Abstract
Because miR-146a is linked to osteoarthritis (OA) and cartilage degeneration is associated with pain, we have characterized the functional role of miR-146a in the regulation of human articular cartilage homeostasis and pain-related factors. Expression of miRNA 146a was analyzed in human articular cartilage and synovium, as well as in dorsal root ganglia (DRG) and spinal cord from a rat model for OA related pain assessment. The functional effects of miR-146a on human chondrocytic, synovialm and microglia cells were studied in cells transfected with miR-146a. Using real-time PCR, we assessed the expression of chondrocyte metabolism related genes in chondrocytes, genes for inflammatory factors in synovial cells, as well as pain-related proteins and ion channels in microglial cells. Previous studies showed that miR-146a is significantly upregulated in human peripheral knee OA joint tissues. Transfection of synthetic miR-146a significantly suppresses extracellular matrix associated proteins (e.g., Aggrecan, MMP-13, ADAMTS-5, collagen II) in human knee joint chondrocytes, and regulates inflammatory cytokines in synovial cells from human knee joints. In contrast, miR-146a is expressed at reduced levels in DRGs and dorsal horn of the spinal cords isolated from rats experiencing OA-induced pain. Exogenous supplementation of synthetic miR-146a significantly modulates inflammatory cytokines and pain-related molecules (e.g. TNFα, COX-2, iNOS, IL-6, IL8, RANTS and ion channel, TRPV1) in human glial cells. Our findings suggest that miR-146a controls knee joint homeostasis and OA associated algesia by balancing inflammatory responses in cartilage and synovium with pain-related factors in glial cells. Hence, miR-146a may be useful for the treatment of both cartilage regeneration and pain symptoms caused by OA.
Keywords: miR-146a, osteoarthritis, pain, chondrocyte, synovial cells, glial cells
1. Introduction
Joint degeneration associated with osteoarthritis (OA) affects a large number of individuals (>100 million) throughout the world and leads to chronic and severe pain in knee joints. Development of OA and one of its key debilitating symptoms, pain, is associated with global changes in gene expression in damaged peripheral tissues and neurons. A mechanistic understanding of OA related pain requires elucidation of how peripheral tissue injury alters gene expression in sensory neurons and how these changes contribute to the development and maintenance of pain in OA patients.
Chronic pain in OA is caused by inflammatory responses that modulate gene expression in a manner dependent of Toll like receptor (TLR) and nuclear factor kappa B (Liu-Bryan and Terkeltaub, 2010). These inflammatory responses are modulated by specific microRNAs, which are small RNAs that bind to 3′-UTR (untranslated region) of target mRNAs to inhibit protein translation and reduce mRNA stability (Taganov et al., 2006). For example, miR-146 has been shown to regulate TLR pathways and NFκB-dependent targets, while expression of miR-146a is transcriptionally induced by inflammatory cytokines (Taganov et al., 2006; Bhaumik et al., 2008). Together, these two processes constitute a negative feedback loop that controls immune activation and may modulate OA related inflammation.
Recent evidence suggests that miR-146a is expressed in OA cartilage at significantly higher levels than in normal cartilage (Yamasaki et al., 2009). Expression of miR-146a/b is induced in response to lipopolysaccharide (LPS) and proinflammatory mediators in THP-1 cells and this induction is regulated by NFκB (Taganov et al., 2006) Furthermore, miR-146a is more strongly expressed in synovial tissues of patients with rheumatoid arthritis (RA) compared to normal individuals, and its expression in synovial fibroblasts from RA patients is stimulated by inflammatory cytokines such as tumor necrosis factor α (TNFα) and Interleukin-1 β (IL-1β) (Nakasa et al., 2008). Expression of miRNAs in neural cells is altered after spinal cord injury (Liu et al., 2009). Bioinformatics analysis of potential targets for these miRNAs reveals that genes involved in inflammation, oxidation, and apoptosis may contribute to the pathogenesis of spinal cord injury. In a mammalian spinal nerve ligation model for chronic neuropathic pain, changes in miRNA expression contribute to pain sensation through translational regulation of genes relevant to pain-related pathways(Aldrich et al., 2009). In this study, we examined pathological links between miR146a, knee OA and neural pathways associated with OA-induced pain. While altered expression of miR146a in degenerating cartilage is associated with OA and controls inflammatory responses, we find that miR146a is also expressed in neural cells and perhaps may modulate genes involved in OA-related pain.
2. Materials and Methods
2.1. Generation of a rat model for knee joint OA
We generated an animal model for OA –induced pain by monosodium iodoacetate (MIA) injection as described previously (Im et al., 2010). This MIA-induced OA pain model demonstrates pathological features similar to those observed in human knee joint OA, including symptomatic chronic pain, cellular alterations in synovial tissues and disorganization of chondrocytes. Briefly, Sprague Dawley rats were anesthetized with isoflurane (Abbott Laboratories, North Chicago, IL, USA) in oxygen and given a percutaneous single intra-articular injection of 0.5 mg of MIA (Sigma, St. Louis, MO, USA; cat #I2512) or saline vehicle through the infra-patellar ligament of the left knee (N=8 for each group). MIA was dissolved in physiologic saline and administered in a volume of 25 μl using a 26-gauge, 0.5-inch needle. The right contra-lateral knee was used as a behavioral and histological control. Animal behavioral tests (Knee pressure hyperalgesia, Knee extension hyperalgesia, Mechanical allodynia (von Frey) and Knee Edema) were performed to confirm that the MIA injection increased knee joint discomfort in rats as described previously (Im et al., 2010)
2.2. General tissue preparation
Human articular synovial tissues from knees were obtained from donors through the Gift of Hope Organ and Tissue Donor Network (normal tissue specimens) and Rush Orthopedic Depository Studies (surgically removed OA tissues). For normal tissues, each donor specimen was graded for gross degenerative changes based on a modified version of the 5-point scale of Collins (Muehleman et al., 1997). At 2 weeks and 4 weeks post-MIA or saline injection, the animals were euthanized with halothane anesthesia. Bilateral lumbar DRGs and dorsal horn of the spinal cords were harvested under the light microscope for further analyses (e.g. RT-PCR).
2.3 Cell culture and Transfection
Human articular chondrocytes and synoviocytes were released by enzymatic digestion as previously described (Im et al., 2003). For monolayer cultures, isolated chondrocytes were counted and plated onto 12-well plates at 8×105 cells/cm2 as previously described (Im et al., 2007). Human synovial tissues were finely minced and tissue suspensions were seeded into culture dishes with medium. After 4 weeks, when the synovial fibroblasts were at 80% confluence, cells were divided into 12 well plates at 2×105 cells/cm2. Human astrocytic cells (Belanger et al., 2010) were plated at 5×105 cells/cm2. Cells were transiently transfected with 10–20 pmol of miR-146a (pre-miRNA mimics, Applied Biosystems) using Lipofectamine plus (Invitrogen, Carlsbad, CA). After 48 hrs, cells were harvested and subjected to total RNA and protein extraction for further experimentation.
2.4. Total RNA isolation; Reverse Transcription and Real-Time Polymerase Chain Reaction
Total RNA was isolated using Trizol reagent (Invitrogen). Reverse transcription (RT) was carried out with 1 μg total cellular RNA using the ThermoScript TM RT-PCR system (Invitrogen) for first strand cDNA synthesis in a reaction volume 50 μl. For real-time PCR, cDNA was amplified using the MyiQ Real-Time PCR Detection System (Bio-Rad Hercules, CA). The RT product was subjected to real-time PCR in a 20 μl total reaction mixture containing 10 μl Bio-Rad iQ™ SYBR Green supermix (Bio-Rad,), 1 μl of 10 μM sense and antisense primers, and 1 μl of template cDNA. A threshold cycle (CT value) was obtained from each amplification curve using iQ5 Optical System Software provided by the manufacturer. Relative mRNA expression was determined using the ΔΔCT method, as detailed in guidelines provided by the manufacturer (Bio-Rad). GADPH was used as internal control in the reaction for normalization. The primer sequences and their conditions for use are summarized in Table 1.
Table 1.
| Primer | Sequences | Tm | NCBI Reference No. |
|---|---|---|---|
| TNF-α | Forward: 5′-ACCAGCTAAGAGGGAGAGAAGCAA-3′ Reverse: 5′-TCAGTGCTCATGGTGTCCTTTCCA-3′ |
60°C | NM_000594 |
| Aggrecan | Forward: 5′ TCTTGGAGAAGGGAGTCCAACTCT--3′ Reverse: 5′-ACAGCTGCAGTGATGACCCTCAGA-3′ |
60°C | NM_013227 |
| MMP13 | Forward: 5′-ACCCTGGAGCACTCATGTTTCCTA-3′ Reverse: 5′-TGGCATCAAGGGATAAGGAAGGGT-3′ |
55°C | NM_002427 |
| Col2A1 | Forward: 5′-GGGCCTCAAGGATTTCAAGGCAAT-3′ Reverse: 5′-TCACCATCATCACCAGGCTTTCCA -3′ |
58°C | NM_001844 |
| ADAMTS5 | Forward: 5′-CTGTGACGGCATCATTGGCTCAAA -3′ Reverse: 5′-TTCAGGAATCCTCACCACGTCAGT-3′ |
60°C | NM_007038 |
| COX2 | Forward: 5′-TTCCATTGACCAGAGCAGGCAGAT-3′ Reverse: 5′-GCATCGATGTCACCATAGAGTGCT-3′ |
55°C | NM_000963 |
| iNOS | Forward: ATCACACGCCCACAGAGATCCA Reverse: GCTTCAGGCTGTTGAGCCATGT |
55°C | NM_000625 |
| IL-6 | Forward: AAGCCAGAGCTGTGCAGATGAGTA Reverse: TTCGTCAGCAGGCTGGCATTTGT |
60°C | NM_000600 |
| IL-8 | Forward: TCTTGGCAGCCTTCCTGATTTCTG Reverse: GGGTGGAAAGGTTTGGAGTATGTC |
55°C | NM_000584 |
| IRAK1 | Forward: TACCTGCCCGAGGAGTACATCAA Reverse: TCCTCTTCCACCAGGTCTTTCAGA |
55°C | NM_001569 |
| TRAF6 | Forward: 5′-AATGTTGGCCCAGGCTGTTCATAG-3′ Reverse: 5′-TAAGGCGACCCTCTAACTGGTGAAT-3′ |
55°C | NM_145803 |
| GAPDH | Forward: 5′-TCGACAGTCAGCCGCATCTTCTTT -3′ Reverse: 5′-GCCCAATACGACCAAATCCGTTGA -3′ |
55°C | NM_031144 |
| RANTS | Forward: 5′-ACCAGCCTGGCCAACATGATGAAA-3′ Reverse: 5′-TTCACGCCATTCTCCTGCCTCA -3′ |
60°C | NM_002985 |
| TRPV1 | Forward: 5′-CCGACAACACGAAGTTTGTGACGA-3′ Reverse: 5′-TTCCCTTCTTGTTGGTGAGCTCCT -3′ |
60°C | NM_080704 |
2.5. Micro-RNA 146 expression
Micro-RNA 146a expression was examined with the TaqMan MicroRNA Assay Kit. MultiScribe Reverse Transcriptase was used for RT and TaqMan primers for has-miR-146a (assay ID 000468) were used to monitor micro-RNA expression. RUN48 (assay ID 001006) was used as internal control for human RNA and U6 (assay ID 001973) was used as internal control for rat RNA.
2.6. Western blotting
Human articular chondrocyte isolated from knee cartilage (grade 0–1) in monolayer were transfected with synthetic miR-146a(10 and 20 pmol). IL-1 was administered in parallel to provoke a catabolic response. After 48 hours of tansfection, cell lysates were prepared using modified RIPA buffer. Total protein concentrations of media were determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). In each case, an equal amount of protein was resolved in 8.5% SDS-polyacrylamide gels and transferred to nitrocellulose membrane for immunoblot analyses as described previously (Im et al., 2007). Immunoreactivity was visualized using the ECL system (Amersham Biosciences, Piscataway, NJ) and the Signal Visual Enhancer system (Pierce), which magnifies the signal.
2.7. Statistical Analysis
All results are expressed as mean values ± S.E.M. (standard error of mean). The evaluation of real-time PCR data was done by one-way ANOVA with a post-hoc Tukey’s test using 2-ΔΔct values of each sample. A value of P<0.05 was considered significant.
3. Results
3.1. Exogenous supplementation of synthetic miR-146a regulates extracellular matrix-associated proteins and cartilage degrading enzymes in human knee joint articular chondrocytes
To assess the biological role of miR-146a in human articular cartilage, chondrocytes isolated from knee cartilage (grade 0–1) in monolayer were transfected with synthetic miR-146a (or scrambled miR as a negative control). IL-1 was administered in parallel to provoke a catabolic response (Eger et al., 2002). Alterations in the expression levels of matrix genes, such as aggrecan, collagen type II, as well as key pathological proteases, such as collagenases (MMP1, MMP13) and aggrecanases (ADAMTS4, ADAMTS5) were assessed by either qPCR or western blotting analyses.
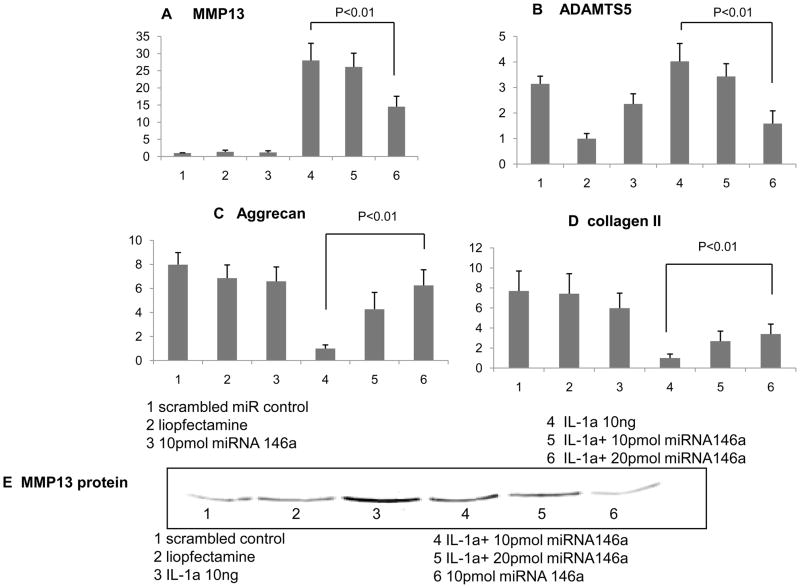
The presence of IL-1 (10 ng/ml) markedly decreased the mRNA levels of collagen type II and aggrecan, a major proteoglycan component, as expected based on previous findings (Eger et al., 2002; Li et al., 2009). Transfection of miR-146a dose-dependently antagonized IL-1-mediated suppression of both aggrecan and collagen type II expression (both p<0.01 at a concentration of 20 pmol) (Figs. 1A and B). Next, we evaluated the biological effects of miR-146a on cartilage degrading enzymes. The presence of IL-1 significantly stimulated collagenases and aggrecanases as previously reported in articular chondrocytes (Rowan and Young, 2007). Upon transfection of miR-146a at a concentration of 10 pmol, we did not observe any significant changes in the expression levels of both MMP1 and ADAMTS4 (data not shown). However, expression levels of MMP13 and ADAMTS5 were significantly suppressed by miR-146a (Figs. 1C and D). This finding indicates that miR-146a supresses of these target proteases. Changes in MMP13 protein levels upon transfection of miR-146a were further evaluated by immunoblot analyses. These studies revealed that the changes in MMP13 mRNA are reflected by corresponding changes in MMP13 protein levels and that miR-146a antagonizes IL-1-induced production of MMP13 in a dose-dependent manner (Fig. 1E).
Fig 1.
Human articular chondrocyte isolated from knee cartilage (grade 0–1) in monolayer were transfected with synthetic miR-146a(10 and 20 pmol). IL-1 was administered in parallel to provoke a catabolic response. After 48 hours of tansfection, the total RNA was isolated and analyzed of MMP13, ADANTS5, Aggrecan and collage II gene were analyzed by real-time RT-PCR(A,B,C,D). The total protein was isolated and analyzed for MMP13 protein expression by western blotting (E).
3.2. Regulation of pain-associated inflammatory factors, matrix metalloproteinases and aggrecanases by miR-146a in human knee joint synoviocytes
In OA, the inflammation of the synovial membrane that occurs in both the early and late phases of OA is associated with alterations in the adjacent cartilage that are similar to those seen in rheumatoid arthritis. Catabolic and proinflammatory mediators such as cytokines, prostaglandin E2 and neuropeptides are produced by the inflamed synovium and alter the balance of cartilage matrix degradation and repair, leading to excess production of the proteolytic enzymes responsible for cartilage breakdown (Sellam and Berenbaum, 2010). Expression of miR-146a is highly upregulated not only in cartilage but also in synovium from OA patients compared to normal tissues. Therefore we examined the biological impact of miR-146a on human knee joint synoviocytes.
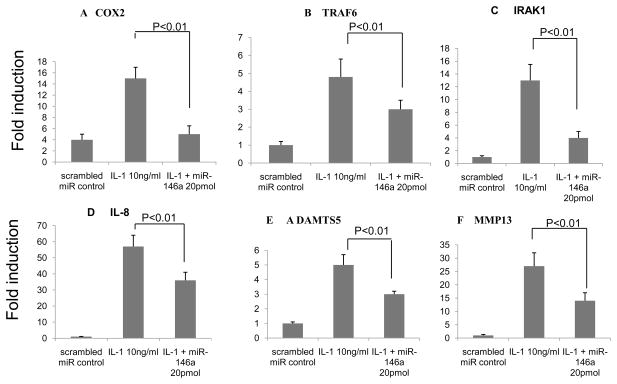
Human knee synoviocytes were transiently tansfected with miR-146a and incubated with IL-1 10 ng/ml and TNF receptor associated factor 6 (TRAF 6) and IRAK1 were evaluated. Compared to control (scrambled miRNA), the presence of IL-1 significantly increased expression levels of the inflammatory factors such as COX-2 (3.5 fold) and IL-8 (55 fold), and this IL-1-mediated induction of COX-2 and IL-8 was markedly suppressed in the presence of miR-146a (20 pmol) (Fig. 2; p<0.01). Similarly, transfection of miR-146a (20 pmol) reduced expression of the known miR-146a target genes TRAF6 and IRAK1 by 60% to 70% (Fig. 2; p<0.01) compared to IL-1 only. Similarly, expression of the genes for collagenase (MMP13) and aggrecanase (ADAMTS5) also increased in synoviocytes when treated with IL-1 and this IL-1-mediated induction of MMP13 and ADAMTS5 was markedly suppressed in the presence of miR-146a (20 pmol) (Fig. 2; p<0.01).
Fig 2.
Human articular synovial cells from knee cartilage (grade 0–1) were transfected with synthetic miR-146a(10 and 20 pmol). IL-1 was administered in parallel to provoke a catabolic response. After 48 hours of transfection, the total RNA was isolated and COX2 (A), TRAF6(B) IRAK1(C), IL-8(D), ADAMTS5(E) and MMP13(F) gene were analyzed by real-time RT-PCR
3.3. Expression of miR-146a is significantly reduced in DRGs and the dorsal horn of spinal cords from rats with induced OA and severe knee joint pain
Pain is major clinical symptom of OA. Using a rat model for OA that is induced by intra-articular injection of MIA, we have previously shown that OA pain is centralized through communication between peripheral OA nociceptors and the central sensory system (Im et al., 2010). Interestingly, target prediction algorithms indicate that there is a conserved set of approximately 70 different genes in human, mouse and rat that may be directly controlled by miR-146a (Supplemental Figures S1, S2). While miR146a clearly controls expression of inflammation related genes (e.g., TRAF6, IRAK1), a subset of these putative target genes is involved in neuronal differentiation and/or has a function associated with synapses (Supplemental Figures S1, S2). Thus, miR-146a may also contribute to neuronal processes in OA.
To examine a possible role for miR-146a in neuronal cells, we investigated whether miR-146a is altered in peripheral (DRG) or central (dorsal horn of the spinal cords) nervous systems of animals with OA pain. Rats received a single intra-articular injection of MIA (2 mg) or saline (sham control) and were euthanized at week 2 or week 4. Ipsilateral lumbar DRG (L3/4, L4/L5, L5/6) and the lumbar dorsal horn of spinal cords were harvested and the levels of miR-146a were analyzed.
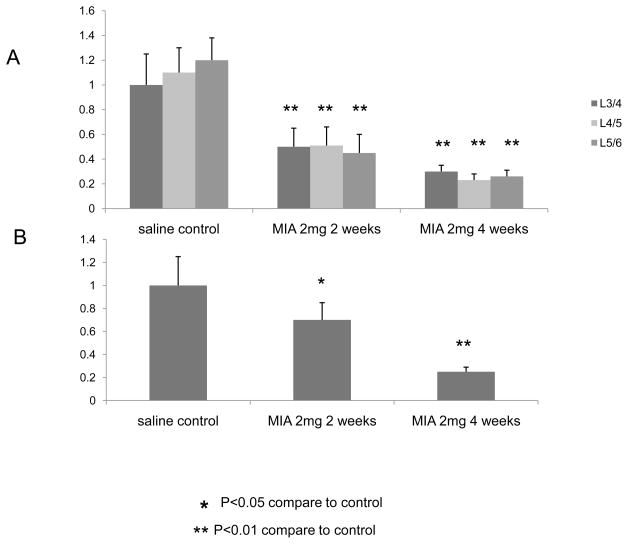
Expression of miR-146a in both lumbar DRG and spinal dorsal horns significantly decreases in animals with OA knee joint pain compared to sham controls. Specifically, the level of miR-146a in the ipsilateral lumbar DRG from the animals with OA-provoked pain is decreased to 50–60 % (P<0.01) and 60–70 % of the sham control after 2 and 4 weeks of OA induction, respectively (Fig. 3A). Similarly, the level of miR-146a in the dorsal horn of the spinal cords from animals with OA pain was decreased to 70 % (p<0.05) of the sham control after 2 weeks of OA induction, and this reduction was further accelerated up to 20–30% (p<0.01) of the sham control at week 4 (Fig. 3B).
Fig 3.
Rats received a single intra-articular injection of MIA (0.5 mg) or saline (control) were euthanized at week 2 and week 4. Left lumbar DRGs at levels of 3/4, 4/5 and 5/6 ( A ) and dorsal horn of the spinal cords (B) were harvested and relative expression of micro-RNA 146a were analyzed using real-time PCR
3.4. Regulation of pain-related inflammatory modulators by miR-146a in human astroglial cells
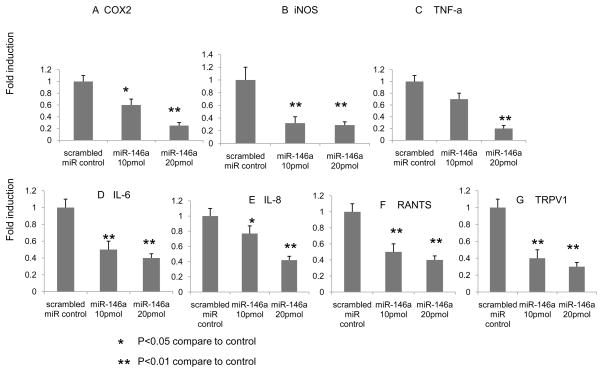
Accumulating evidence suggests that glial cells (e.g., microglial and astroglial cells) play a key role in the development and maintenance of persistent chronic pain (Nakagawa and Kaneko; Nakagawa and Kaneko, 2010). Our results demonstrate that the level of miR-146a is significantly altered in peripheral- and central nervous system suggesting that regulation of miR-146a by glial cells governs expression of pain-modulators. Thus, we further investigated whether miR-146a expression in astrocytes controls pain modulators that influence centralized pain perception. Human astrocytes were transiently transfected with two different doses of miR-146a (10 and 20 pmol) for 24 hrs. Total RNA was analyzed by qRT-PCR for expression of a panel of mRNAs, including TNFα, COX-2, iNOS, IL-6, IL8, RANTS and ion channel, TRPV1, that are involved in pain perception (Fernandes et al.; Im et al., 2010). The results show that transfection of miR-146a significantly decreases expression of these pain-related target molecules in a dose-dependent manner (Fig. 4).
Fig 4.
Human astroglial cells were transfected with micro RNA 146a (10 and 20 pmol). After 48 hours of transfection, the total RNA was isolated and COX2 (A), iNOS (B), TNF-α (C), IL-6(D), IL-8(E) RATNS(F) and TRPV1(G) gene were analyzed by real-time RT-PCR.
4. Discussion
Our results reveal a significant correlation between arthritic joint pathogenesis and miR-146a by demonstrating that miR-146a regulates cartilage degrading proteases and pain-associated inflammatory molecules in the peripheral knee joint tissues including articular chondrocytes and synoviocytes. We find that miR-146a functions in an anti-catabolic manner in articular cartilage by antagonizing the IL-1 induced expression of cartilage-degrading enzymes MMP13 and ADAMTS5, while simultaneously antagonizing IL-1 induced suppressing expression of extracellular matrix proteins such as aggregan and collagen type II. Furthermore, miR-146a regulates TRAF6 and IRAK1, essential factors that mediate receptor signaling in response to ligands of the TNFα superfamily and inflammatory cytokines (IL-1/Toll superfamily) in knee joint synoviocytes. In addition, miR-146a regulates MMP13 and ADAMTS5 gene expression in knee joint synoviocytes. Our transfection studies suggest that upregulation of miR-146a may occur as a component of a cellular response to repair damaged tissue, which is compromised in the disease condition. Using an animal model for OA related knee joint pain, we provide evidence for the novel concept that miR-146a may be involved in pain by modulating inflammatory cytokines and pain-related molecules in astrocytes residing in the central and peripheral nervous systems.
It has been shown that miR-146a expression is regulated by transcription factor NFκB and that miR-146a may control TLRs and cytokine signaling through a negative feedback regulation loop involving down-regulation of IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) protein levels (Taganov et al., 2006). Lentiviral-mediated expression of miR-146a/b significantly downregulated interleukin (IL)-1 receptor-associated kinase and TRAF6, two key adaptor/scaffold proteins in the IL-1 and TLR signaling pathway, known to positively regulate NFκB activity (Bhaumik et al., 2008). Furthermore, miR-146a targets TRAF6 which is key components in NFκB and TLR pathways (Pauley et al., 2008). These pathways are also essential for positive feedback of cytokine expression, and consequently production of cartilage–degrading enzymes, such as MMPs and ADAMTS. Expression analysis of miRNAs in OA and normal cartilage (Jones et al., 2009) identified 17 miRNAs that showed a greater than 4-fold difference in expression in OA cartilage and 30 miRNAs that showed greater than 4-fold differential expression in OA bone. Functional pathway analysis of the predicted gene targets for miR-9 and miR-98, which are both upregulated in OA bone and cartilage tissue, as well as miR-146, which is downregulated in OA cartilage, suggest that these miRNAs mediate inflammatory functions and pathways. Transfection of miR-9, miR-98 or miR-146a in isolated human chondrocytes reduces IL-1β induced TNF-α production. Furthermore, inhibition and transfection of miR-9 modulated MMP13 secretion. Recent reports have also shown increased miR-155, miR-146a and miR-146b expression following activation of the innate immune response in monocytes/macrophages (Taganov et al., 2007). Our in vitro gain-of-function analyses by introduction of synthetic miR-146a showed that transfection of miR-146a reduces both basal and IL-1 stimulated production of MMP13 and ADAMTS5. Recent reports using transfected human chondrocytes revealed that miR-140 down-regulates IL-1β–induced ADAMTS5 expression and rescues the IL-1β–dependent repression of aggrecan gene expression (Miyaki et al., 2009). Also, transfection of human chondrocytic HCS-2/8 cells and chicken normal chondrocytes with miR-1 leads to repressed expression of aggrecan (Sumiyoshi et al., 2010). Our data that transfection of human chondrocytes with miR-146a rescues IL-1–dependent repression of aggrecan and collagen II gene expression, indicates that compromised miR-146a expression in OA correlates with an imbalance in anabolic–catabolic responses.
The synovial membrane, which contains metabolically highly active cells (synoviocytes), is physiologically important as it both nourishes chondrocytes via the synovial fluid and joint space, and removes metabolites and products of matrix degradation (Sellam and Berenbaum, 2010). Inflammation of the synovium (synovitis) that occurs in OA results in the production of two major cytokines involved in the pathogenesis of OA, IL-1β and TNFα, by activated synoviocytes, mononuclear cells and articular cartilage(Furuzawa-Carballeda et al., 2008). These cytokines can also stimulate chondrocytes and synovial cells to produce other cytokines and PGE2, and together these proinflammatory cytokines are thought to diffuse into the synovial fluid. Chronic synovitis is associated with marked changes in the central connections of sensory nerves, and with changes in the synthesis and release of neurotransmitters and neuron-modulators (Kidd et al., 2004; Sellam and Berenbaum, 2010). Our study shows that transfection of miR-146a does not change TNFα gene expression but decreases expression of TRAF6. Hence, miR-146a may modulate inflammation by targeting TRAF6 in human synovium.
In a rat model of chronic neuropathic pain, the expression levels of miR-96, -182, and -183 are significantly reduced in injured DRG neurons (Aldrich et al., 2009). Our finding now provide evidence that miR-146a is not only expressed in peripheral tissues but also highly expressed in the sensory neurons in DRGs and spinal cord. More importantly, miR-146a levels are decreased in DRGs from OA-generated knee joint and spinal cord in an OA animal model. These data suggest that miR-146a has potential dual roles in peripheral (knee joint) and central sensitization for pain transmission in OA-induced nociceptive pathways.
Cross-talk between glial cells and neurons also may influence central sensitization and neuronal inflammatory responses. At the level of the spinal cord, glial cells and the factors they produce play a key role in the development and maintenance of persistent chronic pain. Glial cells dynamically modulate the functions of the sensory system under pathological conditions, including rheumatoid arthritis(Levin et al., 2008; Gwak and Hulsebosch, 2009). Upon activation, glial cells release inflammatory cytokines and pain molecules leading to hyper-sensitivity. Thus, pharmacological attenuation of glial activity may represent a novel approach for controlling neuron-inflammatory diseases and neuropathic pain associated with OA.
Elevation of miR-146a levels significantly inhibits the activation of glioma migration and invasion by directly mediating translational suppression of MMP16 (Xia et al., 2009). MMP16 is a critical activator of MMP2, which is associated with chronic pain by central sensitization (Ji and Suter, 2007) In the MIA-induced rat OA model, significant induction of pro- inflammatory cytokines with simultaneous reduction of anti-inflammatory cytokines was observed in either DRG or spinal cord, and expression of RANTES was highly elevated in spinal cords upon induction of OA (Im et al., 2010) Our data show that transfection of miR-146 suppresses expression of RANTES, COX2, iNOS and the ion channel TRPV1 in astroglia cells. This finding suggests that pathological elevation of miR-146a may modulate production of pain-induced inflammatory cytokines and pain mediators.
In conclusion, our data support a model in which miR-146a plays a role in knee joint homeostasis and OA-associated pain symptoms, by balancing the inflammatory response and expression of pain-associated factors in cartilage synovium and glial cells. Our data suggests that miR-146a may represent an effective therapeutic agent for the treatment of both cartilage regeneration and treatment of its symptom, pain.
Supplementary Material
Acknowledgments
This work was supported by grants from NIH R01AR053220 (HJ Im), the Arthritis Foundation (HJ Im), and the National Arthritis Research Foundation (HJ Im).
Abbreviations
- OA
osteoarthritis
- RA
rheumatoid arthritis
- DRG
dorsal root ganglia
- MMP
matrix metalloproteinase
- ADAMTS
A Disintegrin and Metalloproteinase with Thrombospondin Motifs
- UTRs
untranslated regions
- TLR
Toll Like Receptor
- NFkB
nuclear factor-kappa B
- LPS
lipopolysaccharide
- IL-1
Interleukin-1
- MIA
monosodium iodoacetate
- RT
Reverse transcription
- PCR
polymerase chain reaction
- BCA
bicinchoninic acid
- TNF
tumor necrosis factor
- TRAF 6
TNF receptor associated factor 6
- IRAK1
IL-1 receptor-associated kinase 1
- COX-2
Cyclooxygenase-2
- iNOS
Inductible Nitric Oxide Synthase
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–23. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Differential effects of pro- and anti-inflammatory cytokines alone or in combinations on the metabolic profile of astrocytes. J Neurochem. 2010 Dec 10; doi: 10.1111/j.1471-4159.2010.07135.x. [DOI] [PubMed] [Google Scholar]
- Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–7. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res. 2002;20:526–34. doi: 10.1016/S0736-0266(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD. A distinct role for TRPA1, in addition to TRPV1, in TNFalpha-induced inflammatory hyperalgesia and CFA-induced mono-arthritis. Arthritis Rheum. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- Furuzawa-Carballeda J, Macip-Rodriguez PM, Cabral AR. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 2008;26:554–60. [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, Desevre J, Kroin JS. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62:2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–21. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–94. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, Needham MR, Read SJ, Newham P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–72. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Kidd BL, Photiou A, Inglis JJ. The role of inflammatory mediators on nociception and pain in arthritis. Novartis Found Symp. 2004;260:122–33. discussion 133–8, 277–9. [PubMed] [Google Scholar]
- Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain. 2008;137:182–201. doi: 10.1016/j.pain.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, Im HJ. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–23. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62:2004–12. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–9. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki S, Nakasa T, Otsuki S, Grogan SP, Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK, Asahara H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–30. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kaneko S. Spinal Astrocytes as Therapeutic Targets for Pathological Pain. J Pharmacol Sci. doi: 10.1254/jphs.10r04cp. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kaneko S. Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci. 2010;114:347–53. doi: 10.1254/jphs.10r04cp. [DOI] [PubMed] [Google Scholar]
- Nakasa T, Miyaki S, Okubo A, Hashimoto M, Nishida K, Ochi M, Asahara H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58:1284–92. doi: 10.1002/art.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan AD, Young DA. Collagenase gene regulation by pro-inflammatory cytokines in cartilage. Front Biosci. 2007;12:536–50. doi: 10.2741/2080. [DOI] [PubMed] [Google Scholar]
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi K, Kubota S, Ohgawara T, Kawata K, Nishida T, Shimo T, Yamashiro T, Takigawa M. Identification of miR-1 as a micro RNA that supports late-stage differentiation of growth cartilage cells. Biochem Biophys Res Commun. 2010;402:286–90. doi: 10.1016/j.bbrc.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26:133–7. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–65. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Nakasa T, Miyaki S, Ishikawa M, Deie M, Adachi N, Yasunaga Y, Asahara H, Ochi M. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum. 2009;60:1035–41. doi: 10.1002/art.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.