Abstract
Interstitial lung disease is a devastating disease in humans that can be further complicated by the development of secondary pulmonary hypertension. Accumulating evidence indicates that the oxidant superoxide can contribute to the pathogenesis of both interstitial lung disease and pulmonary hypertension. We used a model of pulmonary hypertension secondary to bleomycin-induced pulmonary fibrosis to test the hypothesis that an imbalance in extracellular superoxide and its antioxidant defense, extracellular superoxide dismutase, will promote pulmonary vascular remodeling and pulmonary hypertension. We exposed transgenic mice overexpressing lung extracellular superoxide dismutase and wild-type littermates to a single dose of intratracheal bleomycin, and evaluated the mice weekly for up to 35 days. We assessed pulmonary vascular remodeling and the expression of several genes critical to lung fibrosis, as well as pulmonary hypertension and mortality. The overexpression of extracellular superoxide dismutase protected against late remodeling within the medial, adventitial, and intimal layers of the vessel wall after the administration of bleomycin, and attenuated pulmonary hypertension at the same late time point. The overexpression of extracellular superoxide dismutase also blocked the early up-regulation of two key genes in the lung known to be critical in pulmonary fibrosis and vascular remodeling, the transcription factor early growth response–1 and transforming growth factor–β. The overexpression of extracellular superoxide dismutase attenuated late pulmonary hypertension and significantly improved survival after exposure to bleomycin. These data indicate an important role for an extracellular oxidant/antioxidant imbalance in the pathogenesis of pulmonary vascular remodeling associated with secondary pulmonary hypertension attributable to bleomycin-induced lung fibrosis.
Keywords: interstitial lung disease, extracellular superoxide, early growth response-1, transforming growth factor
CLINICAL RELEVANCE.
Interstitial lung disease is a devastating disease affecting adults and children. It can be further complicated by the development of secondary pulmonary hypertension. This study provides new insights into the role of extracellular oxidant/antioxidant imbalance in the development of pulmonary vascular remodeling in a murine model of bleomycin-induced interstitial lung disease with pulmonary hypertension.
Interstitial lung disease is a devastating disease in humans, in which morbidity and mortality can be further complicated by the development of secondary pulmonary hypertension (1). Different disease conditions can lead to lung fibrosis and secondary pulmonary hypertension. These diseases include idiopathic pulmonary fibrosis, connective tissue disorders such as scleroderma, and granulomatous diseases such as sarcoidosis. An important pathologic feature of the chronic forms of pulmonary arterial hypertension involves the development of marked pulmonary artery remodeling, which includes medial wall thickening, adventitial matrix deposition, and the formation of occlusive intimal lesions. The pathogenesis of interstitial lung diseases and pulmonary hypertension is incompletely understood, although a substantial body of literature in both fields implicates a role for oxidant/antioxidant imbalance in the development of these serious lung diseases.
The intratracheal administration of bleomycin to rodents has been extensively used to study the role of oxidative stress and inflammation in lung fibrosis (2–6). Reactive oxygen species can cause direct damage to cellular structures in the lung, or function as signaling molecules to regulate the expression of redox-sensitive genes that contribute to lung fibrosis (7). Recent reports indicate that in addition to producing lung fibrosis, bleomycin also causes pulmonary hypertension (6, 8, 9). A small number of studies implicated the reactive oxygen species superoxide, generated specifically in the extracellular compartment, in the pathogenesis of bleomycin-induced lung fibrosis. Extracellular superoxide is scavenged by the antioxidant enzyme extracellular superoxide dismutase (EC-SOD or SOD3). Bleomycin disrupts the normal distribution of EC-SOD in the extracellular matrix of the lung by activating proteases that cleave the heparan-binding domain of EC-SOD and lead to its release into the alveolar space (10). Mice lacking EC-SOD develop more severe bleomycin-induced lung fibrosis, compared with wild-type mice (11), whereas the overexpression of lung EC-SOD protects against bleomycin-induced lung fibrosis. The impact of EC-SOD on the development of bleomycin-induced pulmonary hypertension, however, is unknown (12).
Based on these observations, we hypothesized that transgenic mice overexpressing lung EC-SOD would be protected against the pulmonary vascular remodeling and pulmonary hypertension associated with bleomycin-induced lung fibrosis. We further hypothesized that the overexpression of EC-SOD would prevent the up-regulation of key genes known to be critical to the fibrotic response. We treated EC-SOD transgenic mice and their wild-type littermates with a single dose of intratracheal bleomycin, and evaluated mice up to 35 days after treatment. We performed detailed morphometric analyses to assess remodeling within each layer of the vessel wall. We evaluated whether the overexpression of EC-SOD blocked the up-regulation of two genes known to be critical to the development of fibrosis, transforming growth factor (TGF-β) and the redox-sensitive transcription factor early growth response (Egr-1). In addition, we tested for the development of pulmonary artery hypertension, and calculated survival for the two strains. The results of our study provide insights into the role of extracellular superoxide in the pathogenesis of pulmonary hypertension secondary to interstitial lung disease.
MATERIALS AND METHODS
Mouse Model
These animal studies were approved by the Institutional Animal Care and Use Committee of the University of Colorado. C57/BL6 mice overexpressing human EC-SOD in the lung (EC-SOD TG) or wild-type littermates (WT) received a single dose (0.1 unit) of intratracheal bleomycin (APP Pharmaceuticals, Schaumburg, IL) or PBS. Mortality was recorded, and mice were killed at 7, 14, 21, 28, or 35 days (see the online supplement for details).
Hemodynamic Measurements and Tissue Harvesting
Right ventricular systolic pressures (RVSPs) were obtained through a closed chest technique, as previously described (5). Lungs were flash-frozen or inflation-fixed. Additional lungs were also vascular-perfused and fixed with paraformaldehyde for morphometric analysis, according to published methods (13) (see online supplement for details).
Immunohistochemistry
For dual staining, lung slides were first stained with the mouse monoclonal α-smooth muscle actin (α-SMA) antibody (1:1,000, clone1A4; Sigma, St. Louis, MO) and Alexa 594 fluorescent secondary antibody (1:200; Invitrogen, Carlsbad, CA). The second antibody pairs were rabbit anti-mouse proliferating cell nuclear antigen (PCNA) antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and biotinylated anti-rabbit secondary antibody (1:100; Vector Laboratories, Burlingame, CA), with streptavidin 488 (1:2,000; Invitrogen) or rabbit anti-human/mouse von Willebrand factor (FVIII, 1:500; Sigma) and Alexa 488 fluorescent anti-rabbit secondary antibody (Invitrogen). Nuclei were counterstained with 4′6-diamidino-2-pheylindole (DAPI) (Vector Laboratories). Additional slides stained for α-SMA were developed with ImmPact DAB diluent (Vector Laboratories) (see online supplement for details).
Determination of Medial Wall Thickness
In sections stained with α-SMA antibody, four measurements of the perpendicular lumen radius and medial wall were determined in small muscularized arteries (<50 μm). Medial wall thickness (MWT) was expressed as the average MWT divided by the average vessel radius.
Collagen Deposition
Sections were stained with trichrome to visualize collagen (blue). The Nikon Elements software package (Nikon Instruments, Melville, NY) calculated the percentage of blue stain in each lung field as well as within the adventitia of small to midsized pulmonary arteries (<200 μm).
Cell Proliferation
In lung sections co-stained with α-SMA and PCNA, the percentages of nuclei within the pulmonary artery wall that were positive for PCNA were calculated.
Analysis of Intimal Lesions
The percentage of small vessels (<100 μm) with occlusive intimal lesions was determined in vascular and airway perfusion-fixed lungs. An occlusive lesion was defined by an intimal layer occupying more than 50% of the lumen + intima diameter.
Quantitative Real-Time PCR
See the online supplement for details and primers. The expression of mRNA was determined for Egr-1, TGF-β, and collagen 1A1 (COL1A1). Gene copy numbers were normalized to hypoxanthine-guaninie phosphoribosly transferase (HPRT), and expressed relative to WT control mice.
Nitrotyrosine Slot Blot
Total nitrated protein concentrations were determined by probing a slot-blot membrane with rabbit polyclonal anti-nitrotyrosine antibody (1:1,000; Upstate Biotechnology, Lake Placid, NY). Normalized values were expressed relative to mean protein expression in control (PBS) WT mice (see online supplement for details).
Immunoblot
The expression of endothelial nitric oxide synthase (eNOS) protein was determined by established methods of immunoblotting, using a rabbit polyclonal eNOS antibody (1:2,000; BD Biosciences).
Statistical Analysis
Data (expressed as mean ± SE) were analyzed according to one-way ANOVA, followed by Bonferroni post hoc analysis, using Prism (GraphPad Software, La Jolle, CA). Significance was defined as P < 0.05.
RESULTS
Bleomycin Caused Marked Remodeling in All Three Layers of the Small Pulmonary Arteries in WT Mice, but Vascular Remodeling and Cell Proliferation Were Absent in Mice Overexpressing Lung EC-SOD
Medial wall thickening.
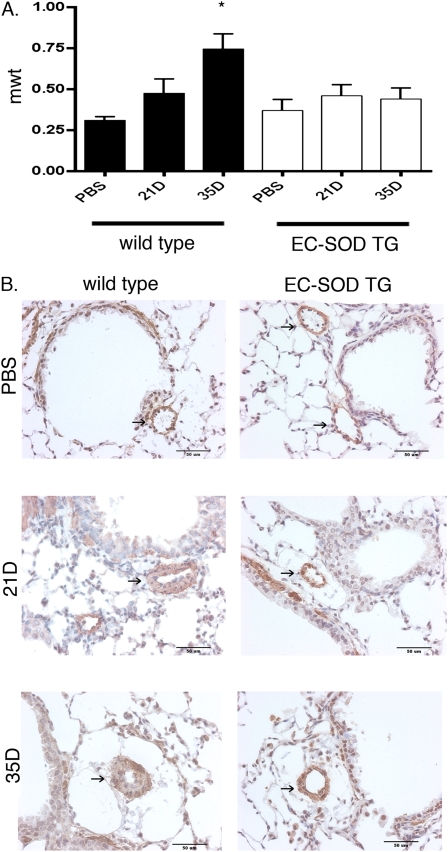
The pulmonary artery MWT was calculated in sections from lungs that were both inflation-fixed and perfusion-fixed to preserve vascular structure, and immunostained with an antibody against α-SMA to identify the medial wall. Significant MWT in the small pulmonary arteries (<50 μm) was evident in bleomycin-treated WT mice compared with PBS-treated control mice at 35 days after treatment. In contrast to WT mice, at 35 days after bleomycin treatment, the EC-SOD TG mice did not develop an increase in MWT, compared with PBS-treated WT or EC-SOD TG mice (Figure 1).
Figure 1.
Pulmonary artery medial wall thickening developed by 35 days after intratracheal bleomycin treatment in wild-type (WT) mice but not in mice overexpressing extracellular superoxide dismutase in the lung (EC-SOD TG). (A) In lung sections stained with α–smooth muscle actin (α-SMA) to define the medial wall, medial wall thickening (mwt) was measured in pulmonary arteries (PAs) < 50 μm in control PBS-treated mice, and 21 and 35 days (21D and 35D, respectively) after bleomycin treatment. *P < 0.05 versus intratracheal control mice by one-way ANOVA. We counted 7–10 vessels per lung, with n = 4 mice per group. (B) Representative images of PAs stained with α- SMA and counterstained with hematoxylin. Arrows indicate pulmonary artery.
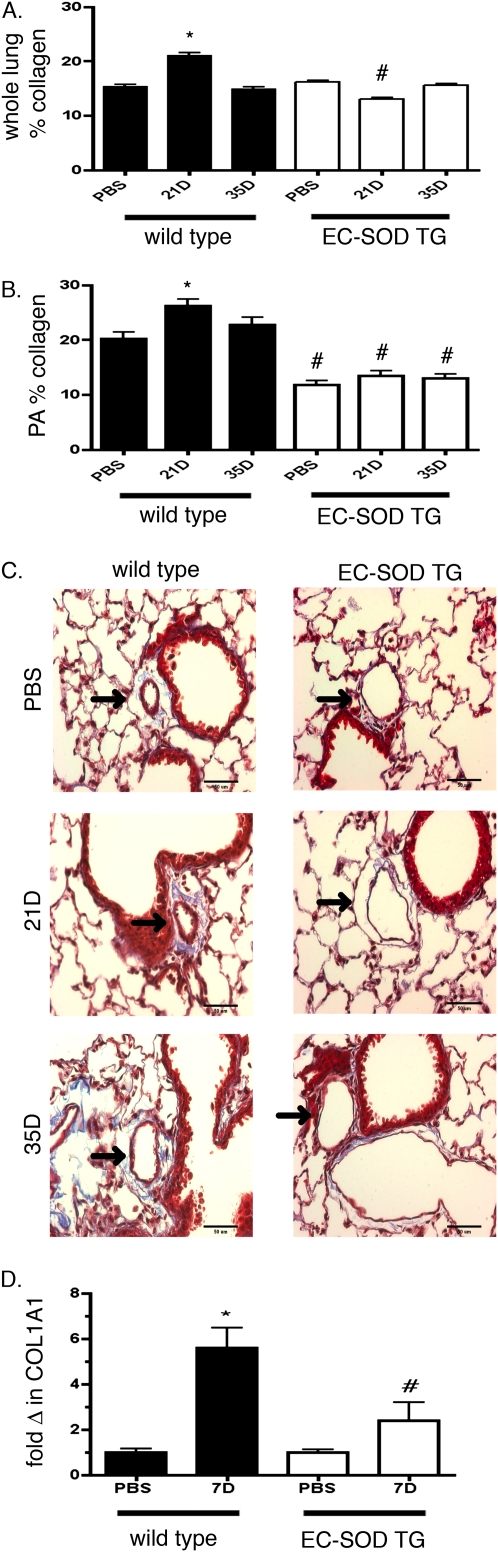
Adventitial collagen deposition.
Computer analysis after trichrome staining enabled a quantitative comparison of both total lung–specific and pulmonary artery–specific collagen deposition in WT and EC-SOD TG mice after bleomycin treatment. The total collagen content in lung sections from WT mice increased at 21 days after bleomycin treatment compared with PBS-treated mice, but was decreased by 35 days (Figure 2). Collagen deposition, specifically around the pulmonary arteries of WT mice, also increased at 21 days after bleomycin treatment, with improvement by 35 days. In contrast, mice overexpressing EC-SOD did not show a significant increase in either total lung collagen or in the accumulation of collagen around their pulmonary arteries at 21 or 35 days after treatment with bleomycin (Figure 2). Although collagen deposition around the pulmonary arteries decreased by 35 days in WT mice, it remained significantly higher than in EC-SOD TG mice at the same time point. We also observed a significant increase in Type 1 collagen, COL1A1, and mRNA in the lungs of WT mice at 7 days compared with sham-treated mice. In contrast, COL1A1 did not significantly increase in EC-SOD TG mice, and its concentrations were also lower than in WT 7-day bleomycin-treated mice (Figure 2).
Figure 2.
Pulmonary artery adventitial collagen deposition was increased 21 days after intratracheal bleomycin treatment in WT mice, and was attenuated in mice overexpressing EC-SOD. Computer analysis determined percent collagen deposition in whole lung (A) and within the pulmonary artery (B), as shown for WT and EC-SOD TG mice 21 days and 35 days after bleomycin treatment. *P < 0.05 according to one-way ANOVA versus PBS-treated mice. #P < 0.05 versus WT mice at comparable time points (n = 3–4 mice per group). (C) Representative images of collagen deposition by trichrome staining around pulmonary artery. Arrows indicate pulmonary artery. (D) mRNA expression for COL1A1 was measured by RT–quantitative PCR in lung homogenates of WT and EC-SOD TG mice 7 days after bleomycin treatment, compared with sham-treated controls (n = 6 in each group). Data are expressed as copies of gene per copy of HPRT standardized to the sham WT. *P < 0.05 compared with PBS-treated WT mice. #P < 0.05 versus WT 7D, according to one-way ANOVA.
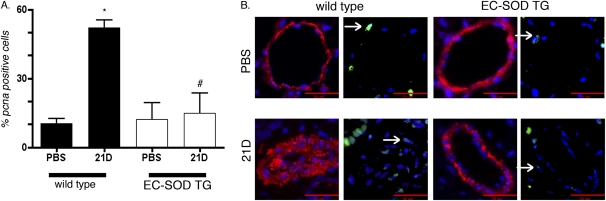
Cell proliferation.
Proliferating cells were identified by positive nuclear PCNA staining. Proliferating cells within the pulmonary artery wall, identified by dual staining for PCNA and α-SMA, were evident at 21 days after treatment with bleomycin, predominantly in the medial wall, with occasional PCNA-positive cells within the intimal layer (Figure 3). Proliferating cells increased within small (<100 μm) pulmonary arteries 21 days after intratracheal treatment with bleomycin in WT mice compared with PBS sham mice. Cell proliferation did not increase in the vessel wall 21 days after treatment with bleomycin in mice overexpressing EC-SOD. No significant increase was evident in proliferation by 35 days after treatment with bleomycin in either the WT or EC-SOD TG mice (data not shown).
Figure 3.
Proliferation was detected within the pulmonary artery wall 21 days after intratracheal bleomycin treatment in WT mice but not in mice overexpressing EC-SOD. (A) Sections were dual-stained with α-SMA and proliferating cell nuclear antigen (PCNA), and counterstained with 4′6-diamidino-2-pheylindole (DAPI). The percentages of proliferating cells within the pulmonary artery, identified by PCNA staining within the nuclei of small pulmonary arteries (< 100 μm), were determined in WT and EC-SOD TG mice at 21 days after bleomycin treatment. *P < 0.05 versus PBS-treated mice. #P < 0.05 versus 21D bleomycin-treated mice according to one-way ANOVA (n = 3–4 mice per group). (B) Representative images of pulmonary arteries co-stained with α-SMA (red) and PCNA (green). Left: α-SMA and DAPI (blue). Right: Same vessel with PCNA and DAPI. Arrows indicate a positive nucleus; scale bar is 50 microns.
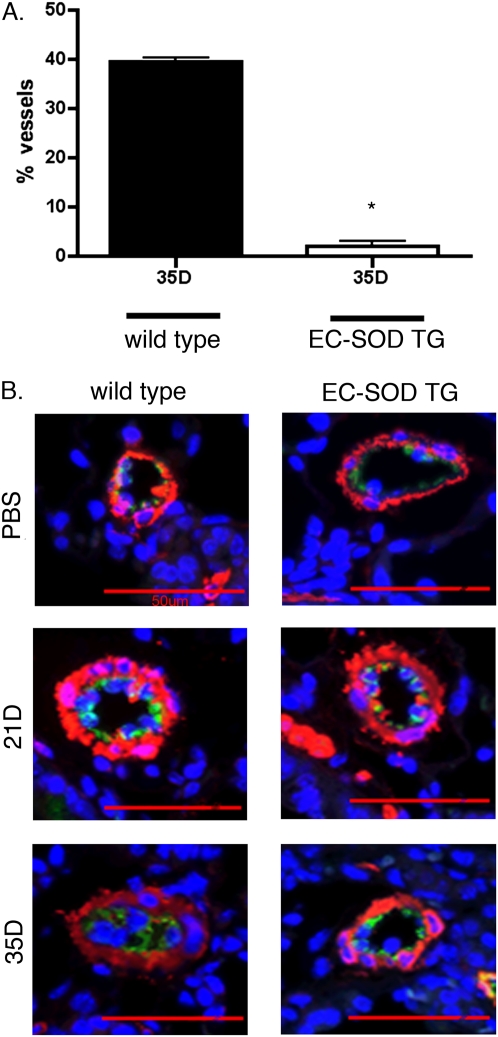
Intimal thickening.
Intimal thickening was evaluated in small pulmonary arteries by co-staining for α-SMA and the endothelial marker FVIII, and evaluating the percentage of vessels with intimal thickening or occlusive lesions. As shown in Figure 4, by 35 days, 39% of small (<100 μm) pulmonary arteries in the lungs of WT bleomycin-treated mice contained significant intimal thickening, defined as lesions occupying at least 50% of the lumen. In contrast, intimal thickening was present in only 2% of pulmonary arteries in mice overexpressing EC-SOD. Intimal changes were not detectable in untreated mice or 21 days after treatment with bleomycin in either strain.
Figure 4.
Pulmonary artery intimal thickening developed by 35 days after intratracheal bleomycin treatment in WT mice, but not in mice overexpressing EC-SOD. (A) Fifty vessels were counted in each lung after co-staining with α-SMA and Von Willebrand factor VIII, to identify the medial and intimal layers. Intimal thickening was defined as an intimal layer occupying more than 50% of the diameter of the intima + lumen. Data are shown for WT and EC-SOD TG mice 35 days after bleomycin treatment. *P < 0.05 versus 35D bleomycin-treated WT mice according to one way ANOVA. n = 3–4 per group. (B) Representative images of lung sections were co-stained with α-SMA (red) and Von Willebrand factor VIII (green), and counterstained with DAPI to identify the nuclei (blue). Arrows indicate pulmonary artery.
The Induction of Two Genes Known to Mediate Bleomycin-Induced Fibrosis, Egr-1 and TGF-β, Was Attenuated at 7 Days in the Lungs of Mice Overexpressing EC-SOD
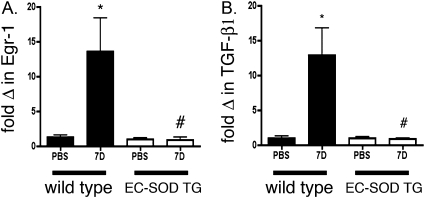
Egr-1 was identified as a key transcription factor for cell proliferation and matrix deposition in diseases, including interstitial lung disease and pulmonary hypertension. In addition, TGF-β is well-known to mediate bleomycin-induced fibrotic responses, and can increase collagen through an Egr-1–dependent mechanism. We therefore measured the time course of lung Egr-1 mRNA expression, and observed a transient increase in mRNA expression that began at 7 days and returned to baseline by 21 days (data not shown). We then focused on the gene expression of TGF-β and on Egr-1 mRNA expression 7 days after treatment with bleomycin in the lungs of WT and EC-SOD TG mice. We observed a significant increase in Egr-1 and TGF-β mRNA in the lungs of WT mice at 7 days, compared with sham-treated mice. In contrast, Egr-1 and TGF-β did not significantly increase in EC-SOD TG mice, and their concentrations were also lower than in WT bleomycin-treated mice at 7 days (Figure 5).
Figure 5.
Redox-sensitive transcription factor early growth response (Egr-1) and transforming growth factor (TGF-β) gene expression increased 7 days after bleomycin treatment in WT lungs, but not in lungs of mice overexpressing EC-SOD. mRNA expression for TGF-β (A) and Egr-1 (B) was measured by RT–quantitative PCR in lung homogenates of WT and EC-SOD TG mice 7 days after bleomycin treatment, compared with sham-treated control mice (n = 6 in each group). Data are expressed as copies of gene per copy of hypoxanthine-guaninie phosphoribosly transferase (HPRT), standardized to sham WT mice. *P < 0.05 compared with PBS-treated WT mice. #P < 0.05 versus WT 7D, according to one-way ANOVA.
EC-SOD TG Mice Exhibited Less Nitrosative Stress Compared with WT Mice
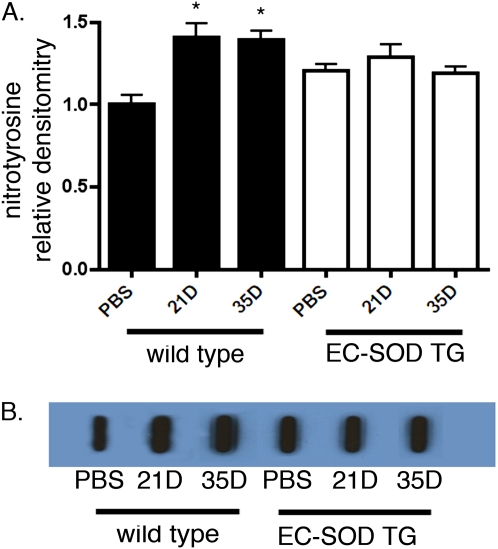
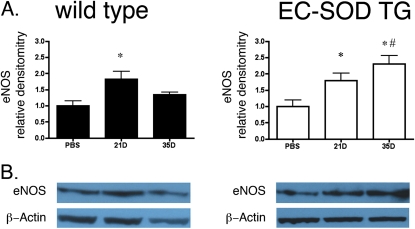
EC-SOD can limit the formation of peroxynitrite by preventing the reaction of superoxide with nitric oxide. Therefore, we measured total lung nitrotyrosine content, a marker of peroxynitrite. Total nitrotyrosine content of the lungs increased at 21 and 35 days in WT mice after treatment with bleomycin. In contrast, mice overexpressing EC-SOD showed statistically similar nitrotyrosine concentrations at baseline compared with WT mice, but the nitrotyrosine content did not increase after bleomycin treatment (Figure 6). Baseline levels of eNOS expression were statistically similar between WT and EC-SOD TG mice (the relative densitometry for EC-SOD/β-actin was 0.52 ± 0.08 for WT mice versus 0.57 ± 0.09 for EC-SOD TG mice, P = 0.69 by unpaired t test; blot not shown). Lung eNOS expression according to immunoblotting increased in WT mice at 21 days, but decreased at 35 days. eNOS also increased in EC-SOD TG mice at 21 days, and further increased at 35 days after treatment with bleomycin, leading to significant differences in eNOS expression between the two mouse strains at 35 days (Figure 7).
Figure 6.
Nitrotyrosine staining increased after bleomycin treatment in WT mice, but not in mice overexpressing EC-SOD. (A) Relative densitometry of nitrotyrosine signal in lung homogenates from WT and EC-SOD TG mice. *P < 0.05 according to one-way ANOVA versus WT PBS-treated mice (n = 6 per group). (B) Representative image of slot blot for lung nitrotyrosine content in WT and EC-SOD TG mice with PBS treatment, and 21 and 35 days after bleomycin treatment.
Figure 7.
eNOS expression decreased in WT mice at the latest time point, but increased in mice overexpressing EC-SOD. (A) Relative densitometry for endothelial nitric oxide synthase (eNOS) protein expression relative to β-actin was determined by immunoblot, using a rabbit polyclonal eNOS antibody (1:2,000). *P < 0.05 according to one-way ANOVA versus WT PBS-treated mice. #P < 0.05 according to one-way ANOVA versus WT 35D mice (n = 4 per group). (B) Representative image of eNOS expression in each group, taken from a single blot.
Late Secondary Pulmonary Hypertension Associated with Bleomycin-Induced Lung Fibrosis Was Attenuated in EC-SOD TG Mice
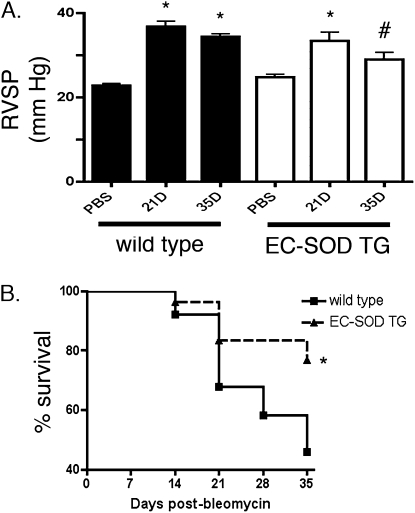
To examine the time course of pulmonary hypertension associated with the bleomycin model of lung fibrosis, mice were studied weekly after a single intratracheal dose of bleomycin or PBS. Pulmonary hypertension, as shown by an increase in RVSP, was present by 7 days in WT mice, progressively increased at 14 and 21 days, and remained elevated at 35 days (Figure 8). The EC-SOD TG mice also exhibited an elevated RSVP, similar to WT mice beginning 7 days after bleomycin treatment. Both strains also demonstrated an increase in right-ventricular hypertrophy at 14 days, as shown by right-ventricular/body weight measurements. Although the RVSP remained elevated at 14 and 21 days after bleomycin treatment, compared with control EC-SOD TG mice, it did not progressively increase on Days 14 or 21, as observed in WT mice (see Figure E1 for entire RVSP time course of WT and EC-SOD TG mice and right-ventricular/body weight on Day 14). By 35 days after intratracheal bleomycin treatment, mice overexpressing lung EC-SOD demonstrated less pulmonary hypertension compared with their WT counterparts, and were no longer statistically different from untreated EC-SOD TG mice (Figure 8A).
Figure 8.
Bleomycin-induced pulmonary hypertension and mortality was attenuated in mice overexpressing EC-SOD. (A) Right ventricular systolic pressures (RVSP; mm HG) in WT mice and EC-SOD TG mice 21 and 35 days after intratracheal treatment with a single dose (0.1 unit) of bleomycin, compared with control mice treated with PBS. *P < 0.05 versus PBS-treated mice according to one-way ANOVA. #P < 0.05 versus WT 35D mice (n = 8–12). (B) A Kaplan-Meier survival curve shows weekly survival over a 35-day period in the WT mice (solid line) and EC-SOD TG mice (dashed line). *P < 0.05 according to log rank test.
Overexpression of EC-SOD Protected against the Mortality Associated with Bleomycin Treatment
Bleomycin-associated mortality was observed by 14 days after treatment in WT mice, with a cumulative mortality of 54% in the 56 WT mice studied at 14, 21, or 35 days. In contrast, in the 46 EC-SOD TG mice studied at the same time points, the cumulative mortality was 23%. A Kaplan-Meier survival curve shows the weekly survival in the two strains, with improved overall survival in the EC-SOD TG mice (P < 0.05 according to log rank test; Figure 8B).
DISCUSSION
In this study, we tested the hypothesis that extracellular superoxide plays an important role in the development of pulmonary vascular remodeling and pulmonary hypertension secondary to interstitial lung disease, using an established model of bleomycin-induced lung fibrosis. We report that the extensive remodeling in the medial, adventitial, and intimal layers of the pulmonary artery wall after bleomycin treatment was markedly attenuated in mice overexpressing lung EC-SOD. We determined that the bleomycin-induced up-regulation of two genes, Egr-1 and TGF-β (well-established as critical molecules involved in cell proliferation, vascular remodeling, and lung fibrosis), was blocked in the lungs of mice overexpressing EC-SOD. The protection against bleomycin-induced pulmonary vascular remodeling in mice overexpressing EC-SOD was accompanied by protection against late pulmonary hypertension and mortality. These data provide new, convincing evidence for the central role of extracellular superoxide and EC-SOD in the pathogenesis of pulmonary vascular remodeling secondary to lung fibrosis, and moreover provide the rationale to develop new strategies for addressing the extracellular oxidant/antioxidant imbalance in this life-threatening disease process.
In addition to producing lung fibrosis, intratracheal treatment with bleomycin caused severe pulmonary artery remodeling in all three layers of the vessel wall. Although medial wall and adventitial thickening in the pulmonary arteries of mice exposed to bleomycin was previously reported, this evidence is the first, to the best of our knowledge, that bleomycin also results in the formation of occlusive intimal lesions (6). These neointimal lesions were not apparent until 35 days, and thus the other experiments that concluded by 21 days would not have shown these structural changes. We did not detect medial wall thickening until the 35-day time point, in contrast to reports of medial and adventitial wall thickening at the 14-day time point (6). Regarding the main methodological difference between our study and previous studies, we both inflation-fixed and perfusion-fixed the pulmonary vasculature in lungs used for morphometric analysis to preserve vessel diameter, which may have accounted for the discrepancy in comparison to lungs processed only with inflation-fixation.
Bleomycin-induced vascular remodeling is a significant finding, because human pulmonary artery hypertension is characterized by severe arteriopathy, including neointimal lesions, whereas classic rodent models of chronic pulmonary hypertension, chronic hypoxia, and monocrotaline only produce changes in the adventitial and medial layers of the vessel, without intimal changes (14). The predominance of vasoconstriction with limited pulmonary vascular remodeling or inflammation in chronic hypoxia or monocrotaline-induced pulmonary hypertension in mice has raised concerns about the relevance of murine models in understanding human disease (15). The bleomycin model, which is relatively simple to implement and is well-characterized by extensive inflammation and fibrosis (see Figure E2), therefore provides new opportunities to use genetically engineered mice to test the role of specific molecules in the pathogenesis of severe arteriopathy, with structural changes more similar to those in the clinical setting, particularly for patients with underlying interstitial lung disease.
The key finding in this study is that the overexpression of lung EC-SOD strongly protected against bleomycin-induced pulmonary vascular remodeling. This observation implicates a role for extracellular superoxide in the pathogenesis of vascular remodeling. We observed remodeling predominantly within small pulmonary arteries, which correlates with an important site of increased pulmonary vascular resistance in pulmonary hypertension. Our findings expand on previous work that described the role of EC-SOD in bleomycin-induced fibrosis and in chronic hypoxic pulmonary hypertension. For example, several laboratories showed that a loss of EC-SOD activity exacerbates lung fibrosis, whereas the overexpression of EC-SOD protects against fibrosis attributable to bleomycin, but the effects of EC-SOD activity on bleomycin-induced pulmonary hypertension have not been tested (10, 12). In addition, we previously reported that the overexpression of EC-SOD protected against chronic hypoxic pulmonary hypertension (5). We have not tested the impact of EC-SOD on vascular reactivity or inflammation, or used other animal models of pulmonary hypertension. Future investigations in these arenas would likely provide further insights into the role of extracellular superoxide and EC-SOD in the development of pulmonary hypertension (14). Treatment with bleomycin is associated with more chronic inflammation and oxidative stress than exposure to chronic hypoxia, which enabled us to identify both common and potentially distinct mechanisms contributing to the development of pulmonary vascular remodeling, and in particular, to focus on secondary pulmonary hypertension attributable to lung fibrosis.
Although most studies of bleomycin-induced interstitial lung disease used endpoints related to inflammation and fibrosis, studies of pulmonary hypertension also included measures of cell proliferation, as observed in relevant cell culture models and in vivo in the early development of pulmonary hypertension. We report a decrease in proliferation in the pulmonary artery wall of EC-SOD TG mice compared with WT mice after treatment with bleomycin. This finding parallels our previous observation that the overexpression of EC-SOD inhibits early (3-day) hypoxia-induced cell proliferation in small pulmonary arteries (5). In our previous study, we did not observe cell proliferation in the vessels of bleomycin-treated mice at 35 days, and we did not detect proliferation in the pulmonary arteries of chronically hypoxic mice at 35 days. A similar paucity of proliferation was reported in the lesions of humans with pulmonary hypertension, although it is unclear why the proliferation is reduced at the time of extensive vascular remodeling. The observation that lung EC-SOD expression modulates cell proliferation provides potential new avenues of investigation in the study of the pathogenesis of pulmonary hypertension.
We are interested in understanding the mechanisms by which lung overexpression of EC-SOD could protect against bleomycin-induced pulmonary vascular remodeling. We therefore measured the bleomycin-induced up-regulation of two key reactive oxygen species (ROS)–regulated genes that are known to mediate lung fibrosis and pulmonary hypertension, Egr-1 and TGF-β, and report that their up-regulation is blocked in EC-SOD TG mice. Egr-1 is a redox-sensitive transcription factor that is increasingly recognized as a key mediator of cell proliferation, fibrosis, and vascular remodeling. Egr-1 was implicated in the pathogenesis of both chronic hypoxic pulmonary hypertension and lung fibrosis (16). We previously reported that the overexpression of lung EC-SOD attenuates the early hypoxia-induced up-regulation of Egr-1 (5). TGF-β has long been recognized as a major molecule responsible for tissue fibrosis and collagen production, including human conditions associated with interstitial lung fibrosis and animal models of bleomycin-induced lung fibrosis. TGF-β induces Egr-1 in both fibroblasts and vascular smooth muscle via a non–SMAD-dependent pathway involving MEK1/2 and ERK1/2, and increases Egr-1 DNA binding activity to the COL1A promoter. Egr-1 was necessary and sufficient to induce COL1A in fibroblasts. In a novel triple-transgenic mouse with inducible overexpression of lung TGF-β concurrent with a knockdown of Egr-1, the absence of Egr-1 protected against the epithelial cell apoptosis and pulmonary fibrosis generated by the induction of TGF-β (17). Although numerous studies showed that TGF-β induces Egr-1 to mediate fibrosis, reports claim that Egr-1 can also induce TGF-β, leading to a positive feedback loop (18–20). Future studies are necessary to determine the mechanism by which extracellular superoxide induces Egr-1 and TGF-β, and how EC-SOD secreted from Type II alveolar epithelial cells protects against vascular remodeling. Secreted EC-SOD may protect the lung and vascular fibroblasts from the increased oxidative stress that leads to the activation of Egr-1. EC-SOD may also modulate the epithelial–mesenchymal transition, which was reported to contribute to bleomycin-induced lung fibrosis (21). These findings provide strong evidence for extracellular superoxide as a signaling molecule in response to injuries associated with oxidative or nitrosative stress, leading to the modulation of key genes.
Bleomycin is well-known to cause the oxidative stress responsible for lung injury. Bleomycin binds to intracellular iron and reduces molecular oxygen to superoxide and hydroxyl radical, to cause oxidative damage in cellular structures. In addition, bleomycin induces a marked inflammatory response, which further potentiates oxidative damage through leukocyte-derived ROS (22). Bleomycin-induced lung injury was attenuated by different antioxidant strategies, including N-acetylcysteine + desferoxamine, hydrogen sulfide, edaravone, carnosine, and epigallocatechin-3–gallate, and was also diminished in mice lacking the p47phox subunit (phagocyte oxidase) of nicotinamide adenine dinucleotide phosphate–reduced oxidase, and in mice overexpressing EC-SOD (23). The phosphodiesterase-5 inhibitor, sildenafil, attenuated injury, along with an inhibition of ROS production (6). EC-SOD may protect against bleomycin-induced injury by limiting extracellular superoxide–mediated toxicity or by preventing the inactivation of NO, thus preserving NO bioactivity (24). We found evidence of increased nitrosative stress in the lung in response to bleomycin, and that stress was attenuated in EC-SOD TG mice. We also found that bleomycin-induced increases in eNOS expression at 21 days were lost by 35 days in WT mice, but not EC-SOD TG mice. Thus, in the face of decreasing eNOS expression, the nitrotyrosine formation seen in the WT mice was not likely attributable to increased NO or superoxide derived from uncoupled NOS, and was more likely attributable to increased superoxide from a different source. Inghilleri and colleagues evaluated the in situ production of oxidative and nitrosative stress, and identified inflammatory cells as well as airway epithelium as the major sites of production of ROS and NO, although Inghilleri and colleagues did not elucidate the source of ROS production (22). Bleomycin-induced pulmonary hypertension is also attenuated by sildenifil, which may also be mediated by its effect on NO bioactivity through its preservation of the half-life of cyclic guanosine monophosphate (cGMP) (25). In a neonatal model of bleomycin-induced pulmonary hypertension and bronchopulmonary dysplasia, chronic treatment with inhaled NO attenuated right ventricular hypertrophy and medial wall thickness (13). Ultimately, the specific contributions of reactive oxygen and nitrogen species, particularly in the extracellular compartment, to bleomycin-induced pulmonary vascular remodeling, and these species' potential role in the up-regulation of redox-sensitive genes, remain to be determined.
Along with pulmonary vascular remodeling, we found that WT mice developed pulmonary hypertension by 7 days, which persisted for the 35-day study period. In mice overexpressing EC-SOD, the most striking finding was a significant attenuation in pulmonary hypertension at the 35-day time point, which coincided with the protection against vascular remodeling. The progressive increase in pulmonary hypertension in WT mice was also blunted in EC-SOD TG mice. Both vasoconstriction and vascular remodeling can contribute to pulmonary hypertension, and pulmonary hypertension in response to bleomycyin was found to be mediated, at least in part, by vasoconstriction. For example, McNamara and colleagues reported that treatment with a Rho kinase inhibitor acutely reversed the elevated pulmonary vascular resistance in bleomycin-treated neonatal rats (13), and Hemnes and colleagues showed how a phosphodiesterase inhibitor that lowered pulmonary vascular resistance improved bleomycin-induced pulmonary hypertension at 14 days (6). Thus EC-SOD does not appear to protect against the early component of bleomycin-induced pulmonary hypertension attributable to vasoconstriction before the onset of vascular remodeling. An improvement in remodeling without an effect on pulmonary hemodynamics was observed in other settings. For example, treatment with rosiglitazone prevented chronic hypoxia-induced pulmonary vascular remodeling, but failed to lower mean pulmonary artery pressures (26). In the clinical arena, patients with pulmonary hypertension who do not respond to vasodilator therapy have worse outcomes, because current clinical therapies do not target arteriopathy, providing strong evidence for the critical role of vascular remodeling in disease outcomes. Nonetheless, EC-SOD would not be expected to exert effects on vascular tone in our study, because other models of vascular dysfunction indicated that EC-SOD can modulate vascular tone through the regulation of nitric oxide bioactivity (24). EC-SOD may also modulate pulmonary vascular tone in part by preserving the up-regulation of eNOS and thus NO production in response to bleomycin. Moreover, the site of EC-SOD overexpression by Type II cells in this particular strain of mouse may provide protection at the site of remodeling, but may not be situated to protect completely against vasoconstriction. WT mice may also exhibit more cardiac dysfunction compared with EC-SOD TG mice, so that similar right ventricular systolic pressure in EC-SOD TG mice might reflect improved pulmonary vascular resistance. Several studies demonstrated that intratracheal bleomycin in WT mice results in a decrease in cardiac output (9). We did not measure cardiac function in this study. However, if bleomycin produces direct cardiotoxicity, we would not expect lung-directed EC-SOD overexpression to protect against cardiac damage. The WT mice dying at 21 and 35 days may have manifested worse pulmonary hypertension, leading us to underestimate the protection by EC-SOD overexpression against pulmonary hypertension at these later time points. Whereas this study focused on the marked protection against remodeling, future studies are needed to address directly the effects of EC-SOD on vascular reactivity in models of pulmonary hypertension, and to determine the contributions of the site of EC-SOD expression on vascular responses.
Along with marked protection against vascular remodeling and late pulmonary hypertension, we report that mice overexpressing lung EC-SOD also had a significant decrease in mortality compared with WT mice. The reported mortality after bleomycin treatment in WT mice has varied in the literature, and is most likely to be determined by dose, length of observation, and method of lung delivery, with administration via tracheotomy associated with early death in contrast to intubation and intratracheal instillation, as performed in this study (6). Our observed mortality in both mouse strains was much higher than reported by Bowler and colleagues, who cited a 3% overall mortality at 14 days in both WT and EC-SOD TG mice (11). The analysis by Bowler and colleagues (11) included mice exposed for only 3, 7, 10, and 14 days, and we similarly found low mortality in both strains when we only examined mice exposed to early time points up to 14 days after exposure, with mortality almost exclusively occurring later. The cause of mortality is likely multifactorial, because previous studies documented severe lung fibrosis with impaired gas exchange, impaired cardiac output, and pulmonary hypertension, each of which can affect outcomes (6). The observation that EC-SOD overexpression protected against pulmonary vascular remodeling and mortality, with little to no impact on early pulmonary hypertension before the development of significant pulmonary vascular remodeling, suggests that pulmonary vascular remodeling is a major factor in the morbidity and mortality of this disease process, and is mediated by an imbalance in the production of extracellular superoxide and scavenging by EC-SOD.
In conclusion, we tested the hypothesis that extracellular superoxide generated in the lung after the administration of bleomycin contributes to pulmonary hypertension and pulmonary vascular remodeling in this model of lung fibrosis. Wild-type mice treated with intratracheal bleomycin developed significant pulmonary vascular remodeling with medial wall thickening, increased COL1A1 mRNA expression, adventitial collagen deposition, proliferation in the vessel wall, and ultimately, occlusive intimal lesions. Two key genes known to contribute to vascular remodeling and lung fibrosis, Egr-1 and TGF-β mRNA, increased in lung homogenates 7 days after the administration of bleomycin. WT mice also developed elevated right ventricular systolic pressures by 7 days that persisted for 35 days, with a mortality rate approaching 50% by 35 days. In contrast, the overexpression of lung EC-SOD significantly protected against bleomycin-induced remodeling in the adventitial, medial, and intimal layers of the pulmonary artery wall, and prevented the up-regulation of Egr-1 and TGF-β. EC-SOD TG mice were protected against late pulmonary hypertension, and achieved a dramatic improvement in mortality. These data provide new insights into the role of EC-SOD and extracellular superoxide in the pathogenesis of pulmonary hypertension, and identify new potential targets of superoxide that can mediate pulmonary vascular remodeling. This study provides a strong foundation for developing novel therapeutic approaches to restore extracellular oxidant/antioxidant balance in the lung and pulmonary circulation, to protect against the pulmonary hypertension complicating interstitial lung diseases.
Supplementary Material
This work was presented in part at the Annual Meeting of the American Thoracic Society in May 2009, in San Diego, California, and at the Annual Meeting of the Society of Free Radical Biology and Medicine in November 2009, in San Francisco, California.
This work was supported by National Institutes of Health/National Heart Lung Blood Institute grant HL086680 (E.N.-G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0065OC on June 10, 2010
Author Disclosure: E.N.-G. received a sponsored grant from the National Institutes of Health for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J 2008;31:1357–1367. [DOI] [PubMed] [Google Scholar]
- 2.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152–L160. [DOI] [PubMed] [Google Scholar]
- 3.Gao F, Kinnula VL, Myllarniemi M, Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal 2008;10:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxic pulmonary hypertension. In: Roach R, Hackett P, editors. Hypoxia and the circulation. Vol 618. New York: Springer Academics, 2007. pp. 101–112. [DOI] [PubMed]
- 5.Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 2008;295:L422–L430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 2008;294:L24–L33. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res 2005;97:967–974. [DOI] [PubMed] [Google Scholar]
- 8.Champion HC, Bivalacqua TJ, D'Souza FM, Ortiz LA, Jeter JR, Toyoda K, Heistad DD, Hyman AL, Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the lung of the mouse in vivo: effect on agonist-induced and flow-mediated vascular responses. Circ Res 1999;84:1422–1432. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz LA, Champion HC, Lasky JA, Gambelli F, Gozal E, Hoyle GW, Beasley MB, Hyman AL, Friedman M, Kadowitz PJ. Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol 2002;282:L1209–L1221. [DOI] [PubMed] [Google Scholar]
- 10.Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med 2001;31:1198–1207. [DOI] [PubMed] [Google Scholar]
- 11.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2002;282:L719–L726. [DOI] [PubMed] [Google Scholar]
- 12.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 2003;35:763–771. [DOI] [PubMed] [Google Scholar]
- 13.McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol 2008;294:L205–L213. [DOI] [PubMed] [Google Scholar]
- 14.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 2009;297:L1013–L1032. [DOI] [PubMed] [Google Scholar]
- 15.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res 2005;97:95–98. [DOI] [PubMed] [Google Scholar]
- 16.Banks MF, Gerasimovskaya EV, Tucker DA, Frid MG, Carpenter TC, Stenmark KR. Egr-1 antisense oligonucleotides inhibit hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. J Appl Physiol 2005;98:732–738. [DOI] [PubMed] [Google Scholar]
- 17.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1–mediated apoptosis is essential for transforming growth factor beta1–induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, Chen SJ, Wu M, Warner-Blankenship M, Ning H, Lakos G, Mori Y, Chang E, Nihijima C, Takehara K, et al. SMAD-independent transforming growth factor–beta regulation of early growth response–1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol 2008;173:1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, Leof E, Varga J. A non-SMAD mechanism of fibroblast activation by transforming growth factor–beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene 2009;28:1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharyya S, Wei J, Melichian DS, Milbrandt J, Takehara K, Varga J. The transcriptional cofactor NAB2 is induced by TGF-beta and suppresses fibroblast activation: physiological roles and impaired expression in scleroderma. PLoS ONE 2009;4:e7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2009;180:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol 2006;125:661–669. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira KC, Soares FS, Rocha LG, Silveira PC, Silva LA, Valenca SS, Dal Pizzol F, Streck EL, Pinho RA. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther 2008;21:309–316. [DOI] [PubMed] [Google Scholar]
- 24.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest 1996;75:617–636. [PubMed] [Google Scholar]
- 25.Yildirim A, Ersoy Y, Ercan F, Atukeren P, Gumustas K, Uslu U, Alican I. Phosphodiesterase-5 inhibition by sildenafil citrate in a rat model of bleomycin-induced lung fibrosis. Pulm Pharmacol Ther 2010;23:215–221. [DOI] [PubMed]
- 26.Crossno JT Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 2007;292:L885–L897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.