Abstract
Objective
To explore race-ethnic differences in the relationship between plasma lipid components and risk of incident myocardial infarction (MI).
Design/Methods
As part of the Northern Manhattan Study, 2738 community residents without cardiovascular disease were prospectively evaluated. Baseline fasting blood samples were collected and lipid panel components were analyzed as continuous and categorical variables. Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for incident MI after adjusting for demographic and cardiovascular risk factors.
Results
The mean age was 68.8±10.4 years; 36.7% men, 19.9% non-Hispanic white, 24.9% non-Hispanic black, and 52.8% Hispanic (over 80% from the Caribbean). Hispanics had lower mean HDL-C, and higher TG/HDL-C. During a mean 8.9 years of follow-up there were 163 incident MIs. In the whole cohort all lipid profile components were associated with risk of MI in the expected directions. However, HDL-C (adjusted HR per 10 mg/dl increase 0.93, 95%CI 0.76–1.12) and TG/HDL-C>2 (adjusted HR 0.89, 95%CI 0.51–1.55) were not predictive of MI among Hispanics, but were predictive among non-Hispanic blacks and whites. TG/HDL-C per unit increase was associated with an 8% higher risk of MI among Hispanics (adjusted HR 1.08, 95%CI 1.04–1.12).
Conclusions
In Hispanics, low HDL-C and TG/HDL-C>2 were not associated with MI risk. Our data suggests that a different TG/HDL ratio cutoff may be needed among Hispanics to predict MI risk.
Introduction
Cardiovascular disease, in particular coronary heart disease (CHD), remains the leading cause of death in the United States for all race-ethnic groups and both sexes1. Conflicting results have been noted regarding CHD mortality by race-ethnicity, with some studies pointing to lower CHD mortality amongst Hispanics despite a higher prevalence of physical inactivity, obesity, and diabetes leading to the so-called “Hispanic paradox”1,2. Given that Hispanics are the fastest growing segment of the population, and that risk factors are more prevalent among Hispanics3, more needs to be understood about possible differential effects of vascular disease risk factors on CHD.
Low levels of high-density lipoprotein cholesterol (HDL-C)4, and elevated total cholesterol5, triglycerides (TG)6, non-HDL-C4, and low-density lipoprotein cholesterol (LDL-C)5 are considered strong independent risk factors for CHD. Ethnic minorities, particularly Hispanics, have been traditionally underrepresented in lipid clinical trials and epidemiologic studies. The levels of lipid profile components vary depending on race-ethnicity, with non-Hispanic blacks having higher average HDL-C levels and lower TG levels, and Hispanics having lower LDL-C, HDL-C, total cholesterol and higher TG levels than non-Hispanic whites7. Hispanics are also less likely to be screened for and be aware that they may have dyslipidemia8. It is not known if lipid panel components differentially affect Hispanics and non-Hispanic blacks and the relationship between lipids and CHD risk.
The purpose of this study was to prospectively examine race-ethnic differences in the longitudinal association between lipid profile components and incident myocardial infarction (MI).
Methods
Recruitment of the Cohort
The Northern Manhattan Study (NOMAS) is a population-based study designed to evaluate the impact of medical, socio-economic, and other risk factors on the incidence of vascular disease in a stroke-free multi-ethnic community cohort. Participants were identified by dual-frame random digit dialing in Northern Manhattan as previously described9, and were eligible if they met the following criteria: (1) had never been diagnosed with a stroke; (2) were over the age of 39 years; and (3) resided in Northern Manhattan for ≥3 months in a household with a telephone. Pre-existing CHD was ascertained via questionnaires capturing self-reported MI, angina, or prior cardiac revascularization. The study was approved by the Institutional Review Boards at Columbia University Medical Center (CUMC). All participants gave informed consent to participate in the study. The study was funded by the National Institutes of Health (NINDSR37NS29993). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Cohort Evaluation
Data regarding baseline status and risk factors were collected through interviews of participants by trained bilingual research assistants. Physical examinations, in-person measurements, and analysis of fasting blood specimens were carried out by study physicians. Race-ethnicity was determined by self-identification in response to a questionnaire modeled after the 2000 U.S. census and conformed to the standard definitions outlined by Directive 1510. The majority of Hispanics in our cohort did not identify with any particular race after self-identifying as Hispanic, and over 80% originated from the Caribbean (Dominican Republic and Puerto Rico). Education was classified as completing high school versus not, and waist circumference was classified as a continuous variable. Standardized questions were adapted from the Behavioral Risk Factor Surveillance System regarding the following conditions: hypertension, diabetes, hypercholesterolemia, cigarette smoking (classified as former smoker, current smoker, or never smoked), and cardiac conditions11. Standard techniques were used to measure blood pressure, height, weight, and fasting glucose and lipid panels as previously described12. Hypertension was defined as systolic blood pressure ≥140mm Hg or diastolic blood pressure ≥90mm Hg based on the average of the two blood pressure measurements, a physician diagnosis of hypertension, or a patient’s self-report of a history of hypertension or anti-hypertensive use. Diabetes mellitus was defined as fasting blood glucose ≥126mg/dl or the patient’s self-report of such a history, or insulin or hypoglycemic use.
Plasma lipids and lipoproteins measurement
Fasting blood samples were obtained and lipid profile components were measured as previously described13. Briefly, total cholesterol (TC) and TG levels were measured using standard enzymatic procedures in an automated spectrometer (Hitachi 705; Boehringer, Mannheim, Germany). Plasma HDL-C cholesterol levels were measured after precipitation of apolipoprotein B containing lipoproteins by phosphotungstic acid. LDL-C concentrations were calculated by the Friedewald formula14. Non-HDL-C was calculated by subtracting the HDL-C level from total cholesterol level.
Follow-up and outcome measures
The subjects were followed annually via phone screening to detect any new cardiac symptoms, as well as to review any interval hospitalizations. In the last 5 years of the study we have been unable to carry out a follow up visit in only 15 participants (less than 1% of participants). Any subject who tested positive for the screen was scheduled for an in-person assessment. Additionally a 10% sample of the cohort was followed annually in-person for 5 years to evaluate for any telephone screen false-negatives; no participants in this sample had an MI adjudicated based on an in-person assessment. MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial requiring at least 2 of the 3 following criteria: (a) ischemic cardiac pain determined to be typical angina; (b) cardiac marker abnormalities defined as abnormal CK-MB fraction or troponin I values; and/or (c) ischemic EKG abnormalities. The presence of an MI was adjudicated by cardiologists independently after review of all the clinical data. (CR, MD, SH).
A total of 3298 participants were recruited between 1993 and 2001. After excluding for a history of MI (n = 244) and CHD without MI (n = 316), 2738 participants were included in this analysis.
Statistical Analysis
Baseline characteristics were compared using Analysis of Variance or 2 sided t-tests for continuous variables, and the χ2 test for proportions. After exploring the distributions of plasma lipid components and risk factors, risk of MI was estimated for HDL-C, LDL-C, TG, non-HDL-C, TC, TC/HDL-C, and TG/HDL-C as both categorical and continuous variables. We used dyslipidemia definitions based on National Cholesterol Education Program Adult Treatment Panel III guidelines, including an LDL-C >130 mg/dl, TG >200 mg/dl, non-HDL-C >160 mg/dl, TC >240 mg/dl, and a sex specific low HDL-C (<40 mg/dl for men and <50 mg/dl for women)15; we used TG/HDL-C ratio >2 16, and the upper quartile of the TC/HDL-C ratio for categorical analyses. We also fitted a model including HDL-C, LDL-C, and TG simultaneously to examine independent effect of each component for the risk of MI. Cox-proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (95%CI) for MI using duration of follow up as the time to event variable. The parameter estimates were calculated adjusting for demographics (age, sex, race-ethnicity, and education), vascular risk factors (hypertension, diabetes mellitus, waist circumference, tobacco use, valvular heart disease, moderate alcohol consumption, and physical inactivity) and cholesterol lowering medications. We tested for interaction by age, sex, and race-ethnicity (non-Hispanic black and non-Hispanic white as reference, and with Hispanic with non-Hispanic white as the reference), and only reported stratified results when there was a statistically significant interaction term for that lipid profile component (p <0.05). All statistical analyses were performed with the use of SAS version 9.1 (SAS Institute, Cary, NC).
Results
Baseline characteristics for the cohort are outlined in table 1. The mean age of the cohort was 68.8±10.4 years; 36.7% were men. The majority of participants (n=1445, 52.8%) were Hispanic, with similar proportions of non-Hispanic blacks and whites making up the rest of the cohort; 45.8% of participants completed high school. There were no significant differences in the proportion of women in each race-ethnicity category. Overall, Hispanics and non-Hispanic blacks had a higher burden of CHD risk factors, including hypertension, diabetes, current smoking, and an increased waist circumference, and were less likely to be physically active.
Table 1.
Baseline characteristics of the Northern Manhattan Cohort (n=2738)
| Overall N=2738* | Hispanic (n=1445) | Non-Hispanic Black (n=681) | Non-Hispanic White (n=545) | p-value for difference | ||
|---|---|---|---|---|---|---|
| Myocardial infarction, n(%) | 163(6.0%) | 68(4.7%) | 33(4.8%) | 58(10.6%) | <0.0001 | |
| Age (Mean +/− SD) | 68.8±10.4 | 65.9±9.5 | 71.6±10.5 | 72.9±10.2 | <0.0001 | |
| Men, n(%) | 1006(36.7%) | 541(37.4%) | 225(33.0%) | 207(38.0%) | 0.1 | |
| Education, completed high school, n (%) | 1253(45.8%) | 321(22.2%) | 432(63.4%) | 453(83.1%) | <0.0001 | |
| Risk factors, n(%) | Hypertension§ | 1967(71.8%) | 1056(73.1%) | 536(78.7%) | 339(62.2%) | <0.0001 |
| Diabetes Mellitus§§ | 555(20.3%) | 318(22.0%) | 154(22.6%) | 72(13.2%) | <0.0001 | |
| Total cholesterol >240 mg/dl | 446(16.9%) | 227(15.7%) | 103(15.1%) | 104(19.1%) | 0.1 | |
| HDL-C (men <40 mg/dl, women <50 mg/dl) | 1483(56.3%) | 926(64.1%) | 276(40.5%) | 256(46.9%) | <0.0001 | |
| LDL-C >130 mg/dl) | 1223(46.8%) | 654(45.3%) | 272(39.9%) | 272(49.9%) | <0.0001 | |
| TG/HDL-C >2 | 1739(66.0%) | 1056(75.7%) | 329(50.3%) | 322(61.2%) | <0.0001 | |
| Treatment with lipid lowering medications | 1172(42.8%) | 730(50.5%) | 237(34.8%) | 185(33.9%) | <0.0001 | |
| Current Smoking | 491(18.0%) | 239(16.5%) | 161(23.6%) | 79(14.5%) | <0.0001 | |
| Waist circumference (inches), mean±SD | 36.6±5.0 | 36.8±4.5 | 36.8±5.5 | 36.1±5.6 | 0.02 | |
| Physically active† | 238(8.7%) | 93(6.4%) | 57(8.4%) | 77(14.1%) | <0.0001 | |
| Moderate alcohol intake†† | 929(34.0 %) | 441(30.5%) | 220(32.3%) | 246(45.1%) | <0.0001 | |
Other race-ethnicity n = 67
Hypertension defined as: systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg based on the average of two measurements, a physician diagnosis of hypertension, or a patient’s self-report of a history of hypertension or anti-hypertensive use.
Diabetes defined as: fasting blood glucose ≥126 mg/dl or the patient’s self-report of such a history, or insulin or hypoglycemic use.
Moderate to heavy intensity leisure-time physical activity
Moderate alcohol intake: 1–2 servings of alcohol per day
Hispanics were more likely to have a low HDL-C than non-Hispanic blacks or whites, while non-Hispanic whites and Hispanics were more likely to have an elevated LDL-C. Mean HDL-C for the cohort was 47.2±14.8 mg/dL. The mean HDL-C was highest in non-Hispanic blacks (52.3mg/dL) and lowest in Hispanics (43.9mg/dL) compared to non-Hispanic whites. Other lipid profile components are summarized in table 2.
Table 2.
Lipid panel components by sex and race-ethnicity in the Northern Manhattan Study (n=2738)
| Mean level (standard deviation) | Total Cholesterol in mg/dl | Triglycerides in mg/dl | High-density lipoprotein Cholesterol in mg/dl | Low-density lipoprotein cholesterol in mg/dl | Non-High-density lipoprotein cholesterol in mg/dl | Total cholesterol/high-density liproprotein cholesterol | Triglycerides/high-density liproprotein cholesterol |
|---|---|---|---|---|---|---|---|
| Total cohort | 203.0(39.9) | 135.4(82.5) | 47.2(14.8) | 129.2(35.9) | 155.8(40.0) | 4.7(1.6) | 3.4(3.4) |
| Men | 192.1(38.5) | 138.6(99.8) | 41.6(12.6) | 123.6(35.1) | 150.6(39.1) | 5.0(1.7) | 4.0(4.6) |
| Women | 209.3(39.4) | 133.5(70.5) | 50.5(14.9) | 132.4(35.9) | 158.8(40.1) | 4.5(1.5) | 3.1(2.5) |
| p-value | <0.0001 | 0.9 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Hispanic | 201.8(40.4) | 146.5(88.1) | 43.9(13.0) | 129.2(35.7) | 158.0(40.2) | 4.9(1.6) | 3.9(3.6) |
| p-value(reference white) | 0.001 | <0.0001 | <0.0001 | 0.04 | 0.6 | <0.0001 | <0.0001 |
| Non-Hispanic black | 201.4(39.4) | 115.7(71.2) | 52.3(16.4) | 126.1(36.2) | 149.1(39.5) | 4.2(1.4) | 2.6(2.8) |
| p-value(reference white) | 0.003 | <0.0001 | 0.0003 | 0.001 | <0.0001 | <0.0001 | <0.0001 |
| Non-Hispanic white | 208.4(39.0) | 132.9(77.7) | 49.3(14.8) | 133.0(35.2) | 159.1(39.0) | 4.6(1.6) | 3.3(3.7) |
Analysis in the total multi-ethnic cohort
Over a mean follow up of 8.9 years, 163 incident MI’s were detected. In univariate analyses using categorical (table 3) and continuous definitions (table 4), almost all of the lipid profile components (total cholesterol, TG, HDL-C, non-HDL-C, TG/HDL-C and TC/HDL-C) were significantly associated with an increased risk of MI. There was a trend, as well, for LDL-C >130mg/dl (HR 1.26, 95%CI 0.92–1.73); LDL-C per 10mg/dl increase was associated with risk of MI (HR 1.06, 95%CI 1.02–1.11).
Table 3.
Hazard ratios (HR) and 95% CI for lipid parameters and risk of myocardial infarction (n =163) using dichotomous definitions
| Plasma lipid panel component | Unadjusted HR and 95%CI | Adjusted* HR and 95%CI | |
|---|---|---|---|
| HDL-C (men <40 mg/dl, women <50 mg/dl)† | 1.39(1.00–1.92) | 1.79(1.25–2.56) | |
| Race-ethnicity | Hispanic | 1.35(0.79–2.31) | |
| Non-Hispanic black | 2.36(1.16–4.81) | ||
| Non-Hispanic whites | 2.03(1.15–3.58) | ||
| Triglycerides >200 mg/dl† | 1.53(1.03–2.28) | 1.80(1.16–2.77) | |
| Race-ethnicity | Hispanic | 1.37(0.74–2.56) | |
| Non-Hispanic black | 1.25(0.29–5.29) | ||
| Non-Hispanic whites | 2.82(1.47–5.42) | ||
| Triglycerides/HDL-C >2† | 1.63(1.13–2.35) | 1.85(1.25–2.74) | |
| Race-ethnicity | Hispanic | 0.89(0.51–1.55) | |
| Non-Hispanic black | 3.31(1.47–7.44) | ||
| Non-Hispanic whites | 2.79(1.42–5.48) | ||
| LDL-C >130 mg/dl | 1.26(0.92–1.73) | 1.59(1.14–2.21) | |
| Non-HDL-C >160 mg/dl | 1.41(1.03–1.93) | 2.02(1.44–2.82) | |
| Total Cholesterol >240 mg/dl | 1.71(1.20–2.43) | 2.36(1.60–3.47) | |
| Total Cholesterol/HDL-C (upper quartile) | 1.86(1.35–2.57) | 2.43(1.71–3.45) | |
adjusted for age, sex, race-ethnicity, education, hypertension, diabetes mellitus, waist circumference, tobacco use, valvular heart disease, moderate alcohol consumption, cholesterol medication use, and physical activity. Not adjusted for other lipid profile components.
Stratified models presented only when interaction term between race-ethnicity and the lipid profile component was statistically significant at p<0.05
Table 4.
Hazard ratios (HR) and 95% CI for lipid parameters and risk of myocardial infarction (n=163) using continuous definitions
| Plasma lipid panel component | Unadjusted HR and 95%CI | Adjusted* HR and 95%CI | |
|---|---|---|---|
| HDL-C per 10mg/dl decrease† | 1.20(1.08–1.37) | 1.27(1.11–1.47) | |
| Race-ethnicity | Hispanic | 1.08(0.89–1.31) | |
| Non-Hispanic black | 1.35(1.04–1.76) | ||
| Non-Hispanic whites | 1.49(1.18–1.88) | ||
| Triglycerides per 10mg/dl increase† | 1.02(1.01–1.04) | 1.03(1.02–1.05) | |
| Race-ethnicity | Hispanic | 1.02(1.01–1.05) | |
| Non-Hispanic black | 1.02(0.98–1.07) | ||
| Non-Hispanic whites | 1.03(1.01–1.06) | ||
| Triglycerides/HDL-C† | 1.05(1.02–1.07) | 1.06(1.04–1.09) | |
| Race-ethnicity | Hispanic | 1.08(1.04–1.12) | |
| Non-Hispanic black | 1.07(0.95–1.20) | ||
| Non-Hispanic whites | 1.05(1.01–1.09) | ||
| LDL-C per 10mg/dl increase | 1.06(1.02–1.11) | 1.12(1.07–1.17) | |
| Non-HDL-C per 10mg/dl increase | 1.07(1.03–1.11) | 1.12(1.08–1.16) | |
| Total Cholesterol per 10 mg/dl increase | 1.05(1.01–1.09) | 1.10(1.06–1.14) | |
| Total Cholesterol/HDL-C | 1.24(1.14–1.34) | 1.36(1.24–1.49) | |
adjusted for age, sex, race-ethnicity, education, hypertension, diabetes mellitus, waist circumference, tobacco use, valvular heart disease, moderate alcohol consumption, cholesterol medication use, and physical activity. Not adjusted for other lipid profile components.
Stratified models presented only when interaction term between race-ethnicity and the lipid profile component was statistically significant at p<0.05
In fully adjusted multivariable models, all plasma lipid components, including LDL-C > 130 mg/dl, remained significantly associated with risk of MI. Using categorical definitions (table 3), we found the strongest association with MI for non-HDL-C > 160 mg/dl (adjusted HR 2.02, 95% CI 1.44–2.82). Low HDL-C was associated with a 79% (adjusted HR 1.79, 95% CI 1.25–2.56) greater risk of MI than high HDL-C, while TG/HDL-C > 2 was associated with an 85% (adjusted HR 1.85, 95% CI 1.25–2.74) higher risk of MI compared to TG/HDL≤ 2 (table 3). A similar pattern was observed using continuous definitions of all the same lipid profile components (table 4). Models including HDL-C, LDL-C, and TG simultaneously showed that LDL-C and HDL-C as both categorical (elevated LDL-C: adjusted HR 1.50, 95% CI 1.08–2.10; low HDL-C: adjusted HR 1.57, 95% CI 1.09–2.28) and continuous variables per 10 mg/dl increase (LDL-C: adjusted HR 1.10, 95% CI 1.05–1.15; HDL-C: adjusted HR 1.19, 95% CI 1.02–1.38) remained independently associated with risk of MI, whereas TG was not (elevated TG: adjusted HR 1.54, 95% CI 0.97–2.42; TG per 10 unit increase: adjusted HR 1.02, 95% CI 1.00–1.05). There were no interactions between lipid profile components with age or sex.
Analyses by race-ethnicity
There were 58 (10.6%) MI’s in non-Hispanic whites, 33 (4.8%) in non-Hispanic blacks, and 68 (4.7%) in Hispanics and 4 (6.0%) in those with another race-ethnicity. The associations between MI and lipid profile components in Hispanics were different from that for non-Hispanic whites. We found an interaction between HDL-C and Hispanic ethnicity (p for interaction = 0.03), such that HDL-C per 10 mg/dl decrease was independently associated with an increasing risk of MI in non-Hispanic whites (adjusted HR 1.49, 95% CI 1.18–1.88), but not among Hispanics (adjusted HR 1.08, 95% CI 0.89–1.31). There was an interaction between TG/HDL-C > 2 and Hispanic ethnicity (versus non-Hispanic white, p for interaction = 0.01) such that TG/HDL-C > 2 was associated with higher risk of MI (adjusted HR 2.79, 95% CI 1.42–5.48) compared to TG/HDL≤ 2 in non-Hispanic whites, but not among Hispanics (adjusted HR 0.89, 95% CI 0.51–1.55). We did not find an interaction between race-ethnicity and the other lipid profile components, including TC/HDL-C.
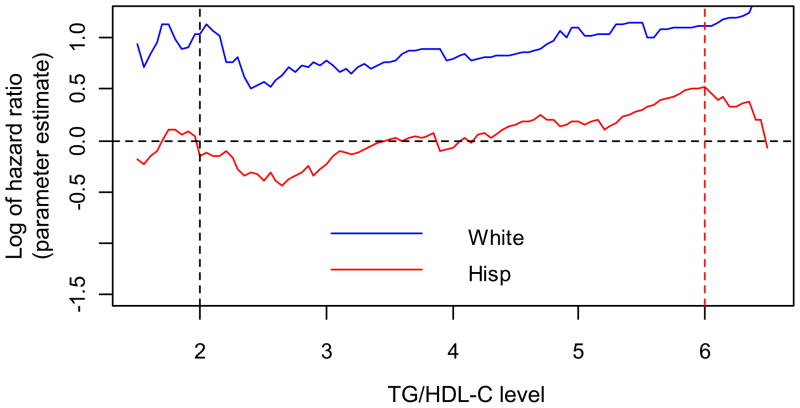
TG/HDL-C as a continuous variable was associated with MI in Hispanics (adjusted HR for TG/HDL per 1 unit change 1.08, 95 CI 1.04 – 1.12), and the magnitude of association was similar to that for non-Hispanic whites (adjusted HR for TG/HDL per 1 unit change 1.05, 95% CI 1.01–1.09), whereas TG/HDL-C > 2 was not associated with risk of MI in Hispanics. The level of TG/HDL-C was greatest in Hispanics (3.9 compared to 3.3 for non-Hispanic whites and 2.9 for non-Hispanic blacks), and the prevalence of an elevated TG/HDL-C, defined according to standard criteria as TG/HDL-C > 2, was highest in Hispanics (75.7 %) compared to non-Hispanic whites (61.2%) or blacks (50.3%). We thus explored whether an alternative threshold might be more appropriate for Hispanics in our population by calculating the log HR (y-axis) for 100 different thresholds of TG/HDL-C (x-axis) and plotting them for Hispanics and non-Hispanic whites (figure). Among Hispanics, the log HR linearly increased for thresholds between two and six, and that for a TG/HDL-C>4 there was no longer a negative parameter estimate. In exploratory analyses, when the TG/HDL-C >6 there was a trend toward association with the risk of MI (adjusted HR 1.54, 95% CI 0.95–2.50), though relatively few Hispanics (n = 201, 14.4%) had a ratio above this threshold.
Figure.
Plot of log hazard ratios (y-axis) in non-Hispanic whites (blue line) and Hispanics (red line) for each unit increase in TG/HDL-C (x-axis)
Discussion
In keeping with prior investigations7, 17, the mean HDL-C was lower and mean TG higher in our study among Hispanics compared to non-Hispanic blacks or whites. Non-Hispanic blacks also had a less atherogenic lipid profile than non-Hispanic whites, though we found that the risk of MI was similar for non-Hispanic whites and non-Hispanic blacks except for the TG. In our main finding of the TG/HDL-C, non-Hispanic blacks and whites were similar. Compared to results from the National Health and Nutrition Examination Survey (NHANES), our sample had a more atherogenic lipid profile as across all race-ethnic groups1, and in the aged 60–69 and >70 samples18. The mean HDL-C values were lower in our cohort (50.5mg/dl in women, 41.6mg/dl in men) compared to the NHANES cohort aged 60–69 (58.3mg/dl in women, 46.8mg/dl in men), and over 70 (59.0mg/dl in women, 46.8mg/dl in men), though our LDL-C values were not appreciably different18. Nonetheless, we found no association between HDL-C and risk of MI among Hispanics, which was also demonstrated in the San Antonio Heart Study19. In our study HDL-C either as a categorical or continuous variable was not associated with risk of MI in Hispanics, arguing against an incorrect threshold. The reasons for the absence of an association are unclear, but may be related to the fact that Hispanics have lower HDL-C levels.7
Although TG/HDL-C as a continuous variable was associated with risk of MI among Hispanics, the commonly recommended categorical TG/HDL-C threshold >2 was not. These exploratory analyses indicated that a TG/HDL-C threshold >2 may be inappropriate for Hispanics, and a higher threshold may be more clinically useful. Our results regarding the TG/HDL-C threshold were exploratory however, and confirmation by others is required. In other populations the cut-off of 2 in the TG/HDL-C ratio appears to have a more important deleterious effect on the risk of MI than the HDL-C alone20. TG/HDL-C may be an indicator of mixed dyslipidemia and more informative than either term alone for predicting risk of CAD and MI6, 21, 22. Several explanations have been posited, including the associations between the TG/HDL-C ratio and insulin resistance16, 23, the metabolic syndrome, or small dense LDL-C particles24. The metabolic syndrome has been associated with incident MI25, and is more prevalent in Hispanics in our cohort26. Hispanics also have higher prevalence of the other clinical components of the metabolic syndrome such as diabetes and obesity26, 27. Few investigators, however, have studied the utility of a TG/HDL-C ratio in Hispanics or older individuals, although one report found that a ratio of 3 predicted insulin resistance in Hispanics and non-Hispanic whites28. Others have reported that the TG/HDL-C is less predictive of insulin resistance in non-Hispanic blacks29.
Our study has important strengths, including analysis of a large predominantly Caribbean Hispanic elderly cohort, among which the association of serum lipids and MI has been less frequently studied. Our sample also includes the largest number of Hispanics to date who have been followed prospectively to examine the role of dyslipidemia on risk of MI.
Our study has several limitations, as well, including a concern that the lipids were measured several years before the MI, and may not reflect levels close to the time of the event. In prior analysis however we have demonstrated that HDL-C and TG remain stable over multiple measures at least one year apart despite treatment with cholesterol lowering medications30. We also may have been underpowered to detect subtle differences in differential effects for the other lipid panel components. In our study Hispanics were more likely to be treated with lipid lowering medications, which may have also reduced our ability to find a differential effect of LDL-C on Hispanics, though we did adjust for lipid lowering treatment in all of our models. Hispanics were additionally less likely to have completed high school, which could have relevant lifestyle and wellness differences that we did not collect in our study. We had a greater proportion of older women, who had both higher HDL-C and LDL-C than the men in our sample, which could have been an important confounder. On the other hand we adjusted for sex in all models, and we found no evidence for an interaction term with any of the lipid profile components. Our findings may not be generalizable to all Hispanics; 88% of our Hispanic sample consists of Caribbean-Hispanics from the Dominican Republic, Cuba, and Puerto Rico. Even though Hispanics have a common language, they have different ancestral origins, cultures, diets, and socioeconomic status which may contribute to different cardiovascular disease outcomes. Our study was not designed to confirm or address the causes of the “Hispanic paradox”, which is a less clearly described phenomenon for Caribbean Hispanics. Confirmation by other investigators using other Hispanic populations is required. Finally, given the number of lipid panel components analyzed, some of our results could be due to chance.
In conclusion, we found that not all components of the lipid panel were associated with an increased risk of MI in Hispanics. In particular, a low HDL-C and the TG/HDL-C >2 ratio were not associated with risk of MI in Hispanics. The TG/HDL-C ratio as a continuous variable was associated with risk of MI in Hispanics, with a trend towards a statistically significant association for a ratio >6. This represents the relative importance of mixed dyslipidemia among Hispanics since Hispanics need a much higher TG/HDL-C ratio to confer risk compared to non-Hispanic whites and blacks. Since there are some variations in lipid expression and metabolism, it is feasible that there could be differences in MI risk. Our findings highlight the importance of developing more accurate risk management and prediction guidelines for Hispanics; at this point further studies are required to determine whether national guidelines for lipids should be adjusted based on race-ethnicity.
Acknowledgments
Funding Sources: This project was supported by NINDSR37NS29993. JZW was funded by NINDST32NS07153. CJR is supported by a Robert Wood Johnson, Harold Amos Medical Faculty Development Award, and a National Heart, Lung, and Blood Institute’s Mentored Patient-Oriented Research Career Development Award (NHLBIK23HL079343-01A2). The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors meet criteria for authorship, including acceptance of responsibility for the scientific content of the manuscript.
Footnotes
Disclosures: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Akerblom JL, Costa R, Luchsinger JA, Manly JJ, Tang MX, Lee JH, et al. Relation of plasma lipids to all-cause mortality in Caucasian, African-American and Hispanic elders. Age Ageing. 2008;37(2):207–13. doi: 10.1093/ageing/afn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic variation in cardiovascular disease risk factors among children and young adults: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1999;281(11):1006–13. doi: 10.1001/jama.281.11.1006. [DOI] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Psaty BM, Anderson M, Kronmal RA, Tracy RP, Orchard T, Fried LP, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: The Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(10):1639–47. doi: 10.1111/j.1532-5415.2004.52455.x. [DOI] [PubMed] [Google Scholar]
- 6.Frost PH, Davis BR, Burlando AJ, Curb JD, Guthrie GP, Jr, Isaacsohn JL, et al. Serum lipids and incidence of coronary heart disease. Findings from the Systolic Hypertension in the Elderly Program (SHEP) Circulation. 1996;94(10):2381–8. doi: 10.1161/01.cir.94.10.2381. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez C, Pablos-Mendez A, Palmas W, Lantigua R, Mayeux R, Berglund L. Comparison of modifiable determinants of lipids and lipoprotein levels among African-Americans, Hispanics, and Non-Hispanic Caucasians > or =65 years of age living in New York City. Am J Cardiol. 2002;89(2):178–83. doi: 10.1016/s0002-9149(01)02197-x. [DOI] [PubMed] [Google Scholar]
- 8.Disparities in screening for and awareness of high blood cholesterol--United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54(5):117–9. [PubMed] [Google Scholar]
- 9.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the NOrthern MAnhattan Study. Stroke. 2004;35(10):2263–9. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 10.Budget. OoMa. Race and ethnic standards for federal statistics and administrative reporting. (Directive no 15). 1978:43 Federal Register 19269.
- 11.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1(6):9–14. [PubMed] [Google Scholar]
- 12.Rodriguez CJ, Lin F, Sacco RL, Jin Z, Boden-Albala B, Homma S, et al. Prognostic implications of left ventricular mass among Hispanics: the Northern Manhattan Study. Hypertension. 2006;48(1):87–92. doi: 10.1161/01.HYP.0000223330.03088.58. [DOI] [PubMed] [Google Scholar]
- 13.Paultre F, Tuck CH, Boden-Albala B, Kargman DE, Todd E, Jones J, et al. Relation of Apo(a) size to carotid atherosclerosis in an elderly multiethnic population. Arterioscler Thromb Vasc Biol. 2002;22(1):141–6. doi: 10.1161/hq0102.101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–9. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, D’Agostino R, Jr, Goff D, Howard B, Festa A, Saad MF, et al. LDL size in African Americans, Hispanics, and non-Hispanic whites : the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 1999;19(9):2234–40. doi: 10.1161/01.atv.19.9.2234. [DOI] [PubMed] [Google Scholar]
- 18.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294(14):1773–81. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 19.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25(7):1177–84. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 20.Arca M, Montali A, Valiante S, Campagna F, Pigna G, Paoletti V, et al. Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. Am J Cardiol. 2007;100(10):1511–6. doi: 10.1016/j.amjcard.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 21.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96(8):2520–5. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 22.Cordero A, Andres E, Ordonez B, Leon M, Laclaustra M, Grima A, et al. Usefulness of triglycerides-to-high-density lipoprotein cholesterol ratio for predicting the first coronary event in men. Am J Cardiol. 2009;104(10):1393–7. doi: 10.1016/j.amjcard.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, Vasan RS, Keyes MJ, Sullivan LM, Robins SJ. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort) Am J Cardiol. 2008;101(4):497–501. doi: 10.1016/j.amjcard.2007.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94(2):219–22. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109(1):42–6. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 26.Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MV, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39(1):30–5. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49(2):109–16. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol. 2008;7:4. doi: 10.1186/1475-2840-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165(12):1395–400. doi: 10.1001/archinte.165.12.1395. [DOI] [PubMed] [Google Scholar]
- 30.Willey JZ, Xu Q, Boden-Albala B, Paik MC, Moon YP, Sacco RL, et al. Lipid profile components and risk of ischemic stroke: the Northern Manhattan Study (NOMAS) Arch Neurol. 2009;66(11):1400–6. doi: 10.1001/archneurol.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]