Abstract
Exposure to cigarette smoke (CS) was shown to impair the capacity of macrophages to clear bacteria and apoptotic cells. Here, we show that both the exposure of macrophages to cigarette smoke extract (CSE) in vitro and an acute single exposure to CS in vivo impair the macrophage clearance of apoptotic polymorphonuclear leukocytes (PMNs). Upon longer periods of exposure to smoke in vivo (4–12 weeks), the impaired capacity of macrophages to clear apoptotic cells persisted after the cessation of smoking, with slow recovery to normality observed 4 weeks later. With respect to the mechanism by which CS impairs the macrophage uptake of apoptotic PMNs, we did not detect altered surface expression of receptors associated with apoptotic cell clearance. We did observe the impaired phosphorylation of the guanine nucleotide exchange factor Vav1 and the downstream inhibition of Ras-related C3 botulinum toxin substrate 1 (Rac1) activation. Consistent with these findings, CS impaired the macrophage cytoskeletal changes observed after stimulation with apoptotic cells. A loss of actin occurred at the leading edge, manifested as impaired ruffling of the cell membrane and a decreased capacity to engulf apoptotic cells. The inability to clear PMNs would lead to a greater release of destructive PMN products, and would diminish the reparative phenotype induced by the macrophage clearance of apoptotic cells.
Keywords: cigarette smoke, alveolar macrophage, phagocytosis and engulfment of apoptotic cells, actin rearrangements, small GTP protein Rac1
CLINICAL RELEVANCE.
This research demonstrated that alveolar macrophages exposed to cigarette smoke (CS) are not efficient in the clearance of apoptotic neutrophils. We found that CS impaired the macrophage cytoskeletal changes observed after stimulation with apoptotic cells, and needed for the engulfment of apoptotic cells. The defective activation of small GTPase Rac was responsible for the defect in actin rearrangement after challenge with CS. Knowledge of the mechanism by which CS affects the clearance of apoptotic cells will contribute to our understanding of the development of disease, and will lead to the design of new approaches to treat chronic obstructive pulmonary disorder.
The inhalation of cigarette smoke (CS) leads to cell injury and death as well as inflammation in neutrophils, macrophages, and T cells, all resulting in the lung destruction and airspace enlargement that defines emphysema, a major component of chronic obstructive pulmonary disorder (COPD). Although the macrophage is strongly implicated in the pathogenesis of COPD via the regulation of the immune response and the production of destructive proteinases, macrophages also serve as the major constitutive professional phagocyte in the lung, responsible for the removal of microorganisms and apoptotic cells. The efficient removal of short-lived apoptotic neutrophils by macrophages prevents secondary necrosis and the release of destructive neutrophil proteinases and oxidants into the extracellular milieu that would worsen the emphysema. In addition, the removal of apoptotic structural cells from the lung by alveolar macrophages promotes the production of anti-inflammatory cytokines (1, 2), antiproteinases (3), and growth factors that promote repair (4, 5).
Evidence exists in cell cultures that the extracellular matrix, modified by CS, interferes with the macrophage uptake of neutrophils (6). Moreover, studies of cell cultures showed that acute exposure of macrophages to CS results in a decreased ability to phagocytose epithelial cells (7), microorganisms (8), and polystyrene beads (9, 10) compared with non–smoke-exposed macrophages. The ability of alveolar macrophages to engulf apoptotic cells was impaired by smoke inhalation in animal models (11). Reversal of the effects of CS on the ability of macrophages to phagocytose apoptotic cells or microorganisms was delayed in proportion to the chronicity of smoke exposure (10, 11).
The small GTPase, Rac, obligatory for the engulfment of apoptotic targets, acts by regulating the rearrangement of the actin cytoskeleton (12). Signaling through another small GTPase of the Rho family, RhoA, inhibits the engulfment of apoptotic cells by promoting stress fiber formation and inhibiting the ruffling of the membrane at the leading edge. Rac antagonizes RhoA activity and ensures the presence of one leading edge by negatively regulating RhoA. The activation of RhoA was shown to be triggered by exposure to CS and oxidants (11, 13, 14).
The purpose of this study was to define in more detail the effects of CS exposure on the capacity of the macrophage to remove apoptotic neutrophils, and the mechanisms responsible for impaired neutrophil uptake. We assessed the effects of both acute and chronic exposure to CS in vitro and in vivo on the ability of macrophages to engulf apoptotic neutrophils. We also explored the mechanism of how CS impairs phagocytosis, by measuring the activity of the Rac1 pathway in CS-exposed macrophages and by studying the effects of CS on actin dynamics in macrophages.
MATERIALS AND METHODS
Mice
We used 8–12-week-old C57BL/6 female mice. All mice were treated according to approved protocols.
Cell Isolation
Cell isolation was performed as described in the online supplement (15, 16).
Induction of Apoptosis and Labeling of Human Polymorphonuclear Leukocytes
Human polymorphonuclear leukocytes (hPMNs) were irradiated with ultraviolet light (17), labeled with Cell-Tracker Green (CTG; Molecular Probes, Eugene, OR), and incubated 3 hours before instillation into the trachea (Figure E2 in the online supplement).
Cigarette Smoke Extract
Cigarette smoke extract (CSE) was prepared as described in the online supplement.
In Vitro Phagocytosis
We treated human monocyte-derived macrophages (hMDMs) with varying concentrations of CSE for designated times, washed and co-incubated with apoptotic hPMNs. Internalized hPMNs were detected by myeloperoxidase staining within macrophages, with hematoxylin as a counterstain. We analyzed 500 hMDMs per sample under the microscope, to calculate phagocytosis.
Acute Exposure to CS and Phagocytosis In Vivo
Mice were exposed, or not exposed, to one cigarette (18). CTG-labeled apoptotic hPMNs were intratracheally installed at the indicated times after exposure to CS (Figure E3). Phagocytosis was quantified using FACS.
FACS Analysis
Cells obtained by bronchoalveolar lavage (BAL) were analyzed by FACS, as described in the online supplement.
Phagocytosis of Multiple hPMNs
Apoptotic hPMNs were stained with CTG or Cell Trace-Far-Red (Molecular Probes), mixed in equal amounts, and instilled intratracheally to CS-exposed and control animals. Two hours later, cells from the BAL were analyzed by FACS.
Long-Term Exposure to CS
Mice were exposed to two cigarettes, twice daily, 5 days a week for 1, 4, or 12 weeks. For some groups, smoking was discontinued for recovery periods. The ability of alveolar macrophages to clear apoptotic hPMNs was analyzed by FACS and myeloperoxidase staining.
Preparation of Liposomes
The preparation of liposomes was performed as described in the online supplement (19).
Vav1 Phosphorylation
Differentiated hMDMs were exposed or not exposed to CSE. Mouse leukaemic monocyte macrophage cell line Raw264.7 were exposed or not exposed to CSE, and were co-incubated or not co-incubated with phosphatidylserine (PC) or phosphatidylserine (PS) liposomes. Cells were lysed in Phosphosafe extraction reagent (Novagen, Darmstadt, Germany), with protease and phosphatase inhibitors (Sigma Chemical Co., St. Louis, MO). Vav was detected using antibodies to phosphorylated (AbCam, Cambridge, MA) and total (Cell Signaling, Danvers, MA) forms of the protein.
Rac Pull-Down
Raw264.7 were exposed to CSE, washed, co-cultured with 100 μM PS liposomes, and lysed in magnesium-containing lysis buffer with protease inhibitors (Sigma Chemical Co.). We used 500–750 μg of proteins for Rac1 pull-down (Millipore, Billerica, MA). Rac1–GTP was visualized with anti-Rac1 antibodies (Millipore).
Real-Time and Confocal Microscopy
Raw264.7, transfected with actin–green fluorescence protein (GFP) (Clontech, Mountain View, CA), were plated on glass-bottomed dishes (MatTek), cultured for 40–48 hours, and moved to a temperature-controlled stage. Dynamics of actin at the leading edge were visualized with an Olympus inverted microscope. Pairs of DICs and fluorescence images were collected every 1 minute, using time-lapse spinning video microscopy (Ultraview; Perkin Elmer).
Raw264.7 were incubated with or without CSE, washed and incubated with or without PS liposomes or apoptotic neutrophils, fixed, and stained with rhodamine–phalloidin (Molecular Probes). Confocal images were obtained using an Olympus Fluoview FV1000 (MidAtlantic Diagnostic, Inc., Mount Laurel, NJ).
Final images and movies were produced using MetaMorph Software (MetaMorph Universal Imaging Corp., Downingtown, PA) and Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
Statistics
Phagocytosis and surface-molecule expression were compared between groups using the Student t test, and among groups using ANOVA followed by the Bonferroni test. Changes in phagocytosis over time in vitro and in vivo were analyzed as an intergroup comparison, using the Bonferroni test. The correlation of macrophage phagocytosis according to the two methods was assessed, using a single regression test.
RESULTS
Acute Exposure to CS Impairs the Uptake of Apoptotic PMNs by hMDMs In Vitro
To assess the effects of acute cigarette smoking on the uptake of apoptotic PMNs by CS-exposed macrophages, we isolated human monocytes and allowed them to differentiate into hMDMs by maintaining them in culture for 8–10 days in the presence of 20% autologous serum. Upon differentiation, hMDMs were exposed to increasing concentrations of CSE (0%, 2%, 5%, and 10%) for 24 hours, and their viability was assessed to ensure that CSE in the concentrations used in the study did not cause cell death (Figure E1A). Apoptotic PMNs were obtained by the ultraviolet irradiation of peripheral blood neutrophils, 3 hours after irradiation (∼ 60% of neutrophils were apoptotic; see Figure E2 for details and explanations).
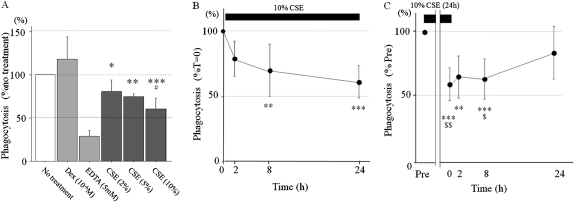
We co-incubated hMDMs with apoptotic PMNs for 2 hours, and the fraction of hMDMs that engulfed apoptotic hPMNs was determined. Exposure to CSE significantly decreased the uptake of apoptotic PMNs by hMDMs in a concentration-dependent manner (Figure 1A) (79.8%, 74.3%, and 60.3%, compared with no treatment; P < 0.01, P < 0.001, and P < 0.0001, respectively; see Table E1 for details). Next, we determined the effects of CSE duration on phagocytosis. We exposed hMDMs to 10% CSE for 2, 8, and 24 hours, and phagocytosis was determined. The ability of macrophages to phagocytose apoptotic PMNs was inversely proportional to the duration of exposure to CSE (Figure 1B and Table E2). To assess the reversibility of CSE's effects on the ability to engulf apoptotic cells, hMDMs were exposed to 10% CSE for 24 hours and allowed to recover for varying periods before co-incubation with apoptotic neutrophils. Figure 1C (see also Table E3) shows that phagocytosis was reduced to 60.3% of its baseline level after 24 hours of CSE treatment, and macrophages did not recover their clearance capacity during the first 8 hours of cigarette withdrawal. Overall, 24 hours after acute exposure to smoke, a statistically significant difference was no longer evident between smoke-exposed and control cells, suggesting a near full recovery of phagocytic capacity (Figure 1C and Table E3).
Figure 1.
Exposure to cigarette smoke (CS) decreases the ability of macrophages to engulf apoptotic cells in vitro. Human monocyte-derived macrophages (hMDMs) were allowed to differentiate in culture for 8–10 days. (A) hMDMs were then treated with indicated concentrations of cigarette smoke extract (CSE) for 24 hours. (B) hMDMs were treated with 10% of CSE for an indicated periods of time. (C) hMDMs were treated with 10% CSE for the first 24 hours an, then allowed to recover in CSE-free medium for indicated periods of time. After CSE treatment, hMDMs were co-incubated with 1.5 × 106 of apoptotic human polymorphonuclear leukocytes (hPMNs) for 1 hour, and hMDMs were fixed and stained for the myeloperoxidase (MPO) activity that results from ingesting apoptotic hPMNs. Phagocytosis was assessed by the presence of intracellular MPO staining in the 500 hMDMs inspected. Values are mean ± SD, representing the average of six duplicates of a representative experiment. *P < 0.01, **P < 0.001, and ***P < 0.0001 versus control group. #P < 0.01 versus CSE (2%). $P < 0.05 and $$P < 0.01 versus 24 hours. hMDMs treated with dexamethasone (10−6 M) for 24 hours before the phagocytosis assay served as a positive control, and hMDMs treated with EDTA (5 mM) during phagocytosis assay served as a negative control.
These studies demonstrate that the ability of hMDMs to phagocytize apoptotic neutrophils is compromised by acute exposure to CSE in vitro. Although the inhibitory effect of CSE was gradually reversible, recovery was slow, leaving the host impaired for a period of time during which further smoking would occur.
Detecting the Uptake of Apoptotic Neutrophils In Vivo by FACS Analysis
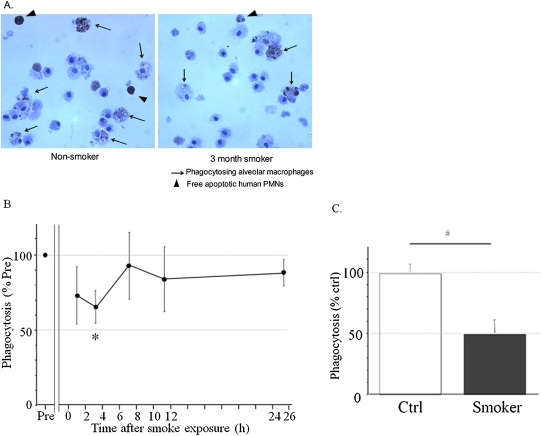
To determine whether CS affects the ability of alveolar macrophages to clear apoptotic PMNs in vivo, we used a murine model of apoptotic cell removal (20, 21), which allowed us to detect the clearance of intratracheally installed human apoptotic neutrophils in mice exposed or not exposed to CS. Apoptotic cells were delivered into murine lungs by intratracheal instillation, as previously described (20, 21), and the delivery of apoptotic neutrophils to the alveolar space was confirmed by their presence in BAL fluid as free apoptotic cells (Figure E4B), and as engulfed by alveolar macrophages detected in BAL cytospin slides (Figure 2A).
Figure 2.
Exposure to CS decreases the ability of macrophages to engulf apoptotic cells in vivo. (A) 1 × 106 apoptotic hPMNs labeled with Cell Tracker Green (CTG) were instilled intratracheally. Bronchoalveolar lavage (BAL) fluid was collected 2 hours later, and BAL cells were fixed and stained for CD11–PECy5. Part of the sample was used for FACS analysis, and the remaining 5 × 104 cells of each sample were used for cytospin. Slides were stained for myeloperoxidase (MPO) to visualize hPMNs. Representative photomicrographs of MPO-stained slides (magnification, ×1,000) are shown. Arrows indicate neutrophils internalized by alveolar macrophages. Arrowheads indicate free neutrophils. PMNs, polymorphonuclear leukocytes. (B) C57BL/6 mice were exposed to smoke from one cigarette. Then 1 × 106 labeled apoptotic neutrophils were instilled at indicated times after exposure to CS. BAL fluid was collected 2 hours later, and phagocytosis in CS-exposed animals was compared with phagocytosis in non–smoke-exposed controls (Pre) by FACS. (C) CTG-labeled apoptotic hPMNs were instilled immediately after smoke exposure. BAL fluid was obtained 6 hours later to allow additional time for engulfment, and was used to determine phagocytosis, as described in A. Values are mean ± SD, and represent average of findings from four mice. *P < 0.05 versus Pre.
To determine the optimal dose and time to evaluate the clearance of apoptotic cells, BAL was performed at different time points after the intratracheal installation of different numbers of apoptotic PMNs (Figure E3). The time point of 2 hours after the instillation of 10 × 106 apoptotic cells was chosen for further analysis, unless otherwise indicated. FACS analysis was used to assay large number of cells in multiple samples.
The population of macrophages was distinguished by CD11 staining (Figure E4A). Apoptotic hPMNs did not react with anti-mouse CD11, and present as a population of small negative cells, whereas macrophages represent a population of large positive cells (Figure E4B) Therefore, CD11-positive cells (FL3) were further analyzed for the presence of the CTG label (FL1), and the percentage of phagocytosis was calculated as the ratio of double-positive cells to CD11-positive cells (Figures E4C and E4D).
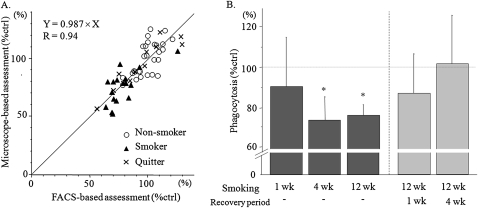
Because alveolar macrophages possess intrinsic fluorescence, which increases with exposure to CS (22, 23), we validated the FACS technique against conventional microscope–based myeloperoxidase (MPO) staining microscopy. The correlation is shown in Figure 4A. The correlation coefficient was 0.94. Based on these results, FACS analysis was used for further experiments.
Figure 4.
Chronic cigarette smoking impairs the ability of alveolar macrophages to engulf apoptotic neutrophils in vivo. Adult C57Bl/6 female mice were exposed to two cigarettes, twice a day, 5 days a week, for indicated periods of time, followed by the 2-hour phagocytosis assay performed 24 hours after the final exposure to CS. After 12 weeks of smoking, mice were allowed to recover for 1 or 4 weeks before the phagocytosis assay was performed. (A) To ensure that autofluorescence did not interfere with our analysis, we compared the engulfment of apoptotic neutrophils by alveolar macrophages assayed by FACS with traditional MPO-staining followed by microscopy. Both assays yielded very similar results. (B) Percentages of macrophages engulfing apoptotic neutrophils in different groups of animals were determined by FACS and compared with age-matched, nonsmoking shams. Values are mean ± SD, and represent the average of 4–6 experiments for each time point. *P < 0.01 versus age-matched, nonsmoking shams.
Acute Exposure to CS Impairs Clearance of Apoptotic PMNs by Macrophages In Vivo
To determine whether CS affects the ability of alveolar macrophages to clear apoptotic PMNs in vivo, naive C57BL/6J female mice were acutely exposed to one cigarette in our smoking chamber. At 2, 8, and 24 hours after smoke inhalation, apoptotic PMNs were instilled intratracheally, and 2 hours later, phagocytosis was assessed in vivo by FACS analysis of BAL, as already described (Figure E4). Phagocytosis in smoke-exposed mice was impaired to a nadir of 64% of that in non–smoke-exposed control mice when apoptotic PMNs were instilled 2 hours after exposure to smoke (P < 0.05). Macrophage phagocytic capacity slowly recovered over 24 hours (Figure 2B and Table E4).
In additional experiments, apoptotic hPMNs were instilled immediately after exposure to smoke, and macrophages were collected by BAL 6 hours later, to allow more time to interact with and engulf apoptotic cells. We found that only 21.1% of CS-exposed macrophages engulfed apoptotic hPMNs, compared with 43.7% of macrophages from non–smoke-exposed control macrophages (P < 0.0001; Figure 2C and Table E5).
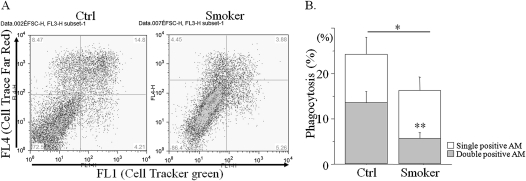
Macrophages are professional phagocytes that likely clear multiple targets at the same time. The number of engulfed target cells is a reflection of macrophage phagocytic capacity. To determine whether CS impairs the capacity to engulf multiple apoptotic PMNs, hPMNs were irradiated with ultraviolet light, labeled with two colors, and co-incubated with macrophages, and phagocytosis was scored by the presence of double-labeled cells inside the macrophage (Figure 3A). Consistent with the result shown in Figure 2B, the total fraction of macrophages containing single, apoptotic hPMNs was decreased after CS exposure to 69.5% of the level in control macrophages (16.0% versus 23.8%, respectively; P < 0.05). Moreover, the fraction of macrophages containing two or more PMNs (i.e., both colors) was even further reduced to 37.6% in smokers, compared with control nonsmokers (5.5% versus 13.3%, respectively; P < 0.01; Figure 3B), demonstrating that the effects of CS in vivo may be even more profound than those estimated via the assessment of engulfment of a single cell.
Figure 3.
Exposure to CS decreases the ability of macrophages to engulf multiple apoptotic targets in vivo. C57BL/6 mice were exposed to smoke from one cigarette. Two hours later, equal amounts of hPMNs stained with CTG and Cell Trace-Far-Red (CTFR) were instilled intratracheally. In 2 hours, macrophages were obtained by BAL, fixed, and stained for CD11 (with PE–Cy5.5 conjugated anti-mouse CD11c antibodies). (A) Macrophages were gated for FSChighFL3(CD11c)high and analyzed for fluorescein (FL1, CTF) and rhodamine (FL4, CTFR). (B) The percentage of macrophages containing single-colored (either FL1highFL4low or FL1lowFL4high) and double-colored apoptotic cells (FL1highFL4high, gray) were calculated from A, where AM refers to alveolar macrophages. Values are mean ± SD, and represent the average of four experiments, where *P < 0.05 and **P < 0.01 versus Ctrl.
Long-Term Exposure to CS Impairs the Ability of Alveolar Macrophages to Clear Apoptotic Neutrophils In Vivo
We tested the effects of chronic cigarette smoking on the ability of alveolar macrophages to clear apoptotic neutrophils in our model of exposure to CS. Mice were exposed to smoke from two cigarettes, twice a day, for 5 days a week, for 1, 4, or 12 weeks. Twenty-four hours after the last exposure to smoke, apoptotic PMNs (stained with fluorescein) were installed intratracheally, and alveolar macrophages were obtained by BAL 2 hours later and analyzed for phagocytosis. Phagocytosis was initially analyzed by two methods: FACS and conventional microscopy (using MPO) to assure that autofluorescence, a result of chronic exposure to CS, did not affect the results obtained by FACS (as already explained). As shown in Figure 4A, both methods yielded similar results (r = 0.94), and confirmed that the autofluorescence of CS-exposed macrophages did not alter our ability to estimate fluorescein-labeled apoptotic PMNs using FACS.
One week of exposure to CS transiently impaired the ability to clear apoptotic cells, but clearance normalized by 24 hours after the last exposure to CS (Figure 4B and Table E6). However, after 4 weeks of exposure to CS, a significant defect was evident in the capacity of macrophages to clear apoptotic neutrophils, even 24 hours after the last exposure to smoke (76% in smoker macrophages vs. nonsmoker macrophages, respectively; P < 0.001; Figure 4B). This effect was maintained but not further enhanced with 12 weeks of exposure to CS.
To assess the reversibility of the CS-induced impairment of macrophage clearance of apoptotic neutrophils after 12 weeks of exposure to CS, smoking was discontinued for 1 and 4 weeks. Mice were then analyzed for the capacity of their alveolar macrophages to engulf apoptotic PMNs. As shown in Figure 4B, after smoking had ceased for 1 week, macrophages partially recovered their ability to clear apoptotic PMNs, whereas the cessation of smoking after 4 weeks resulted in normal phagocytosis (cessation of smoking, 102%, versus nonsmoking, 100%; Figure 4B). Therefore, the effect of CS on the ability of alveolar macrophages to clear apoptotic cells was eventually reversible, even after prolonged cessation of CS in our model.
Exposure to CS Results in Decreased Vav1 Phosphorylation and Rac1 Activation in Macrophages
To determine the molecular basis for the CS-related impairment in the ability of macrophages to clear apoptotic cells, we first assessed the cell-surface expression of nine receptors previously shown to participate in the recognition of apoptotic cells: CD14, CD31, CD32, CD36, CD44, integrin αvβ5, β3, CD91, and Mer tyrosine kinase (MerTK). No effect on the expression of any of them was observed in response to CS (Figure E5).
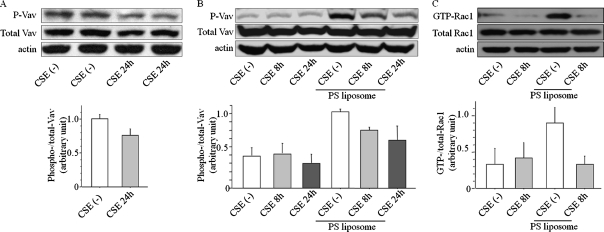
Next, we analyzed early steps in signaling pathways downstream of the apoptotic receptor. After cell-surface interactions with macrophages through apoptotic “cell removal receptors,” apoptotic cells are internalized, triggering a diversity of signals that lead to a rearrangement of the actin cytoskeleton, a step controlled by small GTPase Rac of the Rho/Rac family. We hypothesized that CS can impair guanine diphosphate to guanine triphosphate exchange in small GTPase Rac by inhibiting one of Rac1 guanine nucleotide exchange factor (GEF)–Vav1. We concentrated on Vav1 because MerTK–Vav–Rac1 is one of a few established pathways to mediate the recognition of PS in apoptotic cells (24–26). Because Vav1 is activated by tyrosine phosphorylation, we tested the effect of CS on the phosphorylation of Vav1 in hMDMs. We exposed hMDMs to 10% CSE in vitro for 24 hours, lysed them, and analyzed them for the presence of phosphorylated Vav1. Figure 5A demonstrates that nontreated hMDMs have high basal concentrations of the phosphorylated form of Vav1. This basal concentration (before apoptotic stimulus) was diminished by the addition of CSE.
Figure 5.
Exposure to CS affects Vav1 phosphorylation and Rac1 activation. (A) Differentiated hMDMs were exposed to 10% CSE or control fluid for 24 hours, and then whole-cell lysates were prepared and probed for the presence of the phosphorylated form of Vav1 protein (P-Vav). Total Vav1 and actin were used as loading controls. Below: Quantification of four separate experiments. (B) Raw264.7 macrophage cells were pretreated with 10% CSE for 8 or 24 hours. CSE was washed out, and cells were challenged with phosphatidylserine (PS) liposomes for 60 minutes in fresh Dulbecco's modified Eagle's medium (DMEM). Cell lysates were tested for the presence of phosphorylated Vav1. Total Vav1 and actin were used as loading controls. Below: Quantification of three independent experiments. (C) Raw264.7 cells were pretreated with 10% CSE for 8 hours, and incubated with PS liposomes for 1 hour in fresh DMEM. The active GTP-bound form of Rac1 protein was isolated by pull-down assay and compared with total Rac1, and with actin. Below: Quantification of three separate experiments.
We pursued this finding using the macrophage cell line, Raw 264.7, that exhibits a lower basal activity of Vav1, simplifying the analysis (particularly in detecting upregulation with apoptotic stimuli) (Figures 5B and 5C). Raw cells were challenged with PS-containing liposomes in the presence or absence of CSE (see Figure E1B for Raw cells' viability after CSE). The addition of PS to liposomes mimics the cell surface of apoptotic cells, which contain a negatively charged PS, recognized by phagocytic receptors on macrophages. The incubation of non–smoke-exposed Raw cells with PS liposomes induced Vav1 phosphorylation. The PS-induced Vav1 phosphorylation was inhibited by pretreatment with CSE for 8 and 24 hours (Figure 5B).
We next tested Rac activity in Raw cells challenged with PS liposome in the absence and presence of CSE. The active, GTP-bound form of Rac1 was easily detectable after cells were co-incubated with PS liposomes (Figure 5C). Pretreatment of cells with CSE for 8 hours completely abolished the formation of the active GTP-bound form of Rac1 (Figure 5C). The total amount of Rac1 loaded on the gel was similar among groups, indicating that GDP–GTP exchange was inhibited by CSE.
Actin Rearrangement Induced by Apoptotic Cells Is Inhibited by CS in Macrophages
Because we showed that CS inhibits Rac1 activity, and Rac1 drives actin polymerization at the leading edge during the engulfment of apoptotic cells, we used spontaneously polarized Raw cells as a model system to assess the effects of CS on actin at the leading edge. Raw cells display spontaneous polarization when plated on glass and exhibit active ruffling of the leading edge membrane (27), thus representing an ideal model system for studying the cellular processes essential to leading-edge maintenance and signaling.
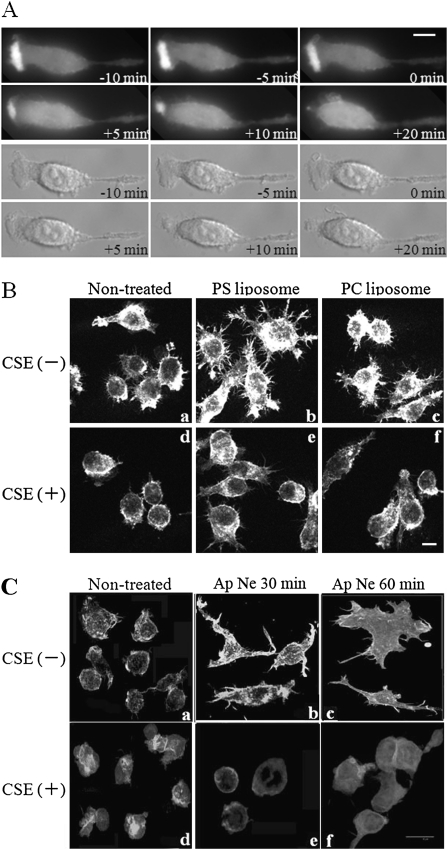
To visualize the effects of CS on actin dynamics, Raw cells were transfected with actin–GFP and polarized on glass-bottomed slides. Spontaneously polarized Raw cells were then incubated with or without CSE on a temperature-controlled stage. Actin rearrangement at the leading edge was followed by time-lapse video microscopy. Figure 6A (see also movie 1 in the online supplement) demonstrates that the formation of the leading edge was marked by the strong expression of actin–GFP at the polarized site of Raw cells. The leading-edge structure was stable and persisted for the entire 1 hour of imaging. The CSE treatment of cells caused a retraction of the leading edge, with a concomitant loss of GFP–actin from the retracting leading edge within 5–10 minutes of adding the CSE (Figure 6A; see also movie 1 in the online supplement). Cells stopped ruffling, and polarization was lost. Therefore, the effect of CSE on actin rearrangement at the leading edge was quick and consistent with the low Rac activity caused by exposure to CS.
Figure 6.
CSE inhibits actin rearrangements, membrane ruffling, and cell spreading in Raw264.7 macrophage cells. (A) Raw264.7 cells were transfected with actin–GFP and polarized on glass-bottomed slides. Afterward, spontaneously polarized Raw264.7 cells were incubated with or without 10% CSE on a temperature-controlled stage. Actin rearrangement at the leading edge was followed by time-lapse video microscopy. Top rows: Actin–GFP. Bottom rows: differential interference contrast microscopy (DIC). Time is shown relative to the addition of CSE. Scale bar = 5 μm. (B) Raw264.7 cells were either pretreated (d, e, and f) or not pretreated (a, b, and c) with 10% CSE for 8 hours, and challenged with either phosphatidylcholine (PC) liposomes or PS liposomes (the latter mimics the engulfment of apoptotic cells, leading to spreading and actin rearrangement. (C) Raw264.7 cells were incubated overnight in DMEM with 1% FBS, either pretreated (d, e, and f) or not pretreated (a, b, and c) with 10% CSE for 3 hours, and challenged with apoptotic neutrophils (Ap Ne) (5 × 105) for 30 minutes (b and e) or for 60 minutes in DMEM (c and f) and fixed. Actin was followed by staining with rhodamine–phalloidin. Images are a projection of six consecutive 0.3-μm optical sections. Scale bar = 10 μm.
To explore the effect of CS on actin dynamics further during the engulfment of apoptotic targets, Raw cells were either pretreated or not pretreated with CSE, and challenged with either PS liposomes or PC liposomes or with apoptotic hPMNs, to induce the actin rearrangement and lamellipodia formation seen during the engulfment of apoptotic cells. Cells were then fixed and stained with rhodamine phalloidin. The co-incubation of Raw cells with PS liposomes or with apoptotic human neutrophils, but not with PC liposomes, induced cell spreading and the formation of lamellipodia-like structures (Figures 6B, b, and 6C, b and c). CSE treatment inhibited both cell spreading and the formation of lamellipodia in cells challenged with both PS liposomes and apoptotic hPMNs (Figures 6B, e, and 6C, e and f). CSE-treated Raw cells were rounder and more shrunken, and had a more ridged cell cortex than control cells. All these findings are consistent with low Rac1 activity.
DISCUSSION
This study confirms in vivo that exposure to CS impairs the capacity of macrophages to engulf and clear apoptotic PMNs, and explores the molecular mechanisms responsible for this effect. We show that exposure of macrophages to CSE in vitro and an acute, single exposure to CS in vivo both impair the macrophage clearance of apoptotic PMNs to a similar degree. Because macrophages have the capacity to engulf multiple target cells, we also confirmed that exposure to CS led to fewer macrophages capable of clearing multiple apoptotic PMNs in vivo. The impaired macrophage phagocytic capacity largely reversed 24 hours after acute exposure to CS. However, upon longer duration of exposure to smoke in vivo (4–12 weeks), CS-exposed macrophages had only 75% of the phagocytic capacity of control macrophages, 24 hours after the discontinuation of smoking. The impaired capacity of macrophages to clear apoptotic cells gradually recovered to a normal level, but only after smoking had ceased for 4 weeks, which may reflect the recruitment of naive bone marrow–derived macrophages. With respect to the mechanism by which CS impairs the macrophage uptake of apoptotic PMNs, we were unable to find any effect on the cell-surface expression of apoptotic cell removal receptors, although we did observe the impaired phosphorylation of Vav1 with a downstream inhibition of Rac1 activation. Consistent with these findings, we found that CS impaired the macrophage cytoskeletal changes observed with the introduction of apoptotic cells. A loss of actin occurred at the leading edge, manifested as impaired ruffling of the cell membrane and a decreased capacity to engulf apoptotic cells.
Cigarette smoking clearly leads to oxidant stress and the proteolytic loss of matrix attachment with subsequent cell death, which contributes to the pathogenesis of emphysema. The clearance of apoptotic cells, however, may be beneficial. Macrophages respond to the clearance of apoptotic cells by generating anti-inflammatory factors such as TGF-β, IL-10 (1, 2), the antiproteinase serine leukocyte proteinase inhibitor (3), and reparative growth factors such as vascular epidermal growth factor (4), and hepatocyte growth factor (5). Moreover, if apoptotic PMNs are not cleared, apoptosis would turn into secondary necrosis, with a release of destructive proteinases such as neutrophil elastase and other harmful proteins and oxygen radicals that allow this professional phagocyte to eliminate pathogens.
Investigators have had a long-standing interest in the role of CS in the impairment of bacterial uptake by host cells. Studies in the early 1990s (8–10) showed that the inhalation of CS led to a transient impairment of alveolar macrophage uptake of latex beads or Candida albicans in mice. Richens and colleagues also found an impaired uptake of apoptotic cells by macrophages upon exposure to CS in vivo (11). That study focused primarily on apoptotic T cells, using a low-dose exposure for multiple hours per day in aged (1 year old) FvBN mice, as opposed to our study, focusing specifically on the clearance of apoptotic PMNs by applying a smoking protocol that represents a shorter burst of larger-dose smoke via the delivery of a burning individual cigarette (mimicking human exposure to CS) to young adult C57BL/6 mice. The protocol of Richens and colleagues (11) led to more permanent changes in macrophages, whereas ours led to slow recovery. It will be interesting to tease out the differences in models that could lead to the basis of potential epigenetic changes in COPD.
Moreover, our two approaches focused on different and complementary mechanistic aspects. Richens and colleagues found high activity of the RhoA pathway in cells exposed to CS (11). We report low Rac signaling because of the defect in activity of Rac GEF–Vav1. Rac was demonstrated to act upstream of Rho during the engulfment of apoptotic cells, and to counterbalance the activity of RhoA (28), which should be low during apoptotic cell uptake. Our finding of low Rac activity may provide a basis for the high Rho activity seen in smokers.
It is very tempting to expose cells in culture to CSE as a means of understanding the effects of smoke on cells in vivo. The translation of “liquid smoke” to in vivo situations is complicated by the different compositions of liquid extract and gaseous smoke, and by an inability to predict the appropriate concentrations and durations of exposure that relate to smoking. That said, it is the only way to distinguish the direct effects of smoke versus the potentially complicated indirect effects in vivo. Our study demonstrated a remarkable similarity in the type and magnitude of response between CSE conditions and conditions in vivo related to acute smoke exposure. Ten percent CSE for 24 hours did not result in impaired cell viability, and led to an approximately 50% decrement in macrophage phagocytic capacity, with near-full recovery over a 24-hour period. These effects were very similar to those observed in the macrophage clearance of apoptotic PMNs of a single cigarette in vivo (Figures 1C and 2B). The gradual return to normal was not fully complete until 24 hours, a time when most smokers will have “lit up” again, suggesting that smokers likely have continuous macrophage dysfunction.
We also used a two-color fluorescence assay of apoptotic cells to demonstrate not only that fewer macrophages took up a single cell after exposure to CS, but that CS impaired the ability of macrophages to take up multiple cells. This finding rules out the possibility that only a few macrophages are affected, with compensation by other macrophages that take up more PMNs (Figures 2B and 2C). Moreover, the two-color uptake assay emphasizes that the effect of CS on macrophage uptake may be more profound than was estimated by assessing the engulfment of a single cell or particle.
Chronic exposure to CS for 4 weeks was required to produce a prolonged impairment in macrophage uptake of apoptotic PMNs. When smoking was discontinued, this impairment lasted more than a week, such that only after 4 weeks of discontinued smoking was macrophage function entirely restored. Restoration could be attributed to the recovery of an individual cell. Alternately, newly recruited monocytes from the bloodstream may be responsible for the recovery. Thus, these are not permanent epigenetic effects on lung macrophages, or else lung macrophages are permanently impaired, but the defect does not extend to bone marrow–derived monocytes. The permanence in the results of Richens and colleagues (11) suggests that in some situations, epigenetic effects of CS on macrophages may indeed occur. Others reported that smokers who quit smoking for more than a year failed to recover macrophage phagocytic capacity (7), an outcome putatively attributed to the epigenetic effects of long-term smoking.
The recognition of apoptotic cells by macrophages begins with the cell-surface presentation of specific ligands by apoptotic cells. One of the earliest changes in the apoptotic cell involves the exposure of PS at the external leaflet of the plasma membrane. Several classes of receptors were implicated in the recognition of PS in apoptotic cells, either via direct contact or by indirect binding through bridging molecules. Hodge and colleagues reported a decrease in the cell-surface expression of CD31, CD44, and CD91 in alveolar macrophages from chronic smokers (7), whereas Kazeros and colleagues did not find any smoking-related decrease in the expression of 13 known apoptotic receptors (including CD68, CD14, CD31, and CD44), but they did detect an increase in expression of MerTK (29). This finding was interpreted to represent an unsuccessful attempt of alveolar macrophages to adjust to an increased demand for cell turnover in lungs of smokers. We did not detect significant changes in the expression of apoptosis-related receptors in hMDMs exposed to acute CSE in culture.
Multiple PS-dependent phagocytic receptors converge on an evolutionarily conserved signaling module that activates Rac1, leading to cytoskeletal remodeling with actin rearrangement, and the formation of lamellipodia resulting in the engulfment and clearance of apoptotic cells. With respect to the activation of Rac1, upon interactions of apoptotic cell PS with macrophage MerTK, Vav1 is phosphorylated, catalyzing the GDP–GTP exchange in Rac1, and setting in motion the cellular changes already described. We demonstrated that Vav1 is phosphorylated in Raw cells upon presentation with apoptotic targets. Exposure to CS inhibited Vav1 phosphorylation both at baseline and upon activation by apoptotic targets. Vav1 is not the only module through which MerTK and other receptors amplify intracellular signals leading to the activation of Rac1. Whether these pathways in addition to Vav1 are relevant in macrophages and are also impaired by CS exposure is unknown. Certainly the fact that Rac1 inhibition was not complete suggests that links remain intact.
Upon challenge with apoptotic targets after Rac1 activation and RhoA inhibition, as recently described by Richens and colleagues (11), we observed the expected classic cytoskeletal changes in macrophages, including actin rearrangement and the protrusion of lamellipodia. These cellular changes were rapidly and profoundly blocked upon incubation with CSE. For example, actin polymerization at the leading edge was greatly diminished 5 minutes after the addition of CS, and was completely lost after 10 minutes. In fact, cells lost their spread morphology and membrane ruffling at the leading edge. No longer polarized, the cells became round, with a very rigid actin cortex surrounding each cell (Figure 6).
In terms of clinical consequences, the CS-impaired uptake of apoptotic PMNs by macrophages would lead to the progression of emphysema. Although the macrophage recently gained notoriety through its participation in proteolytic destruction in COPD, the presence of macrophages is clearly important in removing short-lived, toxic PMNs. Moreover, as already discussed, the uptake of apoptotic cells transforms the macrophage into a reparative phenotype (30–32). Understanding the beneficial and harmful functions of inflammatory cells and how they interact will be important in minimizing pulmonary destruction and maximizing lung repair in COPD and other chronic lung diseases.
Supplementary Material
This work was supported by National Institutes of Health grant RO1 HL082541 (S.D.S.) and by a Japanese Respiratory Foundation Pfizer Fellowship (N.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0463OC on June 4, 2010
Author Disclosure: A.P.-B. received a joint American Thoracic Society/Alpha-1 Foundation research grant ($10,001–$50,000). N.M. received a sponsored grant from the Japanese Respiratory Foundation ($10,001–$50,000). S.D.S. has served on advisory boards for GlaxoSmithKline ($1,001–$5,000) and Boehringer ($1,001–$5,000).
References
- 1.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 2002;109:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odaka C, Mizuochi T, Yang J, Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J Immunol 2003;171:1507–1514. [DOI] [PubMed] [Google Scholar]
- 4.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 2004;18:1716–1718. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608–615. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham PA, Spooner G, Rahman I, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun 2004;318:32–37. [DOI] [PubMed] [Google Scholar]
- 7.Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2007;37:748–755. [DOI] [PubMed] [Google Scholar]
- 8.Ortega E, Barriga C, Rodriguez AB. Decline in the phagocytic function of alveolar macrophages from mice exposed to cigarette smoke. Comp Immunol Microbiol Infect Dis 1994;17:77–84. [DOI] [PubMed] [Google Scholar]
- 9.Ortega E, Hueso F, Collazos ME, Pedrera MI, Barriga C, Rodriguez AB. Phagocytosis of latex beads by alveolar macrophages from mice exposed to cigarette smoke. Comp Immunol Microbiol Infect Dis 1992;15:137–142. [DOI] [PubMed] [Google Scholar]
- 10.Higashimoto Y, Fukuchi Y, Ishida K, Shimada Y, Ohata M, Funasako M, Shu C, Teramoto S, Matsuse T, Sudo E, et al. Effect of chronic tobacco smoke exposure on the function of alveolar macrophages in mice. Respiration 1994;61:23–27. [DOI] [PubMed] [Google Scholar]
- 11.Richens TR, Linderman DJ, Horstmann SA, Lambert C, Xiao YQ, Keith RL, Boe DM, Morimoto K, Bowler RP, Day BJ, et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am J Respir Crit Care Med 2009;179:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimini G, Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat Cell Biol 2000;2:E191–E196. [DOI] [PubMed] [Google Scholar]
- 13.Chiba Y, Murata M, Ushikubo H, Yoshikawa Y, Saitoh A, Sakai H, Kamei J, Misawa M. Effect of cigarette smoke exposure in vivo on bronchial smooth muscle contractility in vitro in rats. Am J Respir Cell Mol Biol 2005;33:574–581. [DOI] [PubMed] [Google Scholar]
- 14.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, Bratton DL, Kang JL, Henson P. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol 2007;178:8117–8126. [DOI] [PubMed] [Google Scholar]
- 15.Boyum A. Isolation of mononuclear cells and granulocytes from human blood: isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest 1968;97:77–89. [PubMed] [Google Scholar]
- 16.McCarthy DA, Perry JD, Holburn CM, Kirk AP, James DW, Moore SR, Holborow EJ. Centrifugation of normal and rheumatoid arthritis blood on Ficoll-Hypaque and Ficoll-Nycodenz solutions. J Immunol Methods 1984;73:415–425. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol 2004;173:6319–6326. [DOI] [PubMed] [Google Scholar]
- 18.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Drake MT, Kornfeld S. ADP–ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc Natl Acad Sci USA 1999;96:5013–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1Q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 2002;169:3978–3986. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol 2006;176:7657–7665. [DOI] [PubMed] [Google Scholar]
- 22.Pankow W, Neumann K, Ruschoff J, von Wichert P. Human alveolar macrophages: comparison of cell size, autofluorescence, and HLA–DR antigen expression in smokers and nonsmokers. Cancer Detect Prev 1995;19:268–273. [PubMed] [Google Scholar]
- 23.Skold CM, Eklund A, Hallden G, Hed J. Autofluorescence in human alveolar macrophages from smokers: relation to cell surface markers and phagocytosis. Exp Lung Res 1989;15:823–835. [DOI] [PubMed] [Google Scholar]
- 24.Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, Landreth GE. Regulation of microglial phagocytosis and inflammatory gene expression by GAS6 acting on the AXL/MER family of tyrosine kinases. J Neuroimmune Pharmacol 2008;3:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001;411:207–211. [DOI] [PubMed] [Google Scholar]
- 26.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the MER receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 2007;109:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci USA 2007;104:16176–16181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol 2003;5:236–241. [DOI] [PubMed] [Google Scholar]
- 29.Kazeros A, Harvey BG, Carolan BJ, Vanni H, Krause A, Crystal RG. Overexpression of apoptotic cell removal receptor MERTK in alveolar macrophages of cigarette smokers. Am J Respir Cell Mol Biol 2008;39:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaykhiev R, Krause A, Salit J, Strulovici-Barel Y, Harvey BG, O'Connor TP, Crystal RG. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol 2009;183:2867–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weigert A, Brune B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide 2008;19:95–102. [DOI] [PubMed] [Google Scholar]
- 32.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J Immunol 2009;182:4415–4422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.