Abstract
Exposure to persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs), dichlorodiphenyldichloroethane (p,p’-DDE), and hexachlorobenzene (HCB) continues to be of concern due to their ubiquitous distribution and high persistence. Current toxicant body burden is still a primary concern within the Akwesasne Mohawk Nation since other studies conducted within the community have shown relationships between these POPs and endocrine disruption.
In this article we describe the levels of these toxicants in young adults of the Akwesasne Mohawk Nation between the ages of 17 and 21 years of age (mean age 18.1 years), and investigate potential influences of their current body-burden. Seventeen congeners in fourteen chromatographic peaks were detected in 50% or more of the individuals sampled (geometric mean [GM] of the sum of these congeners = 0.43 ppb). Congeners 118, 138[+163+164] and 153 had the highest rate of detection (≥ 98%) within the Akwesasne youth. Of the other organochlorines, HCB (GM= 0.04 ppb) and p,p’-DDE (GM=0.38 ppb) were found in 100% and 99% of the sample respectively.
Significantly higher levels of PCBs were found among individuals who were breastfed as infants, were first born, or had consumed local fish within the past year. When compared to levels of p,p’-DDE, HCB, and 13 specific congeners reported by the CDC for youth between the ages of 12 and 19 years, the geometric means of several congeners (CBs 99, 105, 110, and 118) among the Akwesasne were higher than the reported CDC 90th percentile. In contrast, levels of CB 28 in Akwesasne youth were ∼50% or less than those of the CDC cohort. p,p’-DDE and HCB levels were generally higher in the CDC cohort (GM of 0.516 and 0.065 ppb, respectively for Mohawks vs. 2.51 and 0.123, respectively, for CDC). Concentrations of non-persistent PCBs among this sample of Akwesasne young adults were higher than those reported by the CDC suggesting continued exposure, but lower than those associated with severe contamination.
Additional research into the concentration trends of individual PCB congeners within Akwesasne youth and young adults is warranted to further improve our insight into the determinants and influences of organochlorine concentrations within members of the Akwesasne community.
Keywords: PCBs; persistent organic pollutants; HCB; p,p’-DDE; Mohawk; Native American
1. Introduction
Exposure to persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs), dichlorodiphenyldichloroethane (p,p’-DDE), and hexachlorobenzene (HCB) continues to be of concern due to their ubiquitous distribution, persistence in the environment, and long physiological half-lives. All POPs are lipophilic and can bioaccumulate, amplifying readily up the food chain. Many have been shown to disrupt development and functioning of certain endocrine pathways, to alter growth, development, cognitive function, and to exhibit immunotoxicity in experimental animals, biota, and humans (American Council on Science and Health 1997;Brouwer et al. 1999;Denham et al. 2005;Leijs et al. 2009;Newman et al. 2006;Newman et al. 2009;Schell et al. 2009;Schell et al. 2006).
PCBs were originally synthesized for use as lubricants, heat-exchange fluids, and a number of other commercial and industrial uses where high chemical and physical stability was required. Banned in the late 1970’s in the US and many other countries, PCBs persist in the environment with half-lives varying from months to decades (Hansen 1999). PCBs consist of 209 individual congeners which can be divided into groups according to their structure, persistence, and toxicological properties. While routinely found in tissue and body fluid specimens from humans and biota, health risk assessment for PCBs is complex, given the differing proportions of individual PCB congeners in commercial mixtures and their different mechanisms of toxicity. In particular, non-ortho-substituted PCBs are structurally similar to polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) (Hansen 1999). Hence the most common categorization is of “dioxin-like” and “non-dioxin-like” congeners (Hansen 1999).
Prior to the issuance of fish advisories in the late 1980s, the Akwesasne Mohawk Nation relied heavily on subsistence fishing, farming and trapping in and around the St. Lawrence River and its many tributaries. Current toxicant body burden in Akwesasne Mohawks is still a primary concern within the community, since other studies conducted in the Nation have shown associations between these POPs and endocrine disruption(Codru et al. 2007;Goncharov et al. 2008;Goncharov et al. 2009;Schell et al. 2008;Schell et al. 2009).
The aim of the current study is to describe the levels of certain POPs among young adults of the Akwesasne Mohawk Nation (AMN), and to investigate potential influences and/or predictors of their current body-burden. A comparison to levels of specific congeners reported by the Center for Disease Control (Centers for Disease Control 2009) is also presented.
2. Materials and Methods
2.1. Sample
The study was conducted in partnership with the Akwesasne Mohawk Nation (AMN). The Nation is situated on the St. Lawrence River with territory abutting New York State, Ontario and Quebec, Canada.The Akwesasne community is one of several communities comprising the Kahniakehaka/Mohawk nation. Traditionally, the Mohawk are the keepers of the Eastern Door of the Iroquois Confederacy, also known as the Six Nations or the Haudenosaunee Confederacy. Members of the nation live within the boundaries of the AMN and in neighboring communities that are part of traditional Mohawk territory, including Bombay, Fort Covington, Hogansburg, Massena, and Rooseveltownin New York State, and in Cornwall, Ontario, Canada. Though not a federally censused population; reports in the last two decades indicate a population of approximately 12,000 −13,000 (Akwesasne Task Force On The Environment 1997;Fitzgerald et al. 1998;George-Kanentiio 1995).
The area surrounding the St. Lawrence River is a major industrialized region of North America. Industrial facilities located around Cornwall and Massena discharged significant quantities of contaminants to the St. Lawrence River(Sloan and Jock 1990). Industrial development along the St. Lawrence River began in the 1950s, and major industrial dischargers located just upstream of AMN include a National Priority Superfund Site (General Motors Central Foundry Division), and two New York State Superfund Sites (Reynolds Metal Company and Aluminum Company of America). These three sites, one aluminum foundry and two aluminum smelters, have contaminated the St. Lawrence River and its three tributaries with PCBs which have entered the local food chain. The GM Central Foundry is located less than 0.3 km from homes at Akwesasne. Between 1959 and 1974, the facility used PCB-based hydraulic fluids in its die-casting machines, and the predominant PCB type used was Aroclor 1248 (Bush and Kadlec 1995;Fitzgerald et al. 1996).Historical contamination fromp,p’-DDE and HCB is also present. Some local species of fish, birds, amphibians and mammals were found to have PCB levels exceeding the US Food and Drug administration’s tolerance limits for human consumption (Forti et al. 1995;Sloan and Jock 1990), leading to the issuance of fish and game advisories in the late 1980s and early 1990s (Fitzgerald et al. 1995;Fitzgerald et al. 1998). Subsequently, studies of PCB levels in breast milk have reported a decrease consistent with adherence to the advisories and reduced fish consumption (Fitzgerald et al. 2001).
Young adults were eligible for the current study if they had participated in our earlier project, the Mohawk Adolescent Well-Being Study (MAWBs)(Schell et al. 2003), and were now between 17 and 20 years of age. They were ineligible for the current study if they were outside the target age range, were pregnant or had delivered a child, had lactated, or had miscarried within the past six months. However, they could be included six months after any of these events. Of the MAWBs participants who were eligible (n=271), 33.6% were lost to follow-up,9.6% either refused to participate or never completed data collection, resulting in 56.8% who participated in the current study for a sample of 154 individuals. Reasons for refusal included having moved out of the area, and not having enough time to participate. One person was excluded from this analysis because serum organochlorine levels were not available, and another because sociodemographic data was not reported, leaving a final sample of 152.
2.2. Data Collection
The University at Albany, State University of New York’s Institutional Review Board approved all study protocols. Informed consent was obtained from all participants 18 years of age or older. For those under 18, parents provided informed consent and assent was obtained from all participants who were minors.
All data collection was performed by project staff, all of whom were members of the Akwesasne community. All data were collected without prior knowledge of participants’ exposure status. A more detailed description of enrollment and methods was previously published (Schell et al. 2009). In brief, each participant completed interviews and sociodemographic questionnaires providing demographic, lifestyle, and diet information. The interviews included, but were not limited to, information about prescription and over-the-counter medicine use (used in the past year), diet (including locally caught fish and wildlife consumed in the past year), recreational and traditional activities, current cigarette and alcohol use, breastfeeding history (in consultation with their mother; recorded as any or none), sex (males =0, females =1), age (to the nearest month) when blood was drawn, educational status (as the highest year of education completed), body mass index (calculated), weight (kg; self-report), height (cm; self-report), and as a proxy for socioeconomic status, education, current and past employment, and living environment. Birth order was coded as first child, second child, third child, etc.
In addition, information on dietary intake over the past year was collected by interview. The Food Frequency Questionnaire (FFQ), developed by the National Cancer Institute in collaboration with the US Department of Agriculture, asks participants to report the frequency of consumption and portion size of approximately more than a hundred line items retrospectively, representing a person’s long-term average daily intake. Food models were employed to provide guidance on portion sizes. DIETSYS, a nutrient analysis program, was used to calculate total nutrients based on the respondents’ reported frequency of each food combined with the portion size and the nutrient content (the amount of nutrient in 100g of this food).
2.3. Laboratory analysis of toxicants
Organochlorine (OC) pesticide and PCB analyses for the current study were conducted at the University at Albany’s Exposure Assessment Laboratory. Fasting blood specimens were collected at first rising by trained Mohawk staff to provide material for analysis of four toxicants (PCBs, p,p’-DDE, HCB, and mirex).Complete details of the laboratory protocol for PCB analysis have been published (DeCaprio et al. 2000;DeCaprio et al. 2005). In brief, high resolution, ultratrace, congener-specific analysis was performed by parallel dual-column (splitless injection) gas chromatography with electron capture detection on an Agilent 6890 instrument. This method quantitates up to 83 individual PCB congeners and 18 PCB congeners as pairs or triplets, as well as p,p’-DDE, HCB, and mirex (a total of 94 analytical peaks). The analytes include all of the major Aroclor-derived congeners typically present in human samples plus a number of sporadic or rare congeners. The laboratory is accredited by the NYSDOH Clinical Laboratory Evaluation Program and participated in the Arctic Monitoring and Assessment Programme (AMAP) Ring Test for Persistent Organic Pollutants in Human Serum. Individual chlorinated biphenyl (CB) congeners are identified according to the IUPAC numbering system (Ballschmiter and Zell 1980;Guitart et al. 1993). Congeners for which all reported values were below the laboratory method detection limit (mdl) included CBs 3, 6, 63, and 67. These congeners were not included in any calculations. Data were expressed on a whole-weight basis (i.e., not lipid-adjusted) as participants had fasted overnight prior to the blood draw.
2.4. Congener groupings
Any individual datum of an analyte that was below the mdl was substituted with the midpoint value between zero and the mdl (i.e., mdl/2) of each compound or congener. Using this approach, we calculated PCB summary variables for each participant (Table 2, footnote). The rate of detection, the degree of chlorination, and the chlorination pattern of individual PCB congeners are considered in the composition of the PCB groupings. Environmental persistence, bioaccumulation in food chains, distribution in human tissue, and the toxicologic action (i.e., dioxin-like vs. Ah receptor-independent effects) of PCBs depend on the chemical structure of individual congeners (Laden et al. 1999;McFarland and Clarke 1989). All PCB congeners in the sample were included in a summation variable (Total PCBs), as the sum of the 94 congeners (in 85 analytical peaks) that were detected. The fourteen congeners detected in greater than 50% of the sample were used to create three variables for statistical analyses: the sum of all 14 congeners (Σ14PCB50%); the sum of nine persistent congeners (Σ9PersistentPCBs), and the sum of five non-persistent congeners (Σ5NonPersistentPCBs). CBs assigned as persistent were those known or expected to have long physiological half-lives in humans due to high lipid solubility and/or low rates of metabolism (Brown, Jr. 1994;Hansen 1999). Conversely, non-persistent congeners were those known to be more readily metabolized and excreted; theseare indicators of recent and/or ongoing PCB exposure. Mirex was not considered in this analysis; only negligible levels were found in slightly more than 8% of the sample.
Table 2.
Summary and specific PCB congener levels within the Akwesasne Mohawk adolescent population (in ppb; n=152).
| Percentiles |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structure | mdl | Rate of detection |
Mean | GM | SD | 5 | 75 | 95 | MAX | |
| Total PCBsa | - | - | 0.876 | 0.824 | 0.4205 | 0.570 | 0.911 | 1.544 | 4.337 | |
| Σ14PCB50%a,b | - | - | 0.474 | 0.433 | 0.2640 | 0.250 | 0.509 | 0.968 | 2.499 | |
| Σ9 Persistent PCBsa,c | - | - | 0.357 | 0.316 | 0.2291 | 0.158 | 0.392 | 0.829 | 1.914 | |
| Σ5 Non-persistent PCBsa,d | 0.117 | 0.106 | 0.0598 | 0.051 | 0.135 | 0.235 | 0.585 | |||
| p,p’ -DDE (ppb)a | 0.007 | 99.3% | 0.383 | 0.323 | 0.2358 | 0.129 | 0.456 | 0.866 | 1.614 | |
| HCB (ppb)a | 0.004 | 100.0% | 0.036 | 0.033 | 0.0155 | 0.018 | 0.040 | 0.068 | 0.112 | |
| PCB IUPAC#a | ||||||||||
| 118 | 2,3’,4,4’,5 | 0.007 | 100.0% | 0.048 | 0.041 | 0.0416 | 0.021 | 0.051 | 0.089 | 0.419 |
| 138 [+163+164]e | 2,2’,3,4,4’,5’ + 2,3,3’,4’,5’,6 + 2,3,3’,4’,5,6 |
0.011 | 100.0% | 0.070 | 0.061 | 0.0492 | 0.028 | 0. 0 81 | 0.149 | 0.408 |
| 153 | 2,2’,4,4’,5,5’ | 0.024 | 98.0% | 0.079 | 0.067 | 0.0559 | 0.030 | 0.088 | 0.171 | 0.448 |
| 101[+90]e | 2,2’,3,4’,5 + 2,2’4,5,5’ | 0.007 | 97.4% | 0.030 | 0.025 | 0.0162 | 0.009 | 0.036 | 0.060 | 0.123 |
| 99 | 2,2’,4,4’,5 | 0.007 | 91.4% | 0.044 | 0.034 | 0.0278 | 0.004 | 0.053 | 0.093 | 0.217 |
| 87 | 2,2’,3,4,5’ | 0.009 | 90.7% | 0.020 | 0.018 | 0.0097 | 0.005 | 0.024 | 0.034 | 0.071 |
| 52 | 2,2’,5,5’ | 0.005 | 88.1% | 0.029 | 0.022 | 0.0191 | 0.003 | 0.035 | 0.068 | 0.148 |
| 74 | 2,4,4’,5 | 0.004 | 89.4% | 0.021 | 0.014 | 0.0262 | 0.002 | 0.024 | 0.057 | 0.252 |
| 105 | 2,3,3’,4,4’ | 0.002 | 86.1% | 0.022 | 0.013 | 0.0209 | 0.001 | 0.034 | 0.066 | 0.099 |
| 187 | 2,2’,3,4’,5,5’,6 | 0.005 | 82.8% | 0.022 | 0.011 | 0.0213 | 0.001 | 0.028 | 0.055 | 0.183 |
| 110 | 2,3,3’,4’,6 | 0.014 | 77.5% | 0.026 | 0.021 | 0.0199 | 0.007 | 0.035 | 0.061 | 0.138 |
| 180 | 2,2’,3,4,4’,5,5’ | 0.017 | 76.2% | 0.039 | 0.026 | 0.0506 | 0.009 | 0.042 | 0.099 | 0.383 |
| 95 | 2,2’,3,5’,6 | 0.002 | 58.9% | 0.012 | 0.005 | 0.0214 | 0.001 | 0.014 | 0.040 | 0.169 |
| 28 | 2,4,4’ | 0.008 | 55.6% | 0.012 | 0.009 | 0.0099 | 0.004 | 0.020 | 0.032 | 0.054 |
| 156 | 2,3,3’,4,4’,5 | 0.003 | 46.4% | 0.008 | 0.004 | 0.0116 | 0.002 | 0.011 | 0.023 | 0.078 |
| 199 | 2,2’,3,3’,4,5,5’,6’ | 0.002 | 43.7% | 0.008 | 0.003 | 0.0148 | 0.001 | 0.011 | 0.031 | 0.109 |
| 70 | 2,3’,4’,5 | 0.006 | 43.0% | 0.008 | 0.006 | 0.0071 | 0.003 | 0.011 | 0.021 | 0.041 |
| 170 | 2,2’,3,3’,4,4’,5 | 0.005 | 39.7% | 0.012 | 0.008 | 0.0172 | 0.005 | 0.013 | 0.033 | 0.146 |
| 183 | 2,2’,3,4,4’,5’,6 | 0.003 | 39.7% | 0.004 | 0.003 | 0.0043 | 0.002 | 0.005 | 0.012 | 0.029 |
| 123[+149]e | 2,2’,3,4’,5’,6 + 2,3’,4,4’,5’ | 0.010 | 36.4% | 0.016 | 0.009 | 0.0441 | 0.005 | 0.016 | 0.039 | 0.525 |
| 47 | 2,2’,4,4’ +2,3,3’,6 | 0.007 | 35.1% | 0.008 | 0.006 | 0.0079 | 0.004 | 0.011 | 0.023 | 0.051 |
| 146 | 2,2’,3,4’,5,5’ | 0.022 | 35.1% | 0.031 | 0.020 | 0.0379 | 0.011 | 0.047 | 0.092 | 0.249 |
| 114 | 2,3,4,4’,5 | 0.001 | 27.2% | 0.003 | 0.001 | 0.0083 | 0.001 | 0.003 | 0.014 | 0.085 |
| 190 | 2,3,3’,4,4’,5,6 | 0.004 | 25.8% | 0.004 | 0.003 | 0.0056 | 0.002 | 0.004 | 0.012 | 0.050 |
| 66 | 2,3’,4,4’ | 0.004 | 25.8% | 0.005 | 0.003 | 0.0150 | 0.002 | 0.004 | 0.014 | 0.182 |
| 196 | 2,2’,3,3’,4,4’,5,6’ | 0.002 | 21.9% | 0.003 | 0.002 | 0.0079 | 0.001 | 0.001 | 0.014 | 0.076 |
| 84 | 2,2’,3,3’,6 | 0.003 | 21.2% | 0.004 | 0.002 | 0.0080 | 0.002 | 0.002 | 0.015 | 0.077 |
Values below the detection limit have been replaced by the value midway between the detection limit and zero.
Abbreviations: PCB, polychlorinated biphenyls; PPB, parts per billion; IUPAC, International Union of Pure and Applied Chemistry; mdl, method detection limit; GM, geometric mean; SD, standard deviation; MAX, maximum level detected in sample.
Σ14PCB50%: Sum of IUPAC#s 28,52,74,87,95,99,101[+90],105,110,118,138[+163+164],153,180,187
Σ9 Persistent PCBs: Sum of IUPAC#s 28,74,99,105,118,138[+163+164],153,180,187
Σ5 Non-persistent PCBs: Sum of IUPAC#s 52,87,95,101[+90],110
Bracket indicates minor coeluting congener(s) based on Aroclor compositions and expected persistence (Brown, 1994; Hansen, 1998)
In order to compare the toxicant levels among Akwesasne young adults to the CDC data on human body burdens of environmental chemicals(Centers for Disease Control 2009), we did not substitute values below the mdl with the method described above (mdl /2). Instead, using the CDC method for handling values below the mdl, toxicant concentrations less than the mdl were assigned a value equal to the mdl divided by √2 for calculation of means and percentiles. Although using this imputation method made a difference of less than 1% in mean estimates (i.e., 0.437 ppb vs. 0.433ppb for total PCB), we employ it to be consistent with CDC reference values in order to facilitate our comparisons. Note that CDC did not report any geometric means if the proportion of the results below the method detection limit was greater than 40%. Therefore the levels of some congeners found at Akwesasne could not be compared to CDC reference values.
2.5. Statistical analysis
Statistical analyses were conducted with SPSS v.17 (SPSS 2009). All toxicant variables were log transformed to normalize their distributions. Descriptive statistics were calculated to determine the characteristics of the sample; t-tests and bivariate correlations were examined to determine relationships, if any, to potential predictors of toxicant levels (sex, breast feeding status, sociodemographic, and dietary variables). A relationship was considered statistically significant if p ≤ 0.05.
3. Results
3.1. Sociodemographic and behavioral characteristics of the sample
Characteristics of the sample are shown in Table 1. The median age of the sample was 18.1 years; males and females (n= 61, 92 respectively) did not differ significantly in age. More than half of the participants (51%) were seen within 6 months of their 17th birthday (mean = 18.08, SD = 1.10).Forty-nine percent of the participants currently smoke cigarettes (32% have never smoked); 95% consumed alcohol in the last year, with 36% admitted starting at the age of 16 years. Nearly 37% of the participants were first born children (38 females, 17 males). Forty nine percent of the sample had been breast fed; breast feeding history did not differ by sex.
Table 1.
Charactersitics of the Akwesasne Mohawk young adults.
| % |
||||||
|---|---|---|---|---|---|---|
| Mean | Median | SD | Yes | No | ||
| Age (years) | 18.07 | 17.60 | 1.090 | |||
| Alcohol consumption (past year) | 61.7 | 38.3 | ||||
| Birth order (% first-born) | 36.9 | 63.1 | ||||
| Body Mass Index (kg/m2) | 25.71 | 25.37 | 4.831 | |||
| Breastfed (yes/no) | 49.0 | 51.0 | ||||
| Breast feeding duration (months) | 2.75 | 5.240 | ||||
| Consumption of locally caught animal or fowl (past year) | 30.0 | 70.0 | ||||
| Consumption of locally caught fish (past year) | 32.2 | 67.8 | ||||
| Currently smoking (yes/no) | 48.9 | 51.0 | ||||
| Educational level (higest year attained) | 10.79 | 11.00 | 1.412 | |||
| Total caloric intake (kcal/day) | 2067 | 1902 | 904 | |||
| Total fat intake (g/day) | 88.32 | 81.70 | 42.661 | |||
| Total protein intake (g/day) | 77.01 | 71.40 | 35.679 | |||
Thirty percent of the participants reported that in the past year they had eaten some amount of locally caught deer, moose, duck, partridge, rabbit, pheasant and/or muskrat, while 32% reported eating some amount of locally caught fish. Of the fish consumed, approximately 28% were top feeding fish (bass, perch, pike, salmon and trout), and 9.2% bottom feeders (catfish, bullhead, and sturgeon). Slightly more than 5% of the participants reported eating both bottom and top feeding fish.
Seventy-seven percent of the sample at the time of the study attended school; 64% of these were currently in high school. Approximately 4% of the study sample had one or more children, and 6% were employed in full-time positions, while 11% were in part-time positions.
The average body mass index (BMI) was 25.7 kg/m2 and was significantly higher in males (27.6 kg/m2; p≤0.001) than females (24.4 kg/m2). Males had significantly higher intake of calories (mean = 2,535 kilocal), protein (mean = 94 gm), fats (mean= 105 gm), average daily dietary intake of cholesterol (mean = 379 mg) (p≤0.001). Males also consumed more alcohol per month (mean 2.8 times per month; primarily beer) than females. Participation in local fishing and trapping and consumption of the locally caught fish and wild life did not differ between males and females.
3.2. Toxicant levels
Comparisons among subjects
The mean level of PCBs in differentstructural groupings and of individual congeners, as well as rates of detection and percentiles, are shown in Table 2 (individual CBs with detection rates of less than 20% are not shown). CBs 118, 138[+163+164] and 153 accounted for more than 21% of the total PCB body burden (GM: 0.05 ppb, 0.07 ppb, 0.08 ppb, respectively), and 41% of the Σ14PCB50%. Of the other organochlorines, HCB (GM= 0.04 ppb) and p,p’-DDE (GM=0.38 ppb) were found in 100% and 99% of the sample respectively (Table 2). The maximum level of total PCBs was 4.34 ppb (GM0.82 ppb). Only fourteen congeners were detected in 50% or more of the sample, with the highest level (0.45 ppb) noted for CB153. None of the levels of PCB summary variables differed by sex, nor did any individual congener levels. Males were found to have marginally yet significantly higher levels of HCB (t=1.99, p= 0.05).
Breastfed individuals hadsignificantly higher levels of ΣTotal PCBs, Σ14PCB50%, and Σ9PerPCBs (Table 3). Three participants did not report their breast feeding history, reducing the sample size to 149. Levels of six different congeners detected in 50% or more of the sample (CBs138 [+163+164], 153, 52, 74, 187, and 180) were significantly higher in the breastfed sub-sample. Of the 13 congeners detected in less than 50% but more than 20% of the sample, 6 of them (CBs 156, 199, 170 , 183, 66, and 196) were significantly higher (p<0.03-0.01) in breastfed young adults; none were found to be significantly lower. All congener levels were found to be greater in young adults who had been breastfed. Breast feeding duration (in months) was highly, positively correlated with ΣTotal PCBs, Σ14PCB50%, Σ9PerPCBs, and p,p’-DDE, along with many of the persistent individual congeners. Two non-persistent congenerswere found to be related to breast feeding duration, CBs 110 and 84. CB110 was negatively associated with breast feeding duration (r = −0.17, p=0.03), while CB 84 was positively related (r = 0.17, p=0.03).
Table 3.
Organochlorines and specific PCB congener levels in >50% of Akwesasne Mohawk young adults: Breastfed vs. non-breastfed (in ppb)*.
| Non-Breastfed (n=76) | Breastfed (n=73) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | GM | Max | Mean | GM | SD | Max | t | p | ||
| ΣTotal PCBsa,b | 0.81 | 0.78 | 2.64 | 0.94 | 0.87 | 0.516 | 4.34 | –2.10 | 0.04 | |
| Σ14PCB50%a,c | 0.43 | 0.40 | 1.32 | 0.52 | 0.47 | 0.329 | 2.50 | –2.32 | 0.02 | |
| Σ9 Persistent PCBsa,d | 0.31 | 0.29 | 1.09 | 0.41 | 0.35 | 0.286 | 1.91 | –2.77 | <0.01 | |
| Σ5 Non-persistent PCBsa,e | 0.12 | 0.11 | 0.26 | 0.11 | 0.10 | 0.070 | 0.59 | 0.83 | 0.41 | |
| p,p’ -DDEa | 0.35 | 0.32 | 0.93 | 0.42 | 0.33 | 0.295 | 1.61 | –0.40 | 0.69 | |
| HCBa | 0.04 | 0.03 | 0.09 | 0.04 | 0.03 | 0.017 | 0.11 | –1.12 | 0.27 | |
| PCB IUPAC# | ||||||||||
| 118 | 0.05 | 0.04 | 0.42 | 0.05 | 0.04 | 0.027 | 0.181 | 0.17 | 0.87 | |
| 138 [+163+164]f | 0.06 | 0.05 | 0.21 | 0.08 | 0.07 | 0.062 | 0.41 | –2.77 | <0.01 | |
| 153 | 0.06 | 0.06 | 0.21 | 0.09 | 0.08 | 0.071 | 0.45 | –3.52 | <0.01 | |
| 101[+90]f | 0.03 | 0.03 | 0.08 | 0.03 | 0.02 | 0.018 | 0.12 | 1.57 | 0.12 | |
| 99 | 0.04 | 0.04 | 0.13 | 0.04 | 0.03 | 0.033 | 0.22 | 0.72 | 0.47 | |
| 87 | 0.02 | 0.02 | 0.06 | 0.02 | 0.02 | 0.010 | 0.07 | 0.16 | 0.87 | |
| 52 | 0.03 | 0.02 | 0.09 | 0.03 | 0.03 | 0.021 | 0.15 | –2.26 | 0.03 | |
| 74 | 0.01 | 0.01 | 0.06 | 0.03 | 0.02 | 0.035 | 0.25 | –3.01 | <0.01 | |
| 105 | 0.02 | 0.01 | 0.10 | 0.02 | 0.02 | 0.020 | 0.08 | –1.72 | 0.09 | |
| 187 | 0.02 | 0.01 | 0.07 | 0.03 | 0.01 | 0.026 | 0.18 | –2.06 | 0.04 | |
| 110 | 0.03 | 0.02 | 0.10 | 0.03 | 0.02 | 0.023 | 0.14 | 1.36 | 0.18 | |
| 180 | 0.03 | 0.02 | 0.09 | 0.05 | 0.03 | 0.069 | 0.38 | –3.35 | <0.01 | |
| 95 | 0.01 | 0.01 | 0.17 | 0.01 | 0.00 | 0.015 | 0.11 | 1.66 | 0.10 | |
| 28 | 0.01 | 0.01 | 0.05 | 0.01 | 0.01 | 0.009 | 0.03 | 0.89 | 0.38 | |
3 participants did not report breast feeding status.
Abbreviations: PCB, polychlorinated biphenyls; PPB, parts per billion; IUPAC, International Union of Pure and Applied Chemistry; GM, geometric mean; SD, standard deviation; MAX, maximum level detected in sample.
Values below the detection limit have been replaced by the value midway between the detection limit and zero.
ΣTotal PCBs: Sum of all PCB congeners tested
Σ14PCB50%: Sum of IUPAC#s 28,52,74,87,95,99,101 [+90],105,110,118,138[+163+164],153,180,187
Σ9 Persistent PCBs: Sum of IUPAC#s 28,74,99,105,118,138[+163+164],153,180,187
Σ5 Non-persistent PCBs: Sum of IUPAC#s 52,87,95,101 [+90],110
Bracket indicates minor coeluting congener(s) based on Aroclor compositions and expected persistence (Brown, 1994; Hansen, 1998)
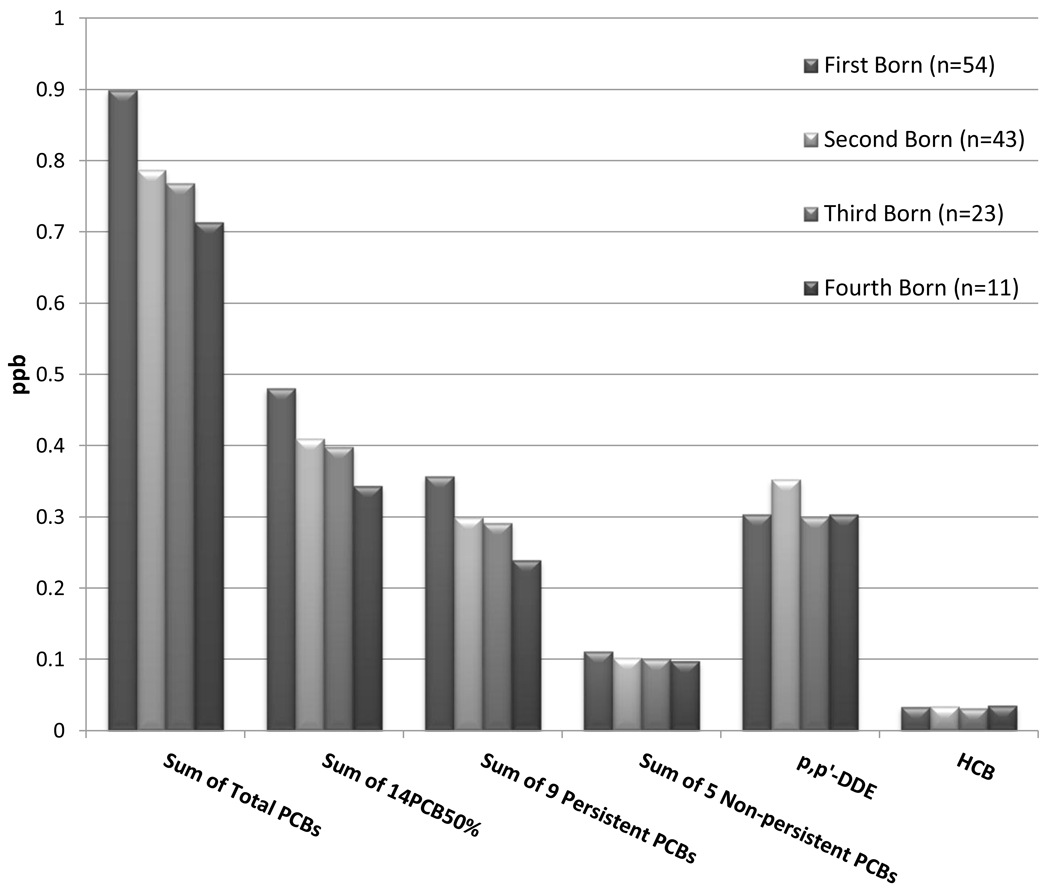
First-born individuals had significantly higher levels of ΣTotal PCBs, and Σ9PerPCBs than subsequent-born (Figure 1). Of the individual congeners found in 50% or more of the sample, only CBs 118 and 28 were significantly higher in the first-born individuals. Levels of the sum of non-persistent PCBs,p,p’-DDE, and HCB did not differ by birth order.
Figure 1.
Difference in toxicant levels by birth order in Akwesasne young adults.
Overall consumption of local foods is low; in the year prior to the interview, participants reported an average of 3 servings of fish and 1½ servings of local wildlife per year. Nevertheless, participants who reported eating locally caught fish during the past year had significantly higher levels of Σ9PersistentPCBs compared to those who did not. No other levels of PCB groupings were found to be significantly different with regard to local fish consumption. Of the nine highly persistent congeners, six (CBs 138, 153, 180, 156, 199, and 183) were individually found to be significantly higher among those who ate locally caught fish, and one congener (CB 123[+149]) was significantly lower. Consumption of bottom-feeding fish was relatively rare (12.8% claimed to have eaten some bottom feeding fish in the past year) and consumption was not associated with higher levels of PCBs. HCB levels increased with consumption of bottom-feeding fish more than one time, but no association was noted with p,p’-DDE. No significant differences in toxicant levels were noted in subjects that consumed locally hunted or trapped wildlife.
Participants who were born in or before 1984, at the time when the New York State Department of Health (NYSDOH)advisory was issued to limit consumption of locally caught fish (Fitzgerald et al. 1998), had significantly higher levels of moderately to highly persistent PCBs, p,p’-DDE, and HCB, than those born after the NYSDOH issued advisory (data are not shown). In contrast, individuals born in or after 1985 had significantly higher levels of most of the non-persistent PCBs. This observation likely reflects ongoing exposure to non-persistent PCBs and the more rapid metabolism of non-persistent PCBs among persons before 1985.
A multiple regression analysis was used to determine the effects of individual factors on the level of each toxicant while controlling for other potential influences on toxicant levels. Multivariate models were made to predict CB 153, the sum of 14 PCBs found in 50% or more of the sample, the sum of 9 persistent PCBs, the sum of 5 non-persistent PCBs, as well as HCB, and p,p’- DDE. All models contained age, sex, breast feeding status (yes, no), body mass index, current smoking status (yes, no), alcohol consumption per day, the highest year of education reached, total cholesterol, caloric, fat and protein intake, consumption of locally caught fish in the past year (yes, no), and birth year before or after issuance of the fish advisory. Significant predictors of CB 153 level were breast feeding status, body mass index and consumption of locally caught fish in the past year. Being born before or after issuance of the fish advisory was marginally significant (stdβ=−0.20, t=−1.88; p=−0.06) (Table 5). Predictors of the level of the sum of 14 PCBs found in 50% or more of the sample and of the levels of 9 persistent PCBs were the same: breast feeding status and consumption of locally caught fish; no other predictor was significant. The level of the five non-persistent PCBs was unrelated to any of the variables in the model. Significant predictors of p,p’-DDE were body mass index, current smoking status, total cholesterol intake, total protein intake and being born before or after the fish advisory. Only three variables were significant predictors of HCB, sex, body mass index, and being born before the fish advisory.
Table 5.
Predictors of toxicant levels in Akwesasne young adults (n=137).
| CB 153 (r2 = 30%) | p,p’ -DDE (r2= 25%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
95% CI |
|||||||||
| Covariate | Std. β |
t | p | Lower | Upper | Std. β |
t | p | Lower | Upper |
| Age (yrs) | 0.15 | 1.34 | 0.18 | –0.04 | 0.19 | 0.08 | 0.70 | 0.49 | –0.09 | 0.18 |
| Sex (0,1) | –0.11 | –1.17 | 0.25 | –0.35 | 0.09 | –0.12 | –1.23 | 0.22 | –0.43 | 0.10 |
| Breast feeding status (0,1) | 0.31 | 3.76 | <0.01 | 0.17 | 0.53 | 0.04 | 0.47 | 0.64 | –0.17 | 0.28 |
| Body Mass Index (kg/m2) | –0.24 | –2.82 | 0.01 | –1.27 | –0.22 | –0.28 | –3.21 | <0.01 | –1.67 | –0.40 |
| Current smoking status (yes/no) | –0.14 | –1.61 | 0.11 | –0.35 | 0.04 | –0.19 | –2.10 | 0.04 | –0.49 | –0.01 |
| Alcohol consumption (per day) | –0.08 | –0.91 | 0.37 | –0.27 | 0.10 | 0.02 | 0.23 | 0.82 | –0.20 | 0.25 |
| Highest year of education reached | 0.00 | –0.01 | 0.99 | –0.08 | 0.08 | 0.04 | 0.43 | 0.67 | –0.07 | 0.12 |
| Total cholesterol intake (mg/day) | 0.26 | 1.55 | 0.12 | –0.07 | 0.59 | 0.42 | 2.46 | 0.02 | 0.10 | 0.90 |
| Total caloric intake (kcal) | –0.12 | –0.33 | 0.74 | –1.11 | 0.79 | 0.35 | 0.95 | 0.35 | –0.61 | 1.71 |
| Total fat intake (g/day) | –0.04 | –0.14 | 0.89 | –0.70 | 0.61 | –0.11 | –0.40 | 0.69 | –0.96 | 0.64 |
| Total protein intake (g/day) | –0.02 | –0.09 | 0.93 | –0.67 | 0.61 | –0.55 | –2.08 | 0.04 | –1.60 | –0.04 |
| Consumption of locally caught fish in the past year (yes/no) |
0.23 | 2.80 | 0.01 | 0.08 | 0.47 | 0.00 | –0.04 | 0.97 | –0.24 | 0.23 |
| Born before or after issuance of fish advisory (yes/no) |
–0.20 | –1.88 | 0.06 | –0.47 | 0.01 | –0.22 | –2.04 | 0.04 | –0.60 | –0.01 |
Additionally, we trimmed each toxicant model to consist of only independent variables that had a p value ≤ 0.20, which resulted in different models for each dependent variable. The variables that were significant predictors were the same as in the full model for CB153, Σ14PCB50%, and HCB. The predictors of Σ9 Persistent PCB remained the same also, except that being born before or after issuance of the fish advisory became an additional significant predictor (stdβ=−0.201, t= −2.47, p= 0.02); being born after the advisory was associated with lower levels, This variable was the only significant predictor of the level of Σ5 Non-persistent PCBs (stdβ=0.177, t= 2.17, p= 0.03) in the trimmed model. Total dietary cholesterol intake was no longer a significant predictor for p,p’ -DDE while the variables significant in the full model remained significant.
3.3. Comparisons with CDC reference population data
We compared published levels reported by the CDC for youth between the ages of 12 and 19 years (n=587–598) to the Akwesasne young adult (n=152) levels of p,p’-DDE, HCB, and 13 of the congeners that comprise Σ14PCB50% (CB95 in this group was not reported by the CDC). Geometric mean concentrations and 50th, 90th, and 95th percentiles of all congeners with the exceptionof CB 28were higher amongst Akwesasne youth than in the CDC 12–19 year old general population age group (Table 4). This increase was noted for both persistent and non-persistent congeners. In contrast, p,p’-DDE and HCB levels were generally higher in the CDC cohort (GM of 0.516 and 0.065 ppb, respectively for Mohawks vs. 2.51 and 0.123, respectively, for CDC). The geometric means of several congeners (CBs 99, 105, 118, and 110) among the Akwesasne were higher than the reported CDC 90thpercentile (Table 4). In contrast, levels of CB 28 in Akwesasne youth were generally ∼50% or less than those of the CDC cohort. CDC did not report total PCB levels for this or any age group, precluding direct comparisons for this parameter. However, summing the GMs for the 13 reported CBs in each cohort indicates that Akwesasne youth had serum PCB levels approximately twice those of the CDC 12–19 year olds (i.e., 0.372 vs. 0.179 ppb).
Table 4.
Comparison of toxicant levels among Akwesasne young adults to CDC National Report.*
| Akwesasne Young adults (n=152, ages 17–20) |
CDC Survey 2003 −2004 (n=587–598; ages 12–19)* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Percentiles | Percentiles | ||||||||
| GM (95% CI) |
50 | 90 | 95 | GM (95% CI) |
50 | 90 | 95 | ||
| p, p, -DDE | 0.324 (0.291–0.360) |
0.336 | 0.618 | 0.866 | 0.516 (0.419–0.635) |
0.456 | 1.69 | 2.51 | |
| HCB | 0.033 (0.032–0.036) |
0.032 | 0.058 | 0.068 | 0.065 (0.062–0.069) |
0.064 | 0.102 | 0.123 | |
| PCB IUPAC# | |||||||||
| 118 | 0.041 (0.038–0.044) |
0.038 | 0.069 | 0.089 | 0.015 (0.014–0.017) |
0.014 | 0.036 | 0.047 | |
| 138 [+163+164]a | 0.061 (0.056–0.066) |
0.058 | 0.115 | 0.149 | 0.025 (0.023–0.028) |
0.023 | 0.062 | 0.079 | |
| 153 | 0.068 (0.061–0.073) |
0.064 | 0.125 | 0.171 | 0.030 (0.027–0.033) |
0.027 | 0.076 | 0.101 | |
| 101[+90] | 0.026 (0.023–0.028) |
0.029 | 0.047 | 0.060 | 0.010 (0.009–0.011) |
0.009 | 0.024 | 0.030 | |
| 99 | 0.035 (0.030–0.039) |
0.042 | 0.076 | 0.093 | 0.012 (0.011–0.013) |
0.012 | 0.025 | 0.032 | |
| 87 | 0.018 (0.002–0.003) |
0.020 | 0.030 | 0.034 | 0.004 (0.003–0.004) |
0.005 | 0.011 | 0.016 | |
| 52 | 0.023 (0.019–0.025) |
0.026 | 0.050 | 0.068 | 0.016 (0.015–0.018) |
0.017 | 0.037 | 0.042 | |
| 74 | 0.014 (0.012–0.017) |
0.015 | 0.042 | 0.057 | 0.011 (0.010–0.012) |
0.011 | 0.021 | 0.026 | |
| 105 | 0.013 (0.010–0.015) |
0.014 | 0.054 | 0.066 | 0.003 (0.003–0.004) |
0.003 | 0.008 | 0.011 | |
| 187 | 0.012 (0.009–0.015) |
0.020 | 0.043 | 0.055 | 0.005 (0.004–0.006) |
0.005 | 0.016 | 0.023 | |
| 110 | 0.022 (0.018–0.023) |
0.022 | 0.048 | 0.061 | 0.007 (0.007–0.008) |
0.007 | 0.019 | 0.025 | |
| 180 | 0.028 (0.023–0.030) |
0.027 | 0.065 | 0.099 | 0.016 (0.014–0.018) |
0.015 | 0.050 | 0.076 | |
| 95 | 0.005 (0.004–0.006) |
0.006 | 0.022 | 0.040 | - | NR | NR | NR | |
| 28 | 0.009 (0.008–0.010) |
0.009 | 0.027 | 0.032 | 0.025 (0.023–0.028) |
0.025 | 0.051 | 0.061 | |
Abbreviations: IUPAC, International Union of Pure and Applied Chemistry; LOD, less than the limit of detection; NR, Not reported.
138+158 for CDC dataset
Centers for Disease Control. Fourth National Report on Human Exposure to Environmental Chemicals. 1–5. 2009. Department of Health and Human Services.
4. Discussion
One of the strengths of the current study is the ability to measure the levels of many individual congeners. This enables the differentiation of persistent and non-persistent congeners as well as other relevant congener groupings and lessens the potential for obscuring important relationships given the differences in biological activity exhibited by specific congeners (Agency for Toxic Substances and Disease Registry 2000). Overall, PCB levels in this sample of Akwesasne young adults are higher than the GM and 50th percentile of the CDC reference values for the US population(Centers for Disease Control 2009), and many are higher than the 90th and 95th percentiles as well. For persistent congeners, cross-sectional serum PCB data reflect both past and recent exposure levels as influenced by the relative persistence of each congener. Our data indicate that Akwesasne youth have sustained higher overall exposure to PCBs than similarly aged subjects from the general U.S. population. In contrast, for non-persistent congeners, serum PCB data reflect primarily recent exposure. There is some evidence of ongoing PCB exposure by the Akwesasne youth based on the detected levels of congeners such as CBs 52 and 110.Both congeners are present at relatively high proportions in Aroclor 1248 (Frame et al. 1996), the predominant PCB mixture emitted to the AMN region(Fitzgerald et al. 1996). This finding is consistent with other reports on serum CB levels in Akwesasne adults (DeCaprio et al. 2005). Finally, the lower p,p’-DDE and HCB levels in Akwesasne as compared to U.S. adolescents probably reflects relatively lower legacy contamination of the AMN by these pesticides and, as a consequence, lower contributions to background body burden.
Our understanding of variation in organochlorine levels benefits from the comprehensive information on socio-demographic, dietary and behavioral characteristics including diet that is available in the current study. A major determinant of organochlorine levels was found to be breast feeding history. Levels of summary groupings of mono- and di-ortho CBs, as well as the sum of nine persistent PCBs differed significantly by history of breast feeding. Levels of moderately to highly chlorinated congenerswere also significantly higher in breastfed individuals. Not surprisingly, no significant effect of breast feeding history on serum levels of five non-persistent CBs was noted. Finally, a trend toward decreasing serum levels of persistent PCBs for first- to fourth-born subjects was identified. No similar trend was observed for the sum of five non-persistent congeners or for the chlorinated pesticides detected in the study. The former observation is consistent with the short half-lives of the non-persistent congeners while the latter suggests that either these pesticides are not efficiently transferred via lactation, or more likely, that there was low overall historical exposure to these pollutants among Akwesasne mothers and their children.
Our breast feeding results are consistent with most of the work on organochlorine body burden reported to date (Den Hond et al. 2009). Lactation has long been considered a major route of elimination of many persistent lipophilic compounds (Abraham et al. 1998;Schecter et al. 1996a), although recent work suggests that this phenomenon may be more complex than previously thought (LaKind et al. 2009). The reduction in maternal PCB body burden due to breast feeding appears to be dependent on the duration of breast feeding and physicochemical characteristic of the individual congener (Clewell and Gearhart 2002;Clewell et al. 2004;Landrigan et al. 2002;Niessen et al. 1984;Schecter et al. 1996b;Schecter et al. 1998). In the present study, nearly 54% of the young adults had been breastfed for at least six months, and 31% of these individuals were breastfed exclusively. Clearly, the most highly persistent congeners are still of environmental and biological consequence in this cohort, since their levels remain significantly elevated even though the participants had been breastfed at least 17 years prior to sampling. To the best of our knowledge, no other study has found differences between levels among breast fed and non-breast fed individuals of this magnitude and duration.
Some of our results can be compared to the levels predicted from models developed by Verner et al. (2008) to describe lifetime exposure to POPs, specifically in terms of levels of CBs 180 and 153, and of HCB. In these models, breastfeeding is associated with a higher level of both PCBs and HCB. The difference is nearly two times higher at young ages and disappears at about age 20. Among Akwesasne young adults averaging 18 years of age, the levels in those breastfed and not breastfed were not equal. They had not disappeared as predicted by the Verner et al. (2008) model, but were 1.3 to 1.5 times higher.
The relationship found between organochlorine levels and year of birth reflects the impact of diminished exposure to a major source of contaminationmost likely due to the adherence of community members to fish advisories issued in the mid-1980s. As a result of this dietary change, current exposures to the more persistent congeners have been reduced among the Akwesasne Mohawk. Furthermore, half-lives of certain PCB congeners in children and young adults have been found to be significantly shorter than in adults (Kerger et al. 2006), since as they age the effect of elimination becomes more important than that of the dilution effect typically reported in adults (Abraham et al. 1996;Abraham et al. 1998;Kerger et al. 2007a;Kerger et al. 2007b).
Consistent with reports that food intake represents the primary current route of human exposure to PCBs (Chiu et al. 2004;Fitzgerald et al. 1996;Schantz et al. 2010), a positive association was evident between consumption of locally caught fish during the previous year and levels of moderately to highly persistent PCB congeners. Studies of Great Lakes fish eaters have demonstrated that with increased consumption of contaminated fish, the body burden of organochlorines increases (Falk et al. 1999;Hanrahan et al. 1999;Knobeloch et al. 2009). In the present study approximately 30% of respondents reported eating some local fish and 30% reported eating some local game in the last year. The lack of a relationship between organochlorine levels and the consumption of local game and wildlifecoupled with a relationship between organochlorine levels and local fish consumption suggests that local fish may be more contaminated than local game.
Although we were able to distinguish many specific congeners, we were not able to calculate TEQ. Consequently, one weakness of the current study is our inability to obtain data for the most potent dioxin-like congeners (CBs 126 and 169). These two congeners are typically present in human blood at levels several orders of magnitude lower than the most prevalent CBs and require either larger specimen sizes or much higher analytical sensitivity to determine accurately. Calculation of a TEQ based only on a measurement of levels of those dioxin-like CBs determined in our study (i.e., CBs 105, 118, and 156) would underestimate dioxin-like exposure by an unknown (but possibly substantial) amount. The results of such an analysis would be uninterpretable. We also considered data analyses based on lipid-adjusted PCB values. However, lack of standardization of laboratory methods and the need for considerable technical expertise in conducting total lipid determination can result in bias and variability in these comparisons (Bernert et al. 2007;Schisterman et al. 2005). Since all of our subjects were fasted prior to blood sampling, we decided not to lipid-adjust our PCB data for this study. Another limitation of the current study is its generalizability. The Akwesasne Mohawk community has not been federally censused, and therefore, it is not possible to know what percentage of the population is reflected in our sample. Nor can we ascertain how representative this sample is of young adults in the community. While this may impact the ability of our summary data to characterize the entire community, it should not affect the validity of our results concerning relationships between levels and lifestyle characteristics.
In conclusion, our results indicate that birth order, consumption of contaminated local fish, and history of breast feeding continue to influence current persistent organochlorine pollutant body burden in this sample of young adults. Also, concentrations of non-persistent PCBs among this sample were higher than those reported by the CDC in the general population, suggesting continued PCB exposure in Mohawks. Additional research into the concentration trends of individual PCB congeners within Akwesasne youth and young adults is warranted to further improve our insight into the determinants and influences of organochlorine concentrations within the Akwesasne community and young adults generally.
Acknowledgements
We would like to acknowledge and thank Maxine Cole, Alice Tarbell, Ken Jock, and Craig Arquette and the Akwesasne Mohawk community for their many contributions, cooperation and participation in this research. We would also like to thank one of the reviewers for their helpful suggestions. This work was supported by grants from the National Institute of Environmental Health Sciences (NIEHS-ESO4913-10; ES10904-06), and the National Center on Minority Health and Health Disparities (NCMHD- 1P20MD003373-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center on Minority Health and Heath Disparities, or the National Institutes of Health.
Abbreviations
- AMN
Akwesasne Mohawk Nation
- p,p’-DDE
p,p’-dichlorophenyldichloroethylene
- HCB
Hexachlorobenzene
- MAWBs
Mohawk Adolescent Well Being study
- PCBs
Polychlorinated Biphenyls
- POPs
Persistent organic pollutants
- ppb
Parts per billion
- ΣTotPCBs
Sum of all PCB congeners tested
- ΣPCB50%
Sum of IUPAC#s 28,52,74,87,95,99,101[90],105,110,118,138[+163+164],153,180,187
- Σ9PersistentPCB
Sum of IUPAC#s 28,74,99,105,118,138[+163+164],153,180,187
- Σ5NonPersistent
Sum of IUPAC#s 52,87,95,101[90],110
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham K, Knoll A, Ende M, Papke O, Helge H. Intake, fecal excretion, and body burden of polychlorinated dibenzo-p-dioxins and dibenzofurans in breast-fed and formula-fed infants. Pediatr. Res. 1996;40:671–679. doi: 10.1203/00006450-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Abraham K, Päpke O, Gross A, Kordonouri O, Wiegand S, Whan U, Helge H. Time course of PCDD/PCDF/PCB concentrations in breast-feeding mothers and their infants. Chemosphere. 1998;37:1731–1741. doi: 10.1016/s0045-6535(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological profile for polychlorinated biphenyls (PCBs) Atlanta, Georgia, U.S: Department of Health and Human Services, Public Health service; 2000. [PubMed] [Google Scholar]
- Akwesasne Task Force On The Environment. Superfund clean-up at Akwesasne: a case study in environmental justice. Int. J. Contemp. Sociol. 1997;34:267–290. [Google Scholar]
- American Council on Science and Health. Position paper of the American Council on Science and Health: Public health concerns about environmental polychlorinated biphenyls (PCBs) Ecotoxicol. Environ. Saf. 1997;38:71–84. doi: 10.1006/eesa.1997.1565. [DOI] [PubMed] [Google Scholar]
- Ballschmiter K, Zell M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Z. Anal. Chem. 1980;302:20–31. [Google Scholar]
- Bernert JT, Turner WE, Patterson DG, Jr, Needham LL. Calculation of serum "total lipid" concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere. 2007;68:824–831. doi: 10.1016/j.chemosphere.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz SL, Winneke G. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ. Health Perspect. 1999;107:639–649. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JF., Jr Determination of PCB metabolic, excretion, and accumulation rates for use as indicators of biological response and relative risk. Environ. Sci. Technol. 1994;28:2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- Bush B, Kadlec MJ. Dynamics of PCBs in teh aquatic environment. Grear Lakes Research Review. 1995;1:24–30. [Google Scholar]
- Centers for Disease Control. Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services; 2009. pp. 1–5. [Google Scholar]
- Chiu A, Beaubier J, Chiu J, Chan L, Gerstenberger S. Epidemiologic studies of PCB congener profiles in North American fish consuming populations. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2004;22:13–36. doi: 10.1081/GNC-120038004. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Gearhart JM. Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure. Environ. Health Perspect. 2002;110:A333–A337. doi: 10.1289/ehp.021100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell HJ, Gentry PR, Covington TR, Sarangapani R, Teeguarden JG. Evaluation of the potential impact of age- and gender-specific pharmacokinetic differences on tissue dosimetry. Toxicol. Sci. 2004;79:381–393. doi: 10.1093/toxsci/kfh109. [DOI] [PubMed] [Google Scholar]
- Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ. Health Perspect. 2007;115:1442–1447. doi: 10.1289/ehp.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ. Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP, Tarbell AM, Bott A, Wagemaker DL, Williams RL, O’Hehir CM. Routine analysis of 101 polychlorinated biphenyl congeners in human serum by parallel dual-column gas chromatography with electron capture detection. J. Anal. Toxicol. 2000;24:403–420. doi: 10.1093/jat/24.6.403. [DOI] [PubMed] [Google Scholar]
- Den Hond E, Govarts E, Bruckers L, Schoeters G. Determinants of polychlorinated aromatic hydrocarbons in serum in three age classes--Methodological implications for human biomonitoring. Environ. Res. 2009;109:495–502. doi: 10.1016/j.envres.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio A. The Akwesasne Task Force on the Environment, 2005. Relationship of Lead, Mercury, Mirex, Dichlorodiphenyldichloroethylene, Hexachlorobenzene, and Polychlorinated Biphenyls to Timing of Menarche Among Akwesasne Mohawk Girls. Pediatrics. 115:e127–e134. doi: 10.1542/peds.2004-1161. [DOI] [PubMed] [Google Scholar]
- Falk C, Hanrahan L, Anderson HA, Kanarek MS, Draheim L, Needham L, Patterson DJ. Body burden levels of dioxin, furans, and PCBs among frequent consumers of Great Lakes sport fish. The Great Lakes Consortium. Environ. Res. 1999;80:S19–S25. doi: 10.1006/enrs.1998.3906. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Brix KA, Deres DA, Hwang S-A, Bush B, Lambert G, Tarbell A. Polychlorinated biphenyl (PCB) and dichlorodiphenyl dichloroethylene (DDE) exposure among Native American men from contaminated Great Lakes fish and wildlife. Toxicol. Ind. Health. 1996;12:361–368. doi: 10.1177/074823379601200308. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang S-A, Brix KA, Bush B, Cook K, Worswick P. Fish PCB concentrations and consumption patterns among Mohawk women at Akwesasne. J. Expo. Anal. Environ. Epidemiol. 1995;5:1–19. [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang S-A, Bush B, Cook K, Worswick P. Fish consumption and breast milk PCB concentrations among Mohawk women at Akwesasne !Lost Data. 1998;148:164–172. doi: 10.1093/oxfordjournals.aje.a009620. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang S-A, Deres DA, Bush B, Cook K, Worswick P. The association between local fish consumption and DDE, mirex, and HCB concentrations in the breast milk of Mohawk women at Akwesasne. J. Expo. Anal. Environ. Epidemiol. 2001;11:381–388. doi: 10.1038/sj.jea.7500180. [DOI] [PubMed] [Google Scholar]
- Forti A, Bogdan KG, Horn E. Health Risk Assessment for the Akwesasne Mohawk Population from Exposure to Chemical Contaminants in Fish and Wildlife. Albany, NY: New York State Department of Health. New York State Department of Health Center for Environmental Health Bureau of Toxic Substance Assessment; 1995. [Google Scholar]
- Frame GM, Cochran JW, Bøwadt SS. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. Journal of High Resolution Chromatography. 1996;119:657–712. [Google Scholar]
- George-Kanentiio D. Iroquois population in 1995. Akwesasne Notes. 1995;1:61. [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, McCaffrey RJ, Rej R, Carpenter DO. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ. Res. 2008;106:226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Rej R, Negoita S, Santiago-Rivera A, Morse G, Carpenter DO. Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men. Environ. Health Perspect. 2009;117:1454–1460. doi: 10.1289/ehp.0800134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart R, Puig P, Gomez-Catalan J. Requirement for a standardized nomenclature criterium for PCBs: Computer-assisted assignment of correct congener denomination and numbering. Chemosphere. 1993;27:1451–1459. [Google Scholar]
- Hanrahan LP, Falk C, Anderson HA, Draheim L, Kanarek MS, Olson J. Serum PCB and DDE levels of frequent Great Lakes sport fish consumers-a first look. The Great Lakes Consortium. Environ. Res. 1999;80:S26–S37. doi: 10.1006/enrs.1998.3914. [DOI] [PubMed] [Google Scholar]
- Hansen LG. The ortho side of PCBs: Occurrence and Disposition. Norwell, MA: Kluwer Academic Publishers; 1999. [Google Scholar]
- Kerger BD, Leung HW, Scott P, Paustenbach DJ, Needham LL, Patterson DG, Jr, Gerthoux PM, Mocarelli P. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Environ. Health Perspect. 2006;114:1596–1602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerger BD, Leung HW, Scott PK, Paustenbach DJ. An adaptable internal dose model for risk assessment of dietary and soil dioxin exposures in young children. Toxicol. Sci. 2007a;100:224–237. doi: 10.1093/toxsci/kfm199. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Leung HW, Scott PK, Paustenbach DJ. Refinements on the age- dependent half-life model for estimating child body burdens of polychlorodibenzodioxins and dibenzofurans. Chemosphere. 2007b;67:S272–S278. doi: 10.1016/j.chemosphere.2006.05.108. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Turyk M, Imm P, Schrank C, Anderson H. Temporal changes in PCB and DDE levels among a cohort of frequent and infrequent consumers of Great Lakes sportfish. Environ Res. 2009;109:66–72. doi: 10.1016/j.envres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, Wolff MS, Hunter DJ. Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environ. Health Perspect. 1999;107:75–81. doi: 10.1289/ehp.9910775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Jr, Sjodin A, Turner W, Wang RY, Needham LL, Paul IM, Stokes JL, Naiman DQ, Patterson DG., Jr Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environ Health Perspect. 2009;117:1625–1631. doi: 10.1289/ehp.0900876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Mattison D, McCally M, Garg A. Chemical contaminants in breast milk and their impacts on children's health: an overview. Environ. Health Perspect. 2002;110:A313–A315. doi: 10.1289/ehp.021100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, Koppe JG, Olie K, van Aalderen WM, de VP, ten Tusscher GW. Effects of dioxins, PCBs, and PBDEs on immunology and hematology in adolescents. Environ. Sci. Technol. 2009;43:7946–7951. doi: 10.1021/es901480f. [DOI] [PubMed] [Google Scholar]
- McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: Considerations for a congener- specific analysis. Environ. Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Aucompaugh A, Schell LM, Denham M, DeCaprio AP, Gallo MV, Ravenscroft J, Kao C-C, Hanover MR, David D, Jacobs A, Tarbell A, Worswick P. Akwesasne Task Force On The Environment, 2006. PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicol. Teratol. 28:439–445. doi: 10.1016/j.ntt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD. Akwesasne Task Force On The Environment, 2009. Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotox. 30:686–696. doi: 10.1016/j.neuro.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen KH, Ramolla J, Binder M, Brügmann G, Hofmann U. Chlorinated hydrocarbons in adipose tissue of infants and toddlers: inventory and studies on their association with intake of mothers' milk. Eur J Pediatr. 1984;142:238–244. doi: 10.1007/BF00540242. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gardiner JC, Aguiar A, Tang X, Gasior DM, Sweeney AM, Peck JD, Gillard D, Kostyniak PJ. Contaminant profiles in Southeast Asian immigrants consuming fish from polluted waters in northeastern Wisconsin. Environ Res. 2010;110:33–39. doi: 10.1016/j.envres.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Kassis I, Papke O. Partitioning of dioxins, dibenzofurans, and coplanar PCBS in blood, milk, adipose tissue, placenta and cord blood from five American women. Chemosphere. 1998;37:1817–1823. doi: 10.1016/s0045-6535(98)00247-1. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Lis A, Ball M, Ryan JJ, Olson JR, Li L, Kessler H. Decrease in milk and blood dioxin levels over two years in a mother nursing twins: estimates of decreased maternal and increased infant dioxin body burden from nursing. Chemosphere. 1996a;32:543–549. doi: 10.1016/0045-6535(95)00248-0. [DOI] [PubMed] [Google Scholar]
- Schecter A, Startin J, Wright C, Papke O, Ball M, Lis A. Concentrations of polychlorinated dibenzo-p-dioxins and dibenzofurans in human placental and fetal tissues from the U.S. and in placentas from Yu-Cheng exposed mothers. Chemosphere. 1996b;32:551–557. doi: 10.1016/0045-6535(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Denham M, Ravenscroft J, DeCaprio AP, Carpenter DO. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p'- DDE, and other toxicants in Akwesasne Mohawk youth. Environ. Health Perspect. 2008;116:806–813. doi: 10.1289/ehp.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Ravenscroft J, DeCaprio AP. Persistent organic pollutants and anti-thyroid peroxidase levels in Akwesasne Mohawk young adults. Environ. Res. 2009;109:86–92. doi: 10.1016/j.envres.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Hubicki LA, DeCaprio AP, Gallo MV, Ravenscroft J, Tarbell A, Jacobs A, David D, Worswick P. Organochlorines, lead, and mercury in Akwesasne Mohawk youth. Environ. Health Perspect. 2003;111:954–961. doi: 10.1289/ehp.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Denham M, Ravenscroft J. Effects of pollution on human growth and development: an introduction. Journal of Physiological Anthropology. 2006;25:103–112. doi: 10.2114/jpa2.25.103. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RJ, Jock K. New York State Department of Environmental Conservation. Albany, NY: New York State Department of Environmental Conservation; 1990. Chemical contaminants in fish from the St. Lawrence River drainage on lands of the Mohawk nation at Akwesasne and near the General Motors Corporation/Central Foundry Divisioin, Massena, NY Plant. [Google Scholar]
- SPSS, Inc. Statistical Package for the Social Sciences. [17] Chicago: Illinois, SPSS, Inc; 2009. [Google Scholar]
- Verner MA, Charbonneau M, Lopez-Carrillo L, Haddad S. Physiologically based pharmacokinetic modeling of persistent organic pollutants for lifetime exposure assessment: a new tool in breast cancer epidemiologic studies. Environ. Health Perspect. 2008;116:886–892. doi: 10.1289/ehp.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]