Abstract
Background
There are limited studies of generalized anxiety disorder (GAD) across pregnancy.
Methods
Women (n=2793) were enrolled in the Yale Pink and Blue study, a cohort enriched with subjects who suffered from major depressive disorder (MDD) within the past five years or used antidepressants in the past year. Subjects were evaluated with the Composite International Diagnostic Interview at three time points: twice in pregnancy and once after delivery. We defined a generalized anxiety disorder (GAD) episode as per DSM IV but with required duration reduced to one month or longer. Course and correlates of GAD were examined in women who had: 1) no GAD during the 6 months prior or in pregnancy (Group A), 2) GAD in the 6 months prior to but not in pregnancy (Group B), 3) GAD in pregnancy only (Group C) and 4) GAD both in the 6 months prior to and during pregnancy (Group D).
Results
9.5% of the cohort suffered from GAD at some point in pregnancy. Anxiety symptoms were highest in the first trimester and decreased across pregnancy. Regression analysis revealed that previous GAD episodes, education, social support and a history of child abuse distinguished between membership in the four groups.
Limitations
The sample may not be representational, as it was enhanced with those at risk, and had relatively low representation of socio-economically disadvantaged women.
Conclusions
Identification of anxious patients during pregnancy may provide an opportunity to engage those in need of psychiatric treatment.
Keywords: anxiety, pregnancy, risk, antenatal, postnatal, mothers
Introduction
Few studies have focused on anxiety disorders in the perinatal period. Most work concludes that there is no difference in rates of anxiety disorders in pregnancy as compared to other times of a reproductive-aged woman’s life. However, rates may vary across pregnancy and the postpartum period. One investigation notes that rates of an anxiety or mood disorder are higher in the third trimester (29.2%) as compared to postpartum period (16.5%). Studies using self report questionnaires find that anxiety peaks in the first and third trimesters.
Risk factors for generalized anxiety disorder (GAD) in pregnancy have also received limited attention. Existing information suggests that the likelihood of suffering from GAD in pregnancy is increased by co-morbid psychiatric disorders, stressful life events and social disadvantage. As GAD is thought to be closely linked to major depressive disorder (MDD) risk factors for GAD may be similar to those of MDD in pregnant and postpartum women but this requires further research.
Importantly, anxiety disorders in pregnancy are associated with postnatal depressive symptoms and adverse child outcomes. Unfortunately anxiety disorders are often missed and untreated. Given the lack of information in the literature and the need to understand the risk for anxiety disorders in pregnancy we elected to examine the rates and correlates of GAD longitudinally across pregnancy. We had two primary aims: 1) to examine the changing nature, if any, of GAD and GAD symptoms in relation to the course of pregnancy; 2) to characterize differences in GAD susceptibility or risk with respect to demographic and potential clinical risk factors.
Methods
Women were recruited from obstetric services throughout Connecticut and Western Massachusetts to participate in the Yale Pink and Blue study. The study received ethics approval from the Yale University and participating hospitals ethics committees. Women were eligible if they had not yet completed 17 weeks of a singleton pregnancy, did not have insulin dependent diabetes, were not planning on moving or terminating the pregnancy, could speak English or Spanish and were able to provide informed consent. Recruitment occurred between 2004 and 2008. All women with a history in the last five years of major depressive disorder (MDD) or post traumatic stress disorder (PTSD), or antidepressant use in the last year were invited to participate. We also randomly selected one out of every three women who did not have these characteristics to participate as controls.
Enrollment
A total of 9525 women were screened and 2793 women were enrolled. The second interview was completed by 2367 (85%) women and 2349 (84%) completed the final interview; 89 (3%) were excluded subsequent to their pregnancy loss.
Procedure
Women were interviewed face to face at enrollment and contacted again by telephone at 30 weeks (+/−2) pregnant and 8 (+/−4) weeks postpartum. Participants received $20 per interview plus a $20 bonus if they completed all interviews. Interviewers underwent a minimum of four days of training, six practice interviews and at least two fully supervised interviews until competency to perform the fully structured interview was obtained. Interviews were audio-taped with permission of the subject and checked and coded with reference as needed. Retraining was performed as needed for interviewers who did not adequately perform the interviews. Additionally, a random sample of 10% was assessed for reliability.
Interviews included the depressive disorders, panic disorders and GAD modules of the World Mental Health Composite International Diagnostic Interview with adjusted time frames for one month periods (WMH-CIDI). The modified PTSD symptom scale (MPSS) was used to diagnose PTSD. At enrollment we asked subjects about GAD symptoms during the six months prior to pregnancy and since they became pregnant. In the subsequent two interviews we elicited information about symptoms during the second and third trimesters. We initially examined those women with a clear diagnosis of DSM IV defined GAD that required symptoms lasting at least six months; only 26 women (1%) met this criteria in the first trimester. Given that we were interested in clinically significant symptoms prior to and during pregnancy, we elected to define GAD as an episode of one month or longer.
The EPDS, a commonly used screening tool in perinatal women measuring past week symptoms, was completed at each time point. Three items form an anxiety subscale which has been found to account for 47% of the variance in EPDS score antenatally, compared to only 38% postnatally.
As the final visit, which collected information on the third trimester and the immediate postnatal period, occurred 4–6 weeks after delivery, there is no EPDS score for third trimester. Additional data included demographic, educational, obstetrical and psychiatric treatment information. Childhood abuse was determined by one or more affirmative responses to the following occurring before the age of 18; “were you purposefully hurt or put down or ridiculed by an adult, or treated in a cold, uncaring way most of the time”. Social support was measured using the modified Kendler Social Support Inventory (MKSSI).
Analysis
GAD was operationalized as per DSM-IV with the exception that symptoms required a minimum of one month rather than six months. A widely held belief is that psychiatric syndromes improve in pregnancy and given that the hormonal milieu of pregnancy may have an impact on the expression of GAD, we devised four, mutually exclusive subgroups of individuals: 1) no GAD diagnosis (n=2420), 2) GAD only pre-pregnancy (n= 105); 3) GAD in pregnancy only (n=152); and 4) GAD in pre-pregnancy and pregnancy (n=116). Differences in categorical demographics between GAD groups were assessed using a chi-square test, while we used the non-parametric Kruskal-Wallis test for the continuous social support scale. Confidence limits for GAD period prevalence were estimated using exact binomial confidence limits. To analyze the risk factors for GAD group we used a generalized logits model. This analysis involves comparison of potential risk factors that included age, race/ethnicity, marital status, education, social support, child abuse, number of prior episodes of GAD, and number of relatives with GAD, to the four-level response variable GAD group. All analyses were performed using SAS 9.1.3.
Results
Characteristics
Characteristics of the study population and study groups are presented in Table One. Groups differed significantly with respect to age, marital status, race, education, drug, smoking, and abuse histories, as well as past and family histories, number of psychiatric co-morbidities, support level, co-morbidities and worries regarding their baby.
Table 1.
Characteristics of the Study Population
| Characteristic | Total N (%) | No GAD N=2420 (Group A) | GAD pre- pregnancy only Group B N=105 | GAD in pregnancy Group C N=152 | Both GAD pre- pregnancy and in pregnancy Group D N=116 | P- value |
|---|---|---|---|---|---|---|
| Age | 0.05 | |||||
| <25 | 472 (17) | 410 (17) | 12 (11) | 20 (13) | 30 (26) | |
| 25–34 | 1570 (56) | 1353 (56) | 66 (63) | 88 (58) | 63 (55) | |
| 35+ | 748 (27) | 656 (27) | 27 (26) | 43 (28) | 22 (19) | |
| Race/ethnicity | 0.001 | |||||
| White | 2053 (74) | 1783 (74) | 87 (83) | 104 (68) | 79 (68) | |
| Black | 216 (8) | 175 (7) | 3 (3) | 21 (14) | 17 (15) | |
| Hispanic | 401 (14) | 350 (14) | 12 (11) | 19 (13) | 20 (17) | |
| Other | 123 (4) | 112 (5) | 3 (3) | 8 (5) | 0 (0) | |
| Married/cohabiting | 2424 (87) | 2118 (88) | 89 (85) | 131 (86) | 86 (74) | 0.001 |
| Education (years) | <.001 | |||||
| <12 | 187 (7) | 156 (6) | 3 (3) | 8 (5) | 20 (17) | |
| 12–15 | 411 (15) | 352 (15) | 17 (16) | 20 (13) | 22 (19) | |
| 13–15 | 638 (23) | 527 (22) | 28 (27) | 48 (32) | 35 (30) | |
| 16+ | 1557 (56) | 1385 (57) | 57 (54) | 76 (50) | 39 (34) | |
| Parity | 0.86 | |||||
| 0 | 1195 (43) | 1036 (43) | 50 (48) | 61 (40) | 48 (41) | |
| 1 | 1001 (36) | 862 (37) | 38 (36) | 58 (38) | 43 (37) | |
| 2+ | 597 (21) | 522 (22) | 17 (16) | 33 (22) | 25 (22) | |
| Child abuse | 395 (17) | 295 (14) | 18 (20) | 39 (28) | 43 (50) | <.001 |
| Domestic violence in pregnancy | 33 (1) | 22 (1) | 1 (1) | 4 (3) | 6 (5) | <.001 |
| Heavy alcohol use | 25 (1) | 23 (1) | 0 (0) | 1 (1) | 1 (1) | 0.77 |
| Smoking | <.001 | |||||
| None in pregnancy | 2366 (85) | 2088 (86) | 84 (80) | 122 (80) | 72 (62) | |
| Trimester 1 only | 191 (7) | 149 (6) | 12 (11) | 9 (6) | 21 (18) | |
| After trimester 1 | 236 (8) | 183 (8) | 9 (9) | 21 (14) | 23 (20) | |
| Illicit drugs | <.001 | |||||
| None | 2573 (92) | 2250 (93) | 95 (90) | 135 (89) | 93 (80) | |
| Marijuana only | 159 (6) | 131 (5) | 6 (6) | 12 (8) | 10 (9) | |
| Other drugs | 61 (2) | 39 (2) | 4 (4) | 5 (3) | 13 (11) | |
| Antidepressant use in pregnancy | 343 (12) | 250 (10) | 33 (31) | 39 (26) | 21 (18) | <.001 |
| # prior GAD episodes | N/A | |||||
| 0 | 2178 (78) | 2114 (87) | 0 (0) | 64 (42) | 0 (0) | |
| 1 | 155 (6) | 106 (4) | 12 (11) | 24 (16) | 13 (12) | |
| 2 | 95 (3) | 59 (2) | 10 (10) | 13 (9) | 13 (12) | |
| 3 | 66 (2) | 40 (2) | 13 (12) | 5 (3) | 8 (7) | |
| 4+ | 293 (11) | 99 (4) | 70 (67) | 45 (30) | 79 (70) | |
| # relatives with GAD | <.001 | |||||
| 0 | 1043 (39) | 964 (41) | 18 (18) | 42 (29) | 19 (17) | |
| 1 | 986 (36) | 860 (37) | 40 (40) | 53 (36) | 33 (29) | |
| 2+ | 679 (25) | 523 (22) | 41 (41) | 52 (35) | 63 (55) | |
| # hours/day mind occupied by new baby | <.001 | |||||
| 0–2 | 769 (33) | 694 (34) | 20 (23) | 38 (28) | 17 (20) | |
| 3–12 | 1421 (60) | 1227 (60) | 60 (69) | 82 (59) | 52 (60) | |
| 13–24 | 168 (7) | 126 (6) | 7 (8) | 18 (13) | 17 (20) | |
| Thoughts about new baby | <.001 | |||||
| Mostly happy | 1163 (49) | 1068 (52) | 30 (34) | 47 (34) | 18 (21) | |
| Mostly worries | 21 (1) | 14 (1) | 1 (1) | 4 (3) | 2 (2) | |
| Mixture of both | 1181 (50) | 970 (47) | 57 (65) | 87 (63) | 67 (77) | |
| Past week frequency of worries about new baby’s health | <.001 | |||||
| None | 768 (32) | 709 (35) | 17 (19) | 21 (15) | 21 (24) | |
| Once | 509 (22) | 451 (22) | 22 (25) | 24 (17) | 12 (14) | |
| More than once | 770 (33) | 660 (32) | 32 (36) | 53 (38) | 25 (29) | |
| Daily | 228 (10) | 174 (8) | 15 (17) | 21 (15) | 18 (21) | |
| Several times a day | 90 (4) | 58 (3) | 2 (2) | 19 (14) | 11 (13) | |
| MDD in pregnancy | 230 (8) | 130 (5) | 10 (10) | 47 (31) | 43 (37) | <.001 |
| PTSD in pregnancy | 136 (5) | 67 (3) | 8 (8) | 27 (18) | 34 (29) | <.001 |
| Panic disorder in pregnancy | 105 (4) | 53 (2) | 10 (10) | 17 (11) | 25 (22) | <.001 |
| # disorders in pregnancy | N/A | |||||
| 0 | 2286 (82) | 2205 (91) | 81 (77) | 0 (0) | 0 (0) | |
| 1 | 342 (12) | 187 (8) | 20 (19) | 84 (55) | 51 (44) | |
| 2 | 109 (4) | 21 (1) | 4 (4) | 48 (32) | 36 (31) | |
| 3 | 45 (2) | 7 (<1) | 0 (0) | 17 (11) | 21 (18) | |
| 4 | 11 (<1) | 0 (0) | 0 (0) | 3 (2) | 8 (7) | |
| GAD in pregnancy by trimester | N/A | |||||
| None in pregnancy | 2525 (90) | 2420 (100) | 105 (100) | 0 (0) | 0 (0) | |
| Trimester 1 only | 152 (5) | 0 (0) | 0 (0) | 58 (38) | 94 (81) | |
| Trimester 2 or 3 | 116 (4) | 0 (0) | 0 (0) | 94 (62) | 22 (19) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Social Support | 3.52 (0.69) | 3.56 (0.68) | 3.40 (0.67) | 3.32 (0.68) | 3.00 (0.66) | <.001 |
Because this sample was selected with an antidepressant use bias, antidepressant use is also presented. Antidepressant agents were used by 343 (12%) women at some time point in pregnancy or after delivery. Women with GAD prior to pregnancy but not in pregnancy (Group B) were the most frequent users of antidepressants (31%) while only 18% of the group with the highest co-morbidities (Group D) used antidepressants. There appeared to be an increase in anxiety/new cases in Group C as pregnancy progressed (Table One) compared to Group D but this may be a function of the way in which groups were defined.
Course in Pregnancy
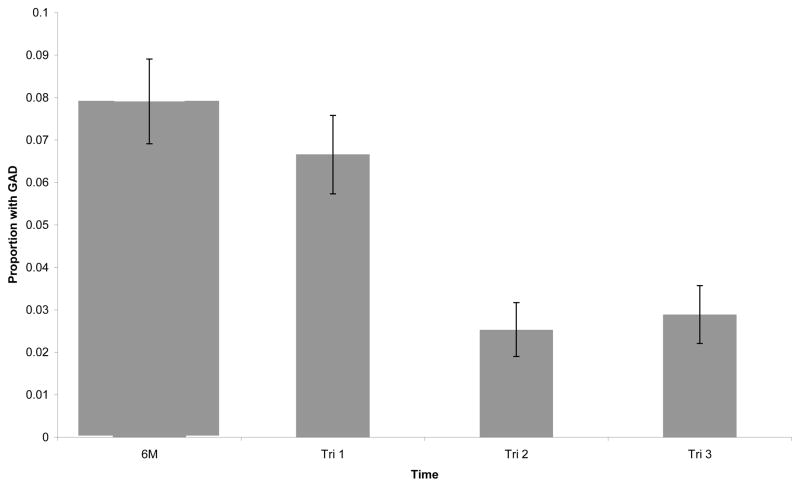
Changes in rates of GAD over pregnancy in the total sample are shown in Figure One. Two-hundred twenty one women (8%) retrospectively qualified for a GAD in the six months prior to pregnancy. In pregnancy, the highest rate of GAD was 7% in the first trimester. In the second trimester, only 2% of women met criteria for a diagnosis. GAD rates in the third trimester were only slightly higher (3%) compared to trimester 2.
Fig 1.
Relative Period Prevalence of GAD Over Time
An examination of anxiety symptoms in the total population (Table Two) suggests that a number of factors peak in the first trimester: duration of worry, impairment due to worry, and somatic symptoms. Most symptoms decreased over time, particularly from trimester 1 to trimester 2. For example, 9% of women were worried for at least 1 month in the first trimester compared to 4% in the second and 5% in the third. Functional impairment and somatic and behavioral symptoms followed similar patterns.
Table 2.
Anxiety Symptoms Over Time (Total Population)
| Symptom | Tri 1 | Tri 2 | Tri 3 |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Worried at least 1 month | 263 (9) | 100 (4) | 107 (5) |
| Functional impairment due to worry | |||
| Emotional distress | 197 (7) | 78 (3) | 85 (4) |
| Interfere with work/social/relationships | 176 (6) | 64 (3) | 78 (3) |
| Unable to carry out daily activities | 184 (7) | 67 (3) | 72 (3) |
| Received professional treatment for worry | 69 (2) | 31 (1) | 47 (2) |
| Somatic/behavioral symptoms | |||
| Jittery | 101 (4) | 37 (2) | 62 (3) |
| Tired | 1954 (70) | 708 (30) | 757 (32) |
| Difficulty Concentrating | 577 (21) | 192 (8) | 268 (11) |
| Trouble Sleeping | 1233 (44) | 905 (38) | 781 (33) |
The EPDS anxiety subscale also showed the tendency to decrease over time. In the over all sample, the anxiety subscale of the EPDS was highest in the first trimester with a mean of 2.39 (SD=2.24). It decreased to a mean of 1.97 (SD=2.03) in the second trimester and decreased again in the early postnatal phase (mean=1.68, SD=1.94).
The severity of GAD episodes also seemed to decrease slightly. The 186 women with first trimester GAD had an average first trimester EPDS anxiety subscale score of 4.91 (SD=2.34). This score was slightly greater than EPDS scores for the 60 women with second trimester GAD (mean=4.58, SD=2.28) and postpartum scores for the 68 women with third trimester GAD (mean=4.10, SD=2.60).
Risk Factors
Adjusted odds ratios examining risk factors for GAD are presented in Table Three. Previous history of GAD was the strongest predictor of GAD subgroup; women with 4 or more episodes were significantly more likely to be in group B (OR=7.30; 95% CI: 5.05–10.56; p <.0001), group C (OR=2.79; 95% CI: 2.01–3.89; p <.0001) and group D (OR=7.44; 95% CI: 4.93–11.21; p <.0001) compared to women with no GAD (Group A). Group C had significantly more black women (OR=1.58; 95% CI: 1.02–2.43; p=0.04); group D had a high representation of black women but this was not significantly higher than the reference (OR=1.62; 95% CI: 0.86–3.04; p=0.13). Group D had more than double the likelihood of child abuse (OR=2.11; 95% CI: 1.17–3.80; p=0.01) compared to women with no GAD (Group A). Groups C and D both had lower social support (OR≤0.73; p≤0.04). Groups B,C and D were somewhat less educated than Group A, particularly group D which had two and a half times as many women with less than 12 years of education as compared to the reference group (OR=2.48; 95% CI: 1.47–4.20; p=0.001).
Table 3.
Adjusted Odds Ratios for GAD Groups by Demographic and Potential Clinical Risk Factors
| Effect | GAD pre- pregnancy only Group A N=105 | GAD in pregnancy Group B N=152 | Both GAD pre- pregnancy and in pregnancy Group C N=116 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age | |||||||||
| <25 | 0.76 | (0.52, 1.12) | 0.16 | 0.75 | (0.56, 1.00) | 0.05 | 0.80 | (0.54, 1.18) | 0.26 |
| 25–34 | REF | ||||||||
| 35+ | 0.65 | (0.36, 1.18) | 0.15 | 0.81 | (0.53, 1.24) | 0.34 | 0.42 | (0.20, 0.89) | 0.02 |
| Race/Ethnicity | |||||||||
| White | REF | ||||||||
| Black | 0.87 | (0.36, 2.07) | 0.75 | 1.58 | (1.02, 2.43) | 0.04 | 1.62 | (0.86, 3.04) | 0.13 |
| Hispanic | 1.03 | (0.54, 1.97) | 0.92 | 0.87 | (0.60, 1.27) | 0.48 | 0.75 | (0.43, 1.31) | 0.31 |
| Education (years) | |||||||||
| <12 | 1.75 | (1.03, 2.97) | 0.04 | 1.05 | (0.68, 1.62) | 0.81 | 2.48 | (1.47, 4.20) | 0.001 |
| 12 | 1.42 | (0.63, 3.21) | 0.39 | 0.83 | (0.42, 1.62) | 0.59 | 1.66 | (0.70, 3.94) | 0.25 |
| 13–15 | 2.32 | (1.23, 4.38) | 0.01 | 2.11 | (1.37, 3.27) | 0.001 | 3.00 | (1.50, 6.00) | 0.002 |
| 16+ | REF | ||||||||
| Social support (one unit increase) | 1.01 | (0.65, 1.56) | 0.98 | 0.73 | (0.54, 0.99) | 0.04 | 0.55 | (0.35, 0.87) | 0.01 |
| History of child abuse | 0.79 | (0.41, 1.51) | 0.47 | 1.31 | (0.82, 2.07) | 0.26 | 2.11 | (1.17, 3.80) | 0.01 |
| Number of prior GAD episodes | |||||||||
| 0–1 | REF | ||||||||
| 2–3 | 2.49 | (1.63, 3.79) | <.0001 | 1.25 | (0.84, 1.88) | 0.27 | 2.29 | (1.42, 3.68) | 0.001 |
| 4+ | 7.30 | (5.05, 10.56) | <.0001 | 2.79 | (2.01, 3.89) | <.0001 | 7.44 | (4.93, 11.21) | <.0001 |
| Number of relatives with GAD | |||||||||
| 0 | REF | ||||||||
| 1 | 1.19 | (0.60, 2.33) | 0.62 | 1.06 | (0.66, 1.69) | 0.81 | 1.36 | (0.60, 3.07) | 0.46 |
| 2+ | 1.12 | (0.74, 1.69) | 0.60 | 1.15 | (0.87, 1.52) | 0.31 | 1.42 | (0.86, 2.33) | 0.17 |
Discussion
On average, both GAD and individual anxiety symptoms decreased across pregnancy and the postpartum period. GAD before and during pregnancy was strongly correlated with previous episodes. Women in pregnancy who were anxious before and during pregnancy seemed most severe and unique, having low levels of support, low educational level and a high likelihood of a child abuse history.
Though population prevalence could not be established from our sample, it is surprising that rates are so low in our cohort given that we used one month rather than six month minimum criteria. It is even more remarkable when one considers that we made a deliberate attempt to recruit women with current or past MDD and women who were undergoing antidepressant treatment. Whilst our sample may not have been representational, Hispanic, Black women and lower SES were included and were of similar (8% vs 9% Black) or higher proportions (14% vs 9% Hispanic) for Connecticut (US 2000 census). Differences in our rates and those in other studies relate to variations in recruitment and methodology as we used diagnostic interviews and others with high rates of anxiety have used self report questionnaires. Recall bias in our study for pre-pregnancy illness or other reports can also be a factor in discrepancies among GAD rates.
Our data suggest that one month duration may be a realistic and reliable criterion given that women who were symptomatic for one month had functional impairment. The reduction in required duration of illness is in keeping with the DSM V working parties proposals (dsm5.org) to reduce the time frame for GAD from six months to three months. We concur with the workgroup that this may improve reliability.
Few studies have examined the course of GAD across pregnancy; two studies (Lee et al., 2007;Teixeira et al., 2009) found anxiety symptoms most prevalent in first and third trimester; in our sample the first trimester only was significantly higher for GAD symptoms. This is similar to the pattern of MDD found in this cohort and may not be surprising given that GAD and MDD commonly co-occur. The stability from third trimester to the postpartum period found in this study is supported by other work. In Lee et al’s (2007) study Chinese women were noted to be more anxious regarding the gender of the child. Multiparity has also been identified as another potential contributor to anxiety in pregnancy.
The decrease in symptoms across pregnancy may be accounted for by a hormonal effect of pregnancy as we have hypothesized elsewhere. It seems however that the main risk of anxiety in pregnancy is for a small group of women (Groups C and D) where biological and environmental risks combine with current lack of support at a time of stress; some of these factors were also found in other high risk samples. The child abuse history in these women needs further consideration; the role of abuse as a risk factor for prolonged severe postnatal depression has been noted. More detailed examination of what constitutes child abuse that would increase risk of GAD is warranted.
Child outcomes of women with perinatal mental illness include emotional, behavioral and cognitive impairment and a higher risk for psychiatric diagnoses as children and adults. Attachment and maternal sensitivity has been noted to be impaired in mothers with postnatal depression as it has been in women with abuse histories. Difficulties in attachment may reflect a mechanism for intergenerational transmission of psychiatric distress with high cortisol noted in children of mothers who suffered from depression or anxiety during pregnancy and after birth. Many of these women are already anxious prior to the pregnancy and a likely epigenetic phenomena encompassing inherited disorders, biological changes in childhood secondary to abuse, as well as poor role modeling and insecure attachment may explain these women’s long standing difficulties.
Whilst most pregnant women will have some worries about pregnancy, more time and mixed worrying was characteristic of women with GAD, particularly Group D. They appeared to have little support and may feel ill equipped to cope as a parent. Worrying about the baby or mothering gives the clinician a potential ‘in’ to a group who may at other times be inaccessible. These questions are not commonly asked in screening and may be particularly relevant to the very high risk group (our Group D).
Limitations
A limitation of this study is the potential confounding effect of antidepressant use that may have ameliorated symptoms of GAD. However, women who took antidepressants were more likely to have current GAD in this sample so at best treatment effects were partial. Group D, arguably the most distressed women, were less likely to be on antidepressants. Factors, such as low income and unfamiliarity with services, has been associated with poor attainment of mental health treatment in minority populations such as this. Consequently there is also a substantial untreated group in addition to the under treated group. However, since many women choose to cease antidepressants in the first trimester, it seems unlikely that antidepressant use in this sample explains the lower anxiety symptoms in the subsequent two trimesters.
Another consideration in our study was the possible role of attrition although follow up rates were high overall (85% and 84%) but lower for Group D (75% and 72%). Finally, people who were very symptomatic may have declined to participate in our study and this may have led to a less representative cohort of women.
Conclusion
GAD symptoms were infrequent in pregnancy, with symptoms most common in the first trimester. Women with past history, low education and support, and child abuse histories were at risk of GAD before and during pregnancy. These women might be well supported by specific interventions helping them address their anxiety about the pregnancy, parenting and their unborn child.
Acknowledgments
Funding
Funding for this study was provided by the National Institute for Health NICHD:5-roi-hd045735. They had no influence on study design
Footnotes
Contribution
Anne Buist wrote the first draft and edited subsequent drafts.
Nathan Gotman analysed data and wrote the analysis sections
Kimberly Yonkers edited and rewrote sections of article.
All authors contributed to the article.
Conflict of Interest:
Anne Buist has received educational and travel grants from Astrazeneca in the previous three years
Kimberly Yonkers has received an investigator initiated grant from Lilly and study medication from Pfizer in the previous three years.
Nathan Gotman – no conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne Buist, Email: a.buist@unimelb.edu.au, Dept Psychiatry, University of Melbourne, Austin Health, 10th Fl Lance Townsend Building, Heidelberg 3081, Victoria, Australia
Nathan Gotman, Email: nathan.gotman@yale.edu, PMS and Postpartum Research Yale University, Suite 301, 142 Temple St, New Haven 06510
Kimberly Ann Yonkers, Email: kimberly.yonkers@yale.edu, PMS and Postpartum Research Yale University, Suite 301, 142 Temple St, New Haven 06510, Ph 203-7646621, Fax: 203-7646766
References
- American Psychiatric Association, A. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM IV-TR) [Google Scholar]
- Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M, Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstetricia et Gynecologica Scandinavica. 2006;85(8):937–944. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Figueiredo B, Guedeney N, Gorman LL, Hayes S, Muzik M, Glatigny-Dallay E, Valoriani V, Kammerer MH, Henshaw CA. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. The British Journal of Psychiatry. 2004;184(46):s31–37. doi: 10.1192/bjp.184.46.s31. [DOI] [PubMed] [Google Scholar]
- Bowen A, Bowen R, Maslany G, Muhajarine N. Anxiety in a socially high-risk sample of pregnant women in Canada. Can J Psychiatry. 2008;53(7):435–440. doi: 10.1177/070674370805300708. [DOI] [PubMed] [Google Scholar]
- Buist A. Childhood abuse, postpartum depression and parenting difficulties: a literature review of associations. Aust N Z J Psychiatry. 1998;32(3):370–378. doi: 10.3109/00048679809065529. [DOI] [PubMed] [Google Scholar]
- Buist A, Janson H. Childhood sexual abuse, parenting and postpartum depression--a 3-year follow-up study. Child Abuse Negl. 2001;25(7):909–921. doi: 10.1016/s0145-2134(01)00246-0. [DOI] [PubMed] [Google Scholar]
- Chen YH, Lin HC, Lee HC. Pregnancy outcomes among women with panic disorder -- Do panic attacks during pregnancy matter? J Affect Disord. 2009;120(1–3):258–262. doi: 10.1016/j.jad.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davis RG, Ressler KJ, Schwartz AC, Stephens KJ, Bradley RG. Treatment barriers for low-income, urban African Americans with undiagnosed posttraumatic stress disorder. Journal of Traumatic Stress. 2008;21(2):218–222. doi: 10.1002/jts.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Miech R, Smider NA. Timing of initial exposure to maternal major depression and children’s mental health symptoms in kindergarten. Br J Psychiatry. 2001;179:151–156. doi: 10.1192/bjp.179.2.151. [DOI] [PubMed] [Google Scholar]
- Falsetti S, Resnick H, Pesick P, Kilpatrick D. The modified PTSD symptom scale: A brief self-report measure of posttraumatic stress disorder. Behavior Therapist. 1993;16:161–162. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, Schanberg S, Kuhn C. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behavior and Development. 2009;33(1):23–29. doi: 10.1016/j.infbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH, Tyer-Viola L. Detection, Treatment, and Referral of Perinatal Depression and Anxiety by Obstetrical Providers. Journal of Women’s Health (15409996) 19(3):477–490. doi: 10.1089/jwh.2008.1352. [DOI] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Austin MP. Maternal anxiety during the transition to parenthood: A prospective study. J Affect Disord. 2008;108(1–2):101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kendler K. Major Depression and Generalised Anxiety Disorder Same Genes, (Partly) Different Environments - Revisted. The British Journal of Psychiatry. 1996;168 (suppl 30):68–75. [PubMed] [Google Scholar]
- Lee AM, Lam SK, Sze Mun Lau SM, Chong CSY, Chui HW, Fong DYT. Prevalence, Course, and Risk Factors for Antenatal Anxiety and Depression. Obstet Gynecol. 2007;110(5):1102–1112. doi: 10.1097/01.AOG.0000287065.59491.70. [DOI] [PubMed] [Google Scholar]
- Lim L, Tze Pin N, Hong Choon C, Peak Chiang C, Won V, Lee T, Fones C, Ee Heok K. Generalised anxiety disorder in Singapore: prevalence, co-morbidity and risk factors in a multi-ethnic population. Soc Psychiatry Psychiatr Epidemiol. 2005;40(12):972–979. doi: 10.1007/s00127-005-0978-y. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Wolfe R, Lyubchik A. Depression and the parenting of young children: making the case for early preventive mental health services. Harvard Review of Psychiatry. 2000;8(3):148–153. [PubMed] [Google Scholar]
- Manassis K, Bradley S, Goldberg S, Hood J, Swinson RP. Behavioural inhibition, attachment and anxiety in children of mothers with anxiety disorders. [Research Support, Non-U.S. Gov’t] Can J Psychiatry. 1995;40(2):87–92. doi: 10.1177/070674379504000206. [DOI] [PubMed] [Google Scholar]
- Martini J, Knappe S, Beesdo-Baum K, Lieb R, Wittchen HU. Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev. 2010;86(5):305–310. doi: 10.1016/j.earlhumdev.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Matthey S, Barnett B, Howie P, Kavanagh DJ. Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? J Affect Disord. 2003;74(2):139–147. doi: 10.1016/s0165-0327(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Moore PS, Whaley S, Sigman M. Interactions between mothers and children. Impact of maternal and child anxiety. J Abnorm Psychol. 2004;113(3):471–476. doi: 10.1037/0021-843X.113.3.471. [DOI] [PubMed] [Google Scholar]
- Moss KM, Skouteris H, Wertheim EH, Paxton SJ, Milgrom J. Depressive and anxiety symptoms through late pregnancy and the first year post birth: an examination of prospective relationships. Arch Women Ment Health. 2009;12(5):345–349. doi: 10.1007/s00737-009-0086-1. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression - a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- Ross LE, Evans SEG, Sellers EM, Romach MK. Measurement issues in postpartum depression part 1: Anxiety as a feature of postpartum depression. Arch Women Ment Health. 2003;6:51–57. doi: 10.1007/s00737-002-0155-1. [DOI] [PubMed] [Google Scholar]
- Smith MV, Poschman K, Cavaleri MA, Howell HB, Yonkers KA. Symptoms of posttraumatic stress disorder in a community sample of low-income pregnant women. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Am J Psychiatry. 2006;163(5):881–884. doi: 10.1176/ajp.2006.163.5.881. [DOI] [PubMed] [Google Scholar]
- Spoozak L, Gotman N, Smith MV, Belanger K, Yonkers KA. Evaluation of a social support measure that may indicate risk of depression during pregnancy. J Affect Disord. 2009;114(1–3):216–223. doi: 10.1016/j.jad.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C, Figueiredo B, Conde A, Pacheco A, Costa R. Anxiety and depression during pregnancy in women and men. J Affect Disord. 2009;119(1–3):142–148. doi: 10.1016/j.jad.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven JM, de Weerth C. Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress. doi: 10.3109/10253890.2010.499485. [DOI] [PubMed] [Google Scholar]
- Uguz F, Gezginc K, Kayhan F, Sari S, Buyukoz D. Is pregnancy associated with mood and anxiety disorders? A cross-sectional study. Gen Hosp Psychiatry. 2009;32(2):213–215. doi: 10.1016/j.genhosppsych.2009.11.002. [DOI] [PubMed] [Google Scholar]
- van Batenburg-Eddes T, de Groot L, Huizink AC, Steegers EAP, Hofman A, Jaddoe VWV, Verhulst FC, Tiemeier H. Maternal Symptoms of Anxiety During Pregnancy Affect Infant Neuromotor Development: The Generation R Study. Developmental Neuropsychology. 2009;34(4):476–493. doi: 10.1080/87565640902964508. [DOI] [PubMed] [Google Scholar]
- van Bussel JC, Spitz B, Demyttenaere K. Women’s mental health before, during, and after pregnancy: a population-based controlled cohort study. Birth. 2006;33(4):297–302. doi: 10.1111/j.1523-536X.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- WHO. Composite International Diagnostic Interview (CIDI, Version 2.1) Geneva, Switzerland: World Health Organization; 1997. (Version 2.1 ed.) [Google Scholar]
- Yonkers KA, Smith MV, Lin H, Howell HB, Shao L, Rosenheck RA. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. [Research Support, N.I.H., Extramural] Psychiatr Serv. 2009;60(3):322–328. doi: 10.1176/appi.ps.60.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]