Abstract
Our studies in the mRen2.Lewis female rat, an angiotensin II- and estrogen-dependent model of hypertension, revealed that chronic activation of estrogen receptor GPR30 markedly reduces blood pressure in ovariectomized females. The present studies measured acute vasodilation to the selective GPR30 agonist G-1 and 17-β-estradiol (10-9 to 10-5.5 M) in isolated aortic rings and mesenteric arteries from intact mRen2.Lewis females. Maximal relaxation was greater in mesenteric vessels versus the aorta for both G-1 (47 ± 8% vs. 80 ± 5% of phenylephrine preconstriction, P < 0.001) and estradiol (42 ± 7% vs. 83 ± 4% of phenylephrine preconstriction, P < 0.001). The GPR30 antagonist G15 attenuated the response to both estradiol and G-1. Removal of the endothelium or pretreatment with L-NAME partially attenuated vasorelaxation. Responses were not altered in mesenteric vessels from ovariectomized females. Immunohistochemical analysis revealed GPR30 expression in mesenteric endothelial and smooth muscle cells, and smooth muscle expression was confirmed in cultured cells. We conclude that estradiol-induced relaxation in conduit and resistance vessels from mRen2.Lewis females may be mediated by the novel estrogen receptor GPR30. The direct vasodilatory response of G-1 in resistance vessels presents one mechanism for the reduction in blood pressure induced by chronic G-1 administration.
Keywords: estrogen, estrogen receptors, GPR30, GPER, vascular reactivity, mRen2.Lewis
INTRODUCTION
Sublingual estradiol decreases peripheral resistance in menopausal women; however the receptor(s) which mediate this immediate vasodilatory response are not known (1,2). Studies using arterial rings from ERα and ERβ knockout mice show that while these classic steroid receptors alter nitric oxide production, their blockade or genetic deletion does not completely inhibit estradiol-induced vasorelaxation (3-6). In fact, aortic rings from ERβ knockout mice exhibit greater relaxation in response to estradiol (7). In addition, the ERα/β antagonist ICI 182,780 (ICI) does not completely attenuate vascular estrogenic effects and induces agonist-like vasodilation when administered alone (7,8). The dependence on GTP-binding for estradiol-induced signaling in endothelial, smooth muscle, neural, and cancer cells clearly suggests that some estrogenic effects are mediated by a G protein-coupled receptor (9-11). The recent identification of the estrogen receptor GPR30 and its localization in human arteries and veins presents the possibility that this receptor may mediate the acute vasorelaxant effects of estradiol (12).
Ongoing studies in our laboratory have focused on the role of endogenous estradiol to modulate various components of the renin-angiotensin system (RAS) in regards to blood pressure regulation and target organ damage (13-16). In the female hypertensive mRen2.Lewis strain, removal of circulating estrogen via ovariectomy markedly exacerbates blood pressure and is associated with increased circulating levels of angiotensin II (Ang II) but a reduction in Ang-(1-7) (17,18). Moreover, chronic estradiol replacement completely attenuates the increase in blood pressure to the same extent as the AT1 receptor antagonist olmesartan (18). We recently provided evidence that GPR30 influences blood pressure and the vascular RAS in estrogen-depleted mRen2.Lewis rats. Chronic administration of the selective GPR30 agonist G-1, which binds at a similar affinity as estradiol (Kd ≈ 10 nM) but essentially shows no binding at ERα or ERβ, reduces blood pressure to the same level as the intact hypertensive female, as well as suppresses vascular expression of the AT1 receptor and angiotensin converting enzyme (ACE) and increases ACE2 (19). Our previous studies also demonstrate that G-1 induces vasorelaxation in isolated aortic rings of the ovariectomized mRen2.Lewis; however, we did not compare the vascular actions of G-1 to estradiol nor assess relaxation in a resistance bed (19,20). To establish whether GPR30 mediates the direct vasorelaxant actions of estrogen, the current study compared G-1 and estradiol-induced vasorelaxation in conduit and resistance vessels of the hypertensive mRen2.Lewis female, as well as assessed the inhibitory actions of the GPR30 antagonist G15.
METHODS
Animals
Heterozygous mRen2.Lewis females were obtained from the Hypertension Center transgenic breeding colony. Rats were housed in the Wake Forest University Animal Resources Facility, an AALAC-approved facility in a temperature-controlled room (22 ± 2°C) with a 12h light/dark cycle, free access to food and water, and daily monitoring by veterinary staff. Ovariectomy (OVX) was performed by bilateral flank incisions under sterile conditions on four week-old animals anesthetized with 4% isoflurane as previously described (18). All methods were approved by the institutional ACUC.
Vascular reactivity
At 15 weeks of age, animals were decapitated and the aorta and mesenteric arcade removed. Vessels were carefully dissected to remove surrounding fat and cut into 2 mm segments. Aortic rings were suspended from isometric force transducers (Grass Technologies, West Warwick, RI) in Radnoti glass organ chambers (Monrovia, CA) and passive tension was set to 2 g, as previously described (19). Second order mesenteric vessels (<200 μm) were mounted in a DMT wire myograph and the internal circumference was normalized to 0.9·IC100, where IC100 is the internal circumference at a transmural pressure of 100 mmHg (21). Chambers were filled with Kreb's solution (in mM): 118 NaCl, 25 NaHCO3, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 0.03 EDTA, and 5.5 glucose, at pH 7.4 bubbled continuously with 95% O2 and 5% CO2 and maintained at 37°C. Vessels were stimulated with phenylephrine (PE; aorta: 1 μM, mesentery: 10 μM) and 1 μM acetylcholine (both from Sigma, St. Louis, MO) and rings with >50% relaxation considered endothelium intact. Some vessels were pretreated with 100 μM L-NAME (Sigma) or 1 μM G15 (22). The responses to increasing concentrations (10-9 to 10-5.5) of G-1 (Cayman Chemical, Ann Arbor, MI) and estradiol (E2; Sigma) were measured in PE-preconstricted vessels. Drugs were added at five minute increments so that the total concentration response curve was completed within a reasonable time frame for vessel viability (~45 min). G-1 was dissolved in DMSO at 10 mM and subsequently diluted in Kreb's. Control vessels were treated with a corresponding concentration of DMSO at each agonist concentration. Responses were recorded using Chart 5 (AD Instruments, Colorado Springs, CO) and are expressed as the percentage (%) of PE contraction.
Immunohistochemistry
During mesenteric vessel isolation, some segments were formalin-fixed overnight and paraffin-embedded. Tissue sections (5 μm) were blocked with 0.1% Tween, 1% BSA, and 5% normal donkey serum. Anti-GPR30 (1:200; MBL #A4272, Woburn, MA) and biotinylated goat anti-rabbit (1:400) were diluted in the blocking buffer. Antibody binding was detected using the Vectastain Elite kit (VectorLabs, Burlingame, CA) and 0.1% diaminobenzene (Sigma, St. Louis, MO). For a negative control, the primary antibody was pre-incubated with the blocking peptide for 1 h at 25°C and centrifuged before being applied to tissue sections. Slides were counterstained with hematoxylin (Sigma). Van Gieson's Solution (Rowley Biochemical, Danvers, MA) was used according to the manufacturer's directions.
Cell Studies
Mesenteric smooth muscle cells were isolated from a 12-week old intact mRen2.Lewis female by enzymatic digestion as previously described, and the smooth muscle phenotype was confirmed by positive immunofluorescent staining for α-actin (23). Cells were maintained in DMEM-F12 containing 10% FBS. For immunocytochemistry, first passage cells were seeded onto glass chamber slides, fixed with 2% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 3% BSA. Slides were incubated with a primary antibody directed against α-actin (1:200, Sigma) or GPR30 (1:100; MBL #A4272, Woburn, MA) and secondary AlexaFluor 488 (1:200; Invitrogen, Carlsbad, CA). Coverslips were mounted using ProLong mounting media with DAPI (Invitrogen). For immunoblotting, 50 μg of total cell lysate was probed for GPR30 as previously described (19).
Statistics
Data were analyzed using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA) and expressed as the mean ± SEM. Two-way ANOVA with Bonferroni post-test was used to analyze concentration response data with P < 0.05 considered significant.
RESULTS
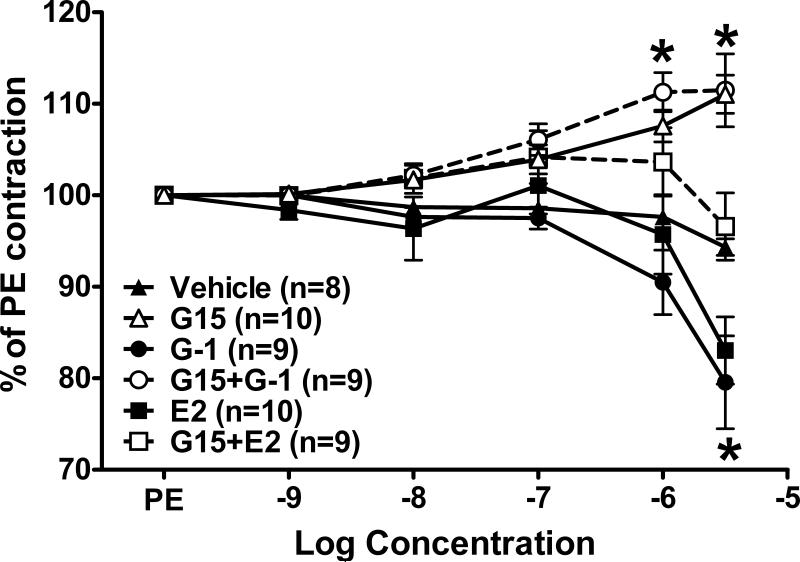
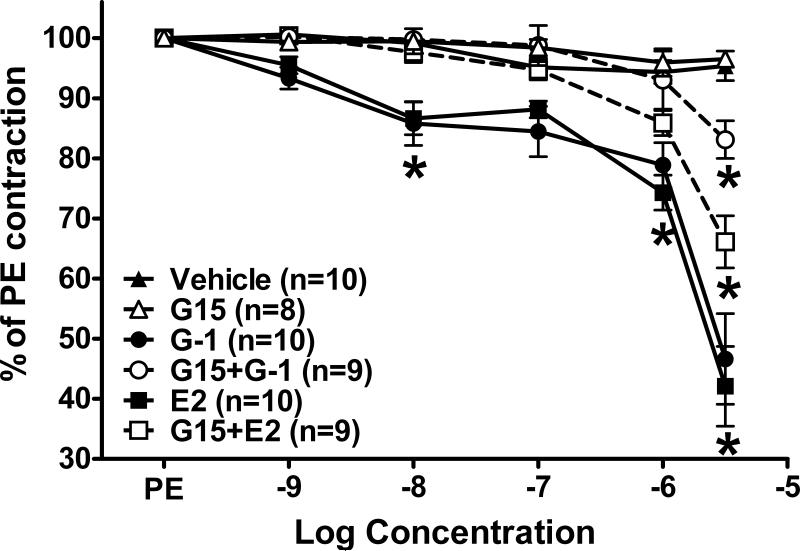
In isolated aortic rings from intact mRen2.Lewis females, the GPR30 agonist G-1 induced vasodilation that was not significantly different from estradiol (Figure 1). Pretreatment with the GPR30 antagonist G15 (1 μM) significantly attenuated both the G-1 and estradiol response. The antagonist alone induced a slight but significant vasoconstrictor response in the aorta. In mesenteric vessels, G-1 and estradiol again achieved similar levels of vasorelaxation (Figure 2). Vasorelaxation in the mesentery reached significance at a much lower concentration (10 nM) in comparison to the aorta (3 μM). In addition, maximal relaxation was greater in mesenteric vessels versus the aorta for both G-1 (47 ± 8% vs. 80 ± 5% of phenylephrine preconstriction, P < 0.001) and estradiol (42 ± 7% vs. 83 ± 4% of phenylephrine preconstriction, P < 0.001). G15 pretreatment abolished both G-1 and estradiol responses up to 1 uM; however, the antagonist only partially blocked the highest concentration of estradiol and G-1 (3 μM). In contrast to the aorta, the same concentration of G15 did not induce constriction in mesenteric vessels (Figure 2).
Figure 1.
Aortic relaxation in response to the GPR30 agonist G-1 and estradiol (E2). *P < 0.05 vs. Vehicle.
Figure 2.
Mesenteric relaxation in response to the GPR30 agonist G-1 and estradiol (E2). *P < 0.05 vs. Vehicle, #P < 0.05 vs. G-1 or E2.
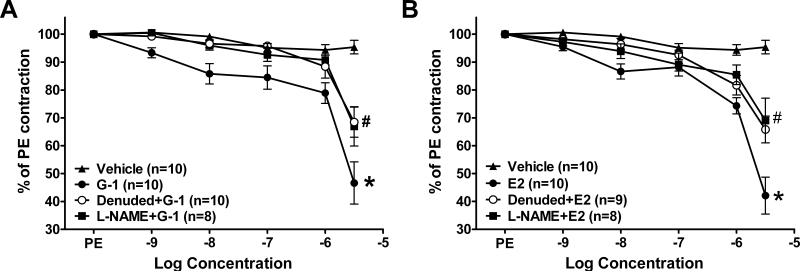
We previously reported that endothelial denuding attenuated the G-1 response in aortic rings (19). In mesenteric resistance vessels, mechanical denuding partially attenuated the responses to both G-1 and estradiol (Figure 3). Pretreatment of intact vessels with the nitric oxide synthase inhibitor L-NAME (100 μM) inhibited vasorelaxation to the same extent as endothelial denuding (Figure 3).
Figure 3.
Role of the endothelium and nitric oxide in mesenteric G-1 and estradiol (E2) vasorelaxation *P < 0.05 vs. Vehicle, #P < 0.05 vs. G-1 or E2.
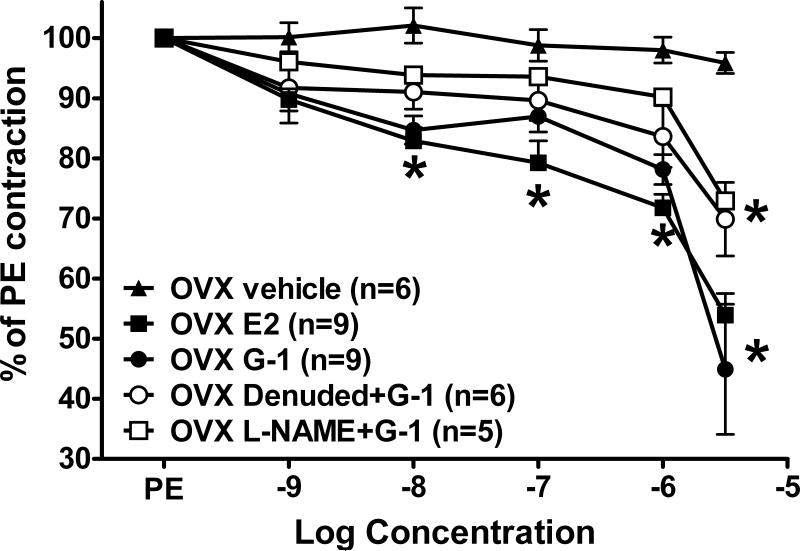
We next assessed whether ovariectomy (OVX) altered the response to either of these estrogen receptor agonists. As previously reported, systolic blood pressure was significantly higher in OVX females (184 ± 5 mmHg) versus intact females (137 ± 7 mmHg; P < 0.001) (18,19). G-1 and estradiol induced concentration-dependent vasorelaxation that was significant in the nanomolar range (Figure 4). Neither G-1 nor estradiol vasodilation was significantly different in OVX versus intact vessels (P > 0.05). As demonstrated in intact vessels, denudation or L-NAME inhibited the G-1 response by ~50%.
Figure 4.
Mesenteric relaxation in response to the GPR30 agonist G-1 and estradiol (E2) in ovariectomized (OVX) females. *P < 0.05 vs. OVX Vehicle.
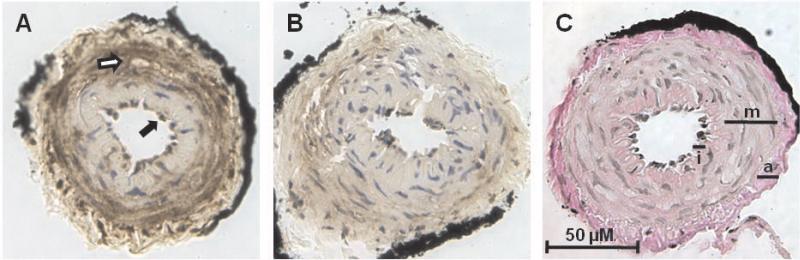
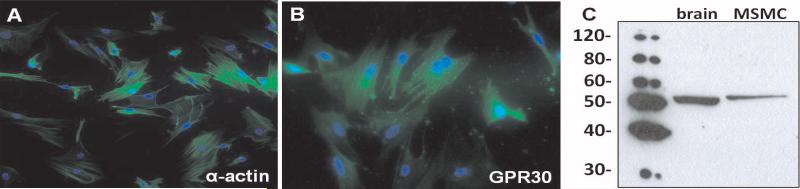
As shown in Figure 5A, immunohistochemical analysis of GPR30 revealed predominant staining in both endothelial and smooth muscle cells in mesenteric vessels. The preabsorption of the GPR30 antibody with the immunogenic peptide attenuated the staining signal throughout the mesenteric tissue (Figure 5B). A serial section stained by the Van Gieson method confirms the location of medial and adventitial layers (Figure 5C). To confirm GPR30 expression in smooth muscle cells, mesenteric vessels from an intact mRen2.Lewis female were enzymatically digested and cultured to obtain an enriched population of cells. Figure 6A shows smooth muscle-specific α-actin expression in all cells, confirming the purity of the smooth muscle cell population. Mesenteric smooth muscle cells also displayed positive immunostaining for GPR30 (Figure 6B). Western blot of mesenteric smooth muscle cell lysate using the same antibody showed a single band for GPR30 at ~50 kDa.
Figure 5.
GPR30 expression in a second-order mesenteric artery from an intact mRen2.Lewis female. A, GPR30 immunostaining in endothelial cells (closed arrows) and smooth muscle cells (open arrows). B, Negative control incubated with pre-adsorbed primary antibody. C, Van Gieson's stain differentiates the intima (I), media (M), and adventitia (A).
Figure 6.
GPR30 expression in cultured mesenteric smooth muscle cells (MSMC) from an intact mRen2.Lewis female. A, Smooth muscle-specific α-actin (green); DAPI (blue). B, GPR30 (green); DAPI (blue). C, GPR30 immunoblot of brain and MSMC total lysate.
DISCUSSION
In the present study, we report that acute activation of the novel estrogen receptor GPR30 induced vasodilation in both conduit and resistance vessels to a similar extent as nonselective activation of estrogen receptors with estradiol. Using isolated vessels from the estrogen-sensitive mRen2.Lewis congenic rat, we found that estradiol and G-1 elicited responses that were significantly greater in mesenteric resistance vessels than aortic rings. Moreover, the responses to G-1 and estradiol in both vessel preparations were significantly attenuated by the GPR30 selective antagonist G15. We also found that vasorelaxation in response to G-1 and estradiol in mesenteric arteries was comprised of both endothelium-dependent and endothelium-independent components, which may reflect the localization of GPR30 in both endothelial and smooth muscle cells. Finally, we show comparable effects of G-1 and estradiol in mesenteric vessels from OVX mRen2.Lewis females, suggesting that the exacerbation of pressure in these animals may, in part, result from the loss of GPR30-mediated estrogenic signaling in the vasculature.
GPR30 is a membrane-bound estrogen receptor, and its presence in the vasculature portends for a novel pathway by which estradiol may influence vascular tone. We and others have recently shown that activation of GPR30 lowers blood pressure in vivo and exhibits vasodilatory actions ex vivo (19,24). The present study is the first to compare vasodilation due to nonselective estrogen receptor activation with estradiol to the GPR30 response using the selective agonist G-1 and the selective antagonist G15. Competitive binding studies show that the affinity of these compounds for GPR30 is in the 5-20 nM range, which is comparable to estradiol (20,22). However, G-1 and G15 display no binding to ERα or ERβ, even when tested at higher concentrations of 1-10 μM. The GPR30 antagonist G15 attenuated both G-1 and estradiol-induced dilation, suggesting that the acute estradiol response is mediated by GPR30. G15 was unable to completely inhibit vasodilation at higher concentrations of the agonist (>1 uM); however, this is likely due to the lower competing concentration of the antagonist (1 uM) and that both compounds exhibit similar affinities for GPR30 (20,22). In aortic rings, G15 (1 uM) induced vasoconstriction that was potentiated by G-1. It is plausible that estradiol was still present in the ex vivo preparation and contributes to endogenous tone in this vessel. Both vascular endothelial and smooth muscle cells express the enzymes necessary for formation of estradiol, suggesting that intracellular production of this hormone may modulate local concentrations and influence vascular responses (25-27). However, the vasoconstrictor response to G15 was not evident in mesenteric vessels, perhaps reflecting a lower level of estradiol synthesis in these vessels. Nonetheless, the similarities between estradiol- and G-1-induced vasodilation and the ability of G15 to attenuate both responses implicate GPR30 as the primary mediator of estrogenic relaxation in the hypertensive mRen2.Lewis female.
Acute administration of estradiol in vivo reduces systemic vascular resistance in women and in animal models (2,28-30). However, while estradiol circulates at nanomolar concentrations, the concentrations required to achieve vasodilation ex vivo are much higher, typically in the micromolar range (8,31-34). In the present study, vessels from hemizygous mRen2.Lewis females exhibited significant relaxation to both estradiol and G-1 at nanomolar concentrations. The maximal response and the IC50 were comparable between these two agonists in both conduit and resistance vessels, suggesting that selective GPR30 activation is equally effective as estradiol-induced vasodilation. While the shape of the concentration response curves from mesenteric vessels suggested a biphasic response, attempts to fit the data to a two-site model were not statistically significant in comparison to a one-site fit. However, our data may be suggestive of a high affinity site which is completely blocked by endothelial denuding or L-NAME and a lower affinity endothelium-independent site.
Estrogen depletion markedly exacerbates hypertension in the female mRen2.Lewis and is reversed by chronic estradiol or G-1 treatment (18,19). However, G-1 does not decrease blood pressure in male mRen2.Lewis rats, implicating sex differences in vascular GPR30 expression. We show here that G-1 and estradiol induce vasorelaxation that is not different in OVX versus intact females, suggesting that vascular GPR30 expression is not altered by estrogen status. These studies are particularly important because of the recent evolution of the “timing hypothesis”, which suggests that the absence of endogenous estrogens between menopause and initiation of hormone therapy may alter estrogen receptor expression and downstream cardiovascular outcomes (35). The fact that both G-1 and estradiol maintain relaxant effects in isolated vessels from estrogen-depleted mRen2.Lewis suggests that the loss of the GPR30 ligand rather than altered receptor expression or downstream effector pathways such as nitric oxide likely contribute to exacerbated pressure in this hypertensive stain. We previously showed that estradiol replacement reduces renal and circulating angiotensin II and ACE in ovariectomized mRen2.Lewis rats (18). Similarly, G-1 administration in these animals decreases vascular ACE and AT1 expression but increases ACE2 (19). Therefore, it is plausible that the actions of GPR30 encompass both direct vasodilatory effects and long-term genomic modulation of the RAS to produce a sustained overall reduction in blood pressure. Additional studies are required to distinguish the influential actions of GPR30 on blood pressure in the hypertensive mRen2.Lewis female.
We and others have previously shown GPR30 immunostaining in both the intima and media of rat aorta and carotid, although G-1 vasorelaxation was completely endothelium-dependent in these vessels (19,36). In the mesenteric vasculature, GPR30 was also expressed in both endothelial and smooth muscle cells; however, endothelial denuding only inhibited the G-1 response by ~50%. Moreover, addition of the nitric oxide synthase inhibitor L-NAME attenuated the effects of G-1 to a similar extent. These data clearly suggest that at least part of the vasorelaxant actions of GPR30 are linked to the release of nitric oxide. However, the residual vasorelaxation in denuded vessels suggests a potential role for direct signaling on vascular smooth muscle cells in the microcirculation. Indeed, immunofluorescence staining and immunoblotting confirmed GPR30 expression in mesenteric smooth muscle cells from the intact mRen2.Lewis female. Others have reported endothelium-independent vasorelaxation in response to estradiol, which may result from activation of plasma membrane ion channels in vascular smooth muscle (8,37-39). The identification of a nitric oxide-independent pathway in mesenteric vessels will require additional studies in the mRen2.Lewis, as well as the background Lewis strain. At this point, it is not known whether GPR30-dependent signaling pathways are similar in mesenteric vessels from normotensive and hypertensive rats.
CONCLUSION
The current study distinguished the vasodilatory effects mediated by GPR30 from the nonselective response to estradiol. We demonstrate that GPR30 plays a predominant role in estrogen-induced vasodilation ex vivo, and in light of our previous results showing the in vivo antihypertensive effects of G-1, we propose that GPR30 mediates estrogenic vasodilation in the female hypertensive mRen2.Lewis. Due to the existence of multiple receptor estrogen subtypes, the selective activation or inactivation of estrogen receptors may prove to be more beneficial in treating estrogen-dependent diseases, as evidenced by the clinical success of selective estrogen receptor modulators. The assessment and development of new modulators that exploit GPR30 signaling may potentially advance the treatment for postmenopausal cardiovascular disease (40-42).
ACKNOWLEDGEMENTS
Funding provided by the National Heart Lung and Blood Institute, NIH (HL-56973, HL-51952, CA-127731), and the American Heart Association (0825515E and 10BGIA3080005) and unrestricted grants from the Unifi Corporation (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pines A, Fisman EZ, Drory Y, Shapira I, Averbuch M, Eckstein N, Motro M, Levo Y, Ayalon D. The Effects of Sublingual Estradiol on Left Ventricular Function at Rest and Exercise in Postmenopausal Women: an Echocardiographic Assessment. Menopause. 1998;5(2):79–85. [PubMed] [Google Scholar]
- 2.Leonardo F, Medeirus C, Rosano GM, Pereira WI, Sheiban I, Gebara O, Bellotti G, Pileggi F, Chierchia SL. Effect of Acute Administration of Estradiol 17 Beta on Aortic Blood Flow in Menopausal Women. Am J Cardiol. 1997 Sep;80(6):791–793. doi: 10.1016/s0002-9149(97)00520-1. [DOI] [PubMed] [Google Scholar]
- 3.Cruz MN, Douglas G, Gustafsson JA, Poston L, Kublickiene K. Dilatory Responses to Estrogenic Compounds in Small Femoral Arteries of Male and Female Estrogen Receptor-Beta Knockout Mice. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H823–H829. doi: 10.1152/ajpheart.00815.2005. [DOI] [PubMed] [Google Scholar]
- 4.Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol Alters Nitric Oxide Production in the Mouse Aorta Through the Alpha-, but Not Beta-, Estrogen Receptor. Circ Res. 2002 Mar;90(4):413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- 5.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular Estrogen Receptors and Endothelium-Derived Nitric Oxide Production in the Mouse Aorta. Gender Difference and Effect of Estrogen Receptor Gene Disruption. J Clin Invest. 1997 May;99(10):2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freay AD, Curtis SW, Korach KS, Rubanyi GM. Mechanism of Vascular Smooth Muscle Relaxation by Estrogen in Depolarized Rat and Mouse Aorta. Role of Nuclear Estrogen Receptor and Ca2+ Uptake. Circ Res. 1997 Aug;81(2):242–248. doi: 10.1161/01.res.81.2.242. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson BO, Ekblad E, Heine T, Gustafsson JA. Increased Magnitude of Relaxation to Oestrogen in Aorta From Oestrogen Receptor Beta Knock-Out Mice. J Endocrinol. 2000 Aug;166(2):R5–R9. doi: 10.1677/joe.0.166r005. [DOI] [PubMed] [Google Scholar]
- 8.Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK. Non-Genomic Mechanism of 17 Beta-Oestradiol-Induced Inhibition of Contraction in Mammalian Vascular Smooth Muscle. J Physiol. 1997 Mar;499(Pt 2):497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu XD, Cui YH, Lin GP, Wang TH. Non-Genomic Effects of 17beta-Estradiol in Activation of the ERK1/ERK2 Pathway Induces Cell Proliferation Through Upregulation of Cyclin D1 Expression in Bovine Artery Endothelial Cells. Gynecol Endocrinol. 2007 Mar;23(3):131–137. doi: 10.1080/09513590601181457. [DOI] [PubMed] [Google Scholar]
- 10.Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of Cyclic Adenosine 3',5'- Monophosphate Signaling and Pulsatile Neurosecretion by Gi-Coupled Plasma Membrane Estrogen Receptors in Immortalized Gonadotrophin-Releasing Hormone Neurons. Mol Endocrinol. 2003 Dec;17(12):1792–1804. [PubMed] [Google Scholar]
- 11.Lee DY, Chai YG, Lee EB, Kim KW, Nah SY, Oh TH, Rhim H. 17Beta-Estradiol Inhibits High-Voltage-Activated Calcium Channel Currents in Rat Sensory Neurons Via a Non-Genomic Mechanism. Life Sci. 2002 Mar;70(17):2047–2059. doi: 10.1016/s0024-3205(01)01534-x. [DOI] [PubMed] [Google Scholar]
- 12.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential Effects of 17beta-Estradiol on Function and Expression of Estrogen Receptor Alpha, Estrogen Receptor Beta, and GPR30 in Arteries and Veins of Patients With Atherosclerosis. Hypertension. 2007 Jun;49(6):1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 13.Chappell MC, Westwood BM, Yamaleyeva LM. Differential Effects of Sex Steroids in Young and Aged Female MRen2.Lewis Rats: a Model of Estrogen and Salt-Sensitive Hypertension. Gend Med. 2008;5(Suppl A):S65–S75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and Salt Sensitivity in the Female MRen(2).Lewis Rat. Am J Physiol Regul Integr Comp Physiol. 2006 Nov;291(5):R1557–R1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen Regulation of Angiotensin-Converting Enzyme MRNA. Hypertension. 1999 Jan;33(1 Pt 2):323–328. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- 16.Pendergrass KD, Westwood BM, Chappell MC. Differential Expression of Neprilysin and Angiotensin-Converting Enzyme 2 May Contribute to the Gender Disparity in the Hypertensive MRen2.Lewis Strain. Hypertension. 2007 Oct;50:e75–e155. [Google Scholar]
- 17.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex Differences in Circulating and Renal Angiotensins of Hypertensive MRen(2).Lewis but Not Normotensive Lewis Rats. Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 Antagonist Olmesartan Reverses the Development of Profound Hypertension in the Congenic MRen(2).Lewis Rat. Hypertension. 2003 Oct;42(4):781–786. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- 19.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic Treatment With the G Protein-Coupled Receptor 30 Agonist G-1 Decreases Blood Pressure in Ovariectomized MRen2.Lewis Rats. Endocrinology. 2009 Aug;150(8):3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and Biomolecular Screening Converge on a Selective Agonist for GPR30. Nat Chem Biol. 2006 Apr;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 21.Mulvany MJ, Halpern W. Contractile Properties of Small Arterial Resistance Vessels in Spontaneously Hypertensive and Normotensive Rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In Vivo Effects of a GPR30 Antagonist. Nat Chem Biol. 2009 Jun;5(6):421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsey SH, Tribe RM, Songu-Mize E. Cyclic Stretch Decreases TRPC4 Protein and Capacitative Calcium Entry in Rat Vascular Smooth Muscle Cells. Life Sci. 2008 Jul;83(1-2):29–34. doi: 10.1016/j.lfs.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory Role of G Protein-Coupled Estrogen Receptor for Vascular Function and Obesity. Circ Res. 2009 Feb;104(3):288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayard F, Clamens S, Meggetto F, Blaes N, Delsol G, Faye JC. Estrogen Synthesis, Estrogen Metabolism, and Functional Estrogen Receptors in Rat Arterial Smooth Muscle Cells in Culture. Endocrinology. 1995 Apr;136(4):1523–1529. doi: 10.1210/endo.136.4.7895662. [DOI] [PubMed] [Google Scholar]
- 26.Bayard F, Clamens S, Delsol G, Blaes N, Maret A, Faye JC. Oestrogen Synthesis, Oestrogen Metabolism and Functional Oestrogen Receptors in Bovine Aortic Endothelial Cells. Ciba Found Symp. 1995;191:122–132. doi: 10.1002/9780470514757.ch7. [DOI] [PubMed] [Google Scholar]
- 27.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized Expression of Aromatase in Human Vascular Tissues. Circ Res. 1999 Jun;84(11):1285–1291. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 28.Beyer ME, Yu G, Hanke H, Hoffmeister HM. Acute Gender-Specific Hemodynamic and Inotropic Effects of 17beta-Estradiol on Rats. Hypertension. 2001 Nov;38(5):1003–1010. doi: 10.1161/hy1101.093422. [DOI] [PubMed] [Google Scholar]
- 29.Magness RR, Rosenfeld CR. Local and Systemic Estradiol-17 Beta: Effects on Uterine and Systemic Vasodilation. Am J Physiol. 1989 Apr;256(4 Pt 1):E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- 30.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen Induces Vascular Wall Dilation: Mediation Through Kinase Signaling to Nitric Oxide and Estrogen Receptors Alpha and Beta. J Biol Chem. 2005 May;280(20):19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 31.Keung W, Vanhoutte PM, Man RY. Nongenomic Responses to 17beta-Estradiol in Male Rat Mesenteric Arteries Abolish Intrinsic Gender Differences in Vascular Responses. Br J Pharmacol. 2005 Dec;146(8):1148–1155. doi: 10.1038/sj.bjp.0706422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naderali EK, Walker AB, Doyle P, Williams G. Comparable Vasorelaxant Effects of 17alpha- and 17beta-Oestradiol on Rat Mesenteric Resistance Arteries: an Action Independent of the Oestrogen Receptor. Clin Sci (Lond) 1999 Dec;97(6):649–655. [PubMed] [Google Scholar]
- 33.Shaw L, Taggart MJ, Austin C. Mechanisms of 17 Beta-Oestradiol Induced Vasodilatation in Isolated Pressurized Rat Small Arteries. Br J Pharmacol. 2000 Feb;129(3):555–565. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teoh H, Leung SW, Man RY. Short-Term Exposure to Physiological Levels of 17 Beta-Estradiol Enhances Endothelium-Independent Relaxation in Porcine Coronary Artery. Cardiovasc Res. 1999 Apr;42(1):224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- 35.Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM. Observational Studies Analyzed Like Randomized Experiments: an Application to Postmenopausal Hormone Therapy and Coronary Heart Disease. Epidemiology. 2008 Nov;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broughton BR, Miller AA, Sobey CG. Endothelium-Dependent Relaxation by G Protein Coupled Receptor 30 Agonists in Rat Carotid Arteries. Am J Physiol Heart Circ Physiol. 2010 Jan; doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 37.Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium Independent Relaxation of Human Coronary Arteries by 17 Beta-Oestradiol in Vitro. Cardiovasc Res. 1993 Nov;27(11):1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 38.Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-Independent Relaxation of Rabbit Coronary Artery by 17 Beta-Oestradiol in Vitro. Br J Pharmacol. 1991 Dec;104(4):1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salas E, Lopez MG, Villarroya M, Sanchez-Garcia P, De PR, Dixon WR, Garcia AG. Endothelium-Independent Relaxation by 17-Alpha-Estradiol of Pig Coronary Arteries. Eur J Pharmacol. 1994 Jun;258(1-2):47–55. doi: 10.1016/0014-2999(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 40.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002 Jul;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 41.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen Plus Progestin and the Risk of Coronary Heart Disease. N Engl J Med. 2003 Aug;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 42.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory Biomarkers, Hormone Replacement Therapy, and Incident Coronary Heart Disease: Prospective Analysis From the Women's Health Initiative Observational Study. JAMA. 2002 Aug;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]