INTRODUCTION
Although vaccination is most effective when used to prevent disease, cancer vaccine development has focused predominantly on providing therapy against established growing tumors (1). The difficulty in developing prophylactic cancer vaccines is due substantially to the fact that tumor antigens are variations of self and would likely mediate profound autoimmune complications if used in a preventive vaccine setting (2). Here we use several mouse breast cancer models to define a prototypic strategy for prophylactic cancer vaccination. We selected α-lactalbumin as our target vaccine autoantigen because it is a breast specific differentiation protein expressed at high levels in the majority of human breast carcinomas (3-5) and in mammary epithelial cells only during lactation (6-9). We found that immunoreactivity against α-lactalbumin provides significant protection and therapy against growth of autochthonous tumors in MMTV-neu and MMTV-PyVT transgenic mice and against 4T1 transplantable tumors in BALB/c mice. Since α-lactalbumin is conditionally expressed only during lactation, vaccination induced prophylaxis occurs without any detectable inflammation in normal non-lactating breast tissue. Thus, α-lactalbumin vaccination may provide safe and effective protection against the development of breast cancer for women in their post child-bearing pre-menopausal years when lactation is readily avoidable and risk for developing breast cancer is high (10).
RESULTS
Mouse α-lactalbumin cDNA was generated from lactating mouse mammary tissue, expressed as a 6X-His tagged fusion protein, and purified by nickel-nitrilotriacetic acid affinity chromatography followed by reverse phase HPLC to produce an endotoxin-free protein (11; Fig. 1a). Ten days after immunization of female SWXJ mice with recombinant mouse α-lactalbumin in complete Freund’s adjuvant (CFA), lymph node cells (LNC) showed dose-dependent proliferation in recall responses to α-lactalbumin and were unresponsive to recombinant human cochlin generated in a virtually identical manner (12; Fig. 1b). Responsiveness to α-lactalbumin involved both CD4+ and CD8+ T cells (Fig. 1c) and showed a proinflammatory phenotype involving high production of interferon-gamma (IFN-γ) and IL-2 and low production of IL-4, IL-5, and IL-10 (Fig. 1d).
Figure 1. Immunogenicity of recombinant mouse a-lactalbumin.

(a) Western blot showing purified recombinant mouse α-lactalbumin detected with His antibody. LNC taken 10 days after immunization of SWXJ female mice with α-lactalbumin showed recall responses that were: (b) antigen-specific to recombinant mouse α-lactalbumin but not to recombinant human cochlin over a broad dose range; (c) elicited from both purified CD4+ and CD8+ T cells in response to 25 μg/ml α-lactalbumin; and (d) consistent with a proinflammatory type 1 cytokine profile with high production of IFN-γ and IL-2 and low production of the type 2 cytokines, IL-4, IL-5, and IL-10 in response to 25 μg/ml α-lactalbumin. (e) Six weeks after α-lactalbumin immunization CD3+ T cells were detected only as isolated individual surveillance cells in mammary parenchyma of non-lactating α-lactalbumin immunized mice (arrows) and never appeared as part of any inflammatory infiltrate in non-lactating breast tissues. (f) Extensive inflammatory infiltrates of CD3+ T cells were consistently found in mammary parenchyma of lactating α-lactalbumin immunized mice (arrows). (g) CD3+ infiltrates were never observed in mammary tissue from lactating control mice immunized with CFA alone. (h) Flow cytometry analysis of breast infiltrates showed high frequencies of CD3+CD4+ (left panel) and CD3+CD8+ T cells (right panel) expressing the CD44high activated phenotype. (i) Real-time RT-PCR analysis of lactating mammary tissue showed significantly elevated expression levels of IFN-γ (P = 0.001) but not IL-10 (P > 0.10). All error bars show ±SE. Each * indicates significance.
Breast tissue from non-lactating mice immunized with α-lactalbumin never showed any inflammatory infiltration, but instead consistently showed isolated individual CD3+ T cells migrating through breast parenchyma in a classic surveillance pattern (Fig. 1e). In sharp contrast, extensive T cell infiltrates consistently occurred throughout mammary tissue of lactating mice immunized with α-lactalbumin (Fig. 1f). Breast tissue from lactating control mice immunized with CFA alone never showed any inflammatory T cell infiltration (Fig. 1g). Analysis of breast infiltrating T cells by flow cytometry showed high frequencies of CD3+CD4+ T cells and CD3+CD8+ T cells expressing the CD44high activation marker (Fig. 1h). Analysis by quantitative real-time RT-PCR showed that breast tissue from lactating mice immunized with α-lactalbumin had significantly elevated expression levels of IFN-γ (P = 0.001) but not IL-10 (P > 0.10) when compared to levels expressed in breast tissue from untreated normal non-lactating or lactating mice or from lactating mice immunized with CFA alone (Fig. 1i). We subsequently found that the inflammation observed in breasts of lactating mice immunized with α-lactalbumin was T cell mediated and resulted in breast failure characterized by pups showing diminished weight gain and failure-to-thrive often with kwashiorkor-like signs of nutritional deficiency and runting (data not shown). This breast failure phenotype mimics that observed in α-lactalbumin deficient mice (13) and in HER2/neu (ErbB2 or neu) transgenic mice vaccinated with xenogeneic HER2 DNA (14).
Our focus quickly turned to the potential use of α-lactalbumin as a breast cancer vaccine. We examined whether early immunization with α-lactalbumin could prophylactically inhibit growth of autochthonous breast tumors. MMTV-neu mice express the unactivated neu protooncogene under the regulation of the long terminal repeat of mouse mammary tumor virus (MMTV) and show a 50% incidence of spontaneous mammary tumors by 205 days of age (15). We immunized eight week old MMTV-neu mice with either α-lactalbumin in CFA or with CFA alone and euthanized all mice when the first tumor in any mouse reached 17 mm in diameter. Whereas all CFA immunized control mice developed breast tumors when the experiment was terminated at ten months of age, none of the mice immunized with α-lactalbumin had any detectable mammary tumors (P = 0.0004; Fig. 2a). Prophylactic vaccination with α-lactalbumin was also effective against transplantable 4T1 tumors. Highly significant (P = 0.0006) growth inhibition occurred in BALB/c mice immunized with α-lactalbumin 13 days prior to inoculation with 4T1 tumor cells (Fig. 2b).
Figure 2. α-lactalbumin vaccination delays and treats breast tumor growth.
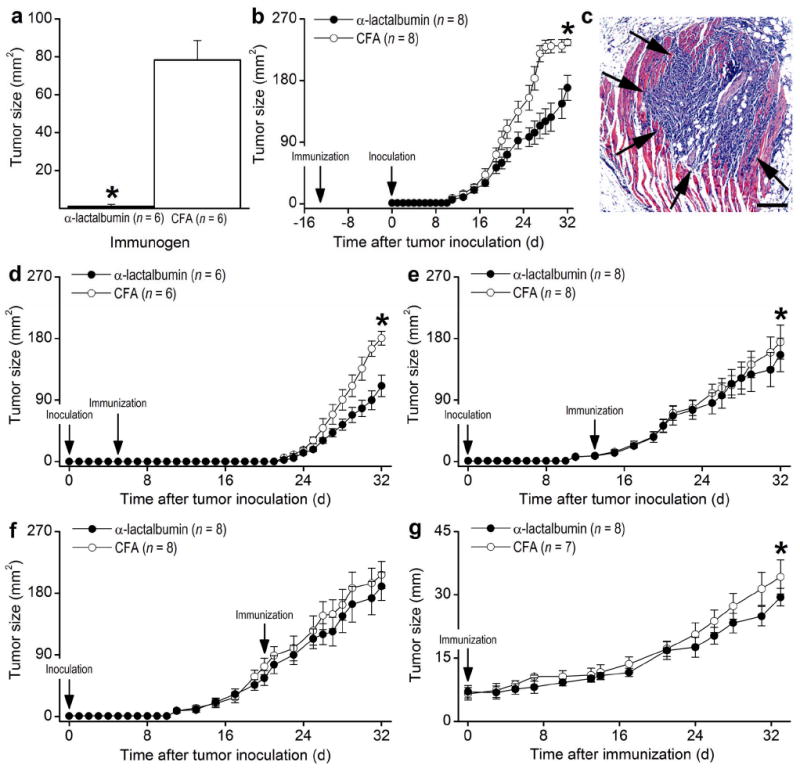
(a) Growth of autochthonous breast tumors was significantly inhibited (P = 0.0004) in ten month old MMTV-neu mice immunized with α-lactalbumin at eight weeks of age. (b) Growth of transplanted 4T1 tumors was significantly inhibited (P = 0.0006) following prophylactic immunization with α-lactalbumin 13 days prior to tumor inoculation. (c) H&E staining of tissue extracted from the flank of a representative mouse 5 days after s.c. inoculation of 2×104 4T1 tumor cells confirms the presence of an established growing tumor (arrows) infiltrating the underlying connective tissue. Size bar = 100 μm. Significant inhibition of 4T1 tumor growth occurred when α-lactalbumin immunization occurred (d) 5 days after tumor inoculation (P < 0.01) and (e) 13 days after tumor inoculation (P < 0.01), (f) but not 21 days after tumor inoculation (P > 0.10). (g) Significant inhibition (P < 0.0006) in the growth of very aggressive autochthonous tumors occurred following α-lactalbumin immunization of MMTV-PyVT transgenic mice at 6 weeks of age. Tumors in MMTV-PyVT mice were amenable to measurement in only one direction. All error bars show ±SE. Each * indicates significance.
We next determined whether α-lactalbumin immunization could therapeutically inhibit growth of established tumors. Although palpable tumors appear by 2-3 weeks following s.c. inoculation of BALB/c mice with 2×104 4T1 tumor cells, tumors are well established within 5 days after inoculation (Fig. 2c). We observed significant inhibition of tumor growth (P < 0.01 in each case) when α-lactalbumin vaccination occurred at 5 days (Fig. 2d) and 13 days (Fig. 2e) but not at 21 days (Fig. 2f) after inoculation with 4T1 tumor cells. However, the lack of tumor growth inhibition in mice immunized 21 days after inoculation may likely be due to the shortened 11 day observation period between the time of immunization and the time when tumors reached the maximum size mandating euthanasia.
We also determined the effectiveness of α-lactalbumin vaccination on the growth of established autochthonous tumors. MMTV-PyVT transgenic mice constitutively express activated neu in breast tissues and develop very rapidly growing mammary tumors palpable by 5 weeks of age (16). We observed significant inhibition (P < 0.0006) in the growth of very aggressive established autochthonous tumors in MMTV-PyVT transgenic mice vaccinated at 6 weeks of age with α-lactalbumin (Fig. 2g). Our results indicate that α-lactalbumin vaccination provides effective protection and therapy against breast tumor growth and is particularly effective when immunization occurs prior to the appearance of palpable tumors.
Tumors taken 32 days after inoculation with 4T1 tumor cells and immunization with α-lactalbumin showed extensive infiltration of CD3+ T cells (Fig. 3a). Such inflammatory infiltrates did not occur in tumors from CFA immunized control mice (Fig. 3b). Flow cytometry analysis of tumor infiltrating lymphocytes (TILs) showed a predominance of CD4+ (64.3%) over CD8+ (14.4%) T cells (Fig. 3c) and a type-1 proinflammatory phenotype involving high production of IFN-γ rather than IL-5 or IL-10 measured by ELISA in response to α-lactalbumin (Fig. 3d). ELISPOT analysis of TILs showed that CD4+ rather than CD8+ T cells produced the IFN-γ since its secretion by cultured T cells was inhibited by treatment with class II but not class I specific antibodies (Fig. 3e). However, CD8+ T cells mediated 4T1 specific cytotoxicity since death of cultured 4T1 tumor cells was inhibited by treatment of cultured α-lactalbumin primed LNC with antibodies specific for mouse CD8 but not CD4 (Fig. 3f).
Figure 3. α-lactalbumin specific T cells induce tumor inflammation and cytotoxicity.

(a) Thirty-two days after BALB/c mice were vaccinated with α-lactalbumin and inoculated with 4T1 cells, tumors were extensively infiltrated with CD3+ T cells. (b) T cell infiltrates were never observed in control inoculated mice immunized with CFA alone. (c) Flow cytometry analysis of TILs showed a predominance of CD4+ (64.3%) over CD8+ (14.4%) T cells. (d) TILs showed a type-1 proinflammatory phenotype involving high production of IFN-γ in response to 50 μg/ml α-lactalbumin. (e) ELISPOT analysis of TILs showed that CD4+ rather than CD8+ T cells produced IFN-γ in response to 50 μg/ml α-lactalbumin since its secretion by cultured T cells was inhibited by treatment with class II but not class I antibodies. (f) CD8+ T cells mediated 4T1 specific cytotoxicity since death of cultured 4T1 tumor cells was inhibited by treatment of cultured α-lactalbumin primed LNC with antibodies specific for mouse CD8 but not CD4. All data shown are representative of three experiments providing similar results. All error bars show ±SE.
Transfer of α-lactalbumin primed T cells but not primed B cells or sera induced breast failure in naive recipients (data not shown). Similarly, transfer of α-lactalbumin primed LNC into naive recipient BALB/c mice on the same day as inoculation with 4T1 tumors resulted in highly significant (P < 0.0001) inhibition of tumor growth (Fig. 4a), significantly decreased (P < 0.03) incidence of tumor bearing mice (Figure 4b), and significantly decreased (P < 0.0008) final tumor weight (Fig. 4c). We also found that significant tumor growth inhibition occurred in naive mice receiving either CD4+ (Fig. 4d, left; P = 0.002) or CD8+ (Fig. 4d, right; P = 0.003) T cells enriched by magnetic bead separation from α-lactalbumin primed LNC. Overall, our data indicate that activated CD4+ and CD8+ TILs mediate the protective and therapeutic effects of α-lactalbumin vaccination on breast tumor growth.
Figure 4. Inhibition of tumor growth by α-lactalbumin vaccination is mediated by T cells.
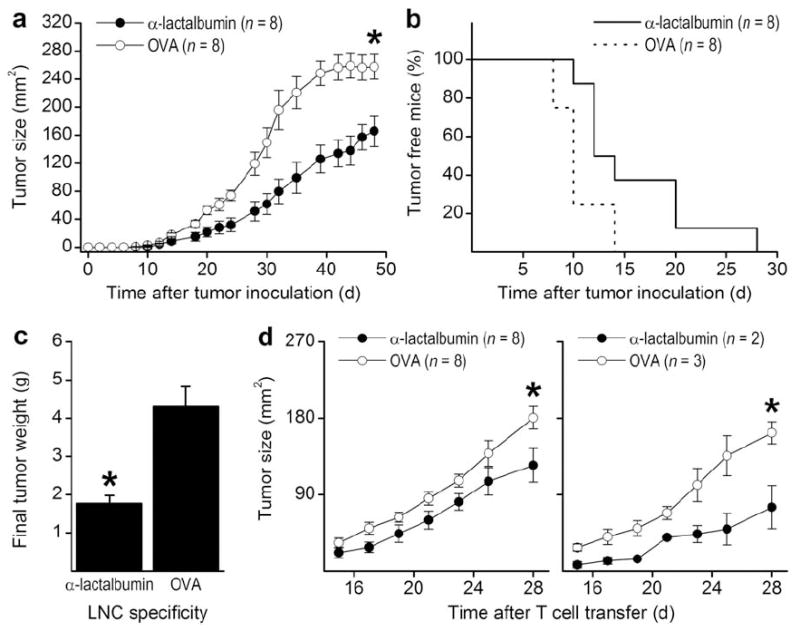
Transfer of α-lactalbumin primed LNC into naive recipient BALB/c mice on the same day as inoculation with 4T1 tumors resulted in (a) highly significant (P < 0.0001) inhibition of tumor growth, (b) significantly decreased (P < 0.03) incidence of tumor bearing mice, and (c) significantly decreased (P < 0.0008) final tumor weight. (d) Significant tumor growth inhibition occurred in naive mice receiving either CD4+ (left panel; P = 0.002) or CD8+ (right panel; P = 0.003) T cells enriched by magnetic bead separation from α-lactalbumin primed LNC. All error bars show ±SE. Each * indicates significance.
DISCUSSION
Our study indicates that induction of autoimmune mediated breast failure following a single immunization with α-lactalbumin provides effective breast cancer vaccination. However, the most remarkable implication of our study is that α-lactalbumin vaccination provides prophylaxis against breast cancer in the absence of any detectable autoimmune induced breast inflammation. Thus, our results may serve as a rational basis for the development of a safe and effective prophylactic vaccine against human breast cancer. In a broader sense, our study defines the criteria for antigen selection in the development of any targeted prophylactic cancer vaccine: 1) the antigen must be constitutively overexpressed in the majority of targeted tumors; 2) expression of the target antigen in normal tissue must be conditional; and 3) the condition determining expression of the target antigen in normal tissue must be readily avoidable. The lactation-dependent milk protein, α-lactalbumin, meets these criteria for prophylactic breast cancer vaccination.
Relative to most organs, the breast is metabolically dormant until lactation occurs, at which time the milk proteins, including α-lactalbumin, are overexpressed. Thus, α-lactalbumin availability in normal non-lactating breast tissue is insufficient to target any autoimmune inflammation. This unique conditional expression of a differentiation protein provides an opportunity for prophylactic breast cancer vaccination of normal healthy women who are either willing to avoid lactation or are past their child-bearing years and at ever increasing risk for breast cancer. Thus, in a broader perspective, it is not difficult to envision an adult vaccination plan patterned on the childhood vaccine strategy that would provide prophylaxis against adult onset diseases including, but not limited to, breast cancer.
For successful cancer vaccination, it is essential that the selected immunogen is overexpressed in the targeted tumor. Although one early study was unable to show α-lactalbumin mRNA in human breast tumors (17), and other studies have shown that expression of α-lactalbumin protein by breast tumors may not render any substantial diagnostic or prognostic usefulness (3-5), several reports have shown that the α-lactalbumin protein is produced in the majority of human breast malignancies at levels sufficient for detection by immunocytochemical analysis (3-5). Moreover, recent studies involving adenoviral mediated gene delivery have shown that the α-lactalbumin promoter can be used for specifically targeting human breast tumors for expression of several toxic factors including the tumoricidal E1A transcriptional regulator (18) and the suicide genes, thymidine kinase (19) and cytosine deaminase (20). Although these studies indicate that α-lactalbumin is expressed in appreciable amounts in most human breast tumors, the ability of α-lactalbumin to target effective breast cancer vaccination also depends on whether it is sufficiently immunogenic in humans. Our preliminary in vitro priming data clearly indicate that women have a substantial proinflammatory T cell repertoire available for responding to recombinant human α-lactalbumin (data not shown).
Although CD4+ T cells often may be more efficient at tumor rejection than CD8+ T cells (21), optimal tumoricidal activity typically occurs when tumor responses involve both CD4+ and CD8+ T cells (22). Thus, it is worth noting that the response to α-lactalbumin involves both CD4+ and CD8+ T cells. Nevertheless, it is not likely that our current data reflect an optimized cancer vaccination strategy. Incorporation of a dendritic cell vaccine approach (23) offers the potential for in vitro shaping of the autoimmune anti-tumor T cell repertoire perhaps by enhancement of the autoaggressive Th17 lineage (24) and/or by selective costimulatory/coinhibitory manipulation (25). A complementary and perhaps synergistic strategy may also involve partial inhibition or ablation of FoxP3+ regulatory T cells that inhibit autoimmunity (26). In any event, our data provide experimental support for developing safe and effective protection against breast tumors, and potentially tumors derived from other organs, by targeted vaccination against conditionally expressed tissue specific differentiation proteins.
MATERIALS AND METHODS
Recombinant mouse α-lactalbumin
α-lactalbumin cDNA generated from lactating mouse mammary tissue was inserted into the pQE-82L expression vector (Qiagen, Valencia, CA) for producing a 6X-His tagged fusion protein in SG13009 E. coli (Stratagene, La Jolla, CA). His-tagged α-lactalbumin was purified by nickel-nitrilotriacetic acid affinity chromatography followed by reverse phase HPLC to yield endotoxin-free protein (11).
Mice and immunization
Female SWXJ (H-2q.s), MMTV-neu, MMTV-PyVT, and BALB/cJ mice were purchased commercially (Jackson, Bar Harbor, ME) and immunized by s.c. injection in the abdominal flank with 100 μg α-lactalbumin in 200 μl of an emulsion of equal volumes of water and complete Freund’s adjuvant (CFA; Difco, Detroit, MI). All protocols were approved by the IACUC of the Cleveland Clinic.
Proliferation assays
3×105 primed LNC were cultured in triplicate in 200 μl of DMEM (Mediatech Cellgro, Herndon, VA) supplemented as described previously (27) in 96-well flat-bottomed microtiter Falcon plates (BD Labware, Franklin Lakes, NJ) in the presence of serial 10-fold dilutions of immunogen. Cultures were pulsed at 72 hours with 3H-thymidine, and the stimulation index was determined as mean scintillation counts per minute from cultures with antigen divided by those from cultures without antigen. CD4+ and CD8+ T cells were positively enriched (> 90%) from primed LNC by magnetic bead separation using a MidiMACS cell separator (Miltenyi, Auburn, CA). 3×105 purified CD4+ and CD8+ T cells/well were cultured as described above in the presence of 2.5×105 γ-irradiated (2000 rads) naive syngeneic splenocytes serving as antigen-presenting cells.
Adoptive transfer
Ten day primed LNC or CD4+ and CD8+ T cells enriched (> 90%) by magnetic bead separation (Miltenyi) from primed LNC were cultured at 4-5×106 cells/well in supplemented DMEM at 2 ml/well in 24 well plates (BD Labware) containing immunogen at 25 μg/ml. Wells containing purified CD4+ or CD8+ T cells were supplemented with 4×106 γ-irradiated (2000 rads) naive syngeneic splenocytes as antigen-presenting cells. At 72 hours, 2-3×107 washed cells were injected i.p. into naive recipients in 200 μl PBS.
Tumor inoculation
4T1 mouse mammary carcinoma cells (ATCC CRL-2539; Rockville, MD) were cultured in 75 cm2 tissue culture flasks (BD Labware) in RPMI 1640 (Mediatech CellGro) supplemented and incubated as described above. At 70-75% confluence, cells were harvested by treatment with 0.25% trypsin and 0.02% EDTA (Sigma Aldrich, St. Louis, MO), and 2×104 washed cells were inoculated s.c. in the abdominal flank of 7-10 week old BALB/c females. Mice were weighed and tumors were measured by Vernier caliper daily. Tumor area was calculated as length × breadth. Mice were euthanized 32 days after 4T1 inoculation. In tumor prophylaxis experiments involving MMTV-neu mice, all mice were euthanized on the day when the first tumor on any mouse reached a length of 17 mm. Due to massive multifocal tumor growth, tumors in MMTV-PyVT mice were amenable to measurement in only one direction. The length on all ten MMTV-PyVT tumors were added to calculate total tumor load in mm on each day.
Isolation of breast and tumor infiltrating lymphocytes
Lymphocytes were isolated from lactating breasts or 4T1 tumors by digestion of minced tissue for 30 minutes at 37°C in HBSS containing 50 KU of DNase I (Sigma Aldrich) and 0.2 mg/ml collagenase II (Life Technologies, Carlsbad, CA). Cells were collected by discontinuous gradient centrifugation and further enriched for T cells by positive selection using Thy1.2 antibody coated magnetic beads and a MidiMACS cell separator (Miltenyi). These enriched cells were used for flow cytometry analysis and in ELISA and ELISPOT assays.
Flow cytometry analysis
Enriched T cells obtained from digestion of lactating breast tissue were triple stained with CD3-FITC and CD44-Cy5 as well as either CD4-PE or CD8-PE antibodies (BD Biosciences). Enriched TILs were double stained with CD3-FITC and either CD4-PE or CD8-PE antibodies. Data collected on 30,000 total events were analyzed using FlowJo software (BD Biosciences) after gating on the CD3+ population.
ELISA and ELISPOT assays
As described previously (27), ELISAs were used to measure cytokine concentrations on 48 hour supernatants of 10-day-primed LNC cultured in supplemented DMEM at 4-5×106 cells/well in 24-well flat-bottom Falcon plates (BD Labware) in the presence of 25 μg/ml α-lactalbumin in a final volume of 2 ml/well. Purified capture/detection antibody pairs and recombinant cytokine standards were obtained commercially (BD Biosciences). ELISAs were also used to measure cytokine concentrations in microtiter well cultures containing 1.5×105 Thy1.2 enriched TILs, 2×104 γ-irradiated (2000 rads) syngeneic splenocyte feeder cells, and 50 μg/ml α-lactalbumin in a total volume of 200 μl/well supplemented DMEM (Mediatech Cellgro). Identical culture conditions using ELISPOT plates (Millipore, Billerica, MA) and capture/detection mouse IFN-γ antibody pairs were used to determine frequencies of IFN-γ producing TILs in response to 50 μg/ml α-lactalbumin or grade VII OVA (Sigma Aldrich) as previously described (12). Some ELISPOT cultures were treated at initiation with 20 μg/ml class I specific (H-2Kd and H-2Dd) or class II specific (IAd and IEd) blocking antibodies or their IgG isotype controls (eBiosciences, San Diego, CA).
T cell cytotoxicity assay
2×104 LNC from BALB/c mice immunized 10 days prior with α-lactalbumin were co-cultured in triplicate microtiter wells containing 200 μl/well supplemented DMEM in the presence of 1×104 4T1 tumor cells pretreated with 50 μg/ml colchicine (Sigma Aldrich) to arrest cell division. Cytotoxicity was determined at 96 hours as percent survival relative to maximum survival determined from wells containing 1×104 4T1 cells alone using the CellTiter 96 aqueous one solution cell proliferation assay (Promega, Madison, WI). Some cultures were treated at initiation with 20 μg/ml of blocking antibodies specific for mouse CD4 or CD8 (eBiosciences).
Real time RT-PCR
Breast tissue RNA was converted to cDNA and analyzed for gene expression by real-time RT-PCR using the following primer pairs: IFN-γ forward 5′-TCAAGTGGCATAGATGTGGAAGAA-3′, reverse 5′-TGGCTCTGCAGGATTTTCATG-3′; IL-10 forward 5′-GGTTGCCAAGCCTTATCGGA-3′, reverse 5′-ACCTGCTCCACTGCCTTGCT-3′; and GAPDH forward 5′-TTCACCACCATGGAGAAGGGC-3′, reverse 5′-GGCATCGACTGTCATGA-3′. Relative gene-expression was determined as the ratio of test gene expression to GAPDH gene expression for each tissue using the comparative threshold cycle method.
Immunocytochemistry
Unmasked and blocked formalin-fixed paraffin-embedded tissues at 6 μm were treated with a 1:50 dilution of rat anti-mouse CD3 (Novacastra, Newcastle Upon Tyne, UK) followed by a 1:100 dilution of mouse-adsorbed biotinylated goat anti-rat IgG (BD Biosciences). Slides were developed conventionally using streptavidin-horseradish peroxidase complex (Vector, Burlingame, CA).
Biostatistical analysis
Differences between mRNA expression levels, mean tumor weights, and mean tumor areas were compared using the Student’s t-test. Differences between tumor growth curves were compared by unweighted one-way ANOVA for correlated samples. Differences between Kaplan-Meier curves were compared using the logrank test.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI-51837 and R01CA-14035 (V.K. Tuohy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard abbreviations used
- CFA
complete Freund’s adjuvant
- ErbB2, HER2, HER2/neu, or neu
epidermal growth factor receptor tyrosine kinase
- GAPDH
glyceraldehyde phosphate dehydrogenase
- IFN-γ
interferon-gamma
- LNC
lymph node cells
- MMTV
mouse mammary tumor virus
- TILs
tumor infiltrating lymphocytes
Footnotes
AUTHOR CONTRIBUTIONS R.J. and P.K. contributed equally to this work. R.J. performed the adoptive tumor immunotherapy studies, the TIL analysis, and evaluated the effects of α-lactalbumin vaccination in MMTV-PyVT mice. P.K. evaluated the phenotype of autoimmune mediated breast failure and performed the initial tumor immunotherapy studies. J.M.J. participated in the cloning and generation of α-lactalbumin. C.Z.A and D.J-w. provided technical assistance with molecular and immunocytochemical assays, and V.K.T. designed the experiments, supervised and obtained funding for the project, analyzed the data, and wrote the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
The authors have no conflicting or competing financial interests regarding publication of this manuscript.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 3.Lee AK, et al. Tumor marker expression in breast carcinomas and relationship to prognosis. An immunohistochemical study. Am J Clin Pathol. 1985;84:687–696. doi: 10.1093/ajcp/84.6.687. [DOI] [PubMed] [Google Scholar]
- 4.Wrba F, et al. Prognostic significance of immunohistochemical parameters in breast carcinomas. Pathol Res Pract. 1988;183:277–283. doi: 10.1016/S0344-0338(88)80122-5. [DOI] [PubMed] [Google Scholar]
- 5.Cohen C, Sharkey FE, Shulman G, Uthman EO, Budgeon LR. Tumor-associated antigens in breast carcinomas. Prognostic significance. Cancer. 1987;60:1294–1298. doi: 10.1002/1097-0142(19870915)60:6<1294::aid-cncr2820600622>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Nagamatsu Y, Oka T. Purification and characterization of mouse alpha-lactalbumin and preparation of its antibody. Biochem J. 1980;185:227–237. doi: 10.1042/bj1850227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren J, Stuart DI, Acharya KR. Alpha-lactalbumin possesses a distinct zinc binding site. J Biol Chem. 1993;268:19292–19298. [PubMed] [Google Scholar]
- 8.Vilotte JL, Soulier S. Isolation and characterization of the mouse alpha-lactalbumin-encoding gene: interspecies comparison, tissue-and stage-specific expression. Gene. 1992;119:287–292. doi: 10.1016/0378-1119(92)90285-w. [DOI] [PubMed] [Google Scholar]
- 9.Vilotte JL, Soulier S, Mercier JC. Sequence of the murine α-lactalbumin-encoding cDNA: interspecies comparison of the coding frame and deduced pre-protein. Gene. 1992;112:251–255. doi: 10.1016/0378-1119(92)90385-3. [DOI] [PubMed] [Google Scholar]
- 10.Setiawan VW, Feigelson HS, Henderson BE. Epidemiology and risk factors: an update. In: Bonadonna G, Hortobagyi GN, Valagussa P, editors. Textbook of Breast Cancer: A Clinical Guide to Therapy. third edition. Abingdon, England: Informa Healthcare/Taylor & Francis; 2006. pp. 1–16. [Google Scholar]
- 11.Dudley A, McKinstry W, Thomas D, Best J, Jenkins A. Removal of endotoxin by reverse phase HPLC abolishes anti-endothelial cell activity of bacterially expressed plasminogen kringle 5. Biotechniques. 2003;35:724–726. doi: 10.2144/03354st02. [DOI] [PubMed] [Google Scholar]
- 12.Baek M-J, et al. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol. 2006;177:4203–4210. doi: 10.4049/jimmunol.177.6.4203. [DOI] [PubMed] [Google Scholar]
- 13.Stinnakre MG, Vilotte JL, Soulier S, Mercier JC. Creation and phenotypic analysis of alpha-lactalbumin-deficient mice. Proc Natl Acad Sci USA. 1994;5:6544–6548. doi: 10.1073/pnas.91.14.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pupa SM, et al. Inhibition of mammary carcinoma development in HER-2/neu transgenic mice through induction of autoimmunity by xenogeneic DNA vaccination. Cancer Res. 2005;65:1071–1078. [PubMed] [Google Scholar]
- 15.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall L, Craig RK, Davies MS, Ralphs DN, Campbell PN. Alpha-lactalbumin is not a marker of human hormone-dependent breast cancer. Nature. 1981;290:602–604. doi: 10.1038/290602a0. [DOI] [PubMed] [Google Scholar]
- 18.Li X, et al. Transcriptional targeting modalities in breast cancer gene therapy using adenovirus vectors controlled by alpha-lactalbumin promoter. Mol Cancer Ther. 2005;4:1850–1859. doi: 10.1158/1535-7163.MCT-05-0167. [DOI] [PubMed] [Google Scholar]
- 19.Anderson LM, et al. Adenovirus-mediated tissue-targeted expression of the HSVtk gene for the treatment of breast cancer. Gene Ther. 1999;6:854–864. doi: 10.1038/sj.gt.3300909. [DOI] [PubMed] [Google Scholar]
- 20.Anderson LM, Krotz S, Weitzman SA, Thimmapaya B. Breast cancer-specific expression of the Candida albicans cytosine deaminase gene using a transcriptional targeting approach. Cancer Gene Ther. 2000;7:845–852. doi: 10.1038/sj.cgt.7700191. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Diez A, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59:1071–1079. [PubMed] [Google Scholar]
- 23.Ludewig B, et al. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 26.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altuntas CZ, Johnson JM, Tuohy VK. Autoimmune targeted disruption of the pituitary-ovarian axis causes premature ovarian failure. J Immunol. 2006;177:1988–1996. doi: 10.4049/jimmunol.177.3.1988. [DOI] [PubMed] [Google Scholar]


