at CHRCO and 1.3% at CHOP, performed on subjects aged 9-21 years at CHRCO and 11-18 years at CHOP. Non-dominant tibial lengths were obtained in triplicate using a segmometer (Rosscraft-Campbell Caliper 20, Canada) and the average used. A mark was placed on the subject's calf prior to positioning into the scanner at 66% of the distal tibia length. A scout scan was performed first to determine the anatomical landmark for the reference line placement as previously described [17]. The scanner was then programatically set to scan at 3% and 38% of the tibial length proximal to the reference line, using a 0.4mm voxel size and 25 mm/s scan speed. The leg was repositioned to measure the 66% site. Trabecular vBMD (mg/cm3) was determined at the 3% site. At the mid-shaft (38% tibia), cortical thickness (mm), cortical BMC, cortical vBMD, endosteal and periosteal circumferences were measured. Section modulus, an in vivo measure of bone strength, was also calculated. Muscle and fat mass were determined at the 66% site. Analytical thresholds for analysis of the distal metaphyseal site (3%) included bone thresholds of 200 and 600 mg/mm3, with contour mode 1 and peel mode 4. For the diaphyseal site (38%), we used a bone threshold of 710 mg/mm3 and for SSI a threshold of 300 mg/mm3 and separation mode 2. For the 66% site soft tissue analysis, we employed thresholds of −100, and 40 mg/mm3 with contour mode 3, peel mode 2, and to separate marrow and skin we used thresholds of −100 and 2000 mg/mm3 with separation mode 4.
Spine and whole body DXA phantoms were scanned at CHRCO and CHOP during the period of study to determine agreement between the two instruments and calibration equations were developed. Spine and whole body BMC and aBMD performed at CHRCO were adjusted to be consistent with CHO values.
2.4 Statistical Analyses
Z-scores for weight and height were calculated using Epi Info™ Version 3.5.1. For subjects between 20 and 30 years of age, height Z-score was calculated using the oldest age in the reference database. Lean mass index Z-score was calculated using DXA-generated, ethnic specific NHANES reference data [see reference 18]. Lean mass index was calculated first (kg of lean mass/cm height 2), then Z-score calculated according to the following equation:
X= LMI, L=power transformation, M=median value, σ=standard deviation
Z-scores for all DXA aBMD, BMC, and pQCT density and geometric variables were based on reference curves created from a sample of healthy children and young adults (5 – 30 years) evaluated at CHOP (see Leonard et al. 2010 [19] for sample details). The healthy reference sample for DXA was comprised of 1001 subjects; a subset of these (n=762) also had pQCT measurements. This reference data set was comprised of 58% non-African American. We compared the subjects in this study to the non-African American reference subjects (83% Caucasian, 8% Hispanic and 5% Asian) in order to calculate Z-scores. Whole body scans were not performed in the reference data set from adults, therefore whole body BMC Z-score data are missing for patients > 22 years. Spine aBMD reference data were collected using the array mode, whereas data in this report were collected in fast array. Published reports have not found significant differences in absolute values or precision between these 2 scan speeds [20]. Z-scores for all pQCT based variables were calculated relative to age and gender. Height Z-for age score for DXA outcomes and trabecular length for age Z-scores for pQCT outcomes were then included in all models to adjust for size differences by age in the subjects compared to the reference data.
Statistical analyses were conducted using Stata 9.2 (Stata, Inc., College Station, TX). Differences in continuous variables were analyzed using independent two sample t-tests for normally distributed data and Wilcoxin-Mann-Whitney test for non-parametric tests. Differences in categorical variables were analyzed using Pearson's chi-square or Fisher's exact tests for small samples (expected frequency less than 5). Spearman's rank correlation coefficients were used to describe relationships between bone parameters and age. Linear regression was used to create univariate and multivariate models to describe relationships between bone outcomes and factors which may influence it (i.e. diagnosis, Tanner stage, age, gender, height Z-score). Interactions between factors which may influence poor bone outcomes (e.g. age*tanner stage) were also explored. Statistical significance was defined as p<0.05 though a p-value of 0.1 was considered significant for entry of a variable into a multivariate model.
Power Calculation
Power calculations were performed with the limited pQCT data available in patients with Thalassmemia at the time of this study initiation. With a sample size of 22 per group, we anticipated we would be able to observe a 5% difference in trabecular vBMD between the cases and controls given a mean vBMD of 249 ± 29 g/cm3 (mean±SD from healthy controls at our center) based on 80% power, alpha = 0.05, using a 2-sided t-test.
3.0 RESULTS
3.1 Subject characteristics
A total of 25 subjects with thalassemia (Thal, 14 female, 10 to 30.3 years) and 34 healthy control subjects (18 female, 7.7 to 30.1 years) were enrolled (Table 1). The majority of patients with Thal (76%) were currently transfused on a regular basis every 3-4 weeks with 2-4 units of blood to maintain a hemoglobin concentration above 9 g/dL. Five Thal subjects received sub-cutaneous desferrioxamine (DFO) an average of 40-60 mg/kg/d for 5-7 nights per week, an additional 13 subjects received desferisirox (Exjade) on average 26.6±8.7 mg/kg/day (range 10 – 40 mg/kg/d), and one subject received combination chelation therapy with DFO and Exjade. Though there were significantly more subjects of Asian ethnicity and fewer Caucasians in the Thal vs. control group, these statistical differences are likely to have minimal effect on outcomes as all subjects in the ‘other’ category for the controls were of mixed Asian/Caucasian ethnicity. There were no African Americans in either the Thal or Control groups.
Table 1.
Demographic, Disease and Dietary Characteristics of Subjects with Thalassemia compared to Healthy Controls
| Thalassemia (n=25) | Control (n=34) | p-value | |
|---|---|---|---|
| Age, years | 17.3 ± 5.3 | 17.5 ± 6.2 | NS |
|
| |||
| Gender | 44% Male | 44% Male | NS |
| 56% Female | 56% Female | ||
|
| |||
| Diagnosis, ß – Thalassemia | 18, 72% | ||
| E, ß Thalassemia | 2, 8% | N/A | |
| Hb H / Constant Spring | 3, 12% | ||
| Thalassemia Intermedia | 2, 8% | ||
|
| |||
| Number Transfused | 19 of 25 | 0 | |
|
| |||
| Ethnicity, Asian | 16, 64% | 9, 26% | 0.009 |
| Caucasian | 8, 32% | 17, 50% | |
| Other | 1, 4% | 8, 24% | |
|
| |||
| Subjects > 18 yrs | 44% | 44% | NS |
|
| |||
| Average Tanner, Category* |
Prepubertal, 24% | Prepubertal, 21% | NS |
| Peri-pubertal, 24% | Peri-pubertal, 9% | ||
| Post-pubertal, 52% | Post-pubertal, 70% | ||
|
| |||
| Age of Menarche, years | 14.6 ± 2.0 (n=6) |
12.7 ± 1.3 (n=14) |
0.01 |
|
| |||
| Primary amenorrhea | 1 of 6 | 0 of 12 | NS |
|
| |||
| Serum ferritin, ng/mL | 1931 ± 1785 | ND | |
|
| |||
| Calcium supplements | 60% | 21% | 0.003 |
|
| |||
| Vitamin D supplements | 60% | 24% | 0.006 |
|
| |||
| 25-OH vitamin D, ng/mL | 24.8± 11.1 | 32.0 ± 8.7 | 0.007 |
Data are presented as mean ± SD, % or (Number of subjects)
Average Tanner Category: Mean Tanner Stage of Gonad + Hair for each individual patient, categorized as prepubertal: Tanner 1; peri-pubertal: 2,3; or post-pubertal: 4,5 Primary Amenorrhea: females >16 years with no spontaneous menstruation Serum Ferritin normal range= 30-300 ng/mL
Calcium and vitamin D are the percentage of subjects that were taking supplements at the time of the study
In the group as a whole, there were no differences between the subjects with Thal and healthy controls with respect to age, gender or average pubertal category. However, subjects with Thal had more pubertal delay. For Thal females who achieved menarche (n=6), onset of menses occurred later than the control group by two years on average (Table 1). Height and weight Z-scores were lower in subjects with Thal compared to controls, though there were no significant differences in BMI Z-score. Lean mass was significantly lower in the young Thal subjects compared to controls (Table 2). Female Thal subjects had pronounced lean mass deficits compared to healthy controls (26.2±1.6 vs. 37.2±1.9 kg, p=0.002), whereas there were no statistically significant differences between male Thal and control subjects in lean mass or fat mass. Percent body fat by DXA was 3% higher in subjects with Thal compared to controls (Table 2). Bone age was significantly delayed in three of 14 patients with Thal assessed; on average 3.8±1.1 years behind chronological age.
Table 2.
Growth, DXA, pQCT and Bone Turnover Marker Results for Patients with Thalassemia and Healthy Controls
| Thal (n=25) | Control (n=34) | ||||
|---|---|---|---|---|---|
| ≤ 18 yrs | >18 yrs | ≤ 18 yrs | > 18yrs | p-value | |
| Weight-for-Age Z-score | −1.5 ± 1.2 (25) |
−0.1 ± 0.9 (34) |
<0.001 | ||
| −1.7 ± 1.4 (14) |
−1.2 ± 0.9 (11) |
−0.1 ± 0.9 (19) |
−0.2 ± 0.9 (15) |
<0.001, 0.01 | |
| Height-for-Age Z-score | −1.8 ± 1.3 (25) |
0.1 ± 1.1 (34) |
<0.001 | ||
| −2.3 ± 1.5 (14) |
−1.3 ± 0.9 (11) |
0.2 ± 1.2 (19) |
−0.1 ± 1.0 (15) |
<0.001, 0.008 | |
| Body Mass Index Z-score | −0.4 ± 0.7 (25) |
−0.2 ± 0.7 (34) |
NS | ||
| −0.3 ± 0.6 (14) |
−0.5 ± 0.8 (11) |
−0.2 ± 0.8 (19) |
−0.1 ± 0.6 (15) |
NS | |
| Lean Mass Index Z-score |
−1.2 ± 0.8 (25) |
−0.2 ± 0.7 (34) |
0.001 | ||
| −1.2 ± 0.8 (14) |
−1.3 ± 0.9 (11) |
−0.1 ± 0.8 (19) |
−0.4 ± 0.6 (15) |
<0.001, 0.009 | |
| Fat Mass, kg | 10.3 ± 4.6 (25) |
10.2 ± 5.1 (34) |
NS | ||
| 8.2 ± 4.3 (14) |
13.0 ± 3.7 (11) |
9.6 ± 4.7 (19) |
11.1 ± 5.6 (15) |
NS, NS | |
| % Body Fat | 24.9 ± 6.5 (25) |
21.1 ± 5.5 (34) |
0.02 | ||
| 25.5 ± 5.7 (14) |
24.2 ± 7.7 (11) |
20.3 ± 5.5 (19) |
22.2 ± 5.3 (15) |
0.01,NS | |
| Spine aBMD Z-score | −2.1 ± 1.1 (25) |
0.1 ± 0.9 (34) |
<0.001 | ||
| −2.1 ± 1.2 (14) |
−2.1 ± 1.0 (11) |
0.0 ± 1.0 (19) |
0.3 ± 0.6 (15) |
<0.001,<0.001 | |
| WB BMC Z-Score | −1.9 ± 1.4 (22) |
0.5 ± 0.9 (27) |
<0.001 | ||
| −1.8 ± 1.7 (14) |
−2.0 ± 0.9 (n=8) |
0.6 ± 0.9 (19) |
0.3 ± 0.7 (n=8) |
<0.001,<0.001 | |
| Trabecular vBMD Z-Score | −0.3 ± 2.0 (25) |
0.0 ± 1.1 (34) |
NS | ||
| 0.4 ± 2.0 (14) |
−1.2 ± 1.4 (11) |
0.1 ± 1.2 (19) |
−0.2 ± 0.9 (15) |
NS / 0.03 | |
| Cortical vBMD Z-Score | 0.2 ± 1.1 (25) |
0.0 ± 1.0 (34) |
NS | ||
| 0.5 ± 0.9 (14) |
−0.1 ± 1.3 (11) |
0.2 ± 1.1 (19) |
−0.3 ± 0.8 (15) |
NS / NS | |
| Cortical Area Z-Score | −2.1 ± 1.3 (25) |
−0.1 ± 1.1 (34) |
<0.001 | ||
| −2.3 ± 1.4 (14) |
−2.0 ± 1.0 (11) |
0.0 ± 1.1 (19) |
−0.3 ± 1.1 (15) |
<0.001,<0.001 | |
| Cortical BMC Z-Score | −2.1 ± 1.3 (24) |
−0.1 ± 1.1 (33) |
<0.001 | ||
| −2.1 ± 1.4 (14) |
−2.0 ± 1.2 (10) |
0.1 ± 1.1 (19) |
−0.4 ± 1.0 (14) |
<0.001, 0.001 | |
| Endosteal Circumference Z-Score |
0.2 ± 1.1 (25) |
−0.1 ± 1.0 (34) |
NS | ||
| 0.0 ± 1.2 (14) |
0.4 ± 0.9 (11) |
−0.3 ± 1.1 (19) |
0.2 ± 0.8 (15) |
NS, NS | |
| Periosteal Circumference Z-score |
−1.4 ± 1.0 (25) |
−0.1 ± 1.1 (34) |
<0.001 | ||
| −1.5 ± 1.2 (14) |
−1.2 ± 0.8 (11) |
−0.2 ± 1.2 (19) |
−0.1 ± 1.0 (15) |
0.003, 0.003 | |
| Cortical Thickness Z-score |
−2.1 ± 1.2 (24) |
0.0 ± 1.0 (33) |
<0.001 | ||
| −2.2 ± 1.4 (14) |
−2.0 ± 1.0 (10) |
0.3 ± 1.0 (19) |
−0.4 ± 0.9 (14) |
<0.001, 0.001 | |
| Section Modulus Z-Score | −1.6 ± 1.2 (24) |
−0.2 ± 1.1 (33) |
<0.001 | ||
| −1.8 ± 1.4 (14) |
−1.4 ± 0.9 (10) |
−0.2 ± 1.2 (19) |
−0.2 ± 0.9 (14) |
<0.001, 0.004 | |
Mean ±SD (number of subjects)
% body fat by Dual x-ray absorptiometry
pQCT: peripheral quantitative computed tomography, performed on the left tibia at 3%, 38% and 66% proximal to the reference line.
Eight subjects sustained non-digit fractures in each group; 10 males and 6 females had a history of fracture. Two Thal and 3 control subjects sustained multiple fractures in different bones. The majority of the fractures sustained by the Thal subjects occurred subsequent to a fall (n=4), during physical activity (n= 5) or a motor vehicle accident (n=1). Fractures in the control group were sustained from a fall (n=5) or during sports activities (n=6). As expected, subjects with Thal were more likely to have a history of diabetes (n=3), hypothyroidism (n=3), hypogonadism (n=3) and growth hormone deficiency (n=4). More subjects with Thal had deficient levels of vitamin D, defined as 25-OH vitamin D less than 20 ng/mL (40% compared to 5.8%, p=0.001).
3.2 Bone Density
As has been observed in previous studies, and expected in this study given the inclusion criteria, aBMD Z-scores at all sites were lower in the Thal compared to control group (Table 2). There were no differences in aBMD Z-scores by age group (≤18 or >18 years) within either the Thal or control groups.
Z-scores for pQCT parameters mirrored the results from DXA with consistently lower values in adult subjects with Thal compared to healthy adult controls for many of the variables with the exception of cortical density and endosteal circumference (Table 2). Periosteal circumference deficits in Thal resulted in a thinner cortical shell Z-score (p<0.001). As a result of these geometrical differences, section modulus was also lower in subjects with Thal compared to controls.
As bone geometry can differ between males and females, we also explored gender differences between the Thal and control subjects. We observed females with thalassemia to have smaller periosteal circumference Z-score (−1.8±1.0 vs. −0.2±1.0, p<0.001) and cortical thickness Z-score (−2.4±1.3 vs. −0.2±0.8, p<0.001) compared to healthy controls. This likely contributed to the lower section modulus Z-score (−0.6 vs. 0.6, p=0.005) observed in female Thal vs. controls. Male subjects with Thal also had significantly lower cortical thickness Z-score compared to healthy controls (−1.8 vs. 0.3, p<0.001). Despite these differences, when gender was included in the multivariate analyses described below, it proved only to be a significant predictor in the model for spine aBMD Z-score.
Given the known effects of growth, puberty and muscle mass on bone mass, linear regression models were developed to control for many of these variables (Table 3). Deficits in spine aBMD and whole body BMC Z-score persisted in patients with Thal after adjustment for height Z-score and either gender or lean mass index Z-score. Age and Tanner stage were not significant predictors in these models which explained between 62% (spine aBMD) and 75% (whole body BMC) of the variability. Similar models were developed for pQCT bone outcomes (Table 3). Following adjustment for tibial length and muscle mass, cortical content, cortical area and cortical thickness Z-scores remained significantly lower in the subjects with Thal compared to controls. These findings are summarized in Figure 1.
Table 3.
Factors Related to Bone Z-Score Outcome Variables. Multivariate linear regression models in Subjects with Thalassemia and Healthy Controls (n=59)
| Outcome | Predictor Variable | Coefficient | Standard Error | p-value | Adjusted r2 |
|---|---|---|---|---|---|
| Spine aBMD Z-score | Thalassemia diagnosis | −1.79 | 0.31 | <0.001 | 0.62 |
| Male gender | 0.47 | 0.24 | 0.057 | ||
| Height Z-score | 0.23 | 0.10 | 0.028 | ||
|
| |||||
| Whole Body BMC Z-score | Thalassemia diagnosis | −0.76 | 0.35 | 0.034 | 0.75 |
| Height Z-score | 0.59 | 0.1 | <0.001 | ||
| Lean Mass Index Z-score | 0.39 | 0.16 | 0.019 | ||
|
| |||||
| Cortical BMC Z-score | Thalassemia diagnosis | −0.85 | 0.26 | 0.002 | 0.74 |
| Tibial Length Z-score | 0.47 | 0.07 | <0.001 | ||
| Lean mass index Z-score | 0.72 | 0.14 | <0.001 | ||
|
| |||||
| Cortical Area Z-score | Thalassemia diagnosis | −1.10 | 0.25 | <0.001 | 0.74 |
| Tibial Length Z-score | 0.47 | 0.08 | <0.001 | ||
| Lean mass index Z-score | 0.64 | 0.14 | <0.001 | ||
|
| |||||
| Cortical Thickness Z-score | Thalassemia diagnosis | −1.3 | 0.28 | <0.001 | 0.69 |
| Tibial Length Z-score | 0.66 | 0.10 | <0.001 | ||
| Tibial Muscle Z-score | 0.40 | 0.12 | 0.003 | ||
|
| |||||
| Periosteal Circumference Z-score | Tibial Length Z-score | 0.72 | 0.08 | <0.001 | 0.59 |
| Tibial Muscle Z-score | 0.59 | 0.10 | <0.001 | ||
|
| |||||
| Section Modulus Z-score | Tibial Length Z-score | 0.82 | 0.08 | <0.001 | 0.66 |
| Tibial Muscle Z-score | 0.66 | 0.09 | <0.001 | ||
Summary of multivariate linear regression models: endosteal circumference, cortical vBMD and trabecular vBMD Z-scores were not included as outcomes in this table given they were not significantly different between Thal and control in the univariate analyses (Table 2). Predictor variables above were included if univariate relationship existed to any outcome variable of p<0.1, these variables included thalassemia diagnosis, gender (male=reference), age, tanner stage (pre-pubertal=reference), height for age Z-score, lean mass index Z-score, tibial muscle mass Z-score determined at the 66% site, kilocalories as %dietary reference index, calcium intake as a %DRI and serum vitamin D (ng/mL). Only those predictor variables that were significant in each individual multivariate model are included above with the individual coefficients and p-values for the predictor variables, and r2 for the models adjusted for sample size. For example, as predicted in the model above, Whole body BMC Z-score is expected to increase by 0.59 units for every 1 SD increase in height Z-score in the subjects studied.
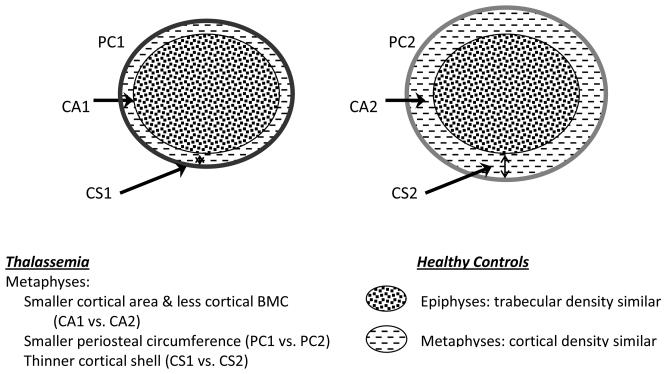
Figure 1. Characterization of Differences between Peripheral Weight-Bearing Bone of Subjects with Thalassemia Compared to Healthy Controls.
Footnote: This figure summarizes the findings from the pQCT variables in the tibia of patients with thalassemia compared to healthy controls. The findings are significant after adjustment for age, gender, Tanner stage, growth (tibial length Z-score) and/or tibial muscle mass. Of note, decreased periosteal circumference and reduced cortical thickness may lead to a mechanically weaker bone. However, in this study after controlling for tibial length and muscle mass, there was no significant differences in the strength parameter observed (Section Modulus Z-score).
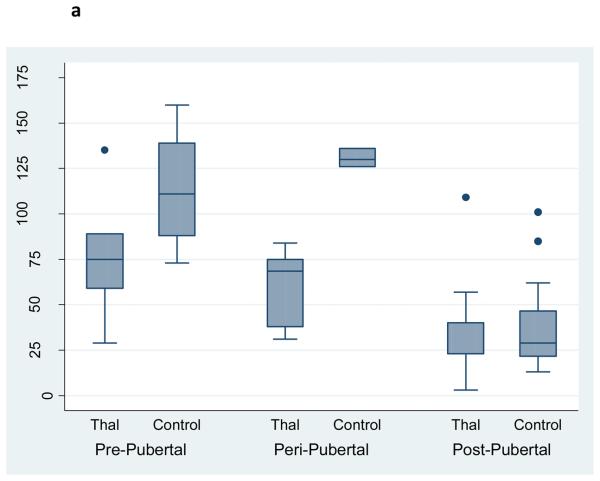
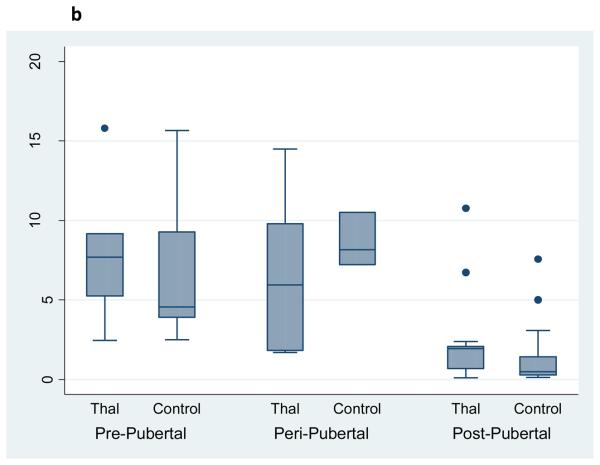
Osteocalcin, a biochemical marker of bone formation, was significantly reduced in young patients with Thal, whereas a bone resorption marker, urinary N-telopeptide, was significantly increased in the older Thal group compared to controls (Figure 2a,b). After controlling for age, Tanner stage and skeletal size (e.g. whole body bone mineral content), only osteocalcin remained significantly lower in Thal compared to controls (Coefficient: 1.4, p=0.029, 95%CI: 1.8, 33.2).
Figure 2a. Serum Osteocalcin by Pubertal Stage and Diagnosis in Subjects with Thalassemia (n=25) and Healthy Controls (n=34).
Footnote: After controlling for age, Tanner stage and whole body BMC, osteocalcin was significantly lower in Thal compared to controls (p=0.029). Subjects were categorized as prepubertal: Tanner stage 1; peri-pubertal: Tanner 2 & 3; post pubertal: tanner 4 & 5. Note the figure presents the raw, unadjusted data.
Figure 2b. Urinary N-telopeptide by Pubertal Stage and Diagnosis in Subjects with Thalassemia (n=25) and Healthy Controls (n=34).
Footnote: Overall higher levels of urinary N-telopeptide by Tanner stage in Thal compared to controls. After controlling for age and whole body BMC, urinary N-telopeptide was no longer significantly different in Thal compared to controls (p=NS). Subjects were categorized as prepubertal: Tanner stage 1; peri-pubertal: Tanner 2 & 3; post pubertal: tanner 4 & 5. Note the figure presents the raw, unadjusted data.
When the group of subjects with Thal were analyzed separately, non-transfused subjects (n=6) had significantly greater cortical area, periosteal circumference and section modulus Z-scores (all p<0.01) compared to transfused subjects (n=19). There were, however, no significant differences with regard to age, fracture history, gonadal history, spine BMD, or whole body BMC Z-scores by DXA or bone turnover markers in non-transfused compared to transfused subjects.
4.0 DISCUSSION
This study is unique because it is the first to examine factors associated with bone deficits using a comprehensive characterization of bone in a group of young subjects with thal, previously identified with low bone mass, compared to a healthy control cohort from the same region. DXA was used to assess spine and whole body bone mineral density, and pQCT evaluated peripheral volumetric density and strength parameters. What was particularly novel was the evaluation of deficits after adjustment for growth, pubertal delay and lean mass deficits.
We have shown that whole body bone mineral deficits assessed by DXA are only partially attributable to reduced skeletal size and lean mass deficits in patients with Thal. Specifically, deficits in spine aBMD and whole body BMC Z-score persisted in patients with Thal after adjustments were made for gender, height Z-score, and lean mass index Z-score. In this group of subjects, despite the presence of pubertal delay, Tanner stage alone was not a strong predictive factor in the bone deficits observed. Similarly, the compartmental deficits in cortical BMC, area, and thickness assessed by pQCT in a weight bearing bone (tibia) were only partially influenced by muscle mass deficits. In contrast, the differences observed between the Thal and controls in periosteal circumference and tibial strength (section modulus) were mediated by muscle mass differences. Interestingly, though differences in bone density and other geometric parameters were observed, fracture incidence was similar between the two groups. Differences in exposure to fracture risk, such as physical activity patterns / sports participation, and age-specific fracture incidence were not accounted for in this comparison.
These findings are not consistent with observations made in several other chronic conditions in which low bone mass occurs in the context of significant growth deficits. In pediatric patients with cystic fibrosis, after correction for significant linear growth deficits, the whole body bone mineral deficits disappeared [21]. In patients with nephrotic syndrome on high dose prednisone therapy and short stature, when height deficits were considered, bone mass assessed by DXA was no longer significantly different between patients and healthy controls [22]. Additionally, in patients with Crohn's disease, adjustment for lean mass deficits eliminated the bone mass deficits observed [23]. In other chronic conditions with reduced growth velocity and decreased bone turnover, significant increases in cortical vBMD have been observed [24]. However, in the present study though reduced bone formation was observed in the context of growth failure, we did not observe an effect on cortical vBMD.
Other factors that may have contributed to decreased BMC in patients with Thal include endocrinopathies, increased hematopoietic activity and nutritional deficits. It must be noted that though many of these subjects with Thal were transfused, they were maintained on chronic chelation therapy and few had extremely high current levels of hepatic iron. Despite this treatment, roughly 15% of these young subjects had iron-related hypogonadism, hypothyroidism and/or diabetes, perhaps a reflection of historically elevated body iron. We attempted, through statistical modeling, to control for the effects of pubertal delay on bone, however due to the small sample size we could not include other endocrinopathies into our models. The presence of these endocrinopathies could explain some of the remaining skeletal deficits observed in this group of subjects. Young Thal patients without endocrinopathy have aBMD by DXA within the normal range (25) further supporting the hypothesis that endocrinopathies may contribute to the observed bone deficits.
Recently, Gurevitch et al. used a mouse model to show that chronic blood loss leads to augmentation of the hematopoietic microenvironment and subsequent osteoporosis [26]. Therefore, increased red cell turnover, either in the non-transfused patient, or the transfused patient at the end of the transfusion cycle, may create pressure on the closely linked osteohematopoietic system by increasing the number of osteoclasts, and thereby intensifying resorption of bone tissue [25]. The effects of hyperactive hematopoiesis and oxidative stress were not directly assessed in the present study and could play a role in the etiology of low bone mass in thalassemia.
Deficiency of vitamin D and other bone forming nutrient deficiencies may also play a role in the development of reduced bone mass in thalassemia [8, 27]. More subjects with Thal than controls were consistently taking calcium and vitamin D supplements for their low bone mass. Despite these supplements, more Thal had deficient levels of vitamin D, a finding which was predictive only in the model for tibial trabecular density (data not shown). Simplified assessments of physical activity and inactivity and dietary intake used in this study did not prove to be strong predictors of bone mass in this sample.
Because DXA is a 2-dimensional image and may underestimate bone density in patients with severe growth and skeletal deficits, three-dimensional volumetric measures using pQCT are particularly useful both to distinguish trabecular from cortical bone, and to reduce the influence of patient size on measurements. Others have used pQCT to assess bone in adult patients with thalassemia with somewhat similar results to ours. In 2004 and again in 2008, Ladis et al compared findings at the 4% site of the distal radius in adult patients with thalassemia major and intermedia (21 to 44 years) with scans from healthy adult controls [28,29]. They observed significant reductions in the trabecular bone compartments of adult patients compared to the controls, with the Thal intermedia group being the most severely affected. The trabecular deficits observed at this non-weight-bearing site (radius) in the study by Ladis were similar to those seen in the weight bearing tibia in our study. The lower trabecular vBMD we found in adult subjects with Thal, however, was no longer significant after controlling for tibial length and vitamin D level.
Ladis et al. also reported cortical compartment deficits at the 4% site of the radius. However, at this site the cortical shell is extremely thin, potentially reducing the accuracy of cortical geometrical measurements and strength parameters at this site. We chose to study the tibia in the present study because the risk of partial volume effects is reduced in this peripheral bone (30). Cortical parameters were assessed at the 38% site only. Additionally, the studies by Ladis et al were limited to older patients, and did not compare the deficits observed by pQCT to traditional measures by DXA or to biochemical markers of bone turnover.
pQCT and other volumetric methods provide separate measurements of trabecular and cortical bone compartments. This may allow earlier detection of changes in bone mass within differing compartments in response to disease or therapy. However, both pQCT and high resolution-pQCT (HRpQCT) are more sensitive to movement and require the subject to be still for nearly five minutes for optimal scans; therefore it is difficult to obtain optimal scans in very young children. pQCT is limited to assessment of peripheral sites only, and QCT, HR-QCT and pQCT have limited published pediatric specific reference data. Volumetric assessments using pQCT or QCT may be used more readily in future studies due to recent publications touting the predictive value of vBMD for fracture risk (31,32). However, DXA remains the most widely used and clinically available tool to assess low bone mass, particularly in pediatric populations. Additionally, given we observed similar deficits in both DXA and pQCT in our sample, it seems reasonable to conclude that DXA continue to be used to assess low bone mass in this population of patients, particularly if the interpretation of DXA scans considers adjustment for body size deficits.
Alterations in bone turnover in adult patients with thalassemia have been explored by other investigators, but few have studied bone markers in pediatric populations with adequate controls [33] and all failed to control for pubertal changes or skeletal mass deficits. Most have reported increased bone resorption (serum CTx, urinary NTx, deoxypyridinoline) [1,10,34,35,36], with and without decreases in bone formation markers (bone specific alkaline phosphatase, osteocalcin) [1,32-35]. In 2003, Domrongkitchaiporn et al. analyzed iliac crest bone biopsies and markers of bone turnover in 18 adult patients with thalassemia. Interesting, they observed reductions in trabecular bone volume but no evidence of reduced bone formation or increased bone resorption [37]. We have shown that particularly in young patients with thalassemia, bone formation is reduced, even after correcting for skeletal size and pubertal delay. By contrast, though others have reported elevated bone resorption in older patients with Thal, after controlling for age, puberty and whole body bone content, we no longer observed differences. This suggests that at least for our marker of resorption, urinary NTx, group differences were mediated by skeletal size. The combination of reduced bone formation, particularly in younger patients during periods of rapid bone mineralization leads to reduced whole body bone mass as well as reduced cortical mass in the peripheral bones.
In order to tailor clinical therapies for patients with Thal, there needs to be a comprehensive understanding of the etiology of bone disease. Our results indicate that deficits in both bone mass and geometry are significant even in relatively young patients. Therapies to stimulate bone anabolism such as physical activity, growth hormone and/or nutritional interventions may be warranted in the younger thalassemia patient. For older patients with significant endocrinopathies, the primary defect may be an increase in bone resorption; for these individuals treatment with anti-catabolic agents such as bisphosphonates may be appropriate.
We recognize some limitations of this study. The cohort was relatively small which precluded analysis of multiple clinical variables which could have contributed to skeletal status. The subjects were limited to patients previously identified to have low bone mass by DXA (BMD Z-score <−1.0). For this reason, we could not examine pQCT parameters of less severely affected subjects with this disorder. The decision to include only Thal subjects with low BMD Z-scores excluded only 5 of 62 (8%) screened. Additionally, the Thal group assessed at Oakland represented approximately half of the transfused patients who regularly attend the thalassemia clinic at the Children's Hospital & Research Center, Oakland. The total number of patients with all types of thalassemia syndromes in North America is estimated to be less than 2,000 and roughly half are transfusion dependent [38, 39]. Therefore, we believe the observations from these 25 subjects may be generalizable to other transfusion dependent patients in the U.S. The strengths of this study are a complete characterization of the skeletal manifestations of Thal using DXA, pQCT and markers of bone turnover in both children and young adults. Bone parameters were compared to large healthy contemporary U.S. cohort to derive both DXA and pQCT Z-scores. Ultimately, we have found evidence of skeletal deficits that cannot be dismissed as an artifact of small bone size or delayed maturity alone.
4.1 CONCLUSIONS
Findings from this study suggest that bone mineral and bone size deficits in patients with thalassemia are not completely explained by growth and lean mass deficits. Pubertal delay did not factor significantly into the explanation of bone insufficiency in this population of patients with Thal. However, a reduction in bone formation is a key component to bone mineral deficits, particularly in young patients leading to significant bone mass and strength deficits in older patients. Given that reduced bone density and strength are associated with increased risk of fracture, therapies focused on increasing bone formation and bone size in younger patients and reducing bone loss in adult patients are worthy of further evaluation.
ACKNOWLEDGEMENTS
The authors express their gratitude and appreciation to the subjects and their families who participated in this study, and to the physicians and staff of the Thalassemia Clinical Research Networks at CHRCO and CHOP and the General Clinical Research Center at each center. In addition, we would like to thank Annie Lui for her tireless assistance with the blood collection, storage and sample shipping and processing, to Ginny Gildengorin, PhD for her consultation with the statistical analysis, to Mary Leonard, MD DrPH for graciously allowing us to utilize some of their expansive healthy adult pQCT reference data and to Bo Fan, PhD and John Shepherd, PhD for their assistance with the development of the calibration equations for the DXA systems at CHOP and CHRCO. We also owe our gratitude to the study coordinators at each site who assisted with the data collection, Sage Green, Owen Beams and Jonah Todd-Geddes; as well as to Donna Paulhamus and Gail Jackson for their assistance with subject measurements at CHOP.
This publication was supported in part by the following grants: NIH K23-HL076468, UL1-RR024131 and UL1 RR024134 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vogiatzi M, Mackin E, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Min Res. 2009;24:543–57. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumain A, Prescott E, Hoffbrand AV, Wonke B. High prevalence of low bone mass in thalassemia major. Br J Haematol. 1998;103:911–5. doi: 10.1046/j.1365-2141.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 3.Vichinsky EP. The morbidity of bone disease in thalassemia. Ann NY Acad Sci. 1998;850:344–8. doi: 10.1111/j.1749-6632.1998.tb10491.x. [DOI] [PubMed] [Google Scholar]
- 4.Wonke B, Jensen C, Hanslip JJ, Prescott E, Lalloz M, Layton M, Erten S, Tuck S, Agnew JE, Raja K, Davies K, Hoffbrand AV. Genetic and acquired predisposing factors and treatment of osteoporosis in thalassemia major. J Ped Endocrin Metab. 1998;11:795–801. [PubMed] [Google Scholar]
- 5.Dundar U, Kupesiz A, Ozdem S, et al. Bone metabolism and mineral density in patients with beta-thalassemia major. Saudi Med Journal. 2007;28:1425–9. A. [PubMed] [Google Scholar]
- 6.Mahachoklertwattana P, Pootrakul P, Chuansumrit A, Choubtum L, Sriphrapradang A, Sirisriro R, Rajatanavin R. Association between bone mineral density and erythropoiesis in Thai children and adolescents with thalassemia syndromes. J Bone Mineral Metab. 2006;24:146–52. doi: 10.1007/s00774-005-0661-0. [DOI] [PubMed] [Google Scholar]
- 7.Bielinski BK, Darbyshire PJ, Mathers L, Crabtree NJ, Kirk JMW, Stirling HF, Shaw NJ. Impact of disordered puberty on bone density in B-thalassemia major. British J Haematology. 2003;120:353–358. doi: 10.1046/j.1365-2141.2003.04066.x. [DOI] [PubMed] [Google Scholar]
- 8.Vogiatzi MG, Macklin EA, Trachtenberg FL, Fung EB, Cheung AM, Vichinsky E, Olivieri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm I, Fleisher M, Grady RW, Peterson C, Giardina PJ. Differences in the Prevalence of Growth, Endocrine and Vitamin D abnormalities among the various Thalassemia Syndromes in North America. British Journal of Hematology. 2009;146:546–556. doi: 10.1111/j.1365-2141.2009.07793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karimi M, Ghiam AF, Hashemi A, et al. Bone mineral density in beta-thalassemia major and intermedia. Indian Pediatr. 2007;44(1):29–32. [PubMed] [Google Scholar]
- 10.Angelopoulos NG, Goula A, Katounda E, Rombopoulos G, Kaltzidou V, Kaltsas D, Konstandelou E, Tolis G. Markers of bone metabolism in eugonadal female patients with beta-thalassemia major. Pediatr Hematol Oncol. 2007;24:481–91. doi: 10.1080/08880010701533611. [DOI] [PubMed] [Google Scholar]
- 11.Petit M, Kent K, Leonard MB, McKay H, Zemel BS. In: Analysis. Bone Densitometry in Growing Patients: Guidelines for Clinical Practice. Sawyer AJ, Bachrach LK, Fung EB, editors. Humana Press; Totowa NJ: 2007. pp. 93–114. [Google Scholar]
- 12.Fewtrell MS, Gordon I, Biassoni L, Cole TJ. Dual X-ray abosrptiometry (DXA) of the lumbar spine in a clinical paediatric setting: does the method of size-adjustment matter? Bone. 2005;37:413–419. doi: 10.1016/j.bone.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Yildiz M, Canatan D. Soft tissue density variations in thalassemia major: a possible pitfall in lumbar bone mineral density measurements by dual-energy x-ray absorptiometry. Pediatric Hematology and Oncology. 2005;22:723–726. doi: 10.1080/08880010500278707. [DOI] [PubMed] [Google Scholar]
- 14.Drakonaki EE, Maris TG, Papadakis A, Karantanas AH. Bone marrow changes in beta-thalassemia major: quantitative MR imaging findings and correlation with iron stores. Eur Radiol. 2007;17:2079–87. doi: 10.1007/s00330-006-0504-y. [DOI] [PubMed] [Google Scholar]
- 15.Tanner JM. Growth at Adolescence. Blackwell Press; Oxford, UK: 1962. [Google Scholar]
- 16.Morris N, Udry J. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adol. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 17.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34(6):1044–52. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TL, Wilson KE, Heymsfield SB. Dual Energy x-ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetsteon RJ, Zemel BS. Effects of sex, race and puberty on cortical bone and the functional muscle bone unit in children, adolescents and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouyoucef SE, et al. Cross calibration of a fan-bean X-ray densitometer with pencil beam system. Br J Radiol. 1996;69:522–31. doi: 10.1259/0007-1285-69-822-522. [DOI] [PubMed] [Google Scholar]
- 21.Kelly A, Schall JI, Stallings VA, Zemel BS. Deficits in bone mineral content in children and adolescents with cystic fibrosis are related to height deficits. J Clinical Densitometry. 2008;11:581–9. doi: 10.1016/j.jocd.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephritic syndrome. J Bone Miner Res. 2009;24:503–13. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnham JM, Shults J, Semeao E, Foster B, Zemel BS, Stallings VA, Leonard MB. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation and body composition. J Bone Mineral Res. 2004;19:1961–8. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 24.Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153:484–90. doi: 10.1016/j.jpeds.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christoforidis A, Kazantzidou E, Tsatra I, Tsantali H, Koliakos G, Hatzipantelis E, Katzos G, Anthanassoiu-Metexa M. Normal lumbar bone mineral density in optimally treated children and young adolescents with beta-thalassemia major. Hormones. 2007;6(4):334–40. doi: 10.14310/horm.2002.1111030. [DOI] [PubMed] [Google Scholar]
- 26.Gurevitch O, Khitrin S, Valitov A, Slavin S. Osteoporosis of Hematologic Etiology. Exp Hematol. 2007;35:128–36. doi: 10.1016/j.exphem.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Fung EB. Nutritional Deficiencies in Patients with Thalassemia. Ann New York Academy Sciences. 2010;1202:188–196. doi: 10.1111/j.1749-6632.2010.05578.x. Annals NY Academy Science. [DOI] [PubMed] [Google Scholar]
- 28.Ladis VA, Gandaifis N, Papdopoulos EC, Cavras GM, Papssoririou I, Korres DS, Kattamis CA. Bone density study at the distal radius, using pQCT analysis in Greek thalassemic patients. Pediatr Endocrinol Rev. 2004;2(Suppl2):307–9. [PubMed] [Google Scholar]
- 29.Ladis V, Raptou P, Rigatou E, Chouliaras G, Galanos A, Korres D, Kattamis C. Study of bone density by pQCT analysis in healthy adults and patients with B-thalassemia major and intermedia. Pediatr Endocrinol Rev. 2008;6(Suppl 1):127–31. [PubMed] [Google Scholar]
- 30.Augat P, Gordon CL, Lang TF, Iida H, Genant HK. Accuracy of cortical and trabecular bone measurements with peripheral quantitative computed tomography (pQCT) Phys Med Biol. 1998;43:2873–2883. doi: 10.1088/0031-9155/43/10/015. [DOI] [PubMed] [Google Scholar]
- 31.Cheng S, Xu L, Nicholson PHF, et al. Low volumetric BMD is linked to upper limb fracture in pubertal girls and persist into adulthood: a seven year cohort study. Bone. 2009;45:480–486. doi: 10.1016/j.bone.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Darelid A, Ohlsson C, Rudang R, et al. Trabecular volumetric bone mineral density is associated with previous fracture during childhood and adolescence in males: The Good Study. J Bone Miner Res. 2010;25:537–544. doi: 10.1359/jbmr.090824. [DOI] [PubMed] [Google Scholar]
- 33.Salama OS, al-Tonbary YA, shahin RA, Eldeen OA. Unbalanced bone turnover in children with beta-thalassemia. Hematology. 2006;11(3):197–202. doi: 10.1080/10245330600702851. [DOI] [PubMed] [Google Scholar]
- 34.Morabito N, Gaudio A, Lasco A, Atteritano M, Pizzoleo M.a., Cincotta M, La Rosa M, Guarino R, Meo A, Frisina N. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: new pieces of the puzzle. J Bone Miner Res. 2004;19:722–7. doi: 10.1359/JBMR.040113. [DOI] [PubMed] [Google Scholar]
- 35.Voskaridou E, Maria-Christina K, Evangelos T, et al. Bone resorption is increased in young adults with thalassemia major. British J Haematol. 2001;112:35–41. doi: 10.1046/j.1365-2141.2001.02549.x. [DOI] [PubMed] [Google Scholar]
- 36.Pratelli L, Verri E, Fortini M, Marconi S, Zolezzi C, Fornasari PM, Gamberini MR, De Sanctis V. Chelation therapy and bone metabolism markers in thalassemia major. J Pediatr Endocrinol Metab. 2006;19:1335–42. doi: 10.1515/jpem.2006.19.11.1335. [DOI] [PubMed] [Google Scholar]
- 37.Domrongkitchaiporn S, Sirikulchayanonta V, Angchaisuksiri P, Stitchantrakul W, Kanokkantapong C, Rajatanavin R. Abnormalitites in bone mineral density and bone histology in Thalassemia. J Bone Min Res. 2003;18:1682–88. doi: 10.1359/jbmr.2003.18.9.1682. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Complications of B-thalassemia major in North America. Blood. 2004;104:34–39. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- 39.Vichinsky EP, Macklin EA, Waye JS, Lorey F, Olivieri NF. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116(6):e818–825. doi: 10.1542/peds.2005-0843. [DOI] [PubMed] [Google Scholar]