Abstract
A recent study has indicated that alveolar macrophages from smokers incubated with lipopolysaccharide (LPS) secrete much more IL-1β and TNF-α than those from healthy nonsmokers, but the mechanisms underlying this augmented secretion by cigarette smoke (CS) remain unknown. CS and LPS reportedly promote macrophages' secreting substance P (SP) that could up-regulate these cytokines' secretion from macrophages by acting on neurokinin 1 receptor (NK1R). Moreover, NF-κB from macrophages participates in NK1R intracellular signaling and synthesis of these cytokines. The present in vitro study was undertaken to examine whether CS is able to synergize these cytokines' response to LPS in macrophages, and if so, whether an amplified SP secretion is responsible for this synergistic cytokines' response via a NK1R-driven NF-κB pathway. THP-1–derived and MH-S macrophages were exposed to control medium and CS condensate (CSC) without or with LPS. We found that LPS, CSC, and CSC+LPS significantly increased IL-1β, TNF-α, and SP secretion and that SP secretion markedly preceded cytokines' secretion. CSC+LPS-induced responses were markedly greater than the sum of the responses to CSC and LPS alone, suggesting a synergistic effect. Blocking NK1R reduced the responses of IL-1β, TNF-α, and NF-κB activation to CSC+LPS by 41, 40, and 46%, respectively. NF-κB inhibitors decreased the CSC+LPS–induced cytokines' responses by 70%. Our findings suggest that CS amplifies the LPS-induced macrophages' secretion of IL-1β and TNF-α through synergizing SP secretion, which activates NF-κB via binding with NK1R.
Keywords: cytokines, infection, emphysema, signal pathway of NF-κB
CLINICAL RELEVANCE.
This research reveals a synergistic effect of cigarette smoke on the lipopolysaccharide-evoked IL-1β and TNF-α secretion from macrophages mainly by activating NK1R–NF-κB pathway. Our finding may contribute to better understanding the mechanisms underlying cigarette smoke–exaggerated pulmonary inflammation.
IL-1β and TNF-α are important cytokines participating in the pathogenesis of multiple pulmonary diseases, such as pulmonary infection, fibrosis, cancer, and emphysema (1–4). For example, cigarette smoke (CS)-induced emphysema was diminished up to 83% in mice that were null of receptors for IL-1β and TNF-α (4). Macrophages are one of the important sources these cytokines (5), especially during infection (6). It was reported that pulmonary infection induced by intratracheal instillation of lipopolysaccharide (LPS) substantially promoted cytokine secretion from macrophages (7). A recent study has further indicated that alveolar macrophages from smokers incubated with LPS secrete much more IL-1β and TNF-α than those from healthy nonsmokers (8). This finding, along with a higher secretion of these cytokines in smokers with pulmonary infection than in those without infection (6), implies a synergistic effect of CS exposure on macrophage secretion of these cytokines induced by LPS. However, the exact mechanisms underlying this CS augmented effect on cytokine secretion have not been investigated.
There is evidence demonstrating synergistic impacts of CS on the receptors' expression or mucin secretion. We have observed that alveolar macrophages from mice exposed to CS express more neurokinin 1 receptors (NK1R) in response to substance P (SP) compared with those from control mice (9). Moreover, LPS-induced mucin secretion from pulmonary mucoepidermoid cells was facilitated by CS condensate (CSC) (10). These CS synergistic effects generate a possibility that the direct action of CS on macrophages may lead to a synergistic secretion of IL-1β and TNF-α in response to LPS. Incubating macrophages from mice and humans with SP substantially promoted secretion of IL-1β and TNF-α by selectively acting on NK1R in vitro (11–13), pointing to an important role of SP in up-regulating macrophages' secretion of IL-1β and TNF-α. Stimulating macrophages with LPS or CS in vitro not only increased synthesis and secretion of SP (9, 14) but also promoted secretion of IL-1β and TNF-α (15, 16). However, it remains unclear what the role of the SP-NK1R is in CS synergizing the cytokine responses to LPS if this synergy exists.
NF-κB is reportedly downstream of NK1R activation (17, 18) and is involved in macrophages' synthesis of IL-1β and TNF-α (19). CS and LPS alone could activate NF-κB in macrophages (20, 21) and, more importantly, CS exposure in vivo increased the NF-κB–activating response to LPS (22). In addition, NF-κB is reported to be downstream of phosphatidylinositol 3-kinase (PI3K)/Akt (23, 24). Akt can be more effectively activated by CSC than by LPS (25, 26) and fails to be activated by SP at pathophysiological concentrations (27). These results, and our preliminary observation that macrophage-secreted SP during CSC contributes to the synergistic IL-1β and TNF-α responses to LPS, raised the following two questions: (1) How important is the NF-κB pathway in CS synergizing macrophage secretion of IL-1β and TNF-α induced by LPS? and (2) To what extent does NF-κB activation by CSC coupled with LPS depend on NK1R and Akt activation?
In the present study, we tested four hypotheses: (1) CSC is able to synergize the LPS-evoked IL-1β and TNF-α secretion from macrophages, (2) this cytokines' synergy is achieved largely by an amplified secretion of SP that acts on NK1R, (3) NF-κB activation is central in generating this cytokines' synergy, and (4) this NF-κB activation results from stimulating both the NK1R- and PI3K/Akt-pathways.
MATERIALS AND METHODS
Cell Preparations
The human monocytic (THP-1) and murine alveolar macrophage cell lines (MH-S) from American Type Culture Collection (Manassas, VA) were used in this study. THP-1 and MH-S cells were grown in suspension at 37°C in 5% CO2 in culture medium consisting of RPMI 1640 mixed with 5 mM L-glutamine, 100 U/ml penicillin and streptomycin, and 10% heat-inactivated FBS. For differentiation, THP-1 cells were cultured in the medium with 10 nM phorbol 12-myristate 13-acetate for 48 hours (28), washed three times with PBS, and incubated with culture medium for 5 to 7 days. THP-1–derived and MH-S macrophages were transferred to 12-well polystyrene culture plates at 1 × 106 cells per well, and the cells in each well were incubated with 1 ml of culture medium overnight. After washing twice, cells in each well were resuspended with serum-starved medium (1 ml) and underwent the following study series.
Cell Treatments
Study Series I.
Study series I was designed to test the interaction of CSC and LPS in secreting IL-1β and TNF-α from macrophages. To do so, we first detected the dose dependency of the cytokine responses to CSC and LPS. THP-1–derived and MH-S macrophages were cultured for 12 hours with different doses of CSC (0, 0.1, 1.0, and 10 μg/ml) or LPS (0, 0.01, 0.033, 0.1, 0.33, and 1 μg/ml), respectively. Our pilot studies showed that the threshold CSC concentration was 1 μg/ml. Second, we determined the interaction of CSC and LPS in secreting these cytokines. The two types of cells (THP-1–derived and MH-S macrophages) received four types of treatments, respectively. Each type of cell was incubated with CSC or dimethyl sulfoxide (sham control) for 30 minutes, and then CSC- and dimethyl sulfoxide–treated cells were incubated with LPS for 12 hours to serve as the CSC+LPS and LPS or with vehicle to serve as CSC and control (CON). To define the interaction of this threshold CSC stimulation with LPS, we analyzed the cytokines' responses to CSC alone, to six doses of LPS (0, 0.01, 0.033, 0.1, 0.33, and 1 μg/ml) alone, and to their combination. IL-1β and TNF-α in the supernatant were detected in this study series. Because the threshold CSC (1 μg/ml) and LPS concentration at 0.1 μg/ml began to generate a maximal cytokine response in our pilot studies, this mixture were applied in the following mechanistic studies.
Study Series II.
Study series II was performed to evaluate the role of SP and NK1R in CSC+LPS-induced synergistic cytokine response by conducting three experimental steps in both types of macrophages. First, we tested whether CSC+LPS evoked macrophage SP secretion preceding secretion of IL-1β and TNF-α. Macrophages were incubated with CSC+LPS at nine time points (0, 15, 30, 45, 60 min and 3, 6, 12, and 24 h), and the cytokines and SP in supernatant was measured. Second, we tested whether CSC synergized macrophage SP response to LPS. SP in the supernatant was measured after incubation the cells with CSC, LPS, and CSC+LPS for 30 minutes and 12 hours, respectively. Third, we verified whether NK1R contributes to the CSC+LPS–induced synergy of these cytokines. Macrophages were left untreated or pretreated with NK1R antagonists aprepitant (10−8 M) or CP99,994 (10 ng/ml) (12) applied 30 minutes before the protocols described in Study Series I, and the cytokines were detected.
Study Series III.
Study series III was conducted to evaluate the contribution of NF-κB to CSC+LPS–induced synergistic responses of cytokines and SP. THP-1–derived macrophages were incubated with a specific NF-κB translocation inhibitor SN50 (100 μg/ml) or a selective IκB kinase-2 inhibitor SC-514 (10 μM) for 60 minutes (29) before CSC and/or LPS, similar to the protocols described in Study Series I. IL-1β, TNF-α, and SP in supernatant were measured. The reason for using only THP-1–derived macrophages to elucidate the cellular mechanism is described in Results.
Study Series IV.
Study Series IV was performed to address three issues. First, we defined the involvement of the NK1R pathway in NF-κB activation induced by CSC and/or LPS. THP-1–-derived cells were treated using the same protocols described in the Study Series I with the exception that LPS was incubated for only 60 minutes and that aprepitant (10−8 M) or CP99,994 (10 ng/ml) was applied 30 minutes before CSC and/or LPS. Second, we clarified the role of the PI3K/Akt pathway in NF-κB activation. The protocols were the same as described above with exception that CP99,994 was replaced by PI3K/Akt inhibitor LY294002 (10 μM) (26) or its vehicle 60 minutes before CSC+LPS. In these experiments, nuclear p65 and NF-κB activity were measured. Third, we examined whether cytosolic Akt could be activated by CSC and/or LPS and, if so, whether this modulation depends on NK1R. Akt was detected in the cells exposed to CSC and LPS, respectively, and CSC+LPS without or with NK1R antagonists, for 0, 30, 45, and 60 minutes, respectively.
Measurement of IL-1β, TNF-α, and SP
After supernatant collection, protease inhibitor cocktail (PI cocktail IV, 1:100 dilution; Calbiochem, La Jolla, CA) was added and stored at –80°C. IL-1β and TNF-α protein levels were determined according to the manufacturer's protocol with the ELISA kit (R&D Systems, Minneapolis, MN). SP concentration was detected quantitatively using a commercial enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) as described (30). Results were expressed in picograms per milliliter.
Cell Lysates and Nuclear Extracts
Cell lysates were prepared by suspending cells in lysis buffer as previous described (12). Nuclear extracts were prepared with a NEPER nuclear extraction reagent (Pierce, Rockford, IL) as recommended by the manufacturer. Briefly, THP-1–derived macrophages were placed on ice after different stimulations. After media aspiration, the cells were washed three times with cold PBS. The cells were gently removed by scraping and collected by centrifugation at 500 × g for 3 minutes at 4°C. The cells were incubated in cytoplasmic extraction buffer with protease inhibitor cocktail for 10 minutes followed by vortexing. Then, the preparations were centrifuged at 16,000 × g for 5 minutes at 4°C. The nuclear pellet was resuspended in nuclear extraction buffer with protease inhibitor cocktail and vortexed for 15 seconds every 10 minutes for a total of 40 minutes. The supernatant (nuclear extract) was collected, aliquoted, and frozen at −80°C after centrifugation at 16,000 × g for 10 minutes at 4°C.
Western Blotting
Proteins (20 μg) in the cell lysates or the nuclear extracts were separated by 10% SDS-polyacrylamide gel electrophoresis. The cell lysates and nuclear protein extracts were transferred to a nitrocellulose membrane and probed with phosphorylated Akt (p-Akt) at Ser473 rabbit monoclonal and NF-κB–RelA/p65 rabbit polyclonal antibody (1:1,000), respectively, overnight at 4°C. The blots were exposed to horseradish peroxidase–conjugated goat anti-rabbit IgG (1:1,000) and detected by electrochemiluminescence (Amersham Biosciences, Piscataway, NJ). After detecting p-Akt and NF-κB–RelA/p65, membranes were stripped and reprobed with nonphosphorylated Akt rabbit polyclonal and Lamin B mouse monoclonal antibody (1:1,000), respectively. The antibody-bound proteins were visualized after incubation with horseradish peroxidase–conjugated goat anti-rabbit and mouse IgG (1:1,000), respectively. Bands of p-Akt, Akt, p65, and Lamin B were scanned using a GS-800 imaging densitometer (Bio-Rad, Hercules, CA), and the results were expressed as the relative values to nonphosphorylated Akt or Lamin B signals, respectively.
Electrophoretic Mobility Shift Assay
To prepare an NF-κB probe, oligonucleotides (5′ GGCAACTGGGGACTCTCCCTTT-3′ and 5′-GGCAAAGGGAGAGTCCCCAGTT-3′) with an NF-κB binding site were labeled using the biotin 3′ End DNA labeling kit (Pierce, Rockford, IL) and annealed as reported elsewhere (31). Nuclear extracts (8 μg) were incubated with the biotinylated DNA probe at room temperature for 30 minutes in 50 ng/μl poly (dI.dC), 2.5% glycerol, 5 mM MgCl2, 0.05% NP-40, and 20 fmol biotin–EBNA control DNA (Pierce). The DNA–protein complex was electrophoresed on 4% nondenaturing polyacrylamide gel and transferred onto a nylon membrane. After transferring, the DNA probe was cross-linked to the membrane using an UV cross-linker. The DNA mobility shift due to the binding of the NF-κB complex was detected using the light-shift electrophoretic mobility shift assay kit (Pierce). Bands of NF-κB and Lamin B were scanned using a GS-800 imaging densitometer, and the results were expressed as the relative values to the Lamin B signals.
Reagent
CSC (Murty Pharmaceuticals, Lexington, KY) was purchased from the University of Kentucky Reference Cigarette 3R4F. The smoke particulate matter was dissolved in DMSO at 40 mg/ml, aliquoted into small vials, and stored frozen at −80°C as described previously (32). Aprepitant and CP99,994, selective NK1R antagonists (12, 33), were the gifts from Pfizer and Merck, respectively. LPS from Escherichia coli (Serotype 0111:B4) and 12-myristate 13-acetate were purchased from Sigma-Aldrich (St. Louis, MO). SC-514 (a selective IκB kinase-2 inhibitor) and SN50 (a cell-permeable peptide that blocks nuclear translocation of NF-κB) (29) and PI3K/Akt inhibitor LY294002 were supplied by Calbiochem (San Diego, CA). Akt phosphorylated at Ser473 rabbit monoclonal and nonphosphorylated Akt rabbit polyclonal antibody were from Cell Signaling (Beverly, MA). NF-κB–RelA/p65 rabbit polyclonal and Lamin B mouse monoclonal antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical Analysis
The data were expressed as absolute values with the exception that p-Akt, p65, and NF-κB were presented as the ratio to nonphosphorylated Akt or Lamin B, respectively. All data are presented as mean ± SE. One-way ANOVA was used to compare the responses of the cytokines to different doses of CSC and LPS and of SP and cytokine responses to CSC+LPS during different times. To detect the significant changes in secreting cytokines or SP by CSC and/or LPS or to compare the NF-κB responses to CSC and/or LPS with or without PI3K/Akt inhibitor, two-way ANOVA was used. Factorial ANOVA was used to compare the cytokine and SP responses to CSC and/or LPS with and without NK1R antagonists or NF-κB inhibitors and to compare the Akt responses to CSC and/or LPS at different time points with and without NK1R antagonists. If an overall test was significant, Fisher's post hoc tests were followed for comparing the data from individual groups. To assess the potential for the interactions between CSC and LPS, we included interaction terms in the models. When tests indicated an interaction, 95% confidence intervals for the differences in means were obtained to assess the magnitude of the interaction. In all analyses, P < 0.05 as considered statistically significant.
RESULTS
CSC and LPS Increases IL-1β and TNF-α Secretion from Macrophages in a Dose-Dependent Manner
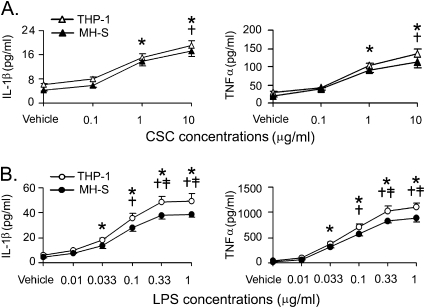
A significant elevation of IL-1β and TNF-α initially occurred at 1 μg/ml for CSC and at 0.033 μg/ml for LPS after THP-1–derived and MH-S macrophages were incubated with different concentrations of these agents for 12 hours (Figure 1). The responses became significantly greater as the concentrations increased. The cytokines reached a plateau response to LPS for 12 hours after 0.33 μg/ml. These responses were similar in both cell lines. Compared with CSC, LPS produced a greater IL-1β and TNF-α responses with a lower threshold concentration, indicating that LPS is more potent in stimulating macrophage secretion of these cytokines than CSC, which is consistent with previous reports in alveolar macrophages (16).
Figure 1.
Effects of different doses of (A) cigarette smoke condensate (CSC) and (B) lipopolysaccharide (LPS) for 12 hours on IL-1β (left) and TNF-α (right) secretion from THP-1–derived and MH-S macrophages. Five independent experiments in each treatment; data are mean ± SE. *P < 0.05 compared with vehicle. †P < 0.05 compared between 1 μg/ml and 10 μg/ml CSC or between 0.033 μg/ml LPS and the higher doses. ‡P < 0.05 compared between 0.1 μg/ml LPS and the higher doses.
CSC Synergizes the LPS-induced Secretion of IL-1β and TNF-α from Macrophages
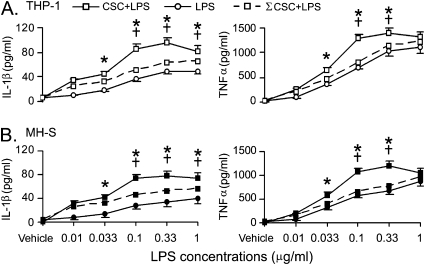
Figure 2 presents the data to reflect a synergistic effect of CSC on macrophage secretion of IL-1β and TNF-α in response to LPS. The full dose-response curves of LPS and CSC+LPS were obtained by plotting cytokine responses to six different LPS doses and to the mixture of CSC (1 μg/ml) and LPS (six individual doses), respectively. The sum of response curve to LPS and CSC alone was calculated by each of the six responses to LPS doses plus the given response to 1 μg/ml CSC. IL-1β responses to CSC+LPS became significantly greater (40–70%) than the sum of the responses to CSC and LPS alone when the cells were incubated with LPS at the concentrations higher than 0.033 μg/ml (Figure 2). TNF-α responses to CSC+LPS were also markedly greater (30–60%) than the sum of the responses to CSC and LPS alone if the concentration of LPS was up to 0.33 μg/ml. In contrast to IL-1β, these TNF-α responses became almost equal to the sum when the cells were incubated with LPS at 1 μg/ml. As reported previously, this decline phenomenon may be due to activation of other cellular inhibitory responses at high concentrations of LPS (10 μg/ml) (34).
Figure 2.
CSC (1 μg/ml) synergistically affects IL-1β and TNF-α secretion from (A) THP-1–derived and (B) MH-S macrophages in response to different doses of LPS for 12 hours. CSC-induced IL-1β (pg/ml) and TNF-α (pg/ml) were 15.1 ± 1.2 and 102.4 ± 6.8 in THP-1–derived macrophages and 13.7 ± 1.4 and 88.8 ± 3.8 in MH-S macrophages, respectively. Data are from five independent experiments in each treatment (mean ± SE). *P < 0.05, CSP+LPS versus ΣCSC+LPS or LPS. †P < 0.05 compared with the responses to CSC+LPS between 0.033 μg/ml LPS and the higher doses. CSC+LPS = combination of CSC and LPS; ΣCSC+LPS = sum of CSC and LPS alone.
SP Secretion Amplified by CSC Contributes to Synergistic IL-1β and TNF-α Secretion
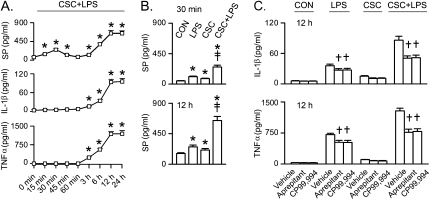
To reveal the role of SP-NK1R in CSC+LPS-induced cytokines' secretion, we first tested whether CSC+LPS–evoked macrophage SP secretion occurred earlier than secretion of IL-1β and TNF-α. Figure 3A compares the kinetics of SP and cytokine responses to CSC+LPS in THP-1–derived macrophages. As depicted, the kinetics of the SP response to CSC+LPS was characterized by two phases. The first phase consisted of an initial increase of SP with the peak response occurring at 30 minutes that declined afterward. The mechanisms underlying this decline are unknown. It may be because the SP secretion becomes relatively less than the decline of free SP molecules through its internalization (35) during this period. The second phase featured a gradual increase over 3 hours and reached a plateau 12 hours after incubation with CSC+LPS. In sharp contrast, the first phase was absent for IL-1β and TNF-α secretion, clearly indicating that SP secretion precedes the secretion of these cytokines. Second, we tested whether CSC synergized macrophage SP response to LPS (Figure 3B). LPS and CSC alone significantly increased the levels of SP at 12 hours. CSC+LPS-produced SP were 40% greater than the sum of the responses to CSC and LPS alone. Third, we verified whether NK1R contributed to the CSC+LPS-induced synergy of these cytokines by using the NK1R antagonists aprepitant and CP99,994. Aprepitant diminished the LPS-induced IL-1β and TNF-α secretion with little effect on CON levels and the CSC-induced responses at 12 hours in THP-1–derived macrophages (Figure 3C). The CSC+LPS–induced secretion of these cytokines was reduced by approximately 41% after using aprepitant or CP99,994. Similar results were observed in MH-S macrophages (data not shown).
Figure 3.
Effects of substance P (SP) on IL-1β and TNF-α responses to CSC (1 μg/ml) and/or LPS (0.1 μg/ml) in THP-1–derived macrophages. (A) Time-course of CSC+LPS-induced secretion of SP, IL-1β, and TNF-α. (B) SP secretion at 30 minutes and 12 hours by CSC and/or LPS. (C) Cytokine response to CSC and/or LPS with or without NK1R antagonists (aprepitant, 10−8 M; CP99,994, 10 ng/ml). The concentrations of the CSC, LPS, and NK1R antagonists used here are the same for the following figures. Data are from five independent experiments in each treatment (mean ± SE). *P < 0.05 compared with 0 minutes or control (CON). †P < 0.05 compared between vehicle and aprepitant or CP99,994. ‡P < 0.05, interaction of CSC and LPS. The same results were observed in MH-S macrophages (not shown).
NF-κB Activation Is Required for CSC Synergizing the LPS-Induced IL-1β, TNF-α, and SP Secretion from Macrophages
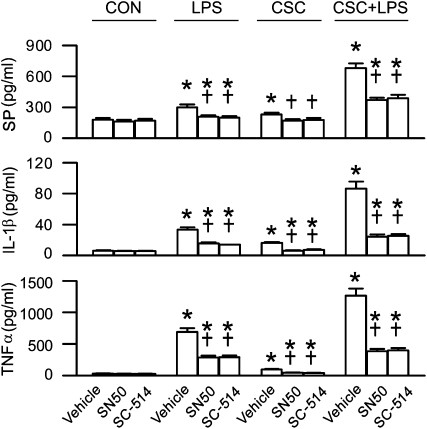
To define the involvement of NF-κB in CSC synergizing the LPS-induced secretion of these cytokines and SP, we compared cytokine and SP responses to CSC and/or LPS before and after the NF-κB inhibitor SN50 or SC-514 in THP-1–derived macrophages. We only used this cell line for intracellular signaling study because the results mentioned above have demonstrated the high similarity of cytokine and SP response to CSC and/or LPS between these two types of cells. Inhibition of NF-κB activation by SN50 did not significantly alter baseline (CON) levels of the cytokines and SP (Figure 4). However, it reduced cytokine (50%) and SP (30%) response to LPS or CSC, and this reduction became even greater for the synergistic responses of the cytokines (70%) and SP (45%) to CSC+LPS. Similar responses were observed when SC-514 was used. These results suggest that NF-κB activation plays an important role in generating the responses of these cytokines and SP to CSC or LPS, especially to CSC+LPS.
Figure 4.
Effects of NF-κB inhibitors (SN50, 100 μg/ml; SC-514, 10 μM) on SP (top), IL-1β (middle), and TNF-α (bottom) responses to 12 hours CSC and/or LPS in THP-1–derived macrophages. Data are from five independent experiments in each treatment (mean ± SE). *P < 0.05 compared with the corresponding CON. †P < 0.05 compared between vehicle and SN50 or SC-514.
NK1R and Akt Participate in NF-κB Responses to CSC and/or LPS
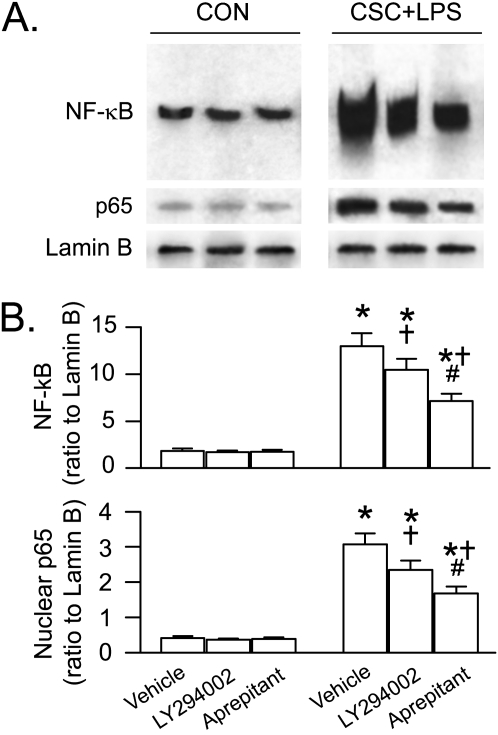
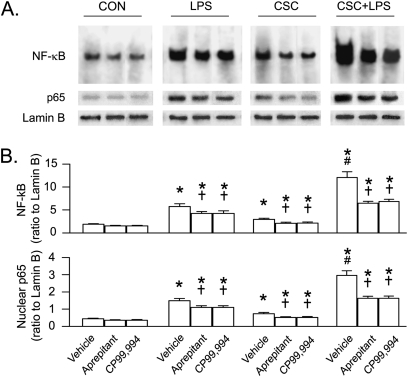
To define the contributions of the NK1R- and PI3K/Akt-pathway to NF-κB activation in responses to CSC and/or LPS, we compared the effects of CSC and/or LPS on NF-κB activation before and after blocking NK1R or inhibiting PI3K/Akt activation in THP-1–derived macrophages. We found that the increased NF-κB DNA binding activity in the macrophages in response to CSC+LPS was much greater than those in response to LPS and CSC alone (Figure 5). All of the responses to CSC or LPS, especially to CSC+LPS, were significantly reduced by approximately 26%, approximately 29%, and approximately 46%, respectively, after blocking NK1R with aprepitant or CP99,994, without significant changes in baseline NF-κB activation. These effects also were observed in the levels of nuclear translocation of p65, one of the subunits of NF-κB that plays a key role in NF-κB translocation related to stimulating IL-1β and TNF-α synthesis (19). We also found that LY294002 did not markedly alter baseline NF-κB activity and p65 nuclear translocation but down-regulated their response to CSC+LPS by approximately 20%, which was significantly smaller than the aprepitant-produced down-regulation (43%) (Figure 6). These data suggest that the NK1R and PI3K/Akt pathways are involved in the NF-κB response to CSC+LPS and that the former appears to be more important.
Figure 5.
The p65 nuclear translocation and NF-κB responses to CSC and/or LPS for 60 minutes with or without blocking NK1R (aprepitant and CP99,994) in THP-1–derived macrophages. The selected and representative NF-κB DNA-binding sites by EMSA, and protein bands of p65 (65 kD) and Lamin B (67 kD) by Western blotting are illustrated in panel A (note: the third lane in each of the gels from a different blot). The corresponding group data are shown in panel B. Five independent experiments for p65 and three independent experiments for NF-κB were performed in each treatment. Data are mean ± SE. *P < 0.05 compared with the corresponding CON. †P < 0.05 compared between vehicle and aprepitant or CP99,994. #P < 0.05 compared between CSC+LPS and LPS or CSC after vehicle treatment.
Figure 6.
Effects of PI3K/Akt inhibitor (LY294002, 10 μM) and NK1R antagonist (aprepitant, 10−8 M) on NF-κB activation and the p65 nuclear translocation in responses to CSC+LPS for 60 minutes in THP-1–derived macrophages. (A) Representative NF-κB DNA-binding sites by electrophoretic mobility shift assay, protein bands of p65 (65 kD), and Lamin B (67 kD) by Western blotting. (B) Corresponding group data. Five independent experiments for p65 and three independent experiments for NF-κB in each treatment. Data are mean ± SE. *P < 0.05 compared with the corresponding CON. †P < 0.05 compared with vehicle. #P < 0.05 compared between LY294002 and aprepitant.
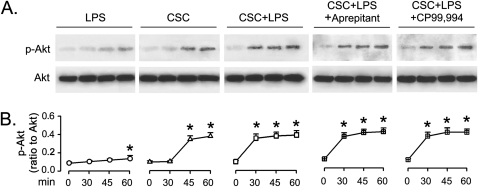
Akt Is Predominantly Activated by CSC without Effect from NK1R
To determine whether Akt could be activated by CSC and/or LPS, and, if so, whether this modulation depends on NK1R, p-Akt was detected in the macrophages exposed to CSC and LPS, respectively, or to CSC+LPS without or with pretreatment of NK1R antagonists. LPS marginally induced Akt activation, indicating LPS as a weak Akt activator (Figure 7). In contrast, CSC potently activated Akt, which was not significantly different from that evoked by CSC+LPS, again confirming CSC but not LPS as a potent activator. We also found that neither of the NK1R antagonists interfered CSC+LPS–induced Akt activation, revealing that the SP/NK1R pathway is dispensable for CSC+LPS–induced Akt activation. Our data are consistent with a lack of SP effect on activating Akt at doses lower than 1 nM (27).
Figure 7.
The time course of Akt responses to LPS and/or CSC with and without pretreatment of NK1R antagonists (aprepitant and CP99,994) in THP-1–derived macrophages. (A) Representative protein bands of phosphorylated Akt (p-Akt, 60 kD) and total Akt (Akt, 60 kD) by Western blotting. (B) Corresponding group data. Five independent experiments were performed in each treatment. Data are mean ± SE. *P < 0.05 compared with 0 minutes in each group. CSC+LPS+Aprepitant or CSC+LPS+CP99,994, pretreating the macrophages with the given NK1R antagonist before incubation with CSC+LPS.
DISCUSSION
CS Directly Stimulates Macrophages, Leading to a Synergized Secretion of IL-1β and TNF-α Induced by LPS
A previous study has shown that LPS induces a much greater secretion of IL-1β and TNF-α from alveolar macrophages in smokers than from those in healthy nonsmokers (8), implying a CS synergistic effect on macrophage secretion of these cytokines. Whether CS directly affects macrophages to cause this phenomenon remains unknown. One of our major findings in this study is that CS directly acts on macrophages to synergize their secretion of IL-1β and TNF-α in response to LPS. In agreement with previous reports (15, 16), we found that LPS and CSC alone significantly promoted macrophages' secretion of IL-1β and TNF-α. The responses of IL-1β and TNF-α to CSC+LPS were 1.8-fold and 1.6-fold higher, respectively, than the sum of their responses to CSC and LPS alone, convincingly showing a synergistic response induced by the direct action of CS on macrophages. CS has been reported to synergize the macrophage NK1R response to SP (9) and LPS-induced pulmonary mucoepidermoid cell secretion of mucin (10). Although the mechanisms underlying the augmented effects on IL-1β and TNF-α in smokers could be more complicated, our finding strongly supports the assumption that direct stimulation of macrophages by CS at least partially contributes to the synergistic effect of CS on the LPS-induced macrophage secretion of IL-1β and TNF-α observed in smokers.
Amplified SP Secretion by Macrophages Contributes to the CS-Induced Synergistic Cytokines' Response to LPS by Acting on NK1R
Previous studies have shown that CSC promotes SP secretion from macrophages (9). Moreover, SP stimulates macrophages to secret IL-1β and TNF-α by acting on NK1R (12, 13). In this study, we tested the role SP-NK1R plays in the CSC-induced macrophage synergistic responses of these cytokines to LPS. There are three lines of evidence in the present study that allow us to conclude that CSC facilitates LPS-mediated macrophage secretion of SP and thereby contributes to the synergistic cytokine responses by acting on NK1R. First, CSC+LPS-induced SP secretion started much earlier than IL-1β and TNF-α secretion, suggesting that the induction of SP may result in cytokine secretion. Second, CSC+LPS-induced SP was 1.4-fold higher than the sum of the responses to CSC and LPS alone, consistent with the up-regulatory impact of CSC or LPS on SP synthesis in and secretion from macrophages (9, 14). Third, and most importantly, blocking NK1R reduced 41% of the synergistic cytokine response to CSC+LPS. This finding is supported by previous results showing that SP promotes the secretion of these cytokines by macrophages (11–13), similar to CS and LPS (5, 16). Because blocking NK1R only reduced 41% of the synergistic effect, other pathways may also participate in the CSC synergistic modulation. In fact, previous studies have demonstrated that nicotine, one of the major components of CS, is able to elevate IL-1β and TNF-α secretion by acting on the nicotinic receptors (36). Similarly, LPS can act on toll-like receptors to induce IL-1β and TNF-α secretion (37). Further studies are required to define whether there is a functional interaction of these two receptors in CS-synergized IL-1β and TNF-α secretion from macrophages.
NF-κB Activation Is Essential for CSC Synergizing the LPS-Induced IL-1β and TNF-α Secretion
NF-κB was reportedly involved in the synthesis of IL-1β and TNF-α by macrophages (19), and its activation was promoted by CSC and LPS (20, 22, 38). Our novel finding in this study is that NF-κB inhibition diminished approximately 70% of the cytokine and approximately 45% of the SP response to CSC+LPS. This finding suggests that although other pathways may be involved in this CSC-induced synergy, the NF-κB pathway is central. The remaining 30% of the cytokine that was not blocked by NF-κB inhibitors might be due to the incomplete pharmacological inhibition of the NF-κB pathway by SN50 or SC-514 or to involvement of other signaling pathways. Because blocking NK1R or inhibiting NF-κB did not eliminate the CSC-synergized responses of the cytokines, other pathways are likely involved in these responses. The known pathways involved in CSC- and LPS-induced cytokine responses, dependent or independent of NF-κB activity, include p38 (18, 22), reactive oxygen species (39, 40), activator protein 1 (37, 41), and nuclear factor of activated T cells (42, 43). In addition, the fact that inhibition of NF-κB reduced SP secretion suggests a mutually regulation between NF-κB and SP (i.e., a positive feedback loop for CSC+LPS-induced cytokine secretion). The role of these intracellular signaling pathways in the CS-synergized cytokine response to LPS should be clarified by further investigations.
NF-κB Response to CSC+LPS Stems from Stimulation of Both NK1R and PI3K/Akt-Pathways
Another important finding in this study is that SP secreted by macrophages significantly contributes to NF-κB activation by acting on NK1R. Our results showed that NK1R blockade reduced NF-κB activation by 46% in response to CSC+LPS. This crucial role of NK1R is supported by previous results that SP activates NF-κB through NK1R (17, 18). We also found that CSC+LPS–induced Akt activation was independent of the NK1R pathway and that PI3K/Akt inhibition down-regulated CSC+LPS–induced NF-κB activity by 20%. The results reveal that PI3K/Akt is another pathway upstream of NF-κB activated by CSC+LPS, although its contribution is smaller than that of the NK1R pathway, which is in agreement with the known role the PI3K/Akt-pathway plays in NF-κB activation (23, 24, 44). Taken together, these results suggest that the NK1R and PI3K/Akt pathways participate in CSC+LPS-induced NF-κB activation, which is a key contributor to the synergistic cytokines' response.
In summary, there are four major findings from the present study: (1) CS directly stimulates macrophages to synergize the LPS-evoked IL-1β and TNF-α secretion; (2) CS amplifies LPS-induced macrophage secretion of SP, which plays an important role in generating this cytokines' synergy via acting on NK1R; (3) NF-κB activation is essential for the genesis of this cytokines' synergy; and (4) both the NK1R and PI3K/Akt pathways are responsible for the NF-κB response to CSC+LPS. This novel mechanism explains, at least in part, the phenomenon that LPS induces a much greater secretion of IL-1β and TNF-α from alveolar macrophages in smokers than from those in healthy nonsmokers.
Acknowledgments
The authors thank Pfizer Inc. and Merck & Co., Inc. for providing the NK1R antagonist as a gift and W. Chen and Y.A. Mebratu for their technical assistance.
Supported by National Heart, Lung, and Blood Institute Grant 74183 and by the Master Tobacco Settlement.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0288OC on February 16, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Reiniger N, Lee MM, Coleman FT, Ray C, Golan DE, Pier GB. Resistance to pseudomonas aeruginosa chronic lung infection requires cystic fibrosis transmembrane conductance regulator-modulated interleukin-1 (IL-1) release and signaling through the IL-1 receptor. Infect Immun 2007;75:1598–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 2008;118:3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000;343:269–280. [DOI] [PubMed] [Google Scholar]
- 4.Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol 2009;40:482–490. [DOI] [PubMed] [Google Scholar]
- 5.Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J Immunol 2007;178:463–473. [DOI] [PubMed] [Google Scholar]
- 6.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stampfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2004;170:1164–1171. [DOI] [PubMed] [Google Scholar]
- 7.Beck-Schimmer B, Schwendener R, Pasch T, Reyes L, Booy C, Schimmer RC. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res 2005;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunella G, Bardelli C, Amoruso A, Viano I, Balbo P, Brunelleschi S. Macrophage-stimulating protein differently affects human alveolar macrophages from smoker and non-smoker patients: evaluation of respiratory burst, cytokine release and NF-kappaB pathway. Br J Pharmacol 2006;148:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Xu F. Role of neurogenic substance p in over-expression of alveolar macrophages' neurokinin 1 receptor in the mice exposed to cigarette smoke. Exp Lung Res 2010;36:243–254. [DOI] [PubMed] [Google Scholar]
- 10.Baginski TK, Dabbagh K, Satjawatcharaphong C, Swinney DC. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am J Respir Cell Mol Biol 2006;35:165–174. [DOI] [PubMed] [Google Scholar]
- 11.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988;241:1218–1221. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Xu F, Barrett E. Metalloelastase in lungs and alveolar macrophages is modulated by extracellular substance p in mice. Am J Physiol 2008;295:L162–L170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bardelli C, Gunella G, Varsaldi F, Balbo P, Del Boca E, Bernardone IS, Amoruso A, Brunelleschi S. Expression of functional nk1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-kappaB pathway. Br J Pharmacol 2005;145:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bost KL, Breeding SA, Pascual DW. Modulation of the mrnas encoding substance p and its receptor in rat macrophages by LPS. Reg Immunol 1992;4:105–112. [PubMed] [Google Scholar]
- 15.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol 2002;72:752–761. [PubMed] [Google Scholar]
- 16.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/myd88 and IL-1r1/myd88 signaling dependent. J Immunol 2008;180:1169–1178. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Tanaka A, Hara M, Nakanishi S. The primary structure and gene organization of human substance p and neuromedin k receptors. Eur J Biochem 1992;204:1025–1033. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance p enhances NF-kappaB transactivation and chemokine response in murine macrophages via erk1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol 2008;294:C1586–C1596. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, Collins RD, Hawiger J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem 2004;279:48434–48442. [DOI] [PubMed] [Google Scholar]
- 20.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via rela/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol 2007;292:L567–L576. [DOI] [PubMed] [Google Scholar]
- 21.Serio KJ, Reddy KV, Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in thp-1 cells via a NF-kappaB and c/ebp-mediated mechanism. Am J Physiol Cell Physiol 2005;288:C1125–C1133. [DOI] [PubMed] [Google Scholar]
- 22.Mochida-Nishimura K, Surewicz K, Cross JV, Hejal R, Templeton D, Rich EA, Toossi Z. Differential activation of MAP kinase signaling pathways and nuclear factor-kappaB in bronchoalveolar cells of smokers and nonsmokers. Mol Med 2001;7:177–185. [PMC free article] [PubMed] [Google Scholar]
- 23.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the akt serine-threonine kinase. Nature 1999;401:82–85. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Chen W, Lin Y. Sensitization of TNF-induced cytotoxicity in lung cancer cells by concurrent suppression of the NF-kappaB and akt pathways. Biochem Biophys Res Commun 2007;355:807–812. [DOI] [PubMed] [Google Scholar]
- 25.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates lps-induced inflammatory responses by activating the phosphoinositide 3-kinase/akt signaling pathway. Proc Natl Acad Sci USA 2007;104:4077–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SE, Thanh Thuy TT, Lee JH, Ro JY, Bae YA, Kong Y, Ahn JY, Lee DS, Oh YM, Lee SD, et al. Simvastatin inhibits induction of matrix metalloproteinase-9 in rat alveolar macrophages exposed to cigarette smoke extract. Exp Mol Med 2009;41:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance p mediates antiapoptotic responses in human colonocytes by akt activation. Proc Natl Acad Sci USA 2007;104:2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson JL, Ansari S, Cameron H, Wang A, Akhtar M, McKay DM. Green tea polyphenol (-)-epigallocatechin gallate blocks epithelial barrier dysfunction provoked by IFN-gamma but not by IL-4. Am J Physiol Gastrointest Liver Physiol 2004;287:G954–G961. [DOI] [PubMed] [Google Scholar]
- 29.Killeen ME, Englert JA, Stolz DB, Song M, Han Y, Delude RL, Kellum JA, Fink MP. The phase 2 enzyme inducers ethacrynic acid, dl-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by raw 264.7 cells. J Pharmacol Exp Ther 2006;316:1070–1079. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Xu F, Wang R, Seagrave J, Lin Y, March TH. Cigarette smoke-induced hypercapnic emphysema in c3h mice is associated with increases of macrophage metalloelastase and substance p in the lungs. Exp Lung Res 2007;33:197–215. [DOI] [PubMed] [Google Scholar]
- 31.Lee BS, Kim YM, Kang HS, Kim HM, Pyun KH, Choi I. Octamer binding protein-1 is involved in inhibition of inducible nitric oxide synthase expression by exogenous nitric oxide in murine liver cells. J Biochem 2001;129:77–86. [DOI] [PubMed] [Google Scholar]
- 32.Narayan S, Jaiswal AS, Kang D, Srivastava P, Das GM, Gairola CG. Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene 2004;23:5880–5889. [DOI] [PubMed] [Google Scholar]
- 33.Herrstedt J. Risk-benefit of antiemetics in prevention and treatment of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf 2004;3:231–248. [DOI] [PubMed] [Google Scholar]
- 34.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol 2008;214:27–37. [DOI] [PubMed] [Google Scholar]
- 35.Sarntinoranont M, Iadarola MJ, Morrison PF. A kinetic analysis of substance p trafficking. J Pharm Sci 2003;92:232–243. [DOI] [PubMed] [Google Scholar]
- 36.Lau PP, Li L, Merched AJ, Zhang AL, Ko KW, Chan L. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor(−/−) mice. Arterioscler Thromb Vasc Biol 2006;26:143–149. [DOI] [PubMed] [Google Scholar]
- 37.Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol 2001;69:1036–1044. [PubMed] [Google Scholar]
- 38.Akira S, Takeda K. Toll-like receptor signalling. Natl Rev 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 39.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein ucp2 in macrophages. Biochem J 2007;402:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB, and pro-inflammatory gene expression. Biochem Pharmacol 2004;68:1255–1267. [DOI] [PubMed] [Google Scholar]
- 41.Chu M, Guo J, Chen CY. Long-term exposure to nicotine, via ras pathway, induces cyclin d1 to stimulate g1 cell cycle transition. J Biol Chem 2005;280:6369–6379. [DOI] [PubMed] [Google Scholar]
- 42.Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-atp-deficient mice. Immunity 1996;4:397–405. [DOI] [PubMed] [Google Scholar]
- 43.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, et al. Cd14 regulates the dendritic cell life cycle after LPS exposure through nfat activation. Nature 2009;460:264–268. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets 2010;14:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]