SUMMARY
Virus entry into cells is typically initiated by binding of virally encoded envelope proteins to specific cell surface receptors. Studying infectivity of lentivirus pseudotypes lacking envelope binding, we still observed high infectivity for some cell types. On further investigation, we discovered that this infectivity is conferred by the soluble bovine protein S in fetal calf serum, or Gas6, its human homologue. Gas6 enhances native infectivity of pseudotypes of multiple viral envelope proteins. Gas6 mediates binding of the virus to target cells by bridging virion envelope phosphatidylserine to Axl, a TAM receptor tyrosine kinase on target cells. Phagocytic clearance of apoptotic cells is known to involve bridging by Gas6. Replication of vaccinia virus, which was previously reported to use apoptotic mimicry to enter cells, is also enhanced by Gas6. These results reveal an alternative molecular mechanism of viral entry that can broaden host range and enhance infectivity of enveloped viruses.
INTRODUCTION
Binding of virus to target cells, the first step of viral infection, is usually mediated by viral proteins that can bind directly to receptors expressed on target cells (Norkin, 2010). The affinity and specificity of receptor-binding viral proteins can determine the efficiency of replication in target cells and the tropism of the virus. Abundant expression of high-affinity receptor-binding molecules on the surface of viruses is an ideal condition for viral replication.
Viruses use several strategies to facilitate efficient binding to target cells. One means is a single viral protein binding to multiple receptors. For example, the HIV envelope protein, gp120, binds to its primary receptor CD4 (Dalgleish et al., 1984) but also C-types lectins, including DC-SIGN (Geijtenbeek et al., 2000) to facilitate gp120-CD4 interaction, resulting in increased HIV replication in target cells expressing both CD4 and DC-SIGN (Lee et al., 2001). Another approach is through use of multiple viral proteins for binding. For example, the IMV (intracellular mature virus) form of vaccinia virus contains at least three viral envelope proteins that bind to the viral receptor, glycosaminoglycans (Smith et al., 2002).
However, viruses can mediate efficient binding to target cells, despite low affinity and density of receptor-binding viral proteins. One means is by utilizing soluble proteins present in bodily fluids to bridge the virus to target cells. For example, amyloid fibrils in semen can enhance HIV replication more than 10-fold by bridging virus to target cells in an envelope protein-independent manner (Munch et al., 2007). Infection of one type of non-envelope virus, adenovirus, can also be enhanced by soluble factors in serum (Shayakhmetov et al., 2005; Waddington et al., 2008). Enhancement of adenoviral transduction by soluble factors was observed during development of targeting vectors that can specifically transduce selected cell types. Binding of adenovirus is mediated by viral penton proteins. Previous attempts to redirect adenovirus vectors via ablation of receptor-binding regions of penton proteins were unable to eliminate their liver tropism. It was recently found that binding of adenovirus vectors to the liver is mediated by factors IX and X, which are serum proteins in the coagulation system, bridging adenovirus to target cells. We developed targeting retroviral vectors, including both γ-retroviral and lentiviral vector types. Binding of a retrovirus is usually mediated by its envelope proteins, but modification of receptor-binding regions of retroviral envelope proteins often results in loss of fusion activity (Zhao et al., 1999). Unlike retroviral envelope proteins, the receptor-binding regions of the Sindbis virus envelope proteins can be modified without losing fusion activity (Ohno et al., 1997). We pseudotyped retroviral vectors with modified Sindbis virus envelope proteins and successfully redirected their tropisms in vitro and in vivo (Morizono et al., 2001; Morizono et al., 2005). Although we eliminated the original tropism of the Sindbis virus envelope protein by mutating its known receptor-binding regions, we still observed significant residual infectivity for certain cell types. Because complete elimination of non-targeted transduction is necessary to minimize adverse effects of lentiviral transduction, we attempted to eliminate the residual infectivity of our targeting vectors. While studying the mechanism of this residual infectivity, we found that a soluble protein, Gas6, enhances binding of envelope viruses by binding to one type of lipid, phosphatidylserine (PtdSer), on virions and the TAM receptors on the cell surface.
RESULTS
Fetal calf serum (FCS) increases transduction efficiencies of lentiviral vectors pseudotyped with various types of envelope proteins
For several years, we have developed pseudotyped lentiviral vectors that can specifically transduce certain target cells due to modification of Sindbis virus envelope pseudotypes , whereby the original receptor-binding regions were ablated. New target tropisms were then conferred to the envelope protein by conjugation with targeting ligands directed to cell surface molecules. During vector development, we noticed that despite ablation of native receptor-binding activity, residual infectivity persisted, typically less than 3%, but as high as 50–70% of wildtype activity in some experimental settings. These observations suggested an unknown interaction between the virus and target cells that contributed to binding and infectivity. We investigated this infectivity further by identifying its source, characterizing its properties, and ultimately determining its molecular identity.
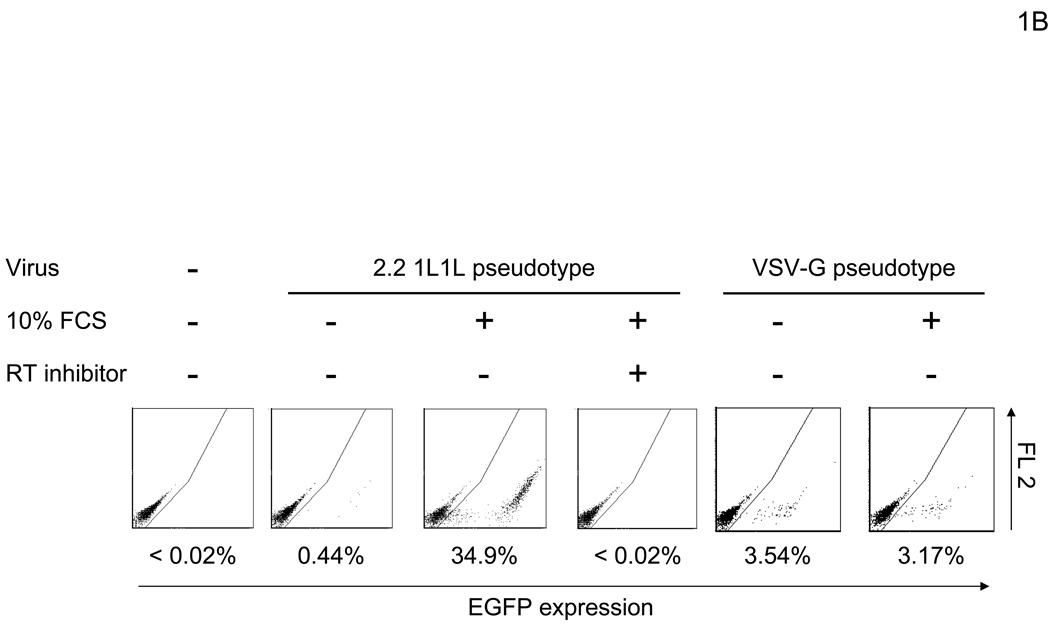
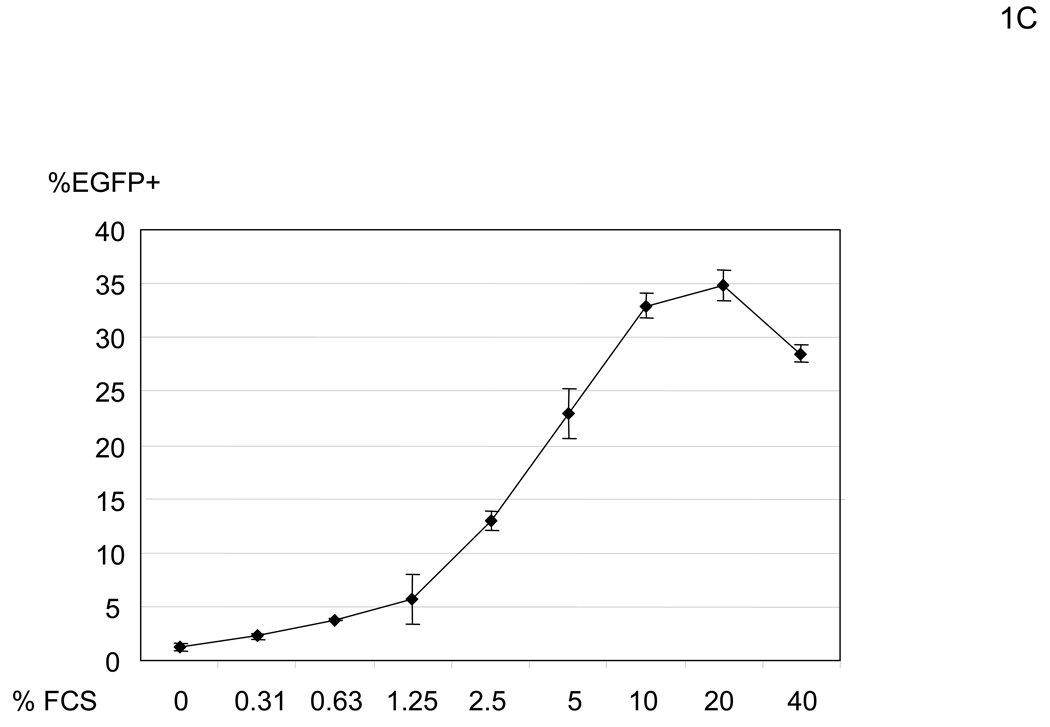
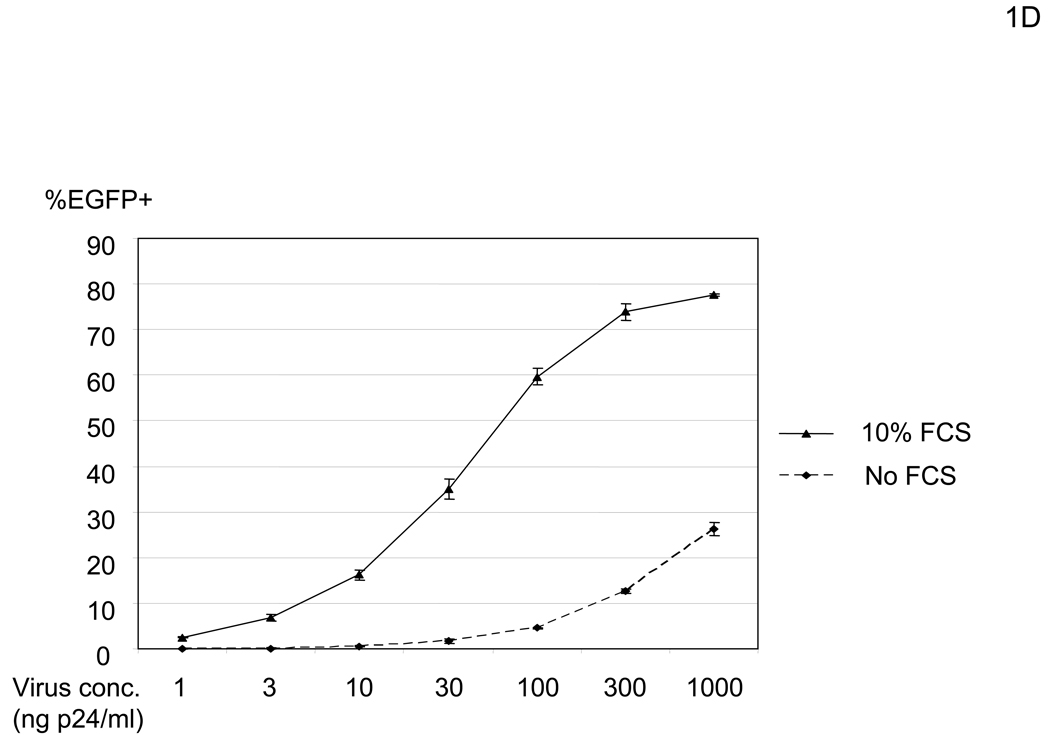
In our initial experimental system, we utilized human microvascular endothelial cells (HMVEC) (Shao and Guo, 2004), since they demonstrate a high level of infectivity even when the native receptor-binding activity is ablated. The titer of the lenvitial vector (containing 1000ng HIV p24/ml) pseudotyped with the vesicular stomatitis G protein (VSV-G), which is commonly used due to its high transduction efficiency, is typically 1×106 EGFP TU (transduction unit)/ml on HMVEC in the presence of 10% FCS. Under the same conditions, the titer of the vector pseudotyped with the wild-type Sindbis virus envelope protein is 4×105 EGFP TU/ml, while the titer of lentiviral vectors pseudotyped with mutant Sindbis virus envelope proteins, which should have minimal receptor binding, is as high as 2×105 EGFP TU/ml. We first determined that the origin of the factor(s) responsible for the residual infectivity was present in FCS. HMVEC were minimally transduced with the vector (2.2 1L1L pseudotype) when cells are preincubated with PBS containing CaCl2, MgCl2, and BSA [PBS (+)] (Figure 1A and B). However, we found that transduction drastically increased when the cells were preincubated with higher concentrations of FCS (Figure 1B). This enhanced transduction was blocked by a reverse transcriptase inhibitor (Nevirapine), demonstrating that EGFP expression is not caused by pseudotransduction mediated via contaminated EGFP proteins in the virus. Transduction by VSV-G pseudotype was not significantly enhanced (Figure 1B), suggesting that enhancement occurred during entry of 2.2 1L1L pseudotype. This enhancement of the 2.2 1L1L pseudotype is concentration-dependent and saturated at 10% FCS (Figure 1C). Enhancement was observed regardless of transducing virus amount, although the extent of enhancement was more obvious with lower virus amounts (Figure 1D). Enhanced viral transduction was observed with five different FCS lots.
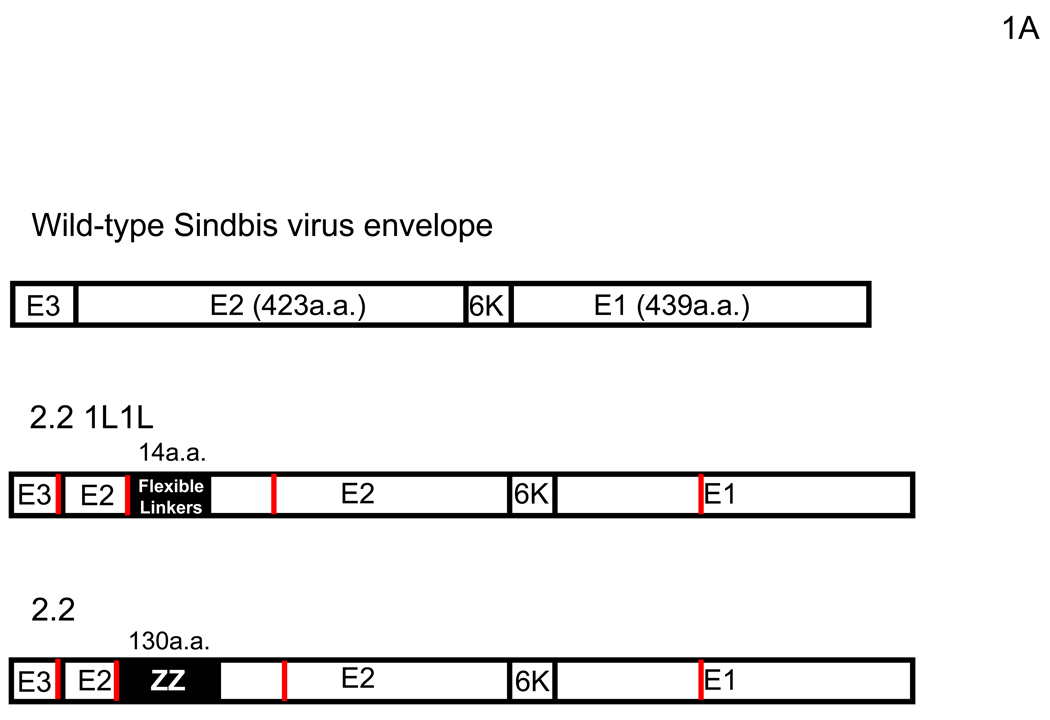
Figure 1.
FCS enhances transduction with lentiviral vectors pseudotyped with various types of envelope proteins. (A) Schematic representation of chimeric Sindbis virus envelope proteins. The Sindbis virus envelope protein is first synthesized as a polypeptide and subsequently cleaved by cellular proteases to generate the E3, E2, 6K, and E1 proteins. E1 and E2 are incorporated into the viral envelope. Both 2.2 and 2.2 1L1L were derived from the wild-type Sindbis virus envelope protein with mutations (shown as red lines) to reduce their binding to original receptors. In addition, 2.2 1L1L has two flexible linkers and 2.2 contains the ZZ domain at the E2 amino acid 70. (B) For all transduction and infection experiments, 1×104 HMVEC/well were seeded into 48-well plates 2 days before infection or transduction. Total infection/transduction volume was 150 µl. HMVEC were incubated with PBS (+) or 10% FCS in PBS (+), followed by transduction with 2.2 1L1L (100 ng p24/ml). Nevirapine (5 µM) was used 30 minutes before, during, and after transduction. VSV-G-pseudotype (10 ng p24/ml) was also used instead of the 2.2 1L1L pseudotype. (C) HMVEC were incubated with different concentrations of FCS in PBS (+) and transduced with the 2.2 1L1L pseudotype (100 ng p24/ml). (D) The enhancing effects of 10% FCS on HMVEC transduction were investigated, using various concentrations of 2.2 1L1L pseudotype. In this manuscript, all error bars and the values after ± represent standard deviations of triplicated experiments.
An FCS factor(s) enhances transduction of vectors pseudotyped with multiple types of envelope proteins
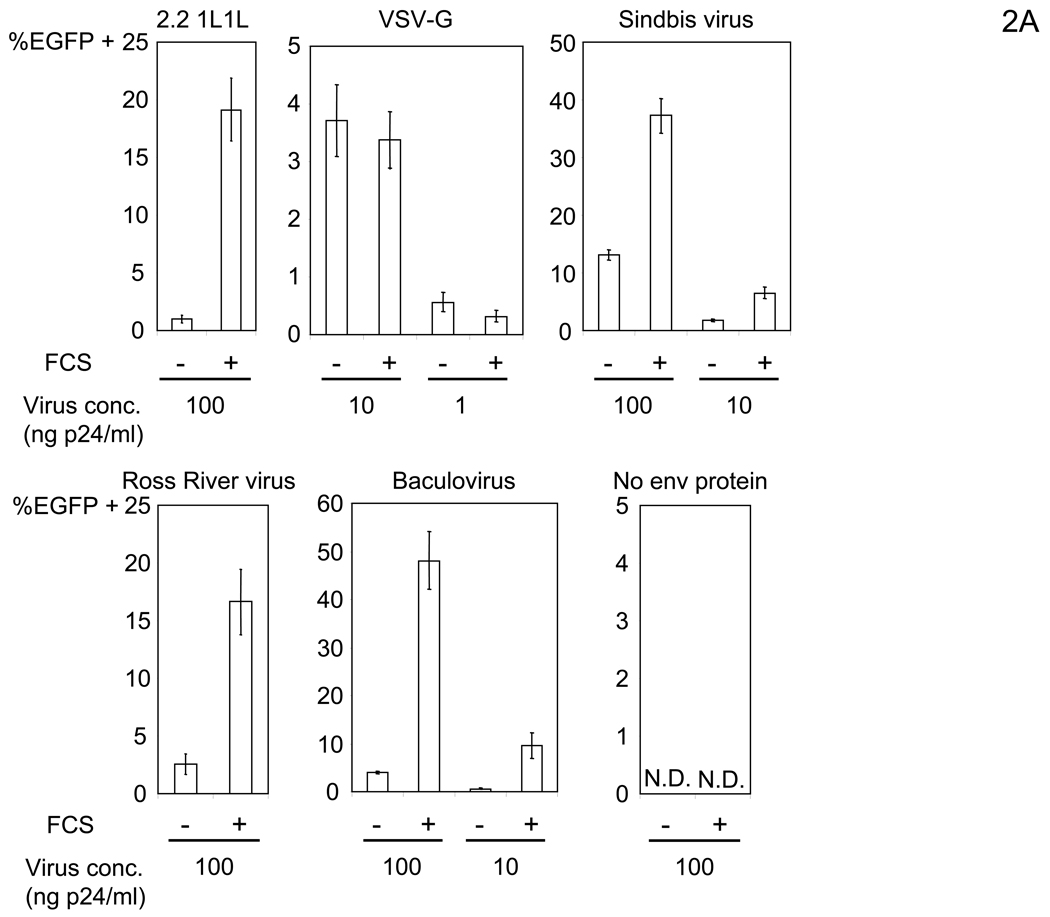
We initially assumed that enhancement of transduction by FCS is specific to viruses containing the envelope proteins derived from Sindbis virus. To test this, we determined the effect on lentiviral vectors pseudotyped with other viral envelope proteins including wild-type Sindbis virus, Ross River virus which belongs to the same alphaviridae as Sindbis virus, and baculovirus which belongs to baculoviridae. Surprisingly, the vectors pseudotyped with the envelope proteins of these viruses were also enhanced (Figure 2A). Thus, the enhancement is not specific to particular types of envelope proteins. Of note, transduction with virus lacking viral envelope protein did not occur, regardless of incubation with FCS (Figure 2A), indicating that the presence of viral envelope proteins is required for enhancement.
Figure 2.
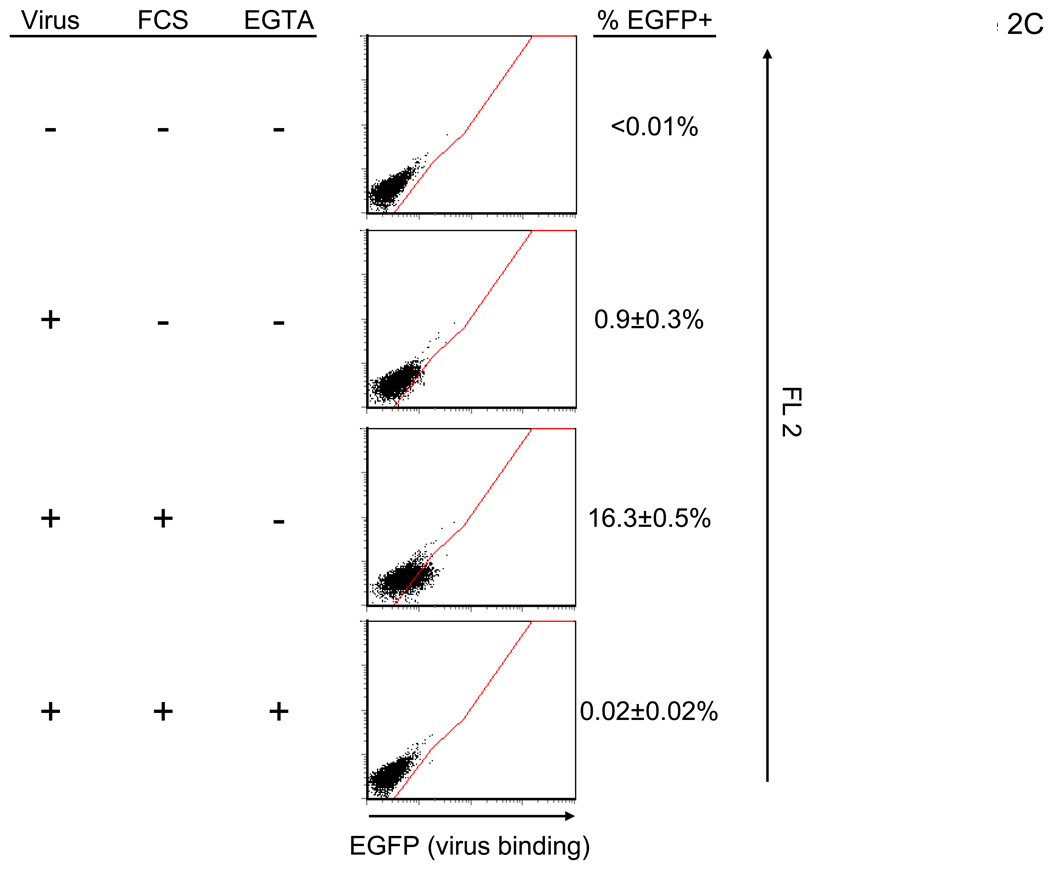
FCS bridges virus to cells in an envelope-protein independent manner. (A) HMVEC were incubated with PBS (+) or 10% FCS in PBS (+) then transduced with various pseudotypes. vEnv(−) was also used for transduction. The concentrations of viruses used for transduction are shown in the figure. N.D.: Not Detected. (B) Left: HMVEC were incubated with 10% FCS in PBS (+) or PBS (EGTA) followed by transduction with the 2.2 pseudotype (100 ng p24/ml) in PBS (+) or PBS (EGTA). Right: HMVEC were transduced with the 2.2 pseudotype (100 ng p24/ml) conjugated with αHLA Ab (2 µg/ml) in PBS (+) or PBS (EGTA). (C) HMVEC were incubated with PBS (+), 10% FCS in PBS (+), or 10% FCS in PBS (EGTA) for 4 hours. The cells were incubated with the 2.2 1L1L pseudotype labeled by EGFP (100 ng p24/ml) for 2 hours. (A–C). (See also Figure S1)
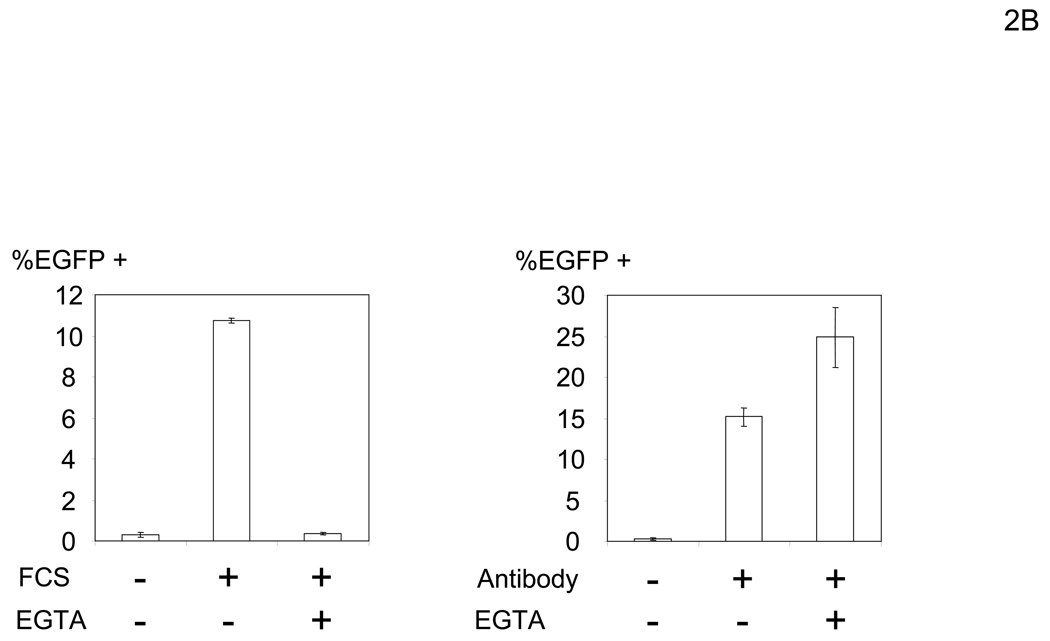
Enhancement of viral transduction by a factor(s) in FCS requires divalent cations
Since it appeared that there was a factor(s) in FCS that can enhance transduction, we elucidated the properties of this factor in preparation for subsequent purification. Initially we investigated whether this enhancement requires Ca2+ and/or Mg2+ which are important for the functions of many proteins. To do so, we used the modified Sindbis virus envelope protein containing the Fc-binding region of protein A (ZZ), designated 2.2 (Figure 1A). We found that FCS-mediated enhancement of transduction with the 2.2 pseudotype is inhibited by EGTA. We previously demonstrated that the tropism of 2.2 pseudotypes can be redirected by conjugation of the ZZ domain with antibodies recognizing chosen cell surface molecules (Pariente et al., 2007). One receptor, HLA Class I expressed ubiquitously on human cells, is efficiently recognized by an anti-HLA Class I antibody (αHLA Ab) and allows binding and transduction of cells (Morizono et al., 2001). A control transduction mediated by HLA Class I on the cell surface was not inhibited by EGTA (Figure 2B). These results demonstrated that binding of the factor to cells and/or virus is Ca2+- and/or Mg2+-dependent.
We determined the step of infection enhanced by the FCS factor(s) by first examining the effect of the factor(s) on viral binding to cells. To quantitate binding of virus, we labeled the 2.2 1L1L pseudotype with EGFP and assessed binding of the labeled virus to cells (Figure 2C). Binding of virus was significantly enhanced when HMVEC were incubated with FCS. FCS-mediated enhancement of viral binding was inhibited by EGTA. These results demonstrated that FCS increases binding of virus to cells in a Ca2+- and/or Mg2+-dependent manner.
An FCS factor(s) enhance viral transduction by bridging virus to cells
There are two possible mechanisms of enhancement of viral binding by FCS: 1) a factor(s) in FCS up-regulates novel binding receptors for virus on the cells and 2) FCS contains a factor(s) that can bridge the virus to cells. To investigate the first hypothesis, before transduction with the 2.2 1L1L pseudotype, we incubated the cells with FCS, either using cycloheximide, or at 4°C instead of 37°C toblock de novo synthesis of proteins, but neither decreased enhancement by FCS (data not shown), suggesting that up-regulation of a receptor is unlikely to be the mechanism for enhanced transduction.
To investigate the possibility that FCS contains a factor that bridges the virus to cells, we first incubated virus with FCS, then purified the virus by ultracentrifugation, and transduced HMVEC with these treated viruses (Figure S1A). The virus incubated with FCS showed increased transduction efficiency. When virus was incubated with FCS in the presence of EGTA, enhancement of transduction was inhibited. These results indicate that FCS enhances viral transduction, most likely through a factor(s) that bridges the virus to HMVEC in a Ca2+- and/or Mg2+-dependent manner.
In Figure 2A, we showed that transduction with the viruses pseudotyped with various envelope proteins was enhanced by FCS, but not by virus lacking viral envelope proteins; therefore, we hypothesized that the factor(s) may bind to viruses independent of viral envelope proteins but still requires viral envelope proteins for subsequent fusion steps. To determine whether the factor(s) binds to virus independently of viral envelope proteins, we used virus lacking viral envelope proteins [vEnv(−)] to compete against the 2.2 1L1L pseudotype for binding by the bridging factor(s) on HMVEC (Figure S1B). In the presence of vEnv(−), enhancement of 2.2 1L1L pseudotype transduction by FCS was inhibited. As a control, transduction of HMVEC (without preincubation with FCS) with the 2.2 pseudotype conjugated with αHLA Ab was not affected by vEnv(−). These results suggest that the bridging factor(s) binds to the virus independent of viral envelope proteins, but dependent on an unknown virion associated factor(s).
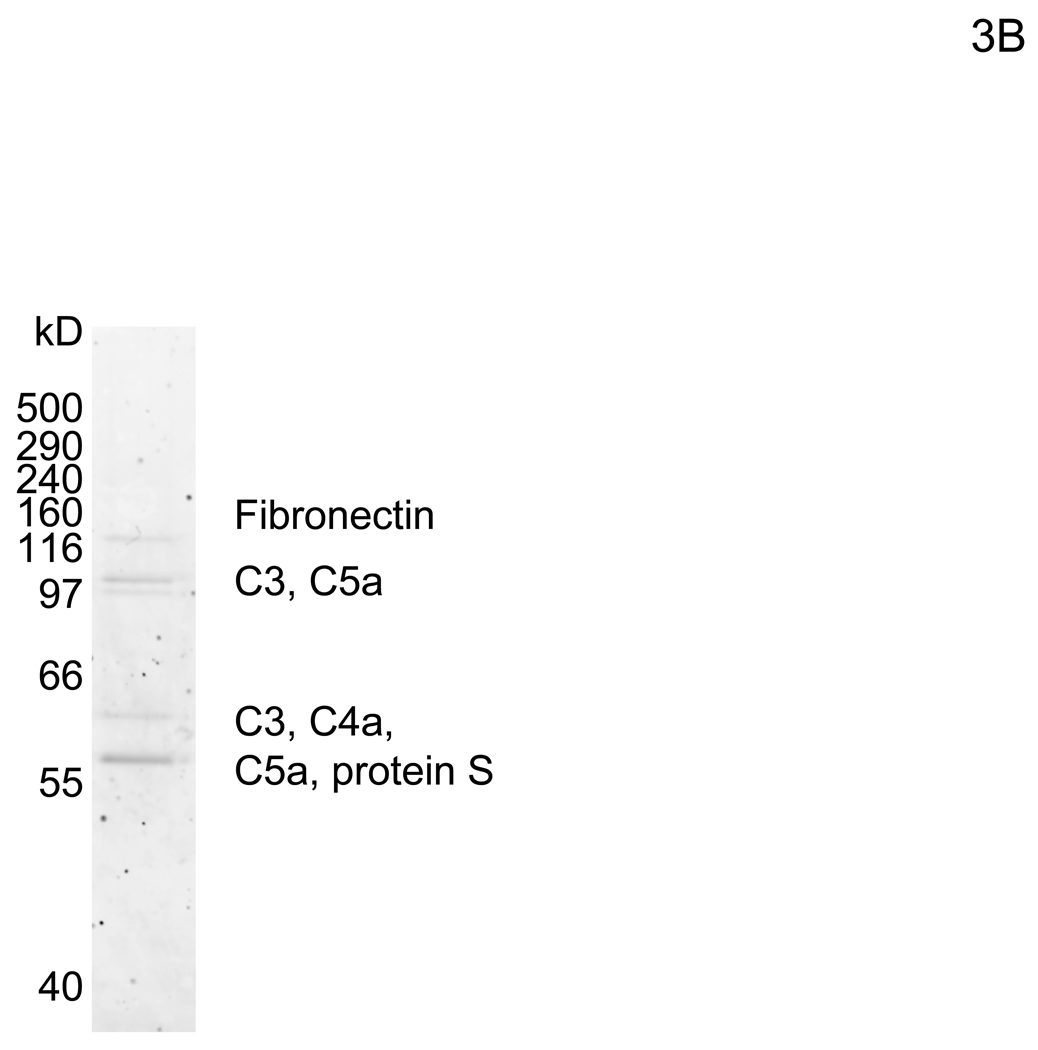
Bovine protein S mediates bridging of virus to cells
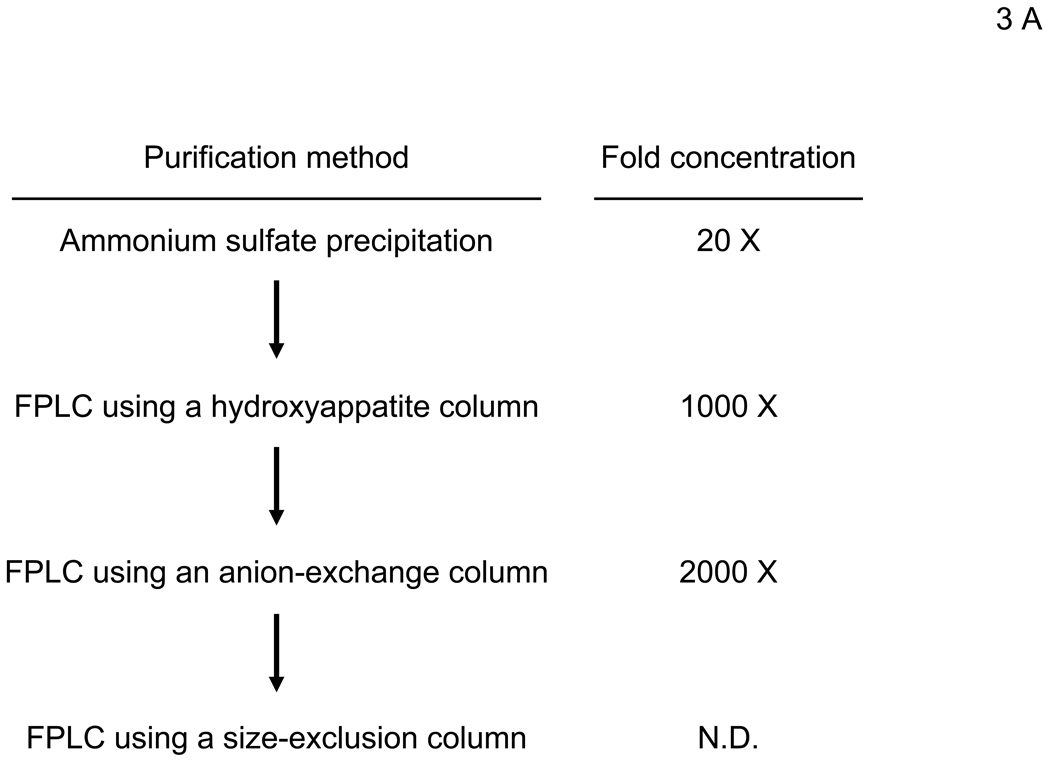
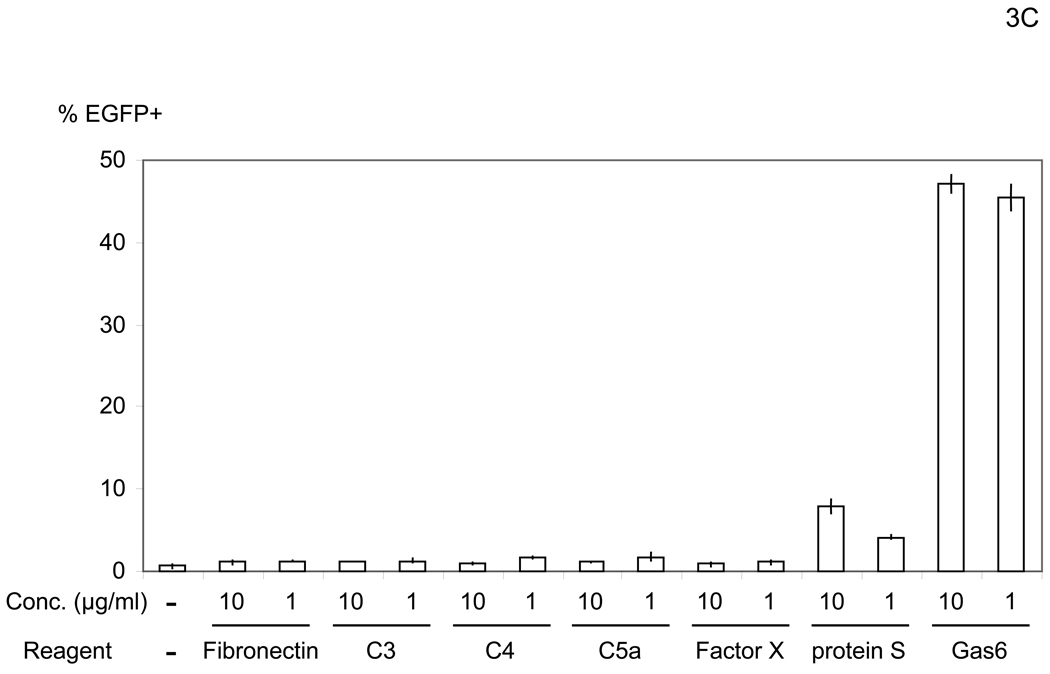
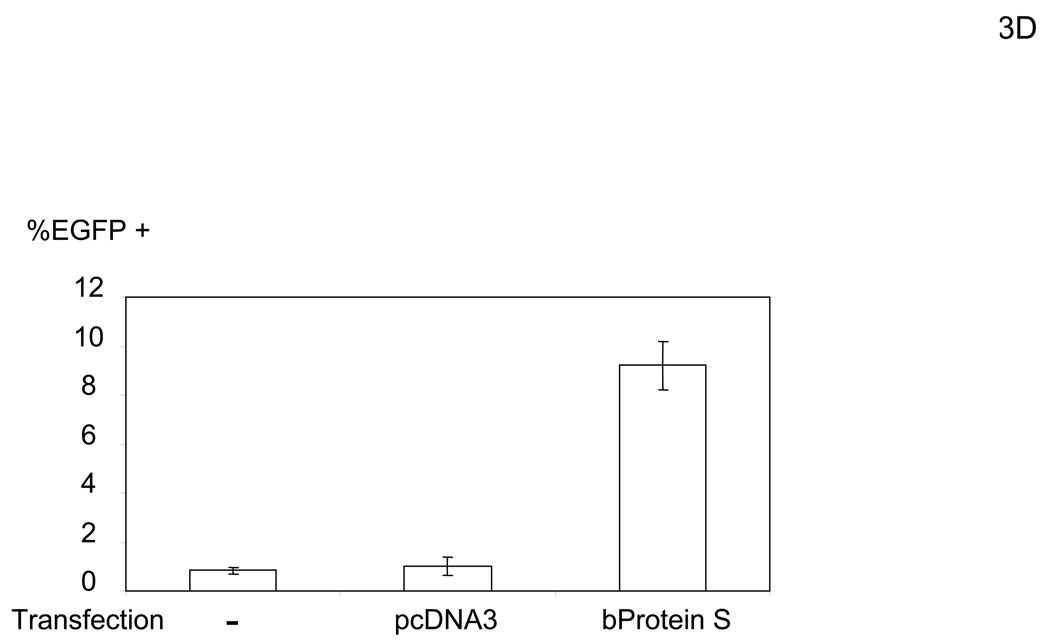
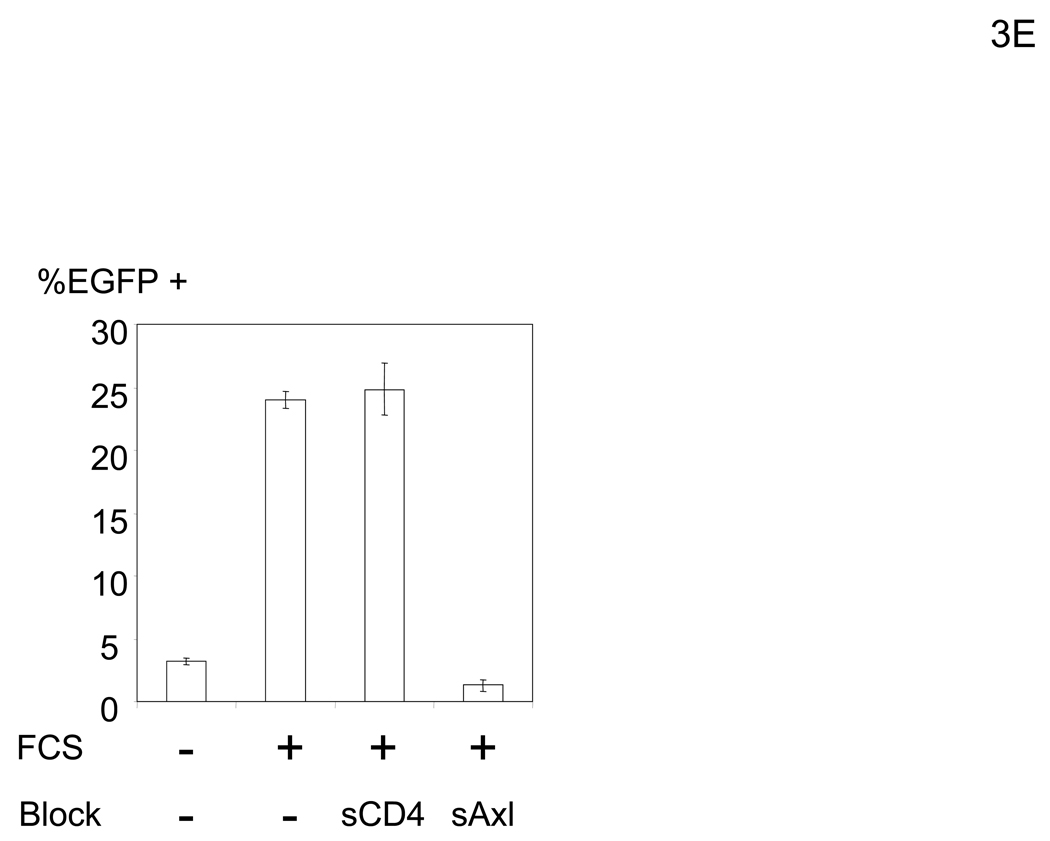
To elucidate the mechanism by which virus bridges to cells, we attempted to isolate the bridging factor from FCS. The enhancing activity of FCS is inactivated by treating FCS with DTT or incubation at 80°C (data not shown), suggesting that the factor is a protein. We used consecutive fractionation methods to purify the factor (Figure 3A). We first used ammonium sulfate precipitation to fractionate FCS, which most efficiently concentrated the factor at a saturation concentration of 33–38% (data not shown). We used this fraction to further purify the factor, using a combination of different types of FPLC in the following order: hydroxylappatite, anion-exchange, and size-exclusion. We assessed the ability of purified fractions to enhance viral transduction as well as the protein concentrations of purified fractions to those of FCS and checked whether the bridging factor was concentrated after each fractionation (Figure 3A). We analyzed the proteins in the final size-exclusion fraction showing the highest activity by SDS-PAGE and SyproRuby staining (Figure 3B). We found five bands in this fraction and identified each band by mass spectrometry. The five bands were identified as fibronectin, C3, CFH, C4, and protein S. Because purified proteins of the human homologues are more readily obtained than bovine proteins, we tested the effects of these proteins on HMVEC transduction with the 2.2 1L1L pseudotype using human homologues. We tested both human Gas6 (hGas6) and protein S (hProtein S) because both are functional homologues of bovine protein S (bProtein S) (Hafizi and Dahlback, 2006). Human protein S and Gas6 increased the titers of the 2.2 1L1L pseudotype, although the effect of hGas6 was much greater than that of hProtein S (Figure 3C). We also confirmed that recombinant bProtein S increased transduction (Figure 3D). Because antibodies that neutralize the function of bProtein S are not available, we used soluble human Axl protein (sAxl), a receptor for protein S and Gas6 lacking transmembrane domain, which can bind bProtein S and potentially neutralize its function, to confirm that the enhancement of virus transduction in FCS is mediated by bProtein S (Figure 3E) (Hafizi and Dahlback, 2006). When FCS was incubated with sAxl before addition to HMVEC, enhancement of viral transduction was completely inhibited, while incubation with soluble human CD4 (sCD4) as a control had no effect. We also tested human factor X, which was previously shown to increase transduction of adenovirus (Parker et al., 2006) and found no effect (Figure 3C). These results demonstrated that bProtein S in FCS and its human homologues bridge the virus to cells.
Figure 3.
The bridging factor in FCS is bProtein S. (A) FCS was fractionated by precipitation with various concentrations of ammonium sulfate (5% increments). The proteins precipitated with 33–38% ammonium sulfate had the highest enhancing activity/mg of protein and was used for subsequent purification by FPLC using hydroxyappatite, anion-exchange, and size exclusion columns. Each fraction was tested for enhancement of transduction of HMVEC with the 2.2 1L1L pseudotype. The fraction(s) showing the highest enhancing activity were used for subsequent purification. The fold concentration of the purified fractions was calculated by the following formula: Protein concentration of FCS necessary for 10-fold enhancement of HMVEC transduction with 2.2 1L1L pseudotype divided by protein concentration of each fraction necessary for the same enhancing activity. Because the protein concentration of the fraction purified by size-exclusion chromatography was too small to measure, fold concentration could not be determined, and is shown as N.D. (B) The size-exclusion chromatography fraction which had the highest enhancing activity was subjected to SDS-PAGE and SyproRuby staining. The protein bands were then subjected to protein identification by mass spectrometry. Since the two bands migrated around 97 kD, we identified the proteins in these two bands together. (C) HMVEC were incubated with the human homologues of the proteins identified in the purified fraction, followed by transduction with the 2.2 1L1L pseudotype (100 ng p24/ml). (D) HMVEC were incubated with the supernatant of 293T cells transfected with an expression vector for bProtein S or control plasmid (pcDNA3) or serum-free medium, followed by transduction with the 2.2 1L1L pseudotype (100 ng p24/ml). (E) 10% FCS was incubated with sAxl or sCD4 (25 µg/ml) in PBS (+) for 1 hour and used to incubate HMVEC for 4 hours. The cells were then infected with the 2.2 1L1L pseudotype (100 ng p24/ml).
Human Gas6 efficiently mediates bridging of virus to cells
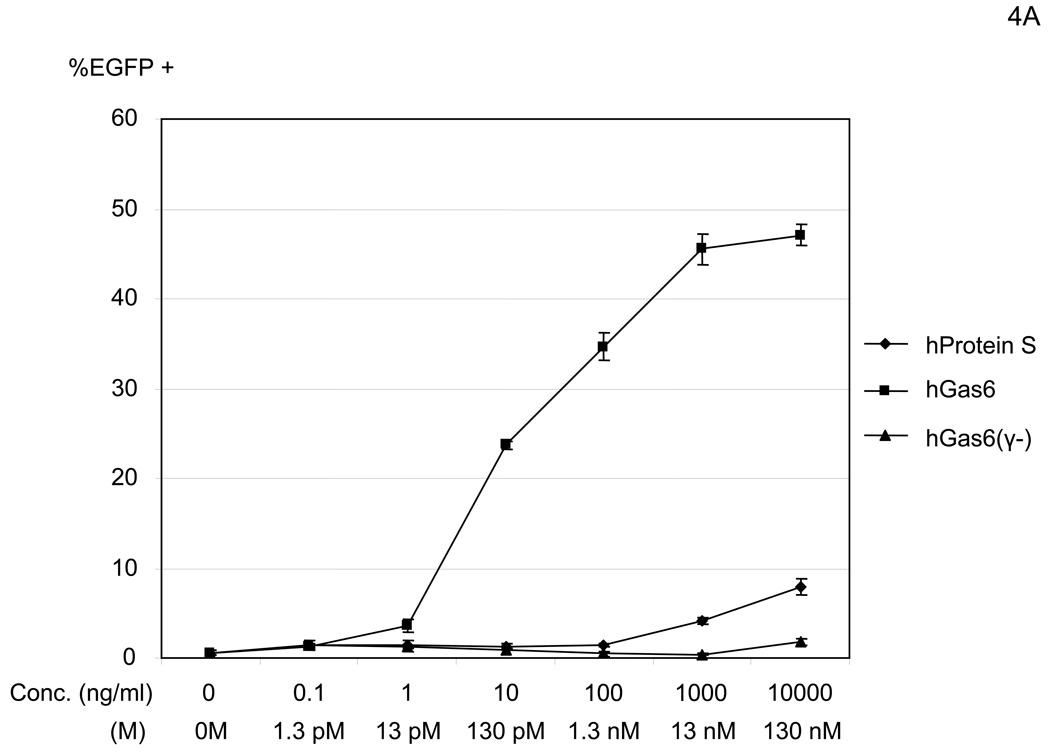
We next tested the dose dependency of enhancement by human protein S and Gas6 for enhancement of 2.2 1L1L transduction (Figure 4A). Although both hGas6 and hProtein S increased transduction, hGas6 enhanced transduction at concentrations 1000- to 10,000-fold lower than hProtein S.
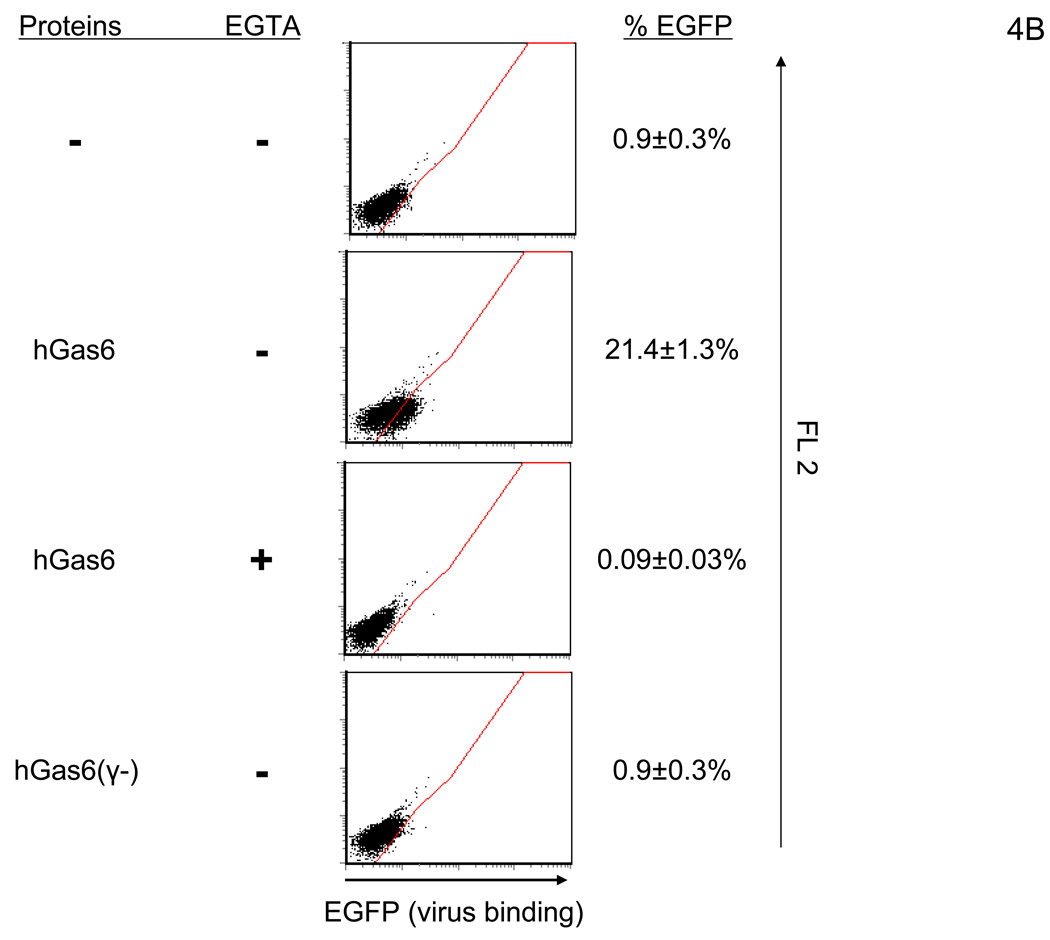
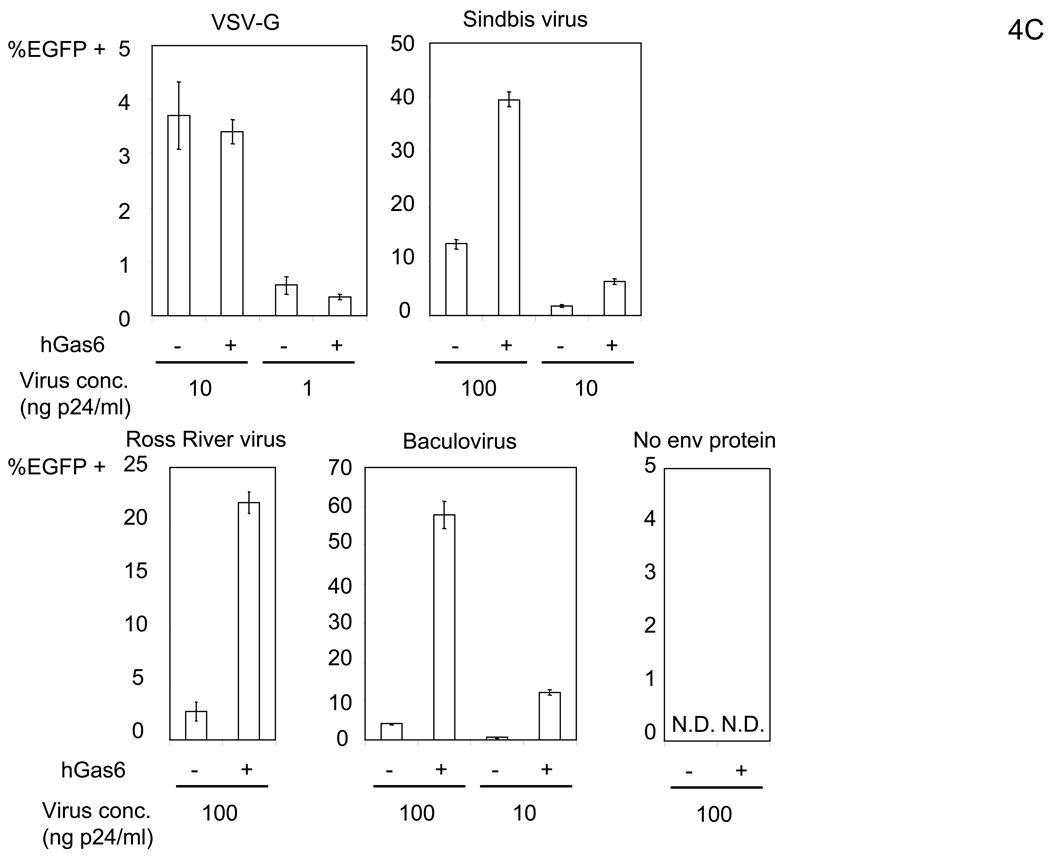
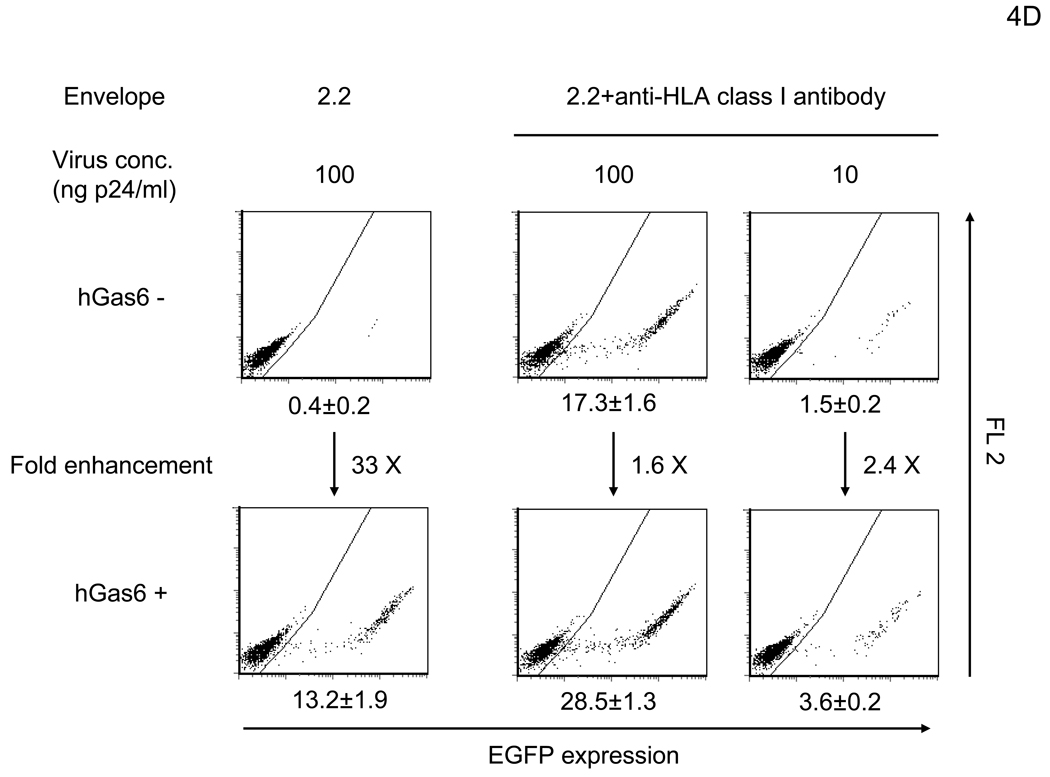
Figure 4.
hGas6 enhances binding and transduction of lentiviral vectors at low concentrations. (A) HMVEC were incubated with hProtein S, hGas6, or hGas6(γ-) at various concentrations (shown by ng/ml and M), followed by transduction with the 2.2 1L1L pseudotype (100 ng p24/ml). (B) 1×105 HMVEC were incubated with 100 ng/ml of hGas6 or hGas6(γ-) in PBS (+), or hGas6 in PBS (EGTA), then incubated with the 2.2 1L1L pseudotype labeled by EGFP (250 µl, 100 ng p24/ml). (C) HMVEC were incubated with PBS (+) or PBS (+) containing hGas6 (100 ng/ml), followed by transduction with various pseudotypes. vEnv(−) was also used for transduction. N.D.: Not Detected. (D) HMVEC were incubated with PBS (+) or hGas6 (100ng/ml) in PBS (+), followed by transduction with the 2.2 pseudotype with or without conjugation with αHLA Ab (2 µg/ml). (See also Figure S2)
The N-terminus region of protein S and Gas6, designated the Gla domain, contains 7 or 8 Ca2+ and can bind to PtdSer (Hafizi and Dahlback, 2006). Binding to PtdSer requires Ca2+ and γ-carboxylation of the Gla domain by vitamin K-dependent (VKD) γ-glutamylcarboxylase. Recombinant hGas6 protein lacking γ-carboxylation [hGas6(γ-)] did not have any enhancing effects (Figure 4A) (Nakano et al., 1997). These results demonstrated that γ-carboxylation is necessary for enhancement of viral transduction and suggested that one of the binding partners for hGas6 may be PtdSer.
Since our experiments with the FCS factor indicated that the factor increases binding of virus, we also investigated whether hGas6 does so (Figure 4B). hGas6 enhanced binding of the 2.2 1L1L pseudotype in a Ca2+-dependent manner. hGas6(γ-) did not have any enhancing effects, demonstrating that γ-carboxylation is necessary for bridging of virus to cells, and suggesting that PtdSer on either cells or virions is involved in the binding.
We further demonstrated entry of virus in the presence of hGas6 using cytoplasmic CCF2 dye and virion-incorporated β-lactamase (Cavrois et al., 2002) (Figure S2A). hGas6 enhanced the binding of EGFP-labeled vEnv(−) (data not shown); however, hGas6 did not mediate the entry of vEnv(−). These results confirmed that hGas6 can mediate binding of virus in an envelope protein-independent manner, but envelope protein is necessary for the subsequent fusion step.
hGas6 increases transduction of various pseudotypes to differing extents
hGas6 also increased transduction of other viral pseudotypes, similar to that observed with FCS (Figure 4C; compare with Figure 2A). When we compared the extent of enhancement of various pseduotypes to their infectivity [EGFP TU per the same amount of virus (1 ng of p24 capsid protein)], we observed that the higher the virus infectivity, the lesser the enhancement (Figure S2B), presumably because stronger binding between the virus and natural receptors with high affinity and/or avidity masks the effects of hGas6. To explore further this observation, we used the 2.2 pesudotype where its infectivity can be increased by conjugation with antibodies recognizing target cells, enabling us to investigate the relationship between viral infectivity and the extent of hGas6-mediated enhancement using the same virus pseudotype. Transduction of HMVEC with the 2.2 pseudotype was enhanced 33-fold by preincubation of HMVEC with hGas6 (Figure 4D). Conjugation of 2.2-pseudotyped vectors with αHLA Ab increased viral titers by 43-fold, while the extent of transduction enhancement by hGas6 decreased both at high (1.6-fold) and low MOIs (2.4-fold) (Figure 4D). Thus, the effect of hGas6 on viral transduction is present among different pseudotypes, but is most obvious when binding of virus is not efficient due to low affinity and/or avidity interaction between viral envelope proteins and its receptors. Of note, the titer (EGFP transduction unit/ 1ml of virus containing 100 µg p24) of 2.2 1L1L with 130 pM hGas6 is 3.3×107 (Figure 4A); while, the wild-type Sindbis pseudotype without hGas6 is 1.8 ×107 (Figure 4C). Therefore, the entry pathway mediated by hGas6 is efficient similar to infection via native receptor binding.
Axl serves as the primary cellular receptor for hGas6-mediated viral transduction
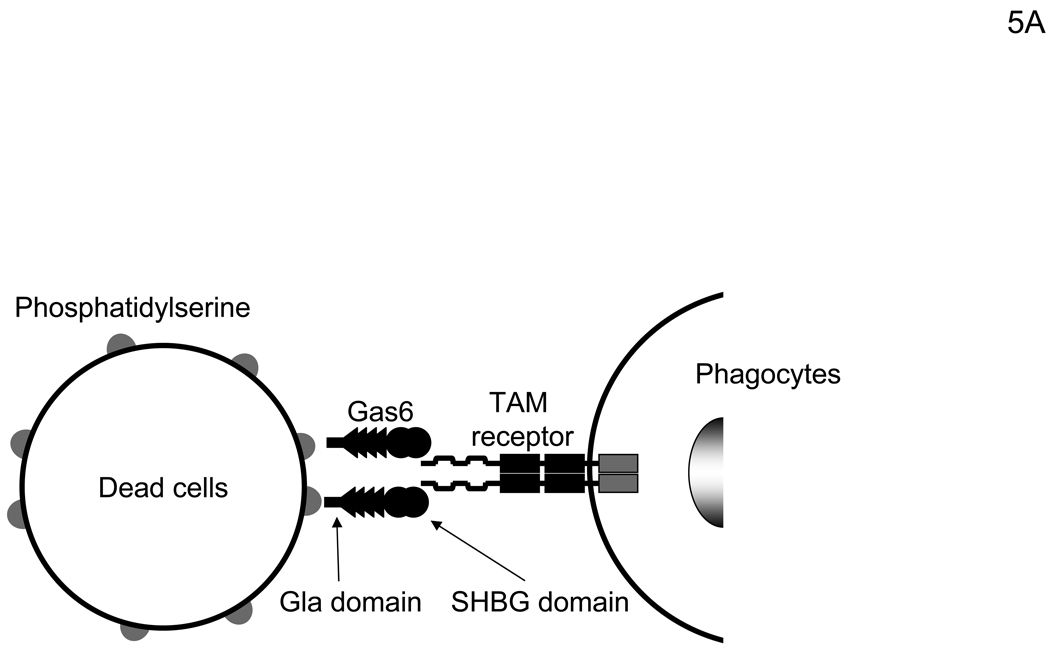
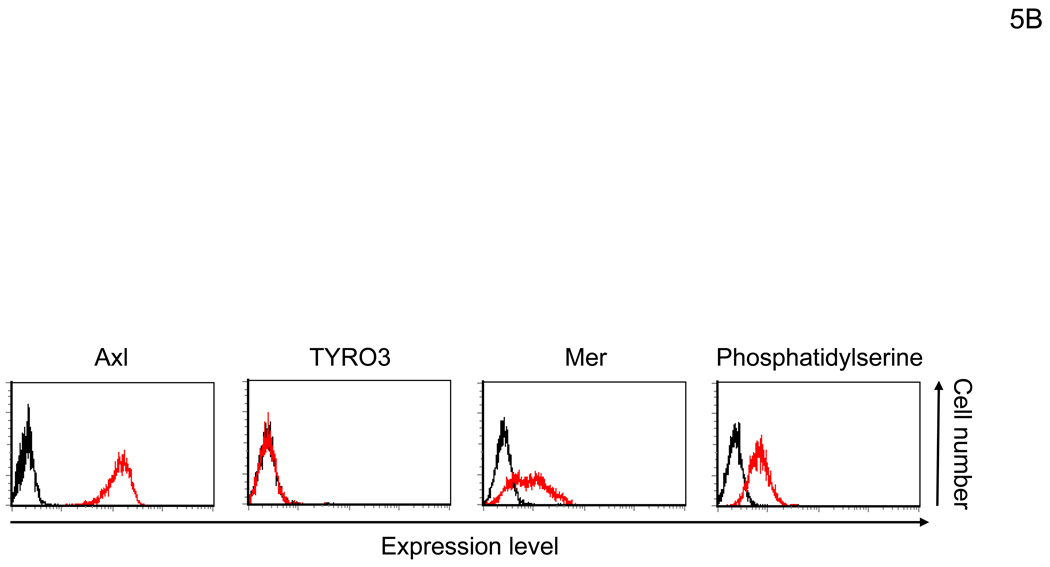
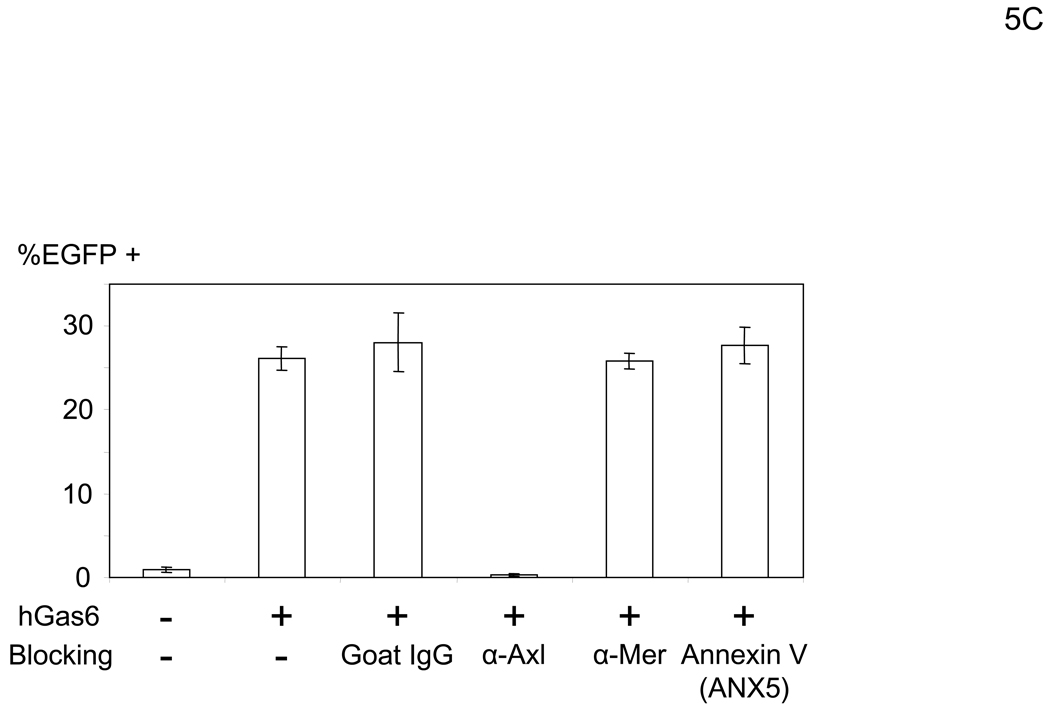
Gas6 and protein S have two ligand-binding regions (Figure 5A) (Hafizi and Dahlback, 2006). The N-terminal Gla domain binds to PtdSer. The C-terminal region has a sex hormone-binding globulin (SHBG)-like structure that contains one Ca2+ and binds to TAM receptors, including Axl, Mer, and TYRO3 (Lemke and Rothlin, 2008). One function of Gas6 and protein S is to bridge apoptotic cells to phagocytes (Figure 5A). Gas6 and protein S bind to PtdSer on apoptotic cells through the Gla domain and TAM receptors on phagocytes using the SHBG-like domains. This binding activates phagocytosis of dead cells by phagocytes. We investigated which receptors for hGas6 are expressed on HMVEC (Figure 5B). HMVEC expressed Axl and PtdSer at significant levels, but only small amounts of Mer and no TYRO3. We then used either anti-Axl or anti-Mer antibody to determine whether these receptors are involved in hGas6-mediated transduction (Figure 5C). The anti-Axl antibody completely blocked hGas6-mediated transduction of HMVEC, while control IgG and anti-Mer antibody did not have an effect. We also used annexin V (ANX5), which binds to PtdSer, to block binding of hGas6 to the cells. Unbound ANX5 was removed by washing prior to virus transduction. We observed no effect of ANX5 on hGas6-mediated transduction. These results show that Axl is the receptor for hGas6 on HMVEC.
Figure 5.
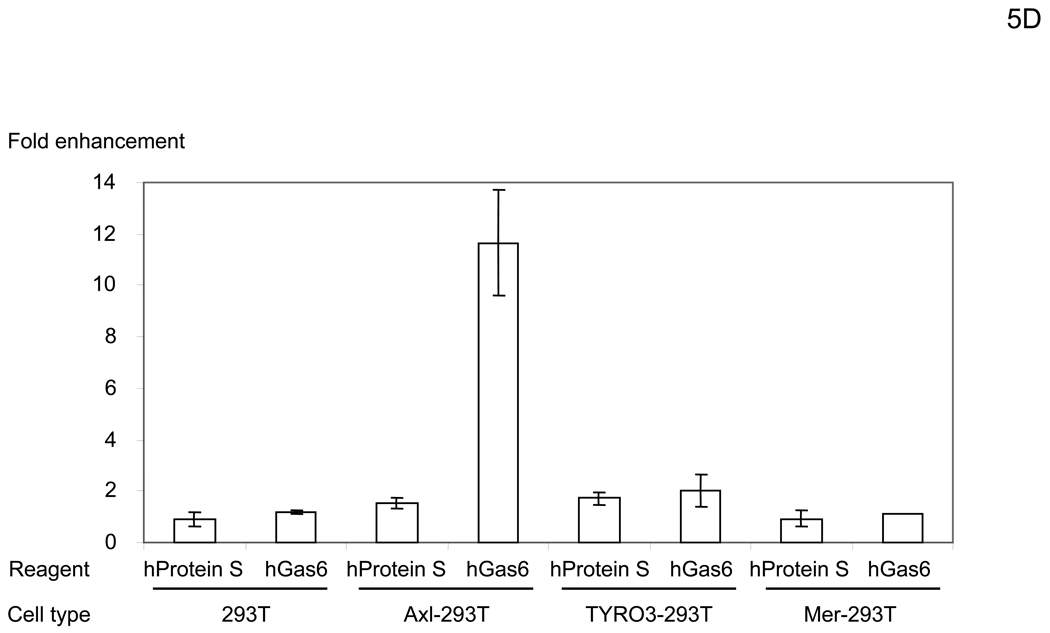
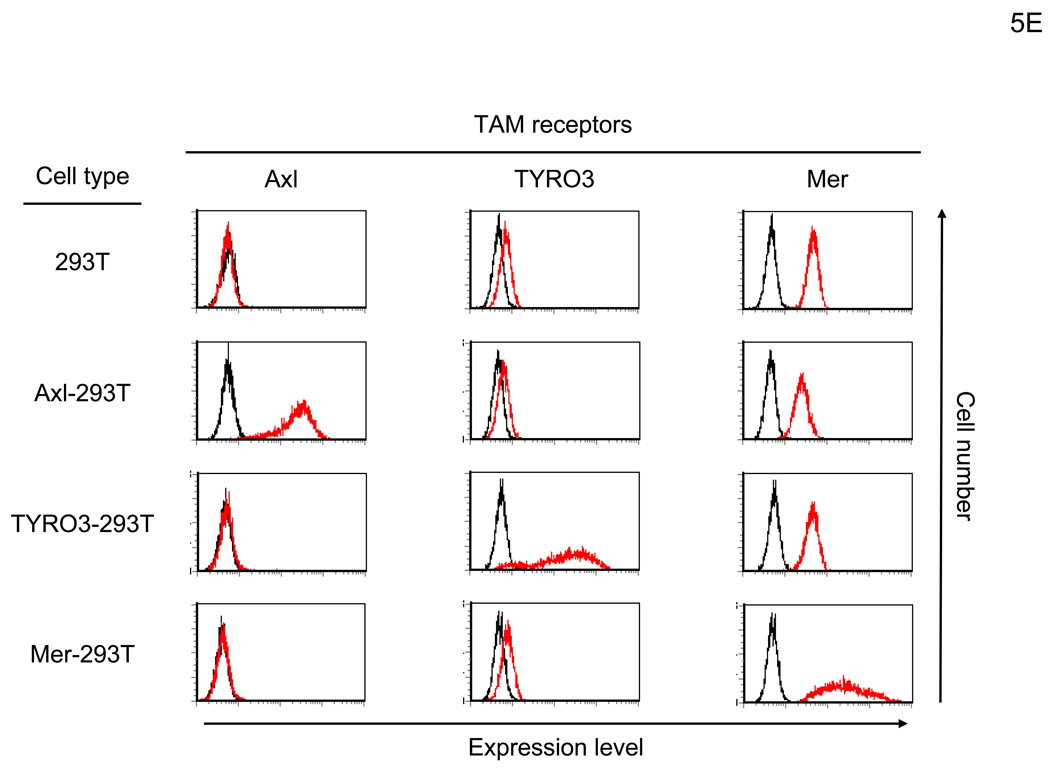
hGas6 binds to Axl on target cells. (A) Schematic representation of the mechanism whereby hGas6 mediates phagocytosis of dead cells. (B) Expression of Axl, TYRO3, Mer, and PtdSer on HMVEC. (C) HMVEC were incubated for 1 hour with PBS (+) or PBS (+) containing one blocking reagent (control IgG, anti-human Axl antibody, anti-human Mer antibody, or ANX5) (25 µg/ml) then incubated with hGas6 (100 ng/ml) in the presence of each blocking reagent for 4 hours. After removing hGas6 and blocking reagents, the cells were transduced with the 2.2 1L1L pseudotype (100 ng p24/ml). (D) Enhancement of viral transduction of 293T, Axl-293T, TYRO3-293T, and Mer-293T cells mediated by hGas6 or hProtein S. 1×105 cells were incubated with PBS (+), PBS (+) containing hProtein S, or hGas6 (100 ng /ml), followed by transduction with the 2.2 1L1L pseudotype (100 ng p24/ml). The fold enhancement was calculated by the following formula: % of EGFP expressing cells preincubated with hProtein S or hGas6 divided by % of EGFP expressing cells preincubated with PBS (+). (E) Expression of Axl, TYRO3, and Mer on 293T, Axl-293T, TYRO3-293T, and Mer-293T cells. Black and red lines are staining with isotype control antibodies and antibodies against each type of TAM receptor, respectively.
Transduction of 293T cells was not enhanced by hGas6, hProtein S, or FCS (Figure 5D and data not shown). Consistent with the above studies showing the role for Axl, 293T cells do not express Axl (Figure 5E). To further demonstrate whether any of the TAM receptors can mediate hGas6- or hProtein S-mediated enhancement of viral transduction, we ectopically expressed each of the TAM receptors on 293T cells (Figure 5E) and tested whether expression could render 293T cells susceptible to hGas6- or hProtein S-mediated viral transduction. Although Mer is originally expressed on 293T cells, we over-expressed Mer to investigate its effects on enhancement of viral transduction. Over-expression of Mer did not render the cells susceptible to hGas6- or hProtein S-mediated enhancement, but ectopic expression of Axl and TYRO3 rendered 293T cells susceptible to hGas6-mediated viral transduction (Figure 5D). Axl was more efficient in mediating enhancement. These results are consistent with the known relative affinities of TAM receptors for hGas6: the higher affinity of Axl, moderate affinity of TYRO3, and low affinity of Mer for hGas6 (Stitt et al., 1995). hProtein S can enhance transduction of Axl- and TYRO3–293T cells to a lesser extent. These results demonstrated that Axl is the primary receptor for hGas6-mediated viral transduction of HMVEC, but TYRO3 can also serve as a receptor.
PtdSer as the receptor for hGas6 on the viral envelope
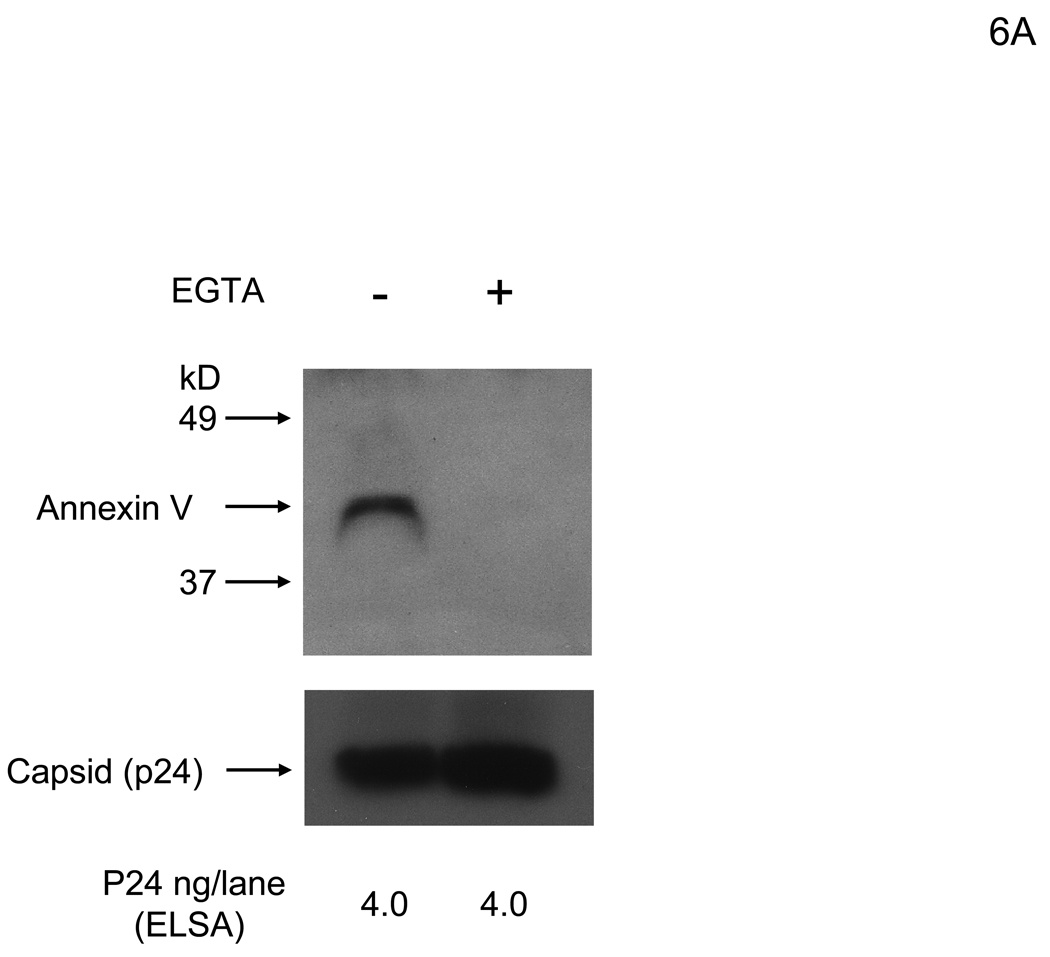
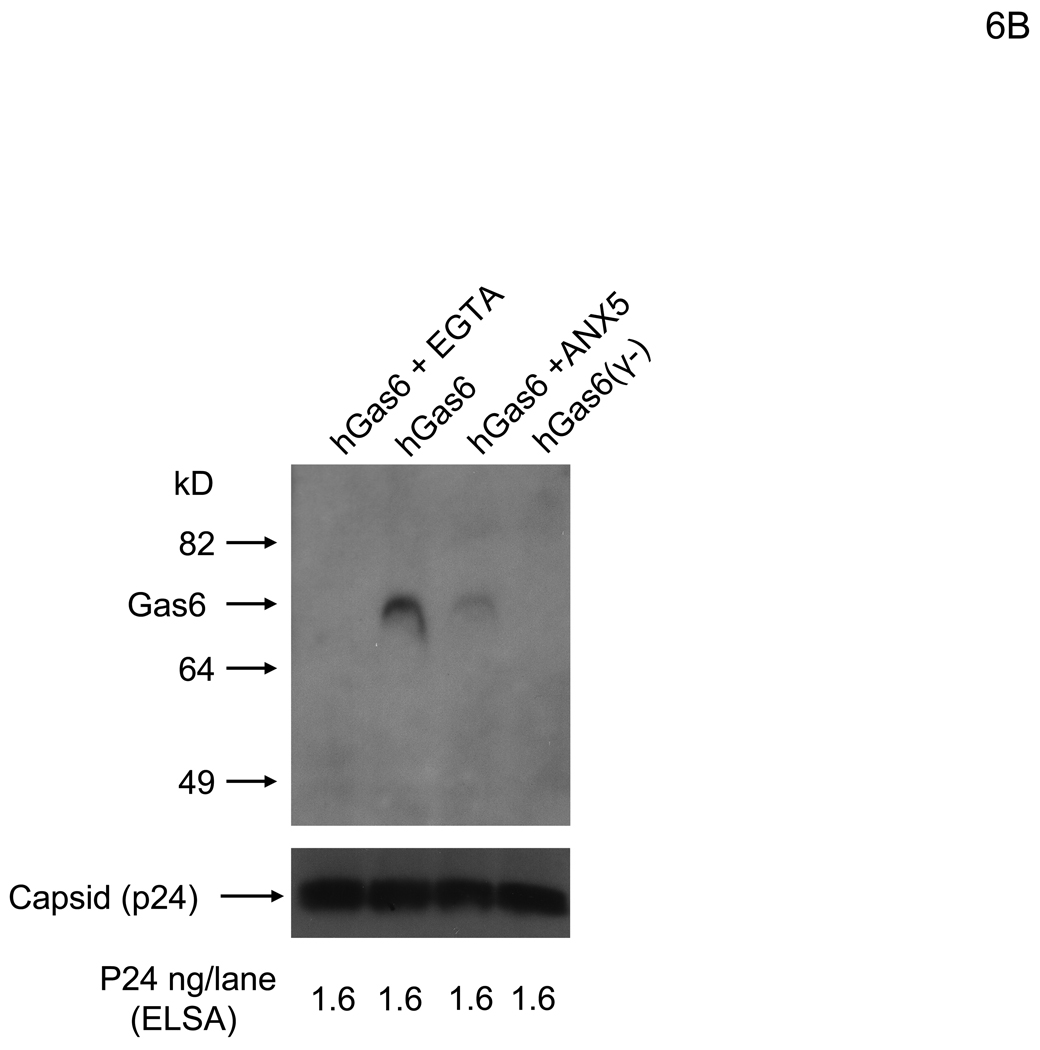
Because Axl is the receptor on HMVEC for hGas6-mediated viral transduction, the other ligand-binding site of Gas6, the Gla domain, is likely to bind to virus. The ligand for the Gla domain is PtdSer, and several viruses, including vaccinia virus and HIV, expose PtdSer on their virion envelopes. Thus, PtdSer is most likely the receptor for Gas6 on the viral envelope. We first investigated whether PtdSer is exposed on the lentiviral vectors by using ANX5 as a probe. The 2.2 1L1L pseudotype was incubated with ANX5, then the virus was purified by ultracentrifugation. The purified virus was analyzed to determine whether ANX5 is co-precipitated with virus. Because the binding between PtdSer and ANX5 is Ca2+-dependent, we performed binding in the presence of Ca2+ or EGTA. Our results demonstrated that ANX5 bound to virus in a Ca2+-dependent manner, indicating that PtdSer is exposed on the envelope of lentiviral vectors (Figure 6A). To confirm that hGas6 binds to virus through PtdSer, we investigated whether ANX5 blocks binding of hGas6 to virus. Virus was preincubated with hGas6 and purified by ultracentrifugation. hGas6 was co-precipitated with virus and the co-precipitation was inhibited by EGTA (Figure 6B). As a control, hGas6(γ-), which does not bind PtdSer, did not bind virus. Preincubation of virus with ANX5 inhibited binding of hGas6 to virus. The infectivity of the virus incubated with hGas6 was 14-fold higher than the virus without hGas6 or the virus incubated with hGas6 in the presence of EGTA (Figure S3). These results demonstrated that hGas6 binds to PtdSer on the viral envelope and the binding is dependent on both Ca2+ and γ-carboxylation of the hGas6 Gla domain.
Figure 6.
hGas6 binds to envelope PtdSer. (A) The 2.2 1L1L pseudotype (200 ng p24/ml) was incubated with biotinylated ANX5 (25 µg/ml) in PBS (+) or PBS (EGTA), followed by purification of virus by ultracentrifugation. The same amount of virus (4 ng p24/lane) was subjected to SDS-PAGE and western blotting. ANX5 was detected using HRP-conjugated streptavidin. (B) The 2.2 1L1L pseudotype (200 ng p24/ml) was incubated with PBS (+), PBS (+) containing ANX5 (25 µg/ml), or PBS (EGTA). The virus was then incubated with hGas 6 or hGas6(γ-) (100 ng/ml) in the conditions used for previous incubations. The virus was purified by ultracentrifugation and the same amount of virus (1.6 ng p24/lane) was subjected to SDS-PAGE and western blotting. hGas6 was detected using biotinylated anti-hGas6 antibody and HRP-conjugated streptavidin. (A and B) The amounts of virus in each lane were confirmed by immunoblotting of p24. (See also Figure S3)
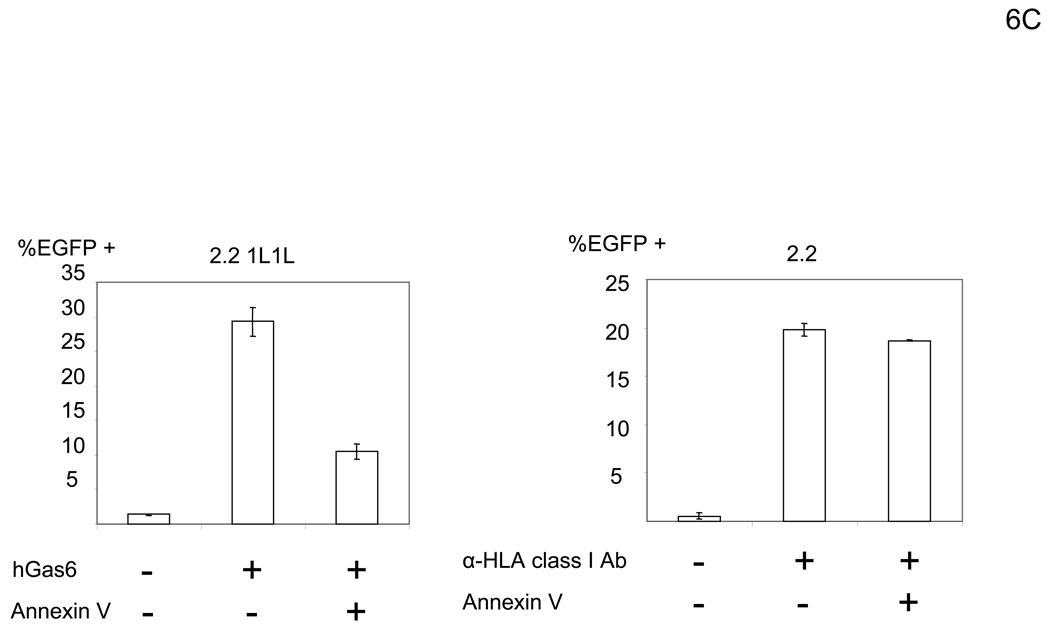
We next demonstrated that blocking envelope PtdSer by ANX5 can block hGas6-mediated enhancement of viral transduction. We incubated the 2.2 1L1L pseudotype with ANX5 before transduction, then transduced HMVEC preincubated with hGas6. Preincubation of the virus with ANX5 inhibited hGas6-mediated enhancement of viral transduction, while transduction of HMVEC (without preincubation with hGas6) with the 2.2 pseudotype conjugated with αHLA Ab was not affected by ANX5 (Figure 6C). These results confirmed that PtdSer on the viral envelope is necessary for hGas6-mediated enhancement of transduction.
hGas6 enhances infection of replication-competent vaccinia virus
Our studies using pseudotyped vectors revealed that the viruses utilize a pathway that is normally used by phagocytes for recognition of apoptotic cells. In that setting, PtdSer exposure of apoptotic cells is recognized by TAM receptors on phagocytes via bridging by Gas6/protein S. Since Gas6 is ubiquitous, we speculate that this pathway for viral infection can be used whenever a virion contains PtdSer and TAM receptors are present on target cells. Vaccinia virus was shown to use its envelope PtdSer to enter into cells, a process referred to as “apoptotic mimicry of viral entry” (Laliberte and Moss, 2009; Mercer and Helenius, 2008; Mercer et al., 2010). The cellular receptor for vaccinia virus envelope PtdSer has not been identified, so we investigated whether Gas6 is involved in PtdSer recognition and enhancement of replication of vaccinia virus.
Vaccinia virus has two different forms, IMV and EEV (extracellular enveloped virus) (Smith et al., 2002). The origin of envelope lipids and the types of envelope proteins differ between IMV and EEV. IMV is produced inside the cells, acquires its envelope inside the cells from an unknown origin, and is released after lysis of cells. EEV is comprised of IMV wrapped by an additional membrane derived from the trans-Golgi network or early endosomes and buds out from the surface of cells.
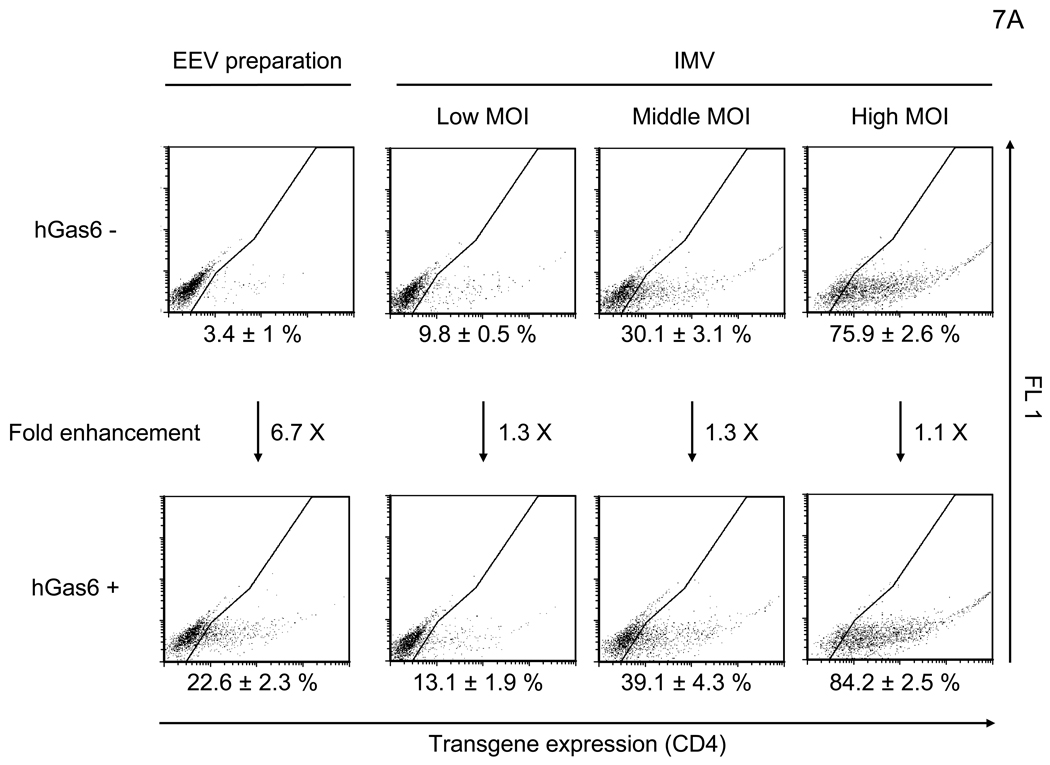
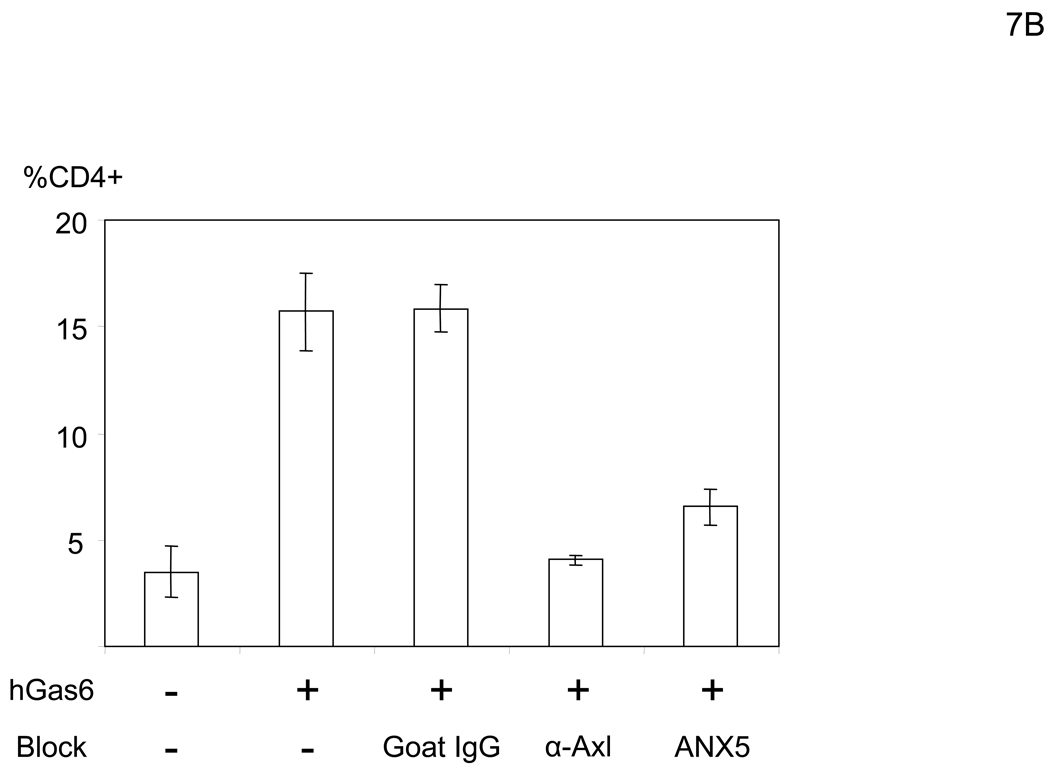
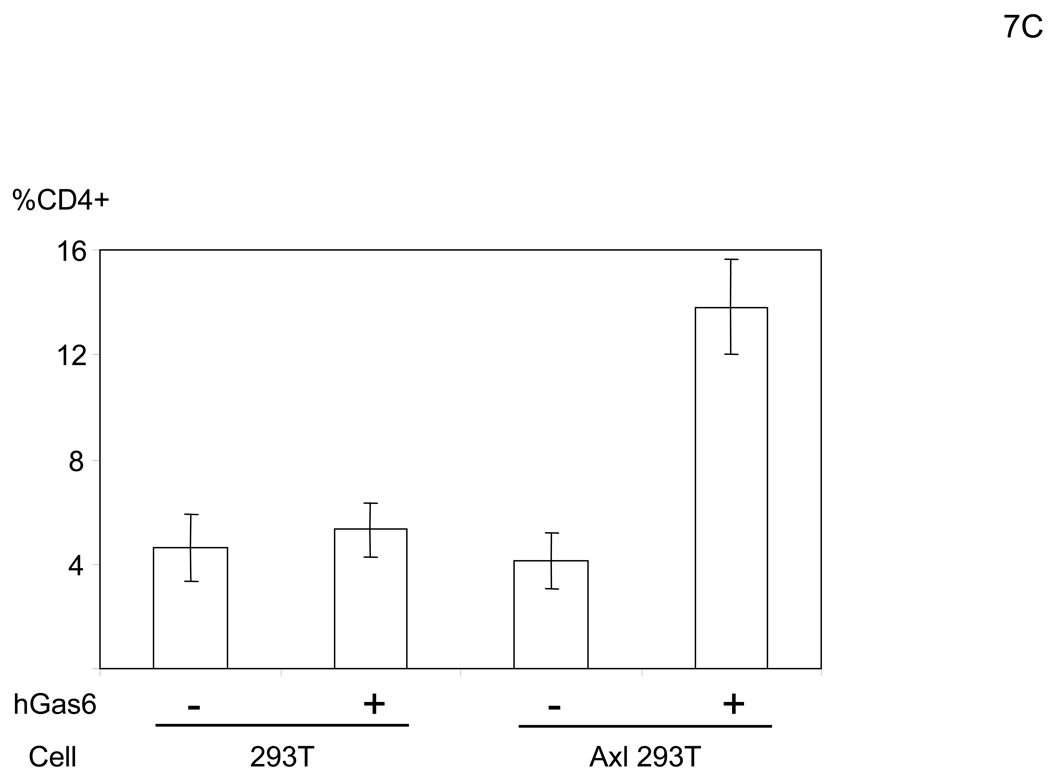
Because the entry mechanisms and envelope structures of IMV and EEV are distinct, we investigated the effect of hGas6 on both IMV and EEV infection. The replication-competent vaccinia virus (WR strain) contains human CD4 as its reporter gene under the control of a synthetic vaccinia strong early/late promoter, which enables detection of infected cells by staining with an anti-CD4 antibody. After infection, the cells were cultured in the presence of cidofovir, which inhibits second-cycle replication of the virus (De Clercq et al., 1987), in order to analyze the effect of hGas6 on a single infection cycle (Li et al., 2010). hGas6 enhanced infection with IMV by only 1.1–1.3 fold, regardless of MOI (Figure 7A). However, hGas6 enhanced infection by EEV preparations 6.7-fold. These results demonstrated that hGas6 can facilitate entry of replication-competent vaccinia virus, predominantly by supporting replication of the EEV form. hGas6-mediated enhancement of infection by EEV preparations was blocked by incubating cells with anti-Axl antibody or incubating virus with ANX5 (Figure 7B). hGas6 did not enhance EEV preparation’s infection of 293T cells but did enhance infection of Axl-293T cells (Figure 7C). hGas6 did not enhance IMV infection of Axl-293T cells (Figure S4B).
Figure 7.
hGas6 enhances infection of the EEV preparation of vaccinia virus. (A) HMVEC were incubated with PBS (+) or PBS (+) containing hGas6 (100 ng/ml). The IMV form was used for infection at MOIs of 0.1, 0.5 and 2.5. The EEV preparation was used for infection at undetermined MOIs. (B) HMVEC were incubated for 1 hour with PBS (+) or PBS (+) containing either normal goat IgG or goat anti-human Axl antibody (25 µg/ml) as a blocking reagent then incubated with hGas6 (100 ng/ml) in the presence of one blocking reagent for 4 hours. The EEV preparation (undiluted) was incubated for 1 hour with the same volume of PBS (+) or PBS (+) containing ANX5 (25 µg/ml) and applied to HMVEC pretreated with hGas6 and blocking reagent. (C) 293T and Axl-293T cells were incubated with AIM-V or AIM-V containing hGas6 (100 ng/ml), followed by infection with the EEV preparation (100-fold diluted supernatant). (A–C) EEV preparations were pretreated with anti-A27L antibody to neutralize possible contamination of IMV (Figure S4) (Ichihashi, 1996).
EEV preparations may contain contaminating IMV and disrupted EEV; thus, we cannot exclude the possibility that the presence of IMV and/or disrupted EEV in EEV preparation might influence the results. Nevertheless, our results demonstrate that EEV preparations obtained by standard methods are significantly enhanced by hGas6. These results demonstrate that vaccinia virus can utilize the same apoptotic mimicry entry pathway as lentiviral vectors.
DISCUSSION
We identified a Gas6-mediated entry mechanism which also revealed the molecular mechanism by which envelope PtdSer facilitates viral replication. This mechanism acts to provide an alternative pathway for viral entry that is not limited by specific interaction between viral envelope proteins and cell receptors. This entry pathway seems to play a major role in viral binding when native binding between virus and cells is weak. Thus, Gas6 and protein S will broaden the tropisms of viruses. Indeed, ectopic expression of Axl and TYRO3 was previously shown to render unsusceptible cells susceptible to infection with Ebola virus by an unknown mechanism (Shimojima et al., 2006). Axl- and TYRO3-mediated infection of Ebola virus might also involve bProtein S in FCS used in cell cultures.
Our data showed that lentiviral vectors pseudotyped with various envelope proteins can be enhanced by hGas6 to differing extents. The differences might be explained by varying affinities and/or avidities of interaction between virus and target cells. Binding by hGas6 can result in drastic increases in infectivity when virus does not bind target cells efficiently (Figure 4D and Figure S2B). Conversely, if viral binding is very efficient due to high affinity and/or avidity interaction between virus envelope proteins and target cells, the contribution of hGas6 to virus binding is negligible. However we cannot rule out the possibility that these differences may be due to differing amounts of exposed PtdSer dependent upon the pseudotyped envelope protein.
Among the viruses known to use envelope PtdSer for its replication, vaccinia virus is the only one clearly shown to use PtdSer for its binding to target cells. This led us to test the effects of hGas6 on vaccinia virus replication. The role of PtdSer in vaccinia virus entry was shown by blocking replication of vaccinia virus using ANX5 and reconstituting the membrane of detergent-extracted virions by PtdSer (Laliberte and Moss, 2009; Mercer and Helenius, 2008; Mercer et al., 2010). These investigations designated PtdSer-mediated virus entry as apoptotic mimicry because exposed PtdSer was known to be a surface marker of apoptotic cells. However, these studies did not show whether the same PtdSer recognition mechanism is used for both phagocytosis of apoptotic cells and entry of vaccinia virus. Our study showed that virus entry can be mediated by the same molecular mechanism used for recognition of dead cells. The differing degrees of enhancement observed between the IMV and EEV preparations of vaccinia virus could be due to several possibilities. 1) IMV and EEV preparations have different envelope proteins on their surfaces, resulting in different affinities of the virus for target cells. 2) IMV and EEV obtain their envelopes from different cytoplasmic organelles, potentially varying the amount of exposed PtdSer. 3) EEV preparations are much less infectious for HMVEC than IMV. Thus, as seen with lentiviral vector pseudotypes, the degree of enhancement is greater when native infectivity is lower. One previous study demonstrated the role of PtdSer in vaccinia virus entry using the IMV form (Mercer and Helenius, 2008). We observed much less of an effect of hGas6 on IMV, potentially due to different cell types used. 4) the EEV outer membrane is fragile, so rupture of its outer membrane may expose its luminal side which contains abundant PtdSer. Although the fragility of the outer envelope of EEV makes analysis difficult, detailed biochemical analysis of both the IMV and EEV envelope will be necessary to address these possibilities.
Gas6 and protein S belong to the family of VKD proteins that include proteins involved in the coagulation system (McCann and Ames, 2009). The proteins of this family undergo γ-carboxylation, a post-translational modification of glutamic acid residues, by VKD γ-glutamylcarboxylase. We found that the Gla domain of hGas6 binds to virus. Interestingly, the Gla domain of factor X was also shown to bind adenovirus (Munch et al., 2007). Although only a few proteins (less than 15) are known to belong to the VKD protein family, four (protein C and Factor VII, IX, and X) were shown to mediate binding of non-envelope virus (adenovirus) (Parker et al., 2006) and two (Gas6 and protein S) mediate binding of envelope virus. These results suggest a common evolutionally convergence where replication of diverse viruses is enhanced by VKD proteins.
Gas6 shares 43% amino-acid sequence homology with Protein S, but there are differences in their expression and functions (Fernandez-Fernandez et al., 2008; Hafizi and Dahlback, 2006). Protein S is mainly produced in the liver, but expression of Gas6 is much lower in the liver than the heart, kidney, and lung. hProtein S has an anticoagulant effect but hGas6 does not. Functions of protein S and Gas6 are also species-dependent. For example, while human and bovine protein S share 82% amino acid sequence identity, only the bovine variant is shown to activate human TYRO3 (Evenas et al., 2000).
PtdSer is known to be exposed not only on vaccinia virus envelope, but also on Pichinde virus (a model of Lassa fever virus) and HIV (Callahan et al., 2003; Soares et al., 2008). Although these studies did not indicate which step of viral replication is supported by PtdSer, both studies clearly demonstrated that exposed PtdSer contributes to viral replication. PtdSer of the plasma membrane is usually present in the inner leaflet, while PtdSer of the membrane of cytoplasmic organelles is present on both leaflets (Boon and Smith, 2002). Asymmetrical distribution of PtdSer in the plasma membrane is maintained by lipid transporters, flippases. When apoptosis or necrosis occurs, flippases are inactivated and scramblase, which can transport PtdSer in both directions, is activated, resulting in exposure of PtdSer. There are three possible mechanisms that can expose PtdSer on the viral envelope: 1) viral infection induces apoptosis of infected cells and budding captures the exposed PtdSer (Soares et al., 2008); 2) virions do not contain flippases, so it cannot maintain asymmetrical distribution of PtdSer (Denard et al., 2009); and 3) virus obtains envelope from cytoplasmic organelles (Chertova et al., 2006). TAM receptors belong to the receptor tyrosine kinase family that contains protein tyrosine kinase activity within the cytoplasmic domain (Lemke and Rothlin, 2008). Signaling via TAM receptors is reported to activate various signaling molecules, including PI3K, STAT3, and Src. This signaling may facilitate endocytic and phagocytic activity of cells, potentially affecting entry of virus. We tested the activity of a tyrosine kinase inhibitor (SKI-606) on hGas6-mediated transduction and observed a 2-fold reduction (data not shown); however, because SKI-606 is a broad tyrosine kinase inhibitor, we are unsure as to the significance of this observation. TAM receptors are expressed on many cell types, including hematopoietic cells, bone-marrow stromal cells, and retinal pigment epithelial cells. Murine macrophages and dendritic cells have been shown to express Axl and TYRO3 in vivo (Seitz et al., 2007). Since envelope PtdSer has been shown to enhance HIV replication in human macrophages (Callahan et al., 2003), we prepared human macrophages by in vitro differentiation of monocytes using GM-CSF (Lee et al., 1999) to study the effects of hGas6 on HIV replication. Unfortunately, we could not detect the expression of Axl and TYRO3 on the macrophages. However, monocyte-derived macrophages do not necessarily represent all types of macrophages in vivo (Krutzik et al., 2005). It was shown that usage of IFN-α for human dendritic cell differentiation from monocytes induces expression of Axl while other differentiation conditions do not (Scutera et al., 2009). Thus, different conditions for macrophage differentiation could induce expression of Axl.
The serum concentration of hGas6 (200 pM) is higher than the concentration required for viral transduction enhancement (13pM) (Hafizi and Dahlback, 2006). However all hGas6 in normal human serum has been shown to be bound with sAxl so it would not be able to mediate bridging of virus to cells (Ekman et al., 2010b). Viral transduction enhancement by hGas6 is expected to be suppressed in normal human serum. We investigated whether human serum can enhance viral transduction using two different lots of human sera and we observed only very weak enhancing activity (data not shown). Increased plasma concentrations of Gas6 have been found in many disease states, including malignancy and inflammation (Ekman et al., 2010a). Importantly, Axl expression on endothelial cells and vascular muscle is upregulated after vessel injury and inflammation (Korshunov et al., 2006; Sharif et al., 2006). It is well known that the virus can efficiently enter into the body via the skin and mucosa when injury and/or inflammation occur (Norkin, 2010). We theorize that injury and inflammation will facilitate virus entry not only by breaks of the barrier function of the skin and mucosa, but also by the upregulation of Axl and Gas6 expression to enhance virus binding. This pathway could be particularly important for viral binding if the expression of native virus receptors is low at portal sites for viruses.
These studies began with efforts to identify factors responsible for nonspecific infectivity of redirected lentiviral vectors. Those efforts focused on both eliminating original receptor-binding regions of the viral protein and conjugating virus with ligands that specifically bind target cells and tissues. Here, we demonstrate that a lipid can mediate binding of virus, which should be considered when eliminating original tropism of viral vectors and/or conferring new tropism by modification of envelope lipids.
Because PtdSer is likely to be a common molecule among many types of envelope viruses, further investigation of the entry pathway mediated by PtdSer may lead to preventive and therapeutic approaches for viral diseases.
EXPERIMENTAL PROCEDURES
Cells, viruses, plasmids, and proteins
See Extended Experimental Procedures.
HMVEC infection with lentiviral vectors
HMVEC were seeded 2 days before transduction for all infection and transduction experiments. See Extended Experimental Procedures for the detailed conditions for lentiviral transduction.
Co-precipitation of the serum factor with the 2.2 1L1L pseudotype was performed by incubating 2.2 1L1L in PBS (+) with 10% FCS followed by ultracentrifugation using a sucrose cushion. The pellet was resuspended in PBS (+) and quantitated by measuring p24 amounts; the same amount of viruses (100 ng p24/ml) was used for transduction of HMVEC.
To confirm bProtein S is the bridging factor, 10% FCS was incubated for 1 hour with sAxl or sCD4 and used to incubate HMVEC for 4 hours.
Purification of the serum factor from FCS and protein identification
See Extended Experimental Procedures.
Binding of the virus to ANX5 and hGas6
To investigate exposure of PtdSer on the viral envelope, 1 ml of the 2.2 1L1L pseudotype (200 ng p24/ml) was incubated with biotinylated ANX5 (2.5 µg/ml) in PBS (+) or PBS containing 2 mM EGTA and 0.3% BSA [PBS (EGTA)], followed by purification of the virus by ultracentrifugation. The amounts of purified virus were measured by quantitating p24 by ELISA. The same amounts of virus (4 ng p24) incubated with ANX5 in PBS (+) or PBS (EGTA) were subjected to SDS-PAGE, western blotting, and staining with streptavidin conjugated with horseradish peroxidase (HRP). To investigate binding of hGas6 to virus, 0.5 ml of the 2.2 1L1L pseudotype (200 ng p24/ml) was incubated with hGas6 (250 ng/ml) in PBS (+) or PBS (EGTA). Viruses were purified by ultracentrifugation. The amounts of purified viruses were quantitated by p24 ELISA. Equal amount of virus was subjected to SDS-PAGE, western blotting, and immunostaining, using biotinylated anti-hGas6 antibody and HRP-conjugated streptavidin. The amounts of virus in each lane were confirmed by immunoblotting, using mouse anti-p24 monoclonal antibody and HRP-conjugated goat anti-mouse IgG.
Binding of the 2.2 1L1L pseudotype with HMVEC
1×105 HMVEC were incubated for 4 hours with hGas6 (100 ng/ml), hGas6(γ-) (100 ng/ml), or FCS (10%) in 200 µl of PBS (+) or PBS (EGTA). The cells were then incubated for 2 hours with 250 µl of the 2.2 1L1L pseudotype (250 µl, 100 ng p24/ml) labeled with EGFP in the same buffer used for the previous incubation.
Vaccinia virus infection
The IMV form and EEV preparations of vaccinia virus were generated by infecting BHK-21 cells with vaccinia virus according to the standard protocol (Law and Smith, 2004). Please see Extended Experimental Procedures for more information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the NIH AIDS Research & Reference Reagent Program, Drs. Bjorn Dahlback, Yoshihiro Kawaoka, Toshio Kitamura, Airi Harui, and Makoto Sato for providing reagents, Drs. Pablo Garcia de Frutos, Jerome A Zack, and Geoffrey Smith for discussion. This work was supported by NIH grants (AI069350, CA120327, NS055212) and a CFAR Mentorship grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boon JM, Smith BD. Chemical control of phospholipid distribution across bilayer membranes. Med Res Rev. 2002;22:251–281. doi: 10.1002/med.10009. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Popernack PM, Tsutsui S, Truong L, Schlegel RA, Henderson AJ. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Jr, Sowder RC, 2nd, Barsov E, Hood BL, Fisher RJ, et al. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holy A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- Denard J, Rundwasser S, Laroudie N, Gonnet F, Naldini L, Radrizzani M, Galy A, Merten OW, Danos O, Svinartchouk F. Quantitative proteomic analysis of lentiviral vectors using 2-DE. Proteomics. 2009;9:3666–3676. doi: 10.1002/pmic.200800747. [DOI] [PubMed] [Google Scholar]
- Ekman C, Linder A, Akesson P, Dahlback B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit Care. 2010a;14:R158. doi: 10.1186/cc9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman C, Stenhoff J, Dahlback B. Gas6 is complexed to the soluble tyrosine kinase receptor Axl in human blood. J Thromb Haemost. 2010b;8:838–844. doi: 10.1111/j.1538-7836.2010.03752.x. [DOI] [PubMed] [Google Scholar]
- Evenas P, Dahlback B, Garcia de Frutos P. The first laminin G-type domain in the SHBG-like region of protein S contains residues essential for activation of the receptor tyrosine kinase sky. Biol Chem. 2000;381:199–209. doi: 10.1515/BC.2000.027. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez L, Bellido-Martin L, Garcia de Frutos P. Growth arrest-specific gene 6 (GAS6). An outline of its role in haemostasis and inflammation. Thromb Haemost. 2008;100:604–610. doi: 10.1160/th08-04-0253. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. Febs J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y. Extracellular enveloped vaccinia virus escapes neutralization. Virology. 1996;217:478–485. doi: 10.1006/viro.1996.0142. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberte JP, Moss B. Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc Natl Acad Sci U S A. 2009;106:17517–17521. doi: 10.1073/pnas.0909376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M, Smith GL. Studying the binding and entry of the intracellular and extracellular enveloped forms of vaccinia virus. Methods Mol Biol. 2004;269:187–204. doi: 10.1385/1-59259-789-0:187. [DOI] [PubMed] [Google Scholar]
- Lee B, Leslie G, Soilleux E, O'Doherty U, Baik S, Levroney E, Flummerfelt K, Swiggard W, Coleman N, Malim M, et al. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol. 2001;75:12028–12038. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ling L, Liu X, Laus R, Delcayre A. A flow cytometry-based immuno-titration assay for rapid and accurate titer determination of modified vaccinia Ankara virus vectors. J Virol Methods. 2010;169:87–94. doi: 10.1016/j.jviromet.2010.07.003. [DOI] [PubMed] [Google Scholar]
- McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009;90:889–907. doi: 10.3945/ajcn.2009.27930. [DOI] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Mercer J, Knebel S, Schmidt FI, Crouse J, Burkard C, Helenius A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc Natl Acad Sci U S A. 2010;107:9346–9351. doi: 10.1073/pnas.1004618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Bristol G, Xie YM, Kung SK, Chen IS. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kawamoto K, Kishino J, Nomura K, Higashino K, Arita H. Requirement of gamma-carboxyglutamic acid residues for the biological activity of Gas6: contribution of endogenous Gas6 to the proliferation of vascular smooth muscle cells. Biochem J. 1997;323(Pt 2):387–392. doi: 10.1042/bj3230387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin LC. Virology, Molecular biology and pathogenesis. ASM press; 2010. [Google Scholar]
- Ohno K, Sawai K, Iijima Y, Levin B, Meruelo D. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat Biotechnol. 1997;15:763–767. doi: 10.1038/nbt0897-763. [DOI] [PubMed] [Google Scholar]
- Pariente N, Morizono K, Virk MS, Petrigliano FA, Reiter RE, Lieberman JR, Chen IS. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol Ther. 2007;15:1973–1981. doi: 10.1038/sj.mt.6300271. [DOI] [PubMed] [Google Scholar]
- Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni S, Lieber A, McVey JH, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- Scutera S, Fraone T, Musso T, Cappello P, Rossi S, Pierobon D, Orinska Z, Paus R, Bulfone-Paus S, Giovarelli M. Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway. J Immunol. 2009;183:3004–3013. doi: 10.4049/jimmunol.0804384. [DOI] [PubMed] [Google Scholar]
- Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004;321:788–794. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhu L, Lee S, Li L, Chang E, Soong NW, Douer D, Anderson WF. Identification of the block in targeted retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1999;96:4005–4010. doi: 10.1073/pnas.96.7.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.