Abstract
A novel series of homo- and heterodimeric ligands containing κ/μ agonist and μ agonist/antagonist pharmacophores joined by a 10-carbon ester linker chain were synthesized and evaluated for their in vitro binding affinity at κ, μ, and δ opioid receptors and their functional activities were determined at κ and μ receptors in [35S]GTPγS functional assays. Most of these compounds had high binding affinity at μ and κ receptors (Ki values less than 1 nM). Compound 15b, which contains butorphan (1) at one end of linking chain and butorphanol (5) at the other end, was the most potent ligand in this series with binding affinity Ki values of 0.089 nM at the μ receptor and 0.073 nM at the κ receptor. All of the morphinan-derived ligands were found to be partial κ and μ agonists; ATPM-derived ligands 12 and 11 were found to be full κ agonists and partial μ agonists.
Introduction
Bivalent ligands have the potential for bridging vicinal receptors. Such bridging should be manifested by a substantial increase in potency due to the high local concentration of the free pharmacophore in the vicinity of the proximal recognition site when the bivalent ligand is bound in a monovalent mode.1,2 Due to homo- and heterooligomerization among the opioid receptors, 3,4,5 a number of bivalent ligands containing opiate or peptide pharmacophores have been designed and synthesized, which proved to possess enhanced binding affinity and selectivity. For example, bivalent ligands containing oxymorphone or naltrexamine pharmacophores with specific spacer lengths have been reported to have enhanced opioid agonist or antagonist potency and selectivity. Portoghese et al.6–11 has also reported a range of homo- and heterodimeric ligands with varying linker lengths designed to investigate pharmacodynamic and organizational features of opioid receptors. These reported heterodimeric ligands were demonstrated to possess significantly greater potency and selectivity compared to their monomer congeners, providing further evidence for the opioid receptor heterooligomerization phenomena.11–13
The development of bivalent ligands that bridge the gap between binding sites on dimerized receptors could lead to a new generation of analgesic drugs that may not cause physical dependence or tolerance with chronic use.14 Behavioral studies suggested that κ opioid agonists with varying activity at the μ receptor effectively reduced cocaine self-administration in nonhuman primates with fewer undesirable side effects than the highly selective κ agonists.15–17 These results encouraged us to further develop novel bivalent ligands targeting both κ and μ receptors. Hence, we investigated a new series of homo- and hetero- bivalent ligands which contain the κ agonist, μ partial agonist butorphanol (5)27 and its analogue 14-methoxybutorphanol (8). The synthesis of a bivalent ligand (11) containing ATPM (10)28, which is a full κ and partial μ agonist, has also been achieved. These ligands were evaluated in vitro for their binding affinity at κ, μ, and δ opioid receptors, and for their pharmacological properties in the [35S]GTPγS binding assay.
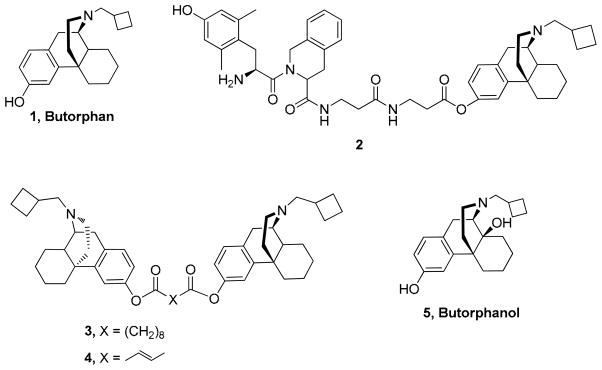
We have previously reported that bivalent ligands incorporating two molecules of butorphan (1) (“homobivalent”) or two distinct (“heterobivalent”) morphinan ligands, when connected by a molecular spacer, can lead to compounds with improved binding affinities and selectivities.18–22 Bivalent ligands 3 and 4, which contain butorphan (1), a mixed κ/μ agonist24 at both ends of the linking ester chain (Figure 1), are the most potent ligands in this series, with κ agonist and partial μ agonist activity.18,19 Ligand 2 (Figure 1) derived from the linkage of a δ selective peptide antagonist Dmt-Tic (2′6′-dimethyl-L-tyrosine-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) and a κ/μ agonist butorphan (1) through a repeated 3-aminopropionyl spacer was found to maintain the same characteristics as the two parent compounds.23 Thus, the binding profile of such bivalent ligands is dependent on the molecular structure of the opioid pharmacophore, the character and length of the connecting spacer, and the connecting site of the two pharmacophores. 6, 9, 18–24, 26
Figure 1.
Structure of homo- and heterobivalent ligands
We reasoned that by varying the particular combination of morphinans in each dimer, we would be able to modulate the combination of selectivity, activity, and potency at κ, μ, and δ opioid receptors for a given ligand. Such dimers, with specifically chosen activity for each receptor subtype, could prove highly useful in studying the biological effects of receptor oligomerization and could also lead to a superior therapeutic agent for the treatment of cocaine abuse. We thus investigated a new series of homo- and hetero- bivalent ligands containing the κ agonist, μ partial agonist butorphanol (5)27 and its analogue 14-methoxybutorphanol (8). The synthesis of the homobivalent ligand (11) which contains ATPM (10)28, a full κ but partial μ agonist, has also been achieved. These ligands were evaluated in vitro for their binding affinity at κ, μ, and δ opioid receptors. These ligands retained or displayed better affinity at κ and μ receptors compared to their parent compounds.
Chemistry
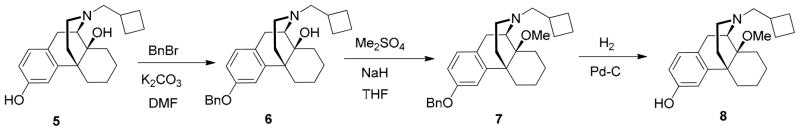
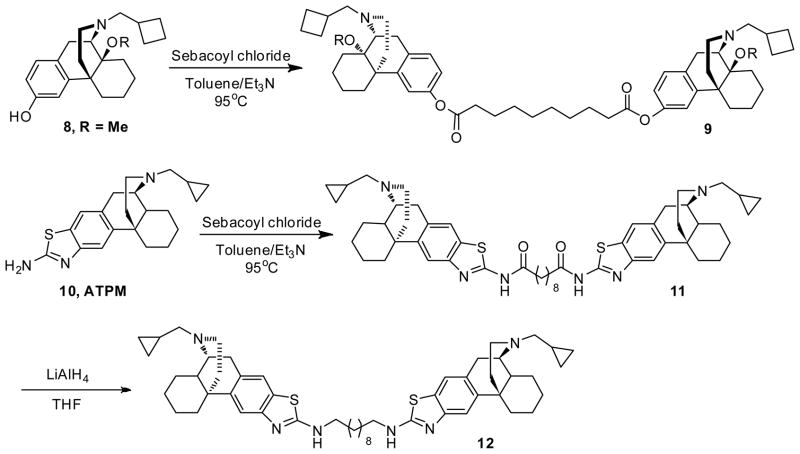
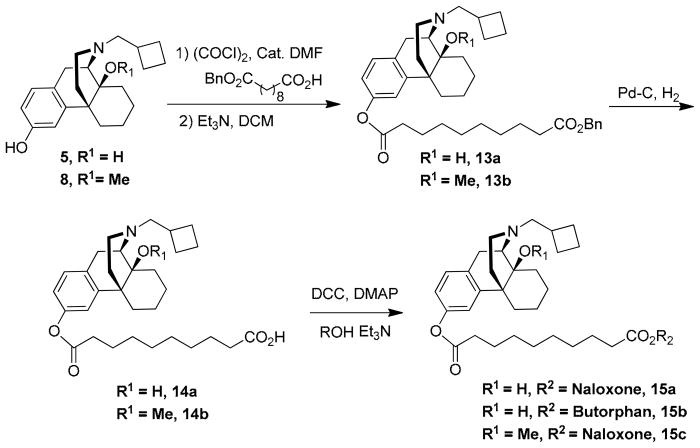
Butorphanol (1) free base was treated with BnBr/K2CO3 to give the corresponding benzyl ether. After treatment with NaH/Me2SO4, the benzyl group was removed to give 14-methoxybutorphanol (8) (Scheme 1) and were pharmacologically characterized at the κ opioid receptor and the μ opioid receptor using the [35GTPγS] assay. Treatment of sebacic acid (1 eq) with oxalyl chloride gave sebacoyl chloride, which was then coupled to the morphinans 8 and 10 (2 eq) to afford the homobivalent compounds 9 and 11, respectively (Scheme 2).19 The heterodimeric ligands were prepared stepwise, by esterifying sebacoyl chloride monobenzyl ester with morphinan 5 or 8 to afford 13, deprotecting the benzyl ether of the latter, and condensing the resulting free acid 14 with 5, 8, or naloxone in the presence of DCC and DMAP, as previously reported (Scheme 3).19
Scheme 1.
Scheme 2.
Scheme 3.
Results and Discussion
All the novel opioid ligands were evaluated for their binding affinities and selectivity for μ, κ and δ opioid receptors with Chinese hamster ovary (CHO) membranes stably expressing one of the human opioid receptors. The data are summarized in Table 1. For comparison purposes, the data for naloxone, butorphan (1), butorphanol (5), ligands 3, 4 and 10 are also included in Table 1. Monovalent compound 13b containing a 10-carbon linking chain, exhibits the same binding affinity at κ and μ receptors as the parent ligand 8 but shows slightly lower affinity at δ receptor. Similarly, 13b shows slightly higher binding affinity at κ and μ receptors, and shows the same affinity at δ receptor compared to homobivalent ligand 9b Heterobivalent ligands 15c and 15a exhibit comparable binding affinity at μ receptor, but have increased binding affinity at both κ and δ receptors when compared to naloxone. In contrast, ligand 15a shows a lower affinity at μ, κ and δ receptors than butorphanol. Ligand 15c also shows lower affinity at μ and δ receptors, but a slightly increased affinity at the κ receptor than monomeric parent ligand 8 It is interesting to note that 15b has enhanced binding affinity both at μ and κ receptors when compared to its parent compounds butorphan (1) and butorphanol (5). Since it has been previously demonstrated that ATPM, a full κ agonist with μ activity, effectively reduced cocaine self-administration in nonhuman primates with fewer undesirable side effects than the highly selective kappa agonists,15–17 we analyzed homodimers of ATPM. It was found that ATPM-derived homodimeric 11 and 12 maintain high binding affinity at κ receptor (less than 1 nM), although they have lower affinity at all three receptors than the parent compound ATPM.
Table 1.
Ki values for the inhibition of μ, κ and δ opioid binding to CHO membranesa
| Compound | Structure | Kia (nM) | Selectivity | ||
|---|---|---|---|---|---|
| [3H]DAMGO μ | [3H]U69,593 κ | [3H]Naltrindole δ | μ : κ : δ | ||
| Naloxoneb |
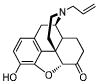
|
0.23 ± 0.05 | 0.25 ± 0.02 | 38 ± 3 | 1/1/152 |
| Butorphanc 1 |
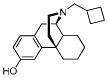
|
0.23 ± 0.01 | 0.079 ± 0.003 | 5.9 ± 0.6 | 3/1/75 |
| Butorphanol 5 |
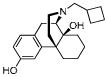
|
0.22 ± 0.012 | 0.12 ± 0.0068 | 12 ± 1.1 | 2/1/100 |
| MCL-691 8 |
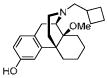
|
0.14 ± 0.014 | 0.20 ± 0.020 | 8.1 ± 0.071 | 1/1/58 |
| MCL-144 3d |
|
0.090 ± 0.004 | 0.049 ± 0.001 | 4.2 ± 0.4 | 2/1/90 |
| MCL-145 4d |
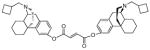
|
0.2 ± 0.03 | 0.08 ± 0.01 | 9.4 ± 0.5 | 3/1/120 |
| MCL-692 9b |
|
0.26 ± 0.037 | 0.37 ± 0.040 | 13 ± 1.0 | 1/1/50 |
| MCL-693 13b |
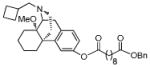
|
0.15 ± 0.014 | 0.23 ± 0.011 | 14 ± 3.8 | 1/1/93 |
| MCL-694 15c |
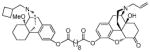
|
0.55 ± 0.10 | 0.14 ± 0.019 | 39 ± 6.1 | 4/1/280 |
| MCL-695 15b |
|
0.089 ± 0.012 | 0.073 ± 0.001 | 8.0 ± 2.4 | 1/1/110 |
| MCL-696 15a |
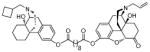
|
0.82 ± 0.11 | 0.28 ± 0.014 | 35 ± 11 | 3/1/130 |
| 10e MCL-147 ATPM |
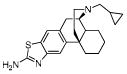
|
1.5 ± 0.21 | 0.049 ± 0.0046 | 29 ± 2 | 31/1/592 |
| MCL-715 11 |
|
8.8 ± 0.66 | 0.37 ± 0.024 | 170 ± 7.1 | 24/1/460 |
| MCL-714 12 |
|
2.5 ± 0.19 | 0.27 ± 0.0023 | 39 ± 4.8 | 9/1/140 |
Membranes from Chinese hamster ovary (CHO) cells, stably expressing either the human κ, μ, or δ opioid receptors, were incubated with 12 different concentrations of the compounds in the presence of receptor-specific radioligands at 25 °C, in a final volume of 1 mL of 50 mM Tris-HCl, pH 7.5. Nonspecific binding was determined using 10 μM naloxone. Data are the mean values ± SEM from three experiments, performed in triplicate.
reference 20
reference 24
reference 18
reference 28
The activities of 14-methoxybutorphanol (8), univalent ligand 13b, homobivalent ligands 9b, 12, 11, and heterobivalent ligands 15c, 15b, and 15a at both κ and μ receptors were assessed using the [35S]GTPγS binding assay, and reported in Tables 2 and 3, respectively.
Table 2.
Pharmacological characterization of morphinan monovalent and bivalent ligands at the kappa opioid receptor using the [35S]GTPγS binding assay
| Compound | Structure | Kappa (AgonMCL-692, MCL-693, ist) | Kappa (Antagonist) | ||
|---|---|---|---|---|---|
| EC50 (nM) | Emax (% maximal stimulation over basal) | IC50 (nM) | Imax (% maximal inhibition) | ||
| MCL-691 8 |
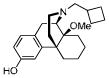
|
2.8 ± 0.90 | 90 ± 3.6 | 22 ± 3.7 | 37 ± 3.0 |
| MCL-692 9b |
|
2.4 ± 0.57 | 83 ± 0.95 | 17 ± 2.9 | 43 ± 4.3 |
| MCL-693 13b |
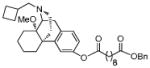
|
2.1 ± 0.076 | 90 ± 6.2 | 6.4 ± 2.7 | 30 ± 4.7 |
| MCL-694 15c |
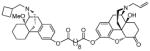
|
18 ± 4.5 | 80 ± 4.8 | 7.7 ± 3.3 | 33 ± 3.8 |
| MCL-695 15b |
|
0.84 ± 0.089 | 100 ± 0.95 | 3.7 ± 1.2 | 23 ± 2.9 |
| MCL-696 15a |
|
12 ± 4.9 | 74 ± 16 | 49 ± 13 | 35 ± 2.2 |
| MCL-715 11 |
|
5.5 ± 0.31 | 130 ± 3.3 | NIa | NIa |
| MCL-714 12 |
|
7.3 ± 0.81 | 130 ± 12 | NIa | NIa |
Membranes from CHO cells that expressed the human κ opioid receptor were incubated with 12 concentrations of the compound in the presence of 0.08 nM [35S]GTPγS for 60 min at 30°C. Nonspecific binding was measured by the inclusion of 10 μM GTPγS. For the inhibition experiments, [35S]GTPγS binding was stimulated by the addition of 100 nM U50,488. Data are the mean ± SEM from three experiments performed in triplicate.
NI = No Inhibition
Table 3.
Pharmacological characterization of morphinan monovalent and bivalent at the mu opioid receptor using the [35S]GTPγS binding assay
| Compound | Structure | Mu (Agonist) | Mu (Antagonist) | ||
|---|---|---|---|---|---|
| EC50 (nM) | Emax (% maximal stimulation over basal) | IC50 (nM) | Imax (%maximal inhibition) | ||
| MCL-691 8 |
|
3.6 ± 1.8 | 50 ± 2.7 | 9.3 ± 3.1 | 50 ± 3.4 |
| MCL-692 9b |
|
1.8 ± 0.36 | 42 ± 2.1 | 260 ± 92 | 44 ± 11 |
| MCL-693 13b |
|
1.7 ± 0.39 | 54 ± 7.0 | 7.6 ± 2.3 | 28 ± 2.2 |
| MCL-694 15c |

|
10 ± 3.0 | 62 ± 3.4 | 19 ± 3.0 | 45 ± 2.8 |
| MCL-695 15b |
|
1.2 ± 0.23 | 46 ± 4.1 | 3.4 ± 0.63 | 50 ± 5.7 |
| MCL-696 15a |
|
2.0 ± 0.42 | 19 ± 3.5 | 34 ± 4.5 | 73 ± 7.7 |
| MCL-715 11 |
|
130 ± 42 | 25 ± 1.8 | NAa | 74 at 10 μM |
| MCL-714 12 |
|
22 ± 5.3 | 38 ± 3.7 | 390 ± 100 | 71 ± 5.3 |
Membranes from CHO cells that expressed the human μ opioid receptor were incubated with 12 concentrations of the compound in the presence of 0.08 nM [35S]GTPγS for 60 min at 30°C. Nonspecific binding was measured by the inclusion of 10 μM GTPγS. For the inhibition experiments, [35S]GTPγS binding was stimulated by the addition of 200 nM DAMGO. Data are the mean ± SEM from three experiments performed in triplicate.
An IC50 value could not be determined because the inhibition curve had not reached a plateau at 10 μM of the compound.
The results indicate that all 14-methoxybutorphanol (8) and butorphanol-derived univalent and bivalent ligands 8, 9b, 13b, 15c, 15b, and 15a acted as partial κ agonists, all having high κ agonist activity (Emax range between 74% and 130% maximal stimulation) and varying degrees of κ antagonist activity (Imax ranged from 23% to 43% maximal inhibition). In contrast, the ATPM-derived homobivalent ligands 11 and 12 were found to be full agonists. All compounds were found to be partial μ agonists in the [35S]GTPγS binding assay (Table 3).
Conclusions
Homo- and heterobivalent ligands, which contain κ/μ agonist butorphanol (5), its analogue 14-methoxybutorphanol (8) and the aminothiazolomorphinan 1028 (ATPM), have been synthesized and their binding affinities evaluated at μ, κ and δ opioid receptors. All of these ligands have been found to have high binding affinity at μ and/or κ opioid receptors. The heterobivalent ligand 15b is more potent than either of its parent compounds butorphan (1) or butorphanol (5) at μ and κ receptors, and was found to be the most potent ligand in this series. With the exception of the two ATPM-derived ligands 11 and 12, all other ligands which were evaluated in this study were partial agonists at the κ receptor (Table 2) and partial agonists at the μ receptor (Table 3). In contrast, the two ATPM derived ligands 11 and 12 were full agonists at the κ receptor, but like the other ligands in this study, were also partial agonists at the μ receptor. These results suggest that appropriate design of bivalent ligands may enhance the binding affinity and selectivity of opioid ligands, and also provide evidence for opioid receptor oligomerization phenomena. These bivalent ligands may be used as chemical and pharmacological tools to elucidate the pharmacodynamic and organizational features of opioid receptors.
Experimental Section
General Synthetic Methods
1H and 13C NMR spectra were recorded at 300 MHz (75 MHz) on a Varian Mercury 300 spectrometer. Chemical shifts are given as δ value (ppm) downfield from tetramethylsilane as an internal reference. Melting points were determined on a Thomas-Hoover capillary tube apparatus and are reported uncorrected. Elemental analyses, performed by Atlantic Microlabs, Atlanta, GA, were within 0.4% of theoretical values. Analytical thin-layer chromatography (TLC) was carried out on 0.2 micrometer Kieselgel 60F-254 silica gel aluminum sheets (EM Science, Newark, NJ). Flash chromatography was used for the routine purification of reaction products. Eluent systems are described for the individual compounds.
(−)-3-benzyloxybutorphanol (6)
To a stirring suspension of butorphanol free base (5, 7.52g, 23.0 mmol) and K2CO3 (9.52 g, 69.0 mmol) in DMF (100 mL) was added BnBr (4.13 g, 24.15 mmol), and the reaction mixture was stirred overnight at room temperature. The next day, EtOAc (200 mL) was added, and the organic layer was washed with water (100 ml×3) and brine (50 mL), dried over Na2SO4, and concentrated under reduced pressure. The crude product was then purified over silica gel (EtOAc/Hexane 1:3) to afford a colorless oil (9.04 g, 94%); 1H NMR (300 MHz, CDCl3) δ 7.37 (m, 5H), 7.01 (d, J = 8.4, 1H), 6.93 – 6.62 (m, 2H), 5.02 (s, 2H), 4.82 – 3.80 (br, 1H), 3.04 (d, J = 18.2, 1H), 2.75 (dd, J = 6.2, 18.2, 1H), 2.63 (d, J = 6.0, 2H), 2.54 –1.37 (m, 19H), 0.99 (d, J = 12.4, 1H); 13C NMR (75 MHz, CDCl3) δ 157.4, 142.8, 137.1, 128.7, 128.5, 128.2, 127.9, 127.6, 112.1, 111.9, 70.0, 69.5, 61.3, 60.5, 44.7, 41.5, 37.0, 33.9, 31.7, 30.2, 27.0, 26.8, 24.9, 21.7, 21.6, 18.7
(−)-3-benzyloxy-14-methoxybutorphanol (7)
To a solution of 3-benzyloxybutorphanol (6, 3.8 g, 9.11mmol) in anhydrous DMF (50 mL) was added NaH (60% in mineral oil, 3.64 g, 91.1 mmol) at room temperature and stirred for 15 min. Next, Me2SO4 (2.6 mL, 27 mmol) was added to the suspension, and the reaction mixture was stirred at room temperature for 1 hour. The reaction was quenched carefully by addition of water at 0°C. Next, EtOAc (200 mL) was added, and the organic layer was washed with water (50 mL ×2) and brine, and dried over Na2SO4. After the solvent was removed under reduced pressure, the crude product was purified on silica gel (gradient: EtOAc/Hexane 1:20 to EtOAc/Hexane 1:3 ) to give a colorless oil (3.88 g, 98%); 1H NMR (300 MHz, CDCl3) δ 7.51 – 7.29 (m, 5H), 7.01 (d, J = 8.4, 1H), 6.87 (d, J = 2.5, 1H), 6.77 (dd, J = 2.4, 8.3, 1H), 5.02 (s, 2H), 3.22 (s, 3H), 3.13 (d, J = 18.2, 1H), 3.00 (d, J = 4.8, 1H), 2.62 – 2.35 (m, 5H), 2.27 – 1.16 (m, 16H), 0.89 (d, J = 12.0, 1H); 13C NMR (75 MHz, CDCl3) δ 157.3, 143.3, 137.1, 129.5, 128.5, 128.0, 127.8, 127.6, 111.9, 111.7, 74.2, 70.0, 61.3, 52.8, 46.9, 45.8, 42.1, 35.8, 34.4, 29.2, 28.1, 27.2, 25.5, 24.7, 21.5, 20.8, 18.8; Anal. Calcd for C29H37NO2·HCl·0.5H2O: C, 73.01; H, 8.24; N, 2.94. Found: C, 72.78; H, 7.97; N, 2.86.
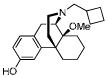
(−)-14-methoxybutorphanol (8)
Pd/C (20 mg) was added to a stirring solution of 3-benzyloxy-11-methoxybutorphanol (230 mg, 0.533 mmol) in MeOH (5 mL), and the suspension was hydrogenated at room temperature over night. The next day, the suspension was filtered off and solvent was removed under reduced pressure to give a colorless oil (170 mg, 93%) which was used directly without purification; 1H NMR (300 MHz, CDCl3) δ 6.56 (d, J = 8.2, 1H), 6.42 (s, 1H), 6.30 (d, J = 8.1, 1H), 6.23 – 5.85 (br, 1H), 2.85 (s, 3H), 2.83 – 2.60 (m, 2H), 2.30 – 0.83 (m, 21H), 0.50 (d, J = 11.9, 1H).; 13C NMR (75 MHz, CDCl3) δ 154.8, 143.1, 128.2, 128.1, 113.3, 112.0, 74.5, 61.0, 52.8, 47.0, 45.9, 41.9, 35.5, 34.2, 29.2, 28.2, 27.3, 25.7, 24.8, 21.6, 20.9, 18.8; m.p. (HCl salt): 195 °C (dec.); Anal. Calcd for C22H31NO2·HCl·H2O: C, 66.73; H, 8.65; N, 3.54. Found: C, 66.40, H, 8.27; N, 3.54.
General procedure for converting morphinan ligands to the HCl salts
To a solution of the free base in a minimal amount of ethyl acetate was added excess ethereal 1N HCl. A precipitate formed, and the resulting solid was filtered, washed with two portions of ether, and dried under vacuum to give the corresponding HCl salt.
Bis((−)-14-methoxy-17-N-cyclobutylmethyl)morphinan-3-yl) decanedioate (9)
(representative procedure): To a solution of sebacic acid (0.0981 mmol, 30.2 mg; 1 eq.) in anhydrous CH2Cl2 (2 mL) was added oxalyl chloride (1.962 mmol, 0.173 mL; 20 eq.) and 2 drops of DMF. Gas evolution was observed and the solution was stirred overnight. The next day, the reaction mixture was concentrated under reduced pressure. The resulting yellow oil was redissolved in anhydrous CH2Cl2 (5 mL), 14-methoxybutorphanol (8) (100 mg, 0.294 mmol; 3 eq.) and Et3N (40 mg, 0.392 mmol; 4 eq.) was added to the solution, and the mixture was stirred at room temperature overnight. The organic layer was washed with NaHCO3 solution, dried over Na2SO4. The solvent was removed and the result crude product was purified on silica gel (EtOAc/Et3N/MeOH 200:1:1) to give the pure compound as colorless oil (70mg, 84%); 1H NMR (300 MHz, CDCl3) δ 7.08 (d, J = 8.3, 2H), 6.92 (d, J = 2.2, 2H), 6.84 (dd, J = 2.3, 8.2, 2H), 3.21 (s, 6H), 3.16 (d, J = 18.8, 2H), 3.01 (d, J = 5.4, 2H), 2.62 – 1.17 (m, 54H), 0.88 (d, J = 10.9, 2H); 13C NMR (75 MHz, CDCl3) δ 172.4, 149.3, 143.4, 134.4, 128.1, 118.6, 118.1, 74.1, 61.3, 52.8, 46.9, 45.6, 42.1, 35.8, 34.4, 29.2, 29.05, 29.02, 28.1, 27.2, 25.5, 25.1, 24.8, 21.4, 20.8, 18.8; m.p. (HCl salt): 154 °C (dec.); Anal. Calcd for C54H76N2O6·2HCl·H2O: C, 68.99; H, 8.58; N, 2.98. Found: C, 69.03, H, 8.58; N, 2.96.
Bis((−)-3-aminothiazolo-N-cyclopropyl-methylmorphinan)sebacamide hydrochloride (11)
A mixture of compound 10 (88 mg, 0.25 mmol), sebacoyl chloride (30 μL, 0.14 mmol) and triethyl amine (45 μL, 0.30mmol) in 2 mL toluene was refluxed overnight. After cooling to room temperature, the reaction mixture was directly purified on silica gel (EtOAc:MeOH : Et3N = 60:1:1). A slightly yellow foam (52 mg, 54%) as product was obtained.; 1H NMR (300 MHz, CDCl3) δ 7.66 (s, 2H), 7.55 (s, 2H), 3.10 (m, 4H), 2.77 (m, 4H), 2.43 (m, 10H), 1.92 (m, 4H), 1.37 (m, 30H), 0.88 (m, 2H), 0.51 (d, J = 8.0, 4H), 0.11 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 171.98, 159.00, 146.92, 139.88, 134.44, 129.24, 119.91, 116.88, 59.96, 55.71, 45.59, 45.02, 42.57, 38.06, 36.87, 36.33, 28.80, 28.74, 26.90, 26.58, 24.95, 24.86, 22.18, 9.40, 4.10, 3.63. m.p. (HCl salt): 270 °C (dec.); Anal. Calc. for C52H68N6O2S2·2HCl·3.5H2O, C, 61.88; H, 7.69; N, 8.33. Found: C, 61.75; H, 7.51; N, 7.94. HPLC analysis indicates a single compound (100%).
(−)-3,3′-(decane-1,10-diaminothiazolo)bis(cyclopropylmethyl)morphinan hydrochloride (12)
To the solution of amide (52 mg, 0.06 mmol) in 3 mL anhydrous THF was added LiAlH4 (10 mg, 0.24 mmol) at room temperature. The mixture was stirred at room temperature for 4h, and then 3 drops water was added to quench the reaction, which was then directly purified on silica gel (EtOAc:MeOH : Et3N = 60:1:1) to give slightly yellow foam (33 mg product, 66%); 1H NMR (300 MHz, CDCl3) δ 7.46 (s, 2H), 7.32 (s, 2H), 5.92 (s, 2H),3.74-3.65 (m, 6H), 3.00 (m, 6H), 2.66 (m, 4H), 2.45 (m, 2H), 2.22 (m, 4H), 1.40 (m, 36H), 0.63 (d, J = 7.9, 4H), 0.29 (d, J = 10.8, 4H). 13C NMR (75 MHz, CDCl3) δ 167.10, 152.11, 137.55, 128.38, 119.46, 115.23, 62.56, 58.99, 56.16, 45.90, 45.40, 37.32, 36.15, 30.03, 29.10, 26.47,26.03, 24.89, 21.87, 4.30, 4.27. m.p. (HCl salt): 252 °C (dec.); Anal. Calc. for C52H72N6S2·4HCl × 2.2H2O, C, 60.17; H, 7.88; N, 8.10. Found: C, 60.27; H, 7.91; N, 7.92.
Benzyl ((−)-14-hydroxy-17-N-cyclobutylmethyl) morphinan-3-yl) decanedioate. (13a)
Method B, see the synthesis of 13b
1H NMR (300 MHz, CDCl3) δ 7.34 (s, 5H), 7.08 (d, J = 8.2, 1H), 6.91 (s, 1H), 6.84 (d, J = 8.2, 1H), 5.10 (s, 2H), 3.07 (d, J = 18.5, 1H), 2.78 (m, 1H), 2.62 (d, J = 5.9, 1H), 2.44 (m, 8H), 1.77 (m, 29H), 0.99 (d, J = 11.9, 1H); 13C NMR (75 MHz, CDCl3) δ 173.6, 172.4, 149.3, 142.9, 136.0, 133.7, 128.5, 128.2, 128.1, 118.7, 118.3, 69.3, 66.0, 61.1, 60.5, 44.5, 41.5, 36.9, 34.3, 34.2, 33.7, 31.5, 30.1, 28.99, 28.96, 26.9, 26.7, 25.2, 24.83, 24.78, 21.6, 18.7; m.p. (HCl salt): 87–92 °C. Anal. Calcd for C38H51NO5·HCl·0.8H2O: C, 69.93; H, 8.28; N, 2.15. Found: C, 70.04; H, 8.21; N, 2.19.
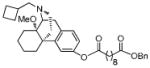
Benzyl (−)-14-methoxy-butorphanol-3-yl decanedioate (13b)
Method A
To the solution of sebacic acid monobenzyl ester (1.08 g, 3.53 mmol) in anhydrous CH2Cl2 (15 mL) was added oxalyl chloride (0.62 mL, 7.04 mmol) and 2 drops of DMF. Gas evolution could be observed and the solution was stirred overnight. Next, CH2Cl2 and excess oxalyl chloride was removed under reduced pressure. The yellow oil was redissolved in anhydrous CH2Cl2. 14-methoxy-butorphanol (8) (1.00g, 2.93 mmol) and Et3N (2.0 mL, 14.1 mmol) was added to the solution and the mixture was stirred overnight. The organic layer was washed with NaHCO3 solution and dried over Na2SO4. After solvent was removed under reduced pressure, the crude product was purified on silica gel (EtOAc:hexanes 1:4 and EtOAc:hexanes 1:1 ) to give a yellow oil (460mg, 25%).
Method B
To the solution of sebacic acid monobenzyl ester (206 mg, 0.676 mmol) and 14-methoxy-butorphanol (192 mg, 0.563 mmol) in anhydrous CH2Cl2 (6 mL) was added DCC (139 mg, 0.676 mmol), and DMAP (7 mg, 0.0563 mmol), and the reaction mixture was stirred overnight. The next day, after solvent was removed under reduced pressure, the residue was redissolved in an equal volume of ethyl acetate and the white solid was filtered off. The organic layer was washed with saturated NaHCO3 solution and brine, and dried over Na2SO4. The crude product was purified on silica gel (EtOAc:hexanes 1:1) to a give yellow oil (110 mg, 31%).
1H NMR (300 MHz, CDCl3) δ 7.35 (m, 5H), 7.09 (d, J = 8.3, 1H), 6.92 (s, 1H), 6.85 (dd, J = 2.3, 8.2, 1H), 5.12 (s, 2H), 3.22 (s, 3H), 3.16 (d, J = 18.5, 1H), 3.02 (d, J = 5.4, 1H), 2.60 – 1.20 (m, 37H), 0.89 (d, J = 11.1, 1H); 13C NMR (75 MHz, CDCl3) δ 173.5, 172.3, 149.3, 143.3, 136.0, 134.2, 128.4, 128.1, 118.5, 118.1, 74.0, 66.0, 61.2, 52.8, 46.9, 45.6, 42.0, 35.6, 34.3, 34.24, 34.19, 29.1, 29.0, 28.9, 28.0, 27.1, 25.4, 25.0, 24.81, 24.78, 21.4, 20.8, 18.7; m.p. (HCl salt): 165–168°C; Anal. Calcd for C39H53NO5·HCl·2.5H2O: C, 67.17; H, 8.53; N, 2.01. Found: C, 67.21; H, 8.35; N, 2.22.
General Procedure for the preparation of morphinans 14a, 14b
Pd/C (42 mg) was added to a solution of benzyl ester 4 (418 mg) in MeOH (20 mL). The reaction mixture was hydrogenated at room temperature overnight. The next day, the suspension was filtered and MeOH was removed under reduced pressure to give the product, which was used directly without purification.
Decanedioic acid (−)-butorphanol-3-yl ester (14a)
Colorless oil (205 mg, 97%); 1H NMR (300 MHz, CD3OD) δ 6.09 (d, J = 8.1, 1H), 5.86 (d, J = 2.1, 1H), 5.77 (dd, J = 2.1, 8.3, 1H), 2.87 –0.14 (m, 41H); 13C NMR (75 MHz, CD3OD) δ 174.6, 171.1, 148.8, 138.6, 128.8, 127.4, 118.5, 117.0, 67.1, 59.8, 55.8, 45.5, 38.3, 31.9, 31.8, 29.2, 29.1, 27.24, 27.20, 27.14, 27.07, 27.00, 25.3, 23.6, 23.0, 22.8, 19.1, 18.5, 16.4
Decanedioic acid (−)-14-methoxyl-butorphanol-3-yl ester (14b)
yellow solid (342 mg, 96%); 1H NMR (300 MHz, CD3OD) δ 7.31 (d, J = 8.4, 1H), 7.08 (d, J = 2.1, 1H), 6.99 (dd, J = 2.1, 8.3, 1H), 3.83 (d, J = 5.8, 1H), 3.55 – 3.09 (m, 4H), 3.37 (s, 3H), 3.04 (d, J = 8.6, 1H), 2.87 –1.14 (m, 34H); 13C NMR (75 MHz, CDCl3) δ 178.5, 172.3, 149.6, 142.5, 132.6, 128.2, 119.2, 118.2, 77.2, 73.8, 60.0, 52.9, 47.5, 45.3, 41.6, 35.8, 34.3, 34.2, 32.9, 29.05, 29.00, 28.2, 27.4, 25.5, 24.8, 21.1, 20.6, 18.7
General Procedure for the Preparation of morphinans 15a, 15b, 15c
(representative procedure): To a solution of morphinan (0.162 mmol; 1 eq.) and sebacic acid monomorphinan ester (0.162 mol; 1 eq.) in anhydrous CH2Cl2 (2 mL) was added DCC (0195 mmol; 1.2 eq.) and DMAP (0.0162 mmol; 0.1 eq.) and the reaction mixture was stirred overnight at room temperature. CH2Cl2 was removed and an equal volume of ethyl acetate was added. The resulting white solid was removed by filtration, and the organic layer was washed with saturated NaHCO3 solution and brine, and dried over Na2SO4. Ethyl acetate was removed to give the crude product, which was purified on silica gel (EtOAc:Et3N 100:1) to give pure product.
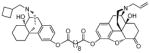
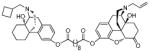
(5α)-17-Allyl-14-hydroxy-6-oxo-4,5-epoxymorphinan-3-yl-17-((−)-N-cyclobutylmethyl-14-hydroxyl-mophinan-3-yl) decanedioate. (15a)
Yellow oil (68 mg, 51%); 1H NMR (300 MHz, CDCl3) δ 7.09 (d, J = 8.3, 1H), 6.87 (ddd, J = 3.3, 8.2, 11.9, 3H), 6.69 (d, J = 8.2, 1H), 5.82 (ddt, J = 6.4, 10.1, 16.5, 1H), 5.21 (m, 2H), 4.69 (s, 1H), 3.25-1.20 (m, 54H), 0.91 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 207.6, 172.4, 171.3, 149.3, 147.7, 142.8, 133.6, 132.5, 129.9, 128.2, 122.9, 119.3, 118.8, 118.2, 90.5, 69.3, 62.0, 61.1, 60.5, 57.6, 50.5, 41.4, 34.3, 29.6, 29.0, 26.7, 22.9, 21.6, 14.1; m.p. (HCl salt): 170–174 °C Anal. Calcd for C50H64N2O8·2HCl·2.4H2O: C, 64.08; H, 7.61; N, 2.99. Found: C, 63.85; H, 7.28; N, 2.98.
((−)-17-N-cyclobutylmethyl)morphinan-3-yl-((−)-14-hydroxyl-17-N-cyclobutylmethyl) morphinan-3-yl) decanedioate (15b)
Colorless oil (52 mg, 42%); 1H NMR (300 MHz, CDCl3) δ 7.09 (d, J = 8.3, 2H), 6.87 (m, 4H), 3.04 (dd, J = 18.4, 28.6, 1H), 2.82 (d, J = 5.8, 1H), 2.65-0.98 (m, 64H); 13C NMR (75 MHz, CDCl3) δ 172.4, 149.3, 149.2, 142.9, 135.1, 133.7, 128.4, 128.2, 118.7, 118.4, 118.3, 118.0, 69.3, 61.4, 60.5, 55.7, 49.0, 45.6, 44.8, 44.5, 41.6, 41.5, 37.7, 36.9, 36.5, 34.8, 34.3, 33.9, 33.7, 31.5, 30.1, 29.02, 28.98, 27.8, 26.9, 26.7, 26.4, 25.6, 25.2, 24.9, 24.8, 24.3, 22.0, 21.6, 18.8, 18.7; m.p. (HCl salt):142–145 °C Anal. Calcd for C52H72N2O5·2HCl·1.8H2O: C, 68.60; H, 8.59; N, 3.08. Found: C, 68.77; H, 8.42; N, 3.34.
(5α)-17-allyl-14-hydroxy-6-oxo-4,5-epoxymorphinan-3-yl-((−)-14-methoxyl-17-N-cyclobutylmethyl)morphinan-3-yl) decanedioate (15c)
yellow oil (36 mg, 51%); 1H NMR (300 MHz, CDCl3) δ 7.09 (d, J = 8.4, 1H), 6.92 (s, 1H), 6.88 – 6.78 (m, 2H), 6.68 (d, J = 8.2, 1H), 5.91 – 5.70 (m, 1H), 5.33 – 5.10 (m, 3H), 4.69 (s, 1H), 3.29 – 2.88 (m, 12H), 2.66 – 1.16 (m, 42H), 0.89 (d, J = 12.3, 1H); 13C NMR (75 MHz, CDCl3) δ 207.7, 172.4, 171.3, 149.3, 147.7, 143.4, 135.0, 134.3, 132.5, 130.0, 129.9, 128.1, 122.9, 119.3, 118.6, 118.2, 118.1, 90.5, 74.1, 70.0, 62.0, 61.2, 57.6, 52.7, 50.5, 46.9, 45.6, 43.1, 42.0, 36.0, 35.7, 34.4, 33.9, 31.1, 30.5, 29.2, 29.0, 28.9, 28.1, 27.2, 25.4, 25.0, 24.8, 24.7, 22.9, 21.4, 20.8, 18.8; m.p. (HCl salt): 175°C (dec.); Anal. Calcd for C51H66N2O8·2HCl·2H2O: C, 64.89; H, 7.69; N, 2.97. Found: C, 65.07; H, 7.61; N, 3.06.
Opioid binding to the human μ, κ, and δ opioid receptors
Chinese hamster ovary (CHO) cells stably transfected with the human κ opioid receptor, δ opioid receptor were obtained from Dr. L.-Y. Liu-Chen (Temple University, Philadelphia, PA) and Dr. L. Toll (SRI International, Palo Alto, CA), respectively. The μ opioid receptor were obtained from Dr. G. Uhl (NIDA Intramural Program, Baltimore, MD). The cells were grown in 100-mm dishes in Dulbecco’s modified Eagle’s media supplemented with 10% fetal bovine serum and penicillin–streptomycin (10,000 U/mL) at 37°C in a 5% CO2 atmosphere. The affinity and selectivity of the compounds for the multiple opioid receptors were determined by incubating the membranes with radiolabeled ligands and 12 different concentrations of the compounds at 25°C in a final volume of 1 mL of 50 mM Tris–HCl, pH 7.5. Incubation times of 60 min were used for the μ-selective peptide [3H]DAMGO and the κ-selective ligand [3H]U69,593. A 3-h incubation was used with the δ-selective antagonist [3H]naltrindole. Nonspecific binding was measured by the inclusion of 10 μM naloxone. Samples were filtered through GF/B glass fiber filters, which were washed three times with 3-ml cold 50 mM Tris-HCl, pH 7,5. The filters were counted in 2 ml of ScintiSafe 30% scintillation fluid. IC50 values were determined by log-probit analysis and were converted to Ki values by the equation of Cheng and Prusoff.29
[35S]GTPγS binding assay to measure pharmacological properties mediated by the kappa and mu opioid receptors
To determine the agonist properties of the compounds at the κ and μ opioid receptors, membranes expressing either the κ or μ receptor were incubated with 12 different concentrations of the compound in 0.5 mL of buffer containing 50 mM Tris-HCl, pH 7.4, 3 μM GDP, 3 mM MgCl2, 0.2 mM EGTA, and 100 mM NaCl. [35S]GTPγS was added at a final concentration of 0.08 nM. Nonspecific binding was measured by the inclusion of 10 μM GTPgS. After a 60-min incubation at 30°C, samples were filtered through GF/B glass fiber filters, which were washed three times with 3-mL of cold 50 mM Tris-HCl, pH 7.5. The filters were counted in 2 mL of ScintSafe 30% scintillation fluid. The EC50 value and Emax values were calculated using SigmaPlot software.
To determine if the compound had antagonistic properties at the κ opioid receptor, membranes were incubated as described above. To stimulate [35S]GTPγS binding, 100 nM U50,488 was added. Twelve different concentrations of the compound were added. To determine if a compound was an antagonist at the μ receptor, membranes expressing the μ opioid receptor were incubated with 12 concentrations of the compound and 200 nM DAMGO to stimulate [35S]GTPγS binding. IC50 and Imax values were calculated with SigmaPlot software.
Acknowledgments
This work was supported by NIH grants R01-DA14251(J. L. N.), K05-DA00360 (J. M. B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dwyer JD, Bloomfield VA. Binding of multivalent ligands to mobile receptors in membranes. Biopolymers. 1981;20:2323–2336. doi: 10.1002/bip.1981.360201104. [DOI] [PubMed] [Google Scholar]
- 2.Erez M, Takemori AE, Portoghese PS. Narcotic antagonistic potency of bivalent ligands which contain .beta.-naltrexamine. Evidence for simultaneous occupation of proximal recognition sites. J Med Chem. 1982;25:847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]
- 3.Jordan BA, Cvejic S, Devi LA. Opioids and their complicated receptor complexes. Neuropsychopharmacology. 2000;23:S5–S18. doi: 10.1016/S0893-133X(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 4.Levac BA, O’Dowd BF, George SR. Oligomerization of opioid receptors: generation of novel signaling units. Curr Opin Pharmacol. 2002;2:76–81. doi: 10.1016/s1471-4892(02)00124-8. [DOI] [PubMed] [Google Scholar]
- 5.Rios CD, Jordan BA, Gomes I, Devi LA. G-protein coupled receptor dimerization: Modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 6.Erez M, Takemori AE, Portoghese PS. Narcotic Antagonistic Potency of Bivalent Ligands Which Contain β-Naltrexamine. Evidence for Bridging between Proximal Recognition Sites. J Med Chem. 1982;25:847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]
- 7.Portoghese PS, Ronsisvalle G, Larson DL, Takemori AE. Synthesis and Opioid Antagonist Potencies of Naltrexamine Bivalent Ligands with Conformationally Restricted Spacers. J Med Chem. 1986;29:1650–1653. doi: 10.1021/jm00159a014. [DOI] [PubMed] [Google Scholar]
- 8.Portoghese PS, Larson DL, Sayre LM, Yim CB, Ronsisvalle G, Tam SW, Takemori AE. Opioid Agonist and Antagonist Bivalent Ligands. The Relationship between Spacer Length and Selectivity at Multiple Opioid Receptors. J Med Chem. 1986;29:1855–1861. doi: 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]
- 9.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, Takemori AE, Rice KC, Tam SW. Stereostructure-Activity Relationship of Opioid Agonist and Antagonist Bivalent Ligands. Evidence for Bridging Between Vicinal Opioid Receptors. J Med Chem. 1985;28:1140–1141. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- 10.Portoghese PS, Nagase H, Takemori AE. Only One Pharmacophore Is Required for the κ Opioid Antagonist Selectivity of Norbinaltorphimine. J Med Chem. 1988;31:1344–1347. doi: 10.1021/jm00402a015. [DOI] [PubMed] [Google Scholar]
- 11.Portoghese PS. From Models to Molecules: Opioid Receptor Dimers, Bivalent Ligands, and Selective Opioid Receptor Probes. J Med Chem. 2001;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- 12.Daniels JD, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A Bivalent Ligand (KDAN-18) Containing δ-Antagonist and κ-Agonist Pharmacophores Bridges δ2 and κ1 Opioid Receptor Phenotypes. J Med Chem. 2005;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 13.Bhushan RJ, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A Bivalent Ligand (KDN-21) Reveals Spinal δ and κ Opioid Receptors Are Organized as Heterodimers That Give Rise to δ1 and κ2 Phenotypes. Selective Targeting of δ–κ Heterodimers. J Med Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 14.Owens J. Bridging the GPCR gap. Nat Rev Drug Disc. 2006;5:105. [Google Scholar]
- 15.Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of κ Opioids on Cocaine Self-administration by Rhesus Monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- 16.Mello NK, Negus SS. Effects of Kappa Opioids Agonists on Schedule-Controlled Behavior and Cocaine Self-Administration by Rhesus Monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- 17.Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of Mixed-Action κ/μ Opioids on Cocaine Self-Administration and Cocaine Discrimination by Rhesus Monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- 18.Neumeyer JL, Zhang A, Xiong W, Gu X, Hilbert JE, Knapp BI, Negus SS, Mello NK, Bidlack JM. Design and Synthesis of Novel Dimeric Morphinan Ligands for κ and μ Opioid Receptors. J Med Chem. 2003;46:5162–5170. doi: 10.1021/jm030139v. [DOI] [PubMed] [Google Scholar]
- 19.Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Synthesis and preliminary in vitro investigation of bivalent ligands containing homo- and heterodimeric pharmacophores at μ, κ, and δ opioid receptors. J Med Chem. 2006;49:256–262. doi: 10.1021/jm050577x. [DOI] [PubMed] [Google Scholar]
- 20.Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Pharmacological Properties of Bivalent Ligands Containing Butorphan Linked to Nalbuphine, Naltrexone, and Naloxone at μ, δ, and κ Opioid Receptors. J Med Chem. 2007;50:2254–2258. doi: 10.1021/jm061327z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker M, Fulton BF, Zhang B, Knapp BI, Bidlack JM, Neumeyer JL. Univalent and Bivalent Ligands of Butorphan: Characteristics of the Linking Chain Determine the Affinity and Potency of Such Opioid Ligands. J Med Chem. 2009;52:7389–7396. doi: 10.1021/jm900379p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathews JL, Peng X, Xiong W, Zhang A, Negus SS, Neumeyer JL, Bidlack JM. Characterization of a novel bivalent morphinan possessing κ agonist and μ agonist/antagonist properties. J Pharmacol Exp Ther. 2005;315:821–827. doi: 10.1124/jpet.105.084343. [DOI] [PubMed] [Google Scholar]
- 23.Neumeyer JL, Peng XM, Knapp BI, Bidlack JM, Lazarus LH, Salvadori S, Trapella C, Bolboni G. New opioid designed multiple ligand form Dmt-Tic and morphinan pharmacophores. J Med Chem. 2006;49:5640–5643. doi: 10.1021/jm0605785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumeyer JL, Bidlack JM, Zong R, Bakthavachalam V, Gao P, Cohen DJ, Negus SS, Mello NK. Synthesis and opioid receptor affinity of morphinan and benzomorphan derivatives: mixed κ agonists and μ agonists/antagonists as potential pharmacotherapeutics for cocaine dependence. J Med Chem. 2000;43:114–122. doi: 10.1021/jm9903343. [DOI] [PubMed] [Google Scholar]
- 25.Gates M, Montzka TA. Some morphine antagonists possessing high analgesic activity. J Med Chem. 1964;7:127–131. doi: 10.1021/jm00332a002. [DOI] [PubMed] [Google Scholar]
- 26.Portoghese PS. From Models to Molecules: Opioid Receptor Dimers, Bivalent Ligands, and Selective Opioid Receptor Probes. J Med Chem. 2000;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- 27.Garner HR, Burke TF, Lawhorn CD, Stoner JM, Wessinger W. Butorphanol-Mediated Antinociception in Mice: Partial Agonist Effects and μ Receptor Involvement. J Pharmacol Exp Ther. 1997;282:1253–1261. [PubMed] [Google Scholar]
- 28.Zhang A, Xiong W, James E, Hilbert JE, DeVita EK, Bidlack JM, Neumeyer JL. 2-Aminothiazole-Derived Opioids. Bioisosteric Replacement of Phenols. J Med Chem. 2004;47:1886–1888. doi: 10.1021/jm049978n. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Y, Prusoff WH. Relationship Between the Inhibition Constant (K1) and the Concentration of Inhibitor Which Causes 50 Per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]