Abstract
Leptin can exert its potent appetite-suppressing effects via activation of hypothalamic proopiomelanocortin (POMC) neurons. It depolarizes POMC neurons via activation of a yet unidentified nonselective cation current. Therefore, we sought to identify the conductance activated by leptin using whole-cell recording in EGFP-POMC neurons from transgenic mice. The TRPC channel blockers SKF96365 (1-[β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole hydrochloride), flufenamic acid, and 2-APB (2-aminoethyl diphenylborinate) potently inhibited the leptin-induced current. Also, lanthanum (La3+) and intracellular Ca2+ potentiated the effects of leptin. Moreover, the diacylglycerol-permeable analog OAG (2-acetyl-1-oleoyl-sn-glycerol) failed to activate any TRPC current. Using a Cs+-gluconate-based internal solution, the leptin-activated current reversed near −20 mV. After replacement of external Na+ and K+ with Cs+, the reversal shifted to near 0 mV, and the I/V curve exhibited a negative slope conductance at voltages more negative than −40 mV. Based on scRT-PCR, TRPC1 and TRPC4–7 mRNA were expressed in POMC neurons, with TRPC5 being the most prevalent. The leptin-induced current was blocked by the Jak2 inhibitor AG490, the PI3 kinase inhibitor wortmannin, and the phospholipase C inhibitors, U73122 and ET-18-OCH3. Notably, we identified PLCγ1 transcripts in the majority of POMC neurons. Therefore, leptin through a Jak2–PI3 kinase–PLCγ pathway activates TRPC channels, and TRPC1, 4, and 5 appear to be the key channels mediating the depolarizing effects of leptin in POMC neurons.
Introduction
Leptin is derived from adipocytes, and it binds to its long-form receptor (LRb), which is expressed in a number of hypothalamic nuclei, including the arcuate nucleus (Elmquist et al., 1998). Within the arcuate nucleus, LRb has been localized in proopiomelanocortin (POMC) and neuropeptide Y neurons. Genetic mutations in leptin or its receptor are associated with profound metabolic and physiological abnormalities such as obesity and infertility.
A rapid but vital action of leptin in acute suppression of food intake is to depolarize and augment firing in POMC neurons (Cowley et al., 2001; Hill et al., 2008). The leptin-induced depolarization of arcuate POMC and paraventricular nucleus neurons is thought to be via the activation of a nonselective cation channel (NSCC) because the depolarization is associated with a small inward current that reverses at approximately −20 mV (Powis et al., 1998; Cowley et al., 2001). However, no one has identified the NSCC channel that is activated by leptin. A potential candidate was a hyperpolarization-activated cation current (Ih) (Ibrahim et al., 2003). However, in our preliminary experiments, the Ih blocker ZD7288 did not abrogate the effects of leptin. Therefore we focused on the potential role of TRPC channels in mediating leptin's effects.
The mammalian TRPC channel family consists of seven members, TRPC1–TRPC7, that appear to function as receptor-operated channels, analogous to the transient receptor potential (TRP) channels involved in Drosophila phototransduction (Clapham et al., 2005). With the exception of TRPC2, these channels are widely distributed in the mammalian brain (Venkatachalam and Montell, 2007). The TRP channels contain subunits with six membrane-spanning domains that coassemble as tetrameric complexes (Clapham et al., 2005), and they appear to coassemble as homomeric or heteromeric channels consisting of either the TRPC1, TRPC4, and TRPC5 subfamily or the TRPC3, TRPC6, and TRPC7 subfamily (Hofmann et al., 2002).
Therefore in the present study, we investigated the potential role of TRPC channels in mediating leptin's depolarizing effects. Indeed, we have identified a Janus 2 tyrosine kinase (Jak 2)-dependent pathway that activated TRPC1, 4, and 5 channels via stimulation of PI3 kinase and PLCγ1.
Materials and Methods
Animals and treatments.
All animal treatments are in accordance with institutional guidelines based on National Institutes of Health standards, and were performed with Institutional Animal Care and Use Committee approval at the Oregon Health & Science University. Female and male EGFP-POMC transgenic mice were selectively bred in-house, and maintained under controlled temperature (25°C) and photoperiod (12/12 h light/dark cycle) conditions with food and water ad libitum. Adult females were ovariectomized under ketamine/xylazine (1 and 0.1 mg/10 g, respectively) anesthesia and allowed to recover for 1 week.
Electrophysiological solutions/drugs.
Normal aCSF (35°C) and normal pipette solution were used in electrophysiological recording (Ibrahim et al., 2003) (supplemental Methods, available at www.jneurosci.org as supplemental material). When the cationic blocker La3+ was added to the bath, a HEPES-buffered CSF solution was used (Zhang et al., 2008). In some experiments, low-Na+ (5 mm) HEPES-buffered CSF solution and Ca2+-free extracellular CSF solution were used, where N-methyl-d-glucamine (NMDG+) replaced Na+, and Mg2+ replaced Ca2+, respectively. For experiments measuring the ramp current–voltages (I–Vs), K+-gluconate in the normal internal solution was replaced with Cs+-gluconate (pH 7.35 with CsOH), and the extracellular solution contained Na+, K+, Ih (HCN), Ca2+, and GABAA channel blockers (in mm: NaCl, 126; 4-aminopyridine, 5; KCl, 2.5; MgCl2, 1.2; CsCl, 2; CaCl2, 1.4; CoCl2, 1; nifedipine, 0.01; HEPES, 20; NaOH, 8; glucose, 10; tetrodotoxin, 0.001; picrotoxin, 0.1). Ramp I–Vs were also recorded in a solution in which both Na+ and K+ were replaced with same concentration of Cs+ (pH 7.35 with CsOH).
All drugs were purchased from Calbiochem unless otherwise specified. Leptin was provided by Dr. Parlow (Harbor-UCLA Medical Center, Torrance, CA) through the National Hormone and Peptide Program. The Jak2 inhibitor (E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)acrylamide (AG 490), the PLC inhibitor U73122, the less active analog U73343 and the PI3 kinase inhibitor wortmannin (Alomone Laboratories) were dissolved in dimethylsulfoxide (DMSO) as stock solutions. The selective PLCγ inhibitor 1-O-octadecyl-2- O-methyl-rac-glycero-3-phosphorylcholine (ET-18-OCH3) was dissolved in H2O. 1,2-Bis-(o-aminophenoxyethane)-N,N,N′,N′-tetra-acetic acid (BAPTA) tetrasodium salt was dissolved in the internal solution at a 10 mm concentration. The ion channel blockers/ activators used were as follows: 1-[β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole hydrochloride (SKF96365), 2-aminoethyl diphenylborinate (2-APB), flufenamic acid (FFA), and 2-acetyl-1-oleoyl-sn-glycerol (OAG) all were dissolved in DMSO. LaCl3 was dissolved in H2O.
Visualized whole-cell patch recording.
Slices were prepared as described previously (Ibrahim et al., 2003) (supplemental Methods, available at www.jneurosci.org as supplemental material). Standard whole-cell patch recording procedures and pharmacological testing were as previously described (Zhang et al., 2008). To display reversal potential and rectification characteristics of the ligand-activated currents, I–V plots constructed by voltage ramps from +100 to −100 mV were applied at 500 ms or 2 s intervals from a holding potential of −60 mV.
Cell harvesting of dispersed mouse POMC neurons and single-cell RT-PCR.
These procedures were as described previously (Ibrahim et al., 2003; Zhang et al., 2008). The primers were as follows: POMC (200 bp product, accession number NM_008895, forward primer 145–164 nt, reverse primer 326–344 nt); PLCγ1 (111 bp product, accession number NM_021280, forward primer 539–558 nt, reverse primer 630–649 nt); PLCγ2 (237 bp product, accession number NM_172285, forward primer 2905–2925 nt, reverse primer 3121–3141 nt). Primers for all TRPC channel subunits were as published previously (Zhang et al., 2008). Each reaction was amplified for 50 cycles using a Bio-Rad C1000 Thermal Cycler (Bio-Rad). All scRT-PCR products were confirmed by sequencing.
Data analysis.
Comparisons between different drug treatments were performed using a one-way ANOVA analysis with the Newman–Keuls post hoc test. Differences were considered statistically significant if p < 0.05. All data are presented as mean ± SEM.
Results
Leptin activates a Ca2+-dependent nonselective cationic channel
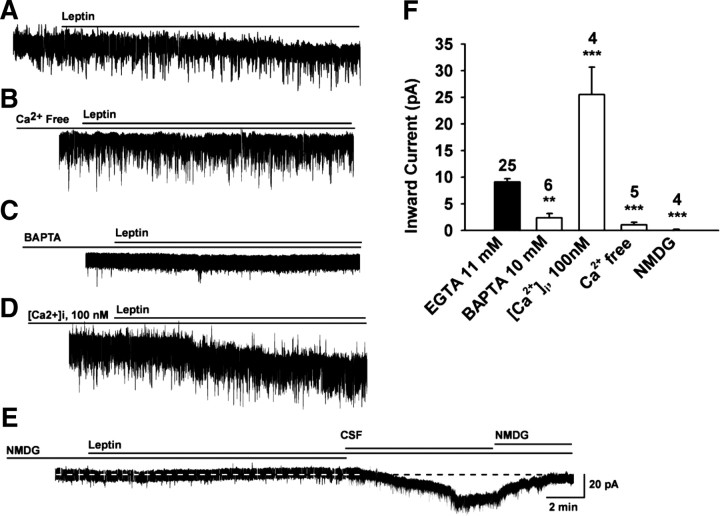
To characterize the ionic mechanism(s) underlying the leptin-induced depolarization, we analyzed the reversal potentials of the leptin-induced currents at a holding potential of −60 mV. A saturating concentration of leptin (100 nm) (Cowley et al., 2001; our preliminary findings) increased the frequency of action potential and induced a depolarization (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) or an inward current with a peak amplitude of 9.1 ± 0.6 pA in 96% of POMC neurons (n = 25, resting membrane potential = −57.3 ± 0.8 mV) (Fig. 1A,F). Replacement of extracellular Ca2+ with Mg2+ attenuated the leptin-induced current (Fig. 1B), reducing the inward current to 1.06 ± 0.49 pA (n = 5, p < 0.001 vs control) (Fig. 1F). An internal solution in which 11 mm EGTA was replaced with 10 mm BAPTA significantly reduced the leptin-induced current (2.4 ± 0.8 pA; n = 6; p < 0.01 vs control) (Fig. 1C,F). After 15 min dialysis with an internal solution containing 0.6 mm Ca2+ and 1 mm EGTA (the intracellular Ca2+ concentration was estimated to be ∼100 nm), the leptin-activated current was increased by 179.8% (25.5 ± 5.1 pA; n = 4; p < 0.001 vs control) (Fig. 1D,F), suggesting that the leptin response was potentiated by the elevation of intracellular Ca2+. Under physiological conditions, the inward current of a NSCC is mainly mediated by extracellular Na+. When the extracellular Na+ concentration was reduced to 5 mm by replacing extracellular Na+ with NMDG+, a large organic cation that does not pass through cationic channels, the leptin-induced inward currents were greatly inhibited (Fig. 1E). The inward current decreased from 9.1 ± 0.6 pA in the control CSF bath to 0.1 ± 0.1 pA (n = 4, p < 0.001 vs control) (Fig. 1F). Therefore, a Na+-dependent NSCC current appears to be the dominant inward current activated by leptin.
Figure 1.
The leptin-induced inward current is mediated by an increase in cationic conductance. A–E, Representative traces of the leptin-induced currents in the presence of a Ca2+-free extracellular solution (B) or an internal solution with 10 mm BAPTA instead of 11 mm EGTA (C) or 100 nm [Ca2+]i (D) or a NMDG solution (E). F, Bar graphs summarizing the effects of BAPTA, Ca2+, and NMDG on the leptin-induced inward currents. Both extracellular Ca2+-free and low-Na+ external solutions attenuated the leptin-induced inward currents, indicative of a cation-selective (e.g., TRPC) channel. Vhold = −60 mV. ***p < 0.001; **p < 0.01, significantly different from the control group (black bar). Cell numbers tested are indicated.
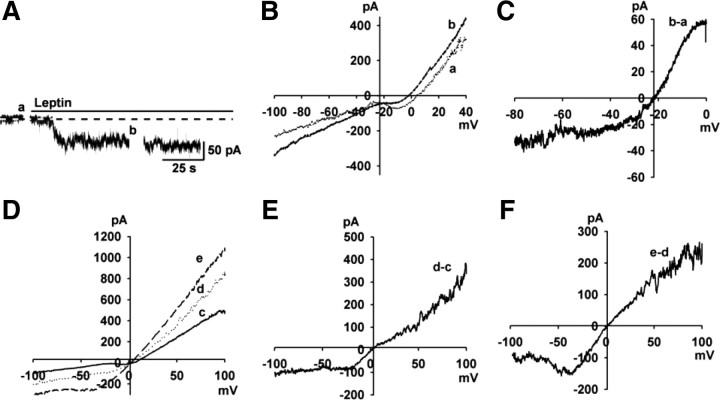
To examine the I–V relationship of the leptin-activated NSCC currents over a wider range of membrane potentials, experiments were conducted using a Cs+-gluconate-based internal solution to block voltage-gated K+ channels. Also, 4-AP (5 mm) and Cs+ (1 mm) were included in a HEPES-buffered CSF to block the A-type K+ and h-currents, respectively. As shown in Figure 2A–C, the leptin-activated NSCC currents reversed near −20 mV (−21.2 ± 1.0 mV, n = 5).
Figure 2.
The leptin-activated inward current and its I–V relationship in POMC neurons. A, Rapid application of 100 nm leptin induced a robust inward current with an internal solution containing 130 mm CsCl. Vhold = −60 mV. B, Voltage ramps from +100 to −100 mV were applied (over 2 s) before (a) and during (b) the treatment with leptin in A. C, The I–V relationship for the leptin-induced current was obtained by digital subtraction in B. D–F, The I–V relationships of NSCC after the exchange of Cs+ for the external Na+ and K+. The I–V relationship was obtained using a voltage ramp from +100 mV to −100 mV for 500 ms. D, Voltage ramps were applied before (c) and after (d) the exchange of Cs+ for the external Na+ and K+, and during treatment with leptin (e). E, The I–V relationship was obtained by subtracting c from d. An inward current was enhanced by Cs+. F, The I–V relationship was obtained by subtracting d from e. Leptin induced an inward current, and the I–V relationship showed a typical doubly rectifying shape.
In heterologous TRPC5 expression systems, Cs+ is a more effective charge carrier (Cs+ > K+ > Na+) (Lee et al., 2003); therefore, we replaced Na+ and K+ with Cs+ in the extracellular solution. As shown in Figure 2D–F, Cs+ increased the baseline conductance (Fig. 2E), suggesting that TRPC channels are constitutively active in POMC neurons. Moreover, the I–V relationship of the leptin-activated currents exhibited a negative slope conductance at voltages around −50 mV (Fig. 2F), similar to I/V relationships for TRPC5 in HEK cells (Strübing et al., 2001).
Pharmacological analysis of TRPC channels in POMC neurons
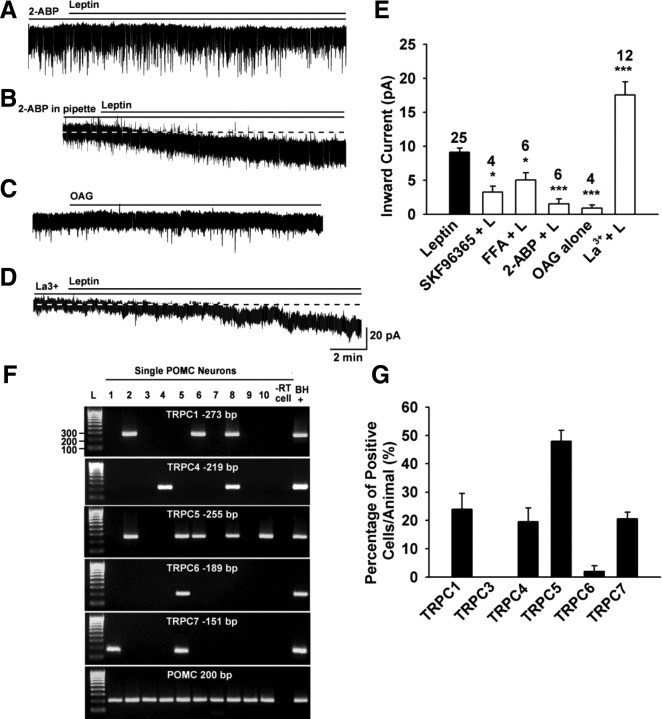
To examine whether the leptin-activated currents were sensitive to TRPC channel blockers, an ensemble of TRPC channel blockers were applied to POMC neurons (Fig. 3A–E). 2-APB is a potent blocker of TRPC3, 4, 5, and 6 (Clapham et al., 2005). Indeed, extracellular 2-APB (100 μm) robustly blocked the leptin-induced currents by 83.4% (1.5 ± 0.7 pA, n = 6 vs control, p < 0.001) (Fig. 3A,E). However, 2-APB is also a blocker of inositol-1,4,5-trisphosphate (IP3) receptors. To determine whether the inhibitory effect of 2-APB was due to a direct blockade of TRPC channels or IP3 receptors, 2-APB (100 μm) was dialyzed into the cells via the patch pipette. Intracellular 2-APB did not significantly affect the amplitude of the leptin-induced inward current (9.2 ± 1.3 pA, n = 4) (Fig. 3B), which indicates that 2-APB acts directly on TRPC channels to block the leptin response.
Figure 3.
TRPC channels are expressed in POMC neurons and activated by leptin. A–D, Representative traces of the leptin-induced currents in the presence or absence of the TRPC channel blocker 2-ABP (100 μm) (A), or 2-ABP (100 μm) in the patch pipette (B) or OAG alone (100 μm) (C) or La3+ (100 μm) (D). E, Summary of the effects of TRPC channel blocker SKF96365 (100 μm), FFA (100 μm), 2-ABP, and La3+ on the leptin-induced inward currents and the effect of OAG on the baseline current. La 3+ (100 μm) augmented the current. Blockers were applied 15 min before the application of leptin (100 nm). Vhold = −60 mV. ***p < 0.001; *p < 0.05, significantly different from the leptin control group. Cell numbers tested are indicated. F, G, TRPC channel transcripts were measured in POMC neurons by scRT-PCR. F, Representative gel images illustrating the mRNA expression of TRPC channel subunits in hypothalamic EGFP-POMC neurons harvested from intact male mice. Ninety-five percent of the harvested EGFP-POMC neurons contained transcripts for POMC. The expected size of PCR products for each TRPC channel subunit is indicated. L is the 100 bp DNA ladder. Negative (−RT) control cell was amplified from a harvested POMC neuron without RT. Positive (BH+) control was amplified using cDNA from mouse basal hypothalamic tissue. G, Bar graph represents the mean ± SEM of the percentage of POMC neurons expressing each TRPC subunit mRNA in 70 cells from five mice. An average of 14 cells were analyzed from each animal.
In addition, SKF96365 blocks TRPC3, TRPC5, TRPC6, and TRPC7 channels (Clapham et al., 2005). When cells were pretreated with 100 μm SKF, the leptin-induced inward current was inhibited by 66.8% (3.0 ± 0.9 pA, n = 4, p < 0.05 vs control) (Fig. 3E). FFA (100 μm), which is another potent blocker of TRPC3, 5, and 7 (Clapham et al., 2005) attenuated the leptin-induced inward current by 44.8% (5.0 ± 2.0 pA, n = 6, p < 0.05 vs control) (Fig. 3E).
In heterologous expression systems, micromolar concentrations of La3+ potentiate TRPC4 and TRPC5 activity (Schaefer et al., 2000; Strübing et al., 2001), but inhibit TRPC1, 3, 6, and 7 channels (Clapham et al., 2005). Indeed, we found that 100 μm La3+ greatly potentiated the leptin-induced current by 92.4% (17.5 ± 1.9 pA, n = 12, p < 0.001 vs control) (Fig. 3D,E).
PLC hydrolyzes PIP2 to yield diacylglycerol (DAG) and IP3, which in turn induces Ca2+ release from the endoplasmic reticulum. Since IP3 receptor antagonism with 2-ABP, did not attenuate the actions of leptin, we examined the effects of DAG. TRPC3, 6, and 7 can be directly activated by OAG, a permeable analog of DAG (Hofmann et al., 1999), but when combined with TRPC1 and TRPC4 or 5, they are at best weakly activated (Strübing et al., 2003). We found that the OAG (100 μm) had no effect on TRPC channel activity (0.9 ± 0.4 pA, n = 4, p < 0.001 vs control) (Fig. 3C,E), indicating that DAG is not the final messenger for leptin signaling in POMC neurons. It also indicates that homomers or heteromers within the TRPC1, 4, and 5 subfamily are probably formed and activated in POMC neurons. Collectively, the pharmacological data would indicate that mainly TRPC4 and 5 channels are involved in the leptin-mediated depolarization of POMC neurons.
TRPC channel transcripts are expressed in POMC neurons
Based on the pharmacological profile of the leptin-evoked inward currents, we predicted that TRPC1, 4, and 5 would be expressed in POMC neurons. Thus, we performed scRT-PCR experiments to examine the expression of TRPC mRNA transcripts in POMC neurons. Five of the six TRPC channel subunits found in the brain (TRPC1 and TRPC4–7) were expressed in POMC neurons, whereas the TRPC3 subunit was not detected (Fig. 3G). TRPC5 was the most prevalent subunit and was expressed in 48 ± 4% of POMC neurons (Fig. 3F,G). Moreover, TRPC1, 4, and 7 were detected in 24 ± 6%, 20 ± 5%, and 21 ± 2% of the neurons, respectively (Fig. 3G). An insignificant number of POMC neurons expressed TRPC6. All negative controls, including cells (n = 5) and tissue RNA processed without RT (reverse transcriptase), were negative following RT-PCR. Therefore, TRPC1, 4, and 5 appear to be the predominant subfamily expressed in POMC neurons.
Leptin receptor-mediated signaling pathway
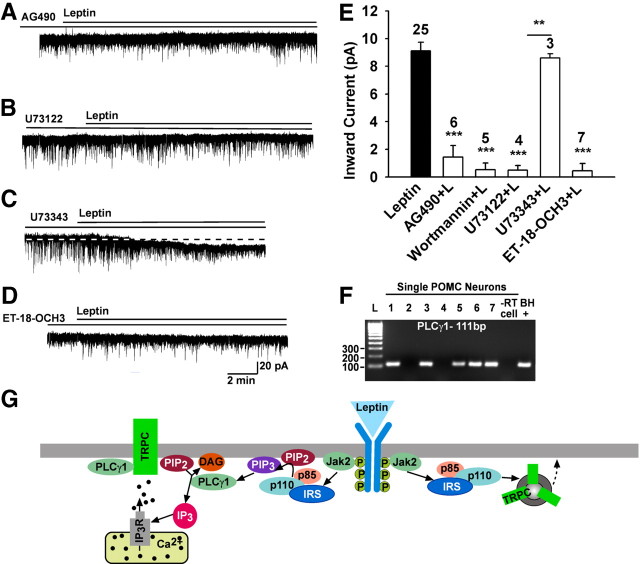
We next explored if the leptin receptor couples to the Jak2–PI3 kinase signaling pathway to affect TRPC channels. Indeed, the Jak inhibitor AG490 (10 μm) potently blocked the effects of leptin (1.4 ± 0.8 pA, n = 6, p < 0.001 vs control) (Fig. 4A,E). PI3 kinase is essential for leptin-induced activation (Hill et al., 2008) but it is also critical for the membrane insertion of TRPC channels (Bezzerides et al., 2004). Therefore, we examined the role of PI3 kinase in the leptin-induced inward current. The selective PI3 kinase inhibitor wortmannin (100 nm) blocked the leptin-induced inward current by 94.2% (0.5 ± 0.5 pA, n = 5, p < 0.001 vs control) (Fig. 4E). When cells were pretreated with the PLC inhibitor U73122 (10 μm), the leptin-induced current was inhibited by 94.5% (0.5 ± 0.3 pA, n = 4, p < 0.001 vs control) (Fig. 4B,E), but the inactive analog U73343 (10 μm) had no effect (8.6 ± 0.3 pA, n = 3, p < 0.01 vs U73122 group) (Fig. 4C,E). This indicates that the LRb couples to Jak2 to activate PLC. Since leptin activates PLCγ2 in human platelets (Dellas et al., 2007), we perfused the selective PLCγ inhibitor ET-18-OCH3 (15 μm) and found that it potently blocked the effects of leptin (0.5 ± 0.5 pA, n = 7, p < 0.001 vs control) (Fig. 4D,E). Finally, to determine whether the LRb is coupled through PLCγ1 or PLCγ2, we used scRT-PCR to identify the expression of these transcripts in POMC neurons (Fig. 4F). We found that the PLCγ1 was expressed in ∼70% of POMC neurons. In contrast, PLCγ2 mRNA was only detected in 1 of 30 neurons. Based on these results, it appears that leptin's coupling to TRPC channels in POMC neurons is through LRb–Jak2–PI3 kinase and PLCγ1 signaling pathways (Fig. 4G).
Figure 4.
The leptin response requires Jak2, PI3 kinase, and PLCγ activation. A–D, Representative traces of the leptin-induced currents in the presence or absence of kinase inhibitors. E, Summary of the effects of the Jak2 inhibitor AG490 (10 μm), PI3 kinase inhibitor wortmannin (100 nm), PLC inhibitor U73122 (20 μm), and its inactive analog U73343 (20 μm), and the PLCγ inhibitor ET-18-OCH3 (15 μm) on the leptin-induced inward current. Blockers were applied for 15 min before the application of leptin (100 nm). Vhold = −60 mV. **p < 0.01, U73122 versus U73343 group; ***p < 0.001, significantly different from the leptin control group. Cell numbers tested are indicated. F, Representative gel illustrating PLCγ1 mRNA expression in POMC neurons. −RT cell and BH+, processed without and with RT. G, A cellular model of leptin's signaling and TRPC channel activation in the POMC neurons. Based on our findings and other published data, we propose that leptin binds to its receptor (LRb) to activate Jak2, which phosphorylates IRS proteins and in turn activates PI3 kinase. PI3 kinase subsequently activates PLCγ1 to augment TRPC channel activity. PI3 kinase also stimulates rapid incorporation of functional TRPC channels into the plasma membrane. All of these signaling events enhance POMC neuronal excitability.
Discussion
We have shown for the first time that leptin potently depolarizes hypothalamic POMC neurons by activating a TRPC conductance. We have concluded this based on several lines of evidence. First, the I–V relationship for the leptin-induced current exhibited a negative slope conductance, outward rectification, and a reversal at ∼0 mV (in Cs+). Second, the leptin current was significantly attenuated by the TRPC channel blockers 2-APB, FFA, and SKF96365. Moreover, the inward current was potentiated over twofold by Ca2+ and La3+, a characteristic of TRPC4 and 5 channels. Third, the leptin-induced inward current was for the most part abolished in low extracellular Na+. Finally, we found that individual POMC neurons express TRPC channel transcripts with TRPC5 being the most prevalent. Therefore, the cumulative evidence supports the conclusion that leptin excites POMC neurons predominantly through the activation of TRPC channels.
An initial indication that the leptin-induced current is mediated by TRPC channels was the characteristic I–V relationship in the presence of a K+ channel blocker (i.e., Cs+). The I–V relationship resembled the I–V relationship of heteromeric complexes of TRPC1 + 4 or TRPC1 + 5 subunits expressed in HEK cells with a negative slope conductance and pronounced outward rectification (Strübing et al., 2001; Clapham, 2003). Similar I–V relationships have been obtained with the kisspeptin-activated currents in gonadotropin-releasing hormone (GnRH) neurons as well as mGluR1- and CCK2-induced currents in basolateral amygdala neurons, which all express a similar complement of TRPC channels as POMC neurons (Meis et al., 2007; Zhang et al., 2008).
The mammalian TRPC channels can be activated by G-protein-coupled receptors and receptor tyrosine kinases (Clapham et al., 2005). In fact, TRPC channels are probably one of the major targets for mGluR1 signaling in the CNS (Tozzi et al., 2003). Interestingly, TRPC1 and 5 are highly expressed in substantia nigra dopamine neurons, and the mGluR1 agonist dihydroxyphenylglycine-induced current exhibits an “S” shape I–V plot (Tozzi et al., 2003), similar to what we see in POMC neurons. In addition, the peptides cholecystokinin via the CCK2 receptor and kisspeptin via the GPR54 receptor also activate TRPC1, 4, and 5 channels in amygdala (Meis et al., 2007) and preoptic GnRH (Zhang et al., 2008) neurons, respectively. The mGluR1, CCK2, and GPR54 receptors are Gq-coupled to PLC activation, and we did find that the PLC inhibitor U73122 inhibited the effects of leptin in POMC neurons. However, OAG had no effect, which fits with our findings that the DAG-sensitive TRPC3, 6, and 7 channels (Clapham et al., 2005) are not highly expressed in POMC neurons. Importantly, La3+ (100 μm) and Ca2+ greatly potentiated the leptin-induced current, which is a hallmark of TRPC4 and 5 activation (Clapham et al., 2005; Blair et al., 2009). In addition, 2-APB and FFA attenuated the effects of leptin in POMC neurons. These blockers have a similar efficacy for the mGluR1 (Tozzi et al., 2003), CCK2 (Meis et al., 2007), and GPR54 (Zhang et al., 2008) activation of TRPC channels in neurons. Therefore, TRPC5 and to a lesser extent TRPC4 appear to be the key players in mediating the effects of leptin in POMC neurons based on the I–V relationship, the pharmacological profile, and the subunit composition.
From a physiological perspective, the pleiotropic effects of leptin in POMC neurons are the most critical for both the short-term and long-term modulation of POMC neuronal activity and the control of energy homeostasis. As has been demonstrated earlier (Hill et al., 2008), we found that the leptin-mediated excitation (inward current) is abrogated by inhibition of PI3 kinase activity, suggesting that the leptin-induced increase in POMC excitability is mediated via PI3 kinase. LRb is a member of the class I cytokine receptor family and signals through activation of Jak2 that mediates leptin signaling via several pathways, including the STAT3, MAPK, AMPK, and mTOR pathways. These pathways act coordinately to form a circuit that fully mediates the leptin response (Morris and Rui, 2009). We found the Jak2 inhibitor AG490 completely blocked the effects of leptin to activate TRPC channels. In addition, leptin via LRb signals through the insulin receptor substrate (IRS)–PI3 kinase pathway (Morton et al., 2005) to increase the activity of PI3 kinase and its association with IRS-2 in the hypothalamus (Niswender et al., 2001; Zhao et al., 2002).
In primary hippocampal neuronal cultures, activation of PI3 kinase increases the rapid membrane insertion of TRPC5 channels from an intracellular vesicular pool (Bezzerides et al., 2004). In addition, activation of PI3 kinase generates phosphatidylinositol-3,4,5-triphosphate (PIP3), which appears to contribute to the translocation and activation of PLCγ at the plasma membrane (Bae et al., 1998; Falasca et al., 1998; Rameh et al., 1998). Through its phospholipase catalytic domains, PLCγ hydrolyzes PIP2 to generate two second messengers, DAG and IP3. Also, PLCγ has enzyme-independent signaling properties acting through its other protein–protein interacting domains (Patterson et al., 2005). In fact, PLCγ1 has a critical role in leptin signaling in POMC neurons, because the selective PLCγ inhibitor Et-18-OCH3 completely blocked leptin's effects. However, all of the steps leading to channel opening following PLC activation are not fully understood (Schaefer et al., 2000), and the importance of PLCγ activation in the overall leptin signaling needs to be determined.
Based on the current findings, we conclude that leptin binds to LRb to activate Jak2, which phosphorylates IRS proteins and in turn activates PI3 kinase; PI3 kinase subsequently activates PLCγ1 to augment TRPC currents and POMC neuronal excitability (Fig. 4G). Since it is evident that leptin is essential for normal regulation of energy homeostasis, these signaling pathways are potential targets for the critical uncoupling events leading to leptin resistance in obesity and type II diabetes.
Footnotes
This work was supported by United States Public Health Service Grants NS 38809, NS 43330, and DK 68098. We thank Elizabeth A. Rick and Dr. Taiping Jia for their expert technical contribution.
References
- Bae YS, Cantley LG, Chen C-S, Kim S-R, Kwon K-S, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009;133:525–546. doi: 10.1085/jgp.200810153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International union of pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Dellas C, Schäfer K, Rohm IK, Lankeit M, Leifheit M, Loskutoff DJ, Hasenfuss G, Konstantinides SV. Leptin signalling and leptin-mediated activation of human platelets: importance of JAK2 and the phospholipases Cγ2 and A2. Thromb Haemost. 2007;98:1063–1071. [PubMed] [Google Scholar]
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose-responsive and express K-ATP channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- Meis S, Munsch T, Sosulina L, Pape H-C. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci. 2007;35:356–367. doi: 10.1016/j.mcn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling: key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Nikolaidis N, Gill DL, Snyder SH. Phospholipase C-γ: diverse roles in receptor-mediated calcium signaling. Trends Biochem Sci. 2005;30:688–697. doi: 10.1016/j.tibs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Powis JE, Bains JS, Ferguson AV. Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol. 1998;274:R1468–R1472. doi: 10.1152/ajpregu.1998.274.5.R1468. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cγ-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-meidated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGLuR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes GnRH neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3kinase-phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]