Abstract
The melanocortin system is well recognized to be involved in the regulation of food intake, body weight, and energy homeostasis. To probe the role of the MC3 in the regulation of food intake, JRH322-18 a mixed MC3 partial agonist/antagonist and MC4 agonist tetrapeptide was examined in wild type (WT) and melanocortin 4 receptor (MC4) knockout mice and shown to reduce food intake in both models. In the wild type mice, 2.0 nmol of JRH322-18 statistically reduced food intake 4hrs post icv treatment into satiated nocturnally feeding wild type mice. The same dose in the MC4KO mice significantly reduced cumulative food intake 24h post treatment. Conditioned taste aversion as well as activity studies support that the decreased food intake was not due to visceral illness. Since these studies resulted in loss-of-function results, the SHU9119 and agouti-related protein (AGRP) melanocortin receptor antagonists were administered to wild type as well as the MC3 and MC4 knockout mice in anticipation of gain-of-function results. The SHU9119 ligand produced an increase in food intake in the wild type mice as anticipated, however no effect was observed in the MC3 and MC4 knockout mice as compared to the saline control. The AGRP ligand however, produced a significant increase in food intake in the wild type as well as the MC3 and MC4 knockout mice and it had a prolonged affect for several days. These data support the hypothesis that the MC3 plays a subtle role in the regulation of food intake, however the mechanism by which this is occurring remains to be determined.
Keywords: melanotropin, MC3R, MC4R, knockout mice, obesity, food intake, feeding, energy homeostasis, AGRP, SHU9119
1. Introduction
The melanocortin system has been implicated in the regulation of a diverse number of physiological processes including pigmentation, feeding behavior, cardiovascular, and sexual function. The melanocortin 1 receptor (MC1) is clearly linked to the melanocortin agonist effects on pigmentation and animal coat coloration (Mountjoy et al., 1992). The melanocortin 4 receptor (MC4) has been demonstrated to be involved in the regulation of food intake (Fan et al., 1997) and therefore has an impact on obesity (Huszar et al., 1997) in rodents and humans. The melanocortin 3 receptor (MC3) is involved in the regulation of energy and fat homeostasis (Butler et al., 2000; Chen et al., 2000) however, its mechanism of action remains to be identified. The double MC3/MC4 knockout mice are extremely obese and hyperphagic (Atalayer et al., 2010) and mounting evidence support the hypothesis regarding a synergistic role between the MC3 and MC4 in the regulation of energy homeostasis and perhaps food intake.
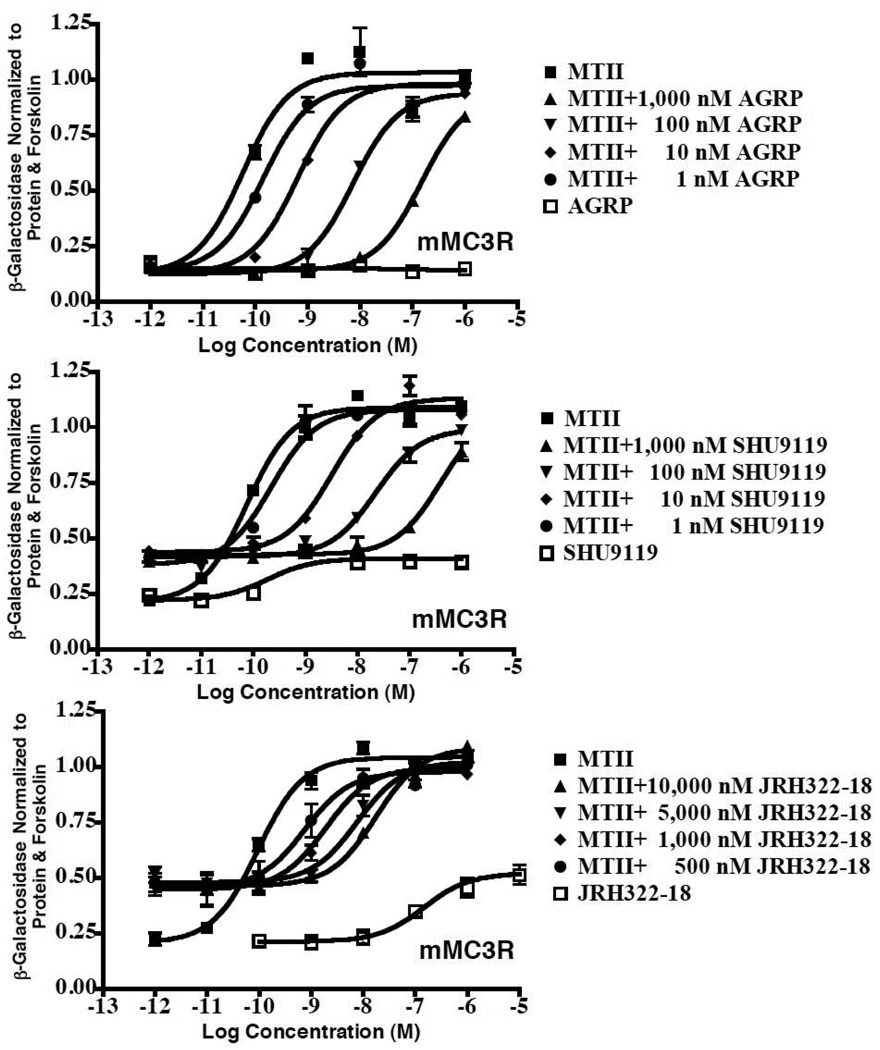
The study reported herein was designed to probe the role of the MC3 in the regulation of food intake and determine if a melanocortin ligand possessing mixed MC3/MC4 agonist/antagonist pharmacology might result in a more desirable anti-obesity therapeutic agent versus a highly selective MC4 agonist that results in increased blood pressure (Greenfield et al., 2009) and erectile function (Wessells et al., 1998). Table 1 summarizes the melanocortin 3 and 4 receptor pharmacology of the peptides examined in this study as well as the reference compounds α-MSH and NDP-MSH (Haskell-Luevano et al., 2001; Haskell-Luevano et al., 2000; Holder et al., 2002; Proneth et al., 2008; Wilczynski et al., 2004a; Wilczynski et al., 2004b). Fig. 1 illustrates the functional antagonist pharmacology of agouti-related protein (AGRP), SHU9119, and JRH322-18 at the mouse MC3R. The AGRP antagonist does not possess any partial agonist activity at the MC3R receptor whereas both SHU9119 and JRH322-12 possess partial agonist as well as antagonist pharmacological profiles.
Table 1.
Summary of in vitro mouse melanocortin receptor pharmacology.
| Name | Structure | Functional Activity (nM) mMC3R |
Binding Affinity (nM) mMC3R |
Functional Activity (nM) mMC4R |
Binding Affinity (nM) mMC4R |
|---|---|---|---|---|---|
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | Agonist 0.80±0.14 |
ND | Agonist 5.40±0.62 |
ND |
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | Agonist 0.098±0.013 |
0.52±0.10 | Agonist 0.21±0.03 |
0.91±0.85 |
| MTII | Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 | Agonist 0.16±0.03 |
1.85±0.35 | Agonist 0.087±0.008 |
0.50±0.13 |
| SHU9119 | Ac-Nle-c[Asp-His-DNal(2’)-Arg-Trp-Lys]-NH2 | Antagonist* 0.32 |
3.2±1.1 | Antagonist 0.040 |
0.38±0.10 |
| JRH887-9 | Ac-His-DPhe-Arg-Trp-NH2 | Agonist 160±9.2 |
ND | Agonist 17±2.8 |
ND |
| JRH322-18 | Ac-His-(pI)DPhe-Arg-Trp-NH2 | Antagonist* 50±1.1 |
270±146 | Agonist 25±9.8 |
4.81±4.5 |
| AGRP(87-132) | Antagonist 1.4 |
2.8±0.4 | Antagonist 0.41 |
3.0±0.3 |
ND indicates values not reported.
Indicates that the antagonists also possess partial agonist activity at the mMC3R. The indicated error represents the standard error of the mean.
Figure 1.
In vitro mouse MC3 receptor pharmacology of the AGRP, SHU9119, and JRH322-18 peptides examined in this study.
2. Materials and Methods
2.1 Peptides
The compounds utilized in this study were purchased from commercial sources MTII and SHU9119 (Bachem, Torrance CA), AGRP(86-132) (Peptides International, Louisville KY) or synthesized as previously reported JRH887-9 and JRH322-18 (Holder et al., 2002). Each compounded was tested at the multiple doses as indicated. Behavior was monitored continuously for up to 6h post treatment for adverse toxic or visceral illness. Such behaviors were only observed for the JRH compounds at the 5 nmol doses, and these data have been excluded, and resulted in a lack of food intake, normal activity and/or death during the duration of data collection.
2.2 Animals
All studies performed were conducted in accord with accepted standards of humane animal care and were approved by the Institutional Animal Care and Use Committee at the University of Florida and at the University of Cincinnati. The MC4 knockout mice on a mixed Bl6/129 background were generously provided by Dr. Dennis Huszar at Millennium Pharmaceuticals (Huszar et al., 1997). The MC3 knockout mice on a mixed Bl6/129 background were provided by Lex Van Der Ploeg at Merck (Chen et al., 2000). A heterozygous breeding strategy was utilized to generate age/litter mate matched mice. Mice were housed in individual cages in a temperature-controlled room (23–25°C) and maintained on a 12 h light/dark cycle (lights off at 1800 h). Animals were given free access to normal chow and water at all times.
2.3 Study design
This study utilized a Latin Square non-fasted feeding paradigm where mice were allowed free access to food at all times. Compound or saline control (1µL in saline) was injected ca 2h before lights out and food intake (standard chow [Harlan Teklad 8604 diet (24% crude protein, 4% crude fat, and 4.5% maximum for crude fiber with a digestible energy of 3.30 kcal/g)]) was measured at X time points. The bedding was inspected for large intact pellets >0.1 g to decrease the error associated with determining food intake. No significant differences between the wild type littermate mice from either the MC4RKO or MC3RKO colonies were observed so the data was combined.
2.4 Cannula surgery and placement validation
Mice were anesthetized with pentobarbital (60 mg/kg) and placed in a stereotaxic apparatus (Cartesian Instruments, Bend, OR). The fur was cut, a midline incision was made, and the exposed area sterilized. A 25-gauge cannula (Plastics One, Roanoke, VA) was inserted into the lateral cerebral ventricle, coordinates: 1.0 mm lateral and 0.46 mm posterior to bregma and 2.3 mm ventral to the surface of the skull (Franklin and Paxinos, 1997). The cannula was fixed to the skull using a liquid adhesive (Loctite 454, Plastics One, Roanoke, VA) applied to the pedestal of the cannula. After the adhesive dried, dental cement was applied to the exposed skull area and allowed to dry. Subsequently, the skin was sutured and the mice allowed to recover for at least seven days post surgery. Cannula placement was verified by administration of 2 µg human PYY(3-36) (Bachem, Torrance, CA.) as described (Marsh et al., 1999a). For the mice examined in this study, the average grams of cumulative food intake 4 h post treatment for the WT (saline=0.73±0.06, PYY(3-36)=1.35±0.08, p<0.001), MC4KO (saline=0.46±0.11, PYY(3-36)=1.41±0.13, p<0.001), and MC3KO (saline=0.76±0.09, PYY(3-36)=1.60±0.06, p<0.001) mice were significantly different. Animals not consuming at least 1 g of food 4 h post injection of PYY(3-36) were excluded from the study.
2.5 c-Fos immunohistochemistry
To study the acute effect of JRH compounds on c-Fos like immunoreactivity in the brain, 3.0 nmol JRH887-9 (n=3), 3.0 nmol JRH322-18 (n=3), and 0.9% NaCl (n=3) was infused into cannulated mice (used previously in feeding studies). Two hours after the infusions, mice were transcardially perfused with 0.9% Phosphate Buffered Saline (PBS) followed by formalin-picric acid mixture (4% paraformaldehyde and 0.4% picric acid in 0.16 M phosphate buffer, pH=6.9, 37°C). Brains were rapidly removed and fixed for 16 h and subsequently rinsed in 0.1 M phosphate buffer. Forty-micrometer forebrain slices were cut in the coronal plane on a vibratome to allow visualization of various hypothalamic nuclei including the PVN, DMH, VMH and ARC. Sections were then rinsed (3X, PBS), incubated for 30 minutes in 0.3% H202, rinsed (3X, PBS), and incubated 1h in 0.3% Triton X-100 and 1% normal goat serum in PBS. Sections were then transferred directly to the primary antibody solution consisting 0.005 g/ml polyclonal rabbit antiserum (Santa Cruz Biotechnology, Santa Cruz, CA), which recognizes residues 3–16 of the c-Fos protein. After 36 h of incubation at 4°C, slices were rinsed (3X, PBS). Slices were then transferred to biotinylated goat anti-rabbit antibody for 1 h, rinsed (3X, PBS), and developed with diaminobenzidine substrate (5 minutes). Slices were rinsed (3X, PBS) mounted on gelatin coated slides and coverslipped with D.P.X mounting medium (Ft. Washington, PA). The number of c-Fos positive nuclei was quantified in specific hypothalamic nuclei using a Leica DC500 imaging system and the SigmaScan Pro 5.0 Image analysis application (SPSS Science, Chicago, IL). Sections examined for c-Fos-like immunoreactivity (c-FLI) included PVN, DMH, VMH and ARC.
2.6 Conditioned taste aversion
Mice (n = 8) were first habituated to 2h daily access to water. During this time, two bottles, each containing unflavored water, were placed on each home cage. After 12 days, all mice received two bottles containing 0.1% saccharin instead of water. Immediately following the 2-h exposure, mice received i.P. saline, LiCl (a volume equivalent to 2% of the mouse body weight of a 0.15M isotonic solution) or drug. On the following day, mice again received 2h access to two bottles with unflavored tap water. On the final day, a two-bottle choice test was administered in which all mice were allowed access to tap water and the saccharin solution in separate bottles. The relative position of the two solutions was counterbalanced across subjects. The ratio of saccharine intake to total liquid intake during test was calculated and used as the index of aversion.
2.7 Locomotion and other behavioral assays
To assess locomotor activity and gross behavioral changes, the CleverSys Topscan and Sidescan programs (CleverSystem, Inc., Reston, VA) were used. Gross behavioral activity was scored by the software, which provides a detailed matrix of behaviors over time, including velocity, distance traveled, sniffing, location, eating and ambulation. Drugs that elicit visceral illness are known also to reduce overall activity (e.g., belly-lying). We can record and analyze this activity at a resolution of approximately 10 seconds. Mice (n = 8) were injected with LiCl, saline, or drug under test and the overall index of activity used was an indicator of illness-like effects. Mice were individually placed in specialized cages equipped with a stainless steel running wheel (Lafayette Instrument Company, Lafayette, IN). All animals had free access to the running wheel which was monitored with infrared beams connected to a computer which transduced revolutions to distance traveled and velocity. Distance was measured in meters (cumulative) over time across 48 h. All animals had ad lib access to food and water while housed in running wheel cages.
2.8 Statistics
Data is represented as the average of the mean ± S.E.M.. For statistical analyses, compound concentration over time were performed using two-way ANOVA followed by Bonferroni post test. Statistical significance is considered if p<0.05.
3. Results
3.1 Experiment 1: validation of MTII decreased food intake in satiated wild type mice
To validate our experimental paradigm (non-fasted and lateral ventricle administration compound) and reproduce previous findings (Fan et al., 1997; Marsh et al., 1999a) we tested the MTII melanocortin agonist in wild type mice. These data are consistent with previous reports and are summarized in Fig. 2.
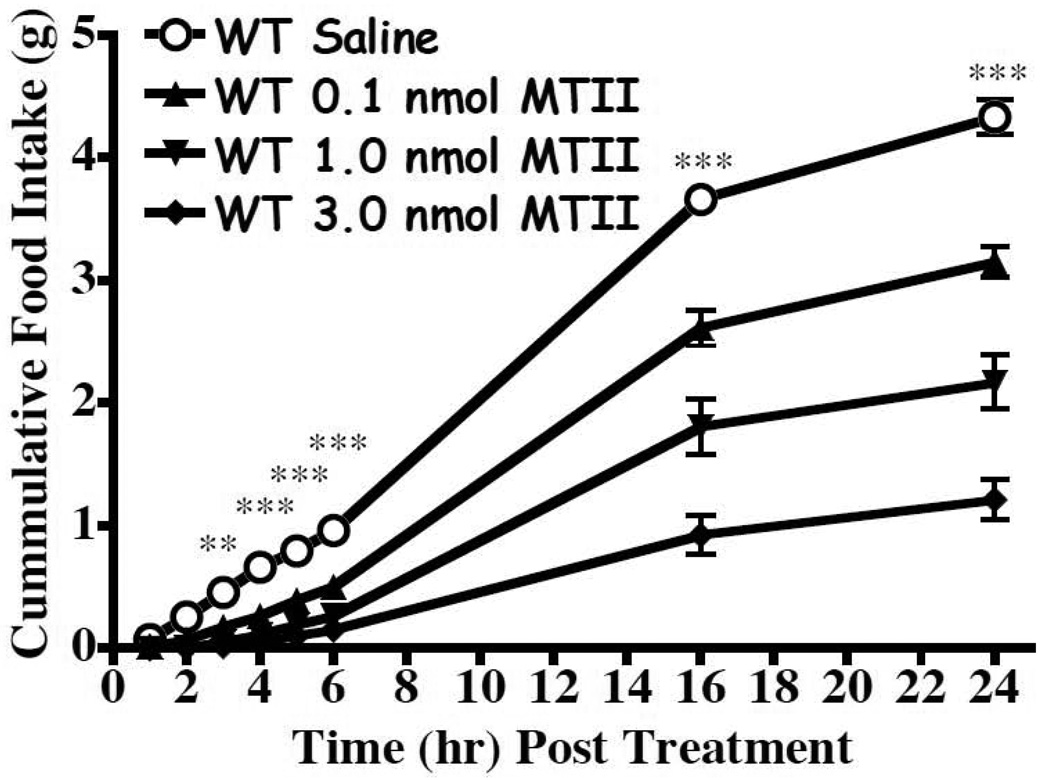
Figure 2.
Effect of MTII on food intake (mean ± S.E.M.) in wild type mice after lateral ventricular administration using a non-fasted paradigm (p<0.0001 n=17/group). The 0.1, 1.0, and 3.0 nmol doses statistically decreased food intake relative to saline starting at t=4h, t=3h, and t=3h, respectively (**p<0.01).
3.2 Experiment 2: effect in wild type and MC4 mice of a mixed MC3 antagonist and MC4R agonist tetrapeptide JRH322-18 and the control MC3R and MC4R tetrapeptide agonist JRH887-9
It is well established that the MC4 is directly involved in the regulation of food intake. However, little is understood about the involvement of the MC3 in food intake or the effects of a mixed MC3 antagonist and MC4 agonist in wild type mice. The JRH887-9 control peptide was included as a control peptide due to similar pharmacological effects on food intake due to the structural similarity. The MC4KO mice were included since it was previously postulated that the melanocortin based feeding effects were only due to the MC4 and not the MC3 (Marsh et al., 1999a), even though the MC3KO mice have been reported to be hypophagic on normal chow (Chen et al., 2000). Thus, for this study, we postulated that we would be able to associate differences in the JRH322-18 mixed receptor pharmacology to that of the MC3. In the wild type littermate mice, as anticipated based upon the in vitro MC4 agonist pharmacology, JRH887-9 decreased food intake 2h post treatment (p<0.05) at the 2.0 nmol dose (Fig. 3). In the MC4KO littermate mice, the 2.0 nmol dose significantly decreased food intake (p<0.001) 16h post treatment. Treatment with the mixed MC3 antagonist/ MC4 agonist JRH322-18 resulted in significantly decreased food intake in the wild type mice at 4 h post treatment (p<0.05) and in the MC4KO mice, 24h post treatment (p<0.05).
Figure 3.
Effect of the JRH tetrapeptides on food intake (mean ± S.E.M.) in the wild type and MC4KO littermate mice (n=10–12 per group wild type and n=6 per group for the MC4KO mice). These compounds significantly decreased food intake at the wild type and MC4KO mice (p<0.0001). The tetrapeptide JRH887-9 in the wild type mice, the 0.1, 0.5, and 2.0 nmol doses statistically decreased food intake relative to saline starting at t=5h, t=4h, and t=3h, respectively (*p<0.05). In the MC4KO mice, the 0.5, and 2.0 nmol doses statistically decreased food intake relative to saline starting at t=16 h (***p<0.001). The tetrapeptide JRH322-18 in the wild type mice, the 0.1, 0.5, and 2.0 nmol doses statistically decreased food intake relative to saline starting at t=16h, t=5h, and t=4h, respectively (p<0.05). In the MC4KO mice, the 0.5 and 2.0 nmol doses statistically decreased food intake relative to saline starting at t=24h (p<0.05).
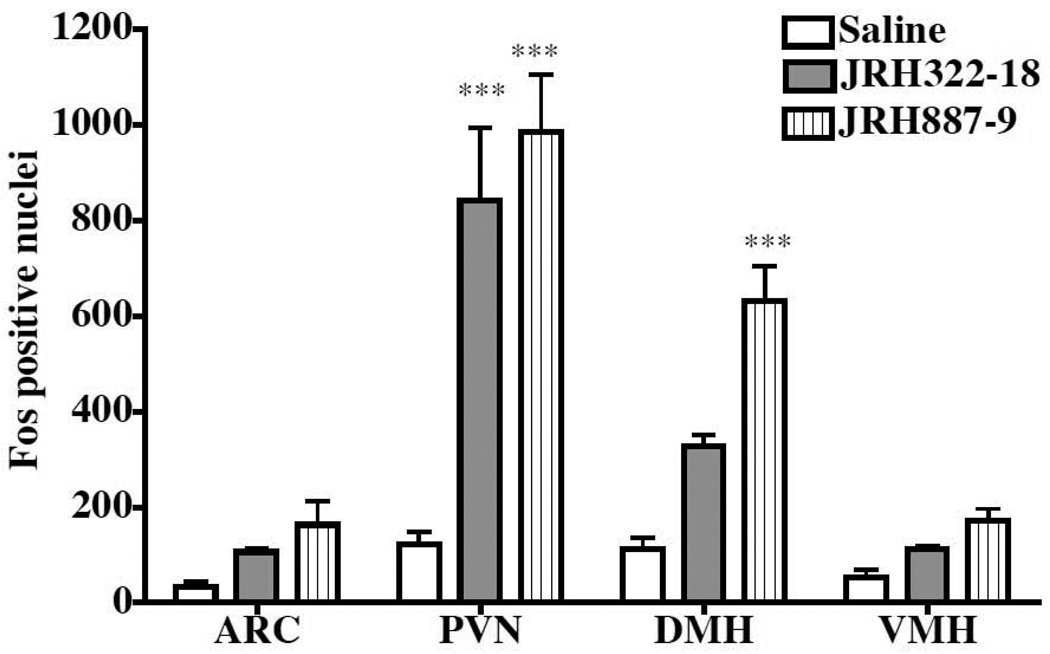
The endogenous POMC melanocortin agonist mRNA is synthesized in the arcuate nucleus (ARC) of the hypothalamus and peptide expressing projections innervate the hypothalamic and brain regions that express the MC3 and MC4 receptors (Low et al., 1994). Previous studies using α-MSH and c-Fos-like immunoreactivity 2h post icv administration identified statistically significant differences in the PVN but not the ARC (McMinn et al., 2000). To determine if the JRH compounds could induce c-Fos-like immunoreactivity in similar or different hypothalamic nuclei, we compared c-Fos-like immunoreactivity following X injection and determined immunoreactivity in the following hypothalamic regions: ARC, PVN, DMH, and VMH regions (Fig. 4). Consistent with previous reports, the JRH compounds did not induce significant c-Fos-like immunoreactivity in the ARC (McMinn et al., 2000). We also did not see a significant effect in the VMH. Significant differences in c-Fos-like immunoreactivity were observed for the JRH compounds as compared to saline in the PVN and DMH (p<0.0001) when compared to saline control injections. Interestingly however, in the DMH, JRH322-18 (MC3 partial agonist and antagonist) did not induce a significant c-Fos-like immunoreactivity response.
Figure 4.
Effect of JRH treatment on hypothalamic c-Fos-like immunoreactivity in wild type mice 2h after 3.0 nmol compound administration (n=3–4 animals per group). Statistical significance is observed for the JRH compounds in the PVN, but only for JRH887-9 in the DMH (***p<0.0001). The JRH322-18 compound is an MC3 antagonist as well as a MC4 agonist.
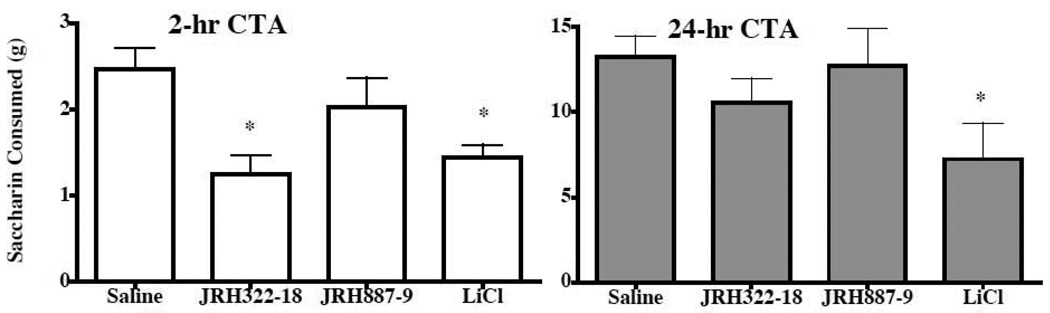
Reductions in food intake can be due to visceral illness induced by the drug treatment. To test for visceral illness, we performed a conditioned taste aversion experiment. Importantly, the MTII agonist has previously been demonstrated to cause a conditioned taste aversion (Benoit et al., 2003; Thiele et al., 1998). Two hour post treatment, JRH322-18 compound had a modest conditioned taste aversion response that was not evident at the 24-h time point (Fig. 5). It is unknown why this compound resulted in the 2h conditioned taste aversion response and it is unclear the affect that this may have on the decreased food intake response observed at the indicated time points. To access visceral illness-like effects and gross behavioral changes mice treated with JRH322-18 and saline were monitored using a locomoter assay (Fig. 6). No statistically notable changes in activity upon compound treatment (1.0 nmol) were observed. However the JRH322-18 treated group demonstrated a slightly increased running wheel activity at the 36 to 48 h post injection time points.
Figure 5.
Effect of JRH treatment (1.0 nmol) in wild type mice in conditioned taste aversion studies (n=4–5 mice per group). At the 2h time point, a difference between saline and JRH322-18 (*p<0.05) treatment in the amount of saccharin consumed is observed. However, it is not observed at the 24hr time point. LiCl is the lithium chloride control.
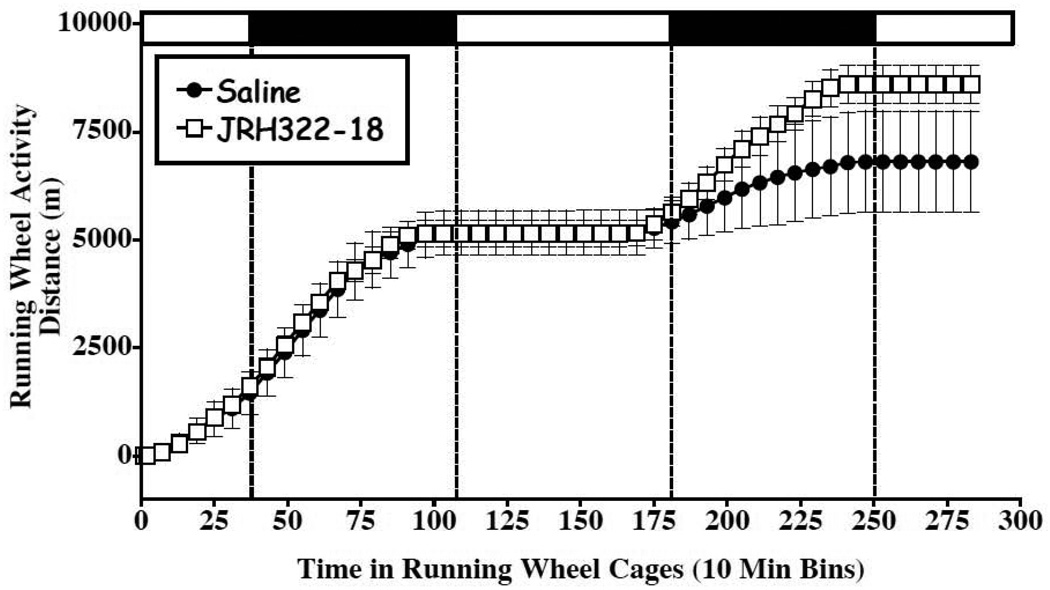
Figure 6.
Locomoter assay to assess visceral illness-like effects and gross behavioral changes of JRH322-18 relative to saline treatment in wild type mice. Mean ± S.E.M. distance traveled (in meters) during 48h in running wheel cages for mice injected into the 3rd ventricle with 1µl of either saline (filled circles, n=5) or JRH322-18 (open box, n=4). X-axis is time spent in running wheel cages in hours, Y-axis is distance traveled in meters. Dashed vertical lines show transition points during light-dark cycle, horizontal bars at top denote light phase (open bar) or dark phase (filled bar). No statistically significant differences resulted and the JRH322-18 treated group demonstrated a slightly increased running wheel activity at the 36 to 48 h post injection time points.
3.3 Experiment 3: effect of AGRP(86-132) and SHU9119 MC3 and MC4 antagonists in wild type littermate and the MC3KO and MC4KO mice
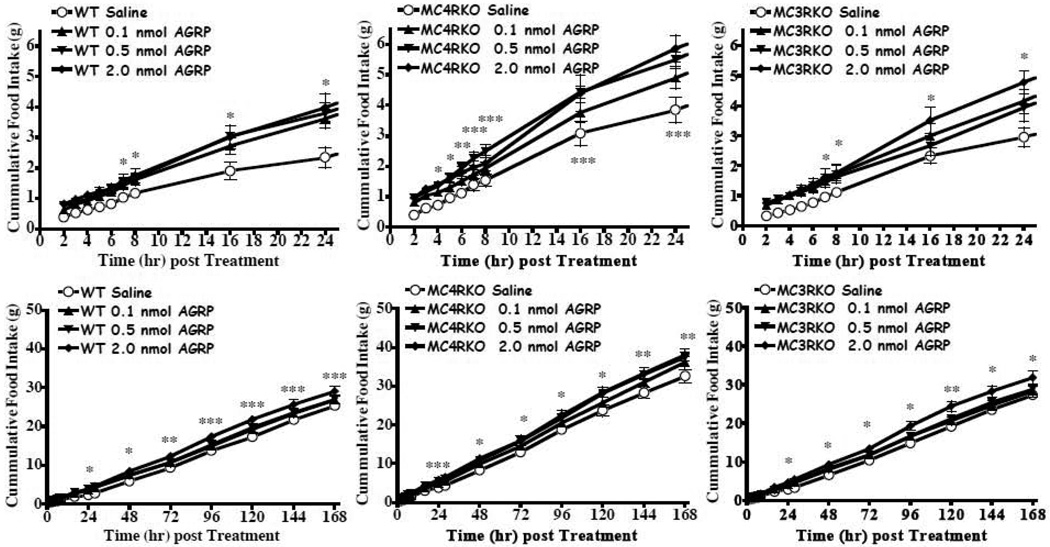
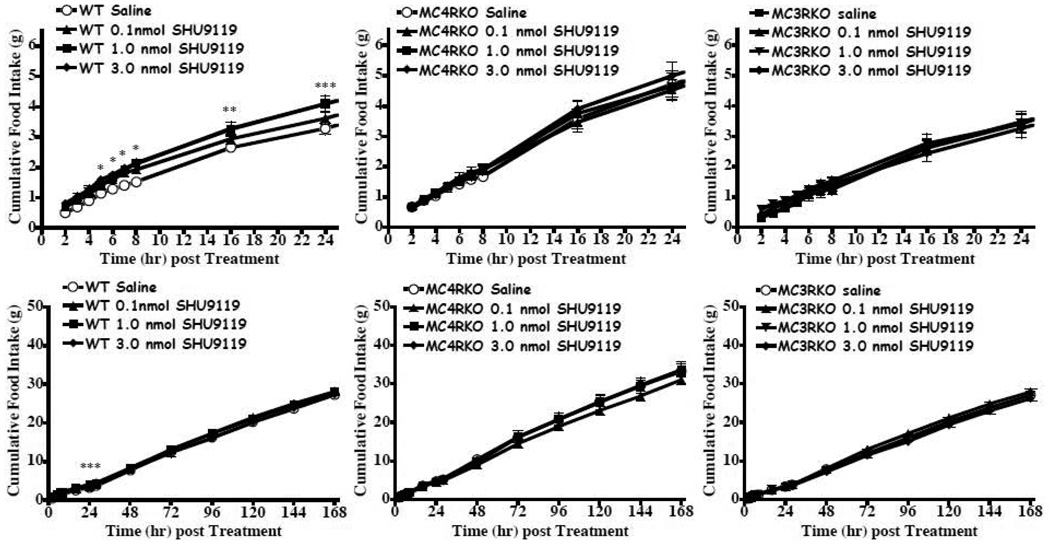
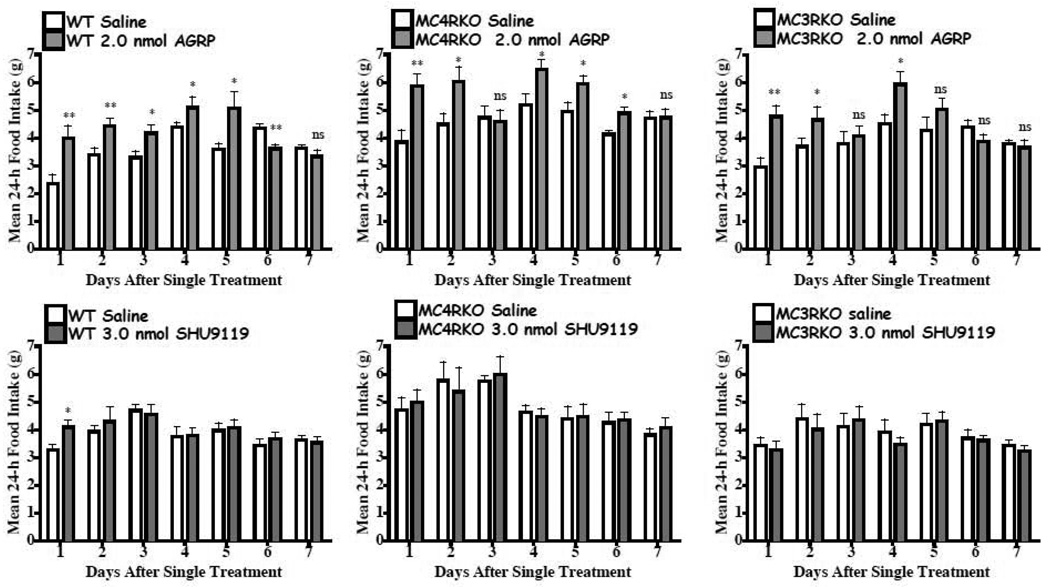
The results of the JRH compounds in the MC4KO mice were unanticipated. A variety of factors could result in decreased food intake, although the conditioned taste aversion and sickness behavior do not appear to be the cause of the reduction in food intake for the JRH compounds in Experiment 2. It has been previously demonstrated that the endogenous AGRP antagonist stimulates food intake over a prolonged (days) period (Hagan et al., 2000). SHU9119 has also been characterized to increase food intake (Fan et al., 1997), although it’s duration has not been previously reported. The goal of this experiment was to reproduce the AGRP increased food intake using our experimental paradigm and characterize the feeding pattern in the melanocortin receptor knock out mice. We hypothesized, based upon previous studies, that in the wild type and MC3KO mice that have intact MC4 receptors would increase their food intake following central AGRP administration. In the MC4KO mice, with intact MC3s we also anticipated an increased food intake response to AGRP based upon previous results by Marsh et al. (Marsh et al., 1999a; Marsh et al., 1999b). For this experiment, we examined the hourly cumulative food intake each between 2 to 8 h post compound administration and daily for up to 7 days. Increased food intake and a prolonged cumulative food intake effect of a single treatment of 2.0 nmol AGRP(86-132) resulted in the wild type, MC3KO and MC4KO mice (Fig. 7). However, for the SHU9119 antagonist, we only observed significantly increased cumulative food intake in the wild type mouse and only for up to 24h post treatment at up to 3.0 nmol concentrations (Fig. 8). Comparison of the average 24h daily intake for mice treated with 2.0 nmol AGRP(87-132) or 3.0 nmol SHU9119 are shown in Fig. 9. Consistent with the previous findings reported by Hagen et al. in the rat (Hagan et al., 2000), AGRP produced a sustained increased food intake for up to six of the seven days measured in the wild type mice. In the MC3KO mice, which has intact MC4s, an increase in food intake was observed for days 1, 2, and 4 post treatment. Interestingly, the MC4KO mice, with intact MC3s, showed prolonged increased food intake, similar to the wild type mice. SHU9119 treatment in the wild type mice only showed increased food intake one day post treatment. No significant prolonged effect for 3.0 nmol SHU9119 treatment was observed in the wild type, MC4KO, or MC3KO mice.
Figure 7.
Effect of AGRP(86-132) on food intake (mean ± S.E.M.) in satiated wild type, MC4KO, and MC3KO littermate mice. For all the mice, statistically increased food intake was observed relative to the saline control (p<0.0001, n=8–9 per group). A) In the wild type mice the 2.0 nmol dose statistically increased food intake relative to saline starting at t=7h and continued to remain statistically significant for the duration of the experiment (*p<0.05). B) In the MC4KO mice, the 0.5 and 2.0 nmol doses statistically increased food intake relative to saline starting at t=5h and t= 4h, respectively, and continued to remain statistically significant for the duration of the experiment (*p<0.05). C) B) In the MC3KO mice, the 2.0 nmol dose statistically increased food intake relative to saline starting at t=7h and continued to remain statistically significant for the duration of the experiment (*p<0.05). *p<0.05, ** p<0.01, ***p<0.001
Figure 8.
Effect of SHU9119 on food intake (mean ± S.E.M.) in satiated wild type, MC4KO, and MC3KO littermate mice (n=10–12 per group). A) In the wild type mice the 1.0 and 3.0 nmol dose statistically increased food intake relative to saline starting at t=7h and t= 5h, respectively, and continued to remain statistically significant up to the 24h post injection time point (*p<0.05). B) In the MC4KO and MC3KO mice, no statistically significant changes in food intake relative to saline was observed.
Figure 9.
Shows the average daily food intake (mean ± S.E.M.) per 24h after a single injection of either AGRP(86-132) or SHU9119 (same groups as in Figures 7 and 8). In the wild type littermate mice, AGRP(86-132) demonstrated significantly increased average daily food intake versus saline for up to the sixth day of the experiment. *p<0.05, **p<0.01, ns=not statistically different.
4. Discussion
The MC4 has been clearly demonstrated to regulate the control of food intake upon icv administration of agonists (decrease food intake) and antagonists (increase food intake) (Fan et al., 1997). Marsh et al. treated the MC4KO mouse with increasing doses of the AGRP antagonist and examined food intake 24 h post treatment and identified that AGRP did increase food intake in MC4KO mice (Marsh et al., 1999b). Chen et al. reported that MC3KO mice possessed a hypophagic response in a gene-dosage effect compared to both the heterozygous and wild type control mice (Chen et al., 2000). Lee et al. reported that 3 day infusion of D-Trp8-γ2-MSH (Grieco et al., 2000), a ca 60-fold mouse MC3 versus MC4 selective agonist, stimulates food intake in rats (Lee et al., 2008). Marks et al. (Marks et al., 2006) reported that i.P. administration of D-Trp8-γ2-MSH at different concentrations and daily versus nocturnal feeding paradigms resulted in both increased and decreased food intake in wild type mice. They also reported that 50 mcg i.p. at 4h post treatment resulted in decreased nocturnal food intake in wild type mice yet the MC4KO mice increased food consumption upon treatment. While previous reports in the literature suggest that MC3 D-Trp8-γ2-MSH based agonists result in increased food intake under certain feeding experimental paradigms (Lee et al., 2008; Marks et al., 2006), this data is at odds with the highly potent non-selective melanocortin receptor agonist MTII which decreases food intake (Fan et al., 1997) in a variety of feeding paradigms as well as in both rats and mice. Clearly the use of purely selective MC3 agonists or antagonists (i.e. devoid of any MC4R and MC5R functional activity) would be a valuable tool to discern the role of the MC3 in the regulation of food intake, however non exist to date. The γ-MSH related analogues are all full agonists at the MC3 and MC4 receptors (albeit with differing degrees of receptor selectivity profiles), the SHU9119 is a MC3 partial agonist and competitive antagonist (Fig. 1) as well as a potent MC4 antagonist, and AGRP is a potent MC3 and MC4 competitive antagonist as well as an inverse agonist at the MC4 (Haskell-Luevano and Monck, 2001; Nijenhuis et al., 2001) with its potential as an MC3 inverse agonist remaining to be documented pharmacologically.
In this study we utilize for the first time, a mixed MC3 partial agonist/antagonist (Fig. 1) and MC4 agonist (JRH322-18) in attempts to further probe the role of the MC3 in the regulation of food intake in combination with the MC4 knockout mice that only have intact MC3s. As anticipated due to is potency and full agonist pharmacology at the MC4, JRH322-18 resulted in a dose-response decrease in food intake in satiated wild type mice starting at 4 h post icv treatment. In the MC4KO mice, at 24h post treatment, a significant (p<0.05) decrease in food intake was observed at the 2.0 nmol concentration (Fig. 3). Additionally the control tetrapeptide JRH887-9 (MC3 and MC4 full agonist) resulted in decreased food intake in wild type mice, and unexpectantly also in the MC4KO mice, albeit at 16 h post 2.0 nmol icv treatment. Interestingly, upon treatment in wild type mice of the JRH compounds possessing different in vitro MC3 and MC3 receptor pharmacological profiles (Table 1), c-Fos- immunoreactivity in the DMH was different (Fig. 4) suggesting that some hypothalamic regions may be stimulated differently, depending upon the ligand pharmacological profile, and thus have variant physiological effects. These data preliminarily support such a hypothesis and further experimental data would need to be generated to either support or disprove this concept.
As the effect of food intake of the JRH compounds in the MC4 knockout mice were unanticipated based upon the hypothesis that only the MC4 was involved in the regulation of food intake (Marsh et al., 1999a), we treated the same experimental mice with a gain of function experiment by treatment with the SHU9119 and AGRP ligands demonstrated previously to increase food intake in wild type rodents (Fan et al., 1997; Hagan et al., 2000). While SHU9119 increased food intake in the wild type mice starting at 5 h post treatment that lasted for only a 24 h duration, in both the MC3KO and MC4KO mice, did not differ in food intake between saline and up to 3.0 nmol SHU9119 concentrations were observed. AGRP however, produced a robust food intake response in the wild type, MC4KO, and MC3KO mice that lasted for several days following a single treatment. These data are consistent with the previous report by Marsh et al (Marsh et al., 1999b) showing an increased food intake response in the MC4KO mice as well as that by Hagen et al reporting a prolonged duration of action (Hagan et al., 2001) in response to AGRP treatment. However the newly provided data demonstrate a prolonged response of AGRP in both the MC3 and MC4 knockout mice could support the hypothesis that a synergistic as well as independent actions of AGRP on the melanocortin receptors increase food intake. Interestingly, using a rat based activity-based anorexia experimental paradigm, Hillebrand et al reported that AGRP, but not SHU9119, increased feeding and body temperature (Hillebrand et al., 2006) which also support this hypothesis using a different rodent model and experimental paradigm.
The MC3 has been postulated to serve as an auto-regulatory factor in a feedback mechanism involving the melanocortin agonists within the arcuate nucleus of the hypothalamus (Cowley et al., 1999). However the role of the MC3 in other expression sites within the brain and periphery remain to be determined. The use of MC3 selective ligands and the melanocortin receptor knockout mice are tools that can be used to study the mechanistic and physiological consequences of different receptor expression profiles in distinct places and tissues.
Conclusions
There is mounting experimental evidence supporting the hypothesis that the MC3 is involved in the regulation of food intake, albeit to a lesser extent than the MC4. However, since the field is lacking specific MC3 agonists and antagonists, the use of the mixed pharmacology ligands and melanocortin knockout mice has been a key experimental paradigm to probe this hypothesis. To date however, the differences in ligand receptor pharmacology (i.e. MC3 partial agonist/antagonist) and selectivity profiles have only resulted in confounding results that do not allow for a clear mechanistic model to be put forward. Nonetheless, the data presented herein examine the effect on food intake for the first time on a mixed MC3 partial agonist/antagonist and MC4 full agonist in the wild type and MC4 knockout mice. Further experiments probing the role of the SHU9119 and AGRP antagonists in wild type, MC3, and MC4 knockout mice demonstrate physiological differences in food intake responses using ligands with differing MC3 and MC3 in vitro receptor pharmacological profiles.
Acknowledgments
This work was supported by NIH Grants DK57080 and DK064250 (CHL). Boman Irani is a recipient of an American Heart Association Predoctoral Fellowship. The authors would like to thank Mr. Gabriel Gaidosh for expert guidance and advice on the c-Fos assay and Dr. Glenn Walter for the use of the Leica DC500 imaging system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atalayer D, Robertson KL, Haskell-Luevano C, Andreasen A, Rowland NE. Food demand and meal size in mice with single or combined disruption of melanocortin type 3 and 4 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1667–R1674. doi: 10.1152/ajpregu.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Sheldon RJ, Air EL, Messerschmidt P, Wilmer KA, Hodge KM, Jones MB, Eckstein DM, McOsker CC, Woods SC, Seeley RJ. Assessment of the aversive consequences of acute and chronic administration of the melanocortin agonist, mtii. Int. J. Obes. Relat. Metab. Disord. 2003;27:550–556. doi: 10.1038/sj.ijo.0802280. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van Der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of npy, agrp, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-amino acid scan of γ-melanocyte-stimulating hormone: Importance of trp(8) on human mc3 receptor selectivity. J. Med. Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Benoit SC, Rushing PA, Pritchard LM, Woods SC, Seeley RJ. Immediate and prolonged patterns of agouti-related peptide-(83--132)-induced c-fos activation in hypothalamic and extrahypothalamic sites. Endocrinology. 2001;142:1050–1056. doi: 10.1210/endo.142.3.8018. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of agrp-(83-132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Cone RD, Monck EK, Wan Y-P. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: Identification of agouti-related protein (agrp), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck EK. Agouti-related protein (agrp) functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regulatory Peptides. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck EK, Wan YP, Schentrup AM. The agouti-related protein decapeptide (yc[crffnafc]y) possesses agonist activity at the murine melanocortin-1 receptor. Peptides. 2000;21:683–689. doi: 10.1016/s0196-9781(00)00194-7. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, Kas MJ, Scheurink AJ, van Dijk G, Adan RA. Agrp(83-132) and SHU9119 differently affect activity-based anorexia. Eur. Neuropsychopharmacol. 2006;16:403–412. doi: 10.1016/j.euroneuro.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors: Part 2 modifications at the Phe position. J. Med. Chem. 2002;45:3073–3081. doi: 10.1021/jm010524p. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Kesterson RA, Boston BA, Fang Q, Berkemeir LR, Gu W, Cone RD, Campfield LA, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim A, Conwell IM, Hruby V, Mayorov A, Cai M, Wardlaw SL. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic pomc expression. Peptides. 2008;29:440–447. doi: 10.1016/j.peptides.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low MJ, Simerly RB, Cone RD. Receptors for the melanocortin peptides in the central nervous system. Curr. Opin. Endocrinol. Diabetes. 1994;1:79–88. [Google Scholar]
- Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999a;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999b;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular α-msh on food intake, adiposity, c-fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA. AGRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol. Endocrinol. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- Proneth B, Pogozheva ID, Portillo FP, Mosberg HI, Haskell-Luevano C. Melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 modified at the para position of the benzyl side chain (DPhe): Importance for mouse melanocortin-3 receptor agonist versus antagonist activity. J. Med. Chem. 2008;51:5585–5593. doi: 10.1021/jm800291b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: Assessment of c- fos expression and taste aversion. Am J Physiol. 1998;274:R248–R254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: Double-blind, placebo controlled crossover study. J. Urol. 1998;160:389–393. [PubMed] [Google Scholar]
- Wilczynski A, Wang XS, Joseph CG, Xiang Z, Bauzo RM, Scott JW, Sorensen NB, Shaw AM, Millard WJ, Richards NG, Haskell-Luevano C. Identification of putative agouti-related protein(87-132)-melanocortin-4 receptor interactions by homology molecular modeling and validation using chimeric peptide ligands. J. Med. Chem. 2004a;47:2194–2207. doi: 10.1021/jm0303608. [DOI] [PubMed] [Google Scholar]
- Wilczynski AM, Wang XS, Bauzo RM, Xiang Z, Shaw AM, Millard WJ, Richards NGJ, Edison AS, Haskell-Luevano C. Structural characterization of a potent (Cys101-Cys119, Cys110-Cys117) bicyclic agouti-related protein (AGRP) melanocortin receptor antagonist. J. Med. Chem. 2004b;47:5662–5673. doi: 10.1021/jm049620r. [DOI] [PubMed] [Google Scholar]