Abstract
Kaposi’s sarcoma-associated herpes virus (KSHV) is the etiological agent of Kaposi’s sarcoma (KS) and at least two B cell lymphoproliferative diseases: primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD). B cells derived from PEL are latently infected, and can be induced to lytic replication by treatment with chemical agents like TPA or butyrate, which have pleiotropic effects on host cell signaling and chromatin structure. Most of these lines also display moderate levels of spontaneous lytic induction, which complicates analysis of latency. Here we describe the creation of latently-infected cell lines derived from SLK endothelial cells that (i) display tight control of KSHV latency, with little spontaneous reactivation and (ii) are efficiently inducible by doxycycline, avoiding the need for pleiotropic inducing agents. These cells produce substantial quantities of infectious KSHV, and should be useful for studies of the latent-lytic switch and the impact of lytic replication on host cell biology.
Keywords: Kaposi’s sarcoma, herpesvirus, latency, reactivation
1. Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), belongs to the gamma-herpesvirus subfamily and shows homology to herpesvirus saimiri and Epstein-Barr virus (EBV). KSHV was first identified based on its association with an endothelial neoplasm called Kaposi’s sarcoma, but was soon discovered to cause at least two lymphoproliferative diseases: multicentric Castleman’s disease (MCD) and primary effusion lymphoma (PEL) (reviewed in (Ganem, 2010)).
PEL cells grow readily in culture, and have been the most widely used lines for the in vitro study of KSHV. In the ground state, they are latently infected: the viral genome is present as a nuclear plasmid, and expresses only a handful of viral genes. However following treatment with certain chemical agents (see below), they can be induced to enter the lytic transcriptional program. In this program, virtually the entire viral genome is transcribed, in a temporally regulated cascade of gene expression – early genes are regulatory and catalytic functions that prepare the cell for viral DNA replication; following genomic replication, the late genes encoding structural components are expressed. Lytic replication is highly cytopathic, and results in the generation of large quantities of infectious virus in the culture supernatant. Subsequently, several labs have shown that many adherent lines in culture can be latently infected following exposure to KSHV virions (Bechtel et al., 2003; Vieira et al., 2001).
Most existing KSHV-infected lines, however, have experimental limitations. For example, most PEL cells have substantial levels of spontaneous lytic reactivation, complicating the analysis of latent gene expression (Renne et al., 1996). Conversely, many adherent cell lines are poorly inducible with chemical agents (Bechtel et al 2003). In addition, the chemicals that are used to induce lytic replication – HDAC inhibitors (e.g. sodium butyrate or valproate) or phorbol esters (e.g TPA) - are pleiotropic, with broad effects on chromatin structure and signal transduction (respectively). As such, it is difficult to rigorously examine the biology of the host cell during the latent-lytic switch, since host cell homeostasis is itself so profoundly affected by the exogenous inducing stimuli.
The principal viral actor in the latent-lytic switch is the protein RTA (replication and transcription activator), encoded by KSHV ORF 50 (Lukac et al., 1999; Lukac et al., 1998; Sun et al., 1998; Xu et al., 2005). RTA is a sequence-specific transcriptional activator that can also interact with host transcription factors to induce genes whose promoters lack canonical RTA response elements (RREs) (Chen et al., 2000; Deng et al., 2002; Liang et al., 2002; Liang and Ganem, 2004; Lukac et al., 2001; Sadler et al., 1999; Song et al., 2001). Ectopic expression of RTA induces cells from latency, and mutant viruses lacking functional RTA cannot reactivate in response to any inducing stimuli (Xu et al., 2005). Thus, RTA expression is both necessary and sufficient to trigger lytic reactivation (Lukac et al., 1999; Lukac et al., 1998; Sun et al., 1998). PEL cells latently infected with KSHV have been transduced with vectors allowing inducible expression of RTA, generating cells that are inducible with doxycycline (Nakamura et al., 2003), thereby obviating the need for pleiotropic inducers. But these cells retain the high rate of spontaneous lytic reactivation of their parental line, and also lack isogenic uninfected counterparts that serve as essential controls for many types of biochemical experiment. Also, since they are of B cell origin they do not allow examination of viral replication in the other important lineage infected by KSHV, the endothelial cell.
In search of endothelial cell lines, which (i) allow efficient KSHV infection, (ii) maintain tight and stable latency when once established and (iii) support robust viral reactivation upon induction, a panel of cell lines were screened. Here we report that SLK cells, which are uninfected endothelial cells derived from a gingival KS lesion of an HIV-negative renal transplant recipient (Siegal et al., 1990), fulfill many of these criteria. To construct SLK derivatives that were inducible without pleiotropic chemicals, we first engineered a derivative of SLK (termed iSLK) that expresses a doxycycline-inducible RTA transgene. To generate iSLK.219 cells, iSLK cells were then latently infected with a recombinant KSHV.219 virus, which constitutively expresses puromycin N-acetyl-transferase and GFP while RFP is expressed during lytic replication. (Vieira and O'Hearn, 2004). The mass culture was inducible by doxycyline, but further induced by addition of HDAc inhibitors. A subclone was derived that displays highly efficient induction by doxycycline alone. These cells provide a reliable source for generating large quantities of rKSHV.219 viruses from endothelial cells without induction by agents like TPA or HDAC inhibitors that disturb host cell physiology.
2. MATERIALS AND METHODS
2.1. Cells and reagents
SLK, Phoenix and Vero76 cells were maintained in DMEM supplemented with 10% FBS and 1% glutamate and penicillin/streptomycin. BJAB, Ramos, Jurkat, SUP-T1 cells were cultured in RPMI 1640 media containing 10% FBS and 1% glutamate and antibiotics. TIME, LEC, BEC, HUVEC, cells were cultured in EGM-2 MV media, supplemented with 2% FBS and various growth factors (Lonza, Allendale, NJ). Hygromycin was purchased from Invitrogen (Carlsbad, CA), Puromycin from Invivogen (San Diego, CA), and G418 from Sigma (St. Louis, MO). QBI293 cells were purchased from QBiogene (Carlsbad, CA).
2.2. Generation of Retroviruses
A retrovirus-based inducible expression system (pRetro-X Tet-ON Advanced Inducible Expression System) was purchased from Clontech (Mountainview, CA). Retroviruses constitutively expressing rtTA (a Tet-On transactivator) were obtained by transfecting pRetroX-Tet-On Advanced into Phoenix cells. Culture supernatants containing retroviral particles were harvested at d2, 3 and 4 post-transfection and filtered through 0.22 µm membrane. RTA, encoded by ORF50, was amplified by PCR (forward primer: GATCGCGGCCGCATGGCGCAAGATGACAAGGG, reverse primer: GGCCGAATTCTCAGTCTCGGAAGTAATTACGCC) and cloned into pRetroX-Tight-Hyg using NotI and EcoRI site. This vector contains 7 copies of the tet operator sequences linked to a minimal CMV promoter, which drives expression of the RTA gene. Sequence was verified (Elim Biopharmaceuticals). Retroviruses, expressing RTA, were generated as described above.
2.3. Generation of iSLK.219
Doxycycline-inducible SLK cells were generated by transducing cells first with retroviruses expressing rtTA, in the presence of polybrene (8 µg/ml) for 3 hours at 2500 RPM. rtTA-expressing SLK cells were selected by G418 at 800 µg/ml for 2 weeks. rtTAexpressing SLK cells were transduced again with retroviruses expressing RTA under tet operator control and transductants were selected by hygromycin at 1200 µg/ml for 2 weeks. Resulting doxycycline-inducible SLK (iSLK) cells were maintained in culture medium containing both G418 and hygromycin. iSLK cells were infected with rKSHV.219 (Vieira and O'Hearn, 2004) and virus-harboring iSLK cells were selected by puromycin at 1 µg/ml for 2 weeks. To increase the virus genome copy number in the resulting cells, iSLK.219 cells were cultured in increasing concentration of puromycin (up to 10 µg/ml) by 1 µg/ml increment. At each puromycin concentration, iSLK.219 cells were passed 3 times before puromycin concentration was increased to the next level. Resulting iSLK.219 cells (iSLK.219p10), selected at 10 µg/ml puromycin, were subcloned at 1000 cells in a 150-mm culture dish and 12 subclones were picked and cultured for subsequent experiments. Doxycycline-inducible stable Vero76 cells, harboring rKSHV.219, were prepared similarly as described above. Inducible cells were maintained in the presence of antibiotics at all times.
2.4. KSHV infection with free virus and viral stock titration
KSHV was delivered to various primary and established cells by spinoculation (2500 RPM for 90 min) (Vieira and O'Hearn, 2004; Yoo et al., 2008) at varying multiplicity of infection (MOI). Unbound viruses were washed off and infected cells were cultured for 48 hr before FACS analysis or selection with puromycin. Virus stocks were prepared from induced Vero cells harboring rKSHV.219 (Vieira and O'Hearn, 2004). Procedure for virus concentration from induced culture supernatant is described elsewhere ((Bechtel et al., 2003) and (Myoung and Ganem, 2011b)). Titers of KSHV stocks were determined by infecting serially diluted stocks on QBI293A cells with subsequent enumeration of GFP-expressing cells by FACS analysis ((Vieira and O'Hearn, 2004) and (Myoung and Ganem, 2011a)).
Flow cytometry
iSLK.219 cells or QBI293A cells were first trypsinized and washed once with incomplete PBS and fixed by 0.5% paraformaldehyde in PBS for 5 min at RT. Cells were washed in cold incomplete PBS and resuspended in FACS staining buffer, containing 4% FBS and 0.09% sodium azide. GFP and/or RFP expression in cells was examined on LSR II (BD Bioscience, San Jose, CA). Data analysis was performed by FlowJo software.
Statistical Analysis
Data represented in this study are either a representative one or shown as the means ± standard deviations (SD) of 2–3 independent experiments. Statistical significance of differences in the mean values was evaluated by paired Student t test. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Generation of doxycycline-inducible SLK cells, harboring rKSHV.219
The following cell lines were first screened for their infectibility by KSHV and their ability to support lytic reactivation at MOI 10 by spinoculation in the absence of polybrene: TIME, FO-1, 293, and SLK cells. TIME cells supported efficient viral entry as assessed by GFP expression (~50%), but the growth of infected TIME cells was very slow, and infected cells eventually died, largely due to spontaneous lytic KSHV reactivation (data not shown). FO-1 and 293 cells demonstrated high levels of rKSHV.219 infection (analyzed by GFP expression), roughly ~30% and 90%, respectively. However, FO-1 cells displayed poor inducubility while 293 cells died of extensive spontaneous lytic replication after infection. In addition, surviving 293 cells were refractory to reactivation by HDAc inhibitors and PMA (data not shown). By contrast, SLK cells demonstrated efficient infection by rKSHV.219, low levels of spontaneous reactivation and (when optimized) could support high levels of infectious rKSHV.219 particle production. Accordingly, these cells were chosen for production of a derivative that could be induced without pleiotropic chemical agents.
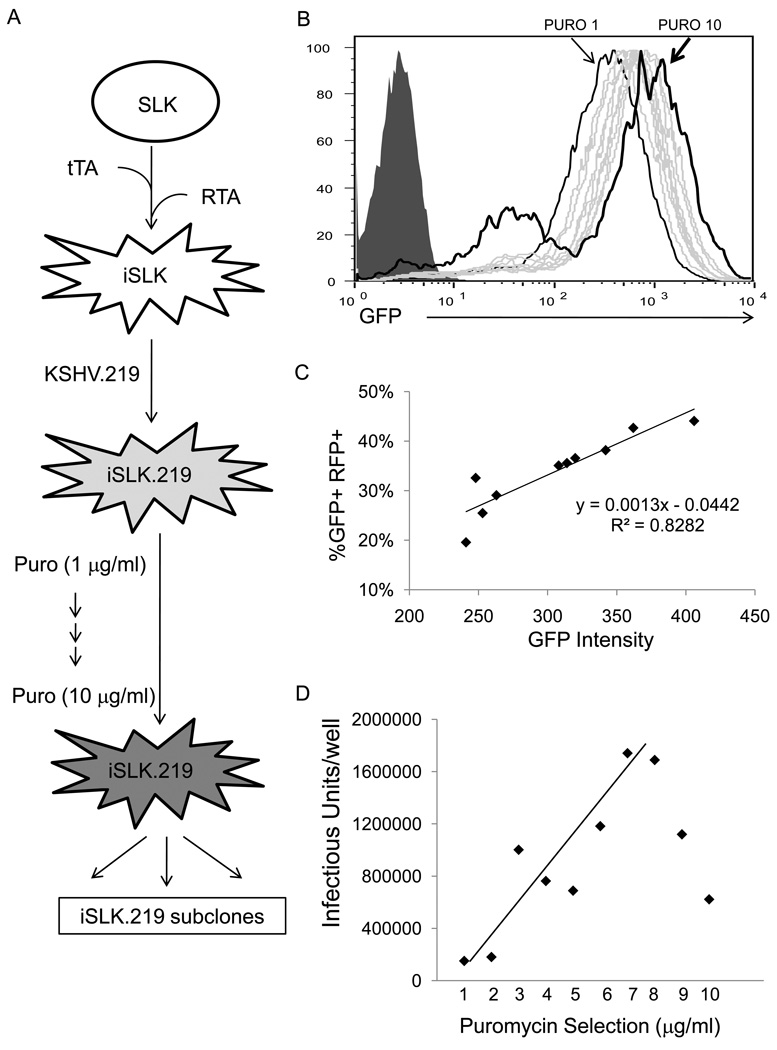
Figure 1A shows how inducible SLK cells harboring rKSHV.219, were generated. As cells could not survive high levels of constitutive RTA expression (unpublished observations), a tightly regulated promoter bearing a tet operator sequence was employed to direct RTA expression; the cells were also transduced with the rtTA (Tet-On) transactivator, which requires doxycycline as a cofactor for activation (Clontech). To dampen basal level expression of RTA, a Kozak consensus sequence, which plays a critical role in efficient translational initiation in eukaryotic cells (Kozak, 1984; Kozak, 1986; Kozak, 1987), was removed from the 5’ UTR of the RTA gene (5′-tgggaggcctatataagcaagctcgtttagtgaaccgtcagatcgcctggagaaggatcccgcggccgcATG-3′, ATG in bold font denotes start codon of RTA). The resulting Dox-inducible RTA-expressing SLK (iSLK) cells looked normal in terms of morphology and growth rate compared to parental SLK cells in the absence of induction, and (surprisingly) did not display signs of unequivocal RTA-mediated cytotoxicity after dox induction. When iSLK cells were infected with rKSHV.219 (to generate iSLK.219), very low levels, if any, of spontaneous lytic replication were detected in terms of both RFP expression and infectious cell-free viruses in the culture supernatant. Virtually all cells remained GFP+ and RFP−negative when no inducing stimuli were provided (RFP+ cells were less than 0.05% of the culture, as judged by FACS).
Figure 1. Generation of RTA-inducible SLK cells (iSLK.219) harboring recombinant KSHV (rKSHV.219).
A) SLK cells were first transduced sequentially with retroviruses expressing rtTA or RTA, resulting in RTA-inducible SLK (iSLK) cells. iSLK cells were infected with rKSHV.219 and subsequently selected in the presence of 1 µ/ml puromycin, generating iSLK.219 cells. Concentration of puromycin was gradually increased up to 10 µ/ml to increase the copy number of KSHV episome. B) GFP intensity of iSLK.219 cells, maintained at 1 µ/ml and 10 µ/ml, as indicated, is plotted. Gray histograms represent intermediate iSLK.219 cells, selected at 2 µ/ml through 9 µ/ml puromycin. Filled histogram represents uninfected iSLK cells. Note that there is a significant increase in GFP− cells among iSLK.219 cells maintained at 8, 9, and especially at 10 µ/ml puromycin. Note that GFP intensity increases when puromycin concentration increases to maintain iSLK.219 cells. PURO represents puromycin. C) iSLK.219 cells were induced with doxycycline (1 µ/ml) for 2 days and GFP intensity vs % GFP+RFP+ is plotted. Note that GFP+ cells were gated on FACS and mean GFP intensity was calculated. D) Infectious units (GFP-transducing untis) in the induced culture supernatants (d3) of iSLK.219 cells, selected at increasing concentrations of puromycin to boost numbers of episomes in the cells, were measured on QBI293A cells. See Materials and Methods for details.
To increase the copy number of viral episomes in a given infected cell, cells were cultured in the presence of increasing concentrations of puromycin (from 1 µg/ml to up to 10 µg/ml). When assessed for their GFP intensity, iSLK.219 cells carried through stepwise elevations of puromycin displayed stepwise increases in mean GFP intensity (cells maintained at 8 µg/ml demonstrated roughly 2 fold higher GFP staining intensity than cells at 1 µg/ml). This pattern suggests that successive increases in viral episomal copy number occurred as cells were carried through rising puromycin concentrations. Oddly, cells maintained in 10 µg/ml puromycin, displayed a substantial percentage of GFP-negative cells in the population, even though those cells that were GFP-positive displayed the highest GFP signal intensity of the series. This pattern implies that recombinational events must have occurred under these conditions, resulting in the loss of GFP expression in a subpopulation of cells (Figure 1B); detailed mechanisms of this phenomenon entail further investigation.
Next, iSLK.219 cells, at puromycin 1 through 10 µg/ml, were induced in the presence of doxycycline (1 µg/ml) for 2 days (Figure 1C), and subjected to FACS analysis for their RFP expression. When GFP+ cells were gated for their RFP expression, very interestingly, cells with higher GFP intensity (presumably due to higher copy number of viral genome (Alt et al., 1978; Kaufman et al., 1978; Schimke et al., 1978)), demonstrated higher levels of RFP expression as well, indicating higher levels of lytic replication. When cell-free viruses were assessed in the induced culture supernatant at d3 post-induction (Figure 1D), higher levels of GFP-transducing units (that is, infectious units, IU) were detected in the cell culture supernatants of cells with higher levels of RFP expression. Not surprisingly, cells with higher levels of GFP-negative cells (i.e. cells selected at cells at puromycin 9 – 10 µg/ml) displayed significant decrease in IU in the culture supernatant, suggesting that their subpopulations of GFP-negative cells were not able to reactivate in response to doxycycline treatment. (Interestingly, when GFP positive cells were gated in this population, they displayed the highest levels of RFP production after induction, indicating that they are highly inducible. For this reason, individual subclones of GFP-positive cells from this highly puromycin-resistant population were selected for the generation of optimal virus-producing lines (see below)).
3.2. iSLK.219 cells are inducible in response to doxycycline and synergistically with HDAC inhibitors
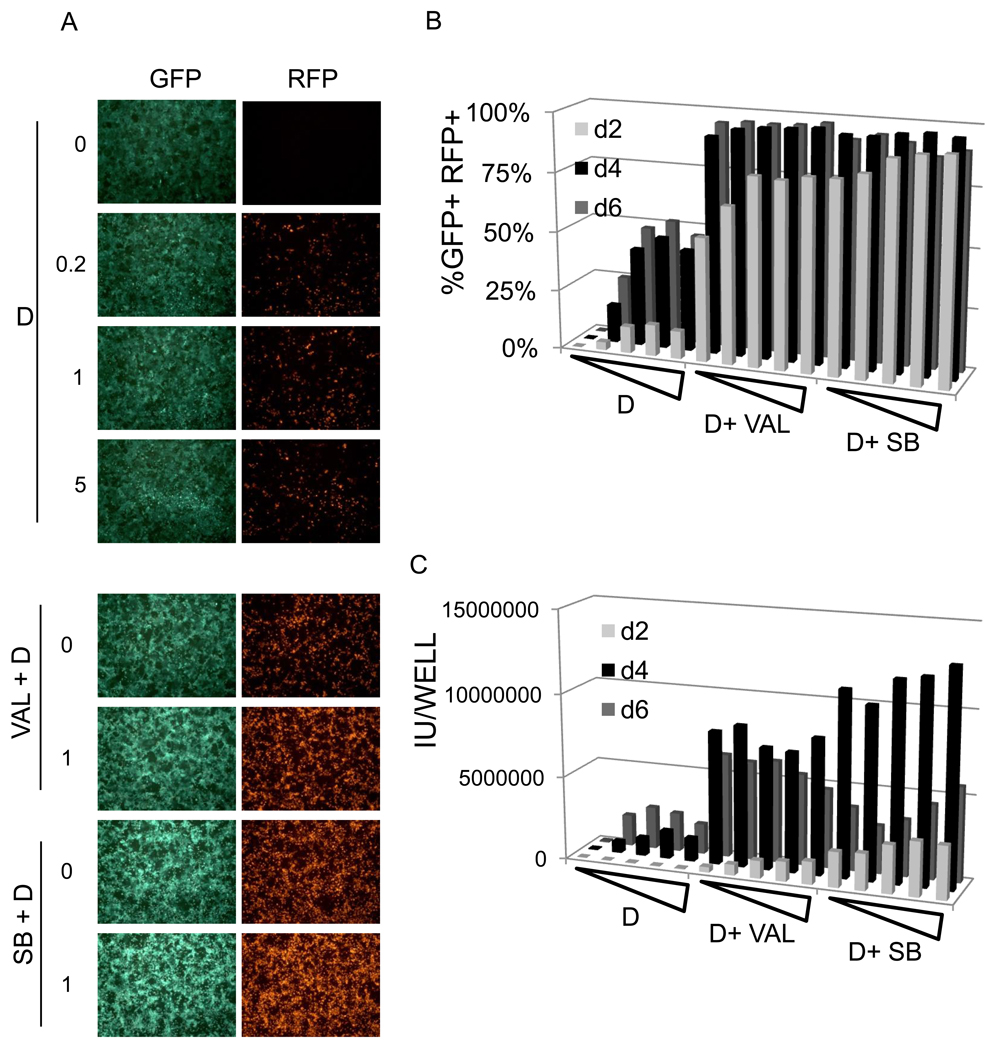
To test whether iSLK.219 cells, maintained at 10 µg/ml puromycin are inducible by doxycycline, cells were induced in the presence of increasing concentration of dox and their GFP/RFP expression was analyzed by either fluorescent microscope (Figure 2A) and flow cytometry (Figure 2B). iSLK.219 cells were activated lytically in a dose-dependent (up to 1 µg/ml doxycyline) and time-dependent manner (up to d6 post-induction). Roughly 10% of cells expressed RFP as early as d2 post-induction; by day 6, 45–50% of iSLK.219 cells became RFP+, suggesting they are highly inducible in response to doxycycline alone. To examine whether the known chemical inducers valproate (Val, 900 µM) and sodium butyrate (SB, 900 µM), could further enhance doxycycline inducibility, cells were treated with doxycycline (1 µg/ml) and/or Val or SB (Figure 2A &B). Both Val and SB enhanced significantly RFP expression in iSLK.219 cells in terms of RFP expression: >95% cells were positive for RFP expression at d4 post-induction. When infectivity in culture supernatants (Fig 2C) was assayed, it rose roughly (albeit not precisely) in proportion to RFP-positivity in most induction settings; however, in the presence of HDAC inhibitors, infectivity fell off after day 4 despite continued RFP-positivity in the monolayer, suggesting that virion inactivation in the culture media occurred at late times. The causes of this inactivation have not been examined.
Figure 2. Inducibility of iSLK.219 cells maintained at 10 µg/ml puromycin (iSLK.219p10).
A) iSLK.219p10 cells were induced by doxycycline alone (at increasing concentrations, 0, 0.04, 0.2, 1, or 5 µ/ml, upper panels) or doxycycline in combinations with either valproate (0 to 1 mM, bottom top 2 panels) or sodium butyrate (0 to 1 mM, bottom 2 panels). Val and SB denotes valproate and sodium butyrate, respectively. B) 105 iSLK.219p10 cells were seeded in a 24-well plate and induced the following day with doxycycline (0, 0.04, 0.2, 1, or 5 µg/ml) alone or doxycycline in combinations with valproate (1 mM) or sodium butyrate (1 mM) for the indicated duration and % GFP+RFP+ is plotted. Note that GFP+ cells were gated and %GFP+RFP+ cells was plotted. C) Infectious units in the culture supernatants were evaluated in the same cultures described in B). IU indicates infectious units.
3.3. A highly inducible subclone of iSLK.219 cells
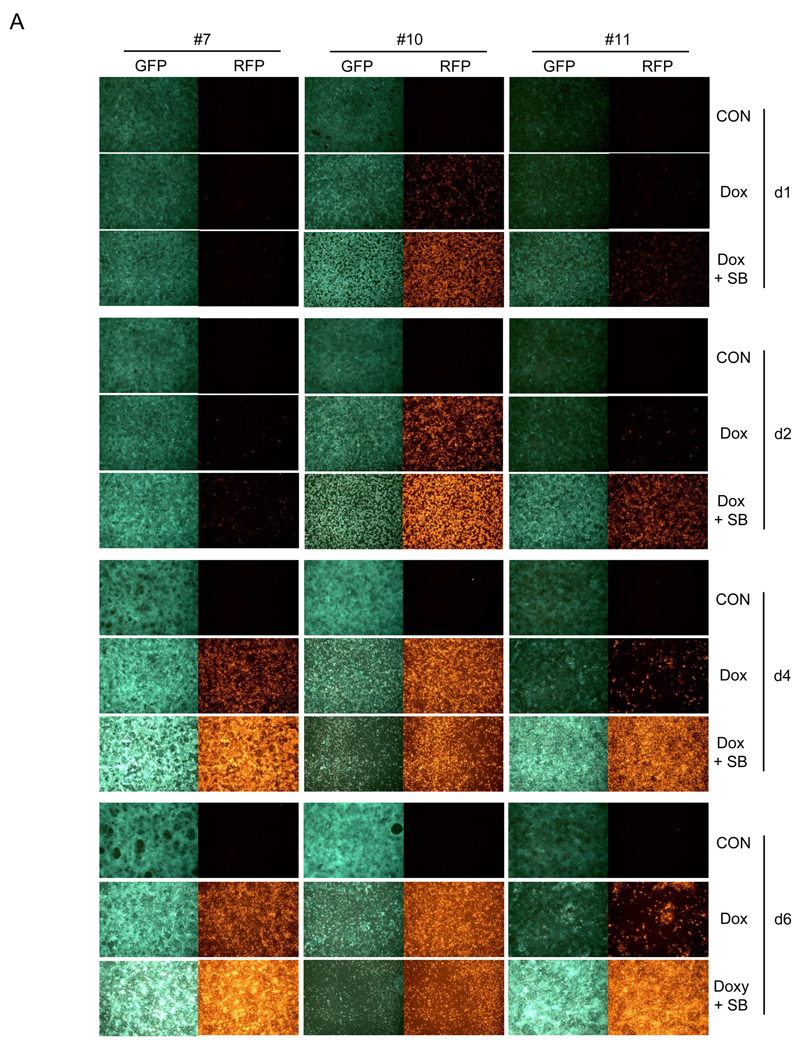
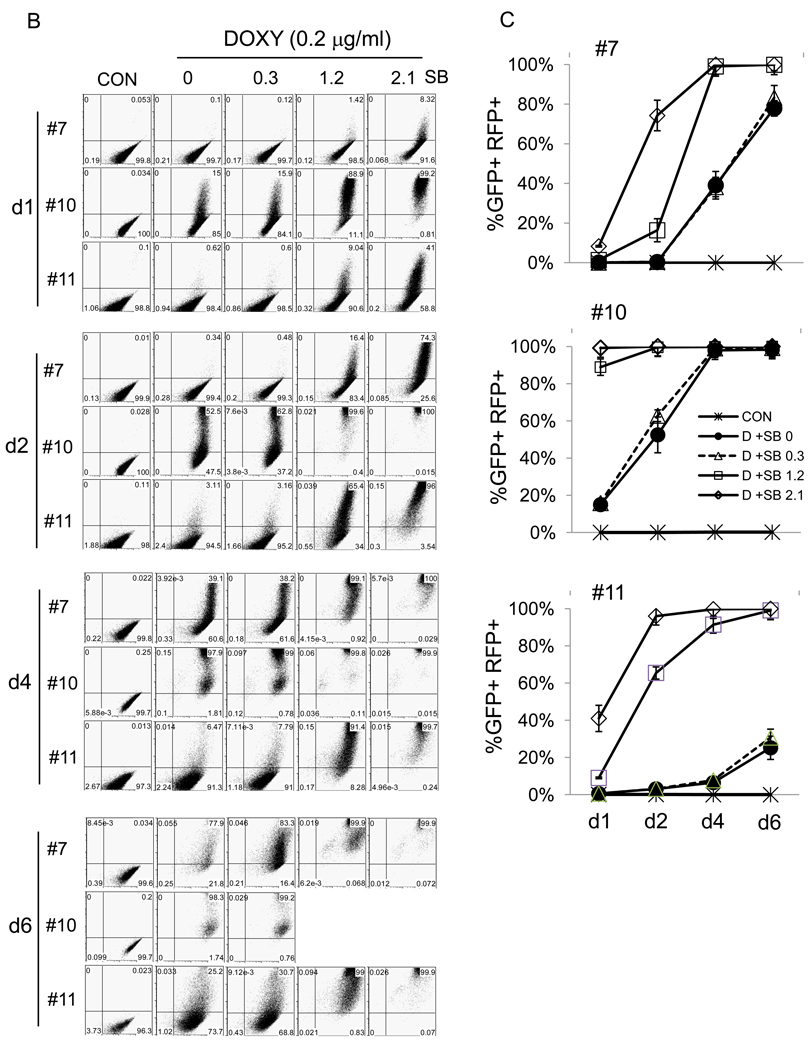
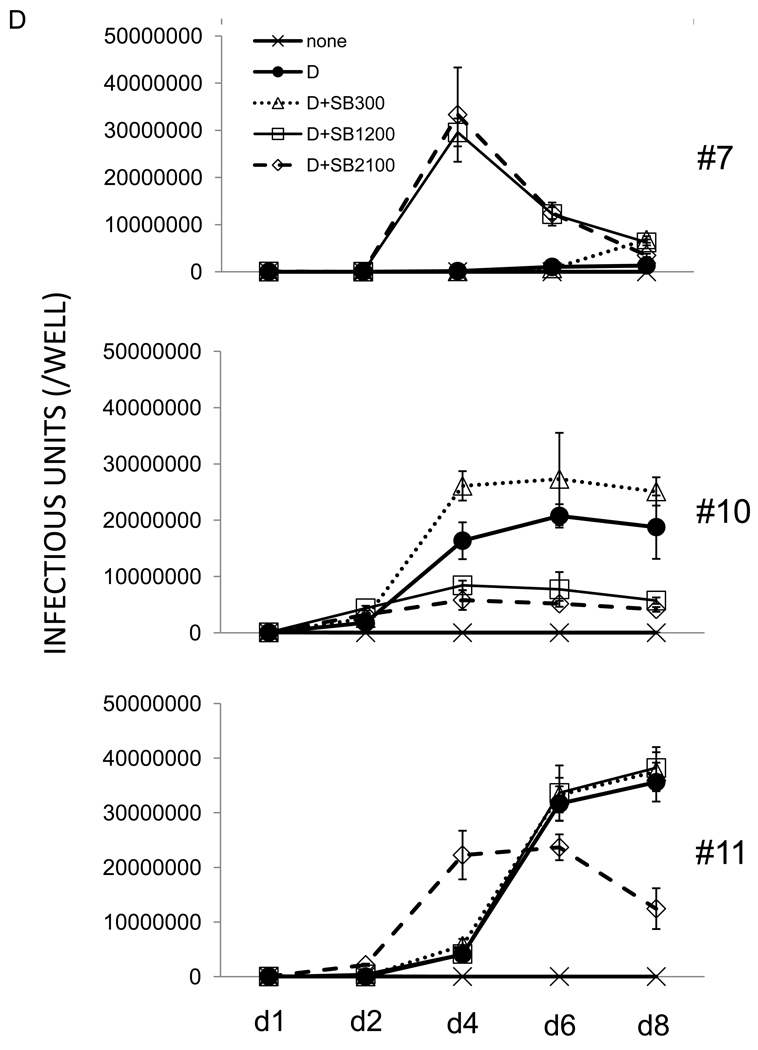
iSLK.219 cells, maintained at 10 µg/ml puromycin were subjected to subcloning and a dozen clones were picked. From these, clones #7, #10, and #11 were selected for further study based on their GFP intensity, growth rate, and inducibility by doxycycline (Table 1). Clone #10 demonstrated high levels of GFP intensity, was highly inducible with doxcycline, and displayed an intermediate growth rate; clone #11 displayed low levels of GFP intensity and low levels of inducible RFP expression, but grew rapidly. Clone #7 showed intermediate levels of GFP and RFP expression. The 3 clones were extensively studied for their inducibility in response to doxycycline alone or in combination with increasing concentration of SB (Figure 3). Notably, doxycycline alone was enough to induce iSLK.219 clone #10 at highest levels, producing high levels of infectious viruses (Figure 3A– D). By day 4 post-induction, doxycycline-induced clone #10 cells were nearly 100% positive for RFP expression, while the drug induced only 40% and 10% RFP+ cells in clone #7 and #11, respectively. Furthermore, doxycyline alone could not induce detectable infectious viruses in clone #7 by day 6 post-induction. However, when induced by doxycycline and sodium butyrate, clone #7 and #11 were highly induced in terms of RFP expression and infectious virus production in the culture supernatant (Figure 3A, B, C, and D), suggesting inhibition of HDAC’s is required for maximal KSHV lytic replication in those clones while it is not in clone #10. It is also worthy to note that there was an imperfect correlation between RFP-positivity and infectivity generation across several clones. For example, at day 6 post dox induction of clone 11, only 20% of cells were RFP+, but infectivity had nearly reached its maximum; in this clone, higher levels of RFP positivity, whether achieved by dox alone or with added HDAC inhibitors (Fig 3C), did not enhance accumulation of infectious particles (Fig 3D). Similarly, in clone 7, the continued rise in RFP-positive cells on days 4 and 6 post dox alone was not correlated with production of infectious virus, which in this clone appeared to require addition of HDAC inhibitors (Fig 3D) and proceeded with peculiar kinetics. Although exact mechanisms of this phenomena are still elusive, it is well to remember that RFP expression in rKSHV.219 primarily reflects RTA production, and many downstream steps in viral gene expression are required for production of infectious virions. So it is not surprising that in some cases RFP production overestimates virion production – in such cells, defects distal to RTA production likely occurred. Similarly, it is likely that once threshold levels of RTA required to trigger lytic replicaton are crossed, further increases in RTA may not result in linear increases in infectious particle production.
Table 1. Charaterization of 12 clones from iSLK.219p10 cells.
Clones were characterized based on their GFP expression, inducibility, growth rate.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GFPa | − | +++ | +++ | +++ | − | +++ | ++ | − | − | +++ | +* | +* |
| Inducibilityb | NA | ++ | ++ | + | NA | + | ++ | NA | NA | +++ | + | + |
| Growth ratec | +++ | + | ++ | ++ | +++ | ++ | ++ | +++ | +++ | ++ | +++ | +++ |
Mean GFP intensity was calculated by FACS analysis. Cells were evaluated based on their GFP intensity; +, lower than 800, ++, between 800 and 1200, +++, greater than 1200
Inducibility of the clones was assessed by inducing cells with 1 µg/ml doxycycline for 3 days. Cells were graded based on %GFP+RFP+; + lower than 50%, ++, between 50% and 80%, +++, greater than 80%
Growth rate was roughly assessed by culturing 1 in 10 diluted cells until they reached 100% confluency. Cell growth rate was evaluated based on how many days it takes to reach 100% confluency in a given culture vessel; +, longer than 7 days, ++, between 4 and 7 days, +++, shorter than 4 days.
10–20% of GFP-negative cells were detected in these clones
Figure 3. Characteristics of 3 representative clones from iSLK.219p10.
A) iSLK.219 #7, #10, and #11clones were left uninduced or induced by doxycycline (Dox, 1 µg/ml) alone or in combination with sodium butyrate (SB, 1.2 mM) for the indicated duration. GFP and RFP expression was examined under a fluorescent microscope and pictures were taken at 10X magnification. B) iSLK.219 subclones, left uninduced or induced as in A) were subjected to flow cytometry and their GFP and RFP expression was analyzed. One representative plot from 3 independent experiments is shown. X axis is for GFP and Y axis for RFP. Note that 2 plots for iSLK.219 #10 cells at d6 of induction by doxycycline and sodium butyrate (1.2 and 2.1 mM) are missing due to extensive cell death. C) Induction studies as in B) were repeated 3 times and the results were plotted. Each data point represents mean ± standard error mean. D) Infectious units in the culture supernatants of iSLK.219 subclones, induced as in C), were titrated on QBI293A cells. One representative data from 3 independent experiments is shown. Each data point represents mean ± standard error mean of triplicates.
3.5. Virus stocks, prepared from inducible Vero (iVero.219) or SLK (iSLK.219) cells, containing rKSHV.219 viruses, displayed similar particle:infectivity ratios
infectious units to particle ratio of a virus stock of many viruses ranges from 1:100 to 1:10.000 (Dittmer et al., 1999). To analyze the ratio of virus stocks, prepared from iVero.219 (Vero cells are a conventional cell line used for KSHV virus stock preparation) and iSLK.219 cells (mass culture), in each experiment, 1 liter of culture supernatants from cells, induced by doxycycline (1 µg/ml) and SB (900 µM), was concentrated by ultracentrifugation as described previously (Bechtel et al., 2003; Vieira and O'Hearn, 2004) and titrated on 293 cells by spinoculation (Yoo et al., 2008) as described in the Materials and Methods. Virus stocks from both iVero.219 and iSLK.219 were produced at comparable titers and seem to have similar particle:IU ratio (Table 2). For stocks from iVero.219 cells, the particle to IU ratio ranged from 174 to 898, and for stocks from iSLK.219 cells, this parameter ranged from 80 to 436, within a factor of two of the values observed in the standard Vero cell line.
Table 2. Comparison of rKSHV.219 stocks prepared from inducible Vero cells (iVero.219), harboring rKSHV.219, and iSLK.219.
4 independent stocks were compared for their infectious units and particle numbers, determined by real-time QPCR. Each stock was prepared by concentrating virus-containing culture supernatants by resuspending virus pellets in fresh medium of the 1/100 original volume. The number of WT KSHV particles, prepared from BCBL-1 cells, is shown for comparison.
| Infectious Units/ml |
Particles/ml | Particle/IU ratio |
||
|---|---|---|---|---|
| rKSHV stock from Vero. 219 | Exp #1 | 1.2*106/ml | 4.99*108/ml | 415.8 |
| Exp #2 | 34.5*106/ml | 60.3*108/ml | 174.7 | |
| Exp #3 | 50.1*106/ml | 450.2*108/ml | 898.6 | |
| Exp #4 | 146.1*106/ml | 560.1*108/ml | 383.3 | |
| rKSHV stock from SLK. 219 | Exp #1 | 1.1*106/ml | 0.88*108/ml | 80.3 |
| Exp #2 | 19.7*106/ml | 86.0*108/ml | 436.5 | |
| Exp #3 | 30.1*106/ml | 121.1*108/ml | 402.3 | |
| Exp #4 | 111.3*106/ml | 352.1*108/ml | 316.3 |
cf) BCBL-1 virus : 873.5*108/ml
3.6. Virus stocks from both iVero.219 and iSLK.219 cells display comparable levels of infectivity in a wide variety of primary and established cell lines
EBV, a human gammaherpesvirus related to KSHV, displays the interesting property that virus produced from cultured epithelial cells infects B lymphocytes 100-fold more efficiently than virus produced from B cells themselves; conversely, B cell derived EBV stocks infect epithelial cells more efficiently than epithelial-derived EBV stocks (Hutt-Fletcher, 2007)). This suggests that virus evolution has maximized the efficiency of transfer of virus between heterologous cell types. In KSHV research, most studies have used virus derived from PEL (B cells); these stocks have uniformly failed to infect other B cell lines. It would be interesting to examine if virus particles produced from endothelial or epithelial lines might differ in their ability to initiate lymphoid infection, or infection of other cell types. Therefore, it was tested if rKSHV.219 derived from two different lines (Vero and SLK) differed in its ability to infect lines derived from a variety of different cell types.
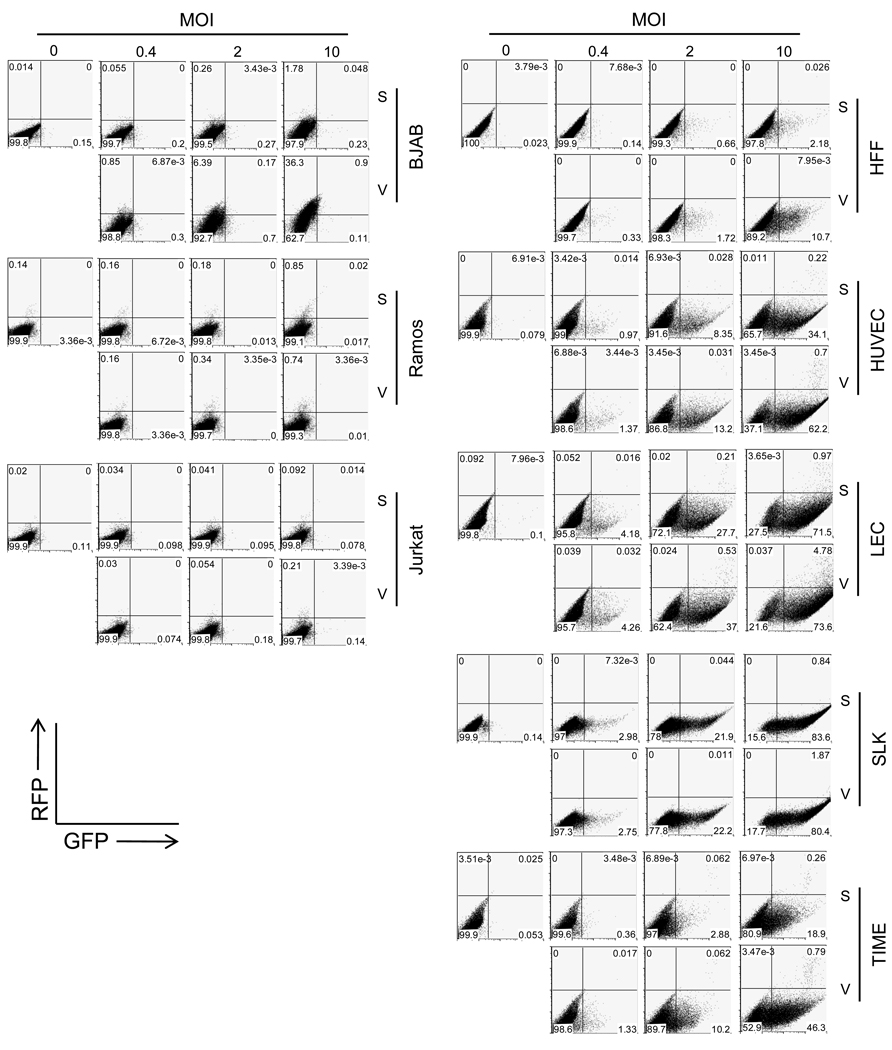
Accordingly, viruses were prepared from either Vero (V) or SLK (S) cells bearing inducible rKSHV.219. These were used to infect (at several different MOIs) B cell lines (BJAB and Ramos) or T cell lines (Jurkat); primary endothelial cells (HUVEC or LEC), immortalized (TIME) or transformed (SLK) endothelial cells, or primary human fibroblast cells (HFF). Infection was judged by GFP and RFP production following infection, as assayed flow cytometrically. rKSHV.219 viruses from either cell background could not infect any of the lymphoblastic cell lines employed (Figure 4, left panels) - exactly as has been described in other studies employing WT viruses prepared from PEL cells (Bechtel et al., 2003; Chandran, 2009; Ganem, 2010). Conversely, rKSHV.219 viruses from both cell types efficiently infected all the adherent cell lines (primary or immortalized) utilized in this study, and did so at comparable efficiencies (Figure 4, right panels). So far, we have not observed differentials in the tropism or infectivity of KSHV stocks based on the cell type on which they were grown. However, it should be acknowledged that a more exhaustive survey on large numbers of different cell types may be required to make a conclusive statement on this issue.
Figure 4. Infectivity of rKSHV.219, prepared from Vero or SLK cells, in established or primary human cells.
rKSHV stocks, prepared from Vero or SLK cells, were infected at increasing MOI in a panel of cells to compare their infectivity in different cell types. At 2 days of post-infection, GFP and RFP expression was analyzed by flow cytometry. S and V represents rKSHV.219 stocks from SLK and Vero cells, respectively.
4. DISCUSSION
Present report demonstrates that doxycycline-inducible iSLK.219 cells, harboring rKSHV.219, provide an efficient and convenient way to prepare recombinant virus stocks and to study many aspects of KSHV biology in cells of endothelial origin. It is believed that iSLK.219 cells will prove useful for a number of types of studies in KSHV research. First, as iSLK.219 cells are strictly latent in the ground state (Figure 2 and Figure 3), with extremely low levels (<0.05%) of lytically reactivated cells, the cells provide a dependable system to study the KSHV latency program. Past efforts to define the transcriptional profile in latency have been bedeviled by the presence of spontaneously-induced lytic cells in 1–5% of the population. Because such cells express large amounts of viral RNA from all over the genome, they can obscure the existence of nonabundant latent transcripts. Indeed, using iSLK.219 cells, Chandriani and Ganem (2010) were recently able to identify several nonabundant mRNAs, including those for K1 and v-IL6, as likely latent gene products.
Second, iSLK.219 clone #10 maintains both tight latency and full inducibility by doxycyline alone, without use of any other chemical stimuli (e.g., Valproate (Shaw et al., 2000), butyrate (Miller et al., 1997), TPA (Renne et al., 1996), ionomycin (Lukac et al., 1999), 5- azacytidine (Chen et al., 2001))- all of which have potent and pleiotropic effects on cellular epigenetics (HDAC inhibitors, 5-azaC) and signal transduction (phorbols, ionomycin). Therefore, this particular iSLK.219 subline will likely be very useful for examining the effects of lytic infection on host epigenetics and signaling, as doxycycline, unlike other inducers, has no known effects of its own on these processes.
Finally, dox-inducible RTA-expressing SLK cells (iSLK) that do not contain KSHV can be used for a variety of other purposes –e.g. (i) the study of RTA modifications and functions; or (ii) as an inducible host for other WT or mutant/recombinant KSHV genomes – for example those derived by BACmid transfection. Recently, iSLK cells have successfully been used to rescue substantial titers of WT KSHV from such a bacmid (C Arias, JM and DG, unpublished).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alt FW, Kellems RE, Bertino JR, Schimke RT. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978;253:1357–1370. [PubMed] [Google Scholar]
- Bechtel JT, Liang Y, Hvidding J, Ganem D. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol. 2003;77:6474–6481. doi: 10.1128/JVI.77.11.6474-6481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran B. Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J Virol. 2009;84:2188–2199. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ueda K, Sakakibara S, Okuno T, Parravicini C, Corbellino M, Yamanishi K. Activation of latent Kaposi's sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc Natl Acad Sci U S A. 2001;98:4119–4124. doi: 10.1073/pnas.051004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ueda K, Sakakibara S, Okuno T, Yamanishi K. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor gene. J Virol. 2000;74:8623–8634. doi: 10.1128/jvi.74.18.8623-8634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Chu JT, Rettig MB, Martinez-Maza O, Sun R. Rta of the human herpesvirus 8/Kaposi sarcoma-associated herpesvirus up-regulates human interleukin-6 gene expression. Blood. 2002;100:1919–1921. doi: 10.1182/blood-2002-01-0015. [DOI] [PubMed] [Google Scholar]
- Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J Exp Med. 1999;190:1857–1868. doi: 10.1084/jem.190.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Invest. 2010;120:939–949. doi: 10.1172/JCI40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher LM. Epstein-Barr virus entry. J Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ, Bertino JR, Schimke RT. Quantitation of dihydrofolate reductase in individual parental and methotrexate-resistant murine cells. Use of a fluorescence activated cell sorter. J Biol Chem. 1978;253:5852–5860. [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984;308:241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Chang J, Lynch SJ, Lukac DM, Ganem D. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 2002;16:1977–1989. doi: 10.1101/gad.996502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ganem D. RBP-J (CSL) is essential for activation of the K14/vGPCR promoter of Kaposi's sarcoma-associated herpesvirus by the lytic switch protein RTA. J Virol. 2004;78:6818–6826. doi: 10.1128/JVI.78.13.6818-6826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Garibyan L, Kirshner JR, Palmeri D, Ganem D. DNA binding by Kaposi's sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J Virol. 2001;75:6786–6799. doi: 10.1128/JVI.75.15.6786-6799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Kirshner JR, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Renne R, Kirshner JR, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoung J, Ganem D. Active lytic infection of human primary tonsillar B cells by KSHV and its noncytolytic control by activated CD4+ T cells. J Clin Invest. 2011a doi: 10.1172/JCI43755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoung J, Ganem D. Infection of primary human tonsillar lymphoid cells by KSHV reveals frequent but abortive infection of T cells. Virology. 2011b doi: 10.1016/j.virol.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J Virol. 2003;77:4205–4220. doi: 10.1128/JVI.77.7.4205-4220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5722–5730. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke RT, Alt FW, Kellems RE, Kaufman RJ, Bertino JR. Amplification of dihydrofolate reductase genes in methotrexate-resistant cultured mouse cells. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):649–657. doi: 10.1101/sqb.1978.042.01.067. [DOI] [PubMed] [Google Scholar]
- Shaw RN, Arbiser JL, Offermann MK. Valproic acid induces human herpesvirus 8 lytic gene expression in BCBL-1 cells. AIDS. 2000;14:899–902. doi: 10.1097/00002030-200005050-00021. [DOI] [PubMed] [Google Scholar]
- Siegal B, Levinton-Kriss S, Schiffer A, Sayar J, Engelberg I, Vonsover A, Ramon Y, Rubinstein E. Kaposi's sarcoma in immunosuppression. Possibly the result of a dual viral infection. Cancer. 1990;65:492–498. doi: 10.1002/1097-0142(19900201)65:3<492::aid-cncr2820650320>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Song MJ, Brown HJ, Wu TT, Sun R. Transcription activation of polyadenylated nuclear rna by rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J Virol. 2001;75:3129–3140. doi: 10.1128/JVI.75.7.3129-3140.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, O'Hearn P, Kimball L, Chandran B, Corey L. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75:1378–1386. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, O'Hearn PM. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology. 2004;325:225–240. doi: 10.1016/j.virol.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Xu Y, AuCoin DP, Huete AR, Cei SA, Hanson LJ, Pari GS. A Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J Virol. 2005;79:3479–3487. doi: 10.1128/JVI.79.6.3479-3487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SM, Ahn AK, Seo T, Hong HB, Chung MA, Jung SD, Cho H, Lee MS. Centrifugal enhancement of Kaposi's sarcoma-associated virus infection of human endothelial cells in vitro. J Virol Methods. 2008;154:160–166. doi: 10.1016/j.jviromet.2008.07.026. [DOI] [PubMed] [Google Scholar]