Abstract
During the past 5 years, 104 angiographic studies were performed in 87 patients (45 children and 42 adults) with 92 transplanted livers for evaluation of possible vascular complications. Seventy percent of the studies were abnormal. Hepatic artery thrombosis was the most common complication (seen in 42% of children studied, compared with only 12% of adults) and was a major complication that frequently resulted in graft failure, usually necessitating retransplantation. In six children, reconstitution of the intrahepatic arteries by collaterals was seen. Three survived without retransplant. Arterial stenosis at the anastomosis or in the donor hepatic artery was observed in 11% of patients. Portal vein thrombosis or stenosis occurred in 13% of patients. Two children and one adult with portal vein thrombosis demonstrated hepatopetal collaterals that reconstituted the intrahepatic portal vessels. Uncommon complications included anastomotic and donor hepatic artery pseudoaneurysms, a hepatic artery–dissecting aneurysm, pancreaticoduodenal mycotic aneurysms, hepatic artery–portal vein fistula, biliary–portal vein fistula, hepatic vein occlusion, and inferior vena cava thrombosis.
Liver transplantation, once an experimental procedure, has become an accepted therapy for end-stage liver disease in both adults and children. With the advent of cyclosporine, 1-year survival rates have steadily risen from 50% in 1980 to 80% in 1984 [1]. Most mortality occurs in the first 6 months after transplant. Four-year survival is 75% in children (≤ 18 years old) and 50% in adult patients, reflecting the poorer first-year statistics of 4 years ago [1]. Most patients enjoy a satisfactory quality of life after transplantation [1–4].
The number of centers performing liver transplants has increased to 11 in the United States and nine in Europe, with a combined total of 992 recipients by June 1984 [1]. Two key ingredients to widespread liver transplantation, immunosuppression with cyclosporine and training of multiple-organ harvest and implantation teams, are quite recent (since 1980 and 1982, respectively). As surgical skill advances and diffuses throughout the medical world, liver transplants will become increasingly common [2, 4–6]. During 1985. 250 liver transplants were performed at our institution.
A vascular complication is a primary diagnostic consideration in the liver-transplant patient with fulminant hepatic failure, bile leak, relapsing bacteremia, gastrointestinal or abdominal bleeding, or hemobilia. Angiography is the test of choice in the evaluation of possible vascular complications. We report our 5-year experience with the angiographic diagnosis and the clinical outcome of patients with vascular complications after liver transplantation.
Materials and Methods
During the 5-year period ending December 1985. 477 patients received 625 liver transplants at the University of Pittsburgh Health Center (Table 1). The four leading indications were cirrhosis, biliary atresia, primary biliary cirrhosis, and inborn metabolic errors. Few transplants were for primary liver tumors [1]. The principle indications for retransplantation were rejection, technical failures, and primary graft nonfunction [5].
TABLE 1.
Liver Transplants and Posttransplant Angiography Performed During the 5-Year Period*
| Liver Transplants | Posttransplant Angiography (%)b | |
|---|---|---|
| Total patients | 477 | 87 (18) |
| Children (≤18 years old) | 204 | 45 (22) |
| Adults (>18 years old) | 273 | 42 (15) |
| Retransplants | 148 | 12 (8) |
| Children | 70 | 9 (13) |
| Adults | 78 | 3 (4) |
| Total transplanted livers | 625 | 92 (15) |
| Female: male | 1:0.8 | 1:0.85 |
Ending December 1985.
Expressed as a total number and as a percentage of the number of liver transplants for each category.
All posthepatic transplant angiograms performed at our institution during the past 5 years were analyzed retrospectively. Adults (>18 years old) and children were grouped separately because complications and prognoses for the two groups are different; children commonly fare better [1]. One hundred four angiographic studies were performed in 87 patients (42 adults and 45 children) with 92 homograft livers; five children studied had two different transplants. The group included 40 males and 47 females, ranging in age from 4 months to 62 years old. Fifteen patients were studied twice, and one patient was studied three times. In 12 patients, the liver represented a retransplant. In one of the 12, the liver was a third transplant. Studies were performed from 2 days to more than 11 years after transplant. Seventy-seven studies (74%) were performed within the first 2 months after transplant.
All studies were performed by conventional angiographic techniques. Adult patients were only lightly sedated. Patients under 12 years old were evaluated under general anesthesia. All angiograms were performed from the femoral artery, except that the left axillary artery was used in one patient with bilateral external iliac artery occlusion. An aortogram was performed in 30 cases. Selective catheterization for evaluation of the hepatic artery or portal vein was performed in 82 cases. In general, angiography was performed on a semiemergent basis, within 4 to 24 hr of the clinical request. Performing the study on an emergent basis (within 2 hr) was rarely necessary.
In all but one patient, liver transplantation was orthotopic (i.e., the donor liver anatomically replaced the recipient’s organ). In the single heterotopic transplant, an auxiliary liver was placed in the right lower abdomen in a patient with situs inversus and polysplenia.
In most patients, hepatic artery revascularization consisted of an end-to-end anastomosis between the donor celiac and recipient common hepatic artery. With anomalies of hepatic arterial supply, techniques were employed to create a single hepatic arterial pedicle [1, 7, 8]. Use of the donor’s aorta or iliac artery for an arterial graft was more commonly necessary in children than in adults [8]. The donor and recipient extrahepatic portal veins were usually anastomosed end-to-end, except in a few patients with an abnormally small or occluded portal vein, for whom revascularization was performed with a venous homograft from the donor’s vena cava, iliac vein, or pulmonary vein [9]. For the inferior vena cava, the infra- and supra- hepatic vena cava segments of the donor were anastomosed end- to-end to the respective vessels of the recipient.
Results
All patients in this study were arbitrarily numbered from 1 through 47 with letters to designate separate livers where applicable. Throughout the Results and Discussion sections these numbers are used for easy reference to Figures 1, 5, and 6. Sixty-four (70%) of the 92 homografts studied were abnormal (Table 2).
Fig. 1.
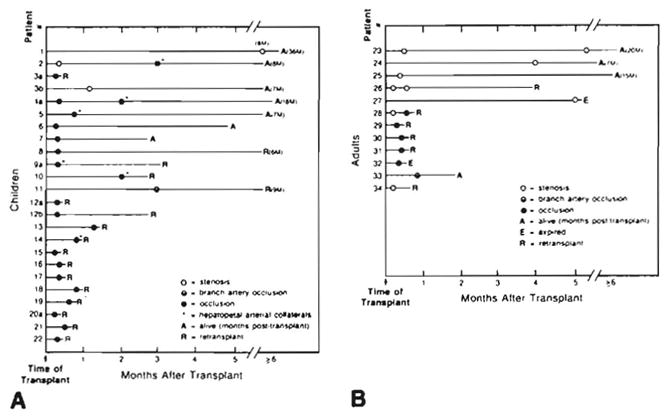
Hepatic artery complications in liver transplants. A. Children. B. Adults. Placement of circles indicates time of angiographic study after transplant. Patients are numbered consecutively, with letters indicating separate transplanted livers. Numbers refer to case numbers in Results and Discussion sections. Numbers followed by M are number of months (e.g., 8M = 8 months).
Fig. 5.
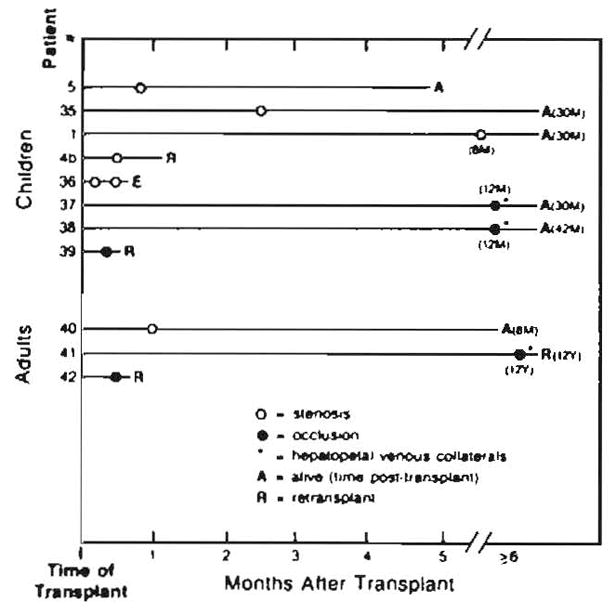
Portal vein complications in liver transplants. Placement of circles indicates time of angiographic study after transplant. Numbers followed by M or Y are number of months or years (e.g., 30M = 30 months).
Fig. 6.
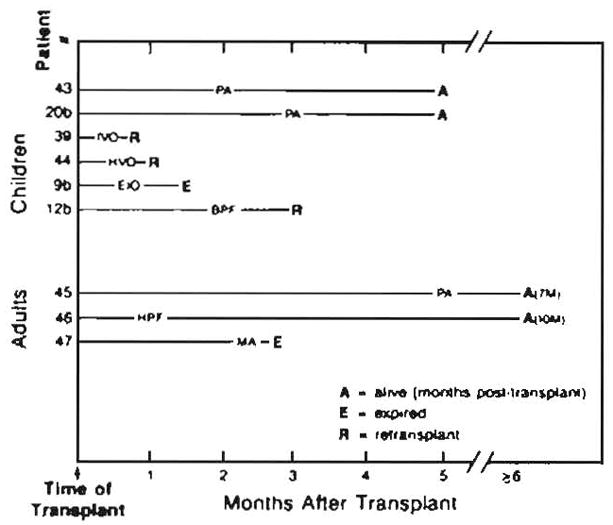
Miscellaneous vascular complications in liver transplants. PA = pseudoaneurysm, IVO = inferior vena cava occlusion, HVO = hepatic vein occlusion, EIO = external iliac artery occlusion, BPF = biliary–portal vein fistula, HPF = hepatic artery—portal vein fistula, MA = mycotic aneurysms. Numbers followed by M are number of months (e.g., 7M = 7 months).
TABLE 2.
Vascular Complications After Liver Transplantation in 87 Patients with 92 Allografts
| Children | Adults | |
|---|---|---|
| Normal | 12 | 16 |
| Hepatic artery | ||
| Complete occlusion | 21 | 5 |
| Branch artery occlusion | 1 | 1 |
| Stenosis | 3 | 7 |
| Portal vein | ||
| Occlusion | 3 | 2 |
| Stenosis | 5 | 1 |
| Miscellaneous findings | 6 | 3 |
| Pseudoaneurysm | 1 | 1 |
| Dissecting aneurysm | 1 | 0 |
| Mycotic aneurysms | 0 | 1 |
| Inferior vena cava occlusion | 1 | 0 |
| Hepatic vein occlusion | 1 | 0 |
| Hepatic artery–portal vein fistula | 0 | 1 |
| External iliac artery occlusion | 1 | 0 |
| Biliary–portal vein fistula | 1 | 0 |
| Possible rejection | 7 | 11 |
Hepatic Artery Complications
The most frequently encountered complication was hepatic artery thrombosis, which occurred in 21 (42%) of 50 pediatric grafts (Fig. 1A), compared with only 5 (12%) of 42 adult grafts (Fig. 1B). Hepatic artery thrombosis was diagnosed from 5 days to 3 months after transplantation. All adults with complete hepatic arterial occlusion required immediate retransplantation, within days (Fig. 1B). However, five (24%) children (cases 2, 4a, 5, 6, 7) survived without retransplant, with follow-up from 3 to 42 months. In three of the five, hepatopetal arterial collaterals were demonstrated (Fig. 2). An additional three organs (cases 8, 9a, 12b) were retransplanted 2 to 5 months after hepatic artery thrombosis. Most children, however, required immediate retransplant. Intraarterial thrombolytic therapy with streptokinase was employed in one patient (case 32), without success.
Fig. 2.
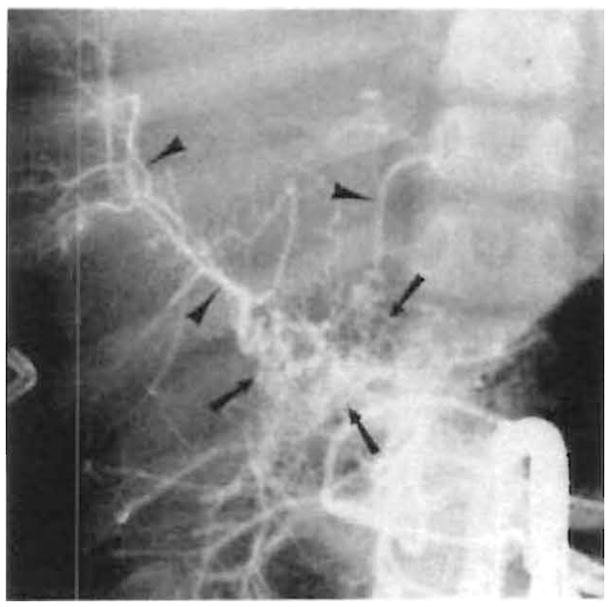
Collateral circulation after hepatic artery thrombosis. Extensive arterial collaterals (arrows), which reconstitute intrahepatic arteries (arrowheads), are demonstrated on selective superior mesenteric arteriogram 2 months after transplant
In one child (case 12b) with hepatic artery occlusion, an intrahepatic biloma developed and was treated by percutaneous drainage. This revealed a biliary–portal vein fistula (Fig. 3). Conservative treatment resulted in fistula resolution, allowing retransplant 1 month later when a suitable donor became available.
Fig. 3.
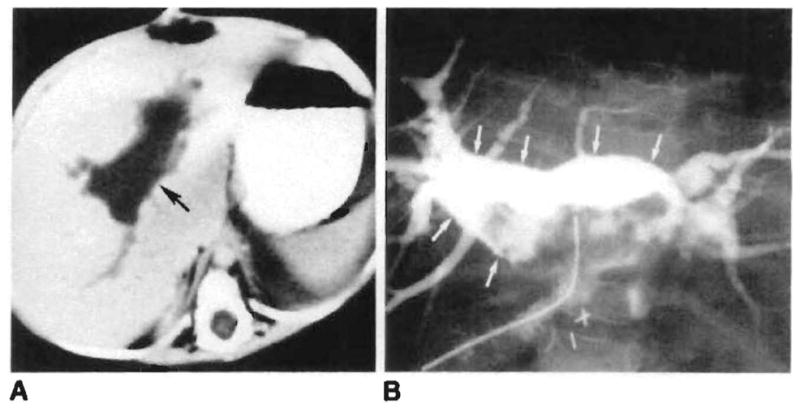
Biliary–portal vein fistula in a 1-year-old girl with hepatic artery thrombosis and recurrent intermittent sepsis. A, Large intrahepatic fluid collection (arrow) is seen on CT 2 months after transplant. B, A biloma (arrows) communicating with intrahepatic portal veins is demonstrated after percutaneous drainage.
Isolated occlusion of the right hepatic artery was seen in two patients (cases 11 and 33). Both showed intrahepatic collaterals (Fig. 4A) and had a benign clinical course. One of these, the child with the auxiliary graft (case 11), required retransplant 6 months later for chronic rejection. The other patient (case 33) demonstrated a right lobe infarct on CT (Fig. 4B). Treatment was conservative, and the patient was asymptomatic 6 weeks later.
Fig. 4.
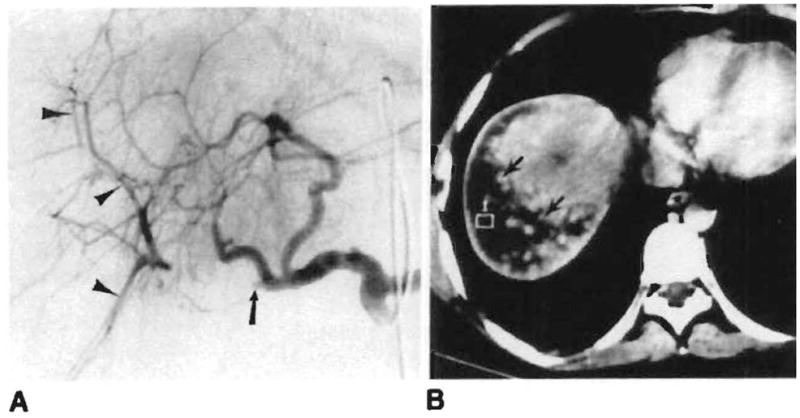
Branch hepatic artery occlusion. A. Occlusion of proximal right hepatic artery (arrow) is seen on celiac arteriogram 3 weeks after transplant. The peripheral branches (arrowheads) are reconstituted by intrahepatic collaterals. B, Probable right hepatic lobe infarct (arrows) seen on abdominal CT performed the same day.
Arterial stenosis occurred in 10 patients (Figs. 1A and 1B). In three children and four adults the stenosis was at the anastomosis. One child with arterial stenosis (case 2) later demonstrated thrombosis. All three children survived without retransplant. In one adult with an anastomotic stenosis (case 23), a follow-up angiogram 5 months later showed almost complete resolution. A second adult patient (case 26) underwent balloon angioplasty but required retransplant for chronic rejection 4 months later. At surgery the hepatic artery was patent. A third adult with a severe anastomotic stenosis (case 27) developed a hepatic abscess and died during retransplant. Presumably the stenosis had progressed to occlusion. In the fourth adult (case 28), an anastomotic stenosis progressed to occlusion after 14 days, and retransplantation was required.
Stenosis within the donor right hepatic artery was seen in two adults. In one (case 24) the stenosis was an incidental finding. The second patient (case 34) required retransplant 3 days later for acute rejection. In one adult (case 25) a Kink was seen in the hepatic artery near the anastomosis. At surgery for revision of the biliary anastomosis the next day, the hepatic arterial pulse was strong. The vessel was straightened but the kink was not thought to be hemodynamically significant. The patient was asymptomatic 1 year later.
Portal Vein Complications
Portal vein thrombosis developed in three children and two adults (Fig. 5). All demonstrated evidence of portal hypertension with massive gastroesophageal varices. Retransplantation was required in one child (case 39). In the other two children (cases 37 and 38), portal vein occlusion was diagnosed 1 year after transplantation. Massive hepatopetal venous collaterals had reconstituted the intrahepatic portal veins. Both patients were treated with endoscopic esophageal sclerotherapy although one eventually required a distal splenorenal shunt for control of portal hypertension. One adult also developed hepatopetal collaterals (case 41), and retransplantation was required for chronic rejection.
Portal vein stenosis at the anastomosis was observed in five children and one adult from 4 days to 8 months after transplantation (Fig. 5). Four presented with upper gastrointestinal tract bleeding from gastroesophageal varices. In the other two (cases 5 and 35), portal vein stenosis was an incidental finding. Varices were not present.
Miscellaneous Vascular Complications
Pseudoaneurysms occurred in two patients (Figs. 6 and 7), and a dissecting aneurysm occurred in one (Fig. 8). In one adult (case 45), abdominal CT for back pain demonstrated an aneurysm adjacent to the abdominal aorta. At angiography, this proved to be an anastomotic pseudoaneurysm. The patient underwent successful revision of the anastomosis. In one child (case 43) angiography was performed for possible hepatic artery thrombosis. A pseudoaneurysm of a branch of the right hepatic artery and a large intrahepatic hematoma were demonstrated (Fig. 7). A hemorrhagic right-lobe infarct was found at surgery. A right hepatic lobectomy was performed. In another child (case 20b), sonography and CT revealed a possible infarct in the posterior right lobe of the liver, indicating possible hepatic artery thrombosis. Angiography revealed a large aneurysm (Fig. 8), which in retrospect was evident on the CT study. At surgery a 4-cm dissecting aneurysm of an aortic graft was resected and a new arterial graft was placed.
Fig. 7.
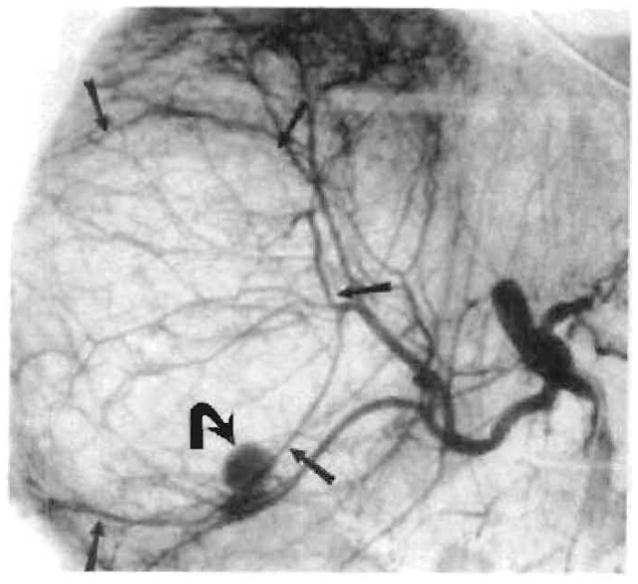
Pseudoaneurysm (curved arrow) with large right-hepatic-lobe hematoma (straight arrows) seen on hepatic arteriogram 7 weeks after transplant
Fig. 8.
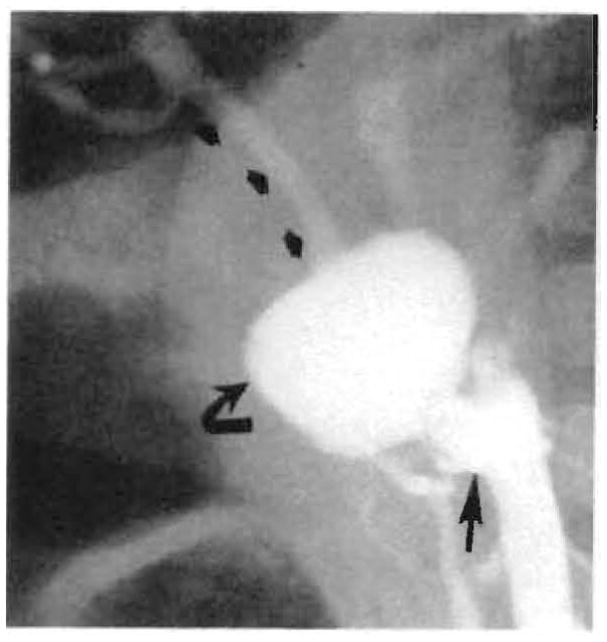
Large aneurysm (curved arrow) just beyond proximal anastomosis (straight arrow) of aortic graft with preservation of distal arterial flow (small arrows) demonstrated on angiogram 3 months after transplant. Surgery showed this to be a dissecting aneurysm starting at proximal anastomosis of graft.
In one patient, abdominal CT was obtained because of an acute decrease in hematocrit. CT demonstrated evidence of intraabdominal hemorrhage that appeared to arise from the proximal anastomosis of an aortic graft (Fig. 9). Angiography was performed to evaluate for a pseudoaneurysm, but the study was normal. Hemorrhage that originated from the anastomosis was found at surgery.
Fig. 9.
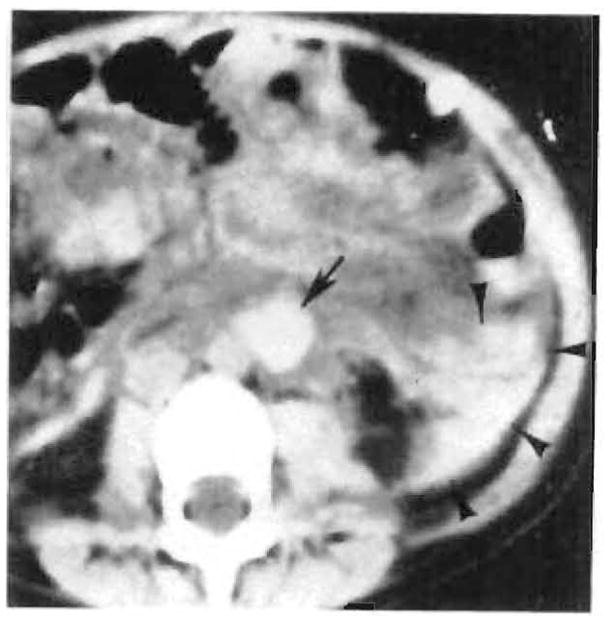
Paraaortic (arrow) and retroperitoneal (arrowheads) hematoma originating from proximal anastomosis of aortic graft seen on nonenhanced abdominal CT scan 1 week after transplant. Angiography was normal.
Unusual complications (Fig. 6) included an adult (case 47) with aneurysms in pancreaticoduodenal arterial collaterals that at laparotomy proved to be mycotic in origin. Progressive hemorrhage resulted in death within 6 days. Another adult (case 46) developed a hepatic artery–portal vein fistula, probably secondary to needle biopsy. This was an incidental finding, with no clinical sequelae. One child (case 9b) developed bilateral external iliac thrombosis, which was initially thought to be secondary to thrombosis of an aortic graft with embolization to the iliac arteries. Angiography confirmed occlusion of the iliac arteries but the graft was patent. The iliac occlusion was attributed to previous indwelling femoral catheters. A second child (case 39) developed inferior vena cava thrombosis and portal vein occlusion, and retransplantation was done the next day. In a third child (case 44), attenuation of the intrahepatic arteries and late portal venous filling were seen on hepatic angiography. The angiographic impression was rejection and hepatic venous occlusion. At retransplant the next day, the large hepatic veins were patent. However, histologic evaluation showed central venous endophlebitis with necrosis and occlusion. Indications of rejection were also present.
In 18 studies, intrahepatic arterial attenuation and narrowing or decreased flow was seen, suggesting rejection. Because the diagnosis of rejection is complex [10, 11], these studies are being analyzed separately and are excluded from the present discussion.
Discussion
By far the most common and most significant complication diagnosed at angiography was hepatic artery thrombosis. It was seen in 42% of pediatric and 12% of adult angiographic studies. The overall prevalence in all liver transplants at our center was 3% of adults and 12% of children between March 1980 and June 1984 [12, 13]. Only 25% were diagnosed by angiography. The remainder were diagnosed during retransplant, exploratory laparotomy, or autopsy. With retransplant the mortality was 27%, while without retransplant the mortality was 73% [13]. Only pediatric patients have survived without retransplantation.
The pathophysiology is often difficult to deduce, because biopsy is not available in all patients. Hepatectomy specimens (at retransplant) frequently are not intact, while autopsy specimens are complicated by supervening factors, such as sepsis, extensive hemorrhage, and hypotension. A clear cause was identified in only three (14%) of 22 cases recently reviewed at our institution [13]. Certain risk factors are, however, clear. Patients at increased risk include children and those requiring complex vascular reconstruction, either because of multiple arterial supply to the liver or because of the small size of donor and recipient vessels [3, 13].
Rejection probably plays a role in hepatic artery thrombosis in some cases. Liver biopsies or pathologic material obtained within 1 week of the angiogram were only available in 10 of the 26 cases of hepatic artery thrombosis. One sample showed no rejection, four showed mild rejection, while marked rejection was seen in five. Arterial lesions of rejection are said to be more evident in medium-sized hepatic vessels [10]. In chronic rejection, there is deposition of subintimal foam cells, myointimal hyperplasia, and intimal sclerosis [10]. The end result is progressive arterial narrowing and slow flow, with subsequent thrombosis.
Other potential causes for hepatic artery occlusion are speculative. Thrombosis may be related to turbulent flow at difficult anastomoses. In three cases infection may have been the cause. In two other patients (cases 2 and 28) stenosis progressed to occlusion. Stenosis probably resulted in slow flow with subsequent thrombosis.
Clinically, hepatic artery thrombosis presents in three forms with equal frequency [12, 13]. Massive hepatic necrosis is a dramatic clinical emergency, requiring rapid retransplantation for survival. A second presentation is delayed biliary leak seen in 7 (32%) of 22 patients with thrombosis [13]. After transplantation, the donor bile duct is entirely dependent on hepatic arterial blood supply, usually the right. Occlusion may result in bile-duct ischemia and necrosis. In our series, five children (cases 6. 8, 9a, 10, 12b) with hepatic artery thrombosis had intrahepatic bilomas (Fig. 3), presumably due to bile-duct necrosis. All but one required retransplantation. A third presentation is intermittent episodes of sepsis without an evident source, probably caused by focal abscesses within infarcted areas of the liver. In these patients, evaluation with CT or sonography may demonstrate bilomas, infarcts, or abscesses, suggesting arterial occlusion [14]. Eighty-six percent of transplant patients with hepatic abscesses or infarcts seen with CT or sonography had hepatic artery thrombosis [15].
Although graft function is usually poor enough to warrant retransplant, the prognosis is not inevitably grim. The patient’s clinical condition dictates management, and if liver function is stable, retransplant is postponed. As Figure 1A illustrates, 24% of children with hepatic artery thrombosis survived without retransplantation. This probably is a manifestation of the ability of the liver to recruit collateral arterial supply (Fig. 2). Collaterals were demonstrated in three cases within 2 weeks of transplant, and, if we judge by patient survival, an additional three patients probably also developed collaterals within 2 weeks of transplant. Three other patients developed collaterals within 3 months of transplant, making a total of nine (43%) of 21 pediatric arterial occlusions.
Collateral development is clearly a characteristic of the immediate posttransplant period. The source of these collaterals was from the superior mesenteric artery (two cases) (Fig. 2), superior mesenteric and splenic arteries (one case), and right inferior phrenic artery (one case). Surgery has shown that collaterals supply the liver from adhesions to the diaphragm, omentum, and bowel [5].
The presence of collaterals has significant implications for survival and for surgical blood loss. Half of the patients with collaterals ultimately did not require retransplant, and in an additional patient who did require retransplant for a hilar abscess, the liver was considered viable at surgery. All adult patients with complete arterial thrombosis ultimately required retransplant, and in none of them were collaterals observed. Surgical blood loss during retransplantation is usually half that of the first transplant, but with collaterals, blood loss typically exceeds the first transplant by a factor of three or more. Thus the actual blood loss at retransplantation may be sixfold or higher, information of significant interest to the surgical team as well as to the blood bank [5]. Actuarial survival after retransplant is lower than for primary transplants. Four-year survival is 60% in children and 40% in adults [5]. Our study population was similar. Eight (57%) of 14 children and one (25%) of four adults survived after retransplantation.
Extrahepatic portal vein complications were unusual, constituting six cases of stenosis and five of thrombosis over the past 5 years (Fig. 5). Potential causes include surgical technique, misalignment or excessive vessel length, hypercoagulable states, thrombus formation from the portal venous bypass cannula used at transplantation, and previous portal vein surgery (R. D. Gordon, unpublished data). Although occasionally diagnosed incidentally, most portal vein complications were accompanied by symptoms of portal hypertension (i.e., varices and upper gastrointestinal hemorrhage). Presentation of these symptoms warrants angiography to evaluate the portal venous system. Other clinical presentations include hepatic failure, intestinal swelling, and massive ascites (R. D. Gordon, unpublished data). In most of our patients, treatment consisted of endoscopic sclerotherapy for esophageal varices, although one eventually required a splenorenal shunt and one patient required retransplantation.
Three of the five cases of portal vein thrombosis demonstrated massive hepatopetal venous collaterals that reconstituted the intrahepatic portal veins. Angiographically, the appearance was similar to cavernous transformation of the portal vein [16]. The mechanism by which these collaterals develop a communication with the intrahepatic vessels is unknown. However, they must arise de novo, since all potential collateral communications are severed at transplantation.
Occlusion of the inferior vena cava or hepatic veins was rare. This contrasts with a recent series of 18 posttransplant studies in which thrombosis was as common in the vena cava as in the hepatic artery [11]. In the same series, arteriovenous fistulae after biopsy were demonstrated twice and thought to compromise the graft. Despite frequent percutaneous transhepatic intervention [17], including cholangiography, biliary drainage, and biopsy during the past 5 years, we have seen only two procedure-related vascular complications. One case (case 46) was an incidental finding of a hepatic artery–portal vein fistula. The other case (case 43) was an intrahepatic pseudoaneurysm with an infarct (Fig. 7). Both were secondary to biopsy.
During the past 5 years, angiography has been the principle technique for the evaluation of possible vascular complications after liver transplantation. CT (even with bolus technique), radionuclide scanning, and real-time sonography have all been disappointing in patency evaluation. CT and sonography, however, are valuable in identifying abscesses, bilomas, and hematomas that may result from vascular complications. CT has been the most useful study for evaluation of possible hepatic infarction after transplant [14]. The identification of pseudoaneurysms may be difficult. CT and sonography both have definite roles in addition to angiography (Fig. 9).
Since 1984, we have increasingly employed duplex sonography (combined real-time and pulsed Doppler) for evaluation of suspected hepatic arterial thrombosis after transplant. In 29 cases, duplex sonography correctly diagnosed thrombosis in eight and patency in 21 [15]. These encouraging results have led the transplant team to commonly request duplex sonography to screen for hepatic artery thrombosis. This has resulted in some decrease in angiography.
The practical treatment of end-stage liver disease with homograft liver transplant is quite new, dating from the availability of cyclosporine in 1980 [1–4, 6]. Surgical technique and intra- and postoperative management of transplant patients are still evolving [1, 4]. Our results cover the first 5 years of the cyclosporine era. As surgical and medical management evolves, the vascular complications observed as well as the clinical outcome may change.
Acknowledgments
We thank Donna Scahill for manuscript preparation.
Footnotes
Presented at the annual meeting of the American Roentgen Ray Society, Washington, DC. April 1986.
References
- 1.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–258. [Google Scholar]
- 2.Iwatsuki S, Shaw BW, Jr, Starzl TE. Five-year survival after liver transplantation. Transplant Proc. 1985;17:259–263. [PMC free article] [PubMed] [Google Scholar]
- 3.Iwatsuki S, Shaw BW, Jr, Starzl TE. Current status of hepatic transplantation. Semin Liver Dis. 1983;3:173–180. doi: 10.1055/s-2008-1040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Analysis of liver transplantation. Hepatology. 1984;4:47S–49S. doi: 10.1002/hep.1840040714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw BW, Jr, Gordon RD, Iwatsuki S, Starzl TE. Hepatic retransplantation. Transplant Proc. 1985;17:264–271. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Report of Colorado-Pittsburgh liver transplantation studies. Transplant Proc. 1983;15:2582–2585. [Google Scholar]
- 7.Gordon RD, Shaw BW, Jr, Iwatsuki S, Todo S, Starzl TE. A simplified technique for revascularization of homografts of the liver with a variant right hepatic artery from the superior mesenteric artery. Surg Gynecol Obstet. 1985;160:474–476. [PMC free article] [PubMed] [Google Scholar]
- 8.Zajko AB, Bron KM, Starzl TE, et al. Angiography of liver transplantation patients. Radiology. 1985;157:305–311. doi: 10.1148/radiology.157.2.3901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw BW, Jr, Iwatsuki S, Bron K, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985;161:66–68. [PMC free article] [PubMed] [Google Scholar]
- 10.Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Dekker A. Pathology of hepatic transplantation. A review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:151–161. [PMC free article] [PubMed] [Google Scholar]
- 11.Cardella JF, Castaneda-Zuniga WR, Hunter D, Young A, Amplatz K. Angiographic and interventional radiologic considerations in liver transplantation. AJR. 1986;146:143–153. doi: 10.2214/ajr.146.1.143. [DOI] [PubMed] [Google Scholar]
- 12.Tzakis AG. The dearterialized liver graft. Semin Liver Dis. 1985;5:375–376. doi: 10.1055/s-2008-1040635. [DOI] [PubMed] [Google Scholar]
- 13.Tzakis AG, Gordon RD, Shaw BW, Jr, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1985;40:667–671. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penkrot RJ. Noninvasive evaluation of complications of orthotopic liver transplantation. Semin Intervent Radiol. 1986;3:120–122. [Google Scholar]
- 15.Segel MC, Zajko AB, Bowen A, et al. Hepatic artery thrombosis after liver transplantation: radiologic evaluation. AJR. 1986;146:137–141. doi: 10.2214/ajr.146.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajko AB, Bron KM. Hepatopetal collaterals after portal vein thrombosis following liver transplantation. Cardiovasc Intervent Radiol. 1986;9:46–48. doi: 10.1007/BF02576985. [DOI] [PubMed] [Google Scholar]
- 17.Zajko AB, Campbell WL, Bron KM, et al. Cholangiography and interventional biliary radiology in adult liver transplantation. AJR. 1985;144:127–133. doi: 10.2214/ajr.144.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]


