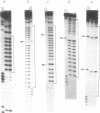
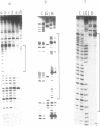
Abstract
We have examined the cleavage of several synthetic DNA sequences by iron(II)-bleomycin. We find that, although bleomycin cuts mixed sequence DNAs with a preference for GC = GT > GA >> GG, it efficiently cleaves regions of (AT)n cutting exclusively at ApT, not TpA. Isolated ApT steps show very little cleavage while blocks of three or more contiguous ATs are cut as efficiently as GpT. This cleavage is specific for (AT)n, since sequences of the type (TAA)n.(TTA)n and (ATT)n.(AAT)n are hardly cut at all. No cleavage is observed at ApC or CpA within sequences of the type (AC)n.(GT)n; regions of An.Tn are also not cut. Although the cobalt-bleomycin complex (which binds to but does not cleave DNA) yields good DNase I footprints at GT and GC sites, no footprints are observed within (AT)n, suggesting that although the cleavage reaction is efficient, the binding affinity is relatively weak. We propose a model in which bleomycin cleavage is determined by local DNA structure, while strong binding requires the presence of a guanine residue.
Full text
PDF
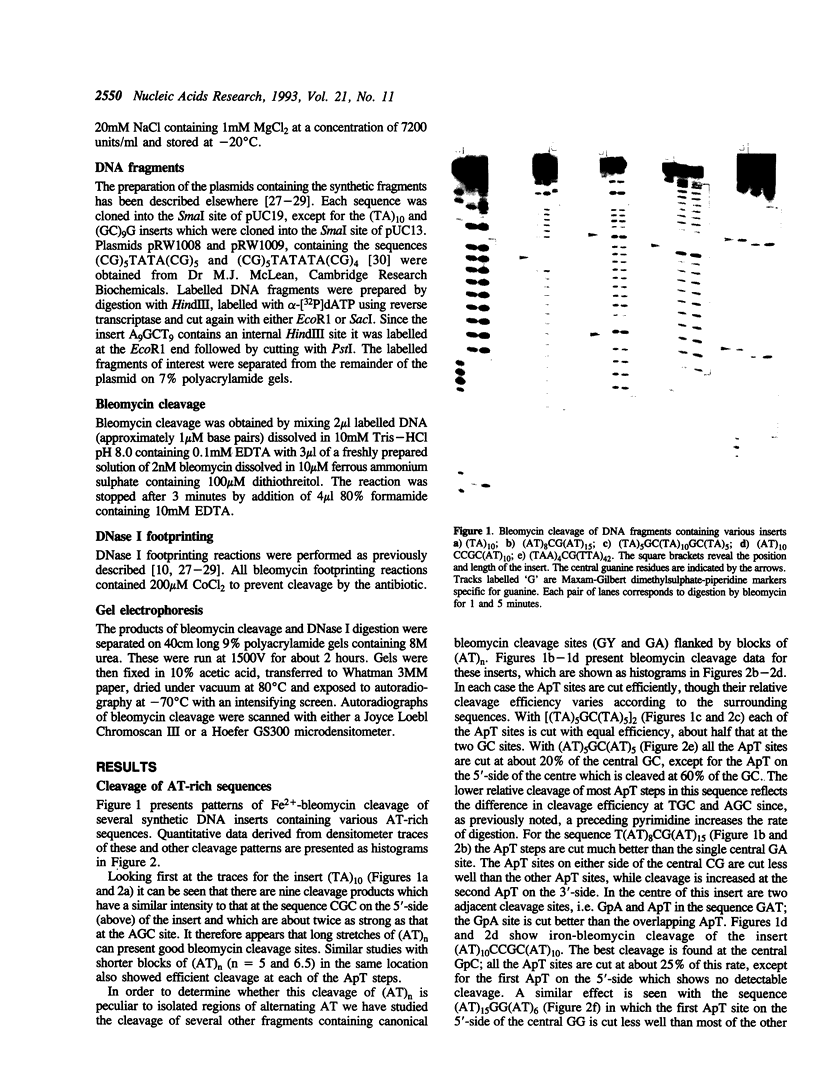
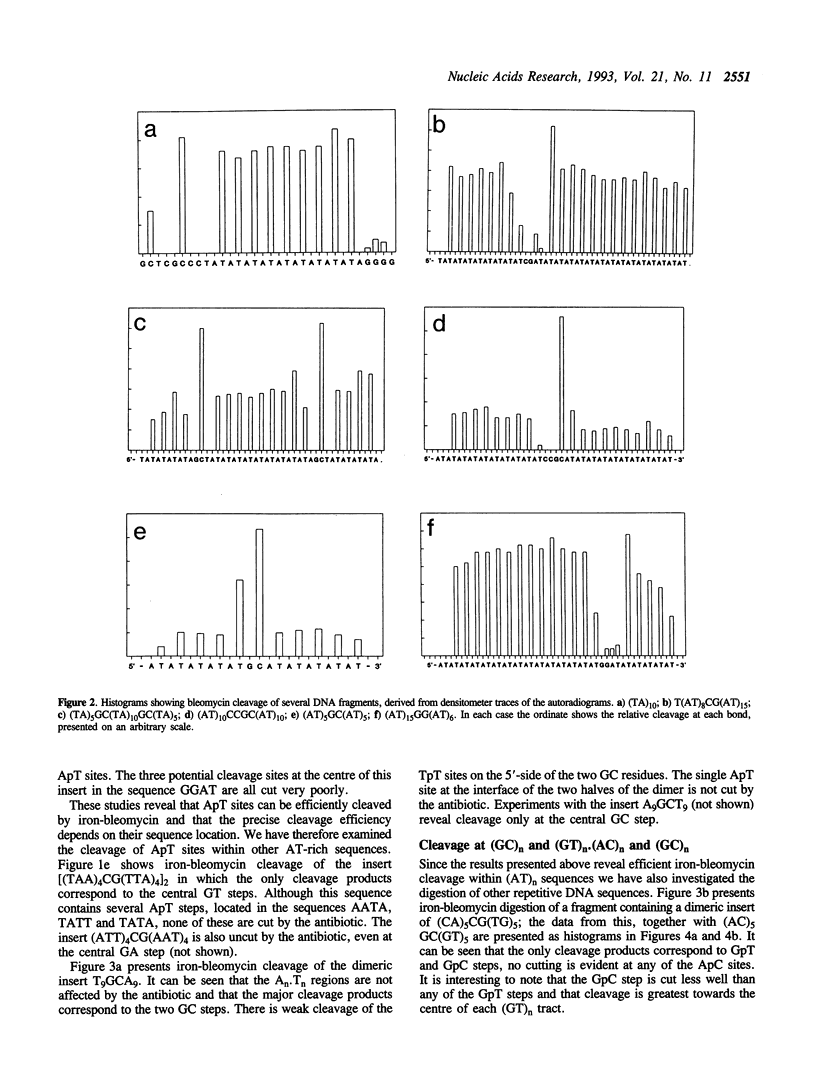

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R. M., Peisach J., Horwitz S. B. Activated bleomycin. A transient complex of drug, iron, and oxygen that degrades DNA. J Biol Chem. 1981 Nov 25;256(22):11636–11644. [PubMed] [Google Scholar]
- Carter B. J., Murty V. S., Reddy K. S., Wang S. N., Hecht S. M. A role for the metal binding domain in determining the DNA sequence selectivity of Fe-bleomycin. J Biol Chem. 1990 Mar 15;265(8):4193–4196. [PubMed] [Google Scholar]
- Carter B. J., de Vroom E., Long E. C., van der Marel G. A., van Boom J. H., Hecht S. M. Site-specific cleavage of RNA by Fe(II).bleomycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9373–9377. doi: 10.1073/pnas.87.23.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. M., Sakai T. T., Glickson J. D., Patel D. J. Bleomycin-A2 complexes with poly(dA--dT): a proton nuclear magnetic resonance study of the nonexchangeable hydrogens. Biochem Biophys Res Commun. 1980 Jan 15;92(1):197–205. doi: 10.1016/0006-291x(80)91539-9. [DOI] [PubMed] [Google Scholar]
- Cons B. M., Fox K. R. The GC-selective ligand mithramycin alters the structure of (AT)n sequences flanking its binding sites. FEBS Lett. 1990 May 7;264(1):100–104. doi: 10.1016/0014-5793(90)80775-e. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R. DNA sequence recognition by bleomycin. Anticancer Drug Des. 1990 Feb;5(1):99–104. [PubMed] [Google Scholar]
- Fox K. R., Grigg G. W., Waring M. J. Sequence-selective binding of phleomycin to DNA. Biochem J. 1987 May 1;243(3):847–851. doi: 10.1042/bj2430847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickson J. D., Pillai R. P., Sakai T. T. Proton NMR studies of the Zn(II)--bleomycin-A2--poly(dA-dT) ternary complex. Proc Natl Acad Sci U S A. 1981 May;78(5):2967–2971. doi: 10.1073/pnas.78.5.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman A. P., Takeshita M. Interactions of bleomycin with DNA. Adv Enzyme Regul. 1980;18:67–83. doi: 10.1016/0065-2571(80)90009-6. [DOI] [PubMed] [Google Scholar]
- Haidle C. W., Weiss K. K., Kuo M. T. Release of free bases from deoxyribonucleic acid after reaction with bleomycin. Mol Pharmacol. 1972 Sep;8(5):531–537. [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Kuwahara J., Sugiura Y. Sequence-specific recognition and cleavage of DNA by metallobleomycin: minor groove binding and possible interaction mode. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2459–2463. doi: 10.1073/pnas.85.8.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Wells R. D. The role of sequence in the stabilization of left-handed DNA helices in vitro and in vivo. Biochim Biophys Acta. 1988 Sep 7;950(3):243–254. doi: 10.1016/0167-4781(88)90120-0. [DOI] [PubMed] [Google Scholar]
- Murray V., Martin R. F. Comparison of the sequence specificity of bleomycin cleavage in two slightly different DNA sequences. Nucleic Acids Res. 1985 Mar 11;13(5):1467–1481. doi: 10.1093/nar/13.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray V., Tan L., Matthews J., Martin R. F. The sequence specificity of bleomycin damage in three cloned DNA sequences that differ by a small number of base substitutions. J Biol Chem. 1988 Sep 15;263(26):12854–12859. [PubMed] [Google Scholar]
- Nightingale K. P., Fox K. R. Interaction of bleomycin with a bent DNA fragment. Biochem J. 1992 Jun 15;284(Pt 3):929–934. doi: 10.1042/bj2840929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Hogan M., Dattagupta N. Binding of bleomycin to DNA: intercalation of the bithiazole rings. Biochemistry. 1979 Jan 9;18(1):96–101. doi: 10.1021/bi00568a015. [DOI] [PubMed] [Google Scholar]
- Shipley J. B., Hecht S. M. Bleomycin congeners exhibiting altered DNA cleavage specificity. Chem Res Toxicol. 1988 Jan-Feb;1(1):25–27. doi: 10.1021/tx00001a004. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Xu C., Murugesan N., Hecht S. M., van der Marel G. A., van Boom J. H. Chemistry of the alkali-labile lesion formed from iron(II) bleomycin and d(CGCTTTAAAGCG). Biochemistry. 1988 Jan 12;27(1):58–67. doi: 10.1021/bi00401a011. [DOI] [PubMed] [Google Scholar]
- Takita T., Muraoka Y., Nakatani T., Fujii A., Iitaka Y., Umezawa H. Chemistry of bleomycin. XXI. Metal-complex of bleomycin and its implication for the mechanism of bleomycin action. J Antibiot (Tokyo) 1978 Oct;31(10):1073–1077. doi: 10.7164/antibiotics.31.1073. [DOI] [PubMed] [Google Scholar]
- Waterloh K., Fox K. R. Interaction of echinomycin with An.Tn. and (AT)n regions flanking its CG binding site. Nucleic Acids Res. 1991 Dec 25;19(24):6719–6724. doi: 10.1093/nar/19.24.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterloh K., Fox K. R. The effects of actinomycin on the structure of dAn.dTn and (dA-dT)n regions surrounding its GC binding site. A footprinting study. J Biol Chem. 1991 Apr 5;266(10):6381–6388. [PubMed] [Google Scholar]
- Wu J. C., Stubbe J., Kozarich J. W. Mechanism of bleomycin: evidence for 4'-ketone formation in poly(dA-dU) associated exclusively with free base release. Biochemistry. 1985 Dec 17;24(26):7569–7573. doi: 10.1021/bi00347a010. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kawanishi S. Enhancement and alteration of bleomycin-catalyzed site-specific DNA cleavage by distamycin A and some minor groove binders. Biochem Biophys Res Commun. 1992 Feb 28;183(1):292–299. doi: 10.1016/0006-291x(92)91642-4. [DOI] [PubMed] [Google Scholar]