Abstract
Names influence how something is perceived. Diagnostic terms (diagnoses) are the names of diseases that are usually derived either from some distinctive characteristic of the disease or include an eponym recognizing someone who elucidated the disease. No matter how logical and appropriate a name may be, if it is not usable and used it is of no lasting value. This brief commentary focuses on the nomenclature of systemic vasculitides, and uses as a prime example Wegener's granulomatosis, which has been renamed recently ‘granulomatosis with polyangiitis’, in part because of concerns about the suitability of Friedrich Wegener as the source of an eponym. The most distinctive pathological feature of Wegener's granulomatosis is multi-focal necrotizing inflammation that has long been called granulomatosis. The systemic variant of Wegener's granulomatosis also is characterized by inflammation in many different vessels or different types, i.e. polyangiitis. Thus, granulomatosis with polyangiitis is a very appropriate alternative term for Wegener's granulomatosis. This term also is in accord with the name for a closely related vasculitis, i.e. microscopic polyangiitis. Terms that indicate aetiology and pathogenesis, when known, are useful to include in names for diseases (diagnoses). Anti-neutrophil cytoplasmic autoantibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA) are implicated in the cause of granulomatosis with polyangiitis and thus also should be specified in the diagnosis (e.g. PR3-ANCA-positive granulomatosis with polyangiitis or MPO-ANCA-positive microscopic polyangiitis). As our understanding of the clinical manifestations, pathogenesis and aetiology of vasculitides change over time, the names and approaches for diagnosing these diseases will change accordingly.
Keywords: ANCA, anti-neutrophil cytoplasmic autoantibodies, microscopic polyangiitis, vasculitis, Wegener's granulomatosis
In Scene 2, Act II, of Shakespeare's Romeo and Juliet, Juliet asks: ‘What's in a name? That which we call a rose by any other name would smell as sweet.’ This states the fact that a particular name does not alter the essential nature of what is being named. However, Juliet also passionately laments that Romeo's family name is Montague and wishes that it could ‘be some other name’. This exemplifies how important a name can be with respect to how something is perceived and treated. In fact, the entire tragedy that befell Romeo and Juliet was precipitated by perceptions and prejudices resulting from their names and classes. Names are not trivial.
In clinical practice and in biomedical research, the name of a disease (i.e. the diagnostic term or diagnosis) derives from prior knowledge of the disease and, importantly, may drive future studies of the disease. Of necessity, a name cannot contain all that is known about a disease but rather should include words that at least conjure up some major clinical or pathophysiological hallmark of the disease. Alternatively, especially if the pathophysiological nature of the disease is unknown or poorly known, an eponym is used in the diagnosis based on a seminal contribution by the source of the name to the recognition or elucidation of the disease. Names for diseases (diagnostic terms) often begin with relatively arbitrary decisions by someone who is involved with the clinical management or pathophysiological study of the disease. Less often, and usually at a juncture when there is a perceived need to alter a commonly used name, a more deliberative approach by a group of clinicians or scientists is used to propose a change in nomenclature for a disease. However, in the final analysis a name is useful only if it is usable and used. No matter how logical and appropriate a name may be based on contemporary knowledge of a disease, if it is not usable and used it is of no lasting value. In this brief commentary, as a case in point, I will focus on Wegener's granulomatosis (WG), recently renamed ‘granulomatosis with polyangiitis’ (GPA) [1,2].
In spring 2011, just prior to the Fifteenth International Vasculitis and ANCA Workshop, the deliberations of a diverse group of clinicians and scientists will result in modifications of the Chapel Hill Consensus Conference (CHCC) nomenclature for systemic vasculitides, which will be based on clinical, pathophysiological and ethical developments since the original CHCC in 1993. The goals of the CHCC were to reach consensus on the names for some of the most common forms of systemic vasculitis and to construct root definitions for these [3]. The success of this effort is evidenced by its wide adoption in both clinical and research settings, and by greater than 17 000 citations in the medical literature. An impact of the recommendations of the CHCC is illustrated in Fig. 1, which shows the use of the diagnostic terms ‘microscopic polyarteritis’versus‘microscopic polyangiitis’ in the titles of papers published in the medical literature before and after the recommendation of the CHCC to use the latter term.
Fig. 1.
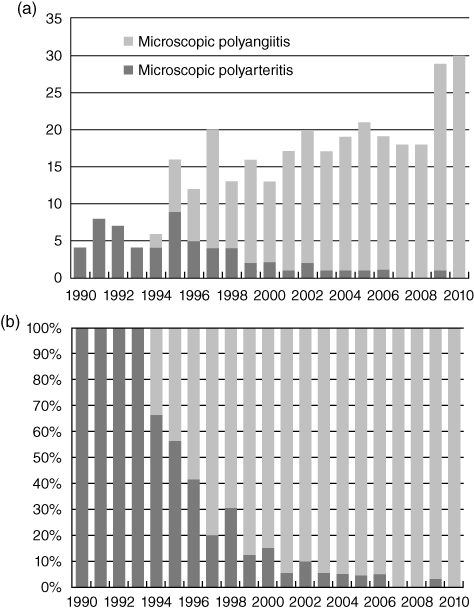
Frequency of the use of the term ‘microscopic polyarteritis’versus‘microscopic polyangiitis’ in the title of articles in PubMed from 1990 to 2010. Graph (a) expresses this as the total number of articles published per year. Graph (b) expresses this as a percentage of all articles that had either term in the title. Note the change in usage after publication of the recommendations of the Chapel Hill Consensus Conference (CHCC) in 1994.
One of the modifications that is anticipated in the 2011 CHCC nomenclature is a recommended change from the diagnostic term ‘Wegner's granulomatosis’ to ‘granulomatosis with polyangiitis’ (GPA), which has already been advocated by some of the 2011 CHCC participants [1,2]. This is justified both on the general rule that diagnostic terms with eponyms are less effective than more descriptive terms that refer to one or more distinctive features of a disease and, in the specific instance of Wegener's granulomatosis, on the evidence that Dr Friedrich Wegener was a member of the Nazi party before and during World War II [4].
The history of the naming of GPA (WG) is illustrative of how a name can influence the understanding of a disease. Instead of ‘rhinogenic granulomatosis’[5], what if Wegener had used the term ‘rhinogenic purulosis’, emphasizing the intense purulent inflammation that is much more conspicuous than true granulomatous inflammation in most acute respiratory tract lesions of GPA (WG) (Fig. 1)? [6,7] Would this emphasis on neutrophilic inflammation in the name have drawn the attention of investigators sooner to the role of neutrophils in the pathogenesis of GPA (WG)?
A granuloma (or granulomatous inflammation) is a lesion characterized histologically by a compact accumulation of predominantly macrophages that, if large enough, grossly appears nodular. Giant cells, which are formed by the coalescence of monocytes and macrophages into multi-nucleated syncytia, may be a component of granulomas, but giant cells are neither sufficient nor required for a diagnosis of granulomatous inflammation. In the most literal sense, granulomatosis indicates a condition characterized by multiple granulomas. Sarcoidosis is an archetype granulomatosis, although the term granulomatosis is rarely used in discussing or writing about sarcoidosis. In fact, the term granulomatosis is most often used in the medical literature in the context of GPA (WG).
Especially in the acute lesions of GPA (WG), the predominant pattern of inflammation is not granulomatous, but purulent. Thus, the inflammation has the appearance of an abscess more than a granuloma (Fig. 2). Often, the only feature in the acute inflammatory lesions that is reminiscent of granulomatous inflammation is the presence of scattered multi-nucleated giant cells. As lesions age, they often develop a central zone of necrosis that seems to evolve from extensive karyorrhectic (leucocytoclastic) debris to a central zone with a slightly basophilic hue, and finally to a central zone of amorphous acidophilic material (Fig. 3). Concurrent with this degeneration of the central zone of neutrophils, the periphery of the lesion accrues palisades of elongated macrophages and scattered multi-nucleated giant cells that justify being called granulomatous inflammation. Mark et al. [6] concluded that in GPA (WG): ‘Micronecrosis, usually with neutrophils (microabscesses), constitutes the early phase in the development of the pathognomonic organized palisading granuloma.’ They suggested that the multi-nucleated giant cells might be a secondary reactive response to the acute necrotizing lesions. This is supported by the finding of engulfed apoptotic and necrotic neutrophil debris that can be seen occasionally within the multi-nucleated giant cells at sites of necrotizing inflammation in GPA (WG) (Fig. 2b).
Fig. 2.
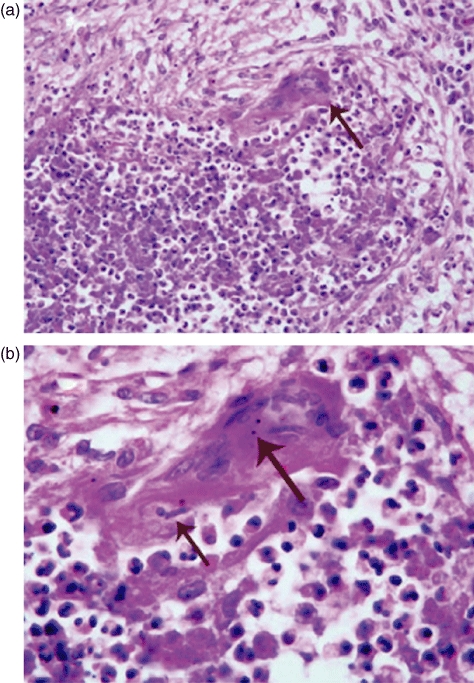
Photomicrographs of an acute necrotizing lesion in the lung of a patient with granulomatosis with polyangiitis (GPA) (Wegener's granulomatosis) showing a central zone of necrosis with intense influx of neutrophils (microabscess formation) with an adjacent multi-nucleated giant cell: arrow in (a). The higher magnification in (b) reveals a phagocytosed neutrophil (small arrow) and phagocytosed apoptotic debris (large arrow) in the cytoplasm of a multi-nucleated giant cell (haematoxylin and eosin stain).
Fig. 3.
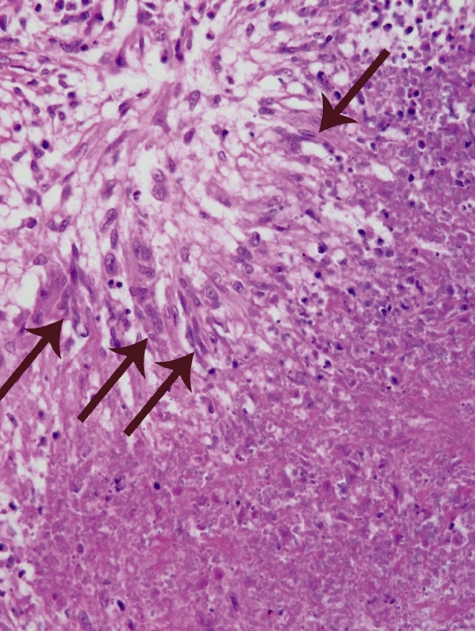
Photomicrographs of the edge of a necrotizing lesion in the lung of a patient with granulomatosis with polyangiitis (GPA) (Wegener's granulomatosis) showing a zone of necrosis in the lower right with a marginal band of palisading elongated macrophages (arrows) in the upper left (haematoxylin and eosin stain).
This prominence of neutrophilic infiltrates (microabscesses) in the acute phase of the disease and the atypicality of the granulomatous inflammation that follows have been reported in detail in the literature [6,7] but probably, in part because of the term ‘granulomatosis’ in the name, concepts and theories about the pathogenesis of the extravascular inflammation in GPA (WG) have drawn analogies to typical granulomatous inflammation as seen in sarcoidosis or tuberculosis, which has little or no resemblance to the granulomatosis of GPA (WG). In a careful pathological study of pulmonary specimens from 35 patients with GPA (WG), Mark et al. [6] concluded that: ‘Compact granulomas of tuberculoid or sarcoidal type did not occur in the cases of Wegener's granulomatosis.’
Although I have been concentrating on the neutrophilic inflammation that is present during the acute phase of the necrotizing inflammation, a distinctive type of multi-focal granulomatous inflammation is unquestionably a hallmark of GPA (WG), and may be the only pattern of inflammation seen with treated chronic disease. This pattern of injury is characterized by an irregular central zone of necrosis containing varying numbers of degenerating neutrophils and necrotic debris surrounded by poorly defined granulomatous inflammation with palisades of elongated macrophages and scattered multi-nucleated giant cells. This pattern of injury with central necrosis and peripheral palisading macrophages was well described in the seminal publications by Wegener [5] and later by Godman and Churg [8]. Some investigators have even concluded that this is essentially pathognomonic for GPA (WG). For example, Mark et al. [6] stated that: ‘Palisading granuloma is virtually pathognomonic of Wegener's granulomatosis whether or not it involves blood vessels.’ They go on to note that: ‘Compact granulomas of tuberculoid or sarcoidal type did not occur in the cases of Wegener's granulomatosis’. They contend that there is a very distinctive special form of granulomatous inflammation in patients with GPA (WG), but it is very different from more typical forms of granulomatous inflammation.
Thus, the pathology of GPA (WG) warrants using the term granulomatosis in the name. As importantly, historical precedent also supports the use of the term granulomatosis in any new name for Wegener's granulomatosis. As noted earlier, granulomatosis has been used in the modern medical literature primarily in the context of Wegener's granulomatosis. Wegener initially used the term ‘rhinogenic granulomatosis’ for this disease. Further, Churg and Strauss used the term ‘allergic granulomatosis’ for what is often called Churg–Strauss syndrome [9], which is related closely to GPA (WG) and shares pathologically similar granulomatosis, polyangiitis and glomerulonephritis with GPA (WG).
Microscopic polyangiitis (MPA) shares a systemic small vessel vasculitis and pauci-immune necrotizing and crescentic glomerulonephritis with GPA (WG) and allergic granulomatosis (Churg–Strauss syndrome). All three diseases are also associated with anti-neutrophil cytoplasmic autoantibodies (ANCA), which appear to have a pathogenic role in these diseases. This substantiates the hypothesis made by Godman and Churg in 1954, based on pathology alone, that these three diseases are closely related and probably share a common pathogenic mechanism [8].
Another issue that will be addressed by the 2011 CHCC is the role, if any, for ANCA serology in the diagnostic terms for GPA (WG), MPA and allergic granulomatosis (Churg–Strauss syndrome). For example, should this group (class) of vasculitides be called ANCA disease or ANCA-associated vasculitis, and should each clinicopathological variant name be prefixed by MPO-ANCA, PR3-ANCA or ‘seronegative’ (e.g. PR3-ANCA GPA, MPO-ANCA MPA, seronegative GPA, etc.)? There are clinical and pathophysiological arguments in favour [1,10] and against [10] this approach.
One thing is certain: as our understanding of the clinical manifestations, pathogenesis and aetiology of these and other vasculitides change over time, the names and approaches for diagnosing these diseases will change accordingly.
Disclosure
None.
References
- 1.Falk RJ, Jennette JC. ANCA disease: where is this field going? J Am Soc Nephrol. 2010;21:745–52. doi: 10.1681/ASN.2009121238. [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. 2011. A joint proposal of the American College of Rheumatology, the American Society of Nephrology, and the European League against Rheumatism, in press. [DOI] [PubMed]
- 3.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: the proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 4.Woywodt A, Haubitz M, Haller H, Matteson EL. Wegener's granulomatosis. Lancet. 2006;367:1362–6. doi: 10.1016/S0140-6736(06)68583-8. [DOI] [PubMed] [Google Scholar]
- 5.Wegener F. Ueber eine eigenartige rhinogene Granulomatose mit besonderer Beteiligung des Arteriensystems und der Nieren. About a peculiar rhinogenic granulomatosis with marked involvement of the arterial system and kidneys. 1939;102:30–68. [Google Scholar]
- 6.Mark EJ, Matsubara O, Tan-Liu NS, Fienberg R. The pulmonary biopsy in the early diagnosis of Wegener's (pathergic) granulomatosis: a study based on 35 open lung biopsies. Hum Pathol. 1988;19:1065–71. doi: 10.1016/s0046-8177(88)80088-1. [DOI] [PubMed] [Google Scholar]
- 7.Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener's granulomatosis. A pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol. 1990;14:555–64. doi: 10.1097/00000478-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Godman GC, Churg J. Wegener's granulomatosis. Pathology and review of the literature. Arch Pathol. 1954;58:533. [PubMed] [Google Scholar]
- 9.Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteriitis nodosa. Am J Pathol. 1951;27:277–94. [PMC free article] [PubMed] [Google Scholar]
- 10.Falk RJ, Hoffman GS. Controversies in small vessel vasculitis – comparing the rheumatology and nephrology views. Curr Opin Rheumatol. 2007;19:1–9. doi: 10.1097/BOR.0b013e328011cb80. [DOI] [PubMed] [Google Scholar]


