Abstract
OBJECTIVE
Trauma survivors’ physiological responses to idiosyncratic trauma reminders may be predictive of later posttraumatic stress disorder (PTSD). The majority of previous studies have been cross-sectional and have produced mixed findings. Sex differences may contribute to this heterogeneity. The present study investigated the predictive validity of heightened physiological responsivity to script-driven imagery and sex for the development of PTSD.
METHODS
Heart rate (HR) and respiratory sinus arrhythmia (RSA) were measured at two weeks post-trauma in 158 assault survivors during baseline and while listening to an idiosyncratic trauma script. At 6 months, 15.2% of male and 28.1 % of female participants met diagnostic criteria for PTSD.
RESULTS
GLM and logistic regression analyses showed that HR response to script-driven imagery and sex interacted in predicting PTSD symptom severity at six months. Women had greater PTSD symptom severities overall. Female HR responders to script driven imagery showed the highest PTSD symptom severities, and were almost three times more likely to develop PTSD at six months compared to men and female nonresponders (OR = 2.72, 95% CI= 1.13-6.57). RSA responder type did not predict PTSD, OR = .64, 95%CI = .30 – 1.33.
CONCLUSION
Female trauma survivors who respond to trauma reminders with increased HR may be at particular risk of developing PTSD. Physiological reactivity to trauma cues may be a useful index for screening and prevention of PTSD.
Keywords: PTSD, anxiety disorder, script driven imagery, heart rate, heart rate reactivity, heart rate variability, psychophysiology, prediction
In the initial days and weeks following a traumatic event, most trauma survivors experience a prolonged physiological fear response, characterized by increased heart rate (HR) and skin conductance, or blood pressure (1, 2). While the majority of survivors recover within days or weeks, some continue to show persistent physiological arousal when exposed to reminders of the event (e.g., 3). Such persisting physiological reactions to trauma reminders are amongst the symptoms of posttraumatic stress disorder (PTSD). They have been interpreted as an effect of fear conditioning, a universal learning process present in both animals and humans (e.g., 4). The traumatic event may act as an unconditioned stimulus that elicits strong unconditioned fear, helplessness and corresponding physiological responses. Stimuli present at the time of the trauma can become associated with the trauma (conditioned stimuli) and subsequently trigger similar reactions, such as increases in heart rate, respiration rate, and electrodermal activity. One hypothesis drawn from this model is that those who adapt successfully to a traumatic event have effectively gone through extinction learning, i.e., they have engaged in new learning which inhibits the initial fear response. Those with PTSD may fail in extinction learning and remain to show conditioned fear responses in the presence of trauma reminders.
An open question is whether early physiological responses to trauma reminders predict later PTSD, and if so, whether they can be used to screen for individuals who need early intervention after trauma (see also 5). One way to investigate responses to trauma reminders in the laboratory is the script-driven imagery, a paradigm developed by Lang and colleagues (6) and adapted to the study of PTSD (7-10). Participants listen to an audiotaped script describing their traumatic event in present tense while their physiological responses are measured. Cross-sectional studies comparing trauma survivors with and without PTSD have tended to find heightened physiological arousal during such personalised trauma-related imagery in those with PTSD (8, 9, 10). Moreover, successful therapy of PTSD was associated with normalised HR response to trauma imagery (11).
While a number of studies investigated whether resting HR shortly after the trauma predicts later PTSD (see 5 for a review, 12), there is a lack of longitudinal studies investigating whether early physiological responses to trauma reminders are predictive. Only few studies found that HR reactivity early after trauma, i.e., one week (12) or 1 to 4 months post-trauma (13) significantly predicted PTSD at follow-up.
Overall, however, the above studies showed considerable heterogeneity, and a few studies reported less psychophysiological reactivity in the PTSD group compared to those without PTSD (e.g., 2, 14, 15, 16). A recent meta-analysis of 22 studies of psychophysiological responses to idiosyncratic trauma cues in trauma survivors with and without PTSD reported a mean association between PTSD and HR reactivity of r = .22 (weighted mean effect size, range: r = −.03 to .70) (17).
The remarkable heterogeneity in response to script-driven imagery in trauma survivors with and without PTSD warrants further exploration. Part of the discrepancies between studies may be due to differences in methodology, but also to sample characteristics, and to the timing of physiological assessment. Most previous studies have been unable to explore variables contributing to heterogeneity as sample sizes were rather small (the median sample size in Pole et al.’s meta analysis was N = 28), or too homogeneous (e.g., a large study of veterans (N > 150), 8).
One candidate moderator of HR responses in PTSD is sex, as acquisition and extinction of fear may differ between men and women according to a number of preclinical and clinical studies (e.g., 18, 19, 20, 21). Women may be more physiologically reactive to stressors than men (22, 23). During trauma, women may experience greater physiological reactivity. Strength of this unconditioned response may be predictive of the conditioned response, and greater peritraumatic physiological reactivity may lead to increased heart rate reactivity to trauma reminders in females and thus may be related to subsequent PTSD. This is in accord with epidemiological studies that found a higher PTSD prevalence in female than in male trauma survivors, with odds ratios ranging from 1.64 to 4.08 (24, 25). Sex differences in psychophysiological responses may contribute to women’s greater risk of developing PTSD, and early physiological responding to trauma reminders may be differentially related to later PTSD in men and women.
The present study investigated whether HR response to script-driven imagery of the trauma at 2 weeks predicts PTSD at 6 months. The sample comprised a relatively large number of male and female assault survivors, permitting an analysis of whether sex interacts with physiological responses to the trauma script in predicting PTSD. Additionally, we explored whether decreased respiratory sinus arrhythmia (RSA), either at baseline or in response to trauma scripts, is related to later PTSD. RSA is one component of heart rate variability, and quantifies the rhythmic oscillation of HR related to phases of breathing. It is associated with vagal efferent effects on the sino-atrial node, and is a well-accepted marker of parasympathetic (or vagal) control of HR (26). The rapid application and withdrawal of vagal inhibition is thought to be an important substrate for flexible behavioural routines (27, 28). Individuals with lower basal RSA or attenuated RSA responsivity may show regulatory deficits, such as compromised heart rate recovery after stress-induced heart rate elevations, for instance in the presence of trauma reminders. It is possible that such a regulatory deficit sustains conditioned fear responses and contributes to enduring reactivity to trauma reminders in PTSD, and thus to a heightened risk of chronic PTSD. Accordingly, low RSA has been been associated with negative mental health outcomes including anxiety (29).
Finally, the study explored associations of sex and physiological responses with socioeconomic, and peritraumatic und posttraumatic variables that have been shown to have robust effects in the prediction of chronic PTSD, namely, socioeconomic status and demographic variables, peritraumatic fear for life, mental defeat (30), dissociation, and social support (31, 32).
Methods
Participants
The study was approved by the local ethics board. A total of 1063 assault survivors who attended the Emergency Department of a large urban teaching hospital in London, UK, from July 2003 to December 2004 were contacted, of whom 389 did not fulfill inclusion criteria and 197 declined to take part. Another 255 were initially interested, but failed to schedule or attend the research session within the designated time period. Two-hundred and twenty-two participants consented to participate in a research session at 2 weeks after the assault, and 158 (101 men, 57 women) took part in the script-driven imagery task. There were no significant differences between the study sample and a random sample of assault survivors drawn from the same A&E department in terms of age, sex or severity of injuries as assessed by triage category. To be eligible for the study, participants had to speak English fluently enough to be able to answer interview questions and fill in questionnaires. Participants with current psychosis, alcohol dependence, ongoing domestic violence and no memory of the assault were excluded. Three participants had to be excluded from the analyses due to potential confounding impact of their current medication on cardiovascular activity (glucosamine, lithium, diazepam, beta-blockers) so that the final sample size was 155 (99 men, 56 women). Nearly all assaults were physical assaults, only 2% were sexual assaults.
Script-driven imagery task
Participants listened to an audiotaped description of their assault that was constructed to last 2 minutes, and included both a description of the event and the participants’ cognitive and bodily responses. Scripts were composed and recorded by the experimenter (BK) in a neutral tone of voice, and described the trauma in the second person and the present tense. They were constructed following guidelines by Blanchard et al. (7), building on a trauma narrative that participants had provided earlier in the session. Participants were instructed to close their eyes, listen carefully to the script, and imagine the event as vividly as possible.
Heart rate recording
HR (in beats per minute, bpm) was recorded continuously for three minutes during baseline, and for two minutes during script-driven imagery, using a Polar S830 HR monitor (Polar Electro, Vantaa, Finland), which consists of a chest band and a wrist receiver. This Polar monitor has been shown to reliably assess consecutive interbeat (IBI) intervals with millisecond accuracy during rest as well as activity (e.g., 33). Recorded data were transferred to PC and subsequently examined using ANSLAB version 4.0 (Wilhelm, F.H., & Peyk, P. (2005). ANSLAB: Autonomic Nervous System Laboratory (Version 4.0). Available at the SPR Software Repository: http://www.sprweb.org.). Artefacts from ectopic or missed beats (<3% of total measurement time) were detected as outliers appearing as spikes from the adjacent IBI curve, deleted, and interpolated from adjacent measurements. HR was calculated as 60000 / IBI. The software also computed RSA as the high frequency (0.15-0.50 Hz) spectral power density (in log ms2) of IBI variability, following established guidelines.
Questionnaire Measures and Clinical Interview
Ratings of responses during script-driven imagery
Immediately after listening to their trauma script, participants rated the extent to which they had relived the experience (1 item), felt irritated (1 item), and had dissociated whilst listening to the tape, each on a scale from 0 (not at all) to 10 (very much) (3 items, α = .85).
General Information Questionnaire
This questionnaire was adapted from Halligan et al. (34) to assess demographic and assault characteristics (age, ethnic background, marital status, socioeconomic status, education, body mass index (BMI), smoking).
Peritraumatic emotions and cognitions
Participants rated how much fear and panic attack symptoms they had felt and the extent to which they had believed that they would die during the assault. Each item was rated on a scale from 0 ‘not at all’ to 4 ‘very strongly’. Mental defeat during the assault was assessed with the Mental Defeat Scale, a self-report questionnaire assessing the perceived loss of all autonomy during the trauma, consisting of 11 items such as “I no longer felt like a human being” or “In my mind, I gave up” that has been shown to predict PTSD (α = .90, 30, 35). Dissociation during the assault was assessed with the State Dissociation Questionnaire (36, α = .88), a 9-item scale assessing different aspects of dissociation such as derealization, depersonalization, detachment, altered time sense, emotional numbing, and reduction of awareness in surroundings. This measure has been shown to predict PTSD after motor vehicle accidents and assault (36). It correlates strongly with the Peritraumatic Dissociation Scale; r = .79 (34).
Social support
Perceived social support was measured using the Crisis Support Scale (CSS, 33), which has been shown to have an adequate internal reliability (38).
Rumination
The responses to Intrusions Questionnaire (RIQ) was used to measure and rumination (α = .84). Participants rated on a scale from 0 (not at all) to 4 (very strongly) how much each statement applied to them. The scales have adequate reliability (α ≥.70) and predictive validity in a range of studies (38,36).
PTSD Symptom Scale–Interview Version
PTSD symptom severity at 6 months was assessed by the experimenter (B.K.) with the PTSD Symptom Scale–Interview Version (PSS-I, 40). The interviewer rated each of the PTSD symptoms on a scale from 0 (not at all) to 3 (5 or more times per week/very much). The total PSS-I score is the sum of the ratings for the 17 items. Interrater reliability for PTSD diagnosis was high (κ = .82, based on 56 interviews).
Posttraumatic Stress Disorder Diagnostic Scale
Severity of initial PTSD symptoms at 1 month was assessed with the Posttraumatic Diagnostic Scale (PDS; 41), a standardized and validated self-report measure of PTSD symptom severity that has been widely used with clinical and non-clinical samples of traumatized individuals. The PDS asks participants to rate 17 items regarding how much they were bothered by each of the PTSD symptoms specified in DSM-IV ranging from 0 (never) to 3 (5 times per week or more/very severely). Internal consistency in this sample was α = .92.
Injury severity
Participants rated the severity of their assault-related injuries on a scale from 0 (‘very mild’) to 10 (very severe/life threatening). Injury severity was also indexed with the Injury Severity Score (ISS) (42), an anatomical scoring system that provides an overall score for patients with multiple injuries. A trained research nurse coded participants’ injuries on the basis of hospital notes.
Procedure
The script-driven imagery task was conducted at the end of an experimental session, after being seated in the laboratory for around 2 hours. During this session, participants had completed questionnaires relating to the assault, an experimental priming task, and an autobiographical memory task; these results are reported elsewhere (43, 44). Participants were given a break before being fitted with the HR recording equipment.
Data analysis
HR reactivity was computed as mean HR during baseline subtracted from the mean HR during the script-driven imagery task. Participants were dichotomised into HR responders (HR response > 0 bpm) versus HR nonresponders (HR response <= 0 bpm). Since RSA is a measure of vagal activity and typically reduced during stressful responding, RSA responders were characterized by RSA decreases during tape compared to baseline, and participants were dichotomised into RSA responders (RSA response < 0 ms2), and RSA nonresponders (RSA response >= 0 ms2). SPSS 15.0 was used to compute univariate general linear models to test for differences in PTSD symptom severity at 6 months depending on responder type (responder vs non-responder) and sex. Variables that differed significantly between male and female participants were included as covariates. Additional logistic regression analyses provided odds ratios for the likelihood of developing PTSD depending on responder type and sex. Alpha levels were set to .05 for all analyses.
Results
Sample characteristics and baseline HR and RSA
Table 1 presents demographic, clinical and assault sample characteristics. Men and women did not differ in these variables, with the exception of age and BMI. Women were on average five years younger than men (p = .01), and had a lower BMI (p = .047). Women showed a significantly higher HR during baseline (M = 70.75, SD = 10.12 vs. M = 65.52, SD = 10.38, respectively, F(158) = 9.22, p = .003). There were no significant sex differences in baseline RSA (M (in log ms2) = 7.27, SD = 1.32). Of the demographic variables shown in Table, 1, nonwhite Ethnicity (r = .23, p= .001), lower income ( r= .27, p<.001), lower education level (r= .31, p<.001), as well as self-reported alcohol/drug intoxication during assault (r= −.24, p= .001), and longer assault duration (r= .29, p< .001) correlated with PTSD severity at 6 months. Furthermore, self-reported dissociation, vividness and sense of irritation during listening to the tape correlated with PTSD symptom severity at 6 months (all r’s <.38, ps <.001).
Table 1.
Demographic, clinical, and assault sample characteristics (N = 155)
| Variable | Men (n = 99) M (SD) or n (%) |
Women (n = 56) M (SD) or n (%) |
Statistical tests | ||
|---|---|---|---|---|---|
| Age, years | 36.2 | (10.9) | 31.5 | (11.5) | F(1, 154) = 6.44, p = .012 |
| BMI1, kg/m2 | 24.1 | (4.4) | 22.5 | (4.4) | F(1, 143) = 4.02, p = .047 |
| Race | |||||
| White | 61 | 61.6% | 35 | 62.5% | Χ2(1) = .01 , p = .527 |
| Other | 38 | 38.4% | 21 | 37.5% | |
| Socio-economic status2 |
|||||
| Very low income (£5,000 or less) | 23 | 23.2% | 18 | 32.1% | Χ2(4) = 5.59, p = .589 |
| Low income (£5,000-£15,000) | 30 | 30.3% | 16 | 28.6% | |
| Moderate income (£15,000- £30,000) | 21 | 21.2% | 14 | 25.0% | |
| High income (over £30.000) | 18 | 18.1% | 5 | 8.9% | |
| No information | 0 | 0% | 3 | 5.4% | |
| Marital status | |||||
| Married/ in long-term relationship | 42 | 42.4% | 19 | 33.9% | Χ2(3) = .28, p = .360 |
| Single / no long-term relationship | 56 | 56.6% | 37 | 66.1% | |
| No information | 1 | 1.0% | 0 | 0% | |
|
| |||||
| Education, years | 15.1 | (6.0) | 14.0 | (5.5) | F(1, 134) = 1.07, p = .302 |
|
| |||||
| Posttraumatic stress disorder at 6 months (n = 146) | |||||
|
| |||||
| Met diagnostic criteria3 | 15 | 15.2% | 16 | 28.1% | Χ2(1) = 4.53, p = .029 |
|
| |||||
| PTSD symptom severity | 9.4 | (10.4) | 12.3 | (10.1) | F (1, 143)= 1.96, p = .164 |
|
| |||||
| Assault duration, min | 7.8 | (8.9) | 9.8 | (9.9) | F (1, 153)= 1.63, p = .204 |
|
| |||||
| Subjective Injury severity, (range: 0-10) | 5.0 | (2.5) | 4.3 | (2.4) | F (1, 137 )= 2.89, p = .092 |
|
| |||||
| Injury Severity Score | 1.4 | (1.4) | 1.1 | (.2) | F(1, 106) = 2.48, p = .118 |
|
| |||||
| Hospital admission4, yes | 18 | 22.8% | 5 | 10.6% | Χ2(1) = 3.35, p = .188 |
|
| |||||
| Weapon used, yes | 47 | 47.5% | 14 | 25.0% | Χ2(1) = 2.79, p = .111 |
| Alcohol intoxication during assault, yes | Χ2(1) = 1.29, p = .167 | ||||
| 46 | 46.9% | 21 | 37.5% | ||
|
| |||||
| Days since assault at script-driven imagery |
18.5 | (7.5) | 17.3 | (7.4) | F(1, 154)= .84, p = .360 |
|
| |||||
| Mental defeat during assault (Range: 0-4) | 1.09 | (1.03) | 1.40 | (1.03) | F (1, 151)= 3.18, p = .076 |
|
| |||||
| Rumination post-assault (Range: 0-24) | 9.45 | (5.24) | 8.39 | (4.54) | F (1, 132)= 1.42, p = .236 |
|
| |||||
| History of anxiety or depression, yes | 34 | 35.1% | 23 | 43.4% | Χ2(1) = .38, p = .203 |
|
| |||||
| Dissociation during tape (Range: 0-10) | 2.5 | (2.6) | 3.1 | (2.4) | F(1, 154) = 1.88, p = .172 |
|
| |||||
| Vividness/sense of re-living during tape (Range: 0-10) | 3.5 | (3.0) | 4.2 | (3.1) | F (1, 154)= 1.95, p = .165 |
|
| |||||
| Sense of irritation during tape (Range: 0-10) | 3.5 | (2.7) | 3.9 | (2.9) | F (1, 154)= .75, p = .387 |
Note: Body mass index,
combined household income,
missing data at follow up: 6 for male, 0 for female subsample,
Hospital admission following treatment at the accident and emergency department was available for 126 participants
Responses to script driven imagery
Men and women did not differ in their subjective responses during script-driven imagery (see Table 1). The mean baseline HR (in bpm) was 67.41, SD = 10.56, and the mean HR during script-driven imagery was 67.07, SD = 10.89. The mean baseline RSA was 7.21, SD = 1.37, and RSA during script-driven imagery was 7.14, SD = 1.38. Thus, for the total sample, participants’ HR during the imagery task was not different from that during baseline, t = .94, p = .34. Women showed a significantly higher HR during script-driven imagery than men (M = 70.33, SD = 11.00 vs. M = 65.23, SD = 10.42, F(158) = 8.24, p = .005). RSA during script-driven imagery was lower than during baseline, t(154) = 2.15, p = .03.
Baseline HR and RSA responses, responses to trauma scripts, sex, psychological variables and PTSD outcome at 6 months
Neither baseline HR, nor baseline RSA predicted PTSD at follow up, in either the male, or the female sample, Male: HR OR= 1.03, 95%CI= .99 – 1.08, p= .19; RSA: OR = .83, 95%CI= .59 – 1.20, p = .33, Female: HR, OR = 1.05, 95%CI= .99 – 1.11 p = .10; RSA: OR= .87, 95%CI= .58- 1.31, p= .50.
Seventy-four participants (47.7%) showed an increased HR whilst listening to the tape compared to baseline (responders’ mean HR response = 2.88, SD = 2.89), and were classified as responders, the remaining participants’ HR during tape was largely unchanged or even reduced during the script, and they were classified as nonresponders (nonresponders’ mean HR response = −3.36, SD = 3.27). Forty-nine men (49.5% of male subsample) were classified as responders, and 25 women (44.6 % of female subsample), χ2 = .34, p = .62.
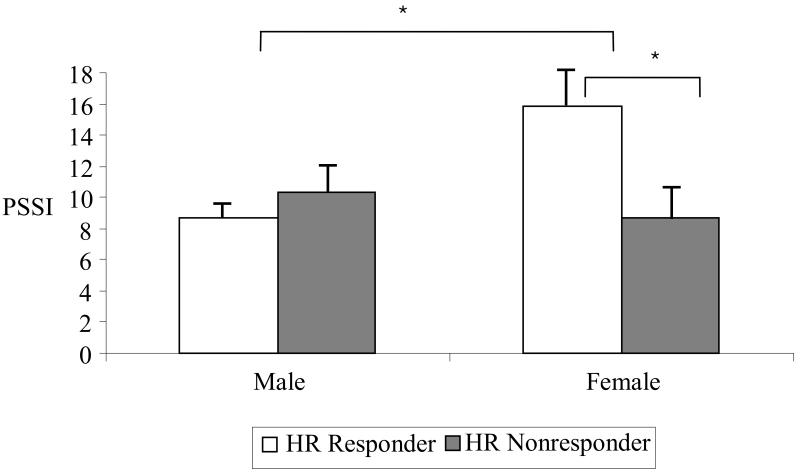
Age, BMI and initial PTSD symptom severity were included as covariates in the following analyses. The GLM of PTSD severity at 6 months with the factors HR Responder Type at 2 weeks, heightened baseline HR (median split) and sex showed no significant main effect of heightened baseline HR, p = .076, HR Responder Type, p = .20, age, p= .053, BMI, p= .43, but a significant effect of initial PTSD symptom severity, F(1,143)= 74.42, p<.001, sex, F(1, 143)= 3.95, p =.049, and a significant HR Responder Type x Sex interaction, F(1, 143) = 4.64, p = .033. The results are shown in Figure 1. Women had higher PTSD symptom severity at 6 months than men, and female HR responders had the highest PTSD symptom severity, higher than all other subgroups, all p-values < .03.
Figure 1.
Mean PTSD symptom severity (PSSI score) and standard error for male and female heart rate responders and non-responders
HR responder = HR increase during script-driven imagery; HR nonresponder = no increase or HR decrease during script-driven imagery; PSSI = PTSD Symptom Scale-Interview Version, * p < .05
As reported previously, mental defeat during trauma, rumination, and a history of anxiety disorders or depression were strong predictors of PTSD severity at 6 months (43). In a separate analysis, we included these psychological predictors, i.e., history of depression or anxiety, peritraumatic mental defeat, and rumination following the assault as factors, controlling for variables from Table 1 that significantly correlated with PTSD symptom severity. The interaction between heart rate responder type and sex remained significant, F = 4.36, p = .040. Other significant factors were history of anxiety or depression, F = 5.15, p = .026, and rumination F = 14.33, p <.001. The interaction between responder type and sex also remained significant when we included emotions during listening to the tape as covariates in a separate analysis, F = 5.78, p = .018.
Logistic regression analyses showed that female HR responders were almost three times more likely to develop PTSD at 6 months compared to the combined rest of the sample (OR = 2.72, 95%CI = 1.13 – 6.57, p = .03), and almost six times more likely to develop PTSD than male responders (OR = 5.80, 95%CI = 1.36 – 24.67, p = .02).
Parallel analyses investigating RSA responses and sex in the prediction of PTSD severity at 6 months revealed no effect of RSA Responder Type, OR = .64, 95%CI = .30 – 1.33, p= .248 , a marginally significant effect of Sex, p= .09, and no interaction between RSA Responder Type and Sex, p= .34. Sixty-three men (63.6 % of male subsample) were classified as responders, and 28 women (50 % of female subsample), χ2 = 2.37, p = .129.
Socioeconomic variables, and peritraumatic cognitive and emotional variables characterizing female HR responders
We compared socioeconomic, peritraumatic cognitive and peritraumatic emotional variables for female responders with those for the remaining subgroups, i.e., female non-responders, male responders and male non-responders. Female responders did not differ from the other groups in terms of socioeconomic status, years of education, social support, smoking, drug and alcohol consumption (all p-values > .09). However, they reported more fear during the assault and more peritraumatic mental defeat, i.e., they were more likely to perceive a complete loss of autonomy during the assault (all p-values < .04). Female responders reported more peritraumatic panic than male responders and nonresponders, ps < .01, but did not differ significantly from female nonresponders, p = .20 Groups did not differ on peritraumatic dissociation, p = .76, or dissociation while listening to the tape, p = .53.
Discussion
This study investigated whether HR responses to script-driven imagery at 2 weeks after assault predict PTSD symptom severity at 6 months. To our knowledge, previous studies have not specifically investigated sex differences in HR responses to script-driven imagery in the prediction of PTSD (17,45). The present study found that HR response to script-driven imagery was predictive of later PTSD in women, but not in men, even when known psychological predictors were controlled for. Being female was related to higher PTSD symptom severity at 6 months. This finding is consistent with epidemiological studies reporting higher PTSD prevalence rates in female than in male trauma survivors (e.g., 46, 47). Female HR responders were almost 3 times more likely to meet diagnostic criteria for PTSD at 6 months compared to the rest of the sample, and almost six times more likely to develop PTSD than male responders. RSA responder type or its interaction with sex was not predictive of later PTSD. The relationship of RSA with cardiac vagal control can be confounded by respiratory variations, which we did not measure and thus were unable to adjust for (48). This may have added error variance in the prediction of PTSD by vagal responding. Moreover, cardiac responses are a function of the combined activity of the two autonomic branches (49). Thus, the observed changes in heart rate reflect the net outcome of both sympathetic and parasympathetic efferents. On the other hand, RSA is an index of only cardiac vagal control. The fact that HR responding to trauma imagery is more predictive of later PTSD than RSA responding may well indicate that sympathetic reactivity, but not parasympathetic reactivity, is important for predicting the development of PTSD. In future studies it may thus be advisable to include a pure index of sympathetic responsivity, such as cardiac pre-ejection period.
Several factors may put female assault survivors with increased HR responses to trauma reminders at heightened PTSD risk. First, female HR responders differed from female nonresponders and male subgroups in a number of peritraumatic responses. They reported more fear and greater mental defeat during the assault, which is in accord with conditioning theories of PTSD that would predict that greater strength of the emotional response during the trauma (the unconditioned response, UR) is associated with more potent conditioning and subsequently greater strength of emotional responses to reminders (the conditioned responses, CRs). Interestingly, there were no group differences in dissociation, neither peritraumatically, nor during script-driven imagery. Thus, dissociation, which has previously been found to be related to decreased HR responses in some studies (50), did not appear to affect HR in this study. Second, there may be sex differences in the ease with which conditioned responses are acquired or extinguished. Differences in fear acquisition do not fully explain the pattern of findings observed in this study as the majority of studies show greater fear conditioning in men than in women (e.g., 18, 51), although female responders in our study reported more peritraumatic fear and panic. Moreover, stress facilitates classical conditioning in male rats, immediately following the stressor as well as 24 hours later, whereas the opposite effect is found in females (52). The more likely explanation are differences in extinction of learned fear responses. Women show slower extinction than men, especially when assessed at mid cycle (18). Female survivors may therefore differ from their male counterparts in unlearning associations between stimuli and feared outcome following their trauma. Milad and colleagues’ findings (18) also highlight the importance of sex hormone levels and their interaction with physiological indices for the effect of stress and trauma on women, and some authors have even proposed that the presence of gonadal hormones during early development may influence the effect of stress later in life, and the direction of that effect (52). The present study did not measure the influence of hormones, or phase of menstrual cycle, so that it would be promising to include these variable in future research exploring sex differences in HR responses to trauma reminders.
Third, coping styles following the acute trauma phase, possibly during the first weeks and months post-trauma, may play an important role. Women with high physiological reactivity may choose more avoidant and less functional coping styles, such as cognitive or behavioral avoidance of trauma reminders, rather than more beneficial coping styles such as positive reframing, venting, and use of emotional support (53), which may over time lead to heightened PTSD symptoms in the subgroup of female responders.
If replicated, our finding of high PTSD rates in female HR responders may have practical implications. First, early HR responses to trauma reminders could potentially help identify those that need intervention after traumatic events (see 5), especially in situations where there is reason to believe that people may overreport or underreport symptoms. Multi-channel psychophysiological assessment in the laboratory is cost- and labour - intensive and may therefore not be feasible in practice (e.g., 54). However, a simple and inexpensive tool, such as the Polar watch used in the current study, may help to identify trauma survivors at risk of later PTSD. Responder type may be assessed by clinicians. In females, this information explained additional variance in later PTSD symptom severity, over and above initial PTSD symptom severity.
Our results may also have specific implications for intervention strategies. If further studies confirm that physiological reactivity to imagery of the traumatic event is related to PTSD risk in female trauma survivors, ways of reducing physiological arousal may be a key strategy for prevention of PTSD in this subgroup. Administration of propranolol after trauma memory retrieval or script driven imagery has recently been shown to reduce physiological responding during subsequent mental imagery of the event (55; 56). This may not be the only biological approach. Other avenues of early intervention include cognitive behaviour therapy including exposure-based approaches (58-60) or exposure to trauma memories early post trauma (61), with the latter awaiting empirical validation in further clinical trials. Such interventions could be delivered specifically to those at risk, such as female trauma survivors with heightened HR reactivity in response to trauma cues.
The present study is not without limitations. Among the strengths is the longitudinal design, the relatively large number of participants, the use of reliable structured interviews to establish PTSD symptom severity, and the early and standardized assessment of script-driven imagery. HR and RSA were the only indices of physiological response to script-driven imagery here; in future studies, assessment of eye-blink startle orbicularis oculi EMG, facial muscle EMG (especially m. corrugator), electrodermal indices, or frontal EEG alpha asymmetry may provide additional predictive information. Moreover, HR was assessed during baseline and script-driven imagery with a small and minimally invasive HR monitor (Polar 830i), which has been shown to reliably assess interbeat-intervals, but more advanced assessment methods, such as a full electrocardiogram may optimize assessment of the HR signal and allow for a more refined analysis of HR variability. Interestingly, a number of participants responded with decreased heart rate in response to the trauma script. This may be related to a disgust reaction to the script. Such responses may in fact be moderated by gender (62) and have often been used to explain such HR decreases, but we did not assess disgust-related responses in the present study. Other important variables that could be included in further studies are childhood trauma history, as well as premorbid and comorbid depression. A control script may also be included to test for the specificity of the effect to the trauma script. Heart rate scores in our sample were lower than in some previous reports, but are still within the range reported across studies (11, 63). As the participants were well familiarized with the laboratory and the experimenter by the time the imagery task was conducted, their baseline heart rates may have been less susceptible to expectation effects compared to studies that measured heart rates soon after arrival in the laboratory. Finally, the study reported on a sample of assault survivors, and it remains to be seen whether the interaction between sex and HR response can be found in other samples of trauma survivors.
In conclusion, we found that, in women, PTSD at 6 months after assault could be predicted from heightened physiological response to script-driven imagery at 2 weeks post-trauma, whereas no such relationship was observed for males. Female responders were at greater risk of heightened PTSD compared to men and women nonresponders. This result may be of practical value as there is yet no consensus as to how to best identify trauma survivors at risk of later PTSD. The current findings draw attention to female HR responders as a subgroup vulnerable to chronic PTSD warranting early intervention.
Acknowledgements
The study was funded by a grant from the Psychiatry Research Trust and a Wellcome Trust Principal Research Fellowship (069777) to Anke Ehlers. We additionally thank Thomas Ehring, Silke Frank, Inga Boellinghaus, Emma Briddon, Anke Weidmann, Ines Sengstock, Johanna Hissbach, Jennifer Baumeister, Stephanie Spengler and the staff of King’s College Accident and Emergency Department for their help.
ACRONYMS
- BMI
Body Mass Index
- PTSD
Posttraumatic Stress Disorder
- SCID
Structured Clinical Interview for DSM-IV
- PDS
Posttraumatic Diagnostic Scale
- PSSI
PTSD Symptom Scale - Interview Version
- RSA
respiratory sinus arrhythmia
- OR
Odds ratio
- CI
confidence interval
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 2.Orr SP, Pitman RK. Psychophysiologic assessment of attempts to simulate posttraumatic stress disorder. Biol Psychiatry. 1993;33(2):127–9. doi: 10.1016/0006-3223(93)90312-2. [DOI] [PubMed] [Google Scholar]
- 3.Tucker PM, Pfefferbaum B, North CS, Kent A, Burgin CE, Parker DE, Hossain A, Jeon-Slaughter H, Trautman RP. Physiologic reactivity despite emotional resilience several years after direct exposure to terrorism. Am J Psychiatry. 2007;164(2):230–5. doi: 10.1176/ajp.2007.164.2.230. [DOI] [PubMed] [Google Scholar]
- 4.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 5.Bryant RA. Longitudinal psychophysiological studies of heart rate: mediating effects and implications for treatment. Ann N Y Acad Sci. 2006;1071:19–26. doi: 10.1196/annals.1364.002. [DOI] [PubMed] [Google Scholar]
- 6.Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol. 1983;92(3):276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard EB, Hickling EJ, Taylor AE, Loos WE, Gerardi RJ. The psychophysiology of motor vehicle accident related posttraumatic stress disorder. Behavior Therapy. 1994;25(3):453–467. doi: 10.1007/BF00999995. [DOI] [PubMed] [Google Scholar]
- 8.Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, Lavori PW. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. J Consult Clin Psychol. 1998;66(6):914–23. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 9.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry. 1987;44:970–5. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 10.Shalev AY, Orr SP, Pitman RK. Psychophysiologic response during script-driven imagery as an outcome measure in posttraumatic stress disorder. J Clin Psychiatry. 1992;53(9):324–6. [PubMed] [Google Scholar]
- 11.Lindauer RT, van Meijel EP, Jalink M, Olff M, Carlier IV, Gersons BP. Heart rate responsivity to script-driven imagery in posttraumatic stress disorder: specificity of response and effects of psychotherapy. Psychosom Med. 2006;68(1):33–40. doi: 10.1097/01.psy.0000188566.35902.e7. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell ML, Creamer M, Elliott P, Bryant R. Tonic and phasic heart rate as predictors of posttraumatic stress disorder. Psychosom Med. 2007;69(3):256–61. doi: 10.1097/PSY.0b013e3180417d04. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Gerardi RJ. The psychophysiology of motor vehicle accident related posttraumatic stress disorder. Behavior Therapy. 1994;25:453–67. [Google Scholar]
- 14.Beckham JC, Vrana SR, Barefoot JC, Feldman ME, Fairbank J, Moore SD. Magnitude and duration of cardiovascular responses to anger in Vietnam veterans with and without posttraumatic stress disorder. J Consult Clin Psychol. 2002;70(1):228–34. doi: 10.1037//0022-006x.70.1.228. [DOI] [PubMed] [Google Scholar]
- 15.Halligan SL, Michael T, Wilhelm FH, Clark DM, Ehlers A. Reduced heart rate responding to trauma reliving in trauma survivors with PTSD: correlates and consequences. J Trauma Stress. 2006;19(5):721–34. doi: 10.1002/jts.20167. [DOI] [PubMed] [Google Scholar]
- 16.Karl A, Malta LS, Alexander J, Blanchard EB. Startle responses in motor vehicle accident survivors: a pilot study. Appl Psychophysiol Biofeedback. 2004;29(3):223–31. doi: 10.1023/b:apbi.0000039060.57276.aa. [DOI] [PubMed] [Google Scholar]
- 17.Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133(5):725–46. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- 18.Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006;120(6):1196–203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 19.Grillon C. Greater sustained anxiety but not phasic fear in women compared to men. Emotion. 2008;8(3):410–3. doi: 10.1037/1528-3542.8.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biological Psychiatry. 2007;62(5):487–95. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toufexis D. Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol. 2007;19(6):461–73. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 22.Stoney CM, Davis MC, Mathews KA. Sex differences in physiological responses to stress and in coronary heart disease- a causal link. Psychophysiology. 1987 Mar;24(2):127–31. doi: 10.1111/j.1469-8986.1987.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 23.Kudielka BM, Hellhammer DH, Kirschbaum C. Sex differences in human stress response. In: Fink G, Chrousos G, Craig I, de Kloet ER, Feuerstein G, McEwen BC, Rose NR, Rubin RT, Steptoe A, editors. Encyclopedia of stress. 2. revised edition Academic Press; Oxford: 2007. pp. 469–473. [Google Scholar]
- 24.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 25.Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54(11):1044–8. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 26.Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosom Med. 1998;60(4):498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 28.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007 Feb;74(2):185–99. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalised anxiety disorder and worry. Biol Psychiatry. 1996 Feb;39(4):255–66. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 30.Dunmore E, Clark DM, Ehlers A. Cognitive factors involved in the onset and maintenance of posttraumatic stress disorder (PTSD) after physical or sexual assault. Behav Res Ther. 1999;37(9):809–29. doi: 10.1016/s0005-7967(98)00181-8. (1999) [DOI] [PubMed] [Google Scholar]
- 31.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 32.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68(5):748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 33.Laukkkanen RMT, Virtanen P. Heart rate monitors: state of the art. Journal of sports sciences. 1998;16(1):3–7. doi: 10.1080/026404198366920. [DOI] [PubMed] [Google Scholar]
- 34.Halligan SL, Michael T, Clark DM, Ehlers A. Posttraumatic stress disorder following assault: The role of cognitive processing, trauma memory, and appraisals. Journal of Consulting & Clinical Psychology. 2003;71(3):419–31. doi: 10.1037/0022-006x.71.3.419. [DOI] [PubMed] [Google Scholar]
- 35.Dunmore E, Clark DM, Ehlers A. A prospective investigation of the role of cognitive factors in persistent posttraumatic stress disorder (PTSD) after physical or sexual assault. Behav Res Ther. 2001;39(9):1063–84. doi: 10.1016/s0005-7967(00)00088-7. (1999) [DOI] [PubMed] [Google Scholar]
- 36.Murray J, Ehlers A, Mayou RA. Dissociation and post-traumatic stress disorder: two prospective studies of road traffic accident survivors. Br J Psychiatry. 2002;180:363–8. doi: 10.1192/bjp.180.4.363. [DOI] [PubMed] [Google Scholar]
- 37.Joseph S, Yule W, Williams R, Andrews B. Crisis support in the aftermath of disaster: a longitudinal perspective. Br J Clin Psychol. 1993;32:177–85. doi: 10.1111/j.2044-8260.1993.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 38.Joseph S, Andrews B, Williams R, Yule W. Crisis support and psychiatric symptomatology in adult survivors of the Jupiter cruise ship disaster. Br J Clin Psychol. 1992;31:63–73. doi: 10.1111/j.2044-8260.1992.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 39.Clohessy S, Ehlers A. PTSD symptoms, response to intrusive memories and coping in ambulance service workers. British Journal of Clinical Psychology. 1999;38:251–265. doi: 10.1348/014466599162836. [DOI] [PubMed] [Google Scholar]
- 40.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity o a brief instrument for assessing posttraumatic stress disorder. Journal of Traumatic Stress. 1997;9(4):445–51. (1993) [Google Scholar]
- 41.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol Assessment. 1997;9:445–451. [Google Scholar]
- 42.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergenxy medical care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 43.Kleim B, Ehlers A, Glucksman E. Early predictors of chronic post-traumatic stress disorder in assault survivors. Psychol Med. 2007 Oct;37(10):1457–67. doi: 10.1017/S0033291707001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleim B, Ehlers A. Reduced autobiographical memory specificity predicts depression and posttraumatic stress disorder after recent trauma. J Consult Clin Psychol. 2008 Apr;76(2):231–42. doi: 10.1037/0022-006X.76.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156(4):575–84. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 46.Foa EB, Tolin DF. Sex Differences in Trauma and Posttraumatic Stress Disorder: A Quantatitive Review of 25 Years of Research. Psychological Bulletin. 2006;(132):959–92. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 47.Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133(2):183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 48.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral funcions. Biol Psychol. 2007;74(2):263–85. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: empirical perspective and conceptual implications. Psych Bull. 1993;114:296–32. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- 50.Griffin MG, Resick PA, Mechanic MB. Objective assessment of peritraumatic dissociation: psychophysiological indicators. Am J Psychiatry. 1997 Aug;154(8):1081–8. doi: 10.1176/ajp.154.8.1081. (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cogn Affect Behav Neurosci. 2005;5(2):191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]
- 52.Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004 Jan;19(1):145–50. doi: 10.1046/j.1460-9568.2003.03065.x. (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly MM, Tyrka AR, Price LH, Carpenter LL. Sex differences in the use of coping strategies: predictors of anxiety and depressive symptoms. Depress Anxiety. 2008;25(10):839–46. doi: 10.1002/da.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. J Affect Disord. 2000 Dec;61(3):225–40. doi: 10.1016/s0165-0327(00)00340-2. (2000) [DOI] [PubMed] [Google Scholar]
- 55.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42(6):503–6. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002 Jan 15;51(2):189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 57.Bisson JI, Brayne M, Ochberg FM, Everly GS., Jr. Early psychosocial intervention following traumatic events. Am J Psychiatry. 2007;164(7):1016–9. doi: 10.1176/ajp.2007.164.7.1016. [DOI] [PubMed] [Google Scholar]
- 58.Ehlers A, Clark DM, Hackmann A, McManus F, Fennell M, Herbert C, Mayou R. A randomized controlled trial of cognitive therapy, self-help booklet, and repeated assessment as early interventions for PTSD. Arch Gen Psychiatry. 2003;60:1024–1032. doi: 10.1001/archpsyc.60.10.1024. [DOI] [PubMed] [Google Scholar]
- 59.Bryant RA, Mastrodomenico J, Felmingham KL, Kenny L, Kandris E, Cahill C, Creamer M. Treatment of acute stress disorder: a randomised controlled trial. Arch Gen Psychiatry. 2008 Jun;65(6):659–667. doi: 10.1001/archpsyc.65.6.659. [DOI] [PubMed] [Google Scholar]
- 60.Ehlers A, Clark DM. Early psychological interventions for survivors of trauma. Biological Psychiatry. 2003;53:817–826. doi: 10.1016/s0006-3223(02)01812-7. [DOI] [PubMed] [Google Scholar]
- 61.Rothbaum BO, Houry D, Heekin M, Leiner AS, Daugherty J, Smith LS, Gerardi M. A pilot study of an exposure-based intervention in the ED designed to prevent posttraumatic stress disorder. Am J Emerg Med. 2008;26(3):326–30. doi: 10.1016/j.ajem.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Olatunji BO, Babson KA, Smith RC, Feldner MT, Connolly KM. Gender as a moderator of the relation between PTSD and disgust: A laboratory test employing individualized script-driven imagery. J Anx Disord. 2009 Dec;23(8):1091–1097. doi: 10.1016/j.janxdis.2009.07.012. (2009) [DOI] [PubMed] [Google Scholar]
- 63.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63:585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]