Abstract
Primary biliary cirrhosis is a frequent indication for liver transplantation. The purpose of this report is to present our experience with liver transplantation for primary biliary cirrhosis. Attention is given to the causes of hepatic dysfunction seen in allografts. In addition, we review the postoperative problems encountered and the quality of life at time of last follow-up in patients with transplants for primary biliary cirrhosis. A total of 97 orthotopic liver transplant procedures were performed in 76 patients with advanced primary biliary cirrhosis at the University of Pittsburgh from March 1980 through September 1985. The transplant operation was relatively easy to perform. The most common technical complications experienced were fragmentation and intramural dissection of the recipient hepatic artery, which required an arterial graft in 20% of the cases. Most of the postoperative mortality occurred in the first 6 mo after transplantation, with an essentially flat actuarial life survival curve from that time point to a projected 5-yr survival of 66%. Common causes of death included rejection and primary graft nonfunction. Thirteen of the 76 patients had some hepatic dysfunction at the time of the last follow-up, although none were jaundiced. Recurrence of primary biliary cirrhosis could not be demonstrated in any of the patients. Antimitochondrial antibody was detected in the serum of almost all of the patients studied postoperatively for it. Most important, almost all of the 52 surviving patients have been rehabilitated socially and vocationally.
Primary biliary cirrhosis (PBC), or chronic nonsuppurative destructive cholangitis, is a slowly progressive disease of unknown etiology that frequently has associations with other autoimmune diseases (1–3). Primary biliary cirrhosis chiefly affects middle-aged women. The diagnosis is established using a combination of clinical findings, particularly chronic cholestasis, the presence of antimitochondrial antibodies (AMAs), increased immunoglobulin M levels, and liver biopsy findings of a chronic inflammatory process, particularly lymphocytic infiltration within portal tracts and interlobular bile ducts (when detected early) or an absolute paucity of bile ducts in the cirrhotic stage of the disease (4,5). Hepatic failure or variceal hemorrhage, or both, are common terminal events (6).
In the past, medical treatment has consisted of the management of symptoms and the prevention, early recognition, and treatment of systemic complications. More recently, orthotopic liver transplantation (OLT) has been offered as a therapeutic modality to patients with advanced disease (7). However, Neuberger et al. (8) have reported the recurrence of PBC in 3 of their first 11 liver transplant recipients operated on for PBC. This report somewhat quieted the initial enthusiastic acceptance of liver transplantation for this particular indication. Herein we report the results of hepatic replacement in 76 patients with PBC who were treated between March 1980 and September 1985 at the University of Pittsburgh using cyclosporine and prednisone as the immunosuppressive agents. In cases that manifested postoperative graft dysfunction, attempts were made to determine whether or not recurrence of PBC was responsible.
Materials and Methods
The records of 450 liver recipients treated between March 1980 and September 1985 at the University of Colorado (until February 1981) and the University of Pittsburgh (since February 1981) were reviewed. Based on preoperative evaluation and pathologic examination of the resected livers, 76 recipients had PBC as the indication for liver transplantation. The immunosuppression used in all cases was cyclosporine and prednisone (7) to which antilymphocyte globulin or, more recently, the monoclonal antibody OKT3 (Orthoclone, ORTHO, Pharmaceutical Corporation, Raritan, N.J.) had been added for short intervals to treat rejection episodes (9). In a few patients with smoldering rejection, low doses of azathioprine were added to the standard combination of cyclosporine and prednisone.
Before OLT, signs and symptoms of PBC had been present for more than 5 yr in 58% of the cases in this series (Table 1). At the time of initial evaluation, their ages ranged from 31 to 68 yr. The female/male ratio was ~10:1. The most common symptoms and signs were jaundice, pruritus, gastrointestinal bleeding, and fatigue. Hepatosplenomegaly, ascites, and bone disease were also common (Table 1).
Table 1.
Characteristics of 76 Liver Transplant Recipients for Primary Biliary Cirrhosis
| Age and sex distribution
|
Time from onset of symptoms to transplantation
|
Clinical features
|
||||||
|---|---|---|---|---|---|---|---|---|
| Age (yr) | Male | Female | Time (yr) | No. of patients (%) | Symptoms | No. of patients (%) | Signs | No. of patients (%) |
| 30–39 | 1 | 14 | 1 | 3 (4.0) | Pruritus | 50 (65.8) | Jaundice | 75 (97) |
| 40–49 | 2 | 41 | 2 | 4 (5.3) | Fatigue | 31 (40.8) | Ascites | 55 (72) |
| 50–59 | 2 | 13 | 3 | 8 (10.7) | Abdominal pain | 17 (23) | Gastrointestinal hemorrhage | 55 (72) |
| ≥60 | 2 | 1 | 4 | 8 (10.7) | ||||
| Total | 7 | 69 | 5 | 9 (12.0) | Encephalopathy | 25 (33) | Hepatomegaly | 52 (69) |
| 6 | 9 (12.0) | Splenomegaly | 39 (52) | |||||
| 7 | 9 (11.8) | Xanthomas | 14 (18.4) | |||||
| 8 | 5 (6.6) | |||||||
| 9 | 8 (10.7) | |||||||
| 10 | 7 (9.3) | |||||||
| >10 | 6 (8.0) | |||||||
Operative Procedures and Findings
Liver transplantation was performed using a technique described previously (10), including venous bypass for the last 61 recipients (11). Most of the excised livers were considerably larger than normal. Although hilar lymphadenopathy and portal hypertension were common findings at surgery, the hepatectomies were easy to perform, particularly when there had been no previous surgical procedures performed in the right upper quadrant of the recipient. In 2 patients the hepatectomy was complicated by the presence of a surgically created portal-systemic shunt performed 3 and 4 yr before OLT.
Special problems with the rearterialization phases of the OLT procedure were common and were caused by the friability of the recipient’s hepatic arteries, which had a tendency for intimal dissection at the site of ligation of arterial branches or where vascular clamps were applied. When dissection was recognized (20% of patients), an interposition graft, using the donor iliac artery (12), was placed proximal to the infrarenal recipient aorta and was tunneled through the retroperitoneum to the graft hilar area, where it was anastomosed to the graft arterial supply (12).
The most common biliary reconstruction used was a duct-to-duct anastomosis (86% of patients) over a T-tube stent. The only commonly used alternative method was a choledochojejunostomy to a Roux-en-Y limb (13% of patients) (7).
Laboratory Data
Total bilirubin, alanine transaminase, alkaline phosphatase, and γ-glutamyl transferase were assayed before transplantation, at multiple times after transplantation, and at the time of last follow-up in all surviving recipients (November 1986). After patients were discharged from the hospital, many different laboratories performed these tests and considered them abnormal only if the result was >1.5 mg/dl for total bilirubin, 37 IU/L for alanine transaminase, 100 IU/L for alkaline phosphatase, and 44 IU/L for γ-glutamyl transferase.
The preoperative mean total bilirubin level was 20.1 mg/dl (range 1.5–51.0). The single patient with a bilirubin of 1.5 mg/dl at the time of OLT was bedridden because of numerous fractures and had had two episodes of variceal gastroesophageal hemorrhage. The mean alkaline phosphatase level before transplantation was 695 IU/L (range 43–2640). The mean alanine transaminase was 163 IU/L with a range from 12 to 1011, and the mean γ-glutamyl transferase was 374 IU/L (range 20–2115).
Preoperative AMA titers were compared to those obtained postoperatively and at last follow-up as of November 1986 for all surviving patients.
Pathologic Studies
Representative sections of the liver were obtained and examined from both lobes of the resected liver. According to protocol, three hilar sections containing major blood vessels, nerve trunks, and bile ducts were obtained and examined (13). All sections were stained with hematoxylin and eosin, trichrome, orcein, and rhodanine and were examined by light microscopy. The orcein and rhodanine stains were used for the detection of copper-binding protein and copper within the tissue, respectively. Histologic staging of the native liver (stages I–IV) was determined using established criteria (5,14).
A similar protocol was used to histologically assess all failed allografts if either autopsy or retransplantation occurred. Posttransplant biopsy specimens from dysfunctional allografts, particularly those obtained beyond 2 mo postoperatively, were evaluated similarly. All histologic specimens were evaluated by a staff pathologist (A.J.D.) for the following: (a) presence of cirrhosis, (b) marginal ductular proliferation, (c) granulomas, (d) portal or septal lymphoid nodules, (e) location of cholestasis (if present), (f) portal or hepatic venous subendothelial infiltration (or both), (g) mononuclear inflammatory cell damage of the bile ducts and ductular loss, (h) chronic arterial or venous lesions (or both) (15), and (i) the presence and location of tissue copper and copper-binding protein accumulation.
Rejection was considered to be present if the patient developed postoperative abnormalities in any of the hepatic injury tests performed, whether or not symptoms such as fever, anorexia, and abdominal pain occurred. In most cases, liver biopsy specimens were obtained to either confirm or refute the clinical impression of rejection. Clinical pathologic correlations were attempted systematically, but for differentiating acute or chronic rejection from recurrent PBC, the morphologic criteria summarized in Table 2 were used.
Table 2.
Assessment of Histologic Findings in Primary Biliary Cirrhosis Compared With Acute and Chronic Rejection
| Endstage PBC | Acute rejection | Chronic rejection | |
|---|---|---|---|
| Granuloma | + | — | — |
| Lymphoid follicles | + + | +/− | + |
| Portal and central vein infiltration | — | + + | + |
| Bile duct damage | + + + | + | + + + |
| Bile duct loss | + + + | +/− | + + + |
| Chronic vascular lesionsa | + | — | + + + |
| Cholestasis | Peripheral | Central | Central |
| Tissue copper deposition | + +/Peripheral | — | — |
| Cirrhosisb | + + | — | +/− |
| Marginal duct proliferation | + + | −/+c | — |
PBC, primary biliary cirrhosis.
Subintimal foam cells in arterial and portal veins, fibrointimal hyperplasia, intimal sclerosis, and rupture and duplication of internal elastic lamina of large arteries are seen in chronic rejection. Mild atherosclerotic changes may be seen in large hilar arteries of PBC livers.
Portal-to-central linkage with regenerative parenchymal nodules.
Normally seen only after treatment of severe acute rejection.
Data Analysis
All data are presented as mean values and range. Patient survival was calculated by the life table method (MOP statistical software, Los Angeles, Calif.). The χ2-test was used to test differences between proportions and the significance of associations. A probability value <0.05 was considered to be significant.
Results
Survival
Fifty-two (68%) of the 76 recipients are alive at follow-ups of 1–6.5 yr. During the 1980–1985 period of case accrual, the survival of patients with PBC was arithmetically better than that of adult recipients with all other indications for the procedure (Figure 1). This difference was due largely to inclusion in the latter curve of many recipients with diseases such as hepatitis B-positive cirrhosis and hepatic malignancy, which have a high incidence of lethal recurrence within 1–3 yr. For patients with PBC, calculated survival curves appear to be flat after the first full year of survival, with an actuarial 5-yr survival of 66% (Figure 1).
Figure 1.
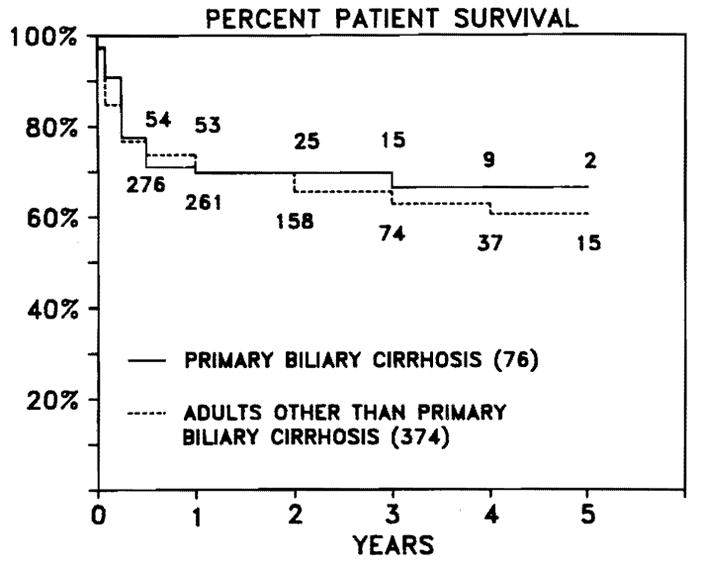
Actuarial survival of adult liver transplant recipients using cyclosporine. The numbers in our graph identify the number of patients alive at each time point. There is no statistical difference between the two groups in survival.
Patient survival for the PBC group would have been considerably less favorable without the frequent use of retransplantation. In 19 of the 76 recipients (25%) with PBC, retransplantation was performed within a few days to almost 3 yr after the initial graft. In 2 of the cases, three successive transplant procedures were attempted. It is important to note that 9 of the 19 patients (47%) who required retransplantation are included among the 52 current survivors. This retransplantation rate is somewhat greater than that in all other groups, but was possible because of the better overall general condition of PBC patients before initial transplantation as compared with those transplanted for other indications. Moreover, the level of functioning of other critical (nonhepatic) organs such as the lungs and kidney, and the presence or absence of infection—not the severity of the initial or even subsequent hepatic dysfunction—determine whether or not retransplantation is possible. These other factors are less of a problem in patients transplanted for PBC than they are for those transplanted for other indications.
Table 3 shows the proportion of patients alive at the time of last follow-up segregated according to duration of survival and six clinical parameters frequently used to categorize patients. No difference in either short- or long-term survival was seen between groups of PBC patients based upon age, total bilirubin or albumin levels, the presence or absence of ascites, or a history of gastrointestinal bleeding. However, the survival of patients who had clinically evident hepatic encephalopathy before OLT was reduced (56%) compared with that of those without encephalopathy (78%) (χ2 = 4.186; p < 0.05).
Table 3.
Proportion of Patients Alive at Time of Last Follow-up
| Duration of follow-up
|
||||
|---|---|---|---|---|
| <6 mo (n = 36) | 6 mo–1 yr (n = 14) | 1–5 yr (n = 24) | >5 yr (n = 2) | |
| Age (yr) | ||||
| <50 | 40 | 100 | 75 | 100 |
| ≥50 | 42 | 100 | 100 | 100 |
| Bilirubin (total) (mg/dl) | ||||
| <10 | 29 | 100 | 100 | — |
| ≥10 but <15 | 86 | 100 | 100 | — |
| ≥15 | 58 | 100 | 95 | 100 |
| Albumin (g/dl) | ||||
| <3 | 50 | 100 | 100 | 100 |
| ≥3 | 41 | 100 | 100 | 100 |
| Ascites | ||||
| Yes | 36 | 100 | 100 | 100 |
| No | 55 | 100 | 80 | — |
| Previous gastrointestinal bleeding | ||||
| Yes | 36 | 100 | 100 | 100 |
| No | 55 | 100 | 80 | – |
| Clinical encephalopathy | ||||
| Yes | 15 | 100 | 100 | 100 |
| No | 57 | 100 | 94 | — |
All values are percentages.
Causes for Mortality
The 24 deaths that occurred in this series are summarized in Table 4. Two patients died during the OLT operation as a result of technical difficulties. Postoperatively, rejection was the single most predominant cause of death, either because retransplantation was not possible (3 cases) or unsuccessful (7 cases). Primary graft nonfunction, although uncommon, was associated with a 100% mortality.
Table 4.
Causes of Death in Patients With Primary Biliary Cirrhosis After Liver Transplantation
| No. days (<90, early) | No. days (≥90, late) | |
|---|---|---|
| Rejection | 9 | 1 |
| Graft nonfunction | 3 | — |
| Hepatic artery thrombosis | 1 | 1 |
| Bacterial pneumonia | 2 | — |
| Pneumocystis carinii pneumonitis | — | 2 |
| Intraoperative | 2 | — |
| Othera | 2 | 1 |
One patient died of an intraoperative myocardial infarction during a reduction of a hip fracture, another died of colchicine-induced hepatic necrosis, and a third died of complications of vagotomy and near-total gastrectomy for peptic ulceration and hemorrhage.
Morbidity and Rehabilitation
Excluding retransplantation, the postoperative complications experienced by patients transplanted for PBC and requiring surgical intervention are listed in Table 5. Wound infection was the most common problem followed closely by the need for biliary tract revision.
Table 5.
Postoperative Complications Requiring Additional Operations
| No. | Percentage | |
|---|---|---|
| Wound infection | 10 | 13.3 |
| Biliary fistula | 8 | 10.6 |
| Gastrointestinal hemorrhage | 5 | 6.6 |
| Hemoperitoneum | 5 | 6.6 |
| Hepatic arterial thrombosis | 3 | 4.0 |
| Aortic aneurysm | 2 | 2.6 |
| Portal vein rupture | 2 | 2.6 |
| Pyloric stenosis | 2 | 2.6 |
| Duodenal fistula | 1 | 1.3 |
| Intestinal perforation | 1 | 1.3 |
| Portal vein thrombosis | 1 | 1.3 |
| Common bile duct stricture | 1 | 1.3 |
| Intraabdominal abscess | 1 | 1.3 |
| Total | 42 |
Seventeen of the 52 surviving patients are currently working full time, 4 are working part time, 2 are partially disabled, and 1 is in the hospital with a seizure disorder (Table 6).
Table 6.
Performance Status Determined Preoperatively and Postoperatively at the Time of Last Follow-up
| Classification | Preoperative | Postoperative |
|---|---|---|
| In coma in ICU | 1 | 0 |
| In an ICU | 4 | 0 |
| In hospital | 11 | 1 |
| At home with care | 38 | 2 |
| At home with self-care | 19 | 28 |
| Working part time | 2 | 4 |
| Working full time | 1 | 17 |
| Total | 76 | 52 |
ICU, intensive care unit.
When the performance status of patients before and after OLT (at the time of last follow-up) are compared, the proportion of those successfully functioning postoperatively (94%) (defined as being either at home caring for themselves or working full or part time) increased significantly (preoperatively 29%; χ2 = 43.853; p < 0.001). Moreover, when the proportion of patients who were not hospitalized but required some form of care is examined, it can be seen that only 4% of the patients required postoperative care at home compared with 50% preoperatively (χ2 = 43.853; p < 0.001). Consistent with these data are the data concerning the percentage of patients with either bone pain or fractures during the preoperative period (40%), early postoperative period (30%), and late postoperative period (13%) (χ2 = 7.250; p < 0.05). It should be noted, however, that several patients required prolonged (3–6 mo) postoperative in-hospital rehabilitation because of previous bone disease before their functional status improved postoperatively.
Liver Function
Thirteen of the 52 surviving patients continue to have some abnormalities of liver function (Table 7). However, the hepatic dysfunction is minor and no patients are jaundiced.
Table 7.
Biochemical and Serologic Measures of Hepatic Dysfunction at Last Follow-up in the Patients Studied
| OT | Date of OLT | Total bilirubin (mg/dl) | ALT (IU) | GGT (IU) | AP (IU) | AMA
|
Diagnosis | Date of biopsy | |
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | ||||||||
| 230 | 4–82 | 0.6 | 34 | 112 | 562 | 1:40 | 1:160 | No biopsy | |
| 280 | 2–83 | 1.2 | 107 | 511 | 473 | 1:1000 | 1:80 | Mild chronic rejection | 9–83 |
| 319 | 9–83 | 0.7 | 16 | 95 | 165 | 1:100 | Neg | No biopsy | |
| 350 | 1–84 | 0.5 | 96 | 309 | 114 | 1:100 | 1:160 | Reticuloendothelial and parenchymal cell iron deposition | 12–85 |
| 359 | 1—84 | 1.3 | 41 | 87 | 66 | 1:60 | Neg | No biopsy | |
| 361 | 2–84 | 0.8 | 125 | 142 | 198 | 1:40 | 1:40 | No biopsy | |
| 497 | 2–85 | 0.6 | 59 | 164 | 133 | 1:1000 | 1:100 | Chronic rejection; portal fibrosis | 4–85 |
| 507 | 3–85 | 0.3 | 107 | 58 | 201 | 1:10 | 1:10 | Acute rejection | 11–86 |
| 519 | 4–85 | 1.1 | 86 | 1510 | 622 | 1:160 | Neg | Chronic rejection; disappearing ducts | 7–85 |
| 550 | 6–85 | 1.3 | 423 | 232 | 140 | — | — | Fatty deposition | 4–86 |
| 585 | 8–85 | 0.7 | 42 | 225 | 88 | 1:300 | 1:50 | Mild cholestasis | 8–85 |
| 588 | 8–85 | 0.7 | 29 | 71 | 163 | 1:200 | 1:40 | Acute rejection | 9–85 |
| 596 | 8–85 | 0.8 | 31 | 96 | 270 | 1:10 | Neg | No biopsy | |
| Upper normals | 1.5 | 37 | 44 | 100 | <1:10 | ||||
ALT, alanine transaminase; AP, alkaline phosphatase; AMA, antimitochondrial antibody; GGT, y-glutamyl transferase; OLT, orthotopic liver transplantation; OT, the number given to liver transplant recipients.
Antimitochondrial Antibody Titers
Sixty-four of the 76 recipients were tested for the presence of AMA before transplantation. Sixty-three (98%) of these patients were found to be positive for this antibody; in 40 the initial titer was >1:80.
Although complete AMA titer data could not be obtained for all of the survivors, in 43 of 45 cases evaluated AMAs continued to be detected throughout at the same level or were found to decline relative to the initial preoperative value (Figure 2). No relationship between the height of the initial or follow-up AMA titer and the quality of postoperative liver function} is evident. Six of the recipients with entirely normal posttransplantation liver function continue to have positive AMA titers as long as 1–4.5 yr after transplantation. Because of the lack of a clinical indication, liver biopsies have not been performed in these 6 individuals.
Figure 2.
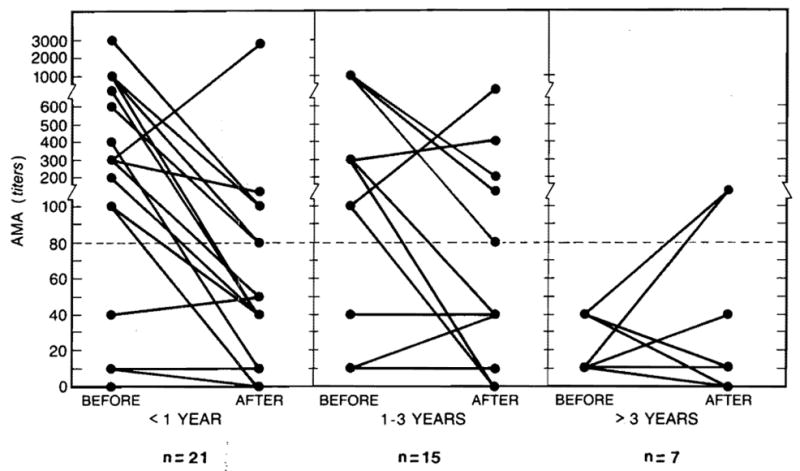
Antimitochondrial antibody titers before and after hepatic transplantation in PBC patients. The majority of the patients have had a decline in the AMA titers.
Special attention has been paid to the 13 recipients with abnormal liver function. The AMA titer in 10 of these patients has either decreased or remained stable at the pretransplant level. In two other patients, the titer has increased, but the bilirubin level has remained normal and both transaminase and alkaline phosphatase activities have not become abnormal. A liver biopsy specimen obtained from 1 of these 2 patients showed increased iron deposition.
Pathologic Observation
Native livers
Ninety percent of the native livers were stage IV, 9% were stage III, and 1% was between stage II and III at the time of hepatectomy. Thus, the vast majority of the specimens demonstrated far advanced disease with either macronodular cirrhosis or advanced fibrosis with a paucity of bile ductules in the fibrous septa (Figures 3A and 3B). Other conspicuous features included the presence of portal granulomata and lymphoid follicles, marked paraseptal cholestasis with an accumulation of tissue copper and copper-binding protein, an irregular margin between the fibrous septa and regenerative nodules, and the occasional findings of Mallory’s hyaline.
Figure 3.
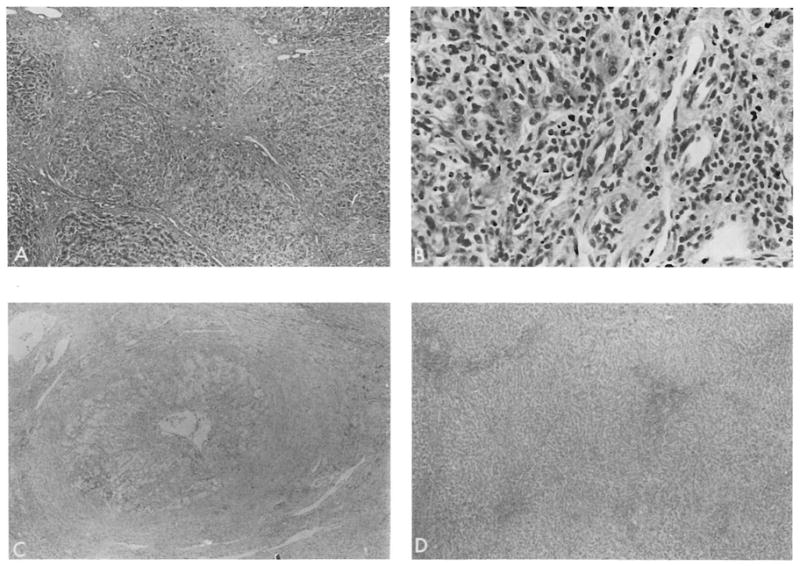
Endstage PBC usually results in a well-developed cirrhosis (A, H&E, raagnifica tion ×40), which is probably a result of activity at the edge of the limiting plate as seen in B (H&E, magnification ×250). A loss of small bile ducts and an obliterative arteropathy with prominent subintimal foam cells is seen in chronic rejection (C, H&E, magnification ×40). A well-developed cirrhosis is uncommon in end-stage liver-rejection (D, H&E, magnification ×40).
Liver homografts
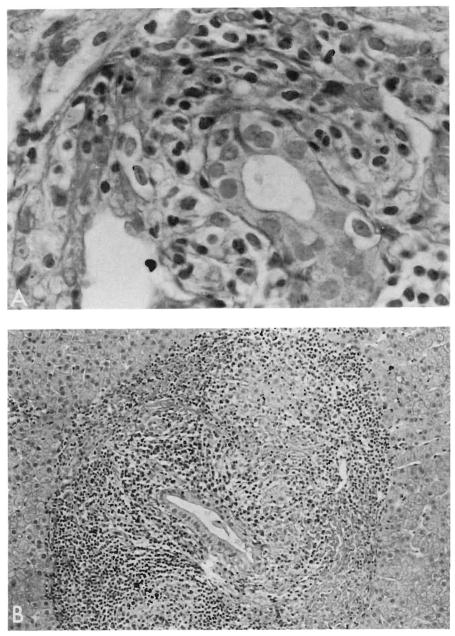
Grafts failing as a result of chronic rejection were easily separable from native livers by light microscopy (Figures 3 and 4). Chronic arterial and venous lesions including subintimal foam cells and myointimal hyperplasia were present only in failed grafts and were never found in native livers (Figure 3C). In failed homografts, fibrosis was seen in portal and central areas, but true cirrhosis was never evident (Figure 3D). In addition to a relative paucity of inflammatory cells, it was found that granulomas, marginal cholangiolar proliferation, and deposition of tissue copper were absent or uncommon in failed grafts but common in native organs. The cholestasis associated with chronic rejection was predominantly centrilobular, whereas in the native liver it occurred predominantly in a paraseptal (paraportal) location. A common feature of both resected native organs and homografts was the presence of a nonsuppurative destructive cholangitis, which in grafts was thought to be a manifestation of rejection rather than recurrent disease (16). Biopsy specimens showing acute rejection differed from those of endstage PBC, principally by the presence of a subintimal accumulation of mononuclear cells in the portal and hepatic veins present in rejecting organs but not in native organs. Both acute rejection and PBC are characterized by a mononuclear portal inflammatory infiltrate with destructive duct lesions; however, the inflammatory infiltrate in PBC often extends well into the adjacent hepatic lobule, whereas this is quite unusual in rejection (Figures 3B and 4).
Figure 4.
Lymphocytic bile duct damage is observed in both PBC and allograft rejection. However, whereas rejection preferentially involves small ducts and ductules (A, H&E. magnification ×400), intermediate-sized ducts are targeted in early PBC in association with granulomas (B, H&E, magnification ×150) and germinal centers, features not seen in rejection.
Discussion
Primary biliary cirrhosis has become the second most common indication for OLT in adults, being exceeded only by posthepatic cirrhosis as a reason for OLT. The decision about the proper timing of OLT for patients with PBC has been made somewhat less difficult than it is for other indications as a result of the prognostic indices that have been developed using common clinical and pathologic criteria available at most centers (17,18). Virtually all of the patients in this series met criteria for advanced disease that predict that without transplantation death is likely within ≤1 yr (6,18). In the future, perhaps many doctors will not allow patients to become mortally ill before performing OLT, as was the case in this series (Tables 1 and 6). Moreover, it is unlikely that as many patients will have previous upper abdominal surgery such as portal systemic shunts performed before transplantation, as was the case in several of our patients.
The liver transplant operation in patients with PBC is easier to perform than it is for most other adult liver diseases, especially in the absence of previous upper abdominal surgical procedures. The enlarged liver, the usual absence of major collaterals in the hepatic suspensory ligaments and bare areas, the usual normal consistency and configuration of both the suprahepatic inferior vena cava and the portal vein, and the presence of a normal recipient common duct are features present in patients with PBC that make the procedure easier to perform. With the use of the venous bypass, liver replacement in patients with PBC can be accomplished with very little blood loss and with little, if any, physiologic derangement during the operative procedure (10,11).
A technical problem unique to transplant recipients with PBC is the fragility of the recipient hepatic arteries. The media and subintima can be separated from the rest of the artery with the slightest trauma including the application of vascular clamps in preparation for an anastomosis and ligation of the gastroduodenal artery or other branches. Intramural dissection of the damaged vessel proximal to or beyond the level of the left gastric and splenic arterial branches can occur. If the diagnosis of a dissection is made, it is necessary to use an iliac graft to prevent subsequent thrombosis (12). Intimal dissection occurred in 20% of the cases in the present series. The acceptance of a suboptimal arterial supply in cases with dissection is often a prelude to tragedy (19).
Because of the greatly improved quality of life experienced by surviving patients, the use of OLT in PBC has become increasingly attractive. Evaluation of the current experience with OLT for this indication demonstrates that survival and particularly the quality of life of patients is enhanced with OLT compared with standard medical therapy (Tables 3 and 6). Most of the mortality experienced after OLT occurs in the first 6 mo with 22 of the 24 deaths occurring during the admission for transplantation. Based on the results reported herein, it appears that transplantation for individuals with advanced PBC is efficacious. Future improvements in the preoperative selection of patients, surgical technique, and postoperative management, as well as more selective or limited use of immunosuppression, should reduce the early surgical mortality and enhance further the acceptability of OLT as a treatment for patients with advanced PBC (9).
The only disquieting observation relevant to the application of OLT for patients with PBC has come from Neuberger et al. (8), who reported clinical and histopathologic evidence for recurrent PBC in 3 of 11 patients who underwent liver replacement for PBC using azathioprine and prednisone. The absence of recurrence in the present series with follow-ups of 1–6.5 yr, however, is encouraging. The many histologic similarities between PBC and rejection, as well as graft-versus-host liver disease, may make it difficult to separate disease recurrence from rejection. Our experience suggests that truly recurrent disease is quite unusual. Similar results were reported by Haagsma et al. (20), who found no evidence of recurrence of PBC in 9 liver transplant recipients followed for at least 1 yr. Attention to the fine details of the type of inflammatory cells found mostly in the portal areas, the degree to which these cells infiltrate the surrounding parenchyma, and the presence or absence of subintimal foamy macrophages and true cirrhosis (regenerative nodules and fibrosis) apparently make it possible to distinguish between these two entities.
It is conceivable that the superior immunosuppression achieved with cyclosporine combined with steroids aborts the underlying autoimmune manifestations of PBC while also controlling the rejection process. The AMA test, which has been considered a relatively specific diagnostic indicator of PBC, does not become negative after transplantation (20,21). Moreover, although the AMA titers tend to decline with time after transplantation (Figure 2), such changes in individual cases have been unpredictable. It may be that future studies of specific subtypes of AMA in posttransplant patients will provide either more information or new insights into the pathogenesis of this disease. It is conceivable that if recurrence of PBC should occur it will be a slow and gradual event. If recurrence were found to be the case, the process would develop at such a slow pace that transplantation for patients with advanced PBC would still be a reasonable course of action.
Much remains to be learned about both PBC and the process of hepatic rejection. We hope that as experience with both increases, the knowledge available about the two conditions will increase, and that newer and better therapies will be developed.
Acknowledgments
This work was supported by research grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland.
Abbreviations used in this paper
- AMA
antimitochondrial antibody
- OLT
orthotopic liver transplantation
- PBC
primary biliary cirrhosis
Footnotes
This paper was presented in part at the meeting of the American Association for the Study of Liver Diseases, Chicago, Illinois, on November 5, 1986.
References
- 1.Dumes SP, Vierling JM, Strober W. The role of the immune response in the pathogenesis of primary biliary cirrhosis. Semin Liver Dis. 1981;1:322–37. doi: 10.1055/s-2008-1040735. [DOI] [PubMed] [Google Scholar]
- 2.Thomas HC. Potential pathogenetic mechanisms in primary biliary cirrhosis. Semin Liver Dis. 1981;1:338–44. doi: 10.1055/s-2008-1040736. [DOI] [PubMed] [Google Scholar]
- 3.Gulp KS, Fleming CR, Daffy J, Baldus WP, Dickson ER. Autoimmune associations in primary biliary cirrhosis. Mayo Clin Proc. 1982;578:365–70. [PubMed] [Google Scholar]
- 4.Christiensen E, Crowe J, Doniach D, et al. Clinical pattern and course of disease in primary biliary cirrhosis based on analysis of 236 patients. Gastroenterology. 1980;78:236–46. [PubMed] [Google Scholar]
- 5.Rubin E, Schaffner F, Popper H. Primary biliary cirrhosis. Chronic nonsuppurative destructive cholangitis. Am J Pathol. 1965;46:387–407. [PMC free article] [PubMed] [Google Scholar]
- 6.Sherlock S. Primary biliary cirrhosis. In: Schiff L, Schiff ER, editors. Diseases of the liver. 5. Philadelphia: JB Lippincott; 1982. pp. 979–1002. [Google Scholar]
- 7.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–36. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuberger J, Portmann B, Macdougall BRD, Calne RY, William R. Recurrence of primary biliary cirrhosis after liver transplantation. N Engl J Med. 1982;306:1–4. doi: 10.1056/NEJM198201073060101. [DOI] [PubMed] [Google Scholar]
- 9.Esquivel CO, Fung JJ, Markus B, et al. OKT3 in the reversal of acute hepatic allograft rejection. Transplant Proc. 1987;19:2443–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Iwatsuki S, Esquivel CO, et al. Refinements in the surgical technique of liver transplantation. Semin Liver Dis. 1985;5:349–56. doi: 10.1055/s-2008-1040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw BW, Jr, Martin DJ, Marquez JM, et al. The advantages of venous bypass during orthotopic transplantation of the liver. Semin Liver Dis. 1985;5:344–8. doi: 10.1055/s-2008-1040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw BW, Jr, Iwatsuki S, Starzl TE. Alternative methods of arterialization of the hepatic graft. Surg Gynecol Obstet. 1984;159:490–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Demetris JA, Lasky S, Van Thiel DH, Starzl TE, Dekker A. Pathology of hepatic transplantation: a review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:115–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Scheuer PM. Liver biopsy interpretation. 2. Baltimore: Williams & Wilkins; 1973. pp. 33–8. [Google Scholar]
- 15.Demetris JA, Lasky S, Van Thiel DH, Starzl TE, Whiteside T. Induction of DR/Ia antigens in human liver allografts: an immunocytochemical and clinicopathologic analysis of twenty failed grafts. Transplantation. 1985;40:504–9. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fennel RH, Shikes RH, Vierling JM. Relationship of pretransplant hepatobiliary disease to bile duct damage occurring in the liver allograft. Hepatology. 1983;3:84–9. doi: 10.1002/hep.1840030114. [DOI] [PubMed] [Google Scholar]
- 17.Neuberger J, Altman DG, Christensen E, Tygstrup N, Williams R. Use of a prognostic index in evaluation of liver transplantation for primary biliary cirrhosis. Transplantation. 1986;41:713–6. doi: 10.1097/00007890-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Roll J, Boyer JL, Barry D, Klatskin G. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med. 1983;308:1–7. doi: 10.1056/NEJM198301063080101. [DOI] [PubMed] [Google Scholar]
- 19.Tzakis AG, Gordon RD, Shaw BW, Jr, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1986;40:667–71. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haagsma EB, Klein R, Huizenga J, et al. Subtypes of antimitochondrial antibodies in primary biliary cirrhosis before and after orthotopic liver transplantation. Hepatology. 1987;7:129–33. doi: 10.1002/hep.1840070125. [DOI] [PubMed] [Google Scholar]
- 21.Van Thiel DH, Gavaler JS. Recurrent disease in patients with liver transplantation. Hepatology. 1987;7:181–3. doi: 10.1002/hep.1840070133. [DOI] [PubMed] [Google Scholar]