Abstract
Pulmonary hypertension is characterized by elevated pulmonary artery pressure and pulmonary artery smooth muscle cell (SMC) proliferation and migration. Clinical and experimental evidence suggests that serotonin (5-HT) is important in these responses. We previously demonstrated the participation of the 5-HT transporter and intracellular 5-HT (5-HTi) in the pulmonary vascular SMC-proliferative response to 5-HT. However, the mechanism underlying the intracellular actions of 5-HT is unknown. We speculated that 5-HTi activates SMC growth by post-translational transamidation of proteins via transglutaminase (TGase) activity, a process referred to as serotonylation. To test this hypothesis, serotonylation of pulmonary artery SMC proteins, and their role in 5-HT–induced proliferative and migratory responses, were assessed. 5-HT caused dose- and time-dependent increase in serotonylation of multiple proteins in both bovine and rat pulmonary artery SMCs. Inhibition of TGase with dansylcadaverin blocked this activity, as well as SMC-proliferative and migratory responses to 5-HT. Serotonylation of proteins also was blocked by 5-HT transporter inhibitors, and was enhanced by inhibition of monoamine oxidase, an enzyme known to degrade 5-HTi, indicating that 5-HTi levels regulate serotonylation. Immunoprecipitation assays and HPLC–mass spectral peptide sequencing revealed that a major protein serotonylated by TGase was fibronectin (FN). 5-HT–stimulated SMC serotonylation and proliferation were blocked by FN small interfering (si) RNA. These findings, together with previous observations that FN expression in the lung strongly correlates with the progression of pulmonary hypertension in both experimental animals and humans, suggest an important role of FN serotonylation in the pathogenesis of this disease.
Keywords: serotonin, transglutaminase, fibronectin, protein serotonylation, smooth muscle cell growth
CLINICAL RELEVANCE.
Serotonylation of pulmonary artery smooth muscle cell proteins may be important in the development of pulmonary hypertension. More knowledge about this process is needed and is provided in this article.
Serotonin (also known as 5-hydroxytryptamine [5-HT]) is one of the most potent naturally occurring pulmonary vasoconstrictors (1). Clinical, experimental, and human genetic data support a relationship between 5-HT, pulmonary artery remodeling, and pulmonary hypertension. Our laboratories and others have demonstrated the importance of both the 5-HT transporter (5-HTT) and 5-HT receptors in pulmonary vascular smooth muscle cell (SMC)–proliferative and migratory responses to 5-HT (2–7). We also have shown that 5-HT transport via the 5-HTT triggers activation of platelet-derived growth factor receptor–β and mitogen-activated protein kinase, which participate in proliferation of pulmonary artery SMCs (2, 8). Furthermore, inhibition of intracellular monoamine oxidase (MAO), which degrades 5-HT, markedly enhances 5-HT–induced proliferation of SMCs (9–11). These data indicate that intracellular 5-HT (5-HTi) is an important signal for SMC proliferation; however, the mechanism underlying this activity is unknown.
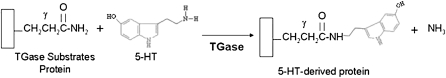
Transglutaminases (TGases), which are abundant in blood and vascular SMCs (12, 13), catalyze post-translational modifications of proteins in a calcium-dependent manner (14, 15). This involves the formation of a covalent bond between the γ-carboxamide group of peptide-bound glutamine residues and either the amino groups of a primary amine substrate of TGases (16) or the ɛ-amine group of peptide-bound lysine residues (17). Modification of TGase substrate proteins by transamidation has been shown to be important in cell survival, apoptosis, and cytoskeleton organization (18, 19). The primary amine, 5-HT, is a TGase substrate. TGase-catalyzed transamidation of proteins with 5-HT is referred to as serotonylation. Serotonylation of platelet procoagulant proteins, including fibrinogens and thrombospondin, facilitates platelet activation and blood clot formation (20, 21). Recent studies have shown that serotonylation of RhoA and smooth muscle β-actin plays an important role in aortic vascular contractility (22, 23). This 5-HT covalent derivative modification reaction is illustrated in Figure 1.
Figure 1.
Schematic illustration of serotonylated modification of proteins by transglutaminase (TGases).
We speculated that serotonylation of TGase substrate proteins modulates their functions in pulmonary artery SMCs, and this was investigated in the present studies. 5-HT was found to initiate serotonylation of several different proteins in bovine and rat pulmonary artery SMCs, including fibronectin (FN). Moreover, inhibition of TGase activity reduced both 5-HT–induced protein serotonylation and proliferation and migration of SMCs. These data suggest that serotonylation of proteins in SMCs is important in 5-HT–induced functional responses. Serotonylation of FN may be particularly relevant, as it has been reported that both 5-HTT expression and FN accumulation in the lung are directly correlated with the progression of pulmonary hypertension (24–26).
MATERIALS AND METHODS
Cell Culture and Functional Evaluation
SMCs from bovine and rat pulmonary artery were isolated as previously described (3, 27). Cells were cultured in Dulbecco's modified Eagle's medium containing 10% FBS, 1% penicillin, and 0.5% streptomycin. Cells from passages 3–9 were used. SMC proliferation was assessed by incorporation of [3H]-thymidine as previously described (28). Cell migration was analyzed in a wound healing assay (8).
Preparation of Whole-Cell Lysates
SMCs were rinsed with ice-cold PBS and then scraped into 200 μl lysis buffer. After 30 minutes at 4°C, lysates were centrifuged at 20,800 × g for 8 minutes, and supernatants containing cell proteins were collected.
Measurement of Protein Serotonylation
Cells were washed with serum free Dulbecco's modified Eagle's medium and incubated with 1 μmol/L 5-HT for 1–60 minutes. Cells were then lysed with RIPA buffer, and 100 μg protein was separated by 4–12% SDS-PAGE gradient gel (Invitrogen, Carlsbad, CA) electrophoresis. Serotonylated proteins were detected by Western blotting with anti-5-HT–BSA conjugate antibody (1:2,000) (Sigma, St. Louis, MO).
Measurement of FN Serotonylation
Serononylated FN was quantified by immunoprecipitation with anti-bovine FN antibody (Santa Cruz Biologicals, Santa Cruz, CA), followed by immunoblotting with anti–5-HT–BSA conjugate antibody (1:2,000). The membrane was stripped and reblotted with anti-FN antibody for normalization.
Transfection of SMCs with FN Small Interfering RNA
Transfection of FN siRNA was performed using Lipofectamine 2,000 from Invitrogen, according to the manufacturer's instructions. Briefly, bovine SMCs were transfected with FN siRNA and control siRNA at final concentrations of 20 or 50 nmol/L. After 48-hour incubation, the cells were treated with 5-HT. Cell proliferation was measured 24 hours later. To confirm the efficiency of FN siRNA transfection, cells were plated in 35-mm plates and transfected with control and FN siRNA for 48 hours. FN expression in cell lysates was assessed by Western blotting analysis with anti-FN antibody.
Tandem Mass Spectrometric Peptide Sequencing
Slices or spots excised from gels were digested with modified trypsin (Promega, Madison, WI). In-gel tryptic digest samples were analyzed by liquid chromatography (LC)–tandem mass spectrometry (MS/MS) at the University of Medicine and Dentistry of New Jersey/Rutgers University Core Facility. Nanospray LC-MS/MS of tryptic digests were conducted using an LTQ linear ion trap mass spectrometer (Thermo Electron, San Jose, CA). A total of 18 Peak list files for MS/MS spectra were generated by Bioworks software (ThermoFinnigan, San Jose, CA) and compared with a bovine database (ENSEMBL 28.35a.1 NCBI35, May 2005) using a local implementation of X! Tandem software. Identified proteins of interest were confirmed manually.
Statistical Analysis
Means (±SE) were calculated, and statistically significant differences between groups were determined by one-way ANOVA followed by Turkey's post hoc comparisons. An effect was considered significant at a P value less than 0.05.
RESULTS
5-HT Induces Transglutamidation of Proteins in Bovine Pulmonary Artery SMC Extracts
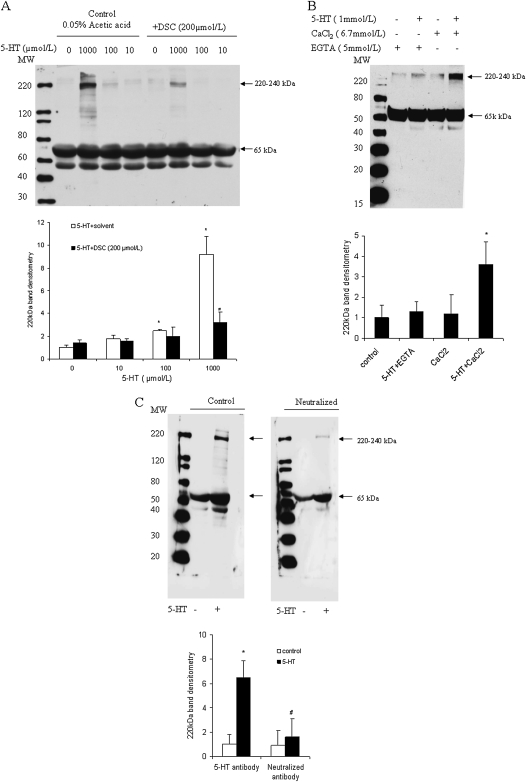
In initial experiments, we analyzed the effects of 5-HT on protein serotonylation in SMC extracts. As shown in Figure 2A, incubation of SMC protein extract with 5-HT at 1,000 μmol/L significantly induced protein binding of 5-HT antibody. Multiple 5-HT binding proteins were noted in the range of 120–240 kD. Lower molecular weight bands in the 50- to 70-kD range were also observed; however, these were unaffected by 5-HT. Prominent protein binding in the 220- 240-kD range was blocked by the TGase inhibitor, dansylcadaverin (DSC), which binds to the enzyme active site of TGase and inactivates the transmidation activity (29–31). Figure 2B shows that the formation of the 220- to 240-kD TGase-modified protein was significantly reduced in the presence of the calcium chelator, EGTA, demonstrating that it is Ca2+ dependent. Preincubation of anti–5-HT–BSA anti-serum with 10 mmol/L 5-HT competitively blocked 5-HT binding to the 220- to 240-kD protein, while minimally affecting the lower molecular weight bands, demonstrating the specificity of binding of the antibody (Figure 2C).
Figure 2.
Serotonin (5-hydroxytryptamine [5-HT])-induced protein serotonylation in bovine pulmonary artery smooth muscle cell (SMC) protein extract. SMCs were washed with Hanks' balanced salt solution, harvested, sonicated at 4°C for 10 seconds, and then centrifuged for 5 minutes at 10,000 × g. Supernatants containing cell extracts then were analyzed for protein serotonylation. (A) Proteins in Tris-buffered saline buffer were incubated with 0–1,000 μmol/L 5-HT at 37°C for 60 minutes in the presence or absence of the TGase inhibitor, dansylcadaverin (DSC; 200 μmol/L). Reactions were terminated by the addition of 5 mmol/L EGTA on ice. Proteins were concentrated and desalted with micro-filter columns (Pierce, Rockford, IL) by centrifugation at 4°C, and then analyzed by Western blotting using anti–5-HT–BSA–conjugated anti-serum (Sigma) for protein serotonylation. (B) Protein serotonylation was induced by 1 mmol/L 5-HT in SMC protein extract for 60 minutes and assessed by Western blotting. (C) Anti–5-HT–BSA anti-serum was preincubated with 10 mmol/L 5-HT for 2 hours to neutralize 5-HT–binding activity. Proteins from control (−) and 5-HT–treated cell lysates (+) were immunoblotted with 0.5 μg/ml 5-HT anti-serum control or neutralized 5-HT anti-serum. Protein molecular weight marker is shown to the left in all figures. Bar graphs represent means (±SD) (n = 3). *Significant difference from the untreated controls at (P < 0.05); #significant difference from 5-HT–treated cells (P < 0.05).
5-HT Stimulates Protein Serotonylation in Intact Primary Pulmonary Artery SMCs
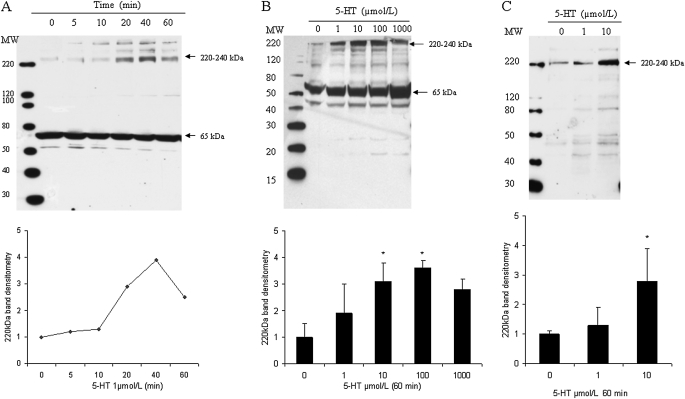
We next analyzed the effects of 5-HT on serotonylation of cellular proteins in intact bovine and rat pulmonary artery SMCs. Binding of 5-HT to the 220–240 kD protein in bovine SMCs was dose and time dependent, reaching a maximal level at 40 minutes and 10–100 μmol/L (Figures 3A and 3B). Similar results were observed in rat SMCs (Figure 3C). Of note, in rat cells, nonspecific 5-HT binding in the 50–70 kD range was not observed.
Figure 3.
5-HT–mediated protein serotonylation in SMCs. Bovine SMCs were washed with serum-free Dulbecco's modified Eagle's medium (DMEM) and then incubated with 5-HT. (A) Cells were incubated for 5–60 minutes with 5-HT (1 μmol/L). (B) Cells were incubated with 5-HT (1–(1,000 μmol/L) for 60 minutes. (C) Rat cells were incubated with 5-HT (0–10 μmol/L) for 60 minutes. After incubation, cell extracts were obtained, electrophoresed, and subjected to immunoblotting, as noted in Materials and Methods. Bar graphs represent means (±SD) (n = 3). *Significant difference from the untreated controls (P < 0.05).
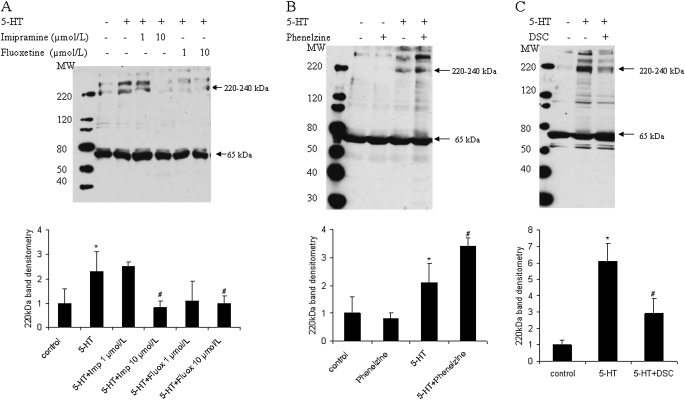
Various cell types, including SMCs, actively transport 5-HT into cells via a 5-HTT (9, 10, 32). We speculated that, once accumulated in the cells, 5-HTi is metabolized via MAO into 5-hydroxyindoleacetic acid, or incorporated into TGase substrate proteins by TGase-mediated transglutamidation. To test this hypothesis, SMCs were pretreated for 30 minutes with the 5-HTT inhibitors, imipramine and fluoxetine, the MAO inhibitor, phenelzine, or the TGase inhibitor, DSC. Figure 4A shows that imipramine and fluoxetine caused a dose-dependent inhibition of 5-HT–induced protein serotonylation of the 220–240 kD band(s). In contrast, inhibition of MAO with phenelzine enhanced protein serotonylation (Figure 4B). As observed in cell extracts, the TGase inhibitor, DSC, blocked protein serotonylation induced by 5-HT in intact cells (Figure 4C).
Figure 4.
Modulation of protein serotonylation in SMCs in culture by 5-HT transporter (5-HTT), monoamine oxidase (MAO), and TGase. Bovine SMCs were washed with serum-free DMEM and then pretreated with (A) 5-HTT inhibitor, imipramine or fluoxetine (1,10 μmol/L) (B) MAO inhibitor, phenelzine (50 μmol/L), or (C) TGase inhibitor, DSC (200 μmol/L) for 30 minutes. Protein serotonylation was then induced by 5-HT (1 μmol/L) for 30 minutes. Cells lysates underwent electrophoresis, and serotonylation was analyzed by Western blotting with anti–5-HT–BSA conjugate antibody. Bar graphs represent means (±SD) for n = 3. *Significant difference from the untreated controls (P < 0.05); #significant difference from 5-HT–treated cells (P < 0.05).
Identification of Major Target Proteins Modified by Serotonylation by MS/MS
Liquid chromatography-mass spectrometry analysis revealed four proteins, including nonmuscle myosin heavy chain IIB (227 kD), FN (257 kD), plakin (195 kD), and filamin B (278 kD), as the major serotonylated proteins in the 220–240 kD band(s) in 5-HT–treated cell lysate (data not shown). Immunoprecipitation and Western blot analysis confirmed that FN was one major protein serotonylated by 5-HT (Figure 5A).
Figure 5.
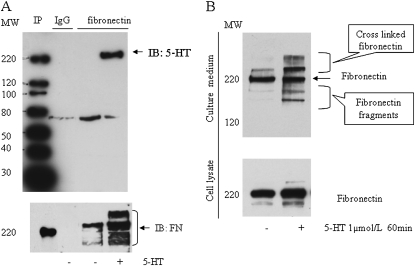
5-HT–induced fibronectin (FN) serotonylation in SMCs. Bovine SMCs were washed with serum-free DMEM and incubated with 5-HT (1 μmol/L) for 1 hour. (A) 5-HT–induced FN serotonylation was assessed by immunoprecipitation (IP) of FN in whole-cell lysates, followed by electrophoresis and immunoblotting (IB) with anti–5-HT–BSA conjugate antibody. Total FN was measured with anti-FN antibody on the stripped membrane (lower panel). (B) Release of FN into the medium. Medium (4 ml) from control (−) and 5-HT–incubated (+) SMCs was concentrated and desalted. FN in the concentrated medium (50 μl) was assessed by Western blotting with anti-FN antibody. Lower panel shows FN in the cell lysate.
As FN is an important extracellular matrix (ECM) protein, we next assessed whether 5-HT treatment resulted in FN accumulation in the medium. A major band in the 220-kD position and lower and higher molecular weight bands were detected in conditioned medium from 5-HT–treated cells, indicating possible degraded fragments and crosslinked products of FN with other extracellular proteins (Figure 5B).
Role of Serotonylation in 5-HT–Induced SMC Proliferation and Migration Responses
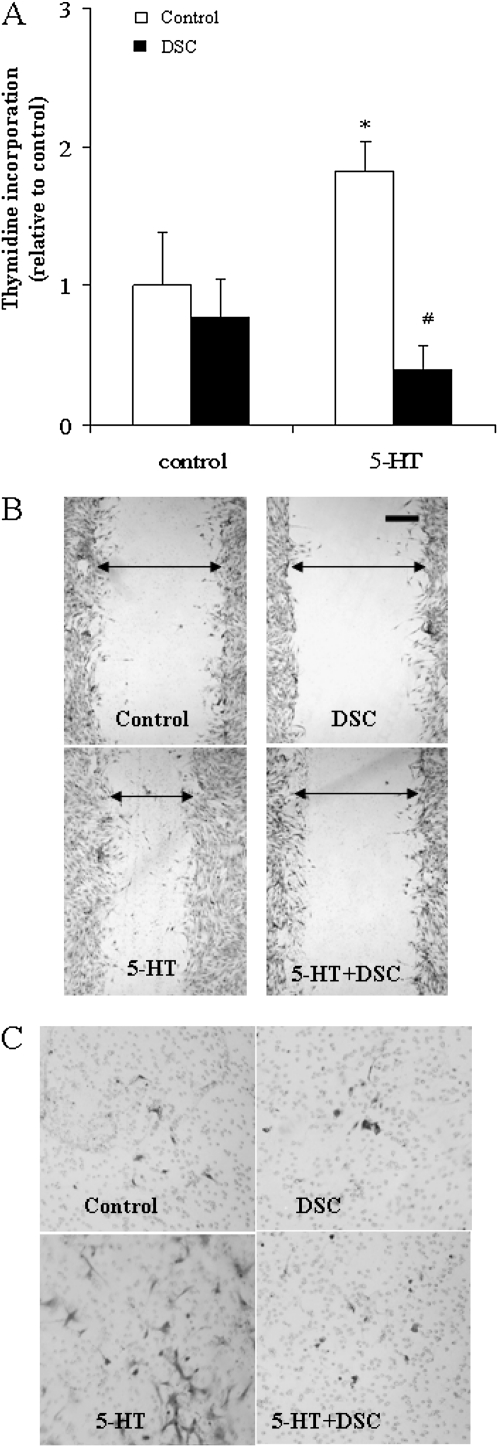
To evaluate the role of protein serotonylation in SMC function, we analyzed the effects of the TGase inhibitor, DSC, on 5-HT–induced proliferation and migration of bovine SMCs. DSC pretreatment (200 μmol/L) caused an inhibition of SMC proliferation, as measured by thymidine incorporation (Figure 6A). Similarly, DSC pretreatment prevented 5-HT–initiated SMC migration, as demonstrated by wound healing and Boyden chamber assays (Figures 6B and 6C).
Figure 6.
Role of TGase in 5-HT–induced SMC proliferation and migration responses. Growth-arrested bovine SMCs were pretreated with TGase inhibitor, DSC (200 μmol/L) for 30 minutes. (A) To test the proliferation response, cells were stimulated with 5-HT (1 μmol/L) for 24 hours. Cell proliferation was analyzed by measuring [3H]-thymidine incorporation (n = 6). (B) Migration was assessed by a wound healing test. Wounding was performed by scraping through the cell monolayer with a sterile 1-ml pipette tip immediately after the addition of 5-HT (10 μmol/L). At 20 hours after 5-HT treatment, cell migration images were taken at four wound sites along the wounding scratch by Nikon phase-contrast microscopy at 100-fold. Triplicate results were obtained in three separate experiments, and a representative experiment is shown. The scale bar shown on the photomicrographs = 0.2 mm. (C) Cell migration was further accessed by Boyden chamber assay. Migrated cells on the bottom of the Transwell filter were stained with crystal violet 20 hours after 5-HT treatment. *Significant difference from control (P < 0.05); #significant difference from 5-HT alone (P < 0.05).
Silencing Cellular FN Inhibits SMC-Proliferative Response to 5-HT
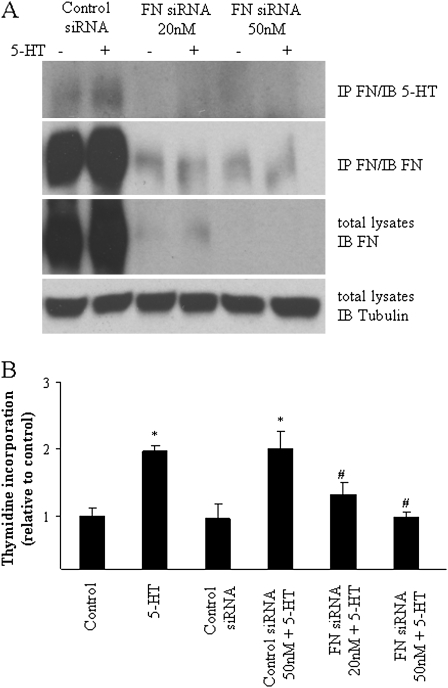
To test the role of FN in SMC proliferation induced by 5-HT, bovine SMCs were transfected with specific FN siRNA. We found that transfection of FN siRNA, but not control siRNA, reduced FN expression to less than 5% of basal level after 72-hour incubation (Figure 7A). FN siRNA also completely blocked SMC proliferation in response to 5-HT (Figure 7B).
Figure 7.
Silencing of cellular FN inhibits SMC-proliferative response to 5-HT. (A) Effects of FN siRNA on its protein expression and serotonylation. Bovine SMCs in 60-mm plates were transfected with FN siRNA and control siRNA using lipofectamine 2,000 for 48 hours. Cells were then treated with 5-HT (1 μmol/L) for 40 minutes. FN serotonylation was evaluated by its immunoprecipitation. FN expression was analyzed in total cell lysates by Western blot and normalized with expression of tubulin on the stripped membrane. (B) Inhibition of 5-HT–induced SMC proliferation by FN siRNA. SMCs transfected with FN siRNA and control siRNA were growth arrested in serum-free medium for 72 hours before treatment with 5-HT (1 μmol/L). Cell proliferation was monitored by [3H]-thymidine incorporation for 24 hours. Data presented are means (±SD) (n = 3). *Significant difference from control (P < 0.05); #significant difference from control siRNA 50 nM + 5-HT (P < 0.05).
DISCUSSION
The process of protein serotonylation was previously demonstrated in platelets, where it was shown that transamidation of surface procoagulant proteins, including fibrinogen and thrombospondin, facilitates activation and blood clot formation (20, 21). Serotonylation of proteins also has been reported to modulate insulin secretion in pancreatic β cells (33). Moreover, Guilluy and colleagues (34) found that transamidation of RhoA by 5-HT led to its activation and depletion in aortic SMCs. Recent studies have demonstrated that serotonylation of contractile and cytoskeleton proteins, including 42-kD smooth muscle actin, is important in isometric contraction of 5-HT–biotin–treated aortic smooth muscle (22).
Cellular internalization of 5-HT through the 5-HTT leads to SMC signaling and proliferation (11, 35). The present studies demonstrate that protein serotonylation participates in the proliferative and migratory response of SMCs to 5-HT. Thus, we found that 5-HT induces serotonylation of several 220- to approximately 240-kD proteins; moreover, TGase inhibition blocks this effect. We also detected approximately 65-kD serotonylated proteins in bovine, but not in rat, SMCs. Antibody neutralization studies demonstrated that the 220- to 240-kD protein(s), but not the approximately 65-kD one(s), specifically bound the 5-HT antibody. It is likely that bovine SMCs express some endogenous BSA precursor protein (69 kD), which reacts with anti–5-HT–BSA antibody (36). Our findings that serotonylation is blocked by 5-HTT inhibitors support a role of 5-HTi in this process. 5-HT, at a concentration of 1 μmol/L, was used in these studies because our previous results showed that this concentration of 5-HT is actively transported by the 5-HTT, whereas higher concentrations enter the cell by diffusion (9, 10). We also found that serotonylation of proteins is increased in the presence of a MAO inhibitor, which prevents degradation of internalized 5-HT. These data indicate that serotonylation of proteins is regulated by the activity of the 5-HTT, MAO, and TGases. Whereas 5-HTT mediates uptake of 5-HT into the cells, both MAO and TGase control 5-HTi metabolism of 5-HT.
The importance of protein serotonylation in the pulmonary artery SMC-proliferative and migratory responses to 5-HT were also investigated. Our findings, that inhibition of TGase blocked these responses, support the concept that this enzymatic process is key to the biologic activity of 5-HT in SMCs.
MS/MS peptide sequencing revealed that the major proteins modified by serotonylation include nonmuscle myosin heavy chain IIB (229 kD), plakin (195 kD), FN (257 kD), and filamin B (278 kD). FN is known to participate in SMC proliferation (34, 41). Immunoprecipitation techniques confirmed that FN was specifically serotonylated by 5-HT. The finding that FN siRNA blocked the proliferative effects of 5-HT on SMCs provides additional support for a role of this protein in SMC function. However, at the present time, it is not clear whether other serotonylated proteins also participate in these mitogenic effects. We demonstrated that serotonylated FN (Figure 5B) and other proteins (data not shown) are present in cell culture medium when SMCs are incubated with 5-HT. We therefore suspected that serotonylated FNs may be released into medium upon cellular interaction with 5-HT. This could be analogous to the process by which it gains access to intercellular matrix and interacts with α5β1-, αIIbβ3-, α4β1-, and α4β7-integrins, leading to differentiation, proliferation, migration, and apoptosis (37–41). The intact FN molecule has four glutamine residue sites sensitive to tissue TGases. The glutamine residues at positions 3 and 4 of the FN amino terminus have been identified as the transglutamination sites for plasma and tissue TGase (42).
In the hypertensive pulmonary vessel wall, vascular SMCs acquire a proliferative, synthetic phenotype, and migrate into the subendothelium (43–45). It has been reported that increased expression of FN not only contributes to the migration of vascular SMCs, but also induces the transformation of these cells into a proliferative phenotype (38, 46). Furthermore, FN deposition in lung is highly correlated with the severity of pulmonary hypertension in rodents with chronic hypoxia and in patients with pulmonary hypertensive (24, 25). Recent studies demonstrate that interactions between αIIbβ3-integrin and 5-HTT regulate 5-HT transport and platelet aggregation in mice and humans, indicating cross-talk between 5-HTT signaling and FN/integrin signaling (47).
Our studies provide evidence of crosslinking of cellular FN and FN fragments in culture medium from 5-HT–treated SMCs. FN crosslinking with other ECM components is a critical step in ECM assembly, and numerous reports have demonstrated that some FN fragments resulting from enzymatic degradation are potent inducers of cell migration (48, 49). Conformational change of the FN molecule may expose certain hidden glutamine residues important in the formation of covalent bonds between FN and the ɛ-amino group of a lysine residue in fibrin. Serotonylated modification of FN may cause a conformational change in the FN that subsequently triggers FN crosslinking with ECM proteins, leading to SMC growth.
In summary, the present studies demonstrate that tissue TGases use 5-HTi to modify substrate proteins (in particular, FN) post-translationally, and that this is important in regulating 5-HT–induced proliferation and migration of pulmonary artery SMCs. Furthermore, this action may play an important role in the development of pulmonary hypertension.
Acknowledgments
The authors thank Dr. H. Zheng and other members of the Biological Mass Spectrometry Facility of University of Medicine and Dentistry of New Jersey/Robert Wood Johnson Medical Center for tandem mass spectrometric peptide sequencing analysis.
This work was supported by National Institute of Health grants HL085260-01 (B.L.F), GM034310, ES004738, CA132624, AR055073, and ES005022 (D.L.L), and by American Heart Association grant 0725961-H (Y.L.).
Originally Published in Press as DOI: 10.1165/rcmb.2010-0078OC on June 17, 2010
Author Disclosure: Y.L. received a sponsored grant from the American Heart Association for $10,001–$50,000; B.L.F. received a sponsored grant from the National Institute of Health for more than $100,001; neither of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rudolph AM, Paul MH. Pulmonary and systemic vascular response to continuous infusion of 5-hydroxytryptamine (serotonin) in the dog. Am J Physiol 1957;189:263–268. [DOI] [PubMed] [Google Scholar]
- 2.Lee SL, Simon AR, Wang WW, Fanburg BLH. (2)O(2) signals 5-HT–induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol 2001;281:L646–L652. [DOI] [PubMed] [Google Scholar]
- 3.Lee SL, Wang WW, Lanzillo JJ, Fanburg BL. Regulation of serotonin-induced DNA synthesis of bovine pulmonary artery smooth muscle cells. Am J Physiol 1994;266:L53–L60. [DOI] [PubMed] [Google Scholar]
- 4.Coatrieux C, Sanson M, Negre-Salvayre A, Parini A, Hannun Y, Itohara S, Salvayre R, Auge N. MAO-A–induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic Biol Med 2007;43:80–89. [DOI] [PubMed] [Google Scholar]
- 5.Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 2006;113:1857–1864. [DOI] [PubMed] [Google Scholar]
- 6.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 2006;98:818–827. [DOI] [PubMed] [Google Scholar]
- 7.Day RM, Agyeman AS, Segel MJ, Chevere RD, Angelosanto JM, Suzuki YJ, Fanburg BL. Serotonin induces pulmonary artery smooth muscle cell migration. Biochem Pharmacol 2006;71:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGFbeta receptor in pulmonary artery smooth muscle cells. FASEB J 2007;21:2725–2734. [DOI] [PubMed] [Google Scholar]
- 9.Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. I. Characterization. Am J Physiol 1986;250:C761–C765. [DOI] [PubMed] [Google Scholar]
- 10.Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol 1986;250:C766–C770. [DOI] [PubMed] [Google Scholar]
- 11.Lee SL, Fanburg BL. Serotonin produces a configurational change of cultured smooth muscle cells that is associated with elevation of intracellular camp. J Cell Physiol 1992;150:396–405. [DOI] [PubMed] [Google Scholar]
- 12.Fesus L, Thomazy V. Searching for the function of tissue transglutaminase: its possible involvement in the biochemical pathway of programmed cell death. Adv Exp Med Biol 1988;231:119–134. [DOI] [PubMed] [Google Scholar]
- 13.Thomazy V, Fesus L. Differential expression of tissue transglutaminase in human cells: an immunohistochemical study. Cell Tissue Res 1989;255:215–224. [DOI] [PubMed] [Google Scholar]
- 14.Lorand L. Crosslinks in blood: transglutaminase and beyond. FASEB J 2007;21:1627–1632. [DOI] [PubMed] [Google Scholar]
- 15.Lorand JB, Urayama T, Lorand L. Transglutaminase as a blood clotting enzyme. Biochem Biophys Res Commun 1966;23:828–834. [DOI] [PubMed] [Google Scholar]
- 16.Lorand L, Conrad SM. Transglutaminases. Mol Cell Biochem 1984;58:9–35. [DOI] [PubMed] [Google Scholar]
- 17.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Natl Rev 2003;4:140–156. [DOI] [PubMed] [Google Scholar]
- 18.Esposito C, Caputo I. Mammalian transglutaminases: identification of substrates as a key to physiological function and physiopathological relevance. FEBS J 2005;272:615–631. [DOI] [PubMed] [Google Scholar]
- 19.Porta R, Esposito C, Metafora S, Malorni A, Pucci P, Siciliano R, Marino G. Mass spectrometric identification of the amino donor and acceptor sites in a transglutaminase protein substrate secreted from rat seminal vesicles. Biochemistry 1991;30:3114–3120. [DOI] [PubMed] [Google Scholar]
- 20.Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemost 2005;3:2185–2192. [DOI] [PubMed] [Google Scholar]
- 21.Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 2002;415:175–179. [DOI] [PubMed] [Google Scholar]
- 22.Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS ONE 2009;4:e5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilluy C, Rolli-Derkinderen M, Tharaux PL, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem 2007;282:2918–2928. [DOI] [PubMed] [Google Scholar]
- 24.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 1997;150:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Shiraishi K, Mori M, Motomiya M. Changes of fibronectin in the right and left ventricles of rats exposed to chronic normobaric hypoxia. Tohoku J Exp Med 1992;168:573–582. [DOI] [PubMed] [Google Scholar]
- 26.Olson TP, Snyder EM, Frantz RP, Turner ST, Johnson BD. Repeat length polymorphism of the serotonin transporter gene influences pulmonary artery pressure in heart failure. Am Heart J 2007;153:426–432. [DOI] [PubMed] [Google Scholar]
- 27.Preston IR, Hill NS, Warburton RR, Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol 2006;290:L367–L374. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 2004;95:579–586. [DOI] [PubMed] [Google Scholar]
- 29.Ray E, Samanta AK. Dansyl cadaverine regulates ligand induced endocytosis of interleukin-8 receptor in human polymorphonuclear neutrophils. FEBS Lett 1996;378:235–239. [DOI] [PubMed] [Google Scholar]
- 30.Ueki S, Takagi J, Saito Y. Dual functions of transglutaminase in novel cell adhesion. J Cell Sci 1996;109:2727–2735. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Johnson BR, Bjornsson TD. Pharmacologic inhibition of transglutaminase-induced cross-linking of alzheimer's amyloid beta-peptide. Life Sci 1997;60:2323–2332. [DOI] [PubMed] [Google Scholar]
- 32.Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol 2008;154:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol 2009;7:e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, et al. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med 2009;179:1151–1158. [DOI] [PubMed] [Google Scholar]
- 35.Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res 1991;68:1362–1368. [DOI] [PubMed] [Google Scholar]
- 36.Holderman MT, Miller KP, Dangott LJ, Ramos KS. Identification of albumin precursor protein, Phi AP3, and alpha-smooth muscle actin as novel components of redox sensing machinery in vascular smooth muscle cells. Mol Pharmacol 2002;61:1174–1183. [DOI] [PubMed] [Google Scholar]
- 37.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front Biosci 1997;2:d126–d146. [DOI] [PubMed] [Google Scholar]
- 38.Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 1998;18:1363–1370. [DOI] [PubMed] [Google Scholar]
- 39.Mosher DF, Furcht LT. Fibronectin: review of its structure and possible functions. J Invest Dermatol 1981;77:175–180. [DOI] [PubMed] [Google Scholar]
- 40.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol 2001;189:1–13. [DOI] [PubMed] [Google Scholar]
- 41.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002;115:3861–3863. [DOI] [PubMed] [Google Scholar]
- 42.Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem 1997;272:24999–25005. [DOI] [PubMed] [Google Scholar]
- 43.Lee KM, Tsai KY, Wang N, Ingber DE. Extracellular matrix and pulmonary hypertension: control of vascular smooth muscle cell contractility. Am J Physiol 1998;274:H76–H82. [DOI] [PubMed] [Google Scholar]
- 44.Mitani Y, Ueda M, Komatsu R, Maruyama K, Nagai R, Matsumura M, Sakurai M. Vascular smooth muscle cell phenotypes in primary pulmonary hypertension. Eur Respir J 2001;17:316–320. [DOI] [PubMed] [Google Scholar]
- 45.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 2000;190:300–309. [DOI] [PubMed] [Google Scholar]
- 46.Rabinovitch M. Elastase and the pathobiology of unexplained pulmonary hypertension. Chest 1998;114(3 Suppl):213S–224S. [DOI] [PubMed] [Google Scholar]
- 47.Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest 2008;118:1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikari Y, Yee KO, Schwartz SM. Role of alpha5beta1 and alphaVbeta3 integrins on smooth muscle cell spreading and migration in fibrin gels. Thromb Haemost 2000;84:701–705. [PubMed] [Google Scholar]
- 49.Powell RJ, Carruth JA, Basson MD, Bloodgood R, Sumpio BE. Matrix-specific effect of endothelial control of smooth muscle cell migration. J Vasc Surg 1996;24:51–57. [DOI] [PubMed] [Google Scholar]