Abstract
Thrombospondin-1 (TSP-1) is an extracellular protein critical to normal lung homeostasis, and is reported to activate latent transforming growth factor-β (TGF-β). Because active TGF-β is causally involved in lung fibrosis after bleomycin challenge, alterations in TSP-1 may be relevant to pulmonary fibrosis. We sought to determine the effects of TSP-1 deficiency on the susceptibility to bleomycin-induced pulmonary fibrosis in a murine model. Age-matched and sex-matched C57BL/6 wild-type (WT) and TSP-1–deficient mice were treated twice weekly for 4 weeks with intraperitoneal bleomycin (0.035 U/g) or PBS, and were allowed to rest 1 week before being killed. Their lungs were inflated with PBS, fixed in formalin, paraffin-embedded, and sectioned. A certified veterinary pathologist blindly scored each slide for inflammation and fibrosis. Lungs were homogenized to obtain RNA and protein for the real-time RT-PCR analysis of connective tissue growth factor (CTGF) and collagen I, and for Western blotting to detect phospho-Smad2, or total Smad2/3, respectively. In response to bleomycin treatment, measures of fibrosis and inflammation, along with CTGF and collagen I mRNA concentrations, were increased in TSP-1–deficient mice compared with WT mice. Notably, Smad 2/3 signaling was of equal strength in WT and TSP-1 knockout mice treated with bleomycin, suggesting that TSP-1 is not required for the activation of TGF-β. These results demonstrate that TSP-1 deficiency does not protect mice from systemic bleomycin challenge, and that TSP-1 deficiency is associated with increased expression of lung collagen and CTGF.
Keywords: TSP-1, pulmonary fibrosis, TGF-β, bleomycin
CLINICAL RELEVANCE.
Pulmonary fibrosis is a complex and often devastating disease, and fibrogenesis is understood to be important in its pathogenesis. In this study, thrombospondin-1 deficiency appears to worsen lung fibrosis in response to bleomycin. Thrombospondin-1 deficiency is also associated with an increased expression of lung collagen and connective tissue growth factor.
Pulmonary fibrosis is a complex disease that likely represents abnormal lung repair after injury. Idiopathic pulmonary fibrosis in humans is characterized by a dense distribution of paraseptal and subpleural collagen and matrix, with a minor component of interstitial inflammation (1). In the past, the primary focus of research in pulmonary fibrosis was the role of inflammation in the disease. More recently, the role of fibrogenesis has emerged as an important concept in the pathogenesis of the disease (2).
A key molecular regulator of lung fibrosis in animal models and humans with disease is active transforming growth factor-β (TGF-β). Active TGF-β and its downstream gene product, connective tissue growth factor (CTGF), are implicated in the genesis and progression of pulmonary fibrosis (3–6). Previous reports suggest that the activation of TGF-β in vivo relies on interaction between thrombospondin-1 (TSP-1) and latent TGF-β (7). The activation of TGF-β then leads to the production of CTGF, a CCN (CTGF, CEF10/Cyr61, and Nov) family member important in organ fibrosis. In addition to the potential for TGF-β activation by TSP-1, the αvβ6 integrin appears responsible for activating TGF-β in response to intratracheal bleomycin (8). Recent work confirms the importance of the integrin-based activation of TGF-β in murine models of inflammation, because mice with mutations in their integrin-binding region of latency-associated peptide (LAP) suffer organ inflammation reminiscent of that seen in TGF-β–deficient mice (9). These data suggest that the integrin-based activation of the TGF-β family provides a critical biological signal to regulate TGF-β activity, leaving the in vivo role of TSP-1 in lung fibrosis unanswered.
TSP-1 is an extracellular protein critical to normal lung homeostasis, and is involved in a number of physiologic processes (10). First discovered as the major component of α granules of platelets as a “thrombin-sensitive protein,” TSP-1 is involved in the coagulation pathway and in wound-healing (11). TSP-1 is important in regulating cellular apoptosis (12), cellular proliferation (13, 14), angiogenesis (15–20), and tumor growth through specific interactions with growth factors, cytokines, and matrix components.
TSP-1 is thought to be a major regulator of latent TGF-β activation (7) and of macrophage recruitment (21), raising the possibility that TSP-1 augments the activation of TGF-β by recruiting cells involved in its production. However, recent data suggest that TSP-1 is not necessary for the biological properties ascribed to active TGF-β1 (9). A wound model was used to understand the role and regulation of TSP-1 in tissue repair and remodeling (22). In that model, platelets are the initial source of TSP-1 activity. After the initial contribution by platelets, monocytes and macrophages appear to be critical sources (22–26). The contributions of monocytes and macrophages extend to alveolar macrophages, where TSP-1, CD36, and TGF-β localize to regulate the activity of TGF-β (27–31). We recently found that TSP-1 is important in the biological activity of the LAP component of latent TGF-β, providing an additional role for TSP-1 in the biology of the latent TGF-β complex (32).
The pathways underlying matrix interactions governing human pulmonary fibrosis are not clear. Human lung samples are being collected and stored to analyze the molecular and biochemical mechanisms involved. Although this resource is valuable, it cannot be manipulated to uncover causal relationships between targets. To facilitate this testing, animal models of human disease were developed, including murine models of pulmonary fibrosis. The most common murine model of pulmonary fibrosis involves the use of bleomycin, a chemotherapeutic agent well-recognized for causing fibrotic lung disease in humans. Although the model does not mirror fibrotic lung disease in humans, the injection of bleomycin intraperitoneally into mice results in a peripheral, subpleural distribution of the pulmonary fibrosis evident in human idiopathic pulmonary fibrosis (33). In this study, we investigated the effects of TSP-1 deficiency on bleomycin-induced pulmonary fibrosis in mice.
MATERIALS AND METHODS
Animal Model of Pulmonary Fibrosis
C57BL/6 wild-type mice (Jackson Laboratory, Bar Harbor, ME) and TSP-1–deficient mice were maintained in pathogen-free conditions. To induce pulmonary fibrosis, 8-week-old mice were challenged with intraperitoneal bleomycin at 0.035 U/g twice weekly for 4 weeks. Control animals received intraperitoneal PBS. Animals were killed by carbon monoxide inhalation, 1 week after the final injection.
Histologic and Immunohistochemical Analyses
Longitudinal sections of the lung from each mouse were evaluated for fibrosis in a blinded fashion by a board-certified veterinary pathologist. The degree of fibrosis was graded, according to a modified Ashcroft score, on a scale from 0–4. Similarly, inflammation was graded on a 0–4 scale for worsening inflammation, as previously described (33).
The antibodies used for immunohistochemical analyses included CD3, F4/80, and neutrophil stain MCA771G (AbD Serotec, Raleigh, NC). Images from each slide were obtained, and the average numbers of pixels corresponding to specific stains were quantitated using Adobe Photoshop software (San Jose, CA).
Collagen Expression
To determine collagen expression by PCR, cDNA was subjected to real-time PCR on an ABI 7700 machine (Applied Biosystems, Foster City, CA), using primers for collagen I (SABiosciences Corp., Fredrick, MD) and SYBR Green (Invitrogen, Carlsbad, CA), with an internal control of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Collagen content was also measured as soluble collagen with a Sircol Assay Kit (Biocolor, Ltd., Belfast, Northern Ireland, UK). Measurements were performed using the procedure outlined by the manufacturer.
Western Blot Analysis
Western blot analysis was performed for the measurement of phospho-Smad2 and total Smad2/3. Results were quantitated using densitometry.
Analysis of Smad Activity from TSP-1–Deficient Bone Marrow–Derived Macrophages
LPS is known to activate endogenous TGF-β, and was used to determine whether TSP-1 was necessary for endogenous TGF-β activation. Bone marrow–derived macrophages (BMMs) were derived from TSP-1–deficient and wild-type (WT) mice by growth in the presence of macrophage colony-stimulating factor (M-CSF) (20 ng/ml) for 5 days, as previously described (32). Cells were serum-starved, stimulated with 100 ng/ml of LPS, and collected at various time points. The collected supernatants were analyzed by the transformed mink lung epithelial cell (TMLC) assay for active TGF-β (34, 35).
Statistical Analysis
For all ELISA and real-time PCR analyses, we used Minitab software (Minitab, State College, PA) with ANOVA and Tukey post hoc testing. The Student t test was used for analyses of cell numbers from murine bronchoalveolar lavage (BAL). These analyses were completed using SAS for Windows, version 9.1 (SAS Institute, Cary, NC). A P value of less than 0.05 was considered statistically significant for all comparisons.
The Western blot proteins (pSMAD/total SMAD, and pSMAD/PBS control) were analyzed using ANOVA. A group was defined by the combination of genotype (WT or TSP-1−/−) and treatment (PBS or bleomycin). If an overall F-test was statistically significant, four comparisons were tested for each protein: WT PBS versus WT bleomycin, TSP-1−/− PBS versus TSP-1−/− bleomycin, WT bleomycin versus TSP-1−/− bleomycin, and WT PBS versus TSP-1−/− PBS. All P values were adjusted using the Holm procedure to conserve Type I error. These analyses were performed using Stata 10.0 (Stata Corp., College Station, TX).
RESULTS
TSP-1−/− Mice Manifest Greater Amounts of Fibrosis than WT Mice after Bleomycin Challenge
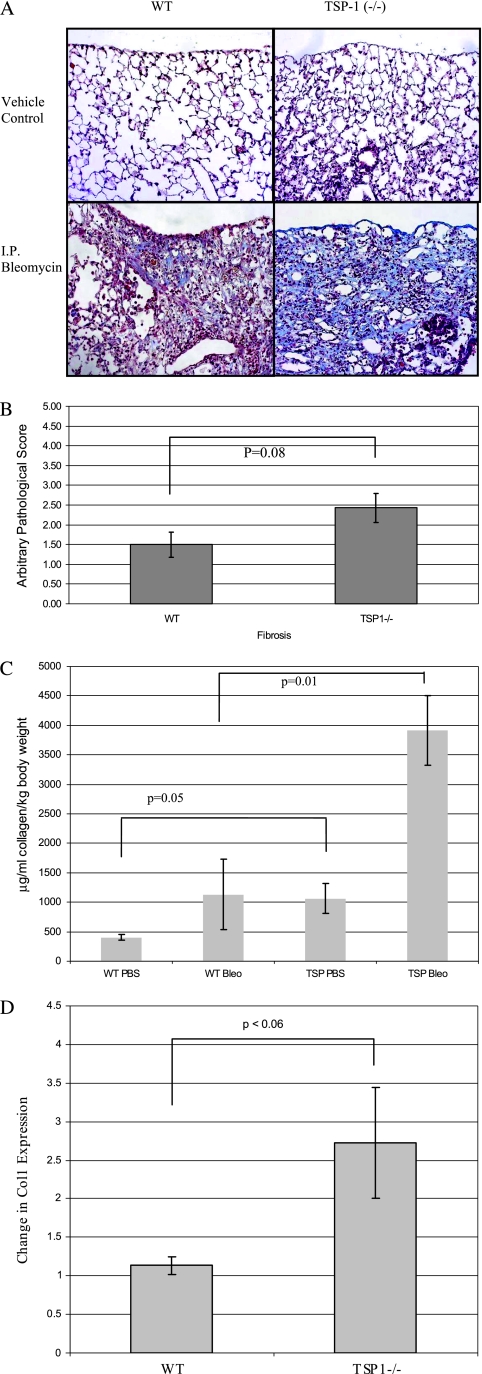
Similar to our previous findings (33), pulmonary fibrosis predominated in subpleural areas of TSP-1–deficient and WT mice after intraperitoneal bleomycin challenge (Figure 1A). According to light microscopy, pulmonary fibrosis appeared to be more severe in TSP-1−/− mice (Figure 1A). Importantly, the pathologic fibrosis score of TSP-1−/− mice treated with bleomycin tended to be higher than of bleomycin-treated WT mice quantified in a blinded fashion by a board-certified veterinary pathologist (Figure 1B). No fibrosis was evident in the PBS (vehicle)-treated control mice (data not shown). Thus, in contrast to our initial hypothesis, TSP-1–deficient mice were not protected against fibrosis after bleomycin challenge compared with WT animals, and in fact, appeared to manifest more fibrosis than control mice.
Figure 1.
Analysis of bleomycin-induced fibrosis in thrombospondin-1 (TSP-1)−/− mice. (A) TSP-1−/− mice suffer more fibrosis than do wild-type (WT) animals after intraperitoneal (I.P.) bleomycin challenge. Representative trichrome stains of these lungs are shown. Data are representative of six mice per group. (B) TSP-1–deficient mice tended to manifest more fibrosis after bleomycin challenge. A certified veterinary pathologist blindly scored each slide for fibrosis. The average pathology scores ± SEMs for six mice per group are shown. No fibrosis was evident in vehicle-treated control mice (data not shown). (C) TSP-1−/− lungs contain more collagen according to Sircol assay after bleomycin challenge. Lungs were homogenized with acetic acid, and Sircol dye was added to each sample to bind collagen. Alkali reagent was added to release the bound dye into solution, and absorbance at a 540-nm wavelength was obtained. Results were normalized to the dead weight of each mouse. The average data ± SEMs are shown for a minimum of four animals per treatment group. P = 0.05, comparing bleomycin-treated WT mice (WT Bleo) versus TSP-1–deficient mice. (D) Bleomycin-challenged TSP-1−/− mice exhibited more collagen I mRNA than did WT animals in whole-lung preparations after bleomycin treatment. RNA was extracted and subjected to real-time RT-PCR analysis, using SYBR Green. Shown is the average fold increase of Collagen I (Col1) expression over Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) ± SEMs in untreated versus bleomycin-treated mice.
TSP-1−/− Mice Manifest More Collagen in Response to Bleomycin Challenge than WT Animals
We analyzed lungs from TSP-1−/− and WT mice to quantify the effects of TSP-1 on bleomycin-induced collagen production. Lungs from TSP-1−/− mice tended to contain more collagen according to Sircol assay after bleomycin challenge than lungs from bleomycin-treated WT mice (Figure 1C). Bleomycin-challenged TSP-1−/− animals also had more collagen I mRNA than WT animals in whole-lung preparations after bleomycin treatment (Figure 1D).
Lung Inflammation in TSP-1−/− and WT Animals after Bleomycin Challenge
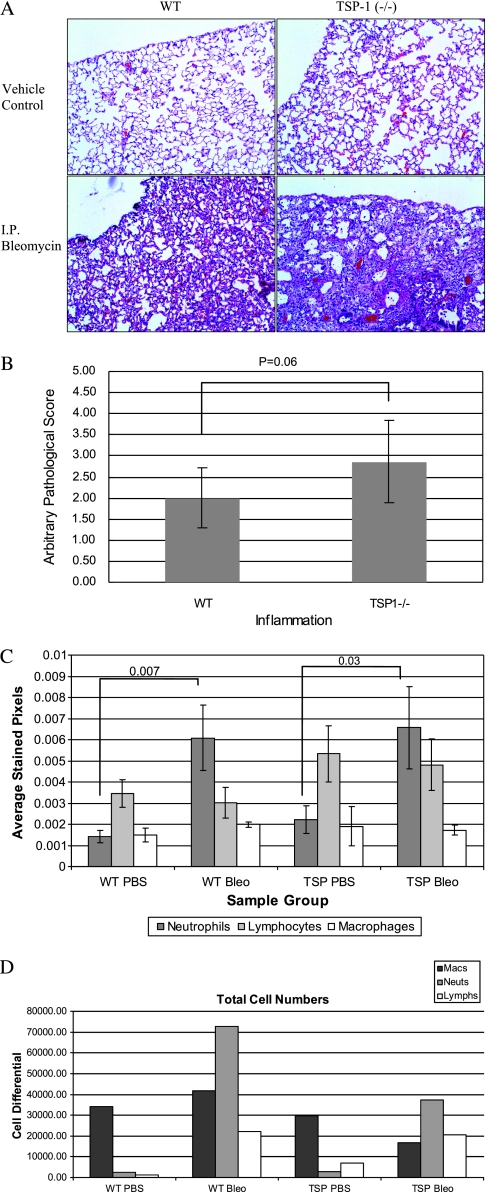
TSP-1−/− and WT mice both suffered inflammation after bleomycin treatment (Figure 2A). Inflammation scores were higher in the lungs of TSP-1−/− bleomycin-treated mice compared with WT-treated mice, as quantitated in a blinded fashion by a board-certified veterinary pathologist (Figure 2B), and tended toward significance. According to the immunohistochemical staining of lung tissue, no difference was evident in neutrophil or macrophage numbers in TSP-1−/− murine lungs compared with the lungs of WT mice after bleomycin treatment (Figure 2C). However, TSP-1−/− mice tended to manifest more lung lymphocytes at baseline and after bleomycin treatment than WT mice after anti-CD3 immunohistochemical staining of lung sections from these mice (P value of 0.09 for TSP-1−/− versus WT). This finding is consistent with previous observations of leukocyte infiltration into the lungs, pancreas, and spleens of these animals (36). Interestingly, upon examination of BAL fluid, TSP-1−/− animals had fewer total cells than WT mice after bleomycin challenge (Figure 2D), suggesting a key difference in tissue-bound inflammation versus lower airway/alveolar inflammation in these animals.
Figure 2.
Analysis of bleomycin-induced inflammation in TSP-1−/− mice. (A) TSP-1−/− and WT mice suffer inflammation after bleomycin injection. Representative hematoxylin-and-eosin stains of these lungs are shown. Data are representative of six mice per group. (B) TSP-1−/− mice tended to exhibit more inflammation after bleomycin challenge. A certified veterinary pathologist blindly scored each slide for inflammation. Shown is the average pathology score ± SEM for six mice per group. No inflammation was evident in vehicle-treated control mice (data not shown). (C) Analysis of cellular recruitment. Slides were subjected to immunohistochemical analysis. From each slide, 10 images were obtained using an Olympus microscope with digital camera (Center Valley, PA). Average numbers of pixels corresponding to specific stains were quantitated using Adobe Photoshop software. Average data ± SEMs are shown for a minimum of five animals per treatment group. All P values not shown are > 0.20. (D) Fresh lungs were flushed with 1.5 ml of sterile PBS. Cell pellets were obtained and resuspended in 200 μl PBS, and cells were counted using a hemacytometer. Cells in PBS were cytospun and stained. One hundred cells were counted to obtain cell differential percentages, and total cell-line numbers were obtained by multiplying the differential by the total cell count. Macs, macrophages; Lymphs, lymphocytes; Neuts, neutrophils.
TSP-1−/− Mice Manifest More CTGF mRNA than Do WT Mice after Bleomycin Challenge
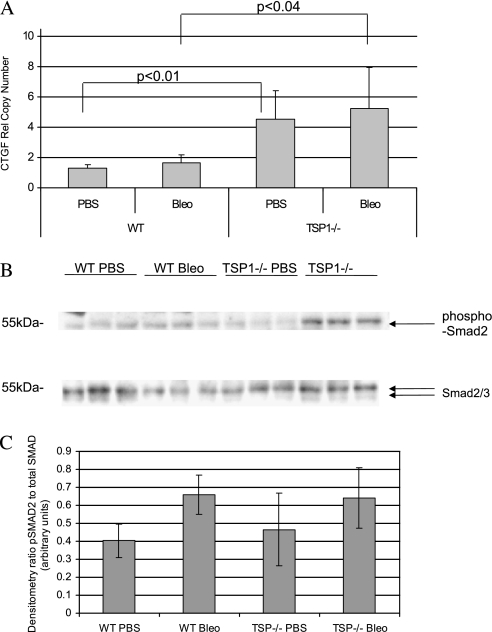
Because collagen expression was elevated in TSP-1−/− mice after bleomycin treatment, we assayed CTGF, a growth factor that induces collagen synthesis. TSP-1−/− animals exhibited a higher expression of lung CTGF mRNA than did WT animals with or without bleomycin treatment (Figure 3A).
Figure 3.
Phospho-Smad2 is induced in TSP-1−/− animals treated with bleomycin, which is accompanied by increased expression of connective tissue growth factor (CTGF). (A) Real-time PCR for CTGF expression. RNA was isolated from lung tissue and subjected to real-time PCR analysis, using SYBR Green. The relative copy number ± SEM, compared with GAPDH for CTGF, is shown. CTGF mRNA expression is increased in TSP-1–deficient mice. (B) Western blot for endogenous Smad activity after bleomycin challenge in TSP-1−/− or WT murine lungs after bleomycin challenge. Lungs were homogenized to obtain protein from WT or TSP-1−/− mice treated with either PBS or bleomycin. Equal concentrations of protein were subjected to Western blot analysis for phospho-Smad2, or total Smad2/3. (C) Densitometry for Smad activity. Western blots for phospho-Smad2 and Smad2/3 were quantitated by densitometry to average numbers of pixels for untreated WT mice. Shown is the average ratio ± SD. For the overall F-test, the P value was 0.031. All comparison P values were greater than the F-test P value.
TSP-1−/− and WT Mice Manifest Similar Concentrations of Active TGF-β and Smad Activity after Bleomycin Challenge
Because TGF-β is important in lung fibrosis and the expression of CTGF, we wanted a direct assessment of the role of TSP-1 in activating TGF-β. We performed an active TGF-β ELISA on BAL fluid from the mice. The amount of active TGF-β was below detectable levels in the majority of samples tested from both PBS and bleomycin-treated animals. Therefore, because Smad2/3 phosphorylation is an endogenous downstream activation event driven by active TGF-β, we examined the phosphorylation of Smad2/3 from vehicle-treated and bleomycin-treated lung tissue in TSP-1−/− and WT mice (Figure 3B). The phosphorylation of Smad2/3 occurred equally in TSP-1−/− mice and in WT mice treated with bleomycin (Figure 3C).
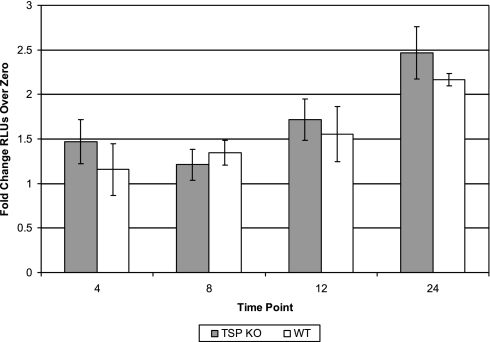
Because we previously reported that macrophages are important in the pathogenesis of pulmonary fibrosis (33), we were interested in understanding whether macrophages from the TSP-1−/− mice were able to produce active TGF-β. To confirm our in vivo observation, we harvested macrophages from TSP-1−/− and WT mice and challenged these cells in vitro with LPS, to evaluate differences in the generation of active TGF-β. Active TGF-β production, as measured by the TMLC assay, was similar in LPS-stimulated TSP-1−/− and WT macrophages (Figure 4).
Figure 4.
Active TGF-β production in vitro by TSP-1−/− macrophages stimulated with LPS is similar to that by WT macrophages. Bone marrow–derived macrophages (BMMs) were derived from TSP-1−/− (TSP KO) and WT mice were treated with 20 ng/ml of M-CSF for 5 days. Cells were serum-starved, stimulated with 100 ng/ml LPS, and collected at 4-hour, 8-hour, 12-hour, and 24-hour time points. The collected supernatants were analyzed by TMLC assay for active TGF-β and relative light units (RLUs) were determined. Active TGF-β concentrations were equivalent in WT and TSP-1−/− mice at zero hours. The fold change in active TGF-β from TSP-1−/− BMMs is the same as that from WT BMMs (n = 4).
DISCUSSION
This report demonstrates that TSP-1 deficiency conferred no protection against bleomycin-induced pulmonary fibrosis in a murine model. Our initial hypothesis that TSP-1 deficiency would protect mice against bleomycin-induced pulmonary fibrosis was based on the reported role of TSP-1 as a major activator of latent TGF-β. Because active TGF-β plays a causal role in murine bleomycin-induced pulmonary fibrosis, we anticipated that a deficiency of TSP-1 would reduce the activation of TGF-β. As a reflection of endogenous TGF-β activity in the lungs of bleomycin-treated TSP-1−/− or WT mice, we measured the activation of Smad2/3, and found no differences in Smad2/3 activation in bleomycin-treated murine lung tissue or in LPS-stimulated macrophages from TSP-1−/− mice compared with WT mice. Similarly, we found no differences in fibrosis or neutrophil or macrophage recruitment to the lungs between bleomycin-challenged TSP-1−/− and WT mice. We did find more lung lymphocytes in TSP-1–deficient mice after treatment with bleomycin, demonstrating a role for TSP-1 in lymphocyte trafficking after lung injury. Finally, we found that TSP-1−/− mice manifested increased amounts of lung collagen according to a Sircol assay, and also displayed increased collagen I and CTGF mRNA after the administration of bleomycin, compared with WT mice.
TSP-1 is an extracellular protein critical to normal lung homeostasis, and is involved in a number of physiologic processes (36). TSP-1 is an important regulator of TGF-β activation (7) and macrophage recruitment (21), raising the possibility that in some environments, TSP-1 augments the activation of TGF-β by recruiting cells involved in its production. Much of the information about TSP-1 comes from the TSP-1−/− mouse (36). Phenotypically, these animals exhibit hyperplasic epithelia and leukocyte infiltration in many organs, with acute and chronic inflammatory changes in the lung and pancreas (36). Because the phenotype of TSP-1−/− mice resembles that of TGF-β1–deficient animals, and because TSP-1 can regulate latent TGF-β activation in vitro, TSP-1 was hypothesized to act as a critical physiologic activator of TGF-β (7). However, we found that the activation of Smad2/3 was normal in TSP-1−/− murine lungs in vivo and in BMMs in vitro. We also found that TSP-1−/− mice manifested a higher expression of lung collagen and CTGF after bleomycin challenge than did WT mice. Interestingly, the amount of collagen measured by the Sircol assay was similar in WT mice receiving bleomycin and in TSP-1−/− mice treated only with vehicle. This finding suggests that the mice possess some background defect in collagen production. TSP-1 may, in fact, limit lung inflammation and fibrosis after an injurious challenge, and our data raise new questions of how TSP-1 affects lung homeostasis.
Other reports demonstrated that integrins are important in regulating lung fibrosis after bleomycin challenge. Specifically, the integrin αvβ6 is critically important in the pathogenesis of pulmonary fibrosis and the activation of TGF-β after intratracheal bleomycin challenge (8). Interestingly, a mutation of the Arg-Gly-Asp (RGD) binding site on LAP, blocking interaction with integrins, recapitulates the basal inflammatory phenotype of TGF-β1–deficient mice (9). We showed that RGD binding sites on LAP are not required for interacting with TSP-1, raising the likelihood that TSP-1 is dispensable for the binding of LAP, to facilitate the activation of latent TGF-β (32). We previously reported that TSP-1 is critical for the independent biological activity of LAP, independent of the ability of LAP to sequester active TGF-β (32). In vivo, LAP independently reduces ear inflammation in a delayed-type hypersensitivity response (DTHR) injury model and induces the recruitment of macrophages, both requiring TSP-1 (32).
Our data suggest that TSP-1 is dispensable for the activation of TGF-β, but may limit fibrosis in a murine model of lung injury and inflammation. We observed that the expression of collagen and CTGF increased after bleomycin challenge in TSP-1−/− mice compared with WT mice. Growing evidence indicates that CTGF plays a critical role in organ fibrosis, including pulmonary fibrosis and cirrhosis. In models of repair and remodeling versus organ fibrosis, the relationship of CTGF to TGF-β was surmised to play an important role in the reversibility of fibrosis. In situations where fibrosis is reversible, a well-orchestrated serial production of active TGF-β appears to occur, followed by CTGF. In sustained fibrosis, the parallel expression of active TGF-β and CTGF appears to occur, which may drive and sustain fibrosis (3). However, the presence of CTGF alone may not be sufficient for fibrosis to occur (3). Instead, we speculate that the presence of CTGF with active TGF-β is important in tissue fibrosis. Furthermore, the non–TGF-β activation of CTGF is certainly a possibility, as previously described (37). CTGF can be induced independent of TGF-β by vascular endothelial growth factor, among other factors (38). In addition, posttranscriptional events also appear to be involved, given that CTGF is a matrix-bound protein. Although we observed an increase in CTGF mRNA, no significant change was evident in protein concentrations between WT and TSP-1−/− mice at baseline. Because we observed significant increases in the protein concentrations of CTGF after bleomycin treatment in both kinds of mice, the system may be primed, but may require bleomycin treatment to facilitate the production of CTGF.
The relationship between TSP-1 and CTGF was previously reported in a murine model of erosive arthritis. TSP-1 was shown to play a role in regulating CTGF gene and protein expression in synovial tissue. Reduced concentrations of TSP-1 with increased expression of CTGF were evident in synovial tissue. Treatment with a TSP-1 peptide downregulated the expression of CTGF (39). In human pulmonary fibrosis, increased CTGF gene expression and elevated concentrations of CTGF protein in isolated fibroblasts occur (4). In an animal model of bleomycin-induced lung fibrosis, antibodies targeting CTGF were protective against the development of lung fibrosis (3). Interestingly, basal CTGF expression was elevated in TSP-1−/− murine lungs, suggesting that TSP-1 may in fact basally suppress CTGF expression. In our study, TSP-1 deficiency appeared to worsen lung fibrosis in response to bleomycin. A greater deposition of collagen and expression of CTGF occurred in TSP-1−/− mice treated with bleomycin than in WT mice, but this finding appeared to be independent of active TGF-β.
This work was supported by National Institutes of Health grants HL067176, HL63800, and HL66108 (C.B.M.), and by the Flight Attendant Medical Research Institute (M.E.E.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0019OC on June 25, 2010
Author Disclosure: D.B. has a patent for heparin-binding growth factor polypeptides, and receives royalties from FibroGen for $10,001–$50,000. D.B. also owns stocks in FibroGen for $1,001–$5,000, and received a sponsored grant from the National Institutes of Health for more than $100,001. M.E.E. received sponsored grants from the National Institutes of Health for $50,001–$100,000 and the Flight Attendant Medical Research Institute for more than $100,001. C.B.M. received a sponsored grant from the Battelle Memorial Institute for more than $100,001, and has three patents pending from Ohio State University and one patent pending from the Battelle Memorial Institute. C.B.M. also received a sponsored grant from the National Institutes of Health for more than $100,001. M.G.P. received a sponsored grant from the Battelle Memorial Institute for more than $100,001, and has three patents pending from Ohio State University and one patent pending from the Battelle Memorial Institute. M.G.P. also received sponsored grants from the National Institutes of Health for more than $100,001, a grant from the American Thoracic Society for $50,001–$100,000, and a grant from the Francis Family for $50,001–$100,000. N.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.A.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.M.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 2.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE Jr, Leinwand LA, et al. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2002;166:236–246. [DOI] [PubMed] [Google Scholar]
- 3.Bonniaud P, Martin G, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol 2004;31:510–516. [DOI] [PubMed] [Google Scholar]
- 4.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol 1998;275:L365–L371. [DOI] [PubMed] [Google Scholar]
- 5.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 2006;3:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 2000;342:1350–1358. [DOI] [PubMed] [Google Scholar]
- 7.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 1998;93:1159–1170. [DOI] [PubMed] [Google Scholar]
- 8.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha V beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol 2007;176:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol 2000;19:597–614. [DOI] [PubMed] [Google Scholar]
- 11.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double–TSP1/TSP2–null mice. Am J Pathol 2002;161:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedl P, Vischer P, Freyberg MA. The role of thrombospondin-1 in apoptosis. Cell Mol Life Sci 2002;59:1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JC. Thrombospondin-1. Int J Biochem Cell Biol 1997;29:861–865. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein RL. The face of TSR revealed: an extracellular signaling domain is exposed. J Cell Biol 2002;159:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol 2003;22:63–71. [DOI] [PubMed] [Google Scholar]
- 16.Bouma-ter Steege JC, Mayo KH, Griffioen AW. Angiostatic proteins and peptides. Crit Rev Eukaryot Gene Expr 2001;11:319–334. [PubMed] [Google Scholar]
- 17.Carpizo D, Iruela-Arispe ML. Endogenous regulators of angiogenesis: emphasis on proteins with thrombospondin-Type I motifs. Cancer Metastasis Rev 2000;19:159–165. [DOI] [PubMed] [Google Scholar]
- 18.Detmar M. Tumor angiogenesis. J Investig Dermatol Symp Proc 2000;5:20–23. [DOI] [PubMed] [Google Scholar]
- 19.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 2002;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawler J. The functions of thrombospondin-1 and -2. Curr Opin Cell Biol 2000;12:634–640. [DOI] [PubMed] [Google Scholar]
- 21.Moodley Y, Rigby P, Bundell C, Bunt S, Hayashi H, Misso N, McAnulty R, Laurent G, Scaffidi A, Thompson P, et al. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol 2003;162:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- 23.Barbul A. Immune aspects of wound repair. Clin Plast Surg 1990;17:433–442. [PubMed] [Google Scholar]
- 24.Diegelmann RF, Cohen IK, Kaplan AM. The role of macrophages in wound repair: a review. Plast Reconstr Surg 1981;68:107–113. [DOI] [PubMed] [Google Scholar]
- 25.Leibovich SJ, Wiseman DM. Macrophages, wound repair and angiogenesis. Prog Clin Biol Res 1988;266:131–145. [PubMed] [Google Scholar]
- 26.Leibovich SJ, Ross R. The role of the macrophage in wound repair: a study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 27.Adamson IY, Letourneau HL, Bowden DH. Enhanced macrophage–fibroblast interactions in the pulmonary interstitium increases fibrosis after silica injection to monocyte-depleted mice. Am J Pathol 1989;134:411–418. [PMC free article] [PubMed] [Google Scholar]
- 28.Agostini C, Siviero M, Semenzato G. Immune effector cells in idiopathic pulmonary fibrosis. Curr Opin Pulm Med 1997;3:348–355. [DOI] [PubMed] [Google Scholar]
- 29.Bowden DH. The alveolar macrophage. Environ Health Perspect 1984;55:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalil N, Greenberg AH. The role of TGF-beta in pulmonary fibrosis. Ciba Found Symp 1991;157:194–207. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura H, Fujishima S, Waki Y, Urano T, Sayama K, Sakamaki F, Terashima T, Soejima K, Tasaka S, Ishizaka A, et al. Priming of alveolar macrophages for interleukin-8 production in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1995;152:1579–1586. [DOI] [PubMed] [Google Scholar]
- 32.Ali NA, Gaughan AA, Orosz CG, Baran CP, McMaken S, Wang Y, Eubank TD, Hunter M, Lichtenberger FJ, Flavahan NA, et al. Latency associated peptide has in vitro and in vivo immune effects independent of TGF-beta1. PLoS One 2008;3:e1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM Jr, Hunter MG, Bringardner BD, Monick MM, Brigstock DR, Stromberg PC, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor–1 promoter–luciferase construct. Anal Biochem 1994;216:276–284. [DOI] [PubMed] [Google Scholar]
- 35.Lichtenberger FJ, Montague C, Hunter M, Frambach G, Marsh CB. NAC and DTT promote TGF-beta1 monomer formation: demonstration of competitive binding. J Inflamm (Lond) 2006;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 1998;101:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gauer S, Segitz V, Goppelt-Struebe M. Aldosterone induces CTGF in mesangial cells by activation of the glucocorticoid receptor. Nephrol Dial Transplant 2007;22:3154–3159. [DOI] [PubMed] [Google Scholar]
- 38.Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, King GL. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3–kinase–Akt–dependent pathways in retinal vascular cells. J Biol Chem 2000;275:40725–40731. [DOI] [PubMed] [Google Scholar]
- 39.Manns JM, Uknis AB, Rico MC, Agelan A, Castaneda J, Arango I, Barbe MF, Safadi FF, Popoff SN, De La Cadena RA. A peptide from thrombospondin 1 modulates experimental erosive arthritis by regulating connective tissue growth factor. Arthritis Rheum 2006;54:2415–2422. [DOI] [PubMed] [Google Scholar]