Abstract
Here, we provide a comprehensive insight into current advances in the use of nanogel-mediated chemotherapy for cancer treatment. Nanogels are composed of cross-linked three-dimensional polymer chain networks that are formed via covalent linkages or self-assembly processes. The porosity between the cross-linked networks of nanogels not only provides an ideal reservoir for loading drugs, oligonucleotides and imaging agents, but also protects them from environmental degradation and hazards. Here, we focus mainly on novel synthetic strategies and key considerations in the design of nanogel-based drug delivery systems for controlled and targeted cancer therapeutic applications.
Introduction
In addition to debulking surgery, chemotherapy is a major treatment modality in cancer therapy [1]. Many chemotherapeutic and chemopreventive agents can simultaneously improve the survival rate of patients with cancer while resulting in adverse effects, owing to a lack of specificity. Hence, the administration of these chemotherapeutic agents in other forms (i.e. encapsulated, conjugated, entrapped and loaded) is required to target the tumors [2]. Controlled and targeted drug delivery systems can act at a tumor site for prolonged periods of time as a result of their specific cancer cell surface interactions, without affecting normal tissues [3,4]. Most tumors are characterized by the overexpression of cancer-specific antigen(s) or receptor(s) on their cell surfaces, which are essential for the growth of tumor cells. Therefore, the use of nanocarrier-based delivery systems to target these antigen(s) or receptor(s) is being investigated extensively as an important modality of treatment. Given that polymer nanoparticles are less stable in biological fluids and that there are no specific responsive groups, as such, on their surfaces, there is still a need to explore novel formulations of treatment modalities.
Nanotechnology versus nanogel technology in cancer therapeutics
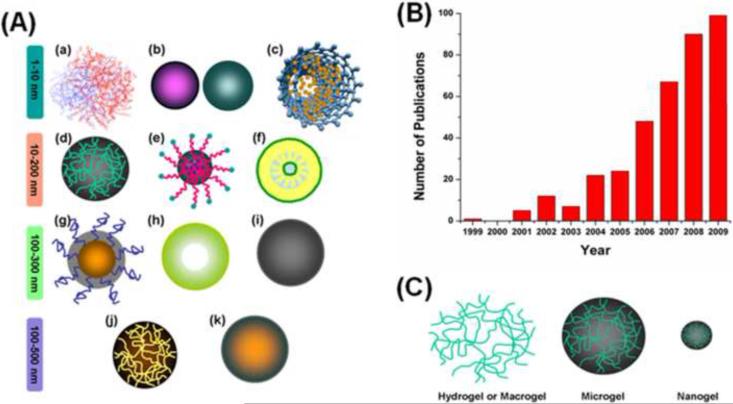
Generally, oral or intravenous chemotherapeutic agents, drugs, enzymes, proteins and biomolecules in aqueous solution do not offer ideal pharmacokinetics in the physiological environment. Anticancer drugs have toxic effects on normal tissue and have a very narrow therapeutic window. Prominent nanotherapeutic approaches treat different types of cancer and have opened up a new era of cancer chemotherapy with beneficial contributions to health care [5,6]. Various types of nanosystem are widely used in drug delivery applications (Figure 1a). The delivery of nanoparticle-mediated anticancer drugs, small interfering RNA (siRNA), DNA, proteins and other biomolecules to tumors can be achieved through passive targeting and active targeting pathways. However, stability and short systemic circulation times have often limited their use in vivo and the continuous development of novel nanocarriers has resulted in a new nanomaterial, so-called `nanogels'. The significance of these nanogel systems has been increasing year on year (Figure 1b). The internal structure of nanogels is similar to that of hydrogels or microgels, but varies in size and responsiveness (Figure 1c) [7].
Figure 1.
Types of nanoparticle used in drug delivery. (a) Schematic structural variation of various drug delivery nanocarriers, such as (i) dendrimer, (ii) metal or metal oxide nanoparticles, (iii) carbon nanotube, (iv) nanogel, (v) micelle, (vi) lipid/liposome nanoparticles, (vii) polymer nanoparticle, (viii) hollow nanoparticle, (ix) solid nanoparticle, (x) microgel, and (xi) microparticles. (b) Number of publications investigating nanogel carriers for drug, protein, enzyme and DNA delivery during the past decade. Data obtained from Wiley, Elsevier, American Chemical Society, Royal Society of Chemistry and PubMed. (c) Schematic representation of the network construction of hydrogels, microgels and nanogels.
Until recently, there was no worldwide consensus on the definition of nanogels; however, based on the results from various research groups, gel nanoparticles <200 nm in size are now considered to be nanogels. Unlike poly(lactic acid) (PLA), poly(caprolactum) (PCL) or poly(lactide-co-glycolide) (PLGA) nanoparticles, nanogels containing hydrophilic functional groups, such as hydroxyl (−OH), carboxyl (−COOH), amino (−NH2), amide and sulfonic (−SO3H) groups, are being promoted for biomedical applications [7,8]. Drug release from nanogel networks depends on the interaction of hydrophobic, hydrogen, complexation and/or coordination of drug molecules with the polymer chain networks. Given that nanogels are both diverse and contain stimuli-responsive or smart-responsive characteristics, we discuss here their possible use in cancer therapy.
Design of nanogels
Hydrogels (macrogels), microgels and nanogels are highly water-absorbed materials that remain insoluble in aqueous solutions owing to the internal chemical or physical cross-linking of their macromolecular chains, which vary in size and structure [9]. An ideal nanogel drug delivery carrier should have a few common features including, but not limited to: a smaller particle size (10–200 nm), biodegradability and/or biocompatibility, prolonged blood circulation time, higher amount of drug or enzyme loading and/or entrapment and protection of molecules from the immune system of the body [8]. The multifunctional properties of nanogels can be achieved by altering the cross-linking density, chemical functional groups, surface-active, and stimuli-responsive constituents.
Nanogel systems have rings and loops within their gel macromolecular chains and require additional attention in choosing the composition of their different constituents [10]. Macrogelation (critical gelation) is an obstacle in nanogel preparation, and ultimately promotes the formation of either a microgel or hydrogel. This can be avoided by selecting a solvent whose solubility parameter matches for polymerization or using a chain transfer agent that controls macrogelation and favors nanogel formation. Nanogels produced from natural polymers are appropriate for pathogens but evoke immune and/or inflammatory responses [11,12]. By contrast, nanogels made from synthetic polymers offer well-defined morphologies that can be customized to gel networks with biocompatible and degradable properties. The first criterion for a nanogel carrier with widespread biomedical applications is to have good stability in biological fluids, which would prohibit aggregation. Colloidal particle stability is evaluated from the thermodynamic relationship (i.e. ΔGfloc = ΔHfloc − TΔSfloc [13]). Gel nanoparticles are stable in a good solvent owing to the negligible van der Waals forces of attraction between the gel nanoparticles; this results in ΔHfloc << TΔSfloc and, therefore, the total ΔGfloc becomes positive (i.e. is a thermodynamically feasible process for flocculation). Given that swollen particles in water exhibit a continuous phase, the solvent and particles phases are perfectly matched. In this situation, the Hamaker constants for the solvent and particles are more-or-less equal and there are no driving forces for the aggregation of the gel nanoparticles. Therefore, gel nanoparticles are stable in aqueous media owing to the negligible van der Waals forces of attraction between them [13].
Nanogel networks formed of stimuli responsive units extensively regulate the drug release profile. However, the transport properties of drug molecules depend on the diffusion coefficient value, which is always influenced by the molecular size of the drug molecules and the network structure of the nanogels [7–9]. The drug transport property varies with, for example, the cross-link density of the gel network, the molecular weight of the polymer, the gel network degradation rate and the interaction of the drug–biomacromolecule with the polymeric chains in the gel network.
Cross-linked nanogels
Cross-linking reactions are suitable preparation methods for nanoscopic gels with porous or micellar network structures. Different cross-linking methods, such as ionic cross-linking, self-assembly, crystallization, cross-linking polymerization, radiation cross-linking, and functional group cross-linking, can be used to prepare nanogels. The basic principles of preparing nanogels have been described elsewhere [7].
Physically cross-linked nanogels
Physical cross-linked nanogel formation occurs via non-covalent attractive forces, such as hydrophilic–hydrophilic, hydrophobic–hydrophobic, ionic interactions and/or hydrogen bonding [14]. These systems are sensitive and this sensitivity depends on polymer composition, temperature, ionic strength of the medium, concentrations of the polymer and of the cross-linking agent.
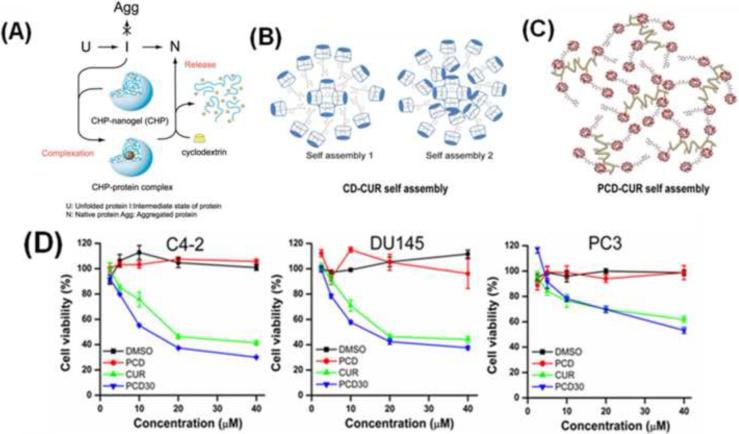
The association of amphiphilic block copolymers and complexation of oppositely charged polymeric chains results in the formation of micro- and nanogels in only a few minutes [15]. The Akiyoshi group [16] modified polysaccharides with hydrophobic groups [e.g. cholesteryl-group modified pullulans (CHP)], which instantaneously formed highly monodispersed nanogel structures, 20–30 nm in size, by intermolecular self-aggregations in water (Figure 2a). The same methodology was also applied to modify various hydrophilic polymers of poly(N-isopropylacryamide) (PNIPAM), chitosan, dextran and poly(amino acids) using hydrophobic moieties, such as cholesteryl, deoxycholic acid, bile acid and so on [17]. These CHP-modified nanogels can entrap or capture drug molecules, proteins, enzymes and DNA by supramolecular self-assembly [18]. A novel approach to encapsulate curcumin in β-cyclodextrin or poly(β-cyclodextrin) via a nano-self assembly process (Figure 2b,c) has been shown to increase curcumin uptake, resulting in greater therapeutic effects in cancer cells (Figure 2d) through various molecular mechanisms [19,20].
Figure 2.
Self-assembly formation of (a) CHP and protein, (b) β-cyclodextrin-curcumin, (c) poly(β-cyclodextrin)-curcumin and (d) an MTT assay of prostate (C4-2, DU145 and PC3) cancer cells treated with either a CUR or poly(β-cyclodextrin)-curcumin (PCD30) formulation. PCD30 resulted in greater therapeutic effects in all cancer cells, although the release of curcumin was only 40–50%. Reproduced, with permission, from [18–20].
A few micelle nanogels are currently in clinical trials [21]. Micelle nanogels significantly promote the solubility of highly hydrophobic (lipophilic) drugs up to 30 000-fold [22]. Among the various choices of hydrophobic core-forming biocompatible and biodegradable polymer micelles, PLA, PLGA, PCL, poly(propylene oxide) (PPO), poly(hydroxybutyrate) (PHB), and poly(γ-benzyl L-asparate) are widely used. For example, nanogels carrying modified heparin with poly(ethylene glycol) (PEG) results in disulfide linkages throughout the nanogels that can be reducible for the intracellular delivery of free heparin [23]. Poly(ethyleneimine) (PEI) cross-linked poly(ethylene oxide) (PEO) systems have been found to be biocompatible and degradable nanogel systems that can be used to deliver nucleoside analogs, nucleosides and DNA [24,25]. These PEI-cl-PEO gel particles enable loading of the nanogel with drug molecules up to 30–33% by weight of the drug–nanogel formulation.
Chemically cross-linked nanogels
Chemically cross-linked nanogels are constructed with several cross-linking points throughout a backbone of polymeric chains. It has been demonstrated that cross-linkers have a vital role in tailoring the swelling, pore size and morphology of the gel macromolecules to make ideal matrices that, in turn, are responsible for obtaining predetermined release kinetics of the entrapped drug molecules [26]. Chain growth polymerization primarily produces hydrophilic functional nanogels. A variety of monomer, co-monomers and cross-linkers have been used to obtain cross-linked nanogels during the past two decades. N,N′-methylenebisacrylamide (MBA), divinylbenzene, diallyl phthalate and PEG diacrylate are commonly used as cross-linkers. They can control the formation of gel nanoparticles from 5 nm to 400 nm, followed by different strategies [27].
To achieve the degradability of nanogel networks, degradable bonds, such as ester, carbonate, amide, anhydride, phosphazene and phosphate esters, need to be inserted either in cross-linkers or in polymeric chains. These nanogel networks follow degradation through solubilization, enzymatic and hydrolysis mechanisms. Peptide cross-linkers containing nanogels have been reported recently [28]. Another route to designing a biodegradable network gel involves polymeric chains cross-linked with albumin or a novel acid labile ortho ester-based cross-linker [29,30].
The synthesis of small molecular cross-linkers is simple and a study of the fundamental properties of degradability of the final nanogel networks produced could lead to better solubility, inherent biocompatibility and desired molecular structures [31,32]. The potential cross-linkers are N,N′-cystaminebisacrylamide (CBA) and N,N′-(dihydroxyethylene)bisacrylamide (DHEA). These cross-linked networks in nanogels are cleaved by chemical reducing agents, such as dithiothreitol (DTT) or sodium periodate (NaIO4) or enzymatically. N,N′-bisacryloylcystamine (BAC) is another cross-linker used to produce intramolecular cross-linked poly(acrylamide) nanogels in a green approach [33].
Thermo-sensitive nanogels
Nanogels containing a large portion of water within their network structures create opportunities for biomedical applications because of their responsiveness to temperature [27]. PNIPAM is a model example and a well-studied gel particle attributed to the reversible formation and breakage of the hydrogen bond interaction between the polymer and water molecules [34]. To synthesize smaller nanogels, the useful concentration of sodium dodecylsulfate (SDS) lies between 0.2–4 mM [35]. Structurally homogeneous 50-nm PNIPAM gel particles were made with 5.3 mM SDS, 3.8 g NIPAM and 0.132 g MBA; however, their dynamic light scattering (DLS) and small angle neutron scattering (SANS) results suggest they were polydispersed in nature [36].
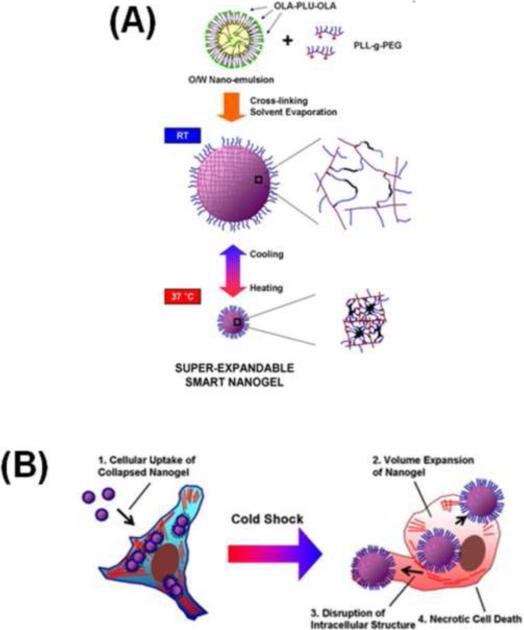
Synthesis of poly(L-lysine-g-ethylene glycol) (PLL-g-PEG)/oligo(lactic acid)-b-pluronic F127-b-oligo(lactic acid) nanogels was achieved by oil-in-water nanoemulsion via cross-linked solvent evaporation. The collapsed nanogels at 37°C (~150 nm) were endocytosed within the cells and prevalently entrapped inside the endosomal/lysosomal vesicular compartments with few escaping into the cytosolic space [37]. Upon cold shock exposure, the intracellular nanogels rapidly expanded by absorbing adjacent aqueous solution to become highly swollen gels with a size greater than 1.4 μm, which induced cell death owing to the sudden spatial development of physical stresses within the cell (Figure 3). However, similar necrotic cell death was not noticed when the pluronic/PEI nanogels changed in size from 95.2 to 332.5 nm upon cold shock. Multistage free-radical polymerization has been used to create thermosensitive core and ionic/non-ionic shell-containing nanogel systems [38].
Figure 3.
Please add title. (a) Schematic representation of a PLL-g-PEG/oligo(lactic acid)-b-pluronic F127-b-oligo(lactic acid) nanogel prepared by cross-linked solvent evaporation using the nano-emulsion method. A clear swell and collapse in networks can be viewed at room temperature and 37.0°C, respectively. (b) Schematic illustration of nanogel uptake when in a collapsed network stage and after internalization expanded their networks and necrotic properties are exhibited on the cells following cold shock process. Reproduced, with permission, from [37].
Yong Li et al. [39] expanded the use of novel thermosensitive micellar nanogel particles for drug delivery. These hydrophobically modified pullulan PNIPAM gels are 20–50 nm in size [40], whereas poly(NIPAM-co-HEMA) comb-shaped (graft) copolymer and poly[(N-isopropylacrylamide-co-2-hydroxyethylmethacrylate terminated oligo(L-lactide)] and poly(D-lactide) self-assembling in water form micelles ranging in size from 100 nm to 160 nm [41].
pH-sensitive nanogels
Nanogels constructed with polymer chains containing ionisable repeating groups are suitable for pH-dependant release [42]. The construction of nanogel networks with poly(acrylic/methacrylic) P(AA/MAA) units and PNIPAM chains underlies a rapid increase in the hydrophilicity and LCST of the copolymer at all the pH ranges, particularly <pH 5 [43]. Polyampholytic or zwitterionic polymeric gel particles have also received priority owing to their interior structural features. These features enable a response under all pH conditions owing to their effectiveness at a wide range of isoelectric points [44]. MMA-diethyl acrylate-DP nanogels stabilized by PEG methyl ether methacrylate (PEGMEM) resulted in varied sizes under different pH conditions, with size following an order of pH 9>2>5 [45]. Sequential investigations of macrogels (20–20 and nanogels (~200 nm) containing PNIPAM and poly(vinylimidazole) (PVI) chains looked at the pH and ionic responses [46]. Considerable variation exists in the equilibrium kinetics (pH 10–2) of plain PNIPAM microgel and ionic PNIPAM-VI micro-nano gels. These results indicate that particle size varies in different pH solutions. The observed results propose categories of mechanisms based on the proton uptake by the gel particles with their ionizable groups: (i) fast binding of ions to the surface of the gel particles; and (ii) diffusion of bound ions into the gel networks. A consequence of this method, in which a thermoresponsive core (PNIPAM) is chemically bound with poly(2-vinylpyridine) chains, is that pendent arms form as shells [47]. These arms are more stable upon heating and at lower pH conditions.
Functional, composite and multi-functional nanogels
The introduction of specific functional groups into nanogel network interiors is an interesting way to direct targeting approaches. For example, a 50-nm multifunctional gel particle formulation made up of NIPAM, AA, N-vinylpyrrolidone (NVP) had a micellar structure with mucoadhesive characteristics [48] and a hydrophobic drug-loading capacity of up to 25% of the combined weight. It has also been useful for bio-conjugation reactions with −COOH functional groups. A few biocompatible polymers have been identified and the addition of these hydrophilic polymeric segments onto nanogel surfaces often tends to enhance their duration in the bloodstream and to reduce cell and protein adhesion [49]. Such biodegradable functional polymer coatings on nanogels or nanoparticles made from PNIPAM, PLGA, PCL, and their copolymers provides an excellent improvement to the conjugation.
Metal colloidal particles or quantum dots incorporated into polymer or nanogel network structures render them with unique properties, such as rheological, optical, magnetic or electrical properties feasible for use in a range of therapeutic and diagnostic applications [50]. Core-shell nanogel particles are also used as templates to produce metal nanoparticles inside the shell networks [51]. Clinical researchers are focused on biocompatible and biodegradable iron oxide (Fe3O4) nanosystem formulations owing to their metabolization as elemental iron and oxygen by enzymes present in the body. Iron oxide nanoparticles were used as the first magnetic resonance imaging (MRI) agent during the 1980s and are currently being approved by the US Food and Drug Administration (FDA) for clinical imaging applications [52]. Investigations revealed that superparamagnetic iron oxide nanoparticles (SPIONs) coated with, or incorporated into, biodegradable polymers or nanogels serve the dual purpose of contrast agents as well as directing biodegradable nanoparticles to the targeted sites [50]. A large number of coating methods use small surfactant molecules and macromolecules (biodegradable polymers) but the resulting biodegradable-magnetic nanocomposite saturation magnetization values decrease by two to three times [53]. It is possible to obtain higher magnetization values in the formulations when the SPIONs are encapsulated before incorporation. In addition, this process must be protected from oxidation during the storage and sonication processes. Another approach was reported for the entrapment of 10–20-nm magnetite particles in thermosensitive hydrogels, resulting in a thermosensitive magnetite nanocomposite used for enzyme immobilization; these magnetic supports can be re-used many times [54].
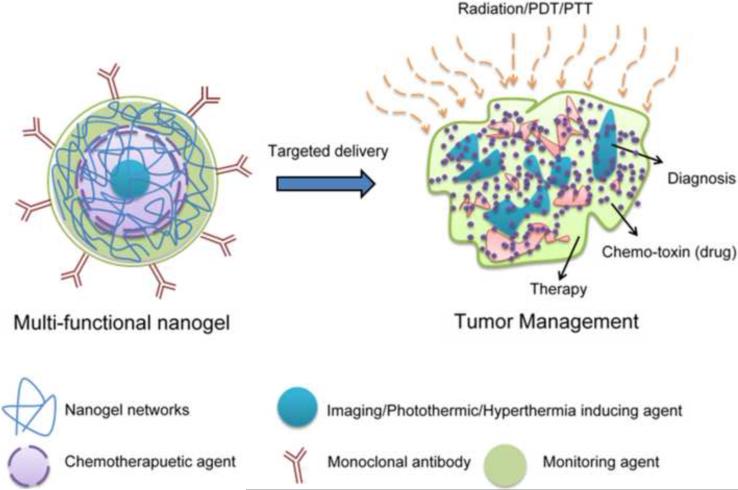
Developing nanogels containing magnetic nanoparticle, contrast or diagnostic agents is a promising alternative approach to gaining different biological functions (Figure 4). These novel composite formulations can be used for multipurpose applications such as combinations of MRI, visible targeting, magnetically targeted photodynamic therapy, targeted thermosensitive chemotherapy and luminescence, near-infrared or multi-model imaging applications.
Figure 4.
Novel multifunctional nanogel formulation for cancer therapy and diagnostic use.
Concluding remarks and future perspectives
Here, we have discussed various aspects of nanoparticulate systems and their superiority in terms of their inherent physico-chemical properties. The nanogel field is a new area of research and, therefore, rapid developments occur almost on a daily basis. Although nanogels such as PLGA, PLA and other naturally occurring degradable polymer-based nanogels were not reviewed in detail here, this review not only provides the reader with an insight into the new synthetic developments, but also reveals the richness of novel nanogel material applications in the biomedical field. Developing multi-targeted nanosystems will result in superior cancer therapeutics and diagnostics.
The higher expression of cancer-associated antigens, such as mucins (MUC1, MUC4, MUC5AC, MUC13 and MUC16), in tumors compared with the surrounding normal tissues can be exploited for the purpose of targeted delivery of nanogels and other nanocarriers [55,56]. Owing to the availability of recently generated antibodies and progress in characterizing the role of tumor antigens, the antibody field has had a major role in cancer diagnosis and treatment in recent years. Nevertheless, more research into the use of antibodies in the field of nanotechnology is required to provide improved therapies for cancer treatment.
Antibody-conjugated nanoparticles have recently been developed for the targeted delivery of anticancer drugs. These antibody-conjugated nanoparticles efficiently targeted cancer cells in cell culture models [57,58]. However, targeting one cancer antigen is unlikely to be adequate for an optimal cancer therapy outcome because of the heterogeneous expression of cancer antigens in tumors. It has been shown that multiple antigen targeting improves the labeling efficiency of cancer cells in ovarian tumors [59]. Therefore, future multitargeted nanosystems must be developed for superior cancer therapeutics and diagnostics. Nanogels will therefore be an ideal choice of nanosystem because of their unique physicochemical properties for antibody conjugation.
Acknowledgments
We thank Cathy Christopherson for editorial assistance. This work was partially supported by grants from Sanford Research/USD, PC073887, PC073643, Governor's Cancer 2010, and NIH RO1 CA142736.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shore S, et al. Review article: chemotherapy for pancreatic cancer. Aliment. Pharmacol. Ther. 2003;18:1049–1069. doi: 10.1111/j.1365-2036.2003.01781.x. [DOI] [PubMed] [Google Scholar]
- 2.Sarembock IJ. From systemic shotgun to site-specific nanoparticle-targeted delivery: a new paradigm for drug delivery. Arterioscler. Thromb. Vasc. Biol. 2008;28:1879–1881. doi: 10.1161/ATVBAHA.108.175190. [DOI] [PubMed] [Google Scholar]
- 3.Jabr-Milane L, et al. Multi-functional nanocarriers for targeted delivery of drugs and genes. J. Control. Release. 2008;130:121–128. doi: 10.1016/j.jconrel.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Jabr-Milane LS, et al. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat. Rev. 2008;34:592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006;3:205–216. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Yallapu MM, et al. Nanogels: chemistry to drug delivery. In: Labhasetwar V, Pelecky LDL, editors. Biomedical Applications of Nanotechnology. John Wiley & Sons; 2007. pp. 131–172. [Google Scholar]
- 8.Vinogradov SV, et al. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman AS. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 10.Graham NB, Cameron A. Nanogels and microgels: the new polymeric materials playground. Pure Appl. Chem. 1998;70:1271–1275. [Google Scholar]
- 11.Raemdonck KD, et al. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–715. [Google Scholar]
- 12.Agnihotri SA, et al. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Vincent B. Microgels and core-shell particles. In: Blitz JP, Gun'ko VM, editors. Surface Chemistry in Biomedical and Environmental Science. 2006. pp. 11–22. Publisher. [Google Scholar]
- 14.Hittinger E, et al. Synthesis and characterization of cross-linked conjugated polymer milli-, micro-, and nanoparticles. Angew. Chem. Int. Ed. 2004;43:1808–1811. doi: 10.1002/anie.200352863. [DOI] [PubMed] [Google Scholar]
- 15.Harada A, Kataoka K. Chain length recognition: core-shell supramolecular assembly from oppositely charged block copolymers. Science. 1999;283:65–67. doi: 10.1126/science.283.5398.65. [DOI] [PubMed] [Google Scholar]
- 16.Nomura Y, et al. Thermoresponsive controlled association of protein with a dynamic nanogel of hydrophobized polysaccharide and cyclodextrin: heat shock protein-like activity of artificial molecular chaperone. Biomacromolecules. 2005;6:447–452. doi: 10.1021/bm049501t. [DOI] [PubMed] [Google Scholar]
- 17.Akiyoshi K, et al. Self-association of cholesteryl-bearing poly(L-lysine) in water and control of its secondary structure by host-guest interaction with cyclodextrin. Macromolecules. 2000;33:6752–6756. [Google Scholar]
- 18.Nomura Y, et al. Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett. 2003;553:271–276. doi: 10.1016/s0014-5793(03)01028-7. [DOI] [PubMed] [Google Scholar]
- 19.Yallapu MM, et al. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces. 2010;79:113–125. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Yallapu MM, et al. Poly(beta-cyclodextrin)/curcumin self-assembly: a novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol. Biosci. 2010;10:1141–1151. doi: 10.1002/mabi.201000084. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura Y. MEMS, NANO and Smart Systems. 2004. Basic aspects and clinical trials of micelle carrier system. ICMENS 2004. [Google Scholar]
- 22.Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv. Drug Deliv. Rev. 2002;54:191–202. doi: 10.1016/s0169-409x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- 23.Bae KH, et al. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials. 2008;29:3376–3383. doi: 10.1016/j.biomaterials.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Vinogradov SV, et al. Cross-linked polymeric nanogel formulations of 5′-triphosphates of nucleoside analogues: role of the cellular membrane in drug release. Mol. Pharm. 2005;2:449–461. doi: 10.1021/mp0500364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradov SV, et al. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Control Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichenbaum KDT, et al. Oligo-hydroxy ester cross-linkers: impact of cross-linker structure on biodegradable hydrogel networks. Macromolecules. 2005;38:10757–10762. [Google Scholar]
- 27.Pelton R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000;85:1–33. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 28.Kim SC, et al. Synthetic MMP-13 degradable ECMs based on poly(N-isopropylacrylamide-co-acrylic acid) semi-interpenetrating polymer networks I. Degradation and cell migration. J. Biomater. Res. 2005;75:73–88. doi: 10.1002/jbm.a.30375. [DOI] [PubMed] [Google Scholar]
- 29.Tada DT, et al. Drug release from hydrogel containing albumin as crosslinker. J. Biosci. Bioeng. 2005;100:551–555. doi: 10.1263/jbb.100.551. [DOI] [PubMed] [Google Scholar]
- 30.Huang XD, et al. Novel acid-labile, thermoresponsive poly(methacrylamide)s with pendent ortho ester moieties. Macromol. Rapid Commun. 2007;28:597–603. [Google Scholar]
- 31.Kim S, Healy KE. Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4:1214–1223. doi: 10.1021/bm0340467. [DOI] [PubMed] [Google Scholar]
- 32.Muggli DS, et al. Reaction behavior of biodegradable, photo-cross-linkable polyanhydrides. Macromolecules. 1998;31:4120–4125. [Google Scholar]
- 33.Aliyer HA, et al. Synthesis of polyacrylamide nanogels by intramolecular disulfide cross-linking. J. Bioact. Compat. Polym. 2005;20:169–181. [Google Scholar]
- 34.Rzaev ZMO, et al. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Prog. Polym. Sci. 2007;32:534–595. [Google Scholar]
- 35.McPhee W, et al. Poly(N-isopropylacrylamide) latices prepared with sodium dodecyl sulfate. J. Colloid Interf. Sci. 1993;156:24–30. [Google Scholar]
- 36.Arleth L, et al. Volume transition and internal structures of small poly(N-isopropylacrylamide) microgels. J. Polym. Sci. B. 2005;43:849–860. [Google Scholar]
- 37.Lee Y, et al. Thermally triggered intracellular explosion of volume transition nanogels for necrotic cell death. J. Control Release. 2009;135:89–95. doi: 10.1016/j.jconrel.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Jones CD, et al. Synthesis and characterization of multiresponsive core-shell microgels. Macromolecules. 2000;33:8301–8306. [Google Scholar]
- 39.Li YY, et al. Novel stimuli-responsive micelle self-assembled from Y-shaped P(UA-Y-NIPAAm) copolymer for drug delivery. Biomacromolecules. 2006;7:2956–2960. doi: 10.1021/bm060080k. [DOI] [PubMed] [Google Scholar]
- 40.Akiyoshi K, et al. Controlled association of amphiphilic polymers in water: thermosensitive nanoparticles formed by self-assembly of hydrophobically modified pullulans and poly(N-isopropylacrylamides) Macromolecules. 2000;33:3244–3249. [Google Scholar]
- 41.Hu J, et al. Self-assembly of a polymer pair through poly(lactide) stereocomplexation. Nanotechnology. 2007;18:185607. [Google Scholar]
- 42.Siegel RA. Hydrophobic weak polyelectrolyte gels: studies of swelling equilibria and kinetics. Adv. Polym. Sci. 1993;109:233–267. [Google Scholar]
- 43.Das M, et al. Biofunctionalized pH-responsive microgels for cancer cell targeting: rational design. Adv. Mater. 2006;18:80–83. [Google Scholar]
- 44.Kihara N, et al. Reaction of methyl thioglycolate with chloromethylstyrene microgel: preparation of core-shell-type microgel by chemical modification. J. Appl. Polym. Sci. 1998;69:1863–1873. [Google Scholar]
- 45.Beng HT, et al. poly(L-lysine-g-ethylene glycol)/oligo(lactic acid)-b-pluronic F127-b-oligo(lactic acid) nanogels. Macromol. Rapid. Commun. 2006;27:522–528. [Google Scholar]
- 46.Sergey K, et al. Ion concentration of external solution as a characteristic of micro- and nanogel ionic reservoirs. J. Phys. Chem. B. 2006;110:15107–15116. doi: 10.1021/jp061044i. [DOI] [PubMed] [Google Scholar]
- 47.Dirk K, et al. Preparation of nanogels with temperature-responsive core and pH-responsive arms by photo-cross-linking. Langmuir. 2002;18:4263–4269. [Google Scholar]
- 48.Gupta AK, et al. Ketorolac entrapped in polymeric micelles: preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 2000;209:1–14. doi: 10.1016/s0378-5173(00)00508-1. [DOI] [PubMed] [Google Scholar]
- 49.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Namdeo M, et al. Magnetic nanoparticles for drug delivery applications. J. Nanosci. Nanotechnol. 2008;8:3247–3271. doi: 10.1166/jnn.2008.399. [DOI] [PubMed] [Google Scholar]
- 51.Yan L, et al. Thermosensitive core-shell particles as carriers for Ag nanoparticles: modulating the catalytic activity by a phase transition in networks. Angew. Chem. Int. Ed. 2006;45:813–816. doi: 10.1002/anie.200502731. [DOI] [PubMed] [Google Scholar]
- 52.Corot C, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Leslie L, et al. Magnetic nanoparticle probes. Mater. Today. 2005;8:32–38. [Google Scholar]
- 54.Akihiko K, Hideki F. Preparation of thermo-sensitive magnetic hydrogel microspheres and application to enzyme immobilization. J. Fermentation Bioeng. 1997;84:337–341. [Google Scholar]
- 55.Chauhan SC, et al. Mucins in ovarian cancer diagnosis and therapy. J. Ovarian Res. 2009;2:21. doi: 10.1186/1757-2215-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yallapu MM, et al. Scope of nanotechnology in ovarian cancer therapeutics. J. Ovarian Res. 2010;3:19. doi: 10.1186/1757-2215-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yallapu MM, et al. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J. Ovarian Res. 2010;3:11. doi: 10.1186/1757-2215-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yallapu, et al. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interf. Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan SC, et al. Combined staining of TAG-72, MUC1, and CA125 improves labeling sensitivity in ovarian cancer: antigens for multi-targeted antibody-guided therapy. J. Histochem. Cytochem. 2007;55:867–75. doi: 10.1369/jhc.7A7213.2007. [DOI] [PubMed] [Google Scholar]