Abstract
The purpose of this preliminary study was to assess whether behavioral and psychophysiological correlates of emotional reactivity and regulation are associated with developmental stuttering, as well as determine the feasibility of these methods in preschool-age children. Nine preschool-age children who stutter (CWS) and nine preschool-age children who do not stutter (CWNS) listened to brief background conversations conveying happy, neutral, and angry emotions (a resolution conversation followed the angry conversation), then produced narratives based on a text-free storybook. Electroencephalograms (EEG) recorded during listening examined cortical correlates of emotional reactivity and regulation. Speech disfluencies and observed emotion regulation were measured during a narrative immediately after each background conversation. Results indicated that decreased use of regulatory strategies is related to more stuttering in children who stutter. However, no significant differences were found in EEG measurements of emotional reactivity and regulation between CWS and CWNS or between emotion elicitation conditions. Findings were taken to suggest that use of regulatory strategies may relate to the fluency of preschool-age children’s speech-language output.
Learner Outcomes: The reader will be able to (1) describe emotional reactivity and regulation processes, (2) discuss evidence for or against the relations of emotional reactivity, regulation and stuttering, (3) understand how multiple measures can be used to measure emotional reactivity and regulation.
Keywords: stuttering, preschool, emotional reactivity, emotion regulation, EEG
1. Introduction
Speculation on the potential impact of personality, emotional adjustment, and other psychological constructs on developmental stuttering began as early as the 1930s (e.g., Bender, 1939; Brown & Hull, 1942; Glauber, 1958; Murphy, 1953; Murphy & Fitzsimons, 1960) and continues to receive theoretical, empirical, and clinical attention (e.g. Adams, 1993; Alm, 2004; Conture et al., 2006; Eggers, De Nil, & Van den Bergh, 2008; Peters & Hulstijn, 1984; Weber & Smith, 1990; Yairi, 1997). To date, most empirical studies of emotions and childhood stuttering have focused on stable, trait-like characteristics of children who do (CWS) and do not stutter (CWNS). These studies have provided some evidence that CWS, compared to CWNS, demonstrate higher emotional reactivity in that they may be more sensitive or reactive to environmental changes (Fowlie & Cooper, 1978; Glasner, 1949; Schwenk, Conture, & Walden, 2007), more active and impulsive (Embrechts, Ebben, Franke, & van de Poel, 1998), and more apt to react with negative emotions (Fowlie & Cooper, 1978; Johnson, Walden, Conture, & Karrass, in press; Karrass, Conture, & Walden, 2008; Karrass et al., 2006). There is also some support for the notion that CWS, compared to CWNS, demonstrate lower emotion regulation in that they are less adaptable (Anderson, Pellowski, Conture, & Kelly, 2003), exhibit less inhibitory control (Embrechts et al., 1998), and have less effective emotion and attention regulation (Karrass et al., 2006).
In contrast, one parent-report study indicated that CWS, compared to CWNS, were less negative in their emotions and more adaptable (K. E. Lewis & Goldberg, 1997). Similarly, another parent-report study, indicated that 20% of the CWS, compared to 5% of the CWNS, demonstrated a temperamental constellation of “easy child” (M. J. Williams, 2006), though it is interesting to note that in this study a higher proportion of CWS, compared to CWNS, fit the temperamental constellation of “slow to warm up.” This latter finding appears consistent with previous findings that CWS may be less adaptable to new situations (Anderson et al., 2003).
The variance in findings in empirical studies of emotional reactivity and regulation in CWS may relate to the measures used. Most such studies have used parent-report measures to assess temperament and emotions (Anderson et al., 2003; Embrechts et al., 1998; Fowlie & Cooper, 1978; Glasner, 1949; Karrass et al., 2006; K. E. Lewis & Goldberg, 1997; M. J. Williams, 2006). Although well-crafted parent-report measures have many strengths (Goldsmith & Hewitt, 2003), behavioral coding and psychophysiological measures such as electroencephalography (EEG) – which have sufficient temporal resolution to track moment-to-moment changes in cognitive, emotional and speech-language behavior – may aid in further understanding the relations between stuttering and emotions.
Although psychophysiological measures have not been used to assess emotion in CWS, behavioral coding has been used. For example, one laboratory observation study reported increased reactivity to environmental stimuli and poorer attentional control in CWS, compared to CWNS (Schwenk et al., 2007). This apparent decrease in attentional control may have implications for the ability of CWS to effectively regulate their emotions as regulatory strategies involving the modulation of attention have been found to be effective in decreasing emotional intensity (Derryberry & Rothbart, 1988; Gilliom, Shaw, Beck, Schonberg, & Lukon, 2002).
In another empirical study of emotional reactivity and regulation in which behavioral coding procedures were used, CWS demonstrated more negative reactions than CWNS after receiving a disappointing gift (Johnson et al., in press). Taken together, parent-report and laboratory-based results support further study of emotional reactivity and regulation in young children who stutter.
1.1. Emotional Reactivity and Regulation: Defined
Emotional reactivity refers to the intensity and threshold for both positive and negative emotional responding that individuals tend to experience (Eisenberg & Fabes, 1992). For example a child with a high level of emotional reactivity would be more likely to experience intense levels of negative emotionality during peer conflict (Fabes et al., 1999), intense levels of positive emotionality during peer play interactions (Derryberry & Reed, 2003), or both.
Emotion regulation is the process of monitoring, evaluating, and modifying emotional reactions (Thompson, 1994). There is some debate on how emotion regulation is defined (e.g., Bell & Wolfe, 2004; Bridges, Denham, & Ganiban, 2004; Campos, Frankel, & Camras, 2004; Eisenberg & Spinrad, 2004). For example, some view emotion and emotion regulation as two distinct processes (Bridges et al., 2004; Cole, Martin, & Dennis, 2004; Eisenberg & Spinrad, 2004; Hoeksma, Oosterlaan, & Schipper, 2004), whereas others see emotion and emotion regulation as one unified process (Campos et al., 2004; M. D. Lewis & Stieben, 2004).
Evidence indicates that neural structures responsible for emotional reactions are also responsible for emotion regulation and that these two processes do not occur in discrete temporal windows (M. D. Lewis & Stieben, 2004) or in a linear fashion (Campos et al., 2004). For example, an emotion may be preceded by regulatory processes, such as cognitive reappraisal, that influence the type or strength of response (Campos et al., 2004; Gross, 2002).
Thus, there is some evidence that emotional reactions and emotion regulation are interrelated. However, some argue and have demonstrated that these two constructs can be measured separately by using experimental procedures that activate target emotions, establishing predictable temporal relations between regulatory efforts and changes in activated emotions (Cole et al., 2004; Jackson et al., 2003). Another way to disambiguate emotional reactivity and regulation, as suggested by Cole et al. (2004), is to use multiple, converging measures such as physiological and behavioral methods. For example, if behavioral coding indicates a child is attempting to regulate emotions and physiological measures indicate a decrease in emotional reactivity during or after those putative regulatory attempts, then one might reasonably suggest that at least some degree of emotion regulation occurred. The present study was designed in accordance with these suggestions. After the emotion elicitation procedures (i.e., background conversation stimuli to be described in section 2.2.), participants’ attempts at regulation were coded. In addition, a second, physiological measure, EEG, was used to assess whether there was a decrease in emotional reactivity during or after these regulatory attempts.
Such measurement of changes in emotional reactivity is important as emotion regulation strategies may differ according to their effectiveness. For example, Gilliom, Shaw, Beck, Schonberg, and Lukon (2002) reported that in 3-and-a-half-year old boys during a task in which a cookie was withheld for a period of time, shifting attention away from sources of frustration (i.e., distraction) and seeking information about situational constraints (i.e., cognitive restructuring) were associated with decreased anger. Conversely, focus on the delay or object being withheld was associated with increased anger.
In the case of stuttering, a child may attempt to regulate instances of stuttering by not initiating speech with a peer or not responding to a question. Such avoidance of speaking could be broadly viewed as a regulatory strategy to reduce speech disfluencies. Although this strategy may be effective in decreasing stuttering or emotional reactivity in these situations, such strategies are not likely to encourage communication or social engagement. Just as with attempts to regulate emotions, attempts to regulate the fluency of speech-language output may not be optimal. Thus, the present study did not code as regulatory attempts avoidance behaviors aimed at directly reducing speech disfluencies behaviors (e.g., not responding to narrative elicitation prompts). Furthermore, of the regulatory strategies in the coding system used, such as self-stimulation, they were only coded when they did not coincide with disfluent speech.
1.2. Behavioral Coding of Emotion Regulation
Behavioral coding identifies enactment of putative regulatory behaviors after a specific emotion is elicited (Cole et al., 2004). Such regulatory behaviors have been used to track the use of regulatory strategies in 12–18-month old (Buss & Goldsmith, 1998), 2-year old (Grolnick, Bridges, & Connell, 1996), 2-and-a-half year old (Putnam, Spritz, & Stifter, 2002), and 3-and-a-half year old children (Gilliom et al., 2002). Typically, the types of regulatory behaviors coded have included distraction (e.g., shifting attention to something other than the target activity) and self-stimulation or seeking physical comfort from others, depending on the context (Buss & Goldsmith, 1998; Gilliom et al., 2002; Grolnick et al., 1996). In studies of 2–3-year old children, regulatory behaviors also include cognitive restructuring behaviors, in which the child seeks more information from the experimenter or directs statements to herself such as “I will get a prize when I’m done,” and instrumental coping behaviors in which the child focuses on the target activity and attempts to change something about the situation such as asking for a desired object (Gilliom et al., 2002; Grolnick et al., 1996).
Behavioral coding of regulatory attempts have helped identify patterns of responding in preschool-age children when they were presented with simulations of adult conflict compared to positive or neutral conversations between two adults (e.g., Cummings, 1987). Thus, behavioral coding methods paired with emotion elicitation methods appear to aid in the assessment of emotion regulation from infancy to preschool-age and, therefore, would seem to be useful in examining differences between preschool-age CWS and CWNS.
1.3. Electroencephalography (EEG) as a Measure of Emotions
EEG, particularly asymmetry in EEG alpha rhythm, has also been used to study emotions, as substantiated by over twenty years of research in normally fluent adults and children (for review, see Coan & Allen, 2004). Specifically, greater cortical activation (i.e., less EEG alpha) over the right frontal scalp region appears to be a correlate of negative emotions, whereas greater activation over the left frontal scalp region appears to be a correlate of positive emotions. For example, in a study of 21-month-old infants, Dawson, Panagiotides, Klinger, and Hill (1992), reported more right frontal activation when infants exhibited negative facial expressions. Furthermore, Fox and Davidson (1986) reported that newborn infants had more left than right frontal activation when tasting a pleasant stimulus. An alternative view of frontal asymmetry is an approach-withdrawal model in which right frontal asymmetry indicates a tendency for avoidance and left frontal asymmetry indicates a tendency for approach behaviors (Coan & Allen, 2003; Davidson, 1993). For this study, however, we adopted the traditional valence model (i.e., right frontal asymmetry = negative emotion, left frontal asymmetry = positive emotion).
Heller (1990, 1993) proposed a second dimension to the EEG frontal asymmetry valence model. According to Heller’s model, greater right versus left parietal activation is associated with increased autonomic arousal (see also Davidson, Chapman, & Chapman, 1987; Davidson, Schaffer, & Saron, 1985; Henriques & Davidson, 1990; McManis, Kagan, Snidman, & Woodward, 2002). For example, in a longitudinal study at 4, 14, and 21 months of age, infants and young children classified as being highly reactive based on behavioral observations demonstrated greater brain activity over the right parietal region than children prone to low levels of arousal (McManis et al., 2002).
With regard to stuttering, there is a long history of EEG studies (see Bloodstein & Bernstein Ratner, 2008, pp. 127–135, for review) with many such investigations involving expressive and receptive language tasks in adults who do (AWS) versus adults who do not stutter (AWNS) (e.g., Boberg, Yeudall, Schopflocher, & Bo-Lassen, 1983; W.H. Moore, 1984; Pinsky & McAdam, 1980; Weber-Fox, Spruill, Spencer, & Smith, 2008). EEG studies reporting greater right than left activity (i.e., decreased alpha) during speech-language tasks were interpreted to mean that people who stutter process speech-language stimuli using the right hemisphere, considered non-dominant for speech and language (e.g., Boberg et al., 1983; W.H. Moore & Haynes, 1980; W.H. Moore & Lorendo, 1980). Boberg et al (1983), however, also suggested that greater activity in the right hemisphere could be related to negative emotions conditioned by adverse experiences with speech-language production. Despite this possibility, to the present authors’ knowledge, only three studies have examined EEG correlates of emotions in people who stutter (Douglass, 1952; Knott, Correll, & Shepherd, 1959; Murphy, 1953).
Two of these studies reported cortical differences between AWS and AWNS when presented with emotion-eliciting tasks. For example, Douglass (1952) observed that AWS had greater cortical reactivity (i.e., change in percent alpha) in the dominant hemisphere when presented with emotional compared to non-emotional pictures. Murphy (1953) reported that AWS listening to two passages, one abstract and the other vivid in imagery, had more cortical activation (reduced alpha) in both hemispheres than AWNS in the listening condition relative to pre-listening levels. Findings also indicated that after listening to the passages, AWS demonstrated less recovery to previous alpha levels in both hemispheres than AWNS.
Although there are limitations in how we can interpret their findings relative to emotions, these studies would seem to provide some early psychophysiological clues suggesting different brain responses to emotional stimuli in people who stutter. To date there have been no similar psychophysiological studies of emotion in relation to stuttering in young children, at ages closer to the onset of stuttering, and therefore, less influenced by long-term experience or learning resulting from a stuttering history.
Given the above literature review and apparent needs for empirical study of emotions in young children who stutter, the present study was conducted for two broad purposes. The first purpose was to assess, at a preliminary level, whether stuttering is related to emotional reactivity and regulation in young CWS and CWNS. A secondary purpose was to determine the feasibility of emotion elicitation and psychophysiological measurement of emotion in preschool-age children who stutter.
It was hypothesized that CWS would demonstrate more emotional reactivity and less regulation than CWNS in response to emotion-eliciting stimuli (i.e., background conversations). Given this hypothesis, increased emotional reactivity and decreased regulation while listening to emotionally evocative conversations were expected to be associated with increased speech disfluency. In essence, these authors speculated, if CWS had more emotional reactivity and less regulation than CWNS, and CWS also demonstrated more speech disfluency when there was more emotional reactivity or less regulation, then such changes in CWS’ emotional processes may be related to their difficulty initiating or maintaining normally fluent speech.
2. Method
2.1. Participants
Children Who Stutter (CWS). Nine children between the ages of 3;0 (years;months) and 5;11 (M = 4;5, SD = 9 months), including 6 boys and 3 girls, were classified as CWS because they (a) exhibited three or more stuttering-like disfluencies (SLDs; i.e., sound-syllable repetition = complete or incomplete repetition of syllables within a word, whole word repetition = complete word repetition, audible sound prolongation = fixed posture with audible airflow, and inaudible sound prolongation = fixed posture with inaudible airflow [including those occurring at the beginning and middle of words] (Conture, 2001, Table 1.1, pp. 5–6; Teeson, Packman, & Onslow, 2003) and (b) received a total overall score of 11 or above (i.e., a severity equivalent of at least “mild”) on the Stuttering Severity Instrument-3 (SSI-3, Riley, 1994). Six were right-handed and three were left-handed (based on the Edinburgh Handedness Inventory, Oldfield, 1971).
Children Who Do Not Stutter (CWNS). Nine children matched on age +/− 9 months (M = 4;8, SD = 8 months) and gender (6 boys, 3 girls) to the CWS, were classified as CWNS because they (a) exhibited two or fewer SLDs per 100 words of conversational speech and (b) received a total overall score of 8 or below (i.e., a severity equivalent of less than “mild”) on the SSI-3 (Riley, 1994). Eight were right-handed and 1 was left-handed. Handedness scores did not significantly differ between the two talker groups (i.e., CWS and CWNS), t(16) = −.559, p = .584.
Likewise, chronological age did not significantly differ between the CWS and CWNS, t(16) = −1.075, p = .298.
Participants were paid volunteers naïve to the purposes and methods of the study and were referred to the Vanderbilt Bill Wilkerson Center (Nashville, TN) by their parents, other speech-language pathologists, or by daycare, preschool or school personnel. CWNS were also recruited through a birth records database. Participants were native speakers of American English with no known or reported hearing, neurological, developmental, academic, intellectual or emotional problems, and no history of speech-language therapy. Further criteria for participant inclusion are described in section 2.3 below. Participants were part of an ongoing series of studies of developmental stuttering (e.g., Anderson & Conture, 2004; Byrd, Conture, & Ohde, 2007). The Vanderbilt University Institutional Review Board approved this investigation. Accordingly, the parents gave informed consent and the children gave assent.
2.2. Stimuli
Emotion Elicitation Stimuli. The emotion stimulation paradigm was adapted from studies of children’s reactions to marital distress, particularly background anger in preschool-age children (Cummings, 1987; Cummings, Vogel, Cummings, & El-Sheikh, 1989; El-Sheikh, Cummings, & Goetsch, 1989) and school-age children (e.g., Cummings, Wilson, & Shamir, 2003; Nixon & Cummings, 1999). Cummings and colleagues used background emotion stimuli involving angry, friendly, and neutral adult interactions (Cummings et al., 2003; Nixon & Cummings, 1999). The background conversations for the present study were audio recordings of adult actress dyads. One dyad read an angry conversation and then a resolution conversation in which they resolve their anger, another dyad read a happy conversation, and another dyad read a neutral conversation. A resolution conversation was included because it was thought that the “return to baseline,” shown to occur for children in similar background emotion studies (Cummings, Ballard, El-Sheikh, & Lake, 1991), would reflect regulation of negative emotions elicited by the angry conversation. All background conversation stimuli had the same number of utterances (41 per conversation) and equivalent mean lengths of utterance ranging from 7.22 to 7.27 grammatical morphemes. Background conversation audio recordings, created in an anechoic chamber, were made equivalent according to loudness (−25 dB root mean squared +/− .5 dB).
These affective stimuli were validated using Likert scales in a sample of 29 adults and 15 preschool-age CWNS, who did not participate in the main study (age: M = 4;7, range = 3;1 to 5;4). Adult ratings of the four (i.e., neutral, happy, angry, and resolution) background conversations indicated they were perceived by adults according to the intended emotions and arousal levels. The children rated the stimuli using scales created from pictures of emotional faces (Tottenham, Borscheid, Ellersten, Marcus, & Nelson, 2002). The happy conversation was rated by 87% of the children as “happy” whereas the angry conversation was rated by 93% of the children as “mad.” For the neutral conversation, 40% rated it as “happy” and 27% rated it as “just ok,” indicating it was perceived as mildly positive. The resolution conversation was rated as “happy” by 67% by the children, indicating it was perceived as positive, though not as positively as the happy conversation. There was a statistically significant association between background conversation category and child ratings (Cramér’s V = .533, p < .0001), suggesting that young children typically perceived the background conversations as intended.
Narrative Stimuli. Children’s narratives were elicited using modified versions of Mercer Mayer’s Frog, Where Are You? (Mayer, 1969), A Boy, a Dog, and a Frog (Mayer, 1967), and Frog on His Own (Mayer, 1972) children’s text-free storybook series. Each Microsoft PowerPoint slide show for the storybooks included 15 pictures. On average, children produced 241 words per narrative and 46 words per minute for the first three minutes of each narrative.
2.3. Procedures
Participants completed two visits to the laboratory. The first visit included speech, language, and hearing assessments. All participants were administered the Peabody Picture Vocabulary Test – Third Edition (PPVT-III, Dunn & Dunn, 1997), the Expressive Vocabulary Test (EVT, K. T. Williams, 1997), the Test of Early Language Development Third Edition (TELD-3, Hresko, Reid, & Hamill, 1999) and the Goldman-Fristoe Test of Articulation: Second Edition (G-FTA-2, Goldman & Fristoe, 2000) to assess receptive and expressive vocabulary, receptive and expressive language, and articulation, respectively. Requirements for inclusion were scores at or above the 16th percentile. In addition, all participants passed a bilateral pure tone hearing screening at 1, 2, and 4 kHz at 20 dB HL; a tympanometric screening (ASHA, 1996); and an ipsilateral acoustic reflex screening at 1 and 2 kHz1. In all, 18 children met the inclusion criteria2
Additionally, parents of the participants provided socio-economic status (SES) information using the Four-Factor Index of Social Status (Hollingshead, 1975), which takes into account maternal and paternal education and occupation. There were no significant differences in SES between CWS and CWNS (CWS: M = 42.6, SD = 9.6; CWNS: M = 39.4, SD = 9.4), t(16) = .719, p = .483.
During the second visit, participants completed the experimental task which involved a “story preview – background conversation – child narrative” sequence. The child faced a computer screen and was seated beside a researcher and in a sound-isolated room. After the EEG net was placed on the child’s head, he or she was told “You are going to tell me a story about a boy, a dog, and a frog. Let’s look at the pictures first while you think about the story.” The story preview consisted of the child looking at each of the story’s pictures for 1–2 seconds per slide. Then the researcher asked the child to “wait quietly while I do some paperwork.” Children then looked at a computer screen displaying slow-moving bubbles while a happy, angry, or neutral background conversation played via an overhead speaker at 70–75 SPL. EEG recordings began at the onset of the background conversation and continued until 30 seconds after the background stimulus ended.
The background conversation paradigm was designed to mimic an overheard impromptu conversation between adults. Thus the experimenter did not inform the child that he or she would hear anything. On rare occasion, when a child heard the first background conversation, he or she would ask, “who is that?” When this happened, the experimenter would say “some people talking” and direct the child to continue looking at the slow moving bubbles on the computer screen. In the vast majority of cases, however, children did not inquire about the background conversations.
Once the EEG recording stopped, the computer displayed the previewed storybook pages and the researcher directed the child to tell a story based on the pictures. To elicit the narratives, the researcher provided up to three standard prompts, if needed, until the child produced at least two utterances per text-free storybook picture. Coded behavioral measures of emotion regulation and speech disfluencies were subsequently obtained from audio-video recordings of the first three minutes of participant narratives.
This “story preview – background conversation – child narrative” procedure was repeated using a different text-free storybook each time until the happy, angry, and neutral background conversations were all presented in counterbalanced order. After the angry background conversation and related child narrative, a resolution background conversation was presented to the child while EEG was measured.
2.4. Behavioral Data Collection
The coding system, adapted from Buss and Goldsmith (1998), assessed duration and frequency of emotion regulation strategies for each of the first three minutes of child narrative based on video coding with coders blind to emotion condition. The four regulation strategies coded were distraction, defined as the child occupying attention with something other than the narrative task (e.g., looking in mirror rather than at computer screen); self-stimulation, defined as the child making contact with two different parts of the body in a self-stimulating way or moving individual body parts repetitively (e.g., rubbing hands together); cognitive restructuring, defined as the child asking for an explanation or commenting on positive aspects of the situation (e.g., reminding self how many pages are left in the storybook); and instrumental coping, defined as the child attempting to change the situation (e.g., requesting the next page of the storybook). Frequency and duration for these four regulatory strategy attempts were summed to create one regulation metric for frequency and one for duration for each of the first three minutes of the children’s narratives.
Temporal epochs excluded from final data corpus. Time periods during which instances of stuttering or other speech disfluencies occurred were excluded from regulatory strategy analyses. Additionally, time periods during which either the child’s face or body was not visible on the cameras were excluded from regulatory strategy analysis. Finally, time periods when the child’s attention was directed away from the narrative task by the researcher’s utterances, noises, or movements were excluded from regulatory strategy analyses rather than coded as distraction because the present study’s focus was on regulatory behaviors that were child-initiated rather than other-initiated.
Due to more speech disfluencies being produced by CWS compared to CWNS, the mean number of excluded seconds per minute was greater for CWS (M = 11.09 seconds, SD = 8.73) than CWNS (M = 6.75 seconds, SD = 5.53; t[135] = 3.78, p < .001). Thus, for each of the first three minutes of the children’s narratives, ratios for duration and frequency of regulatory behaviors were calculated by dividing duration and frequency totals by the total number of usable seconds within each minute. This procedure resulted in separate frequency and duration rates for the first minute, second minute, and third minute.
Interjudge measurement reliability for behavioral coding of regulatory attempts was calculated based on all three narratives for three randomly selected CWS and three CWNS (representing 33% of the total data corpus). Two trained coders coded the regulatory behaviors for the same six participants. Due to involvement with determining talker group classification (i.e., CWS or CWNS) the primary coder could not code behaviors while blind to talker group. However, the second coder was blind to talker-group classification and coded as much video as possible with the audio muted before coding with audio input. Coefficient alpha (Cronbach, 1951) assessed reliability of the two coders’ duration and frequency ratings for each of the four regulatory strategies. Alphas ranged between .72 and 1.00, which indicated an acceptable to high degree of interjudge reliability (Cicchetti, 1994).
2.5. Speech Disfluency Data Collection
Stuttering-like disfluencies were counted and categorized using the same system employed to determine talker group classification in section 2.1 (Conture, 2001, Table 1.1, pp. 5–6). Other disfluencies, which were not considered stuttering-like, were also coded. These included phrase repetitions (i.e., repeating phrases of two or more words), interjections (e.g., um, er), and revisions (e.g., The frog is looking for the – The dog is looking for the frog.) (Conture, 2001). Speech disfluencies were counted and categorized from audio-video recordings for each of the first three minutes of child narrative production. This speech disfluency coding was done blind to emotion condition. Measures of speech disfluency calculated for the first three minutes of each narrative were (1) total speech disfluencies per total words (TD/TW) with TD including other disfluencies (i.e., phrase repetitions, interjections, and revisions) and stuttering-like disfluencies (SLD), (2) stuttering-like disfluencies per total words (SLD/TW), and (3), stuttering-like disfluencies per total disfluencies (SLD/TD).
Interjudge measurement reliability for speech disfluency coding was calculated based on all three narrative speech samples from 3 randomly selected CWS and 3 randomly selected CWNS. The first and second coders for this study were certified speech-language pathologists trained in coding speech disfluencies. Coefficient alpha (Cronbach, 1951) assessed reliability of the two coders on the following variables: TD/TW, SLD/TW, and SLD/TD. Alphas ranged between .83 and .99, indicating a high degree of interjudge reliability (Cicchetti, 1994).
2.6. EEG Measurements
EEG Data Collection. The EEG was recorded using 128-channel electrode nets (Electrical Geodesics, Eugene, Oregon, USA). During the recording, data were referenced to vertex (Cz). Channel impedances were kept at less than or equal to 40 kΩ. Sampling rate was set to 250 Hz and the signal was analog bandpass filtered at 0.1–100 Hz.
EEG Data Processing. Manual post-session processing with EMSE Suite v5.1 (Source Signal Imaging, Inc., San Diego, California, USA) eliminated artifacts (e.g., noise due to movement, muscle tension, and eye blinking). Data were re-referenced off-line from vertex to the average of all electrodes (Junghoefer, Elbert, Tucker, & Braun, 1999)3. All artifact-free EEG epochs of at least 50-seconds duration were Hanning filtered. The absolute amplitude value was calculated for each frequency of recorded EEG using Fourier Transform (frequency resolution .5 Hz).
The measure derived from the EEG was power (µV2) in the 8–12 Hz (alpha) and 13–18 Hz (beta) frequency bands (i.e., ranges of frequencies) using procedures outlined in Davidson, Jackson, and Larson (2000). The observed distributions of absolute amplitude values were sorted within each of the 128 channels (i.e., electrodes). The mean absolute amplitude was calculated for each frequency bin (occurring every .5 Hz) and each EEG channel. Amplitudes for each .5 Hz bin were averaged for the two frequency ranges to determine values for alpha and beta. Mean amplitude values (µV) were squared to derive alpha and beta power (µV)2 for each participant in the four regions of interest (ROI): (1) left frontal, (2) right frontal, (3) left parietal, and (4) right parietal areas.
Although data from single electrodes placed in accordance with the International 10–20 system of electrodes (Jasper, 1958) have been used to assess topography in past EEG studies of emotional processes (e.g., Forbes et al., 2006; Hagemann, Naumann, Becker, Maier, & Bartussek, 1998; Jasper, 1958; Tomarken, Davidson, & Henriques, 1990), the use of high density electrode arrays in the present study necessitated a clustering method to reduce the number of electrodes entered in the statistical analysis (Key, Molfese, & Ratajczak, 2006). Data from electrodes corresponding to traditional frontal and parietal scalp locations were averaged for each hemisphere. Reliability of the clustering approach was verified using coefficient alpha (Cronbach, 1951) for each frequency band (i.e., alpha and beta), background conversation emotion (i.e., angry, resolution, happy, and neutral), and ROI (i.e., frontal and parietal) for all 18 participants. Alphas ranged from .915 to .997, indicating high data consistency within electrode clusters.
EEG Asymmetry Scores. Data from the frontal and parietal ROIs were derived by subtracting the natural log of the right hemisphere region from the natural log of its left hemisphere cognate (lnRight-lnLeft), the accepted practice in EEG emotion research (Coan & Allen, 2004). It is also a well-established procedure to interpret change in asymmetry score from one condition to another as a change in emotion (Fox, Bell, & Jones, 1992; Fox & Davidson, 1986; Fox & Davidson, 1988). Thus, to derive an emotion activation scores per condition, they were referenced to the neutral condition by subtracting neutral asymmetry scores from the happy and angry asymmetry scores. Similarly, physiological responses that occur after an emotionally arousing stimulus has ended have been used as a measure of emotion regulation (Jackson et al., 2003). Thus, a change in EEG asymmetry from the background anger condition to the subsequent resolution condition was interpreted as a measure of emotion regulation. Accordingly, the EEG emotion regulation metric consisted of EEG asymmetry scores from the angry condition minus EEG asymmetry scores from the resolution condition.
3. Results
3.1. Descriptive Information
As would be expected based on talker group classification criteria, CWS, during a conversational speech sample with a researcher, exhibited significantly greater mean total (M = 13.36, SD = 5.98), t(16) = 4.16, p = .003, as well as stuttering-like (M = 8.82, SD = 7.01), t(16) = 3.33, p = .01, disfluencies when compared to CWNS (total: M = 4.93, SD = 1.14; stuttering-like: M = 1.00, SD = .71).
Although all 18 participants scored at or above the 16th percentile on standardized speech-language tests, a multivariate analysis of variance (MANOVA) revealed that CWS scored lower than CWNS on the PPVT-III, F (1, 16) = 5.817, p = .028, Cohen’s d = 1.13 (Mean standard scores for CWS = 108 [SD = 10.5], CWNS = 120 [SD = 10.8]). This finding of lower receptive vocabulary in CWS compared to CWNS is consistent with other reports (e.g., Conture, 2000; Ntourou, Conture, & Lipsey, 2010; Pellowski & Conture, 2005). Note that in the present study CWS scored within normal limits on the PPVT-III with their mean standard score being a half standard deviation above the normative sample mean.
3.2. Behavioral Coding Data: Regulatory Attempts
Multi-level modeling (Singer & Willette, 2003) tested the significance of observed differences in behavioral regulatory attempts. Because visual inspection indicated that frequency and duration of regulatory strategies did not typically increase or decrease across the first three minutes of the children’s narratives, the values for the initial minute were analyzed across emotion conditions and between talker groups. Tests for strategy duration revealed a non-significant talker group effect (β = −.148, SE = .086, t = −1.72, p = .087, Cohen’s d = .66), indicating a non-significant tendency for CWNS to have higher durations of regulatory strategy use than CWS during the first minute of their narratives. No significant or marginal background emotion or background emotion×talker group effects were found for strategy duration.
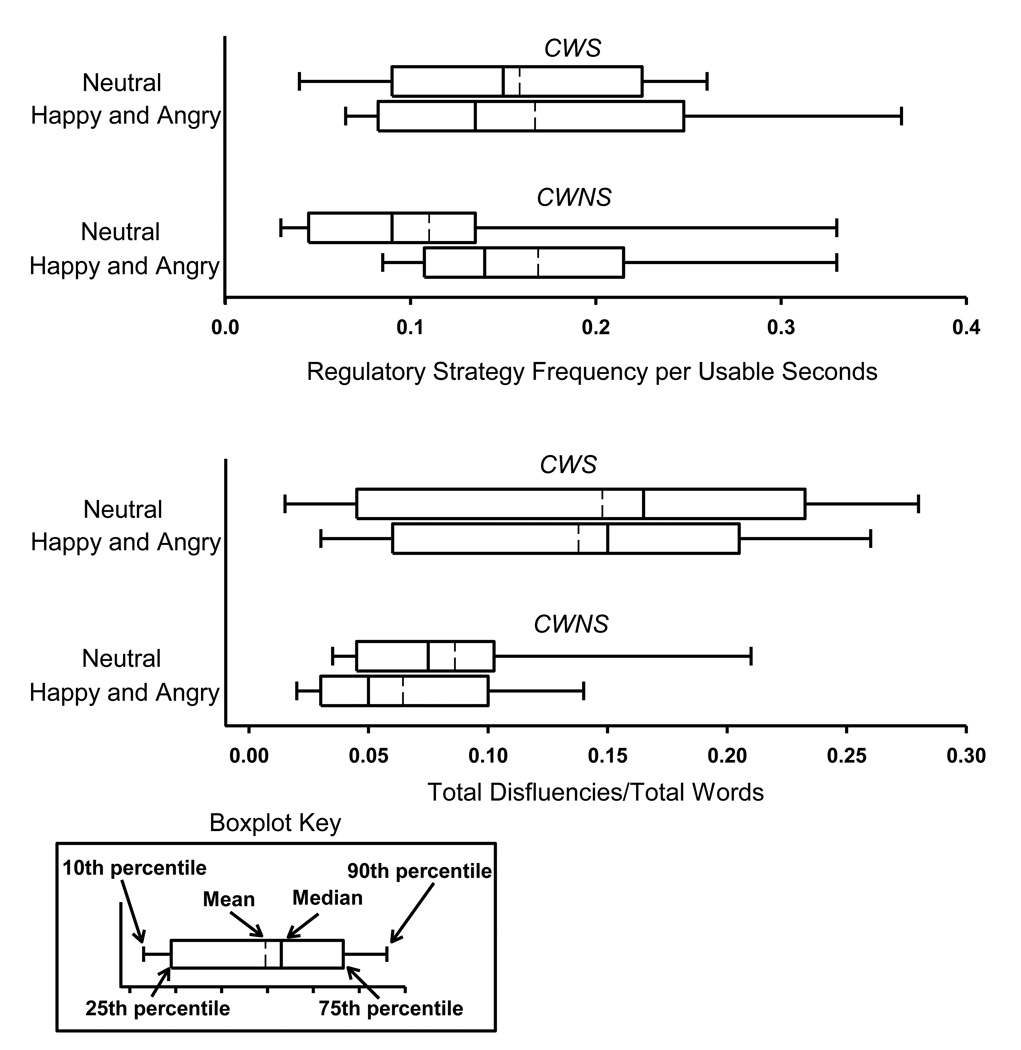
Tests for regulatory strategy frequency did not reveal an effect of talker group, but did result in a significant background emotion effect (β = .039, SE = .019, t = 2.00, p = .047, Cohen’s d = .74), indicating that regulatory strategy use was more frequent in the two emotion conditions, angry and happy, than the neutral condition. Though visual inspection of the data would suggest that regulatory strategy use across emotion conditions differed between the CWS and CWNS, no significant effect of background emotion×talker group was found for regulatory strategy frequency. Figure 1, upper panel, displays results for regulatory strategy frequency.
Figure 1.
For CWS (N = 9) and CWNS (N = 9), regulatory strategy frequency per usable seconds is displayed in a boxplot (Tukey, 1977) for the first minute of each narrative based on maximum likelihood estimates (upper panel). Also, for CWS and CWNS, total disfluencies/total words (TD/TW) for the first minute of each narrative, based on maximum likelihood estimates, are displayed the lower panel.
3.3. Speech Disfluency Measures
Figure 1, lower panel, graphically represents speech disfluency data during children’s narratives. Multi-level modeling was used to test the significance of observed differences in speech disfluencies for the first minute of each condition as there was no apparent trend of increase or decrease in speech disfluencies across the first three minutes of the narrative. As expected, during the first minute of their narratives, CWS had higher SLD/TW and TD/TW ratios than CWNS (β = .082, SE = .016, p < .001). Contrary to expectation, there were no background emotion or background emotion × talker group interactions for either of these speech disfluency variables. Means (and standard deviations) for the SLD/TW ratios were as follows: CWNS neutral: 1.2% (2.1), happy: 0.9% (1.4), and angry: 1.4% (1.1); CWS neutral: 6.5% (5.6), happy: 9.6% (10.2), and angry: 7.8% (7.5). Thus, emotion condition type did not appear to influence either stuttered or total speech disfluencies frequency differentially in CWS versus CWNS.
3.4. Relation of Reactivity and Total Speech Disfluencies
Spearman’s rho correlations were conducted to test the relations of EEG reactivity and speech disfluencies. Separate TD/TW scores for happy and angry were created by averaging across the first three minutes for each condition. Then correlations were conducted between TD/TW and frontal alpha asymmetry scores corresponding to the happy and angry conditions. A positive correlation of asymmetry scores with TD/TW would indicate an association of more left than right frontal activity (i.e., positive emotion) with speech disfluencies. A negative correlation of asymmetry scores with TD/TW would indicate an association of more right than left frontal activity (i.e., negative emotion) with speech disfluencies. Although for the happy condition, there were no significant correlations between TD/TW and frontal alpha asymmetry for either CWS or CWNS, there was a significant negative correlation during the angry condition for CWNS between TD/TW and frontal alpha asymmetry (r = −.683, p = .042), and a non-significant, moderately positive correlation for CWS between the TD/TW and frontal alpha asymmetry (r = .350, p = .356).
To assess whether these correlations differed significantly between CWS and CWNS, a Fisher’s z transformation test was conducted and revealed a marginal difference between CWS and CWNS relative to TD/TW correlations with frontal alpha asymmetry during the angry condition (q = 1.79, p = .07, q > .5 indicates a large effect size; Cohen, 1988). Thus, during the angry condition, CWNS who exhibited greater right than left frontal activation, interpreted as negative emotional reactivity, were apt to have more disfluent speech during their subsequent narrative production. However, this association between frontal asymmetry and speech disfluencies in CWNS was only marginally different from their association in CWS.
3.5. Relation of Regulatory Attempts and Total Speech Disfluencies
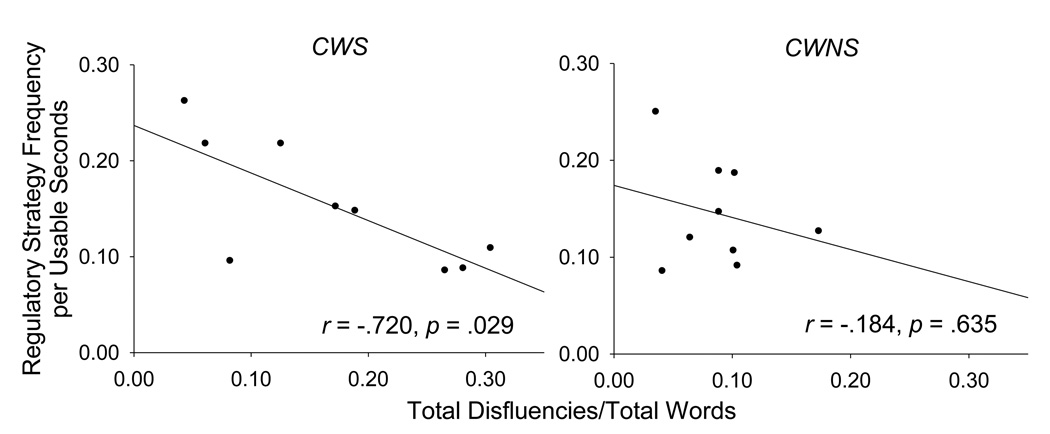
Spearman’s rho correlations tested the relations of regulatory strategy duration and frequency and their corresponding TD/TW during each of the first three minutes of each narrative. Figure 2 graphically represents the relations between regulatory strategy frequency and the corresponding TD/TW for CWNS and CWS. Although regulatory strategy and speech disfluency data were available for each of the first three minutes for each narrative, resulting in nine data points for each variable, data were averaged across the narratives and across the first, second, and third minutes so that each data point used in the correlations represented one participant.
Figure 2.
The relation of regulatory strategy frequency per usable seconds with total disfluencies/total words (TD/TW) is plotted for CWS and CWNS.
Results indicated a significant negative correlation for CWS between TD/TW and regulatory strategy duration (r = −.783, p = .013) as well as between TD/TW and regulatory strategy frequency (r = −.720, p = .029). However, for CWNS there were no significant correlations between TD/TW and regulatory strategy duration (r = −.393, p = .295) or between TD/TW and regulatory strategy frequency (r = −.184, p = .635). Fisher’s z transformation tests revealed no significant differences between CWS and CWNS for TD/TW correlations with regulatory strategy duration (q = .676, p = .50) and regulatory strategy frequency (q = .928, p = .35). Overall, these findings suggest that CWS who less often use regulatory strategies are more apt to exhibit speech disfluencies. However, this association between regulatory strategy use and speech disfluencies in CWS was not significantly different from their association in CWNS.
3.6. Relation of Regulatory Attempts to Stuttering in CWS
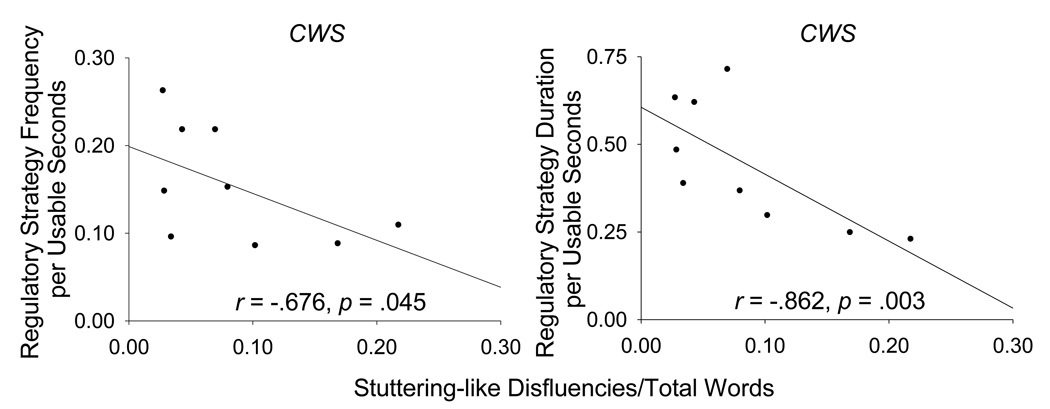
To more specifically test whether CWS exhibited an inverse relation between regulatory strategy use and stuttering, Spearman’s rho correlations were computed between SLD/TW and regulatory strategy duration and frequency averages. Figure 3 graphically represents the relations between regulatory strategy attempts and the corresponding SLD/TW for CWS. Results indicated that for CWS, increased stuttering was significantly related to decreased regulatory strategy duration (r = −.862, p = .003) and regulatory strategy frequency (r = −.676, p = .045). These results further support the notion that CWS with decreased regulatory strategy use are more apt to exhibit increased stuttering.
Figure 3.
The relations of stuttering-like disfluencies per total words (SLD/TW) to regulatory strategy frequency per usable seconds (left panel) and to regulatory strategy duration per usable seconds (right panel) are plotted for CWS.
3.7. EEG Reactivity
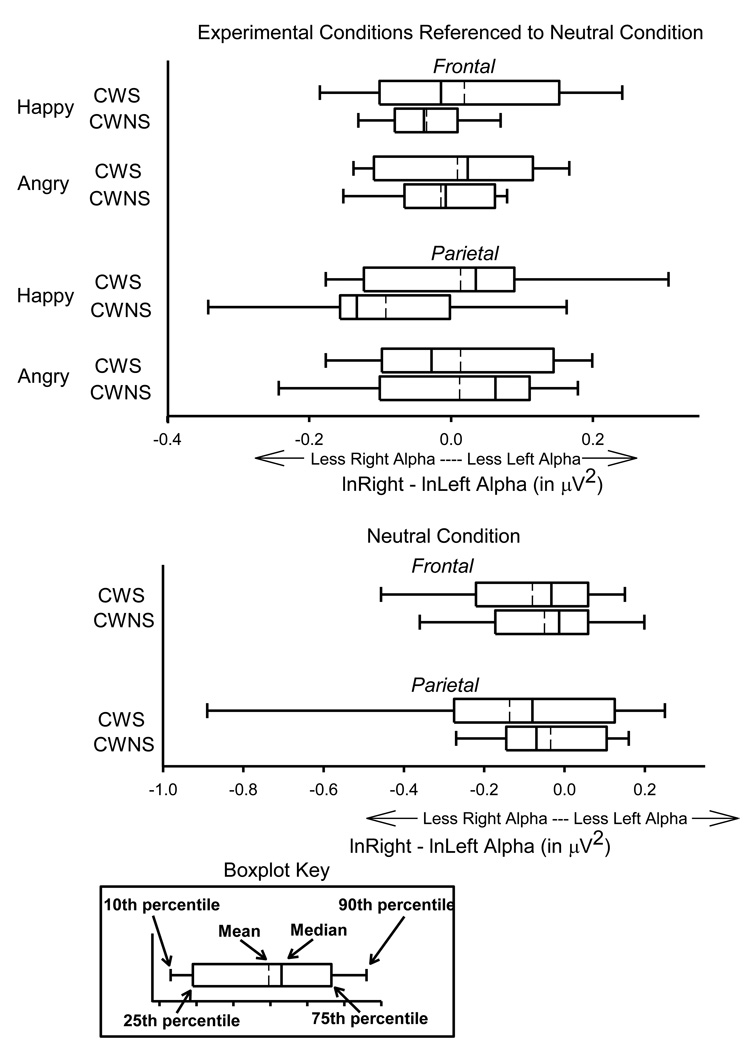
Figure 4 graphically represents results for the alpha band of frequencies recorded during background conversation stimuli. A series of independent samples t-tests was performed to test the significance of observed differences in alpha (8–12 Hz) and beta (13–18 Hz) levels. Because many t-tests were performed for these analyses, a bootstrapping procedure was used, in which outputted p-values are automatically adjusted according to the estimate of risk for type I error (Benjamini & Hochberg, 1995; Westfall, Tobias, Rom, Wolfinger, & Hochberg, 1999; Westfall & Young, 1993). None of the t-tests revealed statistically significant between-group differences for alpha or beta levels (corrected p values ranging from .5 to 1.0).
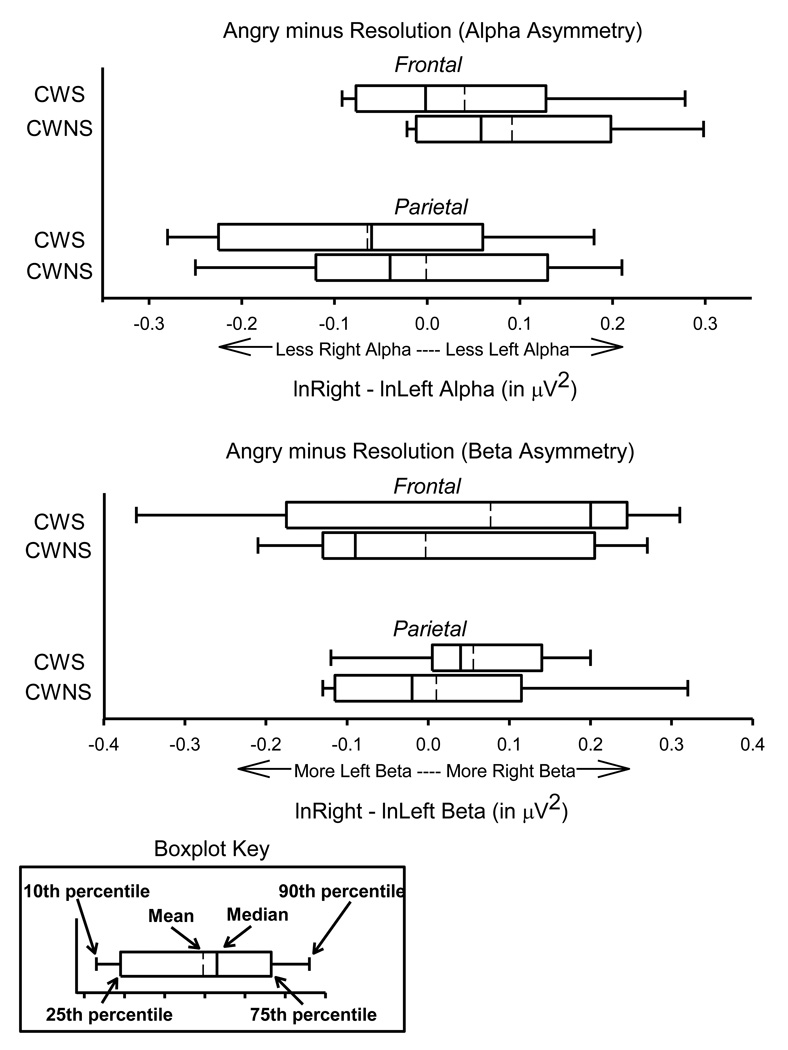
Figure 4.
For CWS (N = 9) and CWNS (N = 9) alpha asymmetry scores for happy and angry conditions, referenced to (i.e., subtracted by) asymmetry scores from the neutral condition, for the frontal and parietal regions are displayed in the upper panel of boxplots. Also for both talker groups, alpha asymmetry scores for the neutral condition are displayed in the lower panel of boxplots.
Visual inspection of the EEG alpha data suggests possible group differences in the neutral condition, which served as the referent for the happy and angry conditions. Because group differences in the neutral condition could undermine the premise of the neutral referencing procedure, a series of between-groups t-tests were performed. None resulted in statistically significant findings (corrected p values ranging from .82 to .99). To further explore the possibility that the neutral results may have confounded results regarding the happy and angry conditions, a mixed-model repeated measures analysis of variance (ANOVA) was performed with the happy, angry, and neutral conditions examined separately. None of these tests revealed statistically significant results (Fs ranging from .061 to 1.788 and p values ranging from .19 to .93). Present findings suggest, therefore, that comparisons of happy and angry results were not confounded by referencing to the neutral condition. Additionally, no significant main effects of emotion condition were found for EEG reactivity within either CWS or CWNS groups, suggesting that the emotions as measured by EEG asymmetry were not robustly elicited by the background conversations presented during the experiment.
3.8. EEG Regulation
Figure 5 graphically represents results for the regulation metric (i.e., angry condition minus resolution condition) for both alpha and beta frequency bands. A series of independent samples t-tests was performed on the EEG data to test the significance of observed differences in alpha and beta levels relative to the regulation metric using the same bootstrapping procedure as above. None of the t-tests revealed statistically significant differences between CWS and CWNS (corrected p values ranging from .84 to .91).
Figure 5.
For CWS (N = 9) and CWNS (N = 9), alpha (upper panel) and beta (lower panel) asymmetry scores for angry minus resolution in the frontal and parietal regions are displayed in boxplots.
3.9. Post-Hoc Analyses
Though no talker group×background emotion interactions were found for regulatory strategy duration and frequency, it was suspected that the main effect of background emotion on regulatory strategy frequency could chiefly be attributed to the CWNS. Because this study’s hypotheses did not distinguish separate effects for positive versus negative emotion, rather predicting that emotion in itself (whether positive or negative) and its regulation influences stuttering, comparison of the two emotion conditions (angry and happy) with the neutral condition was performed. This allowed us to compare the effects of emotional arousal with a condition designed to elicit less emotional arousal. Accordingly, multi-level modeling of regulatory strategy frequency and background emotion was applied to the talker groups separately. Results indicated that CWNS did indeed exhibit significantly higher regulatory strategy frequency in the happy and angry experimental conditions compared to the neutral condition (β = .039, SE = .015, t = 2.07, p = .042). A separate analysis indicated that CWS did not appreciably differentiate regulatory strategy frequency according to emotion condition (β = .005, SE = .020, t = .267, p = .790). Regulatory strategy frequency means for CWS during all conditions, including neutral, were similar to CWNS in the emotional background conversations (i.e., happy and angry). This would suggest that CWS are attempting to regulate even after listening to the neutral background conversation at a similar frequency than CWNS do after emotional background conversations (i.e., happy and angry).
4. Discussion
The present study yielded three main findings. First, behavioral measurement indicated that for children who stutter, decreased use of emotion regulation strategies is associated with more stuttering. Second, EEG measurements of emotional reactivity and regulation did not differentiate talker groups or emotion conditions. Similarly, speech disfluencies did not appreciably differ between emotion conditions for either CWS or CWNS. Third and finally was the unexpected finding that EEG measurements of emotional reactivity during the angry condition were positively correlated with speech disfluencies in children who do not stutter, but failed to indicate a differential pattern of speech disfluencies in relation to the EEG reactivity metric between CWS and CWNS. The following discussion will focus on each finding.
4.1. Emotion Regulation Measured by Behavioral Coding
Results indicated that CWS and CWNS did not appreciably differ in how they attempted to regulate emotions after background conversations. However, when the groups were considered separately, CWNS demonstrated more frequent regulatory strategy attempts in the emotion conditions (i.e., happy and angry) than the neutral condition. This finding would suggest that CWNS responded to background emotions by increasing regulatory strategy attempts.
Though this finding might suggest that the background stimulations were effective elicitors of emotion for CWNS, requiring regulatory behaviors to augment those emotions, such interpretation should be made cautiously given that the present study’s measures of emotional reactivity (i.e., EEG asymmetry) did not differ across emotion conditions. That is, how can emotion regulation occur differently across background conversations when emotional reactivity does not? Consideration of the interactive nature of emotional reactivity and regulation may help to explain this discrepancy. When the regulation of emotions is effective, then emotional reactivity would not differ between conditions. Thus, it is possible that the increase in regulatory strategy use in emotion conditions for CWNS resulted in decreased levels of emotional reactivity for those conditions, making them equivalent to the neutral condition. In order for this to be true, however, effective regulation needed to have occurred prior or during the emotion stimulation, a time period in which regulatory strategies were not coded. Though children may have begun using regulatory strategies early enough in the emotion elicitation procedure to decrease their emotional reactivity, the present study was not designed to detect such early attempts at regulation. Future research in this area, however, may find it fruitful to consider the possibility that regulation may occur prior to the emotion (Campos et al., 2004; Gross, 2002).
It should be noted that CWS did not demonstrate a difference in the frequency of regulatory strategy attempts between the collapsed emotion (i.e., happy and angry) conditions and the neutral condition. These findings do not suggest CWS regulated less than CWNS. Rather, it appears that regulatory frequency for CWS across all conditions was similar to the elevated levels CWNS had in the happy and angry conditions. This result appears consistent with psycho-physiological data from preschool-age CWS indicating little regulatory response from baseline to emotion stimulation, but overall higher regulation across all conditions than CWNS (Buhr, Frankel, Walden, Conture, & Porges, 2010). Perhaps because Buhr et al. (2010) and the present study involved a speech task across all conditions, it could be that CWS needed to regulate emotions elicited by performing these speaking tasks, which for them could have had a greater impact on overall arousal and the need to regulate than the emotional stimulations employed. However, because this study did not compare emotion regulation across speech and non-speech tasks, empirical assessment of this speculation must await further investigation.
4.2. Relation of Emotion Regulation and Speech Disfluency
CWS who used regulatory strategies for shorter durations and with lower frequency were apt to exhibit more speech disfluencies. It may be that decreased use of regulatory strategies allowed unchecked emotional reactions to remain high, resulting in a diversion of attentional resources away from the process of initiating and maintaining reasonably fluent speech-language planning and production. As Johnson et al. (in press) proposed, managing unregulated emotional reactions during speech-language planning and production may constitute what Bosshardt (2006) described as “concurrent attention-demanding cognitive processing.” Bosshardt (2006) reported increases in speech disfluencies during dual-attention tasks with adults who stutter. Thus, for adults who stutter, one task seems to interfere with another, perhaps due to diversion of attentional resources to the concurrent task.
Attentional interference may be most challenging when the tasks to be performed together are problematic than when performed singly. Use of attention to regulate emotions (Eggers et al., 2008; Karrass et al., 2006) and attentional control (Schwenk et al., 2007) appear to be poorer in CWS compared to CWNS. Speech-language planning and production, also appears to be vulnerable in CWS compared to CWNS. Such speech-language vulnerabilities have been reported in both linguistic (e.g., Bernstein Ratner, 1997; Conture, Zackheim, Anderson, & Pellowski, 2004) as well as speech motor domains for people who stutter (e.g., Kleinow & Smith, 2000; van Lieshout, Hulstijn, & Peters, 2004). In short, the attentional resources that would presumably aid CWS in speech-language planning and production may not be robust enough to concurrently attend to regulatory strategies. Difficulty balancing these concurrent tasks might make it difficult for CWS to effectively regulate their emotions or produce fluent speech.
This interpretation should be made cautiously given that, according to EEG asymmetry data for this study, neither group of children exhibited differences in emotional reactivity across emotion conditions. Again, this lack of emotional reactivity could be due to the effective use of regulatory strategies, a possibility that the current paradigm was not designed to test due to the temporal sequence of reactivity and regulation measures.
Another possibility is that regulatory strategy attempts have effects on speech fluency that don’t relate to emotions. That is, children using regulatory strategy attempts more frequently may be diverting attention away from the speech task. These attention diversions may have similar fluency-inducing effects as walking on all fours (Geniesse, 1935), performing a manual tracking task (Arends, Povel, & Kolk, 1988), or playing a simple video game (Vasić & Wijnen, 2005) have on adults who stutter during concurrent speech tasks. One suggestion is that monitoring aspects of these concurrent tasks allow speakers to time word attempts with actions of the task, perhaps simplifying the motor timing of speech-language output (Bloodstein & Bernstein Ratner, 2008, p. 270). Yet another possibility, as Vasić and Wijnen (2005) suggest, is that these putative diversions of attention may disrupt a hyper-vigilant self-monitoring system, resulting in a decrease in speech disfluency.
Could it be that this hypervigilance of the pre-articulatory monitoring system is related to emotional reactivity? For example, a tendency to react strongly to errors might result in increased monitoring of the pre-articulatory plan. If the regulatory strategy of distraction is employed through a non-linguistic concurrent task such as a video game, then those emotional reactions could be regulated, resulting in a less vigilant self-monitor and a decrease in speech disfluency.
It is important to note that concurrent tasks involving increased cognitive-linguistic processing such as silent reading during word repetition (Bosshardt, 2006) or after a cognitively demanding Stroop color word naming test (Caruso, Chodzko-Zajko, & Bidinger, 1994) result in increased, rather than decreased, speech disfluency. Indeed, these cognitively challenging tasks such as the Stroop task can increase autonomic arousal (Caruso et al., 1994; Kleinow & Smith, 2006). Perhaps then when concurrent tasks increase arousal, as a possible result of additional cognitive-linguistic challenges, then stuttering increases. Conversely, if the tasks decrease the level of arousal through the regulatory strategy of distraction, then stuttering may decrease. Indeed, it appears that the relation of attention to stuttering is a complex one, which must be further tested empirically to understand the particular mechanisms, if any, that may impact stuttering.
Speculation regarding this finding thus far has assumed regulatory strategy use impacts speech disfluency. An alternative possibility is that increased speech disfluency impacts regulatory strategy use. That is, could the occurrence of speech disfluencies have made it more difficult for CWS to employ regulatory strategies? Although regulatory strategies were not coded during disfluent speech, it is possible that in a narrative with heightened frequencies of speech disfluencies, attentional resources are less available for regulatory strategies to be employed even during the periods between stuttering instances.
CWS and CWNS did not significantly differ in their correlations between speech disfluency and regulatory strategy use, with CWNS also demonstrating a pattern, one that was not statistically significant, of increased speech disfluency with decreased regulatory strategy duration. Thus, it may be that the mechanism underlying this relation in CWS as also occurring with CWNS, but is not detected given the paucity of speech disfluencies in CWNS.
4.3. Effects of Background Emotions on EEG and Speech Disfluencies
The finding that CWS and CWNS did not appreciably differ on EEG measures of emotional reactivity and regulation was contrary to predictions based on parent-report findings that CWS are more emotionally reactive and less able to regulate emotions than CWNS (e.g., Karrass et al., 2006). These preliminary findings may indicate that CWS and CWNS do not differ in brain measures of emotional reactivity and regulation. This finding is consistent with those of adults who stutter indicating they do not significantly differ from their normally fluent peers in autonomic reactivity (Peters & Hulstijn, 1984; Weber & Smith, 1990).
Even more unexpected were the findings that neither EEG reactivity nor frequency of speech disfluencies differed across background emotion conditions for either group of children. Furthermore, directions of the EEG asymmetry means indicate no tendency in the directions predicted for positive versus negative emotions. Rather, they indicate the opposite pattern. Emotion elicitation should have resulted in at least a main effect of condition for EEG asymmetry effects according to emotion condition. These counterintuitive findings could relate to the effectiveness of the background emotion stimuli in eliciting emotions or to the validity of EEG asymmetry as a measure of emotions for this study.
Consider, for example, the background anger condition. Attempting to keep linguistic content consistent among the various background emotion conversations, the script for the angry condition contained relatively neutral emotional language with only two overt statements of anger such as “I am so mad about this!” and no conflict language between the dyad such as “I am so mad at you.” Rather, actors were instructed to convey anger through dramatic interpretation of the script, resulting in vocal tone and prosodic patterns consistent with anger and conflict. However, the anger conveyed by means of these dramatic interpretations may not have not elicited the quantity and quality of arousal within the participants that would allow for a reasonable test of the relation between situational emotion and stuttering.
The present results could also indicate that although EEG asymmetry has received considerable support in the literature as a measure of emotional responding (for review, see Coan & Allen, 2004), it may not have measured the desired construct reliably for this emotional background conversation paradigm and for preschool-age children who do and do not stutter.
4.4. Relation of Emotional Reactivity and Speech Disfluency
CWNS with higher negative emotional reactivity, as measured by frontal alpha during the angry background conversation, exhibited more disfluent speech during subsequent narratives. These findings suggest that increased emotional reactivity is associated with increased speech disfluency in subsequent speech acts for children who do not stutter when reacting with negative emotions (see Brutten & Shoemaker, 1967 for an early formulation of similar speculation).
What to make of this finding? First, it is important to note that there was no significant difference between the correlations for CWS versus CWNS. Therefore, it cannot be argued that emotions impact speech disfluencies differentially between the two groups. Second, if this effect is only statistically significant for children who do not stutter, it seems difficult to make a case that it has implications for children who stutter.
Also, the EEG asymmetry metric used in the present study did not follow the expected pattern of greater right frontal activation during the angry background conversations and greater left frontal activation during the happy background conversations. As proposed above, the background stimulations may not have effectively elicited emotion as measured by this metric. Given this possibility, any correlation with this metric in the present paradigm must be interpreted with caution.
4.5. Caveats
Present findings point to important methodological considerations when designing similar studies of emotional reactivity and regulation with preschool children who stutter. First, more effective emotion elicitation stimuli would allow questions regarding emotional reactivity and regulation to be better addressed. Second, attention may impact or underlie the relation between regulatory behaviors and stuttering. If this is the case, attentional processes may need to be controlled for or taken into consideration when studying the relation of emotion to stuttering. Third, the findings of this study are subtle and based on a small sample of participants. As a result there was low power and a high risk of Type II error. Fourth, there was a significant difference in receptive vocabulary between CWS and CWNS. However, this study’s CWS scored a half standard deviation above the normative sample mean, suggesting their receptive vocabulary was by no means deficient and would probably not unduly influence the dependent variables. Future researchers, however, may want to consider whether preschoolers’ receptive vocabulary impacts their responses to emotionally-challenging stimuli that are presented auditorily. Finally, although handedness of participants was not matched, inclusion of left-handed participants did not appear to appreciably affect findings when compared to a smaller subset of participants. In summary, similar studies in future may want to take these issues into consideration.
4.7. Conclusion
Results from this study, using behavioral and psychophysiological measures, provide support for the notion that when preschool-age children who stutter use more regulatory strategies during narrative production, they stutter less (Walden et al., 2010). The present authors speculate that decreased emotion regulation, resulting in relatively unchecked or unmodulated emotional reactivity, may require increased attentional resources. Such diversion of attentional resources, especially those needed to efficiently plan and produce fluent speech and language, may be particularly problematic for a vulnerable speech-language planning and production system (speculation similar to that of Conture & Walden, 2010). Perhaps, decreased emotion regulation, together with vulnerabilities in speech-language planning and production and possible difficulties concurrently attending to these two ongoing processes, may contribute to the challenges these children who stutter have establishing and maintaining reasonably fluent speech-language planning and production.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants from the National Institute on Deafness and Other Communication Disorders (NIDCD) to Vanderbilt University (R01 DC006477-01A2, R01 DC000523-14) and a Vanderbilt University Discovery Grant. The research reported herein does not reflect the views of the NIH, NIDCD, Kent State University or Vanderbilt University.
Appendix A. Continuing education
- Emotional _______ refers to the intensity and threshold for both positive and negative emotional responding that individuals tend to experience.
- anxiety
- attention
- disturbance
- reactivity
- regulation
- It is widely accepted that emotional reactivity and regulation are two clearly separable processes.
- True
- False
- Until recently, most studies of emotion-related processes in young children who stutter have been based on what measures?
- physiological
- psychophysiological
- behavioral coding
- brain imaging
- parent-report
- EEG asymmetry is a physiological method for measuring emotion which has been validated in adults and young children.
- True
- False
- If a child uses regulatory strategies and physiological measures indicate a decrease in emotional reactivity during or after those putative regulatory attempts, then one might reasonably suggest which of the following?
- emotional reactivity and regulation cannot be measured independently
- regulatory attempts are effective only after emotional reactions occur
- regulatory attempts used were conducive to communication and social interaction
- some degree of emotion regulation occurred as a result of the strategies
- stuttering decreased as a result of the regulatory strategies
Answer Key: d, b, e, a, d
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
One child refused tympanometric screening and acoustic reflex testing. Because analyses of EEG data with and without this child’s data indicated no appreciable differences in outcome, the child’s data were included in this study’s final data corpus.
A larger pool (N = 46) was considered prior to application of inclusion criteria. Of the original group of 46 participants, 14 (30.4%) did not pass speech-language or hearing screenings; 5 (10.9%) were unable or unwilling to complete the experimental procedure; 5 (10.9%) had, due to movement artifact, less than 50 seconds per condition of usable EEG data; 4 (8.7%) had no gender or age match in the other talker group; and 3 (6.5%) did not meet either talker group’s speech disfluency criteria. In total, 13 CWS, 12 CWNS, and 3 unclassified participants were excluded from the final data corpus.
The average common referencing scheme (Lehmann, 1987) is thought by some (Tucker, 1993) to be the best method for referencing EEG data when it is sampled across 32 or more electrodes spaced evenly over the scalp. This is because variations in voltage and phase across the head should average to approximately zero and thus provide a better approximation of an electrically inactive reference than any single point on the head or body.
References
- Adams MR. The home environment of children who stutter. Seminars in Speech and Language. 1993;14:185–192. [Google Scholar]
- Alm PA. Stuttering, emotions, and heart rate during anticipatory anxiety: A critical review. Journal of Fluency Disorders. 2004;29(2):123–133. doi: 10.1016/j.jfludis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Anderson JD, Conture EG. Sentence-structure priming in young children who do and do not stutter. [Peer Reviewed] Journal of speech, language & hearing research. 2004;47(3):552–571. doi: 10.1044/1092-4388(2004/043). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JD, Pellowski MW, Conture EG, Kelly EM. Temperamental characteristics of young children who stutter. [Feature Article] Journal of Speech, Language, and Hearing Research. 2003;46(5):1221–1233. doi: 10.1044/1092-4388(2003/095). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends N, Povel DJ, Kolk H. Stuttering as an attentional phenomenon. Journal of Fluency Disorders. 1988;13:141–151. [Google Scholar]
- Association, A. S.-L.-H. Guidelines for Audiologic Screening. Rockville, Maryland: ASHA Fulfillment Operations; 1996. [Google Scholar]
- Bell MA, Wolfe CD. Emotion and cognition: An intricately bound developmental process. Child Development. 2004;75:366–370. doi: 10.1111/j.1467-8624.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Bender JF. New York: Pitman Publishing Corp.; 1939. The personality structure of stuttering. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Bernstein Ratner N. Stuttering: a psycholinguistic perspective. In: Curlee R, Siegel GM, editors. Nature and treatment of stuttering: New directions. 2nd ed. Boston, MA: Allyn & Bacon; 1997. pp. 99–127. [Google Scholar]
- Bloodstein O, Bernstein Ratner N. A handbook on stuttering. 6th ed. Clifton Park, NY: Delmar Cengage Learning; 2008. [Google Scholar]
- Boberg E, Yeudall LT, Schopflocher D, Bo-Lassen P. The effect of an intensive behavioral program on the distribution of EEG alpha power in stutterers during the processing of verbal and visuospatial information. Journal of Fluency Disorders. 1983;8:245–263. [Google Scholar]
- Bosshardt H. Cognitive processing load as a determinant of stuttering: Summary of a research programme. Clinical Linguistics and Phonetics. 2006;20:371–385. doi: 10.1080/02699200500074321. [DOI] [PubMed] [Google Scholar]
- Bridges LJ, Denham S, Ganiban JM. Definitional issues in emotion regulation research. Child Development. 2004;75:340–345. doi: 10.1111/j.1467-8624.2004.00675.x. [DOI] [PubMed] [Google Scholar]
- Brown SF, Hull HC. A study of some social attitudes of a group of 59 stutterers. Journal of Speech Disorders. 1942;7:323–324. [Google Scholar]
- Brutten EJ, Shoemaker DJ. The modification of stuttering. Englewood Cliffs, NJ: Prentice-Hall; 1967. [Google Scholar]
- Buhr A, Frankel C, Walden T, Conture E, Porges S. Respiratory Sinus Arrhythmia and Childhood Stuttering. Manuscript submitted for publication. 2010 [Google Scholar]
- Buss KA, Goldsmith HH. Fear and anger regulation in infancy: Effects on the temporal dynamics of affective expression. Child Development. 1998;69:359–374. [PubMed] [Google Scholar]
- Byrd CT, Conture E, Ohde RN. Phonological priming in young children’s picture naming: Holistic versus incremental processing. American Journal of Speech-Language Pathology. 2007;16:43–53. doi: 10.1044/1058-0360(2007/006). [DOI] [PubMed] [Google Scholar]
- Campos JJ, Frankel CB, Camras L. On the nature of emotion regulation. Child Development. 2004;75:377–394. doi: 10.1111/j.1467-8624.2004.00681.x. [DOI] [PubMed] [Google Scholar]
- Caruso AJ, Chodzko-Zajko WJ, Bidinger DA. Adults who stutter: Responses to cognitive stress. Journal of Speech & Hearing Research. 1994;37:746–754. doi: 10.1044/jshr.3704.746. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6:284–290. [Google Scholar]
- Coan J, Allen J. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan J, Allen J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second ed. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Conture E. Dreams of our theoretical nights meet the realities of our empirical days: Stuttering theory and research. In: Bosshardt HG, Peters H, editors. Proceedings: Third World Congress of International Fluency Association. Nijmegen, The Netherlands: University of Nijmegen Press; 2000. [Google Scholar]
- Conture E. Stuttering: Its nature diagnosis and treatment. Boston: Allyn & Bacon; 2001. [Google Scholar]
- Conture E, Walden T. A Dual Diathesis-Stressor Model of Stuttering. 2010 doi: 10.1007/s10802-011-9581-8. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conture E, Walden T, Arnold H, Graham C, Hartfield K, Karrass J. Communicative-emotional model of developmental stuttering. In: Bernstein Ratner N, Tetnowski J, editors. Stuttering Research and Practice Volume 2: Contemporary Issues and Approaches. Mahwah, NJ: Lawrence Erlbaum Assoc.; 2006. pp. 17–46. [Google Scholar]
- Conture E, Zackheim CT, Anderson JD, Pellowski MW. Linguistic processes and childhood stuttering: Many’s a slip between intention and lip. In: Maasan B, van Lieshout P, Hulstijn W, Peters H, editors. Speech Motor Control in Normal and Disordered Speech. Oxford, England: Oxford University Press; 2004. pp. 253–281. [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. [Google Scholar]
- Cummings EM. Coping with background anger in early childhood. Child Development. 1987;58:976–984. doi: 10.1111/j.1467-8624.1987.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Ballard M, El-Sheikh M, Lake M. Resolution and children’s responses to interadult anger. Developmental Psychology. 1991;27:462–470. [Google Scholar]
- Cummings EM, Vogel E, Cummings JS, El-Sheikh M. Children's responses to angry adult behavior as a function of marital distress and history of interparent hostility. Child Development. 1989;60:1392–1404. doi: 10.1111/j.1467-8624.1989.tb03534.x. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Wilson J, Shamir H. Reactions of Chilean and US children to marital discord. International Journal of Behavioral Development. 2003;27:437–444. [Google Scholar]
- Davidson RJ. Cerebral asymmetry and emotion: conceptual and methodological conundrums. Cognition and Emotion. 1993;7:115–138. [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LP. Task-dependent EEG asymmetry discriminates between depressed and non-depressed subjects. Psychophysiology. 1987;24:585. [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd ed. New York: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Davidson RJ, Schaffer CE, Saron CD. Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophysiology. 1985;22:353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Panagiotides H, Klinger LG, Hill D. The role of frontal lobe functioning in the development of infant self-regulatory behavior. Brain and Cognition. 1992;20:152–175. doi: 10.1016/0278-2626(92)90066-u. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Information processing approaches to individual differences in emotional reactivity. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York: Oxford University Press; 2003. [Google Scholar]
- Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. Journal of Personality and Social Psychology. 1988;55:958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- Douglass RL. doctoral dissertation. Los Angeles: University of Southern California; 1952. An experimental electroencephalographic study of stimulus reaction in stutterers. [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd ed. Circle Pines: MN: American Guidance Services, Inc.; 1997. [Google Scholar]
- Eggers K, De Nil L, Van den Bergh B. Temperament and attentional processes in CWS. Paper presented at the European Union Symposium on Fluency Disorders; Antwerp, Belgium. 2008. [Google Scholar]
- Eisenberg N, Fabes RA. Emotion, regulation, and the development of social competence. In: Clark MS, editor. Review of personality and social psychology. Newbury Park, CA: Sage Publications, Inc.; 1992. pp. 119–150. [Google Scholar]
- Eisenberg N, Spinrad TL. Emotion-related regulation: Sharpening the definition. Child Development. 2004;75:334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Cummings EM, Goetsch VL. Coping with adults’ angry behavior: Behavioral, physiological, and verbal responses in preschoolers. Developmental Psychology. 1989;25:490–498. [Google Scholar]
- Embrechts M, Ebben H, Franke P, van de Poel C. Temperament: A comparison between children who stutter and children who do not stutter. In: Healey EC, Peters HMF, editors. Stuttering: Proceedings of the third world congress on fluency disorders. Nijmegen, The Netherlands: University Press Nijmegen; 1998. pp. 557–561. [Google Scholar]
- Fabes RA, Eisenberg N, Jones S, Smith M, Guthrie I, Poulin R, Friedman J. Regulation, emotionality, and preschoolers' socially competent peer interactions. Child Development. 1999;70:432–442. doi: 10.1111/1467-8624.00031. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology & Psychiatry. 2006;47:79–87. doi: 10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Fowlie GM, Cooper EB. Traits attributed to stuttering and nonstuttering children by their mothers. Journal of Fluency Disorders. 1978;3:233–245. [Google Scholar]
- Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology. 1992;8:161–184. [Google Scholar]
- Fox NA, Davidson RJ. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia. 1986;24:417–422. doi: 10.1016/0028-3932(86)90028-x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10-month-old infants. Developmental Psychology. 1988;24:230–236. [Google Scholar]
- Geniesse H. Stuttering. Science. 1935;82:518. doi: 10.1126/science.82.2135.518. [DOI] [PubMed] [Google Scholar]
- Gilliom M, Shaw DS, Beck JE, Schonberg MA, Lukon JL. Anger regulation in disadvantaged preschool boys: Strategies, antecedents, and the development of self-control. Developmental Psychology. 2002;38:222–235. doi: 10.1037//0012-1649.38.2.222. [DOI] [PubMed] [Google Scholar]
- Glasner P. Personality characteristics and emotional problems in stutterers under the age of five. Journal of Speech and Hearing Disorders. 1949;14:135–138. doi: 10.1044/jshd.1402.135. [DOI] [PubMed] [Google Scholar]
- Glauber I. The psychoanalysis of stuttering. In: Eisenson J, editor. Stuttering: A symposium. New York: Harper and Row; 1958. pp. 71–120. [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe Test of Articulation (GFTA) Circle Pines, MN: American Guidance Services, Inc.; 2000. [Google Scholar]
- Goldsmith HH, Hewitt EC. Validity of parental report of temperament: Distinctions and needed research. Infant Behavior and Development. 2003;26:108–111. [Google Scholar]
- Grolnick WS, Bridges LJ, Connell JP. Emotion regulation in two-year-olds: Strategies and emotional expression in four contexts. Child Development. 1996;67:928–941. [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Becker G, Maier S, Bartussek D. Frontal brain asymmetry and affective style: A conceptual replication. Psychophysiology. 1998;35:372–388. [PubMed] [Google Scholar]
- Heller W. The neuropsychology of emotion: Developmental patterns and implications for psychopathology. In: Stein NL, Leventhal B, Trabasso T, editors. Psychology and Biological Approaches to Emotion. Hillsdale, NJ: Lawrence Erlbaum and Associates, Inc. Publishers; 1990. pp. 197–211. [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Hoeksma JB, Oosterlaan J, Schipper EM. Emotion regulation and the dynamics of feelings: A conceptual and methodological approach. Child Development. 2004;75:354–360. doi: 10.1111/j.1467-8624.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hresko W, Reid D, Hamill D. Test of Early Language Development-3 (TELD-3) Austin, TX: Pro-Ed.; 1999. [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Davidson RJ. Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Jasper H. The ten twenty electrode system of the Internation Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Johnson KN, Walden TA, Conture E, Karrass J. Spontaneous regulation of positive and negative emotionality in preschool children. Journal of Speech, Language & Hearing Research. doi: 10.1044/1092-4388(2010/08-0150). (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghoefer M, Elbert T, Tucker D, Braun C. The polar effect of average reference: A bias in estimating the head surface integral in EEG recording. Electroencephalography and Clinical Neurophysiology. 1999;110:1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Karrass J, Conture E, Walden T. Response by Jan Karrass, Edward Conture and Tedra Walden, Vanderbilt University, September 2008. Are children who stammer more sensitive by nature? 2008 Retrieved June 4, 2009, from http://www.stammering.org/sensitivity.html#karrass.
- Karrass J, Walden TA, Conture E, Graham CG, Arnold HS, Hartfield KN, Schwenk KA. Relation of emotional reactivity and regulation to childhood stuttering. Journal of Communication Disorders. 2006;39:402–423. doi: 10.1016/j.jcomdis.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, Molfese DL, Ratajczak E. ERP indicators of learning in adults. Developmental Neuropsychology. 2006;29:379–395. doi: 10.1207/s15326942dn2902_5. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. [Feature Article] Journal of Speech, Language, and Hearing Research. 2000;43(2):548–559. doi: 10.1044/jslhr.4302.548. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Potential interactions among linguistic, autonomic, and motor factors in speech. Developmental Psychobiology. 2006;48:275–287. doi: 10.1002/dev.20141. [DOI] [PubMed] [Google Scholar]
- Knott JR, Correll RE, Shepherd JN. Frequency analysis of electroencephalograms of stutterers and nonstutterers. Journal of Speech and Hearing Research. 1959;2:74–80. doi: 10.1044/jshr.0201.74. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology: Methods of Analysis fo Brain Electrical and Magnetic Signals. Amsterdam: Elsevier; 1987. pp. 309–354. [Google Scholar]
- Lewis KE, Goldberg LL. Measurements of temperament in the identification of children who stutter. European Journal of Disorders of Communication. 1997;32:441–448. doi: 10.3109/13682829709082258. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Stieben J. Emotion regulation in the brain: Conceptual issues and directions for developmental research. Child Development. 2004;75:371–376. doi: 10.1111/j.1467-8624.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- Mayer M. A Boy, A Dog, and a Frog. New York: Dial Books for Young Readers; 1967. [Google Scholar]
- Mayer M. Frog Where Are You? New York: Dial Books for Young Readers; 1969. [Google Scholar]
- Mayer M. Frog on His Own. New York: Dial Books for Young Readers; 1972. [Google Scholar]
- McManis MH, Kagan J, Snidman NC, Woodward SA. EEG asymmetry, power, and temperament in children. Developmental Psychobiology. 2002;41:169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- Moore WH. Central nervous system characteristics of stutterers. In: Curlee RF, Perkins WH, editors. Nature and Treatment of Stuttering: New Directions. San Diego, CA: College-Hill; 1984. [Google Scholar]
- Moore WH, Haynes WO. Alpha hemispheric asymmetry and stuttering: Some support for a segmentation dysfunction hypothesis. Journal of Speech and Hearing Research. 1980;23:229–247. doi: 10.1044/jshr.2302.229. [DOI] [PubMed] [Google Scholar]
- Moore WH, Lorendo LC. Hemispheric alpha asymmetries of stuttering males and nonstuttering males and females for words of high and low imagery. Journal of Fluency Disorders. 1980;5:11–26. [Google Scholar]
- Murphy A. doctoral dissertation. Los Angeles, CA: University of Southern California; 1953. An electroencephalographic study of frustration in stutterers. [Google Scholar]
- Murphy A, Fitzsimons R. Stuttering and Personality Dynamics. New York: The Ronald Press Company; 1960. [Google Scholar]
- Nixon CL, Cummings EM. Sibling disability and children's reactivity to conflicts involving family members. Journal of Family Psychology. 1999;13:274–285. [Google Scholar]
- Ntourou K, Conture E, Lipsey MW. Language abilities of children who stutter: A meta-analytical review. Submitted to a scholarly journal. 2010 doi: 10.1044/1058-0360(2011/09-0102). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pellowski MW, Conture EG. Lexical Priming in Picture Naming of Young Children Who Do and Do Not Stutter. [Feature Article] Journal of Speech, Language, and Hearing Research. 2005;48(2):278–294. doi: 10.1044/1092-4388(2005/019). [DOI] [PubMed] [Google Scholar]
- Peters HFM, Hulstijn W. Stuttering and anxiety: The difference between stutterers and nonstutterers in verbal apprehension and physiologic arousal during the anticipation of speech and non-speech tasks. Journal of Fluency Disorders. 1984;9:67–84. [Google Scholar]
- Pinsky SD, McAdam DW. Electroencephalographic and dichotic indices of cerebral laterality in stutterers. Brain and Language. 1980;11:374–397. doi: 10.1016/0093-934x(80)90134-0. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Spritz BL, Stifter CA. Mother-child coregulation during delay of gratification at 30 months. Infancy. 2002;3:209–225. doi: 10.1207/S15327078IN0302_6. [DOI] [PubMed] [Google Scholar]
- Riley G. Stuttering Severity Instrument for Young Children. 3rd ed. Austin, TX: Pro-Ed.; 1994. [Google Scholar]
- Schwenk K, Conture E, Walden T. Reaction to background stimulation of preschool children who do and do not stutter. Journal of Communication Disorders. 2007;40:129–141. doi: 10.1016/j.jcomdis.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willette JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Teeson K, Packman v, Onslow M. The Lidcombe behavioral language data of stuttering. Journal of Speech, Language & Hearing Research. 2003;46:1009–1015. doi: 10.1044/1092-4388(2003/078). [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59:25–52. [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59:791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellersten K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: Establishing a larger stimulus set. Paper presented at the Cognitive Neuroscience Society Annual Meeting; San Francisco, CA. 2002. [Google Scholar]
- Tucker D. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Reading, Massachusetts: Addison-Wesley; 1977. [Google Scholar]
- van Lieshout P, Hulstijn W, Peters H. Searching for the weak link in the speech production chain of people who stutter: A motor skill approach. In: Maasan B, van Lieshout P, Hulstijn W, Peters H, editors. Speech Motor Control in Normal and Disordered Speech. Oxford, England: Oxford University Press; 2004. pp. 313–355. [Google Scholar]
- Vasić N, Wijnen F. Stuttering as a monitoring deficit. In: Hartsuiker RJ, Bastiaanse R, Postma A, editors. Phonological encoding and monitoring in normal and pathological speech. Hove: Psychology Press; 2005. [Google Scholar]
- Walden T, Frankel C, Buhr A, Johnson K, Conture E, Karrass J. Dual diathesis-stress model of emotional and linguistic contributions to developmental stuttering. Manuscript submitted for publication. 2010 doi: 10.1007/s10802-011-9581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C, Spruill JE, III, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science. 2008;11(2):321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CM, Smith A. Autonomic correlates of stuttering and speech assessed in a range of experimental tasks. [Feature Article] Journal of Speech and Hearing Research. 1990;33:690–706. doi: 10.1044/jshr.3304.690. [DOI] [PubMed] [Google Scholar]
- Westfall P, Tobias R, Rom D, Wolfinger R, Hochberg Y. Multiple Comparisions and Multiple Tests Using the SAS System. Cary, NC: SAS Publishing; 1999. [Google Scholar]
- Westfall P, Young S. Resampling-based multiple testing: examples and methods for P-value adjustment. New York: Wiley; 1993. [Google Scholar]
- Williams KT. Expressive Vocabulary Test (EVT) Circle Pines, MN: American Guidance Services, Inc.; 1997. [Google Scholar]
- Williams MJ. Children who stutter: Easy, difficult, or slow to warm up?. Paper presented at the American Speech, Language, and Hearing Association Convention; Miami, FL. 2006. [Google Scholar]
- Yairi E. Home environments and parent-child interaction in childhood stuttering. In: Curlee RF, Siegel GM, editors. Nature and treatment of stuttering: New directions. Second Edition. Needham Heights, MA: Allyn and Bacon; 1997. pp. 24–48. [Google Scholar]