Abstract
Background
Worsening renal function (WRF) commonly complicates the treatment of acute decompensated heart failure. Despite considerable investigation in this area, it remains unclear to what degree WRF is a reflection of treatment versus patient related factors. We hypothesized that if WRF is significantly influenced by factors intrinsic to the patient than WRF during an index hospitalization should predict WRF during subsequent hospitalization.
Methods
Consecutive admissions to the Hospital of the University of Pennsylvania with a discharge diagnosis of congestive heart failure were reviewed. Patients with >1 hospitalization were retained for analysis.
Results
In total 181 hospitalization pairs met the inclusion criteria. Baseline patient characteristics demonstrated significant correlation between hospitalizations (p≤0.002 for all) but minimal association with WRF. In contrast, variables related to the aggressiveness of diuresis were weakly correlated between hospitalizations but significantly associated with WRF (p≤0.024 for all). Consistent with the primary hypothesis, WRF during the index hospitalization was strongly associated with WRF during subsequent hospitalization (OR=2.7, p=0.003). This association was minimally altered after controlling for traditional baseline characteristics (OR=2.5, p=0.006) and in-hospital treatment related parameters (OR=2.8, p=0.005).
Conclusions
A prior history of WRF is strongly associated with subsequent episodes of WRF, independent of in-hospital treatment received. These results suggest that baseline factors intrinsic to the patient’s cardiorenal pathophysiology have substantial influence on the subsequent development of WRF.
Introduction
Worsening renal function (WRF) complicates the treatment of acute decompensated heart failure (ADHF) in approximately one-third of hospital admissions and has been associated with adverse clinical outcomes including increased mortality.1-8 Despite substantial study of this topic, little is known about the pathophysiologic basis for this phenomenon. Furthermore, multiple studies have demonstrated patient characteristics, hemodynamic parameters, and treatment modalities previously assumed causal to have no association or even an inverse association with WRF.9-13
It remains unclear to what degree WRF is a phenomenon driven by the adverse effects of treatment and/or factors intrinsic to the patient that may have gone unmeasured in prior studies. We hypothesized that if there are in fact specific factors which are intrinsic to the patient (both measured and unmeasured) predisposing to WRF, then WRF during an index ADHF hospitalization should be strongly associated with development of WRF during subsequent hospitalization. We also hypothesized that by employing a repeated measures design, which reduces subject heterogeneity since known and unknown patient related factors are relatively stable between admissions, the signal to noise ratio would improve and potentially allow further delineation of treatment related characteristics responsible for WRF.
Methods
Consecutive admissions from 2004 to 2009 to the cardiology and internal medicine services at the Hospital of the University of Pennsylvania with a primary discharge diagnosis of congestive heart failure were reviewed. Inclusion required an admission B-type natriuretic peptide (BNP) level >100 pg/mL within 24 hours of admission, a length of stay 3 to 14 days, and admission and discharge serum creatinine values. Exclusion criteria included renal replacement therapy or admission to interventional cardiology services (to avoid confounding from contrast nephropathy). Patients with >1 hospitalization were retained in the dataset and the first two consecutive admissions were used for analysis. Estimated glomerular filtration rate (GFR) was calculated by the Modified Diet and Renal Disease equation and WRF was defined as a ≥20% decrease in GFR at any time during the hospitalization.14, 15 Hemoconcentration was defined as the relative percentage increase in hematocrit from admission to peak value during a given hospitalization. Doses of spironolactone ≥50 mg were defined as natriuretic doses. For analyses where a dichotomous variable was necessary, continuous variables (total intravenous loop diuretic dose, peak intravenous loop diuretic dose, net fluid output) were dichotomized about the median value. Odds ratios for age, systolic blood pressure, heart rate, glomerular filtration rate, blood urea nitrogen, and ejection fraction are expressed as a per 10 unit increase, loop diuretic dose per 100 mg increase excluding total loop diuretic which is expressed as per 1000 mg, and B-type natriuretic peptide per 500 pg/mL increase. All other values are reported as per 1 unit increase.
Statistical Methods
Values reported are mean ± standard deviation and percentile unless otherwise noted. Correlations reported are Pearson’s for normally distributed variables, Spearman’s for non-normally distributed variables, and Phi for dichotomous variables. To evaluate the association between prior WRF and the subsequent incidence of WRF, logistic regression using backward selection (likelihood ratio) was employed using WRF during the second hospitalization as the dependent variable and WRF during the index hospitalization as an independent variable. Candidate baseline characteristic covariates were obtained by screening all baseline variables and those with a univariate association with WRF (p≤0.2) were entered in the model. Interaction was formally tested using logistic regression by incorporating terms for the main effect of WRF during the first hospitalization, the main effect of the variable of interest, and the interaction between these variables. All covariates in the above mentioned logistic regression models, aside from WRF during the index hospitalization, represent variables from the second admission. To address the influence of discharge medications on subsequent WRF episodes, discharge medications from the first hospitalization were assessed with respect to their association with WRF during the second hospitalization. All other WRF association analyses where correlated/clustered data were present (repeat hospital admissions with two possible WRF events), were evaluated using generalized estimating equations and reported as odds ratios. An unstructured correlation matrix was assumed for all generalized estimating equation analyses. Statistical analysis was performed with PASW Statistics version 18.0 (SPSS Inc., Chicago, Illinois) and significance defined as 2-tailed p<0.05.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. This work was supported in part by a National Institutes of Health T32 grant.
Results
In total, 181 pairs of hospital admissions met the inclusion criteria. Baseline characteristics of the population are presented in Table 1. Median time between hospitalizations was 175 days (inter quartile range 54 to 378). Worsening renal function occurred in 32.0% (n=58) of patients during the index hospitalization and 33.1% (n=60) of patients during subsequent hospitalization. Overall, baseline patient characteristics were significantly correlated between the two hospital admissions (Table 2). With the exception of inotrope administration, which demonstrated a moderate strength correlation, characteristics related to acute hospital treatment such as length of stay, net fluid output, adjuvant thiazide diuretic use, adjuvant natriuretic dose spironolactone use, and peak and total loop diuretic doses demonstrated very limited correlation between admissions (Table 2). Baseline medications demonstrated moderate strength correlations. However, discharge medications from the index hospitalization were highly correlated with baseline medications from the subsequent hospitalization (Table 2). Of note, the attending physician was different in 87.3% of hospital admission pairs.
Table 1.
Baseline characteristics and their association with worsening renal function
| Characteristics | Association with | ||
|---|---|---|---|
| Overall Cohort |
WRF OR (95% CI) |
p | |
| Demographics | |||
| Age (years) | 59.9 ± 16.0 | 0.9 (0.8 - 1.1) | 0.247 |
| Males | 48.1% | 1.2 (0.7 - 2.0) | 0.450 |
| Black race | 78.5% | 1.0 (0.6 - 1.8) | 0.910 |
| Medical History | |||
| Coronary artery disease | 47.5% | 0.8 (0.5 - 1.3) | 0.458 |
| Hypertension | 80.7% | 1.0 (0.5 - 1.7) | 0.880 |
| Diabetes | 48.1% | 0.9 (0.5 - 1.4) | 0.572 |
| Atrial fibrillation | 34.3% | 0.9 (0.5 - 1.5) | 0.642 |
| Admission Physical Exam | |||
| Systolic blood pressure (mm Hg) | 132.4 ± 28.0 | 1.1 (1.0 - 1.1) | 0.174 |
| Heart rate (bpm) | 88.6 ± 18.5 | 1.1 (1.0 - 1.2) | 0.143 |
| Respiration rate (breaths/min) | 20.6 ± 5.4 | 1.0 (1.0 - 1.0) | 0.767 |
| Crackles > 1/2 lung fields | 9.4% | 1.2 (0.6 - 2.5) | 0.644 |
| Moderate or severe edema | 17.3% | 1.2 (0.7 - 2.2) | 0.460 |
| Jugular venous pressure > 12 cm | 62.5% | 0.9 (0.6 - 1.6) | 0.830 |
| Laboratory Findings | |||
| Hemoglobin (g/dL) | 11.9 ± 2.0 | 1.1 (1.0 - 1.2) | 0.101 |
| Serum sodium (mEq/L) | 138.0 ± 11.1 | 1.0 (1.0 - 1.0) | 0.386 |
| Glomerular filtration rate (mL/min) | 59.6 ± 30.9 | 1.1 (1.1 - 1.2) | <0.001* |
| Serum creatinine (mg/dL) | 1.6 ± 0.9 | 0.6 (0.4 - 0.9) | 0.011* |
| Blood urea nitrogen (mg/dl) | 32.4 ± 24.8 | 0.8 (0.7 - 0.9) | 0.001* |
| B-type natriuretic peptide (pg/mL) | 1834 ± 1281 | 0.9 (0.8 - 1.0) | 0.070 |
| Left Ventricular Function | |||
| Ejection fraction (%) | 30.5 ± 20.5 | 1.0 (0.9 - 1.1) | 0.978 |
| Medications (Baseline) | |||
| Aspirin | 58.9% | 0.8 (0.5 - 1.3) | 0.347 |
| Calcium channel blocker | 20.4% | 1.1 (0.6 - 2.0) | 0.794 |
| β-Blocker | 80.7% | 0.9 (0.6 - 1.5) | 0.770 |
| ACE inhibitor / ARB | 71.8% | 0.9 (0.6 - 1.3) | 0.476 |
| Thiazide diuretic | 7.7% | 1.5 (0.6 - 3.6) | 0.346 |
| Loop diuretic (mg) † | 80 (40-160) | 1.1 (0.8 - 1.4) | 0.612 |
| Digoxin | 26.5% | 1.1 (0.7 - 1.8) | 0.689 |
| Spironolactone | 14.4% | 1.3 (0.7 - 2.4) | 0.421 |
| Medications (Discharge) | |||
| Aspirin | 60.6% | 1.0 (0.5-1.8) | 0.914 |
| Calcium channel blocker | 20.0% | 0.5 (0.2-1.2) | 0.114 |
| β-Blocker | 84.4% | 0.9 (0.4-2.1) | 0.771 |
| ACE inhibitor / ARB | 79.0% | 1.1 (0.5-2.4) | 0.817 |
| Thiazide diuretic | 7.8% | 0.8 (0.2-2.6) | 0.694 |
| Loop diuretic (mg) † | 80 (40-160) | 1.0 (0.7-1.4) | 0.923 |
| Digoxin | 25.6% | 0.8 (0.4-1.7) | 0.629 |
| Spironolactone | 14.4% | 1.6 (0.7-3.7) | 0.294 |
| Oral in hospital medications | |||
| Aspirin | 69.4% | 1.2 (0.7-1.9) | 0.554 |
| Calcium channel blocker | 26.0% | 0.9 (0.5-1.5) | 0.669 |
| β-Blocker | 86.7% | 0.6 (0.3-1.1) | 0.118 |
| ACE inhibitor / ARB | 79.0% | 1.2 (0.7-2.1) | 0.477 |
| Digoxin | 26.7% | 0.8 (0.5-1.3) | 0.382 |
| Acute Treatment Related Parameters | |||
| Length of stay (days) | 5 (3-7) | 1.1 (1.1 - 1.3) | <0.001* |
| Net fluid in/output (L) | 5.4 ± 6.1 | 1.1 (1.0 - 1.1) | 0.001* |
| Hemoconcentration | 45.8% | 1.8 (1.1 - 2.9) | 0.024* |
| Change in hematocrit (% from baseline) | 0.0 (2.3-0.0) | 0.9 (0.8 - 0.9) | 0.002* |
| Thiazide diuretic use | 21.0% | 4.2 (2.5 - 7.3) | <0.001* |
| Maximum 24 hour IV loop diuretic dose (mg)† |
120 (80-240) | 1.3 (1.1 - 1.6) | 0.005* |
| Total IV loop diuretic dose (mg)† | 320 (120-580) | 1.9 (1.2 - 3.0) | 0.004* |
| Inotrope use | 10.5% | 1.0 (0.5 - 2.0) | 0.991 |
| Natriuretic dose spironolactone | 13.0% | 2.3 (0.7 - 8.1) | 0.193 |
Descriptive statistics listed under “Overall Cohort” represent the baseline characteristics from the second hospitalization. Odds ratios for the association between discharge medications and WRF represent discharge medications form hospitalization #1 and the WRF event from hospitalization #2. All other odds ratios are derived using generalized estimating equations using data from both the first and second hospitalizations and are based on 2 potential WRF events. WRF: Worsening Renal Function, OR: Odds ratio. Odds ratios for age, systolic blood pressure, heart rate, glomerular filtration rate, blood urea nitrogen, and ejection fraction are expressed as a per 10 unit increase, loop diuretic dose per 100 mg increase excluding total loop diuretic which is expressed as per 1000 mg, and B-type natriuretic peptide per 500 pg/mL increase. All other values are reported as per 1 unit increase.
Indicates significant p value.
Values represent median (interquartile range).
Table 2.
Correlation between patient and treatment related parameters across the first and second hospitalization
| Characteristics | Correlation Coefficient |
p |
|---|---|---|
| Medical History | ||
| Coronary artery disease | 0.93 | <0.001* |
| Hypertension | 0.86 | <0.001* |
| Diabetes | 0.97 | <0.001* |
| Atrial fibrillation | 0.94 | <0.001* |
| Vital Signs | ||
| Systolic blood pressure | 0.66 | <0.001* |
| Heart rate | 0.41 | <0.001* |
| Laboratory Findings | ||
| Hemoglobin | 0.68 | <0.001* |
| Serum sodium | 0.53 | <0.001* |
| Glomerular filtration rate | 0.9 | <0.001* |
| Serum creatinine | 0.88 | <0.001* |
| Blood urea nitrogen | 0.81 | <0.001* |
| B-type natriuretic peptide | 0.72 | <0.001* |
| Left Ventricular Function | ||
| Ejection fraction † | 0.83 | <0.001* |
| Medications (Baseline to Baseline) |
||
| Calcium channel blocker | 0.57 | <0.001* |
| β-Blocker | 0.32 | <0.001* |
| ACE inhibitor / ARB | 0.31 | <0.001* |
| Thiazide diuretic | 0.29 | <0.001* |
| Loop diuretic | 0.46 | <0.001* |
| Digoxin | 0.64 | <0.001* |
| Spironolactone | 0.47 | <0.001* |
| Medications (Discharge to Baseline) |
||
| Calcium channel blocker | 0.71 | <0.001* |
| β-Blocker | 0.49 | <0.001* |
| ACE inhibitor / ARB | 0.67 | <0.001* |
| Thiazide diuretic | 0.23 | 0.002* |
| Loop diuretic | 0.5 | <0.001* |
| Digoxin | 0.73 | <0.001* |
| Spironolactone | 0.57 | <0.001* |
| Treatment Related Parameters | ||
| Length of stay | 0.13 | 0.079 |
| Net fluid output | 0.26 | 0.008* |
| Hemoconcentration | 0.10 | 0.25 |
| Change in hematocrit (% from baseline) |
0.08 | 0.37 |
| Thiazide diuretic use | 0.10 | 0.185 |
| Maximum 24 hour IV loop diuretic dose |
0.24 | 0.002* |
| Total IV loop diuretic dose | 0.18 | 0.019* |
| Inotrope use | 0.50 | <0.001* |
| Natriuretic dose spironolactone |
0.24 | 0.32 |
OR: Odds ratio. Discharge to baseline medication correlations represent the correlation between medications at discharge from the index hospitalization to medications at baseline from the second hospitalization.
Indicates significant p value.
Derived from the 58.6% of patients with different echocardiograms associated with each hospitalization.
Consistent with findings from prior studies, incident WRF was largely independent of traditional baseline patient characteristics with the notable exception of renal function (Table 1). Inpatient medications, aside from non-potassium sparing diuretics, were not significantly associated with WRF (Table 1). Similarly, discharge medications from the index hospitalization were not associated with WRF during subsequent hospitalization (Table 1). However, diuresis related treatment variables such as 24 hour maximum intravenous loop diuretic dose, total hospital stay intravenous loop diuretic exposure, and the use of adjuvant thiazide diuretics were associated with increased incidence of WRF (Table 1). Diuresis outcome related variables (net fluid output and hemoconcentration) were also significantly associated with WRF (Table 1). Patients with greater numbers of diuresis related variables consistent with aggressive diuresis had a progressively increased risk for WRF with each incremental variable (OR=1.5 per additional variable, 95% CI 1.2-1.8, p<0.001).
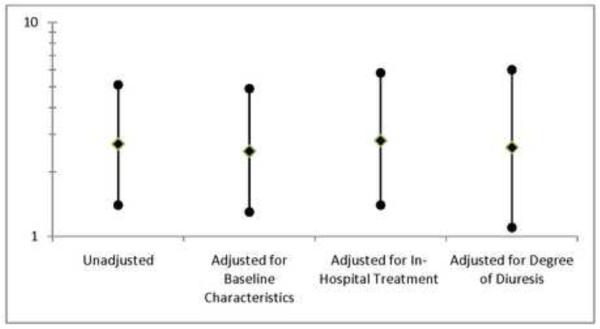
Given that patient characteristics were significantly correlated between admission pairs, whereas acute treatment variables were not, the ability of WRF at the index hospitalization to predict subsequent WRF was investigated as an overall surrogate for (both measured and unmeasured) predisposing patient related factors. WRF during the index hospitalization was significantly associated with subsequent WRF (OR=2.7, p=0.003, Figure 1). Controlling for in-hospital treatment (maximum IV loop diuretic dose given in 24 hours, total loop diuretic dose administered during admission, use of adjuvant thiazide diuretics, inotrope administration, calcium channel blockers, beta blockers, spironolactone, angiotensin converting enzyme inhibitors or receptor blockers, and digoxin use) did not impact the association between WRF during the index hospitalization and subsequent WRF (OR=2.8, p=0.005, Figure 1). Similarly, controlling for factors reflecting the degree of diuresis (net fluid output, length of stay, and hemoconcentration) did not significantly alter the association (OR=2.6, p =0.023, Figure 1). Likewise, controlling for baseline patient characteristics associated with WRF with a p<0.2 (heart rate, systolic blood pressure, glomerular filtration rate, b-type natriuretic peptide, and age) did not significantly alter the association (OR=2.5, p=0.006, Figure 1).
Figure 1.
Association between WRF during the index hospitalization with WRF during subsequent hospitalization. Adjustment for baseline patient characteristics includes: heart rate, systolic blood pressure, glomerular filtration rate, b-type natriuretic peptide, and age. In-hospital treatment: maximum IV loop diuretic dose given in 24 hours, total loop diuretic dose administered during admission, use of adjuvant thiazide diuretics, inotrope administration, calcium channel blockers, beta blockers, spironolactone, angiotensin converting enzyme inhibitors or receptor blockers, and digoxin use. Degree of diuresis: net fluid output, length of stay, and hemoconcentration. WRF: Worsening renal function.
To investigate the possibility that patients with a baseline predisposition to WRF may be more susceptible to deterioration in renal function caused by aggressive diuresis, the interaction between diuresis related variables and a prior history of WRF was formally tested. Although limited by power, no statistically significant interaction could be detected between a prior history of WRF and net fluid output (p=0.53), hemoconcentration (p=0.80), maximum intravenous loop diuretic dose (p=0.96), total intravenous loop diuretic dose (p=0.24), or adjuvant thaizide diuretic use (p=0.50) on the risk of subsequent WRF. Additionally, patients that did not receive adjuvant thiazide diuretics (OR=3.1, p=0.005) and those with values below the median for total loop diuretic dose (OR=3.1, p=0.024), peak loop diuretic dose (OR=2.4, p=0.053), net fluid output (OR=3.3, p=0.051) or degree of hemoconcentration (OR=3.1, p=0.024) demonstrated similar trends for the association between a prior history of WRF and subsequent WRF.
Discussion
The principal finding of this study is the strong association between a prior history of WRF and deterioration in renal function during subsequent hospitalization, independent of traditional baseline patient characteristics and treatment received. Given that in-hospital treatment variables were minimally correlated between hospitalizations, these results suggest that unmeasured patient characteristics have substantial influence on the development of subsequent WRF. Notably, this intrinsic predisposition to WRF appeared be independent of the aggressiveness of diuresis.
Given the substantial negative prognostic implications of WRF, understanding the causative mechanisms responsible for this phenomenon is paramount. Particularly, treatment implications are significantly different if WRF is primarily treatment induced rather than simply a reflection of an underlying patient predisposition to WRF. If WRF is solely caused by overaggressive fluid removal, this would suggest that conservative decongestion of ADHF patients might optimize survival, a strategy that would often be at odds with the overall treatment goals of the hospitalization potentially even increasing mortality.16 The current finding that a prior history of WRF is a risk factor for subsequent WRF, independent of treatment received, argues strongly against adverse treatment effects as the sole determinant of WRF. Additionally, it has been reported that an elevated baseline serum neutrophil gelatinase-associated lipocalin level, a marker of tubular injury, is predictive of subsequent WRF.17 These findings similarly support the notion that baseline cardio-renal pathology can predispose patients to WRF.
Given that both aggressive diuresis and a susceptibility to WRF intrinsic to the patient both appear to be risk factors for WRF, the question remains: if a patient has a baseline predisposition to WRF, is their risk for WRF ultimately dependent on overly aggressive fluid removal? If this is the case, and predisposed patients could be identified, judicious decongestion in this population might be advocated to improve outcomes. However, this scenario is not supported by the current data since controlling for multiple diuresis related factors minimally impacts the risk associated with a prior history of WRF. Additionally, no significant interaction between the risk associated with prior WRF and the risk associated with aggressive diuresis could be detected. Moreover, in patient subgroups with diuresis related parameters below the median (indicative of less aggressive diuresis), prior WRF was still significantly associated with subsequent WRF in the majority of cases.
These findings support the notion that WRF does not identify a homogeneous group of cardio-renal patients, a concept which was highlighted in a recent consensus document by the Acute Dialysis Quality Initiative (ADQI).18 Moreover, this concept was particularly well articulated in a seminal review by Ronco et al. stating “The mechanisms by which the onset of acute HF or acutely decompensated chronic HF leads to AKI are multiple and complex. The clinical importance of each mechanism is likely to vary from patient to patient.”19 The results of the current study provide data in support of the above concepts and further indicate that the generic use of WRF as an endpoint in clinical trials or clinical decision making may be less than ideal.
The direct clinical application of this study is knowledge that patients with a prior history of WRF are at increased risk for recurrent WRF during subsequent hospitalization, a risk which appears to be independent of the aggressiveness of diuresis. Perhaps more importantly, the results of this study raise the question whether a significant proportion of WRF related mortality is secondary the underlying pathophysiology/disease severity responsible for the patient’s cardio-renal fragility and subsequent susceptibility to WRF. Partial support for this hypothesis is provided by a recent sub-analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial where a strong association between surrogates for aggressive decongestion and improved survival was found, despite a substantially increased incidence of WRF in those aggressively diuresed.16 Although the specific factors responsible for this baseline predisposition to WRF cannot be delineated by the current analysis, the knowledge that novel/unmeasured risk factors likely exist and play a role in WRF should facilitate research specifically designed to investigate these factors.
Limitations
The single center retrospective design of this study leads to several potential limitations. Although requisite for the design of the study, selection of patients that survived the index hospitalization and had a second hospital admission available may create a population that is not fully representative of WRF in the general ADHF population. Additionally, physicians were not blinded to changes in renal function or treatments that occurred during prior hospitalization and treatment may have been modified as a result. Although the relationship between in-hospital treatment across admissions was minimal and treatment was controlled for in multivariate models, it is possible that other unidentified treatment related variables were correlated which may exaggerate the influence of patient related factors on WRF. Similarly, the patient’s intrinsic predisposition to WRF is probably not entirely stable over time which may have lead to an underestimation of the effect of a prior history of WRF on subsequent WRF. The evaluation for interaction is significantly limited by power and can exclude only large effect sizes. The primary goal of this exploratory/hypothesis generating study was to provide a comprehensive evaluation of all possible factors related to repeat episodes of WRF which necessitated evaluation of a large number of variables without adjustment for multiple comparisons. However, at the expense of minimizing type II error, this approach may have lead to type I error due to the multiple comparisons.
Conclusion
The phenomenon of WRF during the treatment of acute decompensated heart failure appears to be influenced by both the aggressiveness of diuresis and predisposing factors intrinsic to the patient. However, a baseline susceptibility to WRF does not appear to simply indicate sensitivity to aggressive diuresis. These results raise the possibility that WRF represents more than one distinct clinical entity with different underlying pathophysiologic mechanisms. Future research is necessary to further delineate potentially different subtypes of WRF and to determine if clinical outcomes or treatment implications are different between these groups.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
All authors have nothing to disclose.
References
- 1.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, Burger AJ, Elkayam U. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am. J. Cardiol. 2004;94:957–960. doi: 10.1016/j.amjcard.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 2.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B, Investigators P Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of the prospective outcomes study in heart failure (posh) Eur. Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ, investigators C Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure : Results from the coordinating study evaluating outcome of advising and counseling in heart failure (coach) European journal of heart failure: journal of the Working Group on Heart Failure of the European Society of Cardiology. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 4.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J. Card. Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Logeart D, Tabet J, Hittinger L, Thabut G, Jourdain P, Maison P, Tartiere J, Solal A. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int. J. Cardiol. 2008;127:228–232. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: Clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.van Kimmenade RR, Januzzi JL, Jr., Baggish AL, Lainchbury JG, Bayes-Genis A, Richards AM, Pinto YM. Amino-terminal pro-brain natriuretic peptide, renal function, and outcomes in acute heart failure: Redefining the cardiorenal interaction? J. Am. Coll. Cardiol. 2006;48:1621–1627. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am. Heart J. 2004;147:331–338. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Klein L, Massie BM, Leimberger JD, O’Connor CM, Pina IL, Adams KF, Jr., Califf RM, Gheorghiade M. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: Results from the outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (optime-chf) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 11.Mullens W, Abrahams Z, Francis GS, Skouri HN, Starling RC, Young JB, Taylor DO, Tang WH. Sodium nitroprusside for advanced low-output heart failure. J. Am. Coll. Cardiol. 2008;52:200–207. doi: 10.1016/j.jacc.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 12.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: Insights from the escape trial. J. Am. Coll. Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (ngal) in predicting worsening renal function in acute decompensated heart failure. J. Card. Fail. 2010;16:49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronco C, McCullough PA, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House A, Katz NM, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardiorenal syndromes: An executive summary from the consensus conference of the acute dialysis quality initiative (adqi) Contrib. Nephrol. 2010;165:54–67. doi: 10.1159/000313745. [DOI] [PubMed] [Google Scholar]
- 19.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]