Abstract
The linear growth of 29 patients was evaluated from two to 4⅓ years after liver transplantation. All patients received cyclosporine and low-dose prednisone, light patients (28%) displayed acceleration of linear growth velocity and were above the fifth percentile at the end of the evaluation period. Four patients (14%) grew normally prior to transplantation and continued to grow normally after the surgical procedure. Only four patients (14%) dropped from higher levels to below the fifth percentile. Thirteen patients (45%) were less than the fifth percentile before and after surgery; ten of these 13 patients have attained normal or accelerated growth velocity. Good linear growth has been achieved in more than three fourths of patients who underwent liver transplantation.
Linear growth failure commonly occurs in children with end-stage liver disease, including children who have received an otherwise “successful” portoenterostomy.1 In this study, we examined linear growth in 29 pediatric patients who had undergone orthotopic liver transplantation.
PATIENTS AND METHODS
Patient Population
Forty-nine pediatric patients underwent orthotopic liver transplantation between May 1, 1981, and June 1, 1983. Thirty-two patients (65.3%) survived to Jan 1,1986; one patient subsequently died. Epiphyses had already closed in three of the survivors at the time of their surgery; consequently, these patients were not included in the study. The remaining 29 patients (18 female and 11 male patients) were included.
The indications for transplantation were biliary atresia (13 patients), biliary hypoplasia (one patient), metabolic disease (ten patients), familial cholestasis (three patients), neonatal hepatitis (one patient), and benign liver tumor (one patient). Six patients received two transplants and one patient received three transplants. The ages of the patients at the time of transplantation ranged from 7 months to 13 years (average, 5.2 years). Twenty-eight of the 29 patients were less than 10.5 years of age. All patients were Tanner stage I (prepubertal) at the time of surgery.
Immunosuppression Regimen
Cyclosporine and prednisone2,3 were administered in doses that were tapered as quickly as was consistent with avoidance of rejection,4,5 Patients received 2.5 to 5 mg of prednisone as a single daily dose and an average dosage of 9.4 mg/kg/d of cyclosporine (range, 4.6 to 20 mg/kg/d) divided twice daily. Whole blood cyclosporine levels were measured by high-performance liquid chromatography6 with maintenance of trough levels between 100 to 350 ng/mL (83 to 291 nmol/L).
Twenty-five patients had been receiving 2.5 to 5.0 mg/d of prednisone for an average of 30.3 months. At their last evaluation, only four patients received more than 5 mg/d of prednisone. Three of these patients currently suffer from mild chronic rejection. The fourth patient continues to have elevated levels of serum transaminases secondary to recurrent neurovisceral storage disease with supranuclear ophthalmoplegia.7 The average daily dosage of prednisone for all 29 patients was 0.21 mg/kg/d given as a single morning dose.
Growth Measurements
Pretransplantation data were gathered from growth records of primary physicians and from measurements made during the preoperative evaluation at the Children’s Hospital of Pittsburgh. Postoperative growth data were collected during follow-up visits to our unit as well as to primary referring physicians. All but two patients returned regularly to our clinic for follow-up evaluations. The eight patients less than 3 years of age at the time of surgery were measured with a fiberglass tape measure in the supine position while older children were measured standing on a conventional metal scale. All patients were barefooted when measured. All measurements recorded after July 1985 at the Children’s Hospital of Pittsburgh were obtained with a Harpenden stadiometer. To assess the correlation between the metal scale and stadiometer, we measured 20 children after transplantation using both methods. We failed to find any statistically significant difference between these two methods by both parametric and nonparametric tests (P>.10; correlation, .99).
For those children whose preoperative and postoperative growth lengths plotted below the fifth percentile on the cumulative chart of the National Center of Health Statistics, linear growth velocity was calculated and plotted on the charts created by Tanner and Whitehouse for this purpose.8 The intervals between the first and last measurements postoperatively ranged from 24 to 52 months. The average length of postoperative follow-up was 35.8 months.
RESULTS
Lack of growth was consistently observed in the six- to 12-month period following transplantation. During this interval, patients required multiple surgical procedures and had nutritional requirements that could not always be met adequately. As a rule, the general health of surviving patients improved with time.5 The growth curves of 29 patients were evaluated for at least two years after orthotopic liver transplantation (Fig 1). Eight (four female and four male patients) (28%) of 29 patients displayed accelerated growth velocity and were above the fifth percentile at the end of the evaluation period (Table). Four other patients (14%) had normal height prior to transplantation and maintained normal growth velocity following transplantation. Thus, 12 patients (41%) attained both height above the fifth percentile and normal or accelerated growth velocity two years or more postoperatively. Thirteen patients (45%) were less than the fifth percentile for height or length both before and after transplantation, but most of these patients experienced good linear growth postoperatively (Fig 2).
Fig 1.
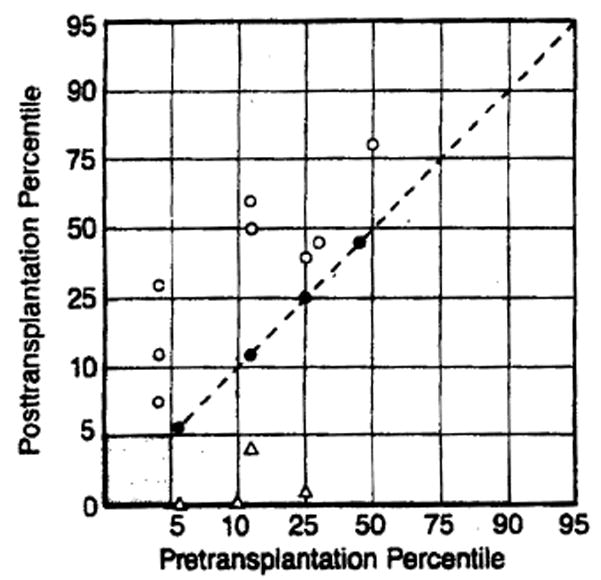
Summary of linear growth data of 29 patients who underwent liver transplantation. In four patients (closed circles), percentiles remained same as before transplantation and are above the fifth percentile. Eight patients (open circles) crossed percentiles in upward direction and are above the fifth percentile. Four patients (open triangles) crossed percentiles in downward direction and dropped below the fifth percentile. Shaded area in lower left corner represents 13 patients whose height before and after transplantation was less than the fifth percentile. Growth velocity curves of these last patients are considered further in Fig 2.
Table 1.
Growth Following Orthotopic Liver Transplantation
| No. (%) of Patients | |
|---|---|
| Total survivors | 29 (100) |
| Crossed percentiles in upward direction and are above the fifth percentile | 8 (28) |
| Normal height prior to transplantation with no percentile change | 4 (14) |
| Less than the fifth percentile before and after transplantation | 13 (45) |
| Crossed percentiles in downward direction falling below the fifth percentile | 4 (14) |
Fig 2.
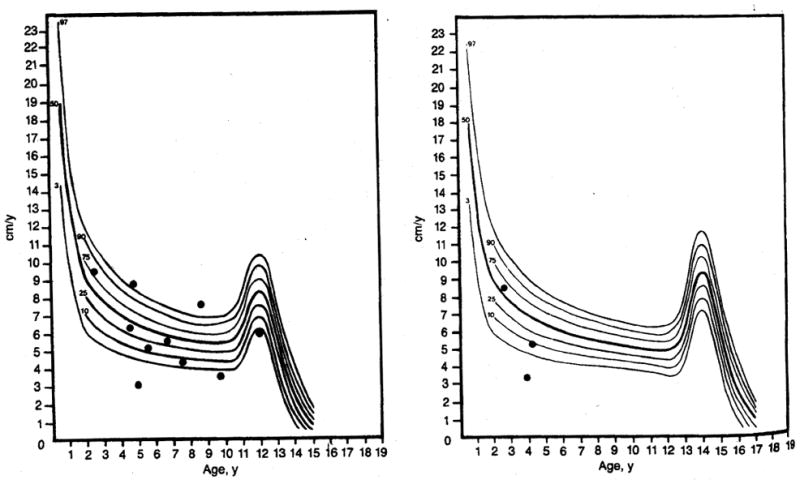
Left, Female growth velocity curve of ten patients whose height before and after transplantation was below the fifth percentile. Note that eight of these patients show normal growth velocity postoperatively. Right, Male growth velocity curve of three patients whose height before and after transplantation was below the fifth percentile. Note that two of these patients show normal growth velocity postoperatively. Curves are based on work of Tanner and Whitehouse as reported by Smith.8
Four patients (14%) were above the fifth percentile preoperatively but dropped to below the fifth percentile following surgery (Table). Of these patients, one child had three transplants and required 10 to 20 mg/d of prednisone for the year following initial transplantation. His growth velocity during the last year has been greater than the 95th percentile, and his linear growth is rapidly approaching the fifth percentile. One patient with hepatolenticular degeneration underwent transplantation at age 13 years; he was more malnourished at the time of transplantation than any other recipient. He weighed 44 kg before onset of the disease but dropped to 22 kg after transplantation. Furthermore, he had chronic renal disease. This patient did not grow for 18 months after liver transplantation, dropping from the 25th to less than the fifth percentile for height. He entered puberty with a brief pubertal growth spurt. A third patient who had neurovisceral storage disease with supranuclear ophthalmoplegia7 developed recurrent disease and was treated with 0.3 mg/kg/d of prednisone; in retrospect, we believe that her elevated levels of liver enzymes were related not to rejection but to recurrent disease. The last patient who experienced a drop in growth percentiles began at the fifth percentile and is now 1.5 cm below the fifth percentile at age 8 years. There is no explanation for his lack of linear growth.
Thirteen (45%) of 29 patients were below the fifth percentile for linear growth prior to transplantation and remained below the fifth percentile following surgery (Fig 1, shaded area). Their growth velocities were plotted on a growth velocity curve to more accurately assess their growth acceleration or deceleration. There were ten female (Fig 2, left) and three male (Fig 2, right) patients. Eight of the ten female patients achieved normal growth velocities or accelerations despite remaining below the fifth percentile (Fig 2, left). One of the two patients with growth failure is 6 years old and has not had any episodes of rejection in two years; however, she is presently receiving 0.3 mg/kg/d of prednisone. The other child with growth failure is 11 years old and has had a recent deceleration in growth velocity. The cause of her growth failure is unknown. Two of the three male patients attained normal growth velocity (Fig 2, right). The male child with growth failure has received two liver transplants and has required extremely high doses of prednisone (0.75 mg/kg/d), as well as frequent steroid pulse therapy, to maintain graft function.
COMMENT
Prior to the use of cyclosporine, the use of transplantation techniques in the pediatric age group was marred by growth retardation and a multitude of other complications attributable to high-dose corticosteroids.9–14 With the use of cyclosporine, high-dose prednisone therapy usually has been unnecessary. Survival of both the patient and graft, as well as quality of life, have been improved with transplantation of kidneys and livers in adults2; similar improvement has been predicted for children.3
Detailed growth studies with the use of cyclosporine and prednisone for immunosuppression have been limited to kidney transplantation recipients. The reports of Conley et al15 and Klare et al16 have been optimistic, whereas Ellis et al17 described poor or absent growth in seven of their eight kidney recipients.
Our study has shown that good growth can be expected in pediatric liver transplant recipients by using cyclosporine with a daily maintenance dose of approximately 0.2 mg/kg of prednisone. Patients experienced a period of six to 12 months of poor growth following transplantation, but growth subsequently improved progressively. In children who did not grow, the failure usually was associated with poor graft function, higher-than-average steroid needs, or multiple operations.
In addition to the abstemious use of steroids, other factors contributed to the good growth of most of these patients: the excellent nutritional status and general well-being that most recipients of liver transplants achieve, the relief of chronic disease state, elimination of the malabsorption associated with liver disease, and amelioration of the metabolic bone disease.
The critical importance of linear growth for the emotional well-being and reintegration of the transplant patient into normal childhood activities has been frequently emphasized.11,12,15 The data reported herein have demonstrated regular achievement of this objective by liver transplantation in children with end-stage liver disease.
Acknowledgments
This investigation was supported by research project grant AM-29961 from the National Institutes of Health, Bethesda, Md.
We are indebted to Susan Gelnett, Melinda Suska, Mary Killian, and Terry Mangan for their secretarial assistance. We thank David N, Fine-gold, MD, for his careful review of this manuscript, and Steven Belle, PhD, for his assistance with statistical analysis.
References
- 1.Burgess DB, Martin HP, Lilly JB. The development status of children undergoing the Kasai procedure for biliary atresia. Pediatrics. 1982;70:624–629. [PubMed] [Google Scholar]
- 2.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1882;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Iwatsuki S, Malatack JJ, et al. Liver and kidney transplantation in children receiving cyclosporine A and steroids. J Pediatr. 1982;100:681–686. doi: 10.1016/s0022-3476(82)80564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malatack JJ, Zitelli BJ, Gartner JC, et al. Pediatric liver transplantation under therapy cyclosporine A and steroids. Transplant Proc. 1983;15:1292–1296. [PMC free article] [PubMed] [Google Scholar]
- 5.Gartner JC, Zitelli BJ, Malatack JJ, et al. Orthotopic liver transplantation in children: Two-year experience with 47 patients. Pediatrics. 1984;74:140–145. [PMC free article] [PubMed] [Google Scholar]
- 6.Sawchuck RJ, Cartier LL. Liquid chromatography determinations of cyclosporine A in blood and plasma. Clin Chem. 1981;27:1368–1371. [PubMed] [Google Scholar]
- 7.Gartner JC, Bergman I, Malatack JJ, et al. Progression of neurovisceral storage disease with supranuclear ophthalmoplegia following orthotopic liver transplantation. Pediatrics. 1986;77:104–106. [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DW. Growth and Its Disorders. Philadelphia: WB Saunders Co; 1977. [Google Scholar]
- 9.Biley CM. Thoughts about kidney homotransplantation in children. J Pediatr. 1964;65:797–800. [Google Scholar]
- 10.MacDougall BRD, Williams B. The indications for orthotopic liver transplantation. Transplant Proc. 1979;11:247–251. [PubMed] [Google Scholar]
- 11.Pennisi AJ, Costin G, Phillips LS, et al. Linear growth in long-term renal allograft recipients. Clin Nephrol. 1977;8:415–421. [PubMed] [Google Scholar]
- 12.Lilly JB, Giles G, Hurwitz B, et al. Renal homotransplantation in pediatric patients. Pediatrics. 1971;47:548–557. [PMC free article] [PubMed] [Google Scholar]
- 13.Fine BN, Malekzadeh MH, Pennisi AJ, et al. Long-term results of renal transplantation in children. Pediatrics. 1978;61:641–650. [PubMed] [Google Scholar]
- 14.Feldhoff C, Goldman AI, Najarian JS, et al. A comparison of alternate day and daily steroid therapy in children following renal transplantation. Int J Pediatr Nephrol. 1984;5:11–14. [PubMed] [Google Scholar]
- 15.Conley SB, Flechner SM, Bose G, et al. Use of cyclosporine in pediatric renal transplant recipients. J Pediatr. 1985;106:45–49. doi: 10.1016/s0022-3476(85)80462-5. [DOI] [PubMed] [Google Scholar]
- 16.Klare B, Walter JV, Hahn H, et al. Cyclosporine in renal transplantation in children. Lancet. 1984;2:692. doi: 10.1016/s0140-6736(84)91243-1. [DOI] [PubMed] [Google Scholar]
- 17.ElMs D, Avner ED, Rosenthal JT, et al. Benal function and somatic growth in pediatric cadaveric renal transplantation with cyclosporine-prednisone immunosuppression. AJDC. 1985;139:1161–1167. doi: 10.1001/archpedi.1985.02140130099040. [DOI] [PubMed] [Google Scholar]


