Abstract
Objective
This study examined the role that easy infant temperament and cumulative environmental risk play in predicting cognitive, language and behavioral outcomes in 3 year-old children at high social risk.
Methods
Subjects were 412 mother-infant dyads, recruited at birth, participating in a longitudinal study examining the effects of prenatal methamphetamine (MA) on child development. This analysis includes a subsample (n=290) of the study with a completed 3 year visit. Temperament was assessed by the Infant Behavior Questionnaire at 12 mos. Factor analysis from well-validated measures generated “easy” and “difficult” temperament profiles, and a profile for high risk environment. Caretaker receptive vocabulary served as a proxy for IQ. Outcomes at 3 years included motor and mental development, behavior problems, and language. Linear regression and hierarchical linear modeling examined the effects of temperament, high risk environment and caregiver receptive language on outcomes adjusting for maternal drug use, demographic, and socioeconomic covariates.
Results
Internalizing and externalizing behaviors were lower in children with easy temperament and higher with increased environmental risk. Easy temperament attenuated behavioral problems only in the setting of lower environmental risk. Caregiver receptive language was associated with lower internalizing scores High risk environment and temperament factors were not related to cognitive or motor outcomes. Prenatal MA exposure was not associated with 3 year-old outcomes, nor did it alter the protective effects of an easier temperament on child behavior.
Conclusions
Children growing up in adverse social environments had increased behavioral problems and compromised language development. Conversely, an easy temperament acts as a protective factor for social-emotional development and could be related to resilience.
Keywords: Prenatal substance exposure, methamphetamine, temperament, child development, child behavior
INTRODUCTION
Temperament refers to individual differences in responsiveness to internal and external stimuli that appear early in life and that play an ongoing role in regulating and modulating emotion, attention, behavior and motor activity (1, 2). Temperament is relatively stable over time (3), but can be influenced by genetics, medical factors, gender, social experiences, and maturation (4–7). Temperament is believed to play an important role in the development of childhood psychopathology, such as ADHD, depression, anxiety and adolescent substance abuse (8–12).
Consensus exists that infants with “difficult” temperaments are less attuned to their environmental demands and might be at greater risk for behavioral difficulties and later childhood psychopathology (13). Conversely, temperamentally “easy” children may be buffered from adverse social experiences and have more positive behavioral outcomes (14–16). The interaction between temperament and the psychosocial-environmental context may be especially important when considering children who are raised in high-risk or high stress social situations, such as poverty, environments with high levels of interpersonal and community violence, and homes where the primary caregiver suffers from mental illness and/or substance abuse. Recently, Belsky (17) and others (18, 19) have described a more complex interaction between individual child characteristics, such as genetic makeup or temperament, and socio-environmental context, labeled “differential vulnerability” or “differential susceptibility”, in which some children are simultaneously liable to the adverse effects of contextual adversity and the beneficial effects of contextual support .
A number of studies have investigated the relationship between prenatal drug and alcohol exposure and infant temperament. In general, these have focused on two issues: 1) whether prenatal drug-exposure is associated with more difficult temperament (6, 20–25) and 2) whether difficult infant temperament is related to less optimal later child behavior (26, 27). However, we were unable to find any literature documenting the relationship between easy infant temperament and cognitive and behavioral outcomes in prenatally drug exposed children, especially the potential protective role that easy infant temperament may confer upon high-risk children.
The current study examines the relationship between infant temperament and later developmental outcomes among children enrolled in the Infant Development, Environment, and Lifestyle (IDEAL) study, the largest controlled, longitudinal study of prenatal methamphetamine (MA) exposure and child development. Although MA use by women of child-bearing age places fetuses at risk for poor outcomes, the medical and developmental consequences of prenatal exposure to MA are still an active area of current research. In a cohort of 65 Swedish children followed since 1976 whose mothers used amphetamines during pregnancy, a variety of adverse physical, cognitive, and emotional problems have been documented, including increased rates of ADHD, learning disabilities, aggression, and school failure (28–30). However, several methodological issues, including the lack of a control group, counfounding drug exposures, and examiners not blinded to exposure status, make interpretation of these studies problematic. Chang et al (31), in a small neuroimaging study, reported lower visual motor integration, attention, and verbal and long-term spatial memory among 13 MA-exposed children compared to 15 age-matched controls. In a larger (N=49 for both MA-exposed and comparison groups) and more recent study, Chang et al (32) replicated their earlier finding of lower visual motor integration scores in MA-exposed children, but found no other group differences in cognitive function or attention. In two other recent neuroimaging studies, Lu et al identified lower verbal memory scores among 14 MA-exposed school-aged children in comparison with 20 age, gender and SES-matched unexposed peers (33), and the same research group, in a slightly larger follow-up study (34), reported lower WISC-IV full-scale intelligence quotient scores among 21 MA-exposed children (FSIQ=97.81; SD 12.77) compared with 25 age, gender and SES-matched unexposed children (FSIQ=110.32 (SD 16.73).
In the larger IDEAL Study cohort, MA-exposed infants were found to be at increased risk of being born small for gestational age (35, 36), to have subtle infant neurobehavioral findings at one-month of age on the NICU Network Neurobehavioral Scale, including poorer quality of movement, lower arousal, and increased physiological stress signs, after adjusting for covariates (37, 38), and to have subtle deficits on Peabody Developmental Motor Scales-2 fine motor performance (grasping) at 12 months of age, with the most heavily exposed children having the poorest performance at 12 and 36 months. No group differences were found in BSID-II cognitive or psychomotor scores at 12, 24 or 36 months (39).
This study examines whether MA exposure, easy infant temperament and cumulative parenting risk contribute to 3 year-old child behavioral and developmental outcomes among the MA-exposed and matched comparison children from the IDEAL Study. The hypotheses are that 1) children in the MA-exposed group would show poorer outcomes than children in the comparison group on measures of behavior and development; and, 2) the effects of MA exposure status on child behavior and development would be attenuated by easy temperament and worsened by difficult temperament and a high-risk environment.
METHODS
Study Design
Mothers and their infants were enrolled at birth in the IDEAL longitudinal study of prenatal MA exposure conducted at 4 clinical sites (University of California, Los Angeles, University of Hawaii, Blank Children’s Hospital-Iowa Health and Universities of Oklahoma and Tulsa). Institutional Review Board approval was obtained at each site and included a federal Certificate of Confidentiality. Detailed recruitment methods have been reported previously (35). Briefly, between September 2002 and November 2004, all women delivering at the four sites were approached, screened for eligibility, and consented to participate. Maternal exclusions were: non-English speaking, <18 years of age, used opiates, LSD, PCP, or cocaine only during pregnancy, institutionalized for retardation or emotional disorders, low cognitive functioning, or current or past psychosis; infant exclusions were: critically ill and unlikely to survive, multiple gestation, life threatening congenital anomaly, chromosomal abnormality associated with mental or neurological deficiency, overt clinical evidence of an intrauterine infection, or sibling previously enrolled in the study.
At recruitment, sociodemographic and substance use information was collected from maternal interviews including the Lifestyle Interview and Substance Use Inventory. Meconium samples were collected and analyzed by a central laboratory (US Drug Testing Laboratory, Des Plaines, IL) for drug metabolites, including MA, cocaine, marijuana and opiates.
MA-exposed infants and mothers (n=204) were matched to unexposed comparison infant- mother pairs (n=208) based on maternal race, birth weight category (<1500 g, 1500–2500 g, >2500 g), private versus public insurance, and education (high school education completed versus not completed). Prenatal exposure to alcohol, tobacco and marijuana existed in both groups and were considered as background variables. MA exposure was determined by self-report and/or a positive meconium screen with Gas Chromatography/Mass Spectroscopy confirmation. Matched comparisons were enrolled based on denial of MA use during pregnancy and a negative meconium screen for MA.
Follow-up assessments were conducted at 1, 12, 24, 30 and 36 months of age. Staff were trained and re-trained regularly to maintain standardization of data collection procedures. Examiners masked to exposure status were trained and certified in the administration and scoring of motor and cognitive assessments. Age of administration was corrected for prematurity for infants born <37 weeks gestation.
Subjects
All subjects who were evaluated at 3 years on at least one of the dependent measures were included. There were 290 subjects overall (n=142 MA exposed and n=148 comparison).
Covariates
The Lifestyle Interview collected the following information: 1) number of prenatal visits; 2) demographics including age, education, occupation, race, marital status, insurance type, and socioeconomic status (SES), calculated using the four-factor Hollingshead Index adapted to single parent and non-nuclear families (40, 41); and 3) details about licit and illicit drug use during pregnancy (coded as “yes” or “no”). The Lifestyle Interview was repeated annually for SES and changes in caretaker. SES is averaged across visits for this study. Caretaker changes are summed. The Substance Use Inventory (SUI) (42) was administered to all participants who used drugs in the Lifestyle Interview, and was repeated annually to assess post-natal drug use. Consistent with published studies of MA exposure (37, 39), heavy MA use was defined as ≥3 days per week across pregnancy. Neighborhood conditions were measured by averaging scores from the Neighborhood Scale (43) at the 1 month and 1, 2 and 3 year visits. Mean Total Neighborhood Quality across the 4 visits was the subscale used. The Early Childhood Home Observation for Measurement of the Environment (HOME) Inventory, a measure of home environment quality including social-emotional and cognitive support was completed at the 30-month home visit (44). An overall summary score, Total Quality of the Home, was computed.
Measures
Independent variables
Temperament was assessed by parent report using a modified version of the Infant Behavior Questionnaire (IBQ) (45) at 12 months (modifications, approved by M. K. Rothbart, included simplification of language and reduction of response scale to five points).
The Brief Symptom Inventory (BSI) is a 53-item questionnaire measuring psychological symptom patterns in 9 primary symptom dimensions and 1 global score (46). The BSI was administered to the caretaker at 1 and 36 months and the Global Severity Score was averaged as the summary measure for analysis. The Conflict Tactics Scale (CTS) is an 80-item questionnaire that asks caretakers about specific behaviors with violent content directed at themselves by spouses or partners, or toward children in the households (47). Household Violence against the Parent was the identified measure. The Child Abuse Potential Inventory (CAPI) is a 160-item questionnaire that screens for potential physical child abuse (48). The summary score for analysis is Total Abuse Potential. Both the CTS and CAPI were administered to the caretaker by interview at 24 months.
The Peabody Picture Vocabulary Test-III (PPVT-III) was administered to the child’s mother or primary caretaker at the 30-month home visit. The PPVT-III is a standardized assessment of receptive vocabulary that serves as a proxy measure of caretaker IQ (49). In this study, higher caregiver receptive language was construed as a measure of protective or positive parenting.
Dependent variables
The Child Behavior Checklist (CBCL) for ages 1.5–5 is a 99-item parental report questionnaire designed to obtain standardized data on social and behavioral functioning in young children, based upon the child’s behavior over the past two months (50). Summary scores reported here are T scores (standardized by age and sex) for Externalizing, Internalizing, and Total Problems. The cut-off point for the normal range of these summary scores is a T score <60, with borderline scores ranging from 60–63 and clinical scores ≥64.
The Bayley Scales of Infant Development II (BSID-II) is a standardized assessment of current developmental functioning of infants including mental and motor scales (51). Two summary scores, the Mental Development Index (MDI) and the Psychomotor Development Index (PDI) were used.
The Preschool Language Scale-4 (PLS-4) directly measures receptive and expressive language in infants and young children (52). The PLS-4 has two subscales, Auditory Comprehension and Expressive Communication, and a score for Total Language.
The CBCL, BSID-II and PLS-4 were administered when the children were 36 +/− 1.5 months of age.
Statistical Methods
Principal component factor analysis (PCFA) was used to define the underlying structure among variables in the analysis, with an objective of condensing information contained in the original variables into a smaller set of factors with minimal information loss (53). The variables that were included in generating these factors were determined by substantive knowledge and the results of the factor analysis. PCFA of the IBQ suggested a two-factor solution. The easy temperament factor (Smiling and Laughter, Soothability, and Duration of Orienting) had an eigenvalue of 1.81 and explained 36.3% of the variance. The difficult (Distress and Latency to Sudden Approach and Novel Stimuli and Distress to Limitations) temperament factor had an eigenvalue of 1.93 and explained 48.3% of the variance. The sixth summary scale, Activity Level, loaded with both derived factors and was not included in the model. Factor analysis based on scores from the BSI, CTS, and the CAPI generated a one-factor solution for high risk environment. The high risk environment factor had an eigenvalue of 1.93 and explained 48.3% of the variance.
Demographic and clinical characteristics for the groups were initially compared using one-way analysis of variance (ANOVA) for continuous variables and Chi-square tests for nonparametric variables. One-way ANOVA also tested associations between MA and heavy MA exposure status, infant temperament, high risk environment, caregiver receptive language and 3 year-old child outcomes from the CBCL, BSID-II and PLS-4.
Multivariate linear regression tested associations between 3 year-old outcomes and hypothesized risk and protective parenting and temperament factors (high risk environment, caregiver receptive language, easy infant temperament, difficult infant temperament), adjusting for covariates selected on the basis of empirical and theoretical criteria, including prenatal drug exposures, low birth weight, small for gestational age (SGA) status, gender, average SES, mean Total Neighborhood Quality, Total Quality of Home, and study site. Study site is included a priori because it is likely to explain the variance in outcomes. Interactions between prenatal MA exposures and covariates were tested and removed if p>0.10.
Hierarchical linear modeling (HLM) tested the relationship between these 3 year-old outcomes and higher and lower levels of easy infant temperament and high risk environment by using dichotomous variables for easy infant temperament and for high risk environment based upon a median split for the entire sample. Site was included to address the nesting structure of children in study sites. Covariates adjusted for in the model included post natal drug exposures, caregiver receptive language, average SES, mean Total Neighborhood Quality, Total Quality of the Home, low birth weight, and SGA.
Significance for all analyses was accepted at p<.05. Analyses were performed using SPSS for Windows (Rel. 17.0.0 2008 Chicago: SPSS Inc.) and SAS for Windows (version 9.1.3; SAS Institute, Cary, NC).
RESULTS
Analyses of selective attrition compared maternal and infant characteristics of subjects included in the study versus those excluded because they were not evaluated at 3 years on at least one of the dependent measures. Comparison of the 290 included to the 122 excluded (Table 1) showed higher gestational age at the 1st prenatal visit in the excluded group. Additionally, there were no differences between included and excluded subjects on the prevalence of MA exposure including heavy MA use.
Table 1.
Maternal and Infant dyads included and not included at the 3 year evaluation
| Mean (SD) / Number (Percent) | |||
|---|---|---|---|
| Maternal Characteristics | Included (N = 290) |
Excluded (N=122) |
P |
| Race | NS | ||
| White | 109 (37.6) | 51 (41.8) | |
| Hispanic | 66 (22.8) | 26 (21.3) | |
| Pacific Islander | 51 (17.6) | 20 (16.4) | |
| Asian | 37 (12.8) | 20 (16.4) | |
| Black | 17 (5.9) | 4 (3.3) | |
| American Indian | 9 (3.1) | 1 (0.8) | |
| Other | 1 (0.3) | 0 (0.0) | |
| Household Income < $10,000 | 71 (26.2) | 35 (32.4) | NS |
| Hollingshead SES V | 61 (21.0) | 32 (26.7) | NS |
| Public Insurance | 229 (81.5) | 106 (86.9) | NS |
| No Partner | 161 (55.5) | 66 (54.1) | NS |
| Education <12 years | 117 (40.3) | 55 (45.8) | NS |
| Age, yr | 25.2 (5.6) | 25.1 (5.5) | NS |
| Gestational Age at 1st Prenatal Visit, wk | 11.3 (7.1) | 13.7 (7.8) | <.01 |
| Weight Gain during this pregnancy, lbs | 42.1 (20.1) | 34.1 (15.8) | NS |
| Prenatal Tobacco Exposure | 148 (51.0) | 70 (57.4) | NS |
| Prenatal Alcohol Exposure | 71 (24.5) | 35 (28.7) | NS |
| Prenatal Marijuana Exposure | 56 (19.3) | 20 (16.4%) | NS |
| Infant Characteristics | |||
| Gestational age, wk | 38.6 (2.2) | 38.7 (1.8) | NS |
| Birth weight, g | 3262.9 (618.9) | 3210.8 (549.5) | NS |
| Length, cm | 50.6 (3.5) | 50.1 (2.8) | NS |
| Head circumference, cm | 33.9 (1.9) | 33.9 (1.7) | NS |
| Low Birth weight, <2500 g | 34 (11.7%) | 13 (10.7%) | NS |
| Apgar 1 minute | 7.8 (1.3) | 8.0 (0.9) | NS |
| Apgar 5 minute | 8.8 (0.5) | 8.9 (0.3) | NS |
| Male, % | 153 (52.8%) | 67 (54.9%) | NS |
Maternal and infant characteristics of the MA-exposed and comparison groups at the time of enrollment at birth are shown in Table 2. As expected by the matched group study design, no differences existed for race, education or public insurance, however mothers in the MA-exposed group were more likely to have no partner, start prenatal care later, experience greater weight gain, live in households earning <$10,000/year and use tobacco, alcohol and marijuana prenatally (all p’s<.001). In addition, by 3 years 53.2% in the exposed group (range 1–3) and 7.4% in the comparison group (range 1–2) had 1 or more caretaker changes ( p<.001). However, the majority of primary caretakers were relatives (e.g. at 3 years, 94.4% exposed, 98.6% comparison).
Table 2.
Maternal and Infant Characteristics at Birth Enrollment*
| Mean (SD) / Number (Percent) | |||
|---|---|---|---|
| Maternal Characteristics | MA Exposed (N = 142) |
Comparison (N=148) |
P |
| Race | NS | ||
| White | 52 (36.6) | 57 (38.5) | |
| Hispanic | 34 (23.9) | 32 (21.6) | |
| Pacific Islander | 26 (18.3) | 25 (16.9) | |
| Asian | 17 (12.0) | 20 (13.5) | |
| Black | 7 (4.9) | 10 (6.8) | |
| American Indian | 6 (4.2) | 3 (2.0) | |
| Other | 0 (0.0) | 1 (0.7) | |
| Household Income < $10,000 | 44 (34.4) | 27 (18.9) | <.01 |
| Hollingshead SES V | 47 (33.1) | 14 (9.5) | <.001 |
| Public Insurance | 116 (85.9) | 113 (77.4) | NS |
| No Partner | 82 (56.4) | 70 (33.7) | <.001 |
| Education <12 years | 63 (44.4) | 54 (36.5) | NS |
| Change in primary caretaker (0–3 years) | 75 (53.2) | 11 (7.4%) | <.001 |
| Age, yr | 25.7 (5.8) | 24.7 (5.5) | NS |
| Gestational Age at 1st Prenatal Visit, wk | 13.8 (8.0) | 8.9 (5.1) | <.001 |
| Weight Gain during this pregnancy, lbs | 43.4 (21.0) | 33.9 (16.4) | <.001 |
| Prenatal Tobacco Exposure | 112 (78.9) | 36 (24.3) | <.001 |
| Prenatal Alcohol Exposure | 51 (35.9) | 20 (13.5) | <.001 |
| Prenatal Marijuana Exposure | 49 (34.5) | 7 (4.7%) | <.001 |
| Infant Characteristics | |||
| Gestational age, wk | 38.4 (2.2) | 38.9 (1.8) | <.01 |
| Birth weight, g | 3206.5 (650.7) | 3317.1 (583.9) | NS |
| Length, cm | 49.9 (3.9) | 51.2 (3.0) | <.001 |
| Head circumference, cm | 33.6 (1.8) | 34.1 (1.9) | NS |
| Low Birth weight, <2500 g | 17 (12.0%) | 17 (11.5%) | NS |
| Apgar 1 minute | 7.7 (1.5) | 8.0 (0.9) | <.05 |
| Apgar 5 minute | 8.8 (0.54) | 9.0 (0.3) | <.01 |
| Male, % | 75 (52.8%) | 78 (52.7%) | NS |
Change in primary caretaker was based on cumulative data from 0–3 years.
The prevalence of low birth weight and males were similar between MA-exposed and comparison infants. MA-exposed infants had lower gestational age, length, and 1- and 5-minute Apgar scores relative to the comparison infants (all p’s<.05).
There were no group differences with respect to protective and high-risk infant temperament and environmental factors (easy infant temperament, difficult infant temperament and high risk environment factors, and caregiver receptive language), although the MA-exposed caregivers had statistically higher scores on one of the measures comprising the high risk environment factor, Household Violence against the Parent (CTS) [p = .007]. There were also no correlations between the derived factor for high risk environment and caregiver ratings of easy or difficult infant temperament (r = 0.09 and 0.11, p=.20 and .10, respectively), or caregiver receptive language and difficult infant temperament (r = −0.05; p=.41), but receptive language and ratings of easy infant temperament were significantly negatively correlated (r = −0.20, p<.01). However, there were positive correlations between the measure for high risk environment and post-natal use of MA, marijuana and tobacco (r=0.28, 0.18 and 0.20, respectively; all p values <.01).
There were no differences in 3 year-old child outcomes (PLS-4, CBCL and BSID-II) by MA or heavy MA exposure status in bivariate analyses (data not shown).
Tables 2–3 show the linear regression results for the associations between 3 year-old child outcomes and potentially protective and high-risk infant temperament and environmental factors (easy and difficult infant temperament, high risk environment, caregiver receptive language) adjusted for covariates. High risk environment was inversely related to Total Language (β=−2.68; p=.037) and borderline inversely related to Expressive Communication (β=−2.32; p=.051), but was not related to 3 year-old MDI or PDI scores. No associations were found between easy or difficult infant temperament and language or cognitive scores. SES averaged from birth to 3 years was positively related to Auditory Comprehension (β=0.37; p=.048), MDI (β=0.38; p=.007) and PDI (β=0.31; p=.010) scores, while a stimulating home environment was positively related to Auditory Comprehension (β=0.99; p=.010) and Total Language (β=0.85; p=.012). Male gender was negatively related to Expressive Communication (β=−4.74; p=.026), MDI (β=−8.62; p<.001) and PDI (β=−4.40; p=.009) scores.
Table 3.
Linear regression model predicting the association between Preschool Language Scale-4 and Bayley Scales of Infant Development-II 36-month child outcomes and protective and high-risk infant temperament and environment factors (Easy Infant Temperament, Difficult Infant Temperament, High-Risk Environment) and PPVT Caregiver Receptive Language (regression coefficients and 95% confidence intervals).
| Preschool Language Scale-4 | Bayley Scales of Infant Development-II |
||||
|---|---|---|---|---|---|
| Variables in the Model |
Auditory Comprehension (n=265) |
Expressive Communication (n=263) |
Total Language (n=263) |
Mental Development Index (n=276) |
Psychomotor Development Index (n=273) |
| Prenatal MA Exposure |
1.75 (−4.82, 8.32) |
2.22 (−3.18, 7.62) |
2.44 (−3.39, 8.26) |
3.14 (−1.91, 8.18) |
2.35 (−1.92, 6.62) |
| High-Risk Environment |
−2.77 (−5.61, 0.08) |
−2.32 (−4.65, 0.02) |
−2.68* (−5.20, −0.17) |
1.06 (−1.18, 3.29) |
−0.16 (−2.06, 1.75) |
| Caregiver Receptive Language |
0.87 (−1.93, 3.67) |
1.60 (−0.69, 3.89) |
1.41 (−1.06, 3.88) |
−0.02 (−2.12, 2.09) |
0.11 (−1.65, 1.86) |
| Easy Infant Temperament |
−0.23 (−2.75, 2.29) |
−1.46 (−3.54, 0.62) |
−1.02 (−3.27, 1.22) |
0.92 (−0.98, 2.82) |
0.18 (−1.42, 1.79) |
| Difficult Infant Temperament |
0.01 (−2.52, 2.54) |
0.49 (−1.60, 2.57) |
0.41 (−1.83, 2.66) |
−1.37 (−3.32, 0.59) |
−1.31 (−2.98, 0.359) |
| Prenatal Tobacco Exposure |
−1.21 (−7.36, 4.93) |
−1.72 (−6.77, 3.32) |
−1.35 (−6.80, 4.09) |
−1.09 (−5.78, 3.61) |
0.71 (−3.27, 4.66) |
| Prenatal Alcohol Exposure |
−2.89 (−9.03, 3.25) |
1.56 (−3.47, 6.59) |
0.07 (−5.35, 5.49) |
0.92 (−3.77, 5.62) |
1.06 (−2.91, 5.04) |
| Prenatal Marijuana Exposure |
2.75 (−4.68, 10.18) |
0.15 (−5.97, 6.27) |
0.58 (−6.02, 7.17) |
−2.55 (−8.26, 3.15) |
−3.93 (−8.77, 0.90) |
| SGA | −3.67 (−13.87, 6.52) |
−7.51 (−16.10, 1.07) |
−6.73 (−15.99, 2.53) |
−3.98 (−11.33, 3.38) |
−3.65 (−9.9, 2.55) |
| Low Birth Weight | −4.80 (−15.88, 6.28) |
−0.60 (−9.78, 8.57) |
−3.32 (−13.22, 6.57) |
−4.26 (−12.03, 3.52) |
−1.02 (−7.58, 5.5) |
| Gender | −.899 (−5.96, 4.16) |
−4.74* (−8.90, −0.58) |
−3.31 (−7.79, 1.18) |
−8.62*** (−12.5, −4.73) |
−4.40** (−7.66, −1.13) |
| Average SES | 0.37* (0.004, 0.72) |
0.16 (−0.14, 0.46) |
0.294 (−.027, 0.61) |
0.38** (0.10, 0.66) |
0.31* (0.08, 0.55) |
| Mean Total Neighborhood Quality |
−0.52 (−4.87, 3.83) |
0.99 (−2.56, 4.55) |
0.55 (−3.29, 4.39) |
1.22 (−2.16, 4.60) |
−0.41 (−3.29, 2.48) |
| Home Total-30 month assessment |
0.99* (0.24, 1.73) |
0.52 (−0.09, 1.13) |
0.85* (0.19, 1.51) |
0.45 (−0.14, 1.03) |
0.06 (−4.34, 0.56) |
| Site | −4.04** (−7.05, −1.02) |
−2.77* (−5.24, −0.31) |
−3.62** (−6.28, −0.96) |
−2.40* (−4.74, −0.04) |
−1.77 (−3.78, 0.212) |
P<.05
P<.01
P<.001
A high risk environment was positively associated with Externalizing (β=4.06), Internalizing (β=4.62) and Total (β=4.75) Problem scores (all P’s<.001), and caregiver receptive language was negatively associated with Internalizing Problems (β=−1.46; p=.041). Easy infant temperament was inversely associated with Externalizing (β=−2.22), Internalizing (β=−2.14) and Total (β =−2.25) Problem scores (all P’s≤.001). There was no significant interaction between the high risk environment and the easy infant temperament factors for any behavioral problem outcome.
The relationship between high versus low environmental risk and easy versus less easy temperament, was explored by first dichotomizing easy infant temperament and high risk environment with a median split yielding four discrete groups: high environmental risk/less easy infant temperament (n=54); high environmental risk/easier infant temperament (n=63); low environmental risk/less easy infant temperament (n=58); and low environmental risk/easier infant temperament (n=50) (Figure 1). Analysis of the group means using HLM showed that children raised with low environmental risk who also had an easier temperament exhibited significantly lower Total Problem scores compared to children with less easy infant temperament (t=−2.67, df=9, p=.025), while for children raised with high environmental risk, level of easy infant temperament was unrelated to Total Problem scores (t=−0.00, df=9, p=.997). However, children with less easy infant temperament raised with low environmental risk also had significantly lower Total Problem scores when compared to easier temperament children raised in high environmental risk (t=3.09, df=9, p=.013). Similar results were seen with Externalizing and Internalizing Problems scores.
Figure 1.
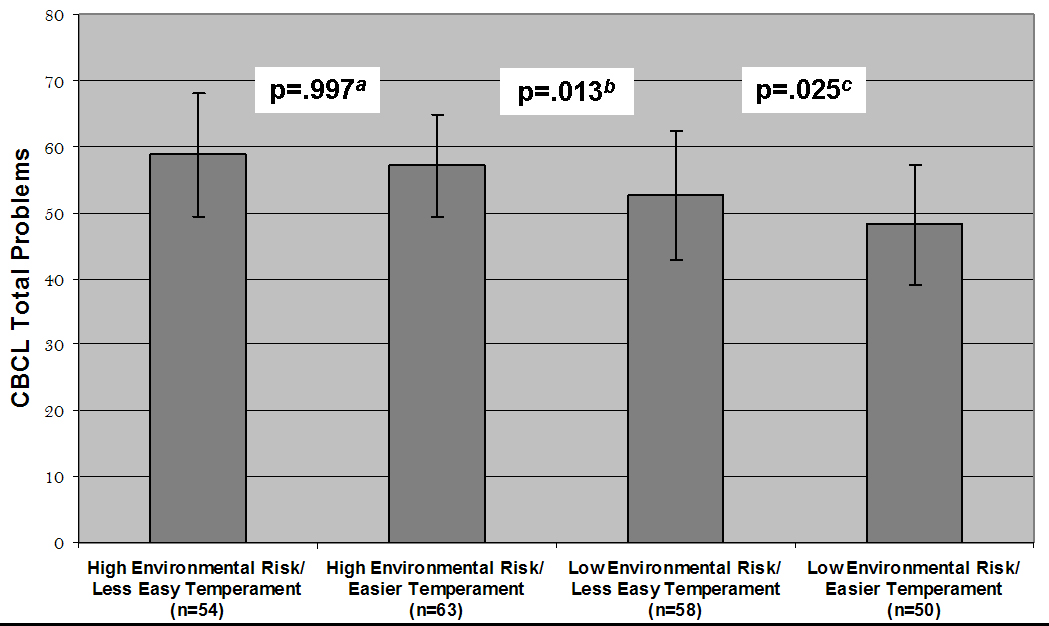
HLM of Child Behavior Checklist (CBCL) Total Problems (T scores + SD) in relation to 4 groups of 50/50 median spit dichotomized High Risk Environment and Easy Infant Temperament factors.
a Comparing High Environmental Risk/Less Easy Infant Temperament with High Environmental Risk/Easier Infant Temperament
b Comparing High Environmental Risk/Easier Infant Temperament with Low Environmental Risk/Less Easy Infant Temperament
c Comparing Low Environmental Risk/Less Easy Infant Temperament with Low Environmental Risk/Easier Infant Temperament
DISCUSSION
This study observed that among children exposed prenatally to MA and a socio-demographically matched comparison group, higher levels of environmental adversity were associated with increased externalizing and internalizing behavioral problems and compromised language development. Conversely, easy infant temperament was associated with reduced externalizing and internalizing behavioral problems. Although prior research has described the association between negative or difficult temperamental traits in prenatally drug-exposed children and later behavioral problems (26, 27), and easy infant temperament has previously been shown to confer resilience in the face of adversity (15, 16, 54, 55), to our knowledge this is the first study to find that easy infant temperament is associated with better behavioral outcomes in a sample that included prenatally drug-exposed children.
Although caregiver ratings of temperament may be confounded by caregiver personality, in this study there was no or only weak correlations between the measured contextual and parental variables, high risk environment and caregiver receptive language, and ratings of easy or difficult infant temperament, a finding consistent with the notion that temperament represents a relatively stable, biologically-based construct (7). The small but significant negative correlation between caregiver receptive language and easy infant temperament is a finding similar to that found by van Bakel (56) where two caregiver-rated positive temperamental traits, interest/persistence and pleasure, were negatively correlated with caregiver educational level and PPVT-derived receptive language. The reasons for this inverse association, however, are not clear.
Also found was the expected and well-documented associations between higher SES, better home environments and improved childhood cognitive and language outcomes (57, 58). Male children also appeared to be at heightened risk for poorer expressive language, cognitive and psychomotor developmental outcomes. Unlike other studies, there was no observed association between difficult infant temperament and poorer 3 year-old child outcomes. This may be because difficult infant temperament effects were obscured by the overall cohort’s multiple and cumulative risks (26). For example, in this study rates of borderline/clinically significant Total scores on the CBCL (≥ 60) were similar between the MA-exposed and comparison groups, but the overall combined group rate was higher than those seen in normative samples. The null findings in the relationship between prenatal MA exposure and 3 year-old child cognitive outcomes is consistent with several longitudinal studies of prenatal cocaine exposure in children up to the age of 3 years (59–61). The lack of observable prenatal MA effects on 3 year-old behavior is also consistent with prior research in which negative behavioral outcomes were related mainly to harsh discipline, maternal psychopathology and environmental risk, but not to prenatal drug exposure (62), although more recent research has reported direct and indirect pathways from prenatal cocaine exposure to 3 and 7 year-old behavioral outcomes (27). Prospective, controlled research on prenatal MA exposure has to date identified either early-appearing but possibly transient neonatal neurobehavioral findings (37, 38), socioenvironmental contextual effects associated with a substance-abusing lifestyle (63), subtle fine motor findings (39), or subtle, higher order executive function deficits (31–34). Therefore, findings of no effects at 3 years of age on global measures of cognitive and behavioral development needs to be taken with caution, given that subtle effects (64) may carry large public health implications, and executive dysfunction may presage later child and adult psychopathology (65, 66).
The exploratory analyses of the temperament and environmental risk groups showed that an easier infant temperament was protective in the face of lower levels of high risk environment, but lost its protective effect as the level of environmental risk increased. In fact, children with easier infant temperament did worse behaviorally in high-risk environments than children with less easy infant temperament did in low risk environments, a finding similar to that described by Sameroff (67). Further, there was no evidence for a “differential susceptibility” interaction (68) between easy infant temperament and high risk environment, where the beneficial effects of easy infant temperament would be most pronounced in a high-risk environment but have little or even detrimental effects under lower risk conditions. However, the IDEAL study was not designed to truly test the concept of differential susceptibility given the focus on adverse (high risk versus lower (but still) high risk) environments and problematic (more versus less) behavioral outcomes, rather than on adverse and nurturing environments, and problematic and normative or enhanced behavioral outcomes. In these analyses, easy infant temperament explained only about 3% of the variance in the 3 year-old Total Problems score; adding in the high risk environment factor to the overall model for the Total Problems score explained an additional 20% of the variance suggesting that cumulative environmental risk is a much more important predictor of behavioral outcome than temperamental traits. This is consistent with Sameroff’s conclusion that “individual resiliencies do not overcome the effects of high environmental risk” (Sameroff, 2006, p.123). This finding, if confirmed, has important implications for prevention science, as it may indicate that the buffering effects of individual resiliency characteristics, such as easy infant temperament, reach a ceiling among conditions of more extreme social adversity (69).
There are several limitations to this study. First, temperament and 3 year-old child behaviors were measured by caregiver report and are therefore subject to reporter bias and possible confounding. More direct measures of temperament and behavior would have been preferable. Despite this, there was no association between the high risk environment measure and caregiver reports of temperament, suggesting that caregivers are assessing child characteristics that are, at least partially, independent of their own level of distress. Second, the observational design of this study makes it difficult to determine directionality of influence. Finally, although the findings add to the literature on temperamental dimensions that appear to promote resilience in the face of high-risk environments, the present study was not designed to uncover any of the underlying biological processes – neural, physiologic, or genetic – that may mediate relationships between temperament, parenting and child behavior.
CONCLUSION
The findings on the associations between high risk environment and easy infant temperament and 3 year-old behavioral and developmental outcomes have a number of potential clinical implications for programs working with substance-abusing families and other groups at elevated social and environmental risk. First, given the modifiable nature of contextual risk factors and the findings of significant associations between post-natal drug use and heightened environmental risk, implementation of evidence-based parenting interventions (70) that reduce coercive parental behaviors and harsh discipline, substance abuse treatment, and other cumulative risk reduction efforts (71, 72) is warranted. Comprehensive mental health treatment is needed to enhance parental well-being, improve access to early prenatal care, and provide job training and adult education. Second, the findings support interventions that more broadly address the social determinants that underlie the excessive cumulative risks facing many children growing up in the U.S today – poverty, toxic neighborhoods, low educational expectations and resources, and endemic violence, to name a few – because these conditions are the medium in which high-risk parenting is nourished (73, 74). Finally, this study calls for an increased understanding of ways to enhance infant and child temperamental characteristics that promote resilience in the face of adversity, by favorably altering a child’s self-regulatory capabilities (75).
Table 4.
Linear regression model predicting the association between Child Behavior Checklist 36-month child outcomes and protective and high-risk infant temperament and environment factors (Easy Infant Temperament, Difficult Infant Temperament, High-Risk Environment) and PPVT Caregiver Receptive Language (regression coefficients and 95% confidence intervals).
| Child Behavior Checklist | |||
|---|---|---|---|
| Variables in the Model |
Externalizing Problems (n=288) |
Internalizing Problems (n=288) |
Total Problems (n=288) |
| Prenatal MA Exposure |
−0.11 (−3.51, 3.29) |
−0.05 (−3.39, 3.28) |
−1.23 (−4.44, 1.98) |
| High-Risk Environment |
4.06*** (2.55, 5.58) |
4.62*** (3.13, 6.10) |
4.75*** (3.32, 6.18) |
| Caregiver Receptive Language |
1.04 (−.378, 2.47) |
−1.46* (−2.86, −0.06) |
0.23 (−1.11, 1.58) |
| Easy Infant Temperament |
−2.22** (−3.48, −0.97) |
−2.14** (−3.37, −0.91) |
−2.25*** (−3.4, −1.06) |
| Difficult Infant Temperament |
0.58 (−0.75, 1.91) |
0.57 (−0.74, 1.88) |
0.71 (−0.55, 1.97) |
| Prenatal Tobacco Exposure |
0.93 (−2.27, 4.12) |
0.14 (−2.99, 3.28) |
1.20 (−1.82, 4.21) |
| Prenatal Alcohol Exposure |
−1.28 (−4.47, 1.91) |
−0.87 (−4.00, 2.26) |
−0.94 (−3.95, 2.07) |
| Prenatal Marijuana Exposure |
−2.78 (−6.69, 1.14) |
1.03 (−2.81, 4.88) |
0.06 (−3.64, 3.76) |
| SGA | 1.34 (−3.79, 6.46) |
0.02 (−5.01, 5.04) |
0.76 (−4.07, 5.60) |
| Low Birth Weight | −4.35 (−9.75, 1.05) |
−3.42 (−8.72, 1.88) |
−3.90 (−8.99, 1.20) |
| Gender | 0.83 (−1.81, 3.47) |
1.14 (−1.45, 3.73) |
1.39 (−1.10, 3.88) |
| Average SES | −0.06 (−0.24, 0.13) |
0.04 (−0.14, 0.23) |
0.002 (−0.17, 0.18) |
| Mean Total Neighborhood Quality |
0.96 (−1.34, 3.26) |
0.93 (−1.33, 3.19) |
0.80 (−1.37, 2.97) |
| Home Total-30 month assessment |
−0.04 (−0.43, 0.36) |
0.23 (−0.16, 0.62) |
0.08 (−0.30, 0.45) |
| Site | −0.86 (−2.45, 0.73) |
−0.07 (−1.63, 1.48) |
−0.50 (−2.00, 0.99) |
P<.05
P<.01
P<.001
ACKNOWLEDGMENTS
The IDEAL study is supported by funding from the National Institutes on Drug Abuse, 2R01DA014948 and 1K23DA020801, and in part by the National Center for Research Resources, 5P20RR011091 and 3M01RR00425. We would like to sincerely thank the journal editors for their helpful critiques and suggestions, and the children, families and staff participating in the IDEAL Study.
Abbreviations
- ANOVA
Analysis of Variance
- BSI
Brief Symptom Inventory
- BSID
Bayley Scales of Infant Development
- CAPI
Child Abuse Potential Inventory
- CBCL
Child Behavior Checklist
- CTS
Conflict Tactics Scale
- HLM
Hierarchical Linear Modeling
- HOME
Home Observation for Measurement of the Environment
- IBQ
Infant Behavior Questionnaire
- MA
Methamphetamine
- MDI
Mental Development Index
- PDI
Psychomotor Development Index
- PLS
Preschool Language Scale
- PPVT
Peabody Picture Vocabulary Test
- PCFA
Principal Component Factor Analysis
- SES
Socioeconomic Status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure and conflict of interest: No author has a financial association with the product or other conflict of interest. There were no commercial sponsors involved in the above matters. Dr. Chris Derauf wrote the first draft of the manuscript and there was no honorarium, grant, or other form of payment given to anyone to produce this manuscript.
REFERENCES
- 1.Fox NA. Temperament and Regulation of Emotion in the First Years of Life. Pediatrics. 1998;102(5 Suppl E):1230–1235. [PubMed] [Google Scholar]
- 2.Rothbart MK, Bates JE. Temperament. In: Damon W, editor. Handbook of Child Psychology. 5th ed. New York, NY: Wiley & Sons; 1998. [Google Scholar]
- 3.Degnan K, Fox NA. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Devel Psychopathol. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- 4.Gorman K, Lourie AE, Choudhury N. Differential patterns of development: the interaction of birth weight, temperament, and maternal behavior. J Dev Behav Pediatr. 2001;22(6):366–375. doi: 10.1097/00004703-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kochanska G, Friesenborg AE, Lange LA, et al. Parents’ personality and infants’ temperament as contributors to their emerging relationship. J Pers Soc Psychol. 2004;86(5):744–759. doi: 10.1037/0022-3514.86.5.744. [DOI] [PubMed] [Google Scholar]
- 6.Sheinkopf SJ, Lester BM, LaGasse LL, et al. Interactions between maternal characteristics and neonatal behavior in the prediction of parenting stress and perception of infant temperament. J Pediatr Psychol. 2006;31(1):27–40. doi: 10.1093/jpepsy/jsj026. [DOI] [PubMed] [Google Scholar]
- 7.Saudino KJ. Behavioral Genetics and Child Temperament. J Dev Behav Pediatr. 2005;26(3):214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crockenberg SC, Leerkes EM. Infant and maternal behavior moderate reactivity to novelty to predict anxious behavior at 2.5 years. Dev Psychopathol. 2006;18(1):17–34. doi: 10.1017/S0954579406060020. [DOI] [PubMed] [Google Scholar]
- 9.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 10.Leve LD, Kim HK, Pears KC. Childhood temperament and family environment as predictors of internalizing and externalizing trajectories from ages 5 to 17. J Abnorm Child Psychol. 2005;33(5):505–520. doi: 10.1007/s10802-005-6734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair C. Early intervention for low birth weight, preterm infants: the role of negative emotionality in the specification of effects. Dev Psychopathol. 2002;14(2):311–332. doi: 10.1017/s0954579402002079. [DOI] [PubMed] [Google Scholar]
- 12.Robinson JL, Acevedo MC. Infant reactivity and reliance on mother during emotion challenges: prediction of cognition and language skills in a low-income sample. Child Dev. 2001;72(2):402–415. doi: 10.1111/1467-8624.00286. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Edgar K, Schmidt LA, Henderson HA, et al. Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology. 2008;33:916–925. doi: 10.1016/j.psyneuen.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim-Cohen J, Moffitt TE, Caspi A, et al. Genetic and environmental processes in young children’s resilience and risk to socioeconomic deprivation. Child Dev. 2004;75:651–668. doi: 10.1111/j.1467-8624.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 15.Tschann JM, Kaiser P, Chesney MA, et al. Resilience and vulnerability among preschool children: family functioning, temperament, and behavior problems. J Am Acad Child Adolesc Psychiatry. 1996;35:184–192. doi: 10.1097/00004583-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Torteya C, Anne Bogat G, von Eye A, et al. Resilience among children exposed to domestic violence: the role of risk and protective factors. Child Dev. 2009;80(2):562–577. doi: 10.1111/j.1467-8624.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 17.Belsky J. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guilford; 2005. pp. 139–163. [Google Scholar]
- 18.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 19.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Research review: genetic vulnerability or differential susceptibility in child development: the case of attachment. J Child Psychol Psychiatry. 2007;48(12):1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson HL, Rosen TS. Mother-infant interaction in a multirisk population. Am J Orthopsychiatry. 1990;60(2):281–288. doi: 10.1037/h0079181. [DOI] [PubMed] [Google Scholar]
- 21.Edmondson R, Smith T. Temperament and behavior problems of infants prenatally exposed to drugs: clinical implications for the mother-infant dyad. Infant Mental Health J. 1994;15:368–379. [Google Scholar]
- 22.Nulman I, Rovet J, Kennedy D, et al. Binge alcohol consumption by non-alcohol-dependent women during pregnancy affects child behaviour, but not general intellectual functioning; a prospective controlled study. Arch Womens Ment Health. 2004;7(3):173–181. doi: 10.1007/s00737-004-0055-7. [DOI] [PubMed] [Google Scholar]
- 23.Dennis T, Bendersky M, Ramsay D, et al. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42(4):688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss SJ, St Jonn-Seed M, Harris-Muchell C. The contribution of fetal drug exposure to temperament: potential teratogenic effects on neuropsychiatric risk. J Child Psychol Psychiatry. 2007;48(8):773–784. doi: 10.1111/j.1469-7610.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 25.Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30(2):96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagner DM, Sheinkopf SJ, Miller-Loncar C, et al. The effect of parenting stress on child behavior problems in high-risk children with prenatal drug exposure. Child Psychiatry Hum Dev. 2009;40(1):73–84. doi: 10.1007/s10578-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lester BM, Bagner DM, Liu J, et al. Infant Neurobehavioral Dysregulation: Behavior Problems in Children With Prenatal Substance Exposure. Pediatrics. 2009;124(5):1355–1362. doi: 10.1542/peds.2008-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billing L, Eriksson M, Jonsson B, et al. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994;18(1):3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 29.Cernerud L, Eriksson M, Jonsson B, et al. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85(2):204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson M, Jonsson B, Steneroth G, et al. Amphetamine abuse during pregnancy: environmental factors and outcome after 14–15 years. Scand J Public Health. 2000;28(2):154–157. doi: 10.1177/140349480002800212. [DOI] [PubMed] [Google Scholar]
- 31.Chang L, Smith LM, LoPresti C, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Chang L, Cloak C, Jiang CS, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48(2):391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu LH, Johnson A, O'Hare ED, et al. Effects of prenatal methamphetamine exposure on verbal memory revealed with functional magnetic resonance imaging. J Dev Behav Pediatr. 2009;30(3):185–192. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowell ER, Leow AD, Bookheimer SY, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci. 2010;30(11):3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith LM, LaGasse LL, Derauf C, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen D, Smith LM, Lagasse LL, et al. Intrauterine growth of infants exposed to prenatal methamphetamine: results from the infant development, environment, and lifestyle study. J Pediatr. 2010;157(2):337–339. doi: 10.1016/j.jpeds.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith LM, Lagasse LL, Derauf C, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30(1):20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagasse LL, Wouldes T, Newman E, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol. 2010 Jul 6; doi: 10.1016/j.ntt.2010.06.009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith LM, LaGasse LL, Derauf C, et al. Motor and Cognitive Outcomes Through Three Years Of Age In Children Exposed To Prenatal Methamphetamine. Neurotoxicol Teratol. doi: 10.1016/j.ntt.2010.10.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- 41.LaGasse L, Seifer R, Wright LL, et al. The Maternal Lifestyle Study (MLS): the caretaking environment of infants exposed to cocaine/opiates [abstract] Pediatr Res. 1999;45:247A. [Google Scholar]
- 42.Della Grotta S, Lagasse LL, Arria AM, et al. Patterns of Methamphetamine Use During Pregnancy: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2010;14(4):519–527. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furstenberg FF, Hughes ME. Social capital and successful development. J Marriage and the Family. 1995;57:580–592. [Google Scholar]
- 44.Caldwell BM, Bradley RH. Home observation for measurement of the environment. Little Rock. AR: U of Arkansas; 1984. [Google Scholar]
- 45.Rothbart MK. Measurement of temperament in infancy. Child Dev. 1981;52:569–578. [Google Scholar]
- 46.Derogatis LR. BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual. 4th Ed. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 47.Straus MA. Measuring intrafamily conflict and violence: The Conflict Tactics Scales. J Marriage Fam. 1979;41(1):75–88. [Google Scholar]
- 48.Milner JS. The Child Abuse Potential Inventory. 2nd ed. Webster, NC: Psytec Corp; 1986. [Google Scholar]
- 49.Dunn LM, Dunn LM. The Peabody Picture Vocabulary Test. 3rd ed. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 50.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- 51.Bayley N. Bayley Scales of Infant Development. 2nd ed. Bayley-II. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 52.Zimmerman IL, Steiner VG, Pond RE. PLS-4: Preschool Language Scale. 4th ed. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- 53.Hair JF, Black WC, Babin BJ, Anderson RE. Multivariate Data Analysis. 7th ed. Upper Saddle River, NJ: Pearson Prentice-Hall; 2010. [Google Scholar]
- 54.Werner EE. Vulnerable but invincible: high-risk children from birth to adulthood. Acta Paediatr Suppl. 1997;422:103–105. doi: 10.1111/j.1651-2227.1997.tb18356.x. [DOI] [PubMed] [Google Scholar]
- 55.Lengua LJ, Wolchik SA, Sandler IN, et al. The additive and interactive effects of temperament and parenting in predicting adjustment problems of children of divorce. J Clin Child Psychol. 2000;29:232–244. doi: 10.1207/S15374424jccp2902_9. [DOI] [PubMed] [Google Scholar]
- 56.van Bakel HJ, Riksen-Walraven JM. Parenting and development of one-year-olds: links with parental, contextual, and child characteristics. Child Dev. 2002;73(1):256–273. doi: 10.1111/1467-8624.00404. [DOI] [PubMed] [Google Scholar]
- 57.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 58.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7(2):55–71. [PubMed] [Google Scholar]
- 59.Messinger DS, Bauer CR, Das A, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113(6):1677–1685. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 60.Alessandri SM, Bendersky M, Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev Psychol. 1998;34(3):565–573. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frank DA, Jacobs RR, Beeghly M, et al. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110(6):1143–1152. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett DS, Bendersky M, Lewis M. Children's intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38(5):648–658. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paz MS, Smith LM, LaGasse LL, et al. Maternal depression and neurobehavior in newborns prenatally exposed to methamphetamine. Neurotoxicol Teratol. 2009;31(3):177–182. doi: 10.1016/j.ntt.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998;282(5389):633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- 65.Nigg JT. On Inhibition/Disinhibition in Developmental Psychology: Views from Cognitive and Personality Psychology and a Working Inhibition Taxonomy. Psychol Bull. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- 66.Ivanov I, Schulz KP, London ED, et al. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am J Drug Alcohol Abuse. 2008;34(3):239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- 67.Sameroff AJ, Rosenblum KL. Psychosocial constraints on the development of resilience. Ann N Y Acad Sci. 2006;1094:116–124. doi: 10.1196/annals.1376.010. [DOI] [PubMed] [Google Scholar]
- 68.Belsky J, Bakermans-Kranenberg MJ, van IJzendoorn MH. For Better and For Worse: Differential Susceptibility to Environmental Influences. Curr Dir Psychol Sci. 2007;16:300–304. [Google Scholar]
- 69.Vanderbilt-Adriance E, Shaw DS. Neighborhood risk and the development of resilience. Ann N Y Acad Sci. 2006;1094:359–362. doi: 10.1196/annals.1376.050. [DOI] [PubMed] [Google Scholar]
- 70.Prinz R. Dissemination of a multilevel evidence-based system of parenting interventions with broad application to child welfare populations. Child Welfare. 2009;88(1):127–132. [PubMed] [Google Scholar]
- 71.Nair P, Schuler ME, Black MM, et al. Cumulative environmental risk in substance abusing women: early intervention, parenting stress, child abuse potential, and child development. Child Abuse Negl. 2003;27:997–1017. doi: 10.1016/s0145-2134(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 72.Schuler ME, Nair P, Kettinger L. Drug-exposed infants and developmental outcome: effects of a home intervention and ongoing maternal drug use. Arch Pediatr Adolesc Med. 2003;157(2):133–138. doi: 10.1001/archpedi.157.2.133. [DOI] [PubMed] [Google Scholar]
- 73.Wright RJ. Moving towards making social toxins mainstream in children's environmental health. Curr Opin Pediatr. 2009;21(2):222–229. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The Prevention Institute. A Time of Opportunity: Local Solutions to Reduce Inequities in Health and Safety. 2009 Available at: http://preventioninstitute.org/documents/IOM_TimeofOpportunity_052209_FINAL_000.pdf.
- 75.Rueda MR, Rothbart MK. The influence of temperament on the development of coping: the role of maturation and experience. New Dir Child Adolesc Dev. 2009;124:19–31. doi: 10.1002/cd.240. [DOI] [PubMed] [Google Scholar]


