Abstract
In this review we first present the anatomical pathways used by the SCN to enforce its rhythmicity onto the body, especially its energy homeostatic system. The experimental data show that by activating the orexin system at the start of the active phase, the biological clock not only ensures that we wake up on time, but also that our glucose metabolism and cardiovascular system are prepared for increased activity. The drawback of such a highly integrated system, however, becomes visible when our daily lives are not fully synchronized with the environment. Thus, in addition to increased physical activity and decreased intake of high-energy food, also a well-lighted and fully resonating biological clock may help to withstand the increasing “diabetogenic” pressure of today’s 24/7 society.
Keywords: Hypothalamus, Autonomic nervous system, Orexin, Glucose, Melatonin, GABA, Liver
Introduction
In the pre-modern world temporal cycles of feeding and fasting matched the patterns of wakefulness and sleep that occurred during the daily periods of light and darkness. In mammals an autonomous endogenous clock developed in the brain to coordinate and anticipate our behaviour and metabolism according to the environmental periodicity induced by the earth’s rotation. A proper entrainment of this endogenous clock mechanism to the outside world is ensured by a number of input signals, of which light, food intake and activity are the most important ones. The biological clock, located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, consists of several clusters of small and densely packed neurons in which various peptidergic transmitters are expressed (1). The entraining signals from light, feeding and locomotor activity are relayed to the SCN via direct projections from the retina, the arcuate nucleus and the raphe nucleus, respectively. The direct projection from the intergeniculate leaflet to the SCN seems to be an important secondary route for all three entraining signals. The afferent projections from these different brain structures use, among other things, glutamate, PACAP, neuropeptide-Y (NPY), neuropeptide FF (NPFF) and serotonin as a neurotransmitter (2). The endogenous clock mechanism consists of interlocking transcriptional-translational feedback loops, and contains genes necessary for oscillator maintenance (“core clock genes”), as well as specific clock controlled output genes that impose their rhythmicity on the rest of the hypothalamus and beyond (3). A few of the peptidergic SCN transmitters, i.e. vasopressin, vasoactive intestinal peptide, cardiotrophin-like cytokine and prokineticin-2, have already been identified as so-called clock-controlled genes (4–7). Subsequently, the rhythmic output of this endogenous clock is conveyed to the endocrine and metabolic systems via efferent projections from the SCN to both neuroendocrine and pre-autonomic neurons in the hypothalamus (8). Here we discuss the hypothesis that in today’s society the output of the biological clock is unbalanced due to insufficient or even contradictory input signals to the SCN, hampering its proper entrainment to the outside world and thus also hampering proper entrainment of its output.
SCN outputs
In 1972, it became clear that the suprachiasmatic nucleus (SCN) in the anterior hypothalamus was the seat of the central biological clock (9). Only a few years after this discovery it was demonstrated that the SCN contains a prominent population of vasopressin-containing neurons (10–11). Due to its pronounced day/night rhythm in the cat cerebral spinal fluid (CSF) (12–13), vasopressin was soon characterized as an output of the SCN. This important finding was followed by reports on vasopressin-containing neurons in the SCN of a large variety of species (14–20), as well as CSF vasopressin rhythms in a number of species, including monkey, rat, guinea pig, goat, sheep and rabbit (21–25). Neither lesions of the PVN, nor hypophysectomy or pinealectomy were able to eliminate this rhythm. The rhythms were even sustained after complete isolation -by circular knife cuts- of the SCN in vivo. Only complete SCN lesions abolished the rhythm and in most cases reduced CSF vasopressin levels to below detection level (26–27). In addition, it was demonstrated that also in vitro the rhythmic release of vasopressin from the SCN is maintained for several days (28–29). Moreover, studies on the circadian fluctuation of mRNA showed an elevation, or poly-A tail elongation, of vasopressin mRNA in the SCN during the light period (30–31), whereas PVN and SON vasopressin mRNA showed no such diurnal fluctuations. Similar observations, i.e., pronounced daily fluctuations in the SCN, but not in the PVN and SON, were made for the extracellular levels of vasopressin in the SCN, PVN and SON (32).
Since then, many SCN transmitters other than vasopressin have come to be recognized (33), most of which also show a clear day/night rhythm in the amount of protein or mRNA expression in the nucleus itself. However, until now VP is still the only SCN output that has been demonstrated to be secreted in a circadian rhythm in vivo. Transplantation and parabiosis experiments have unequivocally demonstrated that non-neuronal mechanisms do not suffice when it comes to reinstating circadian rhythms in all peripheral organs (34–36). Moreover, an elegant experiment by De La Iglesia et al. (37) provided clear functional evidence for the necessity of point-to-point neural connections in order to sustain neuroendocrine rhythms. So where does the rhythmic information that is generated within the SCN go? Information on the distribution of SCN projections was initially obtained from neuro-anatomical studies using tracing, immunocytochemistry, SCN lesions, or a combination of these methods (38–40). All these studies showed that the outflow of SCN information was in fact surprisingly limited and pertained to the medial hypothalamus, in particular to target areas that contain mainly interneurons, such as the MPOA, DMH and subPVN (Fig.1). Although much more scarce, direct connections to neuroendocrine neurons (i.e., CRH-, TRH-, TH- and GnRH-containing) in the PVN, arcuate nucleus and MPOA, and pre-autonomic neurons in the PVN were described as well (41–48). In a series of micro-infusion studies we were able to show how the SCN uses these neural connections to control peripheral rhythms in hormone release and energy metabolism (8, 49–50). The daily fluctuations of vasopressin in the CSF are a result of the day/night rhythm in the firing rate of vasopressin-containing SCN neurons (51) (Fig.2), and the close proximity of the vasopressin containing SCN projections to the ventricular space, i.e., in the medial preoptic area (MPOA), the periventricular and sub-paraventricular nucleus (pePVN and subPVN), the dorsomedial hypothalamus (DMH) and the paraventricular nucleus of the thalamus.
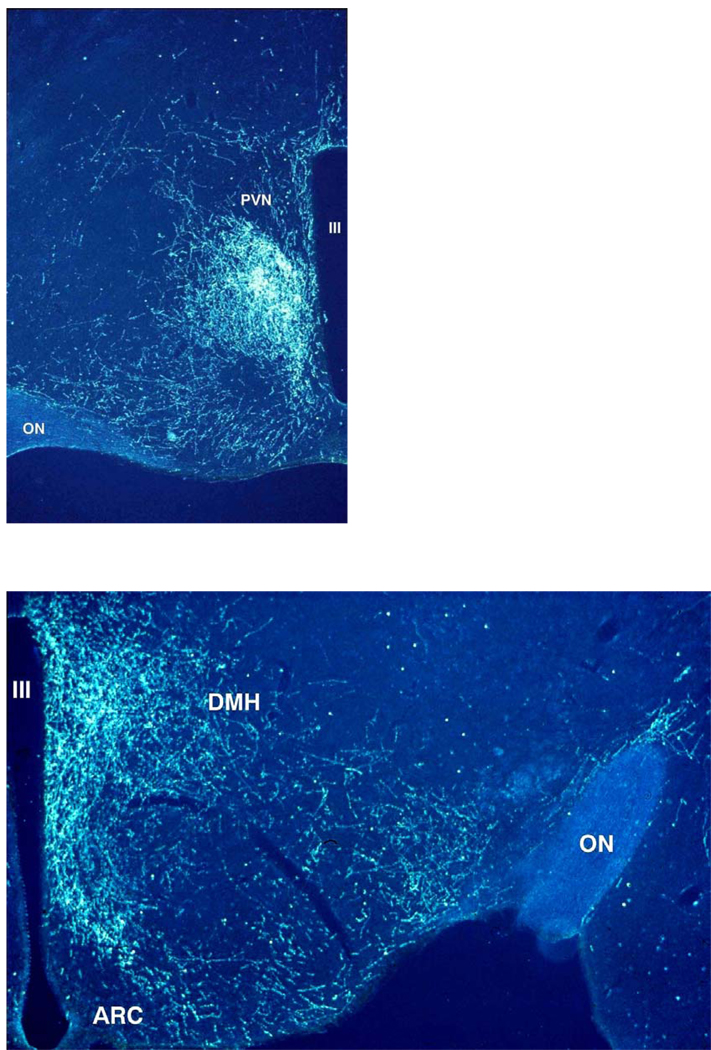
Fig.1. Darkfield illustration of SCN projections into the hypothalamic paraventricular (PVN), dorsomedial (DMH) and ventromedial (VMH) hypothalamus.
After the iontophoretic injection of Phaseolus vulgaris leucoagglutinin (PHA-L) into the SCN a large concentration of labelled fibers can be observed in the area just ventral to the PVN (upper panel), also known as the sub-paraventricular zone or the subPVN. Less dense but also clearly innervated in the upper panel are the periventricular part and the dorsomedial part of the PVN, but note the almost complete lack of labelled fibers in the parvicellular and magnocellular parts of the PVN. Caudal to the PVN (lower panel) a dense terminal field of SCN fibers can be observed in the DMH. In the lower panel also a clear concentration of PHA-L labelled fibers is present in the area between the arcuate nucleus (ARC) and the VMH, as well as the area lateral to the VMH. The third ventricle (III) is on the right side (upper panel) or left side (lower panel).
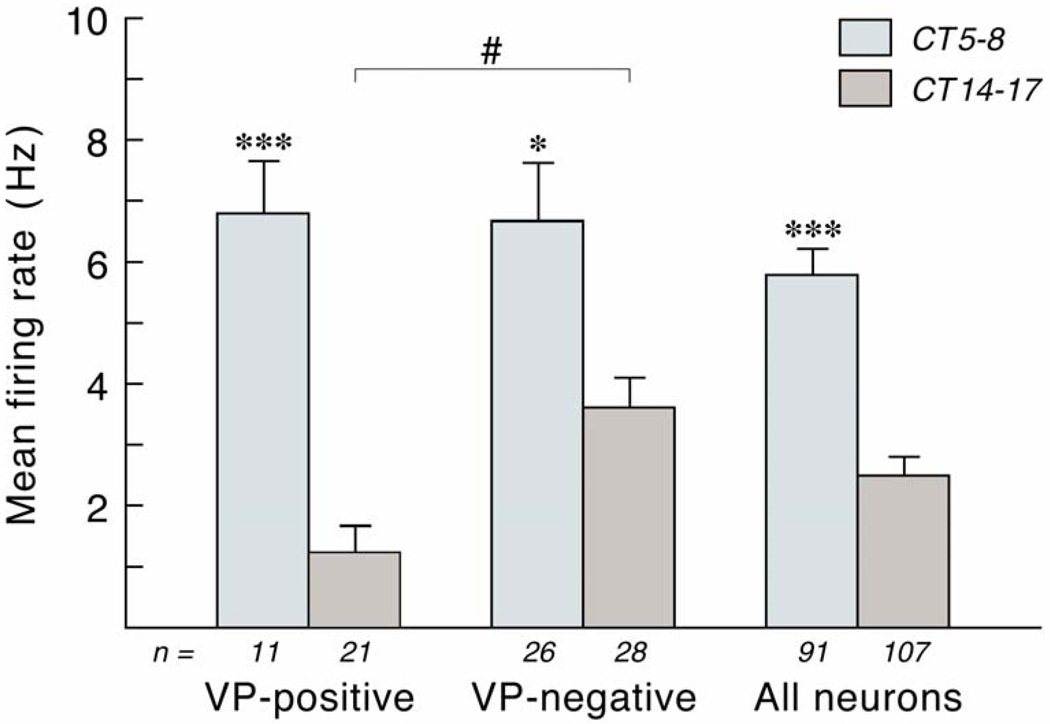
Fig.2. Mean firing frequency of vasopressin-positive, vasopressin-negative, and non-characterized neurons analyzed in the in vitro dorsal SCN of the rat.
The firing frequency was determined in hypothalamic slices at either CT5–8 or CT14–17. All groups showed a significant effect of diurnal timing. However, the difference was only two-fold in the vasopressin-negative neurons, whereas it amounted to five- or six-fold in the vasopressin-positive neurons. Especially the nocturnal firing frequency rate of vasopressin-positive neurons was remarkably lower as compared to the other groups. With permission from (Buijs et al., 2006). ***, p<0.001; *, # p<0.05
SCN control of hormonal rhythms
The data presented above make a strong case for a prominent role of vasopressin as an output signal of the hypothalamic biological clock. In view of all this evidence in favour of an important role for vasopressin in the output from the SCN we began, in 1992, with microinfusions of vasopressin and its antagonist. These first experiments demonstrated that vasopressin released from SCN terminals has a strong inhibitory control over basal plasma corticosterone concentrations (52). Further studies on the relation between the circadian release of vasopressin and the control of the daily rhythm in the activity of the hypothalamo-pituitary-adrenal (HPA)-axis revealed that vasopressin release in the rat DMH is important to ensure low circulating levels of corticosterone during the first half of the light period (53). In addition, the subsequent halt of vasopressin release from these SCN terminals in the DMH is a prerequisite for the daily surge in plasma corticosterone before the onset of the main activity period of the nocturnal rat, i.e., the dark period (54). This series of experiments also clearly showed that vasopressin is not the only SCN signal involved in the control of the daily rhythm in HPA-activity, but that the general principle of SCN control over daily (hormone) rhythms seems to be a push-and-pull or ying-yang mechanism, based upon an alternation of stimulatory and inhibitory inputs to the appropriate target neurons.
The importance of intermediate neurons
In the case of the HPA-axis the most likely target neurons appeared to be the CRH-containing neurons in the PVN. However, some evidence was inconsistent with this as a primary role for CRH neurons. Firstly, a direct effect of vasopressin on the CRH neuron would imply a clear daily rhythm in plasma ACTH concentrations, but this was not observed. Secondly, the observed inhibitory effect of vasopressin was not in line with the usual excitatory effect of vasopressin on its target neurons. Thirdly, contrary to the expected abundant contacts between SCN-derived vasopressin fibers and CRH neurons, only a limited amount of such connections was found (42, 55). A detailed anatomical scheme incorporating all of the above and explaining our current view on the SCN control of the daily rhythm in HPA-activity is shown in Fig.3. The proposed intermediate role of the GABAergic neurons in the subPVN and DMH in rats is supported by electrophysiological in vitro experiments using hypothalamic slices (56). As the image on the right of Fig.3 shows, the proposed important role for intermediate areas such as the subPVN and DMH also provides a good explanation for the mechanism behind the 12-h reversal of certain rhythms in nocturnal and diurnal species (for instance that of HPA-axis activity) (57), when apparently the phase of SCN activity (including vasopressin release) is similar for nocturnal and diurnal species (58–59).
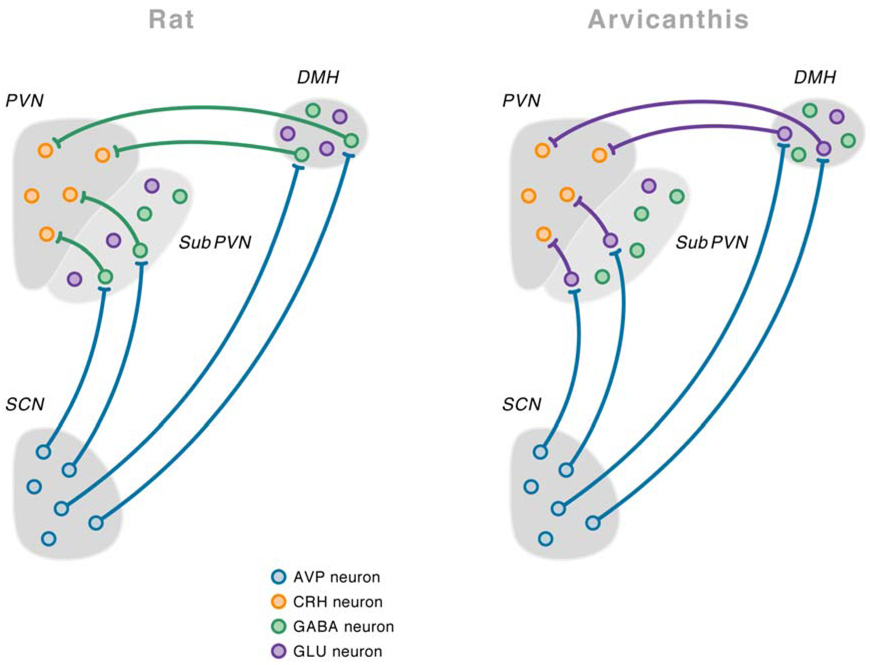
Fig.3. Detailed anatomical scheme of demonstrated and putative* connections of the suprachiasmatic nucleus (SCN) in the rat and Arvicanthis ansorgei brain to explain the opposite effects of vasopressin on the HPA axis in these two species.
Vasopressin is released during the light period, both in the nocturnal rat and the diurnal Arvicanthis ansorgei. In rats vasopressin release during the light period will inhibit the corticotropin-releasing hormone (CRH)-containing neurons in the paraventricular nucleus of the hypothalamus (PVN) by contacting γ-aminobutyric acid (GABA)ergic interneurons in the subPVN and dorsomedial nucleus of the hypothalamus (DMH). On the other hand, in the Arvicanthis ansorgei, vasopressin release during the light period will stimulate CRH-containing neurons because it acts on the glutamatergic, instead of GABAergic, interneurons in the subPVN and DMH. *Only the projections from the subPVN and DMH to the PVN in the Arvicanthis ansorgei have not been formerly confirmed by tracing experiments. With permission from (Kalsbeek et al., 2008).
The sympathetic innervation of the adrenal
An important spin-off of the above-mentioned vasopressin experiments was the insight it provided into the outflow of SCN information to the autonomic nervous system (ANS) as an important mediator for the SCN control of peripheral organs and tissues. The mismatch between plasma ACTH and plasma corticosterone concentrations and responses made us realize that the ANS might be important for setting the sensitivity of the adrenal cortex to ACTH. Transneuronal virus tracing from the adrenal indeed revealed second-order labelling in PVN neurons, and third-order labelling in SCN neurons (60). The functional importance of this multi-synaptic connection between the SCN and the adrenal cortex for the daily rhythm in adrenal corticosterone release was proven later on by an elegant combination of adrenal microdialysis and denervation experiments (61). The SCN thus apparently uses a two-stage mechanism to control daily hormone rhythms: on the one hand it acts on the neuroendocrine motorneurons to influence the release of hypothalamic releasing factors, on the other hand it also acts - through the ANS - on the target tissues to influence the sensitivity to the incoming hormonal message.
SCN control of the sympathetic nervous system
The daily melatonin rhythm
The prime example of circadian control through the autonomic nervous system is the daily rhythm in melatonin release from the pineal gland. Ultimately the synthesis and release of melatonin are controlled by the sympathetic input to the pineal gland (62). A similar pathway, with potential relevance for sleep disturbances, is most likely to be present in humans (63, 64). Based on a series of experiments very much similar to the ones just described for the unravelling of the function of vasopressin in the circadian control of the HPA-axis, we found that the daily rhythm in plasma melatonin concentration is also generated by a combination of stimulatory and inhibitory SCN outputs. We proposed that the activity of the pre-autonomic PVN neurons that are in charge of the sympathetic input to the pineal gland is controlled by a combination of glutamatergic and GABA-ergic inputs from the SCN (65–68). The circadian and light-induced daytime activity of the GABA-ergic SCN projections to the PVN ensures low melatonin levels during the light period. The nocturnal arrest of the inhibitory GABA-ergic inputs, combined with the continuously active glutamatergic inputs, enables the pre-autonomic PVN to become active again and start a new period of melatonin synthesis and release.
Shifting the melatonin rhythm
To further define the subpopulations of SCN neurons responsible for these inhibitory and stimulatory signals we used a combination of two different experimental paradigms, i.e., an 8-h advance of the L/D-cycle and a time-restricted feeding regime. After an 8-h phase advance of the L/D-cycle it takes at least 5 days for the nocturnal peak of melatonin release to re-appear at the appropriate time of day (69). This entrainment is postponed even further when the animals are on a restricted daytime feeding schedule before the shift (70). The abrupt phase-shift induced by the 8-h advance of the L/D-cycle profoundly affects the spatial and temporal distribution of Per clock gene expression in the SCN. We therefore investigated whether the activation of a particular subpopulation of SCN neurons could be correlated with the reappearance of either the onset or the offset of the plasma melatonin peak (71). From the results of these experiments we concluded that there is a small subset of Per expressing neurons located in the central part of the SCN that is responsible for the nocturnal stimulation of melatonin release during the dark period. We propose that these neurons provide the necessary glutamatergic input to the PVN. In addition, an absence of Per expression in the dorsal part of the SCN also seems a necessary prerequisite in order for melatonin levels to increase, i.e., compare the CT14–CT22 Per1 expression in AL and RFs animals (Fig.4). We hypothesize that it is the “activity” of these dorsal SCN neurons (and their sustained release of GABA) in the shifted animals that inhibits the pre-autonomic neurons in the PVN and prevents the reappearance of a new melatonin peak in the shifted dark period.
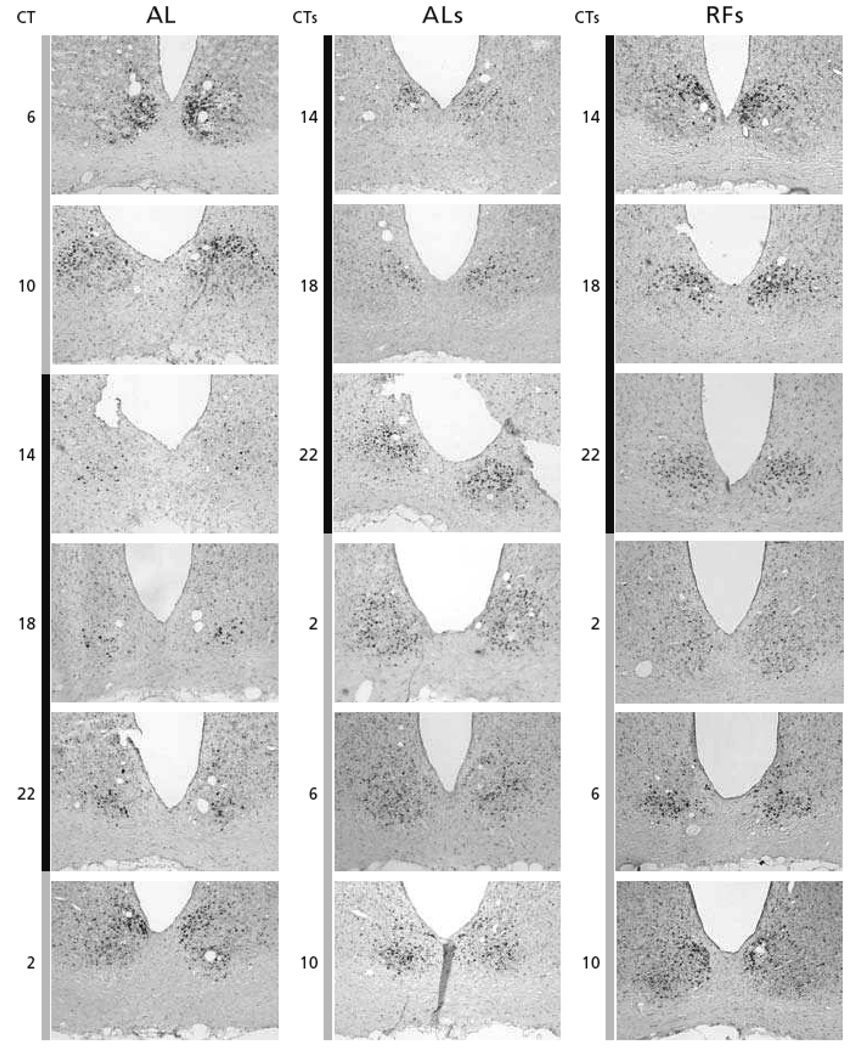
Fig.4. Spatial and temporal distribution of Per1 gene expression in the SCN, throughout the L/D-cycle.
Per1 gene expression is shown for ad libitum fed animals (AL), for ad libitum fed animals that had been phase-shifted (8-hours) 5 days earlier (ALs), and for animals on a restricted-feeding protocol that had been phase-shifted 5 days earlier (RFs). Per1 gene expression was revealed by the non-radioactive immuno-in situ hybridization technique (with the help of Dr. Hugues Dardente, in the laboratory of Dr. Paul Pévet). Gray and black bars on the left side of the figures indicate light and dark portions of the L/D-cycle. Animals on restricted feeding (RF) had daily access to food from ZT4–6. The 8-h phase-advance of the L/D-cycle was realized by shutting down the light at ZT4. The RF animals had been on the RF protocol for 4 weeks before the phase-shift. From the first day of the shift, the animals were provided with food ad libitum. Starting at ZT10 on day 5 after the shift (D5) and ZT2 for the non-shifted groups, the animals of the 4 groups were maintained in constant darkness under red-dim-light until the end of the experiment. The animals of the AL, ALs, and RFs groups were perfused every 4-hours at six different time points starting at CTs14 on D5 (CTs14 indicating CT14 after the shift and corresponding to ZT6 in non-shifted time). For further details see the thesis of Stephanie Perreau-Lenz (2004).
Desynchronizing the melatonin rhythm
Schwartz and de la Iglesias have developed a forced desynchrony protocol in rats that results in a stable dissociation of rhythmic clock gene expression in the ventrolateral and dorsomedial SCN (72). In these animals the rhythms of locomotor activity, sleep, body temperature and melatonin release are internally desynchronized (72–74). These experiments provided a clear example of how the normal phase relation between different compartments of the SCN may be disorganized, thereby profoundly affecting behavioural and hormonal rhythmicity. Whether or not similar internal desynchrony occurs in humans when there is misalignment of the endogenous circadian rhythms of melatonin, temperature, and cortisol with the behavioural sleep/wake and fasting/feeding cycle, remains to be investigated. This would be clinically relevant in relation to the adverse health consequences that night shift workers experience (75).
SCN control of the parasympathetic nervous system
On the basis of a series of retrograde viral tracing studies from adipose tissue (both brown and white), pancreas, stomach, and the heart and intestines, a similar SCN control as just discussed for the adrenal and pineal gland may apply for other peripheral tissues as well (76–79), certainly for tissues involved in energy metabolism. Thus we hypothesized that part of the action of the SCN to prepare our bodies for the alternating periods of sleep and wakefulness would be through the use of its connections with the hypothalamic pre-autonomic neurons to control the daily setting of the sympathetic – parasympathetic balance of autonomic inputs to these peripheral organs. Indeed, in a first series of viral tracing experiments we were able to show a clear separation of the pre-autonomic neurons that control the sympathetic and parasympathetic branch of the autonomic nervous system, up to the level of the second order neurons in the hypothalamus (76, 80–81). Subsequently, we investigated whether one single group of neurons within the biological clock would be dedicated to the control of these sympathetic and parasympathetic pre-autonomic neurons, in other words, whether also within the SCN there is a clear separation of neurons controlling the sympathetic and parasympathetic branches of the autonomic nervous system. Using a combination of double viral tracing and selective organ denervation we were able to demonstrate that the segregation of pre-sympathetic and pre-parasympathetic neurons already starts at the level of the SCN (Fig.5) (82). This high level of differentiation puts the SCN in a unique position to balance the activity of both ANS branches according to the time of day. However, although these neuro-anatomical data provide a nice blueprint for the possible SCN control of energy metabolism and the autonomic balance, the big question remains whether, and if so, to what extent this neuro-anatomical blueprint has any functional significance.
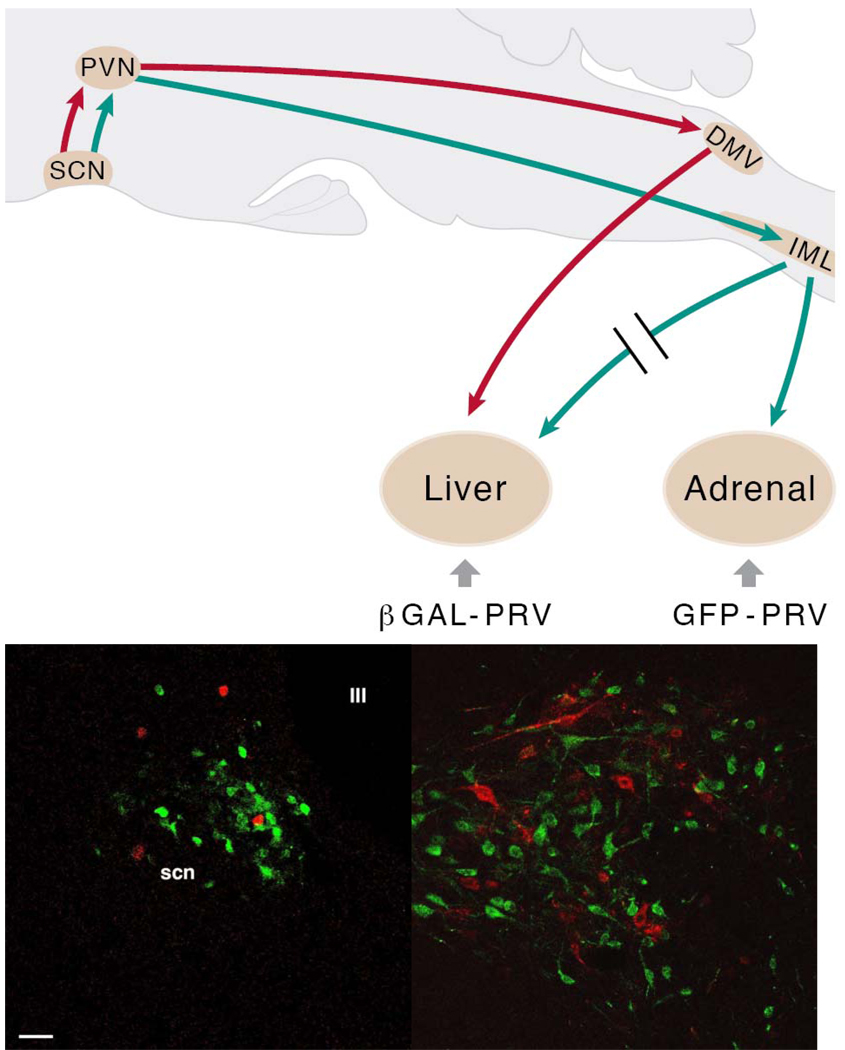
Fig.5. The suprachiasmatic nucleus (SCN) balances sympathetic and parasympathetic output to peripheral organs through separate pre-autonomic neurons.
In the upper panel the experimental setup used to examine the possible separation of sympathetic and parasympathetic pre-autonomic neurons in the hypothalamus is indicated in a schematic drawing of a sagittal section of the rat brain. B-galactoside PRV (βGAL-PRV) was injected into the sympathetic denervated liver, forcing the virus to infect the brain via the vagus nerve (red lines); simultaneously the pre-sympathetic neurons were labelled by an injection of green fluorescent protein (GFP)-PRV into the adrenal (green lines). After the labelling of the first-order neurons in the brainstem and spinal cord, this approach resulted in separate pre-parasympathetic and sympathetic neurons in the PVN (second order), followed by a similar separation of the third-order neurons in the SCN. In the lower panels transverse sections of the hypothalamus in the region of the PVN (left) and SCN (right) show a perfect separation of pre-parasympathetic βGAL-PRV (red) and pre-sympathetic GFP-PRV (green) labelled neurons, as there are no yellow (i.e., double-labelled) neurons. Scale bar = 25 µm. With permission adapted from (Buijs et al., 2003).
A daily rhythm in plasma glucose concentrations
To investigate whether the SCN control of the parasympathetic branch of the autonomic nervous system (ANS) would be comparable to the one just described for the sympathetic branch, we then focused our attention on the daily rhythm in plasma glucose concentrations. Maintaining a constant blood glucose level is essential for normal physiology in the body, particularly for the central nervous system (CNS), as the CNS can neither synthesize nor store the amount of glucose required for its normal cellular function. The liver plays a pivotal role in maintaining optimum glucose levels by balancing glucose entry into, and its removal from, the circulation. From a hypothalamic and chronobiological point of view glucose production by the liver is especially interesting because of the clear involvement of both the sympathetic and parasympathetic input to the liver in glucose metabolism (83–85) and the strong circadian control of (glucose) metabolism in the liver (86–88). Using local intra-hypothalamic administration of GABA and glutamate receptor (ant)agonists we explored the contribution of changes in ANS activity to the daily control of plasma glucose and plasma insulin concentrations. The daily rhythm in plasma glucose concentrations turned out to be controlled according to a mechanism very much similar to the mechanism just described for the SCN control of the daily rhythm in melatonin release, i.e., a combination of rhythmic GABA-ergic inputs and continuous glutamatergic stimulation onto liver-dedicated sympathetic pre-autonomic neurons in the PVN (81, 89). The major difference between the liver-dedicated and pineal-dedicated pre-autonomic neurons seems to be the timing of the GABA-ergic inputs. In the case of the pineal-dedicated pre-autonomic neurons this inhibitory input is present during the major part of the light period with an acrophase around ZT6, whereas for the liver-dedicated pre-autonomic neurons the acrophase of the GABA-ergic inhibition is somewhere around ZT2. Surprisingly, no clear evidence was found for an involvement of the parasympathetic branch of the ANS, as our previous denervation studies clearly showed the daily plasma glucose rhythm to be disrupted, also in parasympathetic liver-denervated animals (90).
The daily glucose rhythm and the perifornical orexin neurons
Plasma glucose concentrations are the result of a glucose influx from the gut and liver, and glucose efflux by its uptake in brain, muscle, and adipose tissue. To investigate in more detail by which glucoregulatory mechanism the just described SCN output mechanism contributes to the increased plasma glucose concentrations at awakening we first performed a series of intravenous glucose tolerance and insulin sensitivity tests in rats. To our surprise these studies revealed that glucose tolerance and insulin sensitivity peak at the onset of the dark period (91). Therefore, the rise in plasma glucose concentrations at the end of the sleep period could not be explained by a diminished glucose uptake at this time of the L/D-cycle. These studies also indicated that glucose production should increase at the end of the sleep period, firstly to compensate for the increased glucose uptake and secondly to explain the increased plasma glucose concentrations. We went on to combine hypothalamic administration studies with systemic infusion of a stable glucose isotope. The use of the stable glucose isotope enabled us to distinguish between changes in glucose production and glucose uptake. These experiments showed that a pronounced increase in hepatic glucose production was caused by the administration of bicuculline (a GABA-A receptor antagonist) in the perifornical area lateral to the DMH, and that orexin- (but not MCH-) containing neurons in this area were strongly activated (92). Subsequent studies revealed that the hyperglycemic effect of bicuculline could indeed be prevented by the concomitant ICV administration of an orexin antagonist and that orexin fibers impinge upon sympathetic preganglionic neurons in the IML of the spinal cord that project to the liver (92, 93). Earlier we had demonstrated that the hyperglycemic effect of a focal blockade of GABA-ergic transmission was very much dependent on the time of day (89), indicating SCN control. Indeed using an approach very much similar to ours, Alam et al. (94) had already demonstrated that perifornical orexin neurons are subject to an increased endogenous GABA-ergic inhibition during sleep. In view of the pronounced day/night-rhythm in orexin release (95, 96) we hypothesized that orexin is the main connection between the biological clock and the daily rhythm in plasma glucose concentrations. To test this hypothesis we measured the rate of glucose appearance (Ra) in ad libitum fed animals during the second half of the light period and the first hours of the dark period, i.e., during the ascending phase of the daily rhythm in plasma glucose. We combined these measurements with the ICV infusion of an orexin antagonist or vehicle (Fig.6). The results of this experiment point to an important role for the orexin system in the control by the biological clock over daily glucose homeostasis, as the ICV orexin-antagonist completely prevented the daily dusk time increase in glucose appearance. The perifornical orexin neurons thus seem to transduce the rhythmic GABA and glutamatergic signals emanating from the SCN into a daily activation of the sympathetic input to the liver, which results in an increased hepatic glucose production at the end of the sleep period in anticipation of a new period of wakefulness.
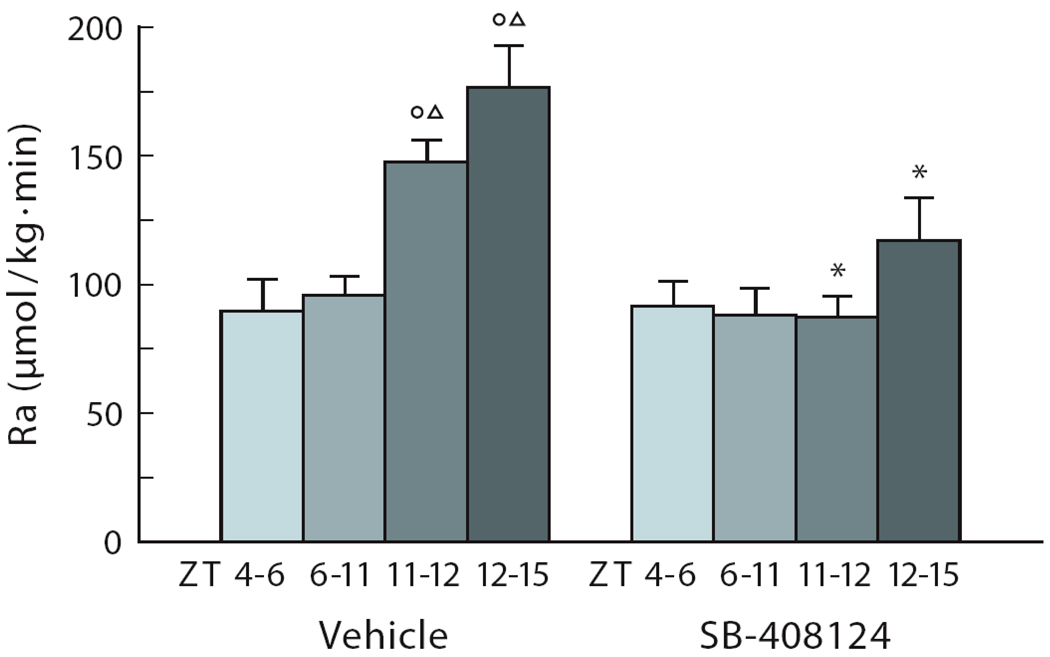
Fig.6. Orexin release is necessary for the endogenous rise in glucose appearance at dusk in nocturnal animals.
The endogenous rise in glucose appearance (Ra) during ICV vehicle administration is shown in the left panel. ICV administration of the orexin antagonist, during the latter part of the light period, prevents the endogenous rise of Ra before the onset of darkness (ZT11–ZT12), and during the onset of the dark period (ZT12–ZT15) (right panel). The orexin antagonist SB-408124 was administered ICV from ZT4 – ZT12. IV administration of the orexin antagonist had no effect on glucose appearance. Food intake was not changed by ICV orexin. O and Δ, P<0.05 compared with ZT4–6 and ZT6–11, respectively. *, P<0.05 compared with comparable time points in the vehicle group.
Remarkably, a recent study by Schiuchi et al. (97) demonstrated that via the VMH and the sympathetic nervous system orexin is able to stimulate glucose uptake in muscle. Thus, orexin might be an important link in the SCN-controlled concomitant increase of glucose production and glucose uptake at the onset of the activity period (91). Together, these results indicate that by a dis-inhibition of the orexin system at the end of the light period the SCN not only promotes arousal, but also causes an increase of endogenous glucose production to ensure adequate concentrations of plasma glucose when the animal awakes. Other studies made it look very likely that the rhythmic activity of the orexin system is also involved in the increased activity of the cardiovascular system at awakening (98, 99). However, a major drawback of such a heavily integrated system may be that, due to their effect on the orexin system, sleep disturbances will result in hypertension (100) and disturbed glucose metabolism (101, 102).
The circadian control of meal-induced insulin responses
As has become evident from the daily variation in meal-induced insulin responses (103), intestinal glucose uptake (104), respiratory functioning (105) and markers of cardiac vagal activity (106–108, 188) the parasympathetic branch of the autonomic nervous system too, is under control of the circadian timing system. Our intra-hypothalamic infusion studies revealed that the daily changes in the activity of the parasympathetic pre-autonomic neurons also involve a combination of GABA-ergic and glutamatergic inputs (89). The inhibition of pre-autonomic neurons, both sympathetic and parasympathetic, by a daily rhythm in GABA release from SCN efferents to the PVN turned out to be a general principle. However, a major difference between the circadian control of parasympathetic and sympathetic pre-autonomic neurons appears to be the origin of the excitatory glutamatergic inputs. SCN-lesion studies proved that the excitatory input to the sympathetic pineal-dedicated pre-autonomic neurons was derived from the SCN neurons (67), but also that the glutamatergic inputs to the parasympathetic pancreas-dedicated pre-autonomic neurons can not be derived from SCN neurons (109). At present, it is not yet clear from which extra-SCN source the glutamatergic inputs to the parasympathetic pancreas-dedicated pre-autonomic neurons originate, but likely candidates are the ventromedial hypothalamic nucleus (VMH) and arcuate nucleus (Fig.7).
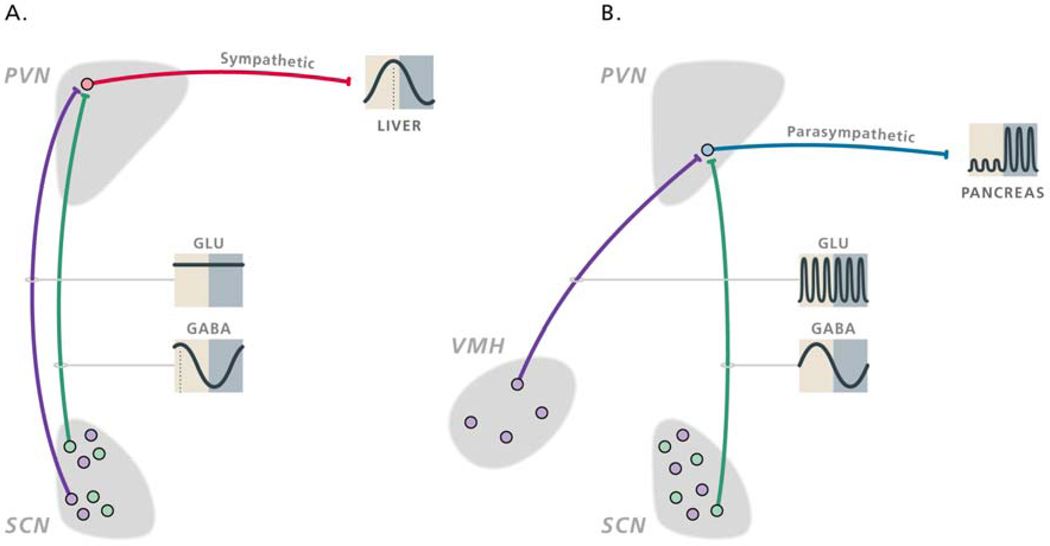
Fig.7. Schematic presentation of the daily activity pattern of hypothalamic populations of GABAergic and glutamatergic neurons implicated in the autonomic control of the daily rhythms in hepatic glucose production (A) and feeding-induced insulin release (B).
The sympathetic and parasympathetic pre-autonomic neurons are inhibited by a rhythmic GABAergic input (green dots and lines) from the SCN that is mainly active during the light period. Sympathetic pre-autonomic neurons are stimulated by glutamatergic inputs (purple dots and lines) from the SCN (A), whereas parasympathetic pre-autonomic neurons are stimulated by glutamatergic inputs from the VMH (B). The glutamatergic stimulation only translates in activity of the pre-autonomic neurons when the inhibitory input from the SCN is absent. The control of the sympathetic pre-autonomic neurons that are involved in the control of the daily melatonin rhythm looks very much similar to that of hepatic glucose production, the only difference being a slight phase delay of the activity of GABAergic SCN neurons that innervate the “pineal-dedicated” pre-autonomic neurons. With permission from (Kalsbeek et al., 2008).
SCN and adipose tissue
Obesity is characterized by excessive accumulation of triglycerides in adipose tissue determined by a net balance of fatty acid uptake and release in favour of fat storage over fat mobilization. The rich innervation of adipose tissue by sympathetic fibers is well-known, and activation of these fibers is associated with enhanced lipolysis (110). Until a few years ago, it was thought that parasympathetic innervation of adipose tissue did not occur and that lipogenesis was merely controlled by hormones, the mass action of free fatty acids and sympathetic withdrawal. In view of the importance of this balance between lipogenesis and lipolysis, and the capacity of the SCN to control the sympathetic/parasympathetic balance in other organs, we re-investigated the existence of parasympathetic input to adipose tissue. This would allow us to test the possibility of SCN control of this lipogenesis/lipolysis balance through the autonomic nervous system. Indeed, as previously reported by others (111), at first we found only very sparse parasympathetic input to white adipose tissue. However, combining the viral tracing technique with a prior selective sympathetic denervation of the targeted fat pad resulted in pronounced labelling of the parasympathetic motorneurons in the brainstem (112). It is not clear what causes this increased visibility of the parasympathetic input, but one possibility is that the parasympathetic fibers are only exposed to the virus when the more active sympathetic fibers have been removed, as previous studies have shown that viral tracing can be modulated by neuronal activity (113). These observations provided the neuro-anatomical substrate for earlier pharmacological observations in human microdialysis studies that showed cholinergic effects on lipolysis (114) and the more recent identification of functional acetylcholine receptors in rat adipocytes (115, 116). In addition, our own functional studies provided clear evidence for the anabolic function of this parasympathetic innervation of adipose tissue. Euglycemic hyperinsulinemic clamp studies revealed a >30% reduction in the insulin-mediated uptake of glucose and FFA’s in adipose tissue as a result of selective removal of its parasympathetic input. Moreover, without parasympathetic input the activity of the catabolic enzyme hormone sensitive lipase (HSL) increased by 51% in the denervated adipose tissue (112). Follow-up studies using two different PRV tracers and selective denervation of the adipose tissue showed the presence of both ‘sympathetic’ and ‘parasympathetic’ adipose neurons in the hypothalamus, including the SCN (117). These results thus provide clear evidence that the SCN may use the ANS to enforce its day/night rhythms upon the endocrine and metabolic functioning of adipose tissue. Indeed, both fat deposition by the key enzyme lipoprotein lipase (LPL) and fat mobilization by HSL show a clear daily rhythm in the white adipose tissue of humans and laboratory animals (118–122). In addition, the circulating plasma levels of a number of adipocytokines, including leptin, as well as their adipose mRNA levels show clear day/night rhythms (123–127), while a SCN-lesion abolishes the diurnal rhythm of plasma leptin (128).
Social jetlag
There is accumulating evidence for an intimate relation between circadian and metabolic cell systems, also at the molecular level (129–130). From the data presented above it is clear that the circadian system has a major influence on energy metabolism. The restricted-feeding paradigm shows that the reverse is also true, i.e., energy metabolism also has a profound effect on the circadian system. Although the central pacemaker in the SCN seems to be relatively protected against the disorganizing effects of restricted feeding during the light period, peripheral clock systems in the liver and many other peripheral organs rapidly entrain to such an aberrant feeding condition (131–134). In keeping with an important role of peripheral circadian clocks in the temporal organization of metabolism, transcriptome profiling in liver and heart has revealed that many clock-controlled genes specify enzymes and regulators involved in the metabolism of food components (87–88, 135). The restricted daytime feeding protocol presents a condition very much similar to that of many night shift workers, i.e., a misalignment of the sleep/wake and fasting/feeding cycles on the one hand and the light/dark cycle on the other. Because of the entrainment of the central pacemaker to the light/dark cycle and the entrainment of peripheral clocks to the feeding/fasting cycle this situation could result in internal desynchrony (136), which would lead to disturbed sleep, reduced cognitive performance, and gastro-intestinal complaints, i.e., symptoms very much similar to those of jet-lag. Not surprisingly, a number of epidemiological studies have indicated a link between shift work and increased risk for cardiovascular disease and/or the metabolic syndrome (137–139).
Shift work is an extreme form of circadian misalignment. In a milder form, circadian misalignment now features in the lives of many as a result of a changing lifestyle. Using a simple questionnaire Roenneberg and coworkers investigated the sleep/wake habits of >25,000 people and determined their chronotype (140, 141). They concluded that not only shift workers but more than half of the population lives with a body clock that is permanently out of phase with environmental time, a condition they coined “social jetlag” (142). Interestingly, many of these lifestyle changes also correlate with an increased prevalence of the metabolic syndrome (143). In their reviews Hastings et al. (144) and Foster & Wulff (145) outlined how the misalignment of biological and social time may cause circadian de-synchrony, possibly resulting in chronic illnesses such as the metabolic syndrome, as well as cardio-vascular and gastro-intestinal diseases.
Balancing the autonomic output of the SCN
Indeed, with the invention of the electric light bulb, the advent of jet travel and the expansion of shift work, the daily cycles of rest and wakefulness and their corresponding periods of fasting and eating, are no longer organized according to the presence of natural light. Therefore, the normal alternation of sleep and wake, or rest and activity, cold/warm, inactive/active and satiety/hunger cycles is likely to be disturbed and the amplitude and coherence of these SCN entraining signals is greatly reduced. We have put forward the hypothesis that as a result of these diminished rhythmic inputs to the SCN, the output of the SCN is diminished as well (146). The normal alternation of rest and activity is accompanied by a shift in the balance of the autonomic nervous system, i.e., the active period is characterized by a predominant sympathetic activity, whereas parasympathetic activity rules the body during the inactive period. This balance of the autonomic nervous system is critically dependent on the output of our biological clock. Figure 4 clearly illustrates how a shift of the L/D-cycle may disorganize SCN output. Subsequently, reduced amplitude, inappropriate timing, or internal desynchrony of the SCN output signals may result in an unbalanced autonomic nervous system and an organism less well prepared for the upcoming demands. This idea is nicely illustrated by the results of Cailotto et al. showing that nocturnal light exposure and a dis-balance, but not a complete absence, of the autonomic inputs to the liver result in a disturbed daily plasma glucose rhythm (90, 147, 148). Also the recent results of Fonken et al. (149), showing that night time light exposure in mice results in increased body mass and reduced glucose tolerance fit in with this idea. We were able to provide further strong supportive evidence for this hypothesis in humans using a forced-desynchrony (FD) protocol. Subjects were subjected to an 11-day protocol consisting of repeated 28-h “days”, with 4 standardized meals per 28-h day. The misalignment between the free-running central circadian pacemaker and the behavioural sleep/wake and fasting/feeding rhythms, resulted in increased plasma glucose and insulin concentrations, increased blood pressure and decreased plasma leptin concentrations (Fig.8) (75). In 3 out of the 10 subjects the circadian disorganization pushed the postprandial glucose response into the pre-diabetic range. This circadian misalignment also led to a complete reversal of the rhythm of plasma cortisol and melatonin across the sleep/wake cycle, which may be involved in impaired glucose metabolism (150–152). Circadian misalignment also results in decreased sleep quality. Although the results of Scheer et al. (75) appeared to be beyond those that could be explained by changes in sleep efficiency per se, sleep quality in itself also has clear effects on energy metabolism. As previously noted there is a significant correlation between sleep duration and the risk of developing hypertension (100), diabetes (153) or obesity (102, 154). Spiegel and colleagues (155) showed in healthy young volunteers that restricting sleep to 4-h a night for 6 nights in a row resulted in reduced glucose clearance, an impaired insulin response and insulin resistance. Partial sleep deprivation has also been demonstrated to result in lower plasma leptin and increased plasma ghrelin concentrations and in increased appetite and food intake (156–158), as well as in a change in the autonomic balance (157).
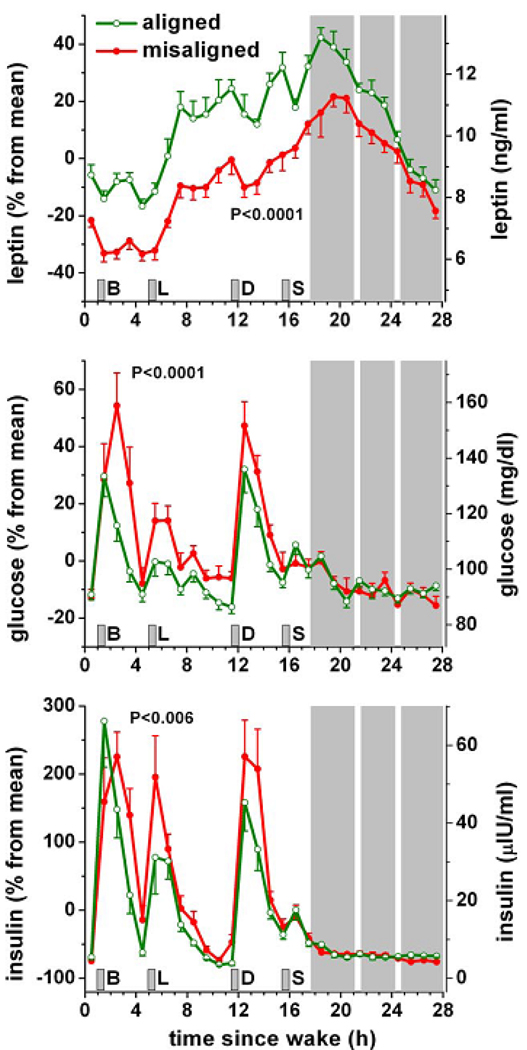
Fig.8. Consequences of circadian misalignment on metabolic and endocrine function.
Data from 10 healthy volunteers are plotted according to time-since-wake, during normal circadian alignment (open green symbols; scheduled awakening at habitual wake time) and during circadian misalignment (filled red symbols; scheduled awakening 12 h out of phase from habitual wake time). P-values, statistical significance for effect of misalignment; gray area, scheduled sleep episode; short vertical gray bars, meal times; B, breakfast; L, lunch; D, dinner; S, snack. Reproduced with permission from Scheer et al. (2009).
A disorganized clock
Is there any direct evidence that a disorganized clock goes hand in hand with metabolic disease? Not really, but there appears to be some circumstantial evidence. The age-related deterioration that has been observed in many daily endocrine, physiological and behavioural rhythms in several species, including human, makes it tempting to hypothesize a causative role for the declining output of the SCN. Interestingly, a marked decrease in the number of vasopressin-containing neurons occurs in subjects between 80 and 100 years of age and in subjects with Alzheimer’s disease (20). Also on the mRNA level vasopressin expression is lower in the SCN of Alzheimer patients (159). The flattening of the daily rhythm in vasopressin abundance was already observed in subjects >50 years of age (160). Similar decreases in the expression of vasopressin in the SCN were also noted in aging experimental animals (161–163). Harper et al. (164) very elegantly demonstrated that the loss of SCN vasopressin-expressing neurons in the human postmortem brain significantly correlated with an increased deterioration of activity rhythms before death. Further functional evidence for disturbances of the SCN in patients from Alzheimer’s disease comes from fractal analysis of actigraphy data, showing breakdown of complexity of scale invariant behaviour (165), very similar to that demonstrated in locomotor activity of experimental animals following lesioning of the SCN (166). A similar situation is apparent in patients with major depression, i.e., disturbed circadian rhythms and a decreased activity of the vasopressinergic SCN neurons (167). Patients with diabetes or coronary heart disease have a flattened melatonin rhythm (168–170). Recently, mutations in the melatonin receptor were linked to an increased risk of type 2 diabetes (150–152), and some large scale genetic studies in humans provided the first evidence for the involvement of disrupted clock gene rhythms in the pathogenesis of obesity and type 2 diabetes. Specifically, haplotypes of the core clock genes Bmal1, Clock and CRY were associated with either obesity or type 2 diabetes (171–175). Finally, a pronounced decline of the immunocytochemical staining for three prominent SCN neuropeptides -including vasopressin- was observed in subjects with a history of primary hypertension (176). This observation is all the more interesting as a clear-cut increase was found in the amount of CRH staining and CRH mRNA expression in the PVN in these same patients (177). The increased activity of CRH-neurons in the PVN of hypertensive patients is probably not only responsible for an increased activity of the HPA-axis, but also for an increased activity of the sympathetic branch of the ANS, which has been implicated in the pathogenesis of some forms of hypertension. The inverse relationship between vasopressin and CRH as reflected by the decreased vasopressin staining in the SCN, and the increased CRH activity in the PVN resembles the inhibitory effect of SCN-derived vasopressin on the HPA-axis as found in the animal experiments. In other words, it is tempting to speculate that one of the mechanisms underlying the increased CRH activity in hypertensive patients is a diminished inhibitory input from the SCN. In line, spontaneously hypertensive rats (SHR) show a change in SCN activity, although in this case it is an increased activity of the VIP neurons (178, 179). The main question that remains is whether these changes are a cause or a consequence of the disease.
Conclusion and perspectives
A key question at this stage is whether a strengthening of the SCN signal could alleviate pathologies such as insulin resistance, obesity and hypertension. Interestingly, the loss of immunoreactivity for SCN neurotransmitters during aging and hypertension is probably not due to a loss of neurons, but to a decreased activity of these neurons. Therefore, an important way to re-vitalize a flattened and disorganized SCN output might be to enhance the rhythmic input signals to the SCN.
Indeed, increased light intensities during the light period appeared to prevent the aging-induced decrease in SCN vasopressin cell number in very old rats (180), as well as the deterioration of their behavioural rhythms (181). Moreover, in a recent double-blind, placebo-controlled randomized study among 189 elderly residents living in 12 different care facilities, long-term treatment with whole-day bright light significantly improved cognitive and non-cognitive symptoms of dementia (182), extending earlier work showing improved rest-activity cycles (183).
An additional way to enhance the rhythmic input to the SCN is the daily timed intake of melatonin. Melatonin is not only one of the most stable hormonal outputs of the biological clock, it also has a profound effect on the electrical activity of SCN neurons (184). Repeated night time administration of exogenous melatonin might therefore help to enhance the amplitude of the SCN rhythm by dampening erroneous night time activity. We conducted a randomized, double-blind, placebo-controlled, crossover study in which 16 men with untreated essential hypertension were treated with oral melatonin (2.5 mg daily; 1 hour before sleep) for three weeks. Repeated melatonin administration reduced blood pressure in hypertensive patients by 4–6 mm Hg, predominantly by a fall in systolic blood pressure during sleep (185). Thus, as with the daily light treatment of elderly people, daily melatonin treatment may enhance the functioning of the biological clock and thus help to improve circadian rhythms in behaviour. Another example may be gathered from studies showing that daily exercise is a very effective way to improve glucose tolerance (186), with the most critical aspect probably being a reduction of the periods of inactivity during the waking hours (187).
The experiments described above provide possible therapeutic strategies to counteract the negative health effects of a chronically reduced SCN output. However, they may not apply for shift workers, as here the circadian misalignment is in constant flux. During working days the shift worker is compelled to shift his/her sleep/wake rhythm to meet the needs of work hours, but during days off he/she reverts back to a normal daytime activity schedule to meet the needs of social life. Therefore, future studies identifying the exact mechanisms of internal desynchronization are justified if we are to propose behavioural strategies capable of minimizing the adverse effects of circadian misalignment.
Acknowledgements
The authors thank Dr. Mariette T. Ackermans at the Academic Medical Center in Amsterdam for her help with the stable isotope measurements, Henk Stoffels for preparation of the images and Wilma Verweij for correction of the manuscript. Special thanks are dedicated to the technicians: Tjitske van der Woude, Joke Wortel, Jan van der Vliet, Caroline van Heijningen, Ewout Foppen and Leslie Eggels, for their superb technical assistance in most of the work just described and to Jilles Timmer for animal husbandry. The authors also would like to sincerely acknowledge Dr. Hugues Dardente for his precious help with the non-radioactive immuno-in situ hybridizations and Dr. Paul Pévet for his great and long-lasting collaboration. F.A.S. was supported by NIH grants P30HL101299 and R01HL094806.
Abbreviations
- ACTH
Adrenocorticotrophic hormone
- AL
Ad libitum
- ANS
Autonomic nervous system
- Bmal
Brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT) like
- CLOCK
Circadian locomotor output cycles kaput
- CRH
Corticotrophin-releasing hormone
- CRY
Cryptochrome
- CSF
Cerebrospinal fluid
- DMH
Dorsomedial nucleus of the hypothalamus
- FD
Forced desynchrony
- FFA
Free fatty acid
- GABA
gamma amino butyric acid
- HPA
Hypothalamo-pituitary-adrenal
- HSL
Hormone sensitive lipase
- IML
intermediolateral column of the spinal cord
- L/D
Light/dark
- LPL
Lipoprotein lipase
- MPOA
Medial preoptic area
- mRNA
messenger RNA
- NPFF
Neuropeptide FF
- NPY
Neuropeptide Y
- PACAP
Pituitary adenylcyclase activating peptide
- pePVN
Periventricular nucleus of the hypothalamus
- Per
Period
- PVN
Paraventricular nucleus of the hypothalamus
- Ra
Rate of (glucose) appearance
- RF
Restricted feeding
- SCN
Suprachiasmatic nuclei
- SHR
Spontaneously hypertensive rat
- SON
Supraoptic nuclei
- subPVN
subparaventricular nucleus of the hypothalamus
- VIP
Vasoactive intestinal peptide
- VMH
Ventromedial nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore RM. Entrainment pathways and the functional organization of the circadian system. Prog. Brain Res. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- 2.Challet E, Pévet P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front. Biosci. 2003;8:S246–S257. doi: 10.2741/1039. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin XW, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 5.Hahm SH, Eiden LE. Five discrete cis-active domains direct cell type-specific transcription of the vasoactive intestinal peptide (VIP) gene. J. Biol. Chem. 1998;273:17086–17094. doi: 10.1074/jbc.273.27.17086. [DOI] [PubMed] [Google Scholar]
- 6.Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat. Neurosci. 2006;9:212–219. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- 7.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 8.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat. Neurosci. Rev. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 9.Weaver DR. The suprachiasmatic nucleus: A 25-year retrospective. J. Biol. Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 10.Swaab DF, Pool CW, Nijveldt F. Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophyseal system. J. Neural. Transm. 1975;36:195–215. doi: 10.1007/BF01253126. [DOI] [PubMed] [Google Scholar]
- 11.Vandesande F, Dierickx K, De Mey J. Identification of the vasopressin-neurophysin producing neurons of the rat suprachiasmatic nuclei. Cell Tiss. Res. 1974;156:377–380. doi: 10.1007/BF00225365. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM, Artman HG, Swaminathan S, Fisher DA. Vasopressin exhibits a rhythmic daily pattern in cerebrospinal fluid but not in blood. Science. 1981;213:1256–1257. doi: 10.1126/science.7268432. [DOI] [PubMed] [Google Scholar]
- 13.Reppert SM, Schwartz WJ, Uhl GR. Arginine vasopressin: a novel peptide rhythm in cerebrospinal fluid. Tr. Neurosci. 1987;10:76–80. [Google Scholar]
- 14.Sofroniew MV, Weindl A. Identification of parvocellular vasopressin and neurophysin neurons in the suprachiasmatic nucleus of a variety of mammals including primates. J. Comp. Neurol. 1980;193:659–675. doi: 10.1002/cne.901930305. [DOI] [PubMed] [Google Scholar]
- 15.Cassone VM, Speh JC, Card JP, Moore RY. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J. Biol. Rhythms. 1988;3:71–91. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- 16.Reuss S, Hurlbut EC, Speh JC, Moore RY. Immunohistochemical evidence for the presence of neuropeptides in the hypothalamic suprachiasmatic nucleus of ground squirrels. Anat. Rec. 1989;225:341–346. doi: 10.1002/ar.1092250410. [DOI] [PubMed] [Google Scholar]
- 17.Goel N, Lee TM, Smale L. Suprachiasmatic nucleus and intergeniculate leaflet in the diurnal rodent Octodon degus: Retinal projections and immunocytochemical characterization. Neuroscience. 1999;92:1491–1509. doi: 10.1016/s0306-4522(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 18.Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from east Africa. J. Comp. Neurol. 1999;403:190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Stopa EG, King JC, Lydic R, Schoene WC. Human brain contains vasopressin and vasoactive intestinal polypeptide neuronal subpopulations in the suprachiasmatic region. Brain Res. 1984;297:159–163. doi: 10.1016/0006-8993(84)90553-5. [DOI] [PubMed] [Google Scholar]
- 20.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 21.Günther O, Landgraf R, Schuart J, Unger H. Vasopressin in cerebrospinal fluid (CSF) and plasma of conscious rabbits - Circadian variations. Exp. Clin. Endocrinol. Diab. 1984;83:367–369. doi: 10.1055/s-0029-1210357. [DOI] [PubMed] [Google Scholar]
- 22.Seckl JR, Lightman SL. Diurnal rhythm of vasopressin but not of oxytocin in the cerebrospinal fluid of the goat: lack of association with plasma cortisol rhythm. J. Endocrinol. 1987;114:477–482. doi: 10.1677/joe.0.1140477. [DOI] [PubMed] [Google Scholar]
- 23.Stark RI, Daniel SS. Circadian rhythm of vasopressin levels in cerebrospinal fluid of the fetus: effect of continous light. Endocrinology. 1989;124:3095–3101. doi: 10.1210/endo-124-6-3095. [DOI] [PubMed] [Google Scholar]
- 24.Forsling ML. Neurohypophysial hormones and circadian rhythm. Ann. NY Acad. Sci. 1993;689:382–395. doi: 10.1111/j.1749-6632.1993.tb55562.x. [DOI] [PubMed] [Google Scholar]
- 25.Robinson ICAF, Coombes JE. Neurohypophysial peptides in cerebrospinal fluid: An update. Ann. NY Acad. Sci. 1993;689:269–283. doi: 10.1111/j.1749-6632.1993.tb55553.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz WJ, Reppert SM. Neural regulation of the circadian vasopressin rhythm in cerebrospinal fluid: A pre-eminent role for the suprachiasmatic nuclei. J. Neurosci. 1985;5:2771–2778. doi: 10.1523/JNEUROSCI.05-10-02771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolkonen J, Tuomisto L, Van Wimersma Greidanus TB, Riekkinen PJ. Vasopressin levels in the cerebrospinal fluid of rats with lesions of the paraventricular and suprachiasmatic nuclei. Neurosci. Lett. 1988;86:184–188. doi: 10.1016/0304-3940(88)90568-x. [DOI] [PubMed] [Google Scholar]
- 28.Earnest DJ, Sladek CD. Circadian rhythms of vasopressin release from individual rat suprachiasmatic explants in vitro. Brain Res. 1986;382:129–133. doi: 10.1016/0006-8993(86)90119-8. [DOI] [PubMed] [Google Scholar]
- 29.Gillette MU, Reppert SM. The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res. Bull. 1987;19:135–139. doi: 10.1016/0361-9230(87)90176-6. [DOI] [PubMed] [Google Scholar]
- 30.Uhl GR, Reppert SM. Suprachiasmatic nucleus vasopressin messenger RNA: circadian variation in normal and Brattleboro rats. Science. 1986;232:390–393. doi: 10.1126/science.3961487. [DOI] [PubMed] [Google Scholar]
- 31.Robinson BG, Frim DM, Schwartz WJ, Majzoub JA. Vasopressin mRNA in the suprachiasmatic nuclei: daily regulation of polyadenylate tail length. Science. 1988;241:342–344. doi: 10.1126/science.3388044. [DOI] [PubMed] [Google Scholar]
- 32.Kalsbeek A, Buijs RM, Engelmann M, Wotjak CT, Landgraf R. In vivo measurement of a diurnal variation in vasopressin release in the rat suprachiasmatic nucleus. Brain Res. 1995;682:75–82. doi: 10.1016/0006-8993(95)00324-j. [DOI] [PubMed] [Google Scholar]
- 33.Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137:1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemcical characterization of the graft and its integration with the host brain. J. Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: Activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J. Neurosci. 2003;23:7412–7414. doi: 10.1523/JNEUROSCI.23-19-07412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoorneman EMD, Buijs RM. Vasopressin fiber pathways in the rat brain following suprachiasmatic nucleus lesioning. Brain Res. 1982;243:235–241. doi: 10.1016/0006-8993(82)90246-3. [DOI] [PubMed] [Google Scholar]
- 39.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II.Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 40.Kalsbeek A, Teclemariam-Mesbah R, Pévet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) J. Comp. Neurol. 1993;332:293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- 41.Hermes MLHJ, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in the rat. J. Physiol. 1996;496:749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrang N, Larsen PJ, Mikkelsen JD. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995;684:61–69. doi: 10.1016/0006-8993(95)00425-p. [DOI] [PubMed] [Google Scholar]
- 43.Vrang N, Mikkelsen JD, Larsen PJ. Direct link from the suprachiasmatic nucleus to hypothalamic neurons projecting to the spinal cord: a combined tracing study using cholera toxin subunit B and Phaseolus vulgaris-leucoagglutinin. Brain Res. Bull. 1997;44:671–680. doi: 10.1016/s0361-9230(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 44.Teclemariam-Mesbah R, Kalsbeek A, Pevet P, Buijs RM. Direct vasoactive intestinal polypeptide-containing projection from the suprachiasmatic nucleus to spinal projecting hypothalamic paraventricular neurons. Brain Res. 1997;748:71–76. doi: 10.1016/s0006-8993(96)01246-2. [DOI] [PubMed] [Google Scholar]
- 45.Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- 46.De La Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6:1715–1722. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Beek EM, Horvath TL, Wiegant VM, Van Den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: Combined tracing and light and electron microscopic immunocytochemical studies. J. Comp. Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Van Der Beek EM, Wiegant VM, Van Der Donk HA, Van Den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J. Neuroendocrinol. 1993;5:137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 49.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tiss. Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 50.Kalsbeek A, Palm IF, La Fleur SE, Scheer FAJL, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 51.Buijs RM, Scheer FA, Kreier F, Yi C-X, Bos N, Goncharuk VD, Kalsbeek A. Organization of circadian functions: interaction with the body. Prog. Brain Res. 2006;153:341–360. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 52.Kalsbeek A, Buijs RM, Van Heerikhuize JJ, Arts M, Van Der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580:62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- 53.Kalsbeek A, Van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J. Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalsbeek A, Van Der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J. Neuroendocrinol. 1996;8:299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- 55.Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdale and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog. Brain Res. 2000;126:117–132. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- 56.Hermes MLHJ, Ruijter JM, Klop A, Buijs RM, Renaud LP. Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. J. Neurophysiol. 2000;83:705–711. doi: 10.1152/jn.2000.83.2.705. [DOI] [PubMed] [Google Scholar]
- 57.Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM, Pévet P. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur. J. Neurosci. 2008;27:818–827. doi: 10.1111/j.1460-9568.2008.06057.x. 2008. [DOI] [PubMed] [Google Scholar]
- 58.Cuesta M, Clesse D, Pévet P, Challet E. From daily behavior to hormonal and neurotransmitters rhythms: comparison between diurnal and nocturnal rat species. Horm. Behav. 2009;55:338–347. doi: 10.1016/j.yhbeh.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Dardente H, Menet JS, Challet E, Tournier BB, Pévet P, Masson-Pévet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Mol. Brain Res. 2004;124:143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MGP, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 61.Jasper MS, Engeland WC. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology. 1994;59:97–109. doi: 10.1159/000126645. [DOI] [PubMed] [Google Scholar]
- 62.Moore RY. Neural control of the pineal gland. Behav. Brain Res. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- 63.Scheer FA, Zeitzer JM, Ayas NT, Brown R, Czeisler CA, Shea SA. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord. 2006;44:78–81. doi: 10.1038/sj.sc.3101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J. Clin. Endocrinol. Metab. 2000;85:2189–2196. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]
- 65.Kalsbeek A, Cutrera RA, Van Heerikhuize JJ, Van Der Vliet J, Buijs RM. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience. 1999;91:453–461. doi: 10.1016/s0306-4522(98)00635-6. [DOI] [PubMed] [Google Scholar]
- 66.Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pévet P, Buijs RM. Melatonin sees the light: Blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 67.Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, Van Der Vliet J, Van Heijningen C, Simonneaux V, Pévet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- 68.Perreau-Lenz S, Kalsbeek A, Pévet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur. J. Neurosci. 2004;19:318–324. doi: 10.1111/j.0953-816x.2003.03132.x. [DOI] [PubMed] [Google Scholar]
- 69.Drijfhout WJ, Brons HF, Oakley N, Hagan RM, Grol CJ, Westerink BH. A microdialysis study on pineal melatonin rhythms in rats after an 8-h phase advance: new characteristics of the underlying pacemaker. Neuroscience. 1997;80:233–239. doi: 10.1016/s0306-4522(97)00080-8. [DOI] [PubMed] [Google Scholar]
- 70.Kalsbeek A, Barassin S, van Heerikhuize JJ, van der Vliet J, Buijs RM. Restricted daytime feeding attenuates reentrainment of the circadian melatonin rhythm after an 8-h phase advance of the light-dark cycle. J. Biol. Rhythms. 2000;15:57–66. doi: 10.1177/074873040001500107. [DOI] [PubMed] [Google Scholar]
- 71.Perreau-Lenz S. Control of the daily melatonin rhythm. A model of time distribution by the biological clock mediated through the autonomic nervous system, Chapter 5, Dissertation. University of Amsterdam; 2004. Feeding conditions affect vasopressin and Per2, but not Per1, gene expression in the rat suprachiasmatic nucleus. [Google Scholar]
- 72.De la Iglesia HO, Cambras T, Schwartz WJ, Díez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr. Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz MD, Wotus C, Liu T, Friesen WO, Borjigin J, Oda GA, De La Iglesia HO. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc. Natl. Acad. Sci. USA. 2009;106:17540–17545. doi: 10.1073/pnas.0906382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cambras T, Weller JR, Anglès-Pujoràs M, Lee ML, Christopher A, Díez-Noguera A, Krueger JM, De La Iglesia HO. Circadian desynchronization of core body temperature and sleep stages in the rat. Proc. Natl. Acad. Sci. USA. 2007;104:7634–7639. doi: 10.1073/pnas.0702424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 77.Kreier F, Kap YS, Mettenleiter T, Van Heijningen C, Van Der Vliet J, Kalsbeek A, Sauerwein H, Fliers E, Romijn JA, Buijs RM. Tracing from fat tissue, liver, and pancreas: A neuroanatomical framework for the role of the brain in Type2 diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 78.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J. Biol. Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 79.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am. J. Physiol. 2001;280:H1391–H1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 80.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- 81.Kalsbeek A, La Fleur SE, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buijs RM, la Fleur SE, Wortel J, Van Heijningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 83.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab. Rev. 1987;3:185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- 84.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 85.Puschel GP. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat. Rec. 2004;280A:854. doi: 10.1002/ar.a.20091. [DOI] [PubMed] [Google Scholar]
- 86.Kita Y, Shiozawa M, Jin WH, Majewski RR, Besharse JC, Greene AS, Jacob HJ. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics. 2002;12:55–65. doi: 10.1097/00008571-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 88.Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, Kobayashi H, Iitaka C, Umehara T, Horikoshi M, Kudo T, Shimizu Y, Yano M, Monden M, Machida K, Matsuda J, Horie S, Todo T, Ishida N. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J. Biol. Chem. 2002;278:41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 89.Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E, Buijs RM. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One. 2008;3:e3194. doi: 10.1371/journal.pone.0003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cailotto C, van Heijningen C, van der Vliet J, van der Plasse G, Habold C, Kalsbeek A, Pévet P, Buijs RM. Daily rhythms in metabolic liver enzymes and plasma glucose require a balance in the autonomic output to the liver. Endocrinology. 2008;149:1914–1925. doi: 10.1210/en.2007-0816. [DOI] [PubMed] [Google Scholar]
- 91.La Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J. Neuroendocrinol. 2003;15:315–322. doi: 10.1046/j.1365-2826.2003.01019.x. [DOI] [PubMed] [Google Scholar]
- 92.Yi C-X, Serlie MJ, Ackermans MT, Foppen E, Buijs RM, Sauerwein HP, Fliers E, Kalsbeek A. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes. 2005;58:1998–2005. doi: 10.2337/db09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies C, Spanswick D. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J. Physiol. 2003;549:809–821. doi: 10.1113/jphysiol.2002.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J. Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM, Mignot E. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27:619–627. doi: 10.1093/sleep/27.4.619. [DOI] [PubMed] [Google Scholar]
- 96.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: Implications for the consolidation of wakefulness. J. Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T, Yanagisawa M, Shioda S, Imoto K, Minokoshi Y. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 98.Zhang W, Zhang N, Sakurai T, Kuwaki T. Orexin neurons in the hypothalamus mediate cardiorespiratory responses induced by disinhibition of the amygdala and bed nucleus of the stria terminalis. Brain Res. 2009;1262:25–37. doi: 10.1016/j.brainres.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 99.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J. Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 100.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 101.Pearson H. Sleep it off. Nature. 2006;443:261–263. doi: 10.1038/443261a. [DOI] [PubMed] [Google Scholar]
- 102.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 103.Kalsbeek A, Strubbe JH. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol. Behav. 1998;63:553–560. doi: 10.1016/s0031-9384(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 104.Houghton SG, Zarroug AE, Duenes JA, Fernandez-Zapico ME, Sarr MG. The diurnal periodicity of hexose transporter mRNA and protein levels in the rat jejunum: role of vagal innervation. Surgery. 2006;139:542–549. doi: 10.1016/j.surg.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J. Neurosci. 2007;27:4359–4365. doi: 10.1523/JNEUROSCI.4131-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am. J. Physiol. 1997;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- 107.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput. Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- 108.Scheer FA, Van Doornen LJ, Buijs RM. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton. Neurosci. 2004;110:44–48. doi: 10.1016/j.autneu.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 109.Strubbe JH, Alingh Prins AJ, Bruggink J, Steffens AB. Daily variation of food-induced changes in blood glucose and insulin in the rat and the control by the suprachiasmatic nucleus and the vagus nerve. J. Auton. Nerv. Syst. 1987;20:113–119. doi: 10.1016/0165-1838(87)90108-1. [DOI] [PubMed] [Google Scholar]
- 110.Weiss B, Maickel RP. Sympathetic nervous control of adipose tissue lipolysis. Int. J. Neuropharmacol. 1968;7:395–403. doi: 10.1016/0028-3908(68)90023-3. [DOI] [PubMed] [Google Scholar]
- 111.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 112.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen C, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein H, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat - functional implications. J. Clin. Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JW, Erskine MS. Pseudorabies virus tracing of neural pathways between the uterine cervix and CNS: effects of survival time, estrogen treatment, rhizotomy, and pelvic nerve transection. J. Comp. Neurol. 2000;418:484–503. [PubMed] [Google Scholar]
- 114.Andersson K, Arner P. Cholinoceptor-mediated effects on glycerol output from human adipose tissue using in situ microdialysis. Br. J. Pharmacol. 1995;115:1155–1162. doi: 10.1111/j.1476-5381.1995.tb15018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J. Pharmacol. Exp. Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- 116.Yang T, Chang C, Tsao C, Hsu Y, Hsu C, Cheng J. Activation of muscarinic M-3 receptor may decrease glucose uptake and lipolysis in adipose tissue of rats. Neurosci. Lett. 2009;451:57–59. doi: 10.1016/j.neulet.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 117.Kreier F. Autonomic nervous control of white adipose tissue, Chapter 4, Dissertation. University of Amsterdam; 2005. Dual sympathetic and parasympathetic hypothalamic output to white adipose tissue. [Google Scholar]
- 118.Cornish S, Cawthorne MA. Fatty acid synthesis in mice during the 24hr cycle and during meal-feeding. Horm. Metab. Res. 1978;10:286–290. doi: 10.1055/s-0028-1093416. [DOI] [PubMed] [Google Scholar]
- 119.Benavides A, Siches M, Llobera M. Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Am. J. Physiol. 1998;275:R811–R817. doi: 10.1152/ajpregu.1998.275.3.R811. [DOI] [PubMed] [Google Scholar]
- 120.Hagström-Toft E, Bolinder J, Ungerstedt U, Arner P. A circadian rhythm in lipid mobilization which is altered in IDDM. Diabetologia. 1997;40:1070–1078. doi: 10.1007/s001250050789. [DOI] [PubMed] [Google Scholar]
- 121.Bergö M, Olivecrona G, Olivecrona T. Diurnal rhythms and effects of fasting and refeeding on rat adipose tissue lipoprotein lipase. Am. J. Physiol. 1996;271:E1092–E1097. doi: 10.1152/ajpendo.1996.271.6.E1092. [DOI] [PubMed] [Google Scholar]
- 122.Hems DA, Rath EA, Verrinder TR. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem. J. 1975;150:167–173. doi: 10.1042/bj1500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J. Clin. Endocrinol. Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 124.Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671–1679. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 125.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 126.Scheer FA, Chan JL, Fargnoli J, Chamberland J, Arampatzi K, Shea SA, Blackburn GL, Mantzoros CS. Day/night variations of high-molecular-weight adiponectin and lipocalin-2 in healthy men studied under fed and fasted conditions. Diabetologia. 2010;53:2401–2405. doi: 10.1007/s00125-010-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J. Clin. Endocrinol. Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 129.Tu BP, McKnight SL. Metabolic cycles as an underlying basis of biological oscillations. Nat. Rev. Mol. Cell. Biol. 2006;7:696–701. doi: 10.1038/nrm1980. [DOI] [PubMed] [Google Scholar]
- 130.Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Tr. Endocrinol. Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 131.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 132.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 134.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 135.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 136.Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obes. Rev. 2009;10:25–36. doi: 10.1111/j.1467-789X.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 137.Knutsson A. Health disorders of shift workers. Occup. Med. (Lond.) 2003;53:103–108. doi: 10.1093/occmed/kqg048. 2003. [DOI] [PubMed] [Google Scholar]
- 138.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 140.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 141.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr. Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 142.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 143.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, DeLuca M, Westfall AO, Allison DB. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int. J. Obesity. 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 144.Hastings MH, Reddy AB, Maywood ES. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 145.Foster RG, Wulff K. The rhythm of rest and excess. Nat. Rev. Neurosci. 2005;6:407–414. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 146.Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pévet P, Buijs RM. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One. 2009;4:e5650. doi: 10.1371/journal.pone.0005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pévet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 2005;22:2531–2540. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- 148.Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, Buijs RM. Hypothesis: Shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes. 2003;52:2652–2656. doi: 10.2337/diabetes.52.11.2652. [DOI] [PubMed] [Google Scholar]
- 149.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci. 2010;107:18664–18649. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 2209;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 153.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel SR. Reduced sleep as an obesity risk factor. Obes. Rev. 2009;10:61–68. doi: 10.1111/j.1467-789X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 155.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 156.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 158.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu RY, Zhou JN, Hoogendijk WJG, Van Heerikhuize J, Kamphorst W, Unmehopa UA, Hofman MA, Swaab DF. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J. Neuropathol. Exp. Neurol. 2000;59:314–322. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- 160.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin- producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 161.Roozendaal B, Van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- 162.Van Der Zee EA, Jansen K, Gerkema MP. Severe loss of vasopressin-immunoreactive cells in the suprachiasmatic nucleus of aging voles coincides with reduced circadian organization of running wheel activity. Brain Res. 1999;816:572–579. doi: 10.1016/s0006-8993(98)01239-6. [DOI] [PubMed] [Google Scholar]
- 163.Cayetanot F, Bentivoglio M, Aujard F. Arginine-vasopressin and vasointestinal polypeptide rhythms in the suprachiasmatic nucleus of the mouse lemur reveal aging-related alterations of circadian pacemaker neurons in a non-human primate. Eur. J. Neurosci. 2005;22:902–910. doi: 10.1111/j.1460-9568.2005.04268.x. [DOI] [PubMed] [Google Scholar]
- 164.Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, Kowall N, Satlin A. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer's disease: Involvement of the circadian pacemaker. Proc. Natl. Acad. Sci. USA. 2009;106:2490–2494. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu K, Scheer FA, Ivanov PCh, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA, Swaab DF. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch. Gen. Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
- 168.Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345:1408. doi: 10.1016/s0140-6736(95)92600-3. [DOI] [PubMed] [Google Scholar]
- 169.O'Brien IAD, Lewin IG, O'Hare JP, Arendt J, Corrall RJM. Abnormal circadian rhythm of melatonin in diabetic autonomic neuropathy. Clinical Endocrinology. 1986;24:359–364. doi: 10.1111/j.1365-2265.1986.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 170.Altun A, Yaprak M, Aktoz M, Vardar A, Betul UA, Ozbay G. Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X. Neurosci. Lett. 2002;327:143–145. doi: 10.1016/s0304-3940(02)00368-3. [DOI] [PubMed] [Google Scholar]
- 171.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L, Maj M. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: Association with higher body mass index. Neurosci. Lett. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 173.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 174.Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 175.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goncharuk VD, Van Heerikhuize JJ, Dai JP, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J. Comp. Neurol. 2001;431:320–330. doi: 10.1002/1096-9861(20010312)431:3<320::aid-cne1073>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 177.Goncharuk VD, vanHeerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: Activation of corticotrophin-releasing hormone neurons. J. Comp. Neurol. 2002;443:321–331. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- 178.Peters RV, Zoeller RT, Hennessey AC, Stopa EG, Anderson G, Albers HE. The control of circadian rhythms and the levels of vasoactive intestinal peptide messenger RNA in the suprachiasmatic nucleus are altered in spontaneously hypertensive rats. Brain Res. 1994;639:217–227. doi: 10.1016/0006-8993(94)91733-7. [DOI] [PubMed] [Google Scholar]
- 179.Avidor R, Eilam R, Malach R, Gozes I. VIP-mRNA is increased in hypertensive rats. Brain Res. 1989;503:304–307. doi: 10.1016/0006-8993(89)91679-x. [DOI] [PubMed] [Google Scholar]
- 180.Lucassen PJ, Hofman MA, Swaab DF. Increased light intensity prevents the age related loss of vasopressin-expressing neurons in the rat suprachiasmatic nucleus. Brain Res. 1995;693:261–266. doi: 10.1016/0006-8993(95)00933-h. [DOI] [PubMed] [Google Scholar]
- 181.Witting W, Mirmiran M, Bos NPA, Swaab DF. Effect of light intensity on diurnal sleep-wake distribution in young and old rats. Brain Res. Bull. 1993;30:157–162. doi: 10.1016/0361-9230(93)90053-e. [DOI] [PubMed] [Google Scholar]
- 182.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 183.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol. Psychiatry. 1997:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 184.Van den Top M, Buijs RM, Ruijter JM, Delagrange P, Spanswick D, Hermes MLHJ. Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. Neuroscience. 2001;107:99–108. doi: 10.1016/s0306-4522(01)00346-3. [DOI] [PubMed] [Google Scholar]
- 185.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 186.Evans JL, Youngren JF, Goldfine ID. Effective treatments for insulin resistance: trim the fat and douse the fire. Tr. Endocrinol. Metab. 2004;15:425–431. doi: 10.1016/j.tem.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 187.Westerterp KR. Pattern and intensity of physical activity. Nature. 2001;410:539. doi: 10.1038/35069142. [DOI] [PubMed] [Google Scholar]
- 188.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. USA. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]