Abstract
Endotoxin and other immunostimulants ubiquitous in ambient air are potent mucosal adjuvants, yet only a minority of individuals develop aeroallergen hypersensitivities, whereas the majority develop tolerance. These investigations were performed to reconcile this paradox. During initial experiments, mice received a primary series of weekly intranasal OVA immunizations (10 vaccination). Selected mice also received intranasal sterile house dust extract (HDE) with each OVA vaccination, at a dose previously found to exert adjuvant activity. A third group of OVA-vaccinated mice received intranasal HDE on a daily basis, but at one seventh the adjuvant dose, beginning 1 week before the first and ending with the last 10 OVA vaccination. Mice were then left untreated for 4 weeks, and then received a secondary series of weekly intranasal OVA immunizations with adjuvant doses of HDE (20 sensitization). Three weeks later, OVA-specific airway challenges and immune responses were assessed. Analogous experiments were conducted with LPS. Mice receiving daily intranasal HDE or LPS during 10 OVA vaccination were highly resistant to 20 sensitization, whereas the mice in other experimental groups readily developed Th2-biased airway hypersensitivity. Tolerance was associated with poor OVA-specific CD4 cell proliferation and with local natural T-regulatory cell (Treg) expansion. Finally, Treg depletion by delivery of the anti-CD25 monoclonal antibody during 10 vaccination attenuated the tolerogenic effects of daily airway HDE exposures. These studies suggest that regular airway immunostimulant exposures selectively increase local Treg numbers and activity in an antigen-independent manner, thereby promoting the development of aeroallergen tolerance.
Keywords: tolerance, asthma, aeroallergen, house dust extract, regulatory T cell
CLINICAL RELEVANCE.
Endotoxin and other immunostimulants ubiquitous in ambient air have proven to be potent mucosal adjuvants in vaccination studies, but only a minority of individuals develop hypersensitivities to aeroallergens, whereas a majority develop tolerance. The present investigation was designed to help explain why semicontinious airway exposures to ambient immunostimulants promote the development of aeroallergen tolerance rather than hypersensitivity.
A variety of molecules known to be ubiquitous in living environments activate cells involved in antigen presentation by receptor-dependent (i.e., Toll-like receptor ligands or TLR ligands) and independent pathways (e.g., diesel exhaust particulate, or DEP) (1, 2). Moreover, many of these ambient immunostimulants have potent mucosal adjuvant activities. In murine models of asthma, several such molecules were found to drive Th1-biased protective immune responses (e.g., ligands for TLR4, TLR7, and TLR9), whereas others functioned as Th2 adjuvants (ligands for TLR2, TLR4, and DEP), priming mice to develop eosinophil-rich airway inflammatory responses upon subsequent allergen challenge (3–6). Despite such observations, the real-world effect of airway exposures to ambient immunostimulatory molecules on the development of physiologic tolerance and pathologic Th2-biased aeroallergen hypersensitivities is far from understood.
Recognizing that exposures to purified molecules do not occur in the real world, we previously characterized the immunomodulatory activities of sterile but unpurified house dust extracts (HDEs) derived from 15 Southern California homes (7–9). Surprisingly, mice intranasally immunized with OVA and HDEs at 3 weekly intervals developed MyD88-dependent, Th2-biased, adaptive responses and airway hypersensitivity responses to allergen challenge (8), suggesting that TLRs play a prominent role in mediating the adjuvant activities of HDEs. These previous experiments may be construed to suggest that many, if not all, living environments intrinsically promote the development of allergic asthma. However, in those investigations, murine airways were exposed to the immunostimulatory contents of HDEs at weekly intervals and at doses likely in great excess of average daily exposures, as evidenced by the neutrophilic airway inflammatory response they induced (8). In contrast, individuals inhale air laced with low concentrations of immunostimulatory molecules on a semicontinuous basis, and the frequency of granulocytes in their airways remains low (10, 11).
For a better model of real-world exposures, additional experiments were performed in which mice received 3 weekly intranasal OVA immunizations, as in previously described studies, whereas low-dose HDE (one seventh the weekly dose) was intranasally delivered daily, beginning 1 week before the first and ending with the last dose of OVA, or weekly with OVA (as in the previous experiments), or according to both schedules (8). After a week of daily low-dose intranasal delivery of HDE, little airway inflammation was evident, and this HDE delivery schedule had little adjuvant effect on OVA-specific responses. More importantly, daily airway HDE exposures prevented mice concurrently receiving weekly intranasal OVA and HDE (adjuvant dose) immunizations from developing allergen-specific, Th2-biased airway hypersensitivities. Similar results were achieved with purified LPS. The present investigations were conducted to expand on those experiments by determining whether intranasal OVA-vaccinated mice concurrently receiving low-dose HDE or LPS on a daily basis developed temporary anergy or long-lived, allergen-specific tolerance. We also sought to elucidate the mechanisms responsible.
MATERIALS AND METHODS
Mice and Commercial Reagents
Investigations received approval from our institution's Animal Welfare Committee. Female mice, aged 4–6 weeks, were used for all studies. BALB/c mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). DO11.10 TCR transgenic (TG), DO11.10 TG × RAG1 knockout (KO) mice, were bred in our animal facility, as were FOXP3egfp C57B/6 mice, a kind gift from Talal Chatila. OVA (Grade VI) and Escherichia coli 026-B6 LPS (1 EU = 0.1 ng) were purchased from Sigma (St. Louis, MO).
Preparation of the HDE Standard
Methods used for the collection and processing of house dust samples were described in detail previously (7). Briefly, with approval from our institution's Human Subjects Committee, dust samples were obtained by vacuuming a single carpeted bedroom in suburban homes in San Diego County, California. Collected house dust was run through a coarse sieve to remove large particulate matter, and suspended in sterile PBS at 100 mg/ml. House dust suspensions were then placed on a rotor at room temperature for 18 hours, and filtered through glass wool and then through 0.22-μm Steriflip filters (Millipore, Bedford, MA) to obtain sterile HDEs. In previous studies, we compared the relative bioactivities of 15 HDEs (7), and subsequently prepared a high bioactivity HDE standard, which was described previously, and which was used for studies described here (9). The endotoxin concentration of the HDE was determined with a QCL-1000 kit (Bio-Whittaker, Walkersville, MD) according to the manufacturer's instructions. Using of this kit, we calculated that the endotoxin concentration of the HDE standard was approximately 33,000 EU/ml (or 3,300 ng/ml).
Immunization and Airway Allergen Challenge
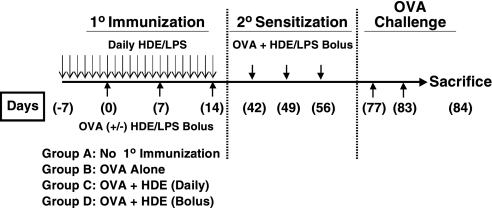
As outlined in Figure 1, naive mice (n = 4/group) received a primary series of 3 weekly intranasal OVA (100 μg) vaccinations (10 vaccinations), followed by a 4-week hiatus and then a secondary series of 3 weekly intranasal OVA (100 μg) vaccinations (20 sensitization). During the 10 vaccination period, some mice also received 21 μl of HDE with each dose of OVA. A third group of mice began receiving intranasal HDE (3 μl) on a daily basis, beginning 7 days before the first and ending with the last OVA vaccination. During 20 sensitization, all mice received OVA in conjunction with adjuvant doses of HDE (21 μl). A fourth experimental murine group only received 20 sensitization. Three weeks after completing 20 sensitization, all mice received two intranasal OVA (10 μg) challenges, delivered 6 days apart, and airway and immunologic responses were assessed the following day. Analogous experiments were performed with purified LPS. Based on the calculated endotoxin concentration of the HDE, we estimated that the 21-μl and 3-μL doses used in the in vivo studies contained approximately 70 ng (700 EU) and 10 ng (70 EU) of LPS, respectively, and these LPS doses were used for all experiments in this study. Mice were lightly anesthetized (isoflurane; Abbott Laboratories, North Chicago, IL) before the intranasal delivery. All reagents were intranasally delivered at a total volume of 30 μl PBS.
Figure 1.
Schedule for vaccination studies. Mice received three intranasal OVA vaccinations at weekly intervals either alone, with bolus high-dose house dust extract (HDE) and LPS, or daily low-dose HDE and LPS (10 Immunization). Four weeks after completing 10 immunization, mice received 3 weekly intranasal vaccinations of OVA with bolus HDE (20 Sensitization). Three weeks later, mice were intranasally challenged with OVA alone, and airway hypersensitivity and immune responses were assessed.
Eighteen hours after the final OVA challenge, the mice were killed, their lungs were lavaged with 800 μl of PBS, and the bronchoalveolar lavage fluid (BALF) was collected. Total BALF cell counts were determined with a hemocytometer. In addition, BALF cytospin slides were prepared, fixed in acetone, and Wright-Giemsa–stained. A blinded observer determined the percentage of eosinophils, neutrophils, and mononuclear cells on each slide by counting a minimum of 150 cells in random high-power fields with a light microscope. Lung tissue was flash-frozen, cryosectioned, acetone-fixed onto poly-L-lysine–coated slides, and stained with hematoxylin–eosin, peroxidase/DAB, and PAS, according to standardized techniques. To quantitate peribronchial inflammation, eosinophil infiltration, and airway mucus production, a scoring system (with 0–5 points) was devised in which a blinded observer scored 4–8 airways per mouse for each parameter. Mean inflammation scores were determined by averaging the total cellular infiltration, eosinophil infiltration, and airway mucus production scores for each murine group. The experimental techniques used for these analyses were previously described in detail (4, 9, 12).
Regulatory T-Cell Depletion Experiments
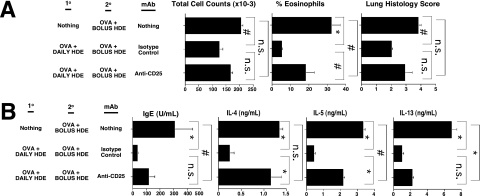
In selected experiments, the CD25 monoclonal antibody (mAb) was delivered to mice during 10 immunization to deplete regulatory T cells (Tregs). In preliminary studies, spleens harvested from mice 2 weeks after an intraperitoneal injection of 500 μg of CD25 mAb (PC61; Leinco Technologies, St. Louis, MO) were found to have fewer than 0.4% FOXP3-positive CD4 cells, an at least 95% reduction compared with untreated mice (data not shown). Therefore, for the experiments presented in Figure 8, mice received 500 μg of PC61 mAb (to deplete them of Tregs) or an isotype control mAb, 4 days before the first intranasal OVA immunization and again 2 weeks later.
Figure 8.
Treg depletion attenuates the allergen-specific tolerance induced by daily intranasal delivery of HDE. BALB/c mice received three intranasal vaccinations of OVA at weekly intervals with daily low-dose HDE, or did not receive 10 OVA immunization, as outlined in Figure 1. Mice receiving 10 OVA immunization were also treated with CD25 monoclonal antibody (mAb) to deplete them of Tregs or an isotype control mAb. Four weeks after completing 10 immunization, all mice received 20 sensitization. Mice were then intranasally challenged with OVA, and 3 weeks later, hypersensitivity and immune responses were assessed, as outlined in Figure 1. Bar graphs present mean values for four mice per group ± SEs (#P ≤ 0.05, *P ≤ 0.01; n.s., P value not significant for the indicated comparison). Similar results were obtained in two independent experiments.
OVA-Specific IgE and Cytokine Responses
When the mice were killed, sera were obtained to measure antigen-specific IgE responses by ELISA. Samples were compared with a high-titer, anti-OVA IgE standard (endpoint titration, 512). To remove IgG and improve the sensitivity of the OVA-specific IgE ELISA, serum samples were preincubated with protein G sepharose beads (Pharmacia, Piscataway, NJ). Subsequent Ig ELISA techniques were routine, as previously described (4, 9, 12). OVA-specific bronchial lymph node (BLN) cytokine responses were also assessed by a previously described method (4, 9, 12). Briefly, BLNs harvested from each group of experimental mice were pooled, and single-cell suspensions were prepared by enzymatic digestion with Collagenase VIII (300 U/ml; Sigma) and DNase-I (1.5 μg/ml; Sigma). BLN cells were cultured in triplicate at 1 × 106 cells/ml in media with or without OVA (50 μg/ml) for 72 hours before harvesting the supernatants. IL-4, IL-5, and IL-13 concentrations in culture supernatants were determined by ELISA, using reagents from Pharmingen (San Diego, CA), according to the manufacturer's recommendations. BLN cytokine responses were calculated by subtracting the background cytokine production (generally equal to or less than 0.05 ng/ml) from responses of BLN cells cultured with OVA.
Adoptive Transfer Experiments
For adoptive transfer experiments, naive BALB/c mice were intranasally immunized with OVA (100 μg) on a single occasion. Selected mice were co-immunized with adjuvant doses of either HDE (21 μl) or LPS (70 ng). Other mice received daily intranasal HDE (3 μl) or LPS (10 ng), beginning 7 days before and ending with intranasal OVA vaccination. DO11.10 TG × RAG-1 KO CD4 cells were isolated from donor spleens with Miltenyi magnetic beads (Auburn, CA), according to the manufacturer's directions, and recipient mice received 1 × 107 cells by an intraperitoneal injection 1 day before intranasal OVA immunization. Ten days after OVA vaccination, mice were intranasally challenged with OVA alone, and BLNs were harvested 24 hours later for cellular analyses. In some experiments, DO11.10 TG × RAG-1 KO CD4 cells were stained with CFSE (Molecular Probes, Carlsbad, CA), according to the manufacturer's recommendations, before adoptive transfer.
Analyses of CD4 Cell Responses to Intranasal Delivery of HDE and LPS
BALB/c or FOXP3egfp C57B/6 mice received intranasal PBS, a single bolus dose of HDE (21 μl), or LPS (70 ng), or 7 days of daily intranasal HDE (3 μl) or LPS (10 ng). Twenty-four hours, 3 days, or 7 days after the final dose of HDE or LPS, the mice were killed. Single-cell suspensions of relevant tissues were prepared by enzymatic digestion, as previously described (4, 8, 9). Cells were subsequently stained for FOXP3 and CD4 expression or CD4 expression alone.
Flow Cytometry
Cell surface staining was performed by standard methods with fluorescent mAbs purchased from Pharmingen, Caltag (Burlingame, CA), and eBiosciences (San Diego, CA). For intracellular FOXP3 staining, a kit from eBiosciences was used according to the manufacturer's instructions. All flow cytometric studies were performed with a FACSCalibur flow cytometry unit, and 10,000–100,000 events of interest were recorded for each sample. Gates based on isotype control staining were used to identify the percentage of cells bearing markers of a specific linage. Flow cytometric analyses were conducted immediately or else samples were fixed in 2% paraformaldehyde, and stored in the dark at 4°C until their analysis. Flow cytometry data were analyzed with FlowJo software (Tree Star, Inc., San Carlos, CA).
Statistical Considerations
Statistical analyses were performed using Statview software, and two-tailed, unpaired Student t tests were used to analyze data. The Bonferroni correction factor was included in the calculation of P values, to account for the increased probability of type I errors when multiple groups are statistically compared.
RESULTS
Daily Intranasal Delivery of HDE and LPS Promotes the Development of Long-Lived Aeroallergen Tolerance
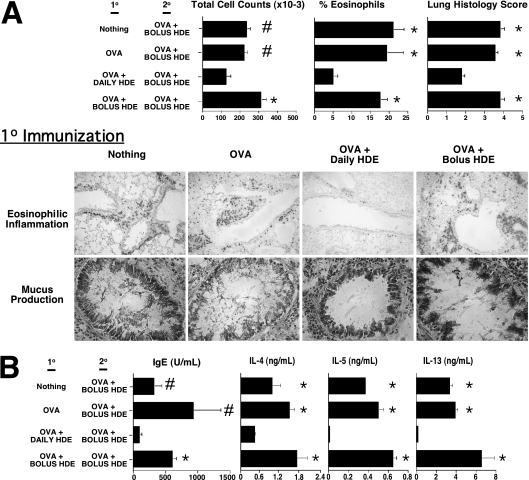
As shown in Figure 1, naive mice received a primary series of 3 weekly intranasal OVA vaccinations (10 vaccinations), followed by a 4-week period of observation and a secondary series of 3 weekly intranasal OVA vaccinations (20 sensitization). During the 10 vaccination period, selected mice also received HDE with each OVA vaccination, at doses previously shown to have adjuvant potential (8). A third murine group began receiving intranasal HDE on a daily basis, at one seventh the adjuvant dose, beginning 7 days before the first and ending with the last OVA vaccination. During 20 sensitization, all mice received weekly adjuvant doses of HDE in conjunction with OVA. A fourth experimental mouse group only received 20 sensitization. Three weeks after completing 20 sensitization, mice were intranasally challenged with OVA alone, and immune and airway hypersensitivity responses were assessed. Mice receiving nothing, OVA alone, or OVA and adjuvant doses of HDE during 10 immunization were found to have significantly exaggerated airway inflammatory responses, compared with those mice receiving daily intranasal HDE (Figure 2A; Figure E1 in the online supplement is a color version of Figure 2). Likewise, mice in these experimental groups displayed far stronger Th2-biased immune responses (Figure 2B). Finally, mice receiving daily HDE during 10 immunization did not display compensatory increases in OVA-specific serum IgG2a or BLN IFN-γ and IL-10 production, compared with mice in other groups (data not shown).
Figure 2.
Daily intranasal delivery of HDE promotes long-lived aeroallergen tolerance. BALB/c mice received 10 immunization and 20 sensitization with HDE, as outlined in Figure 1. Mice were then intranasally challanged with OVA alone, and (A) airway hypersensitivity and (B) immune responses were assessed. Bar graphs present mean values for four mice per group ± SEs (#P ≤ 0.05 and *P ≤ 0.01 for control mice compared with mice receiving daily HDE). Similar results were obtained in three independent experiments. Figure E1 presents the same experimental results with color images of lung histology.
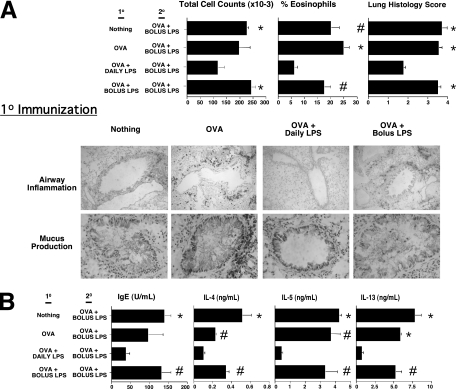
Although HDE adjuvant activities were found to be largely TLR-dependent (8), their molecular contents are inherently ill-defined. Therefore, analogous experiments were conducted with LPS. The endotoxin content of HDE was first measured, and bolus and daily HDE doses were determined to contain the equivalent of approximately 70 ng and 10 ng of LPS, respectively (9). Therefore, these bolus and daily LPS doses were used. Results shown in Figure 3 and Figure E2 demonstrate that mice receiving intranasal bolus and daily doses of LPS during the 10 immunization period had airway allergen challenge and immunologic outcomes analogous to those of mice treated with bolus and daily HDE, respectively. These results establish that regular airway exposures to LPS and other immunostimulants in HDEs and the living environments they represent have the potential to promote long-lived aeroallergen tolerance.
Figure 3.
Daily intranasal delivery of LPS promotes long-lived aeroallergen tolerance. BALB/c mice received 10 immunization and 20 sensitization with LPS, as outlined in Figure 1. Mice were then intranasally challanged with OVA alone, and (A) airway hypersensitivity and (B) immune responses were assessed. Bar graphs present mean values for four mice per group ± SEs (#P ≤ 0.05 and *P ≤ 0.01 for control mice compared with mice receiving daily LPS). Similar results were obtained in three independent experiments. Figure E2 presents the same experimental results with color images of lung histology.
Daily Intranasal Delivery of HDE and LPS Inhibits the Clonal Expansion CD4 Cells, without Promoting Their Differentiation into Adaptive Regulatory Tregs
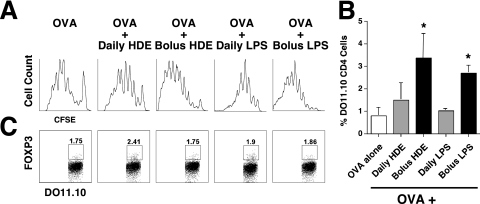
To assess OVA-specific CD4 cell outcomes, CFSE-labeled or unlabeled DO11.10 TG × RAG-1 KO CD4 cells were adoptively transferred to naive mice, 1 day before a single intranasal OVA vaccination. RAG-1 deficiency renders DO11.10 TG × RAG-1 CD4 cells unable to express an endogenous T-cell receptor, and mice with this genotype fail to produce FOXP3-positive Tregs by thymic selection (natural Tregs). However, DO11.10 TG × RAG-1 CD4 cells retain the ability to differentiate into adaptive Tregs upon encountering antigens (13). Selected mice also received daily low-dose HDE, beginning 1 week before and ending with OVA delivery, or as a single adjuvant dose delivered with OVA. Ten days later, mice were intranasally challenged with OVA alone, and 24 hours afterward, BLNs were harvested. Compared with BLNs from mice receiving intranasal OVA alone, BLNs from mice co-immunized with high-dose HDE displayed marked increases in the proliferation (Figure 4A) and expansion (Figure 4B) of adoptively transferred DO11.10 TG × RAG KO CD4 cells, whereas BLNs from mice receiving daily low-dose HDE did not. Experimental group differences in OVA-specific CD4 cell death were not found (data not shown), perhaps because dead and dying CD4 cells were endogenously cleared from mice before they were killed. Moreover, analyses of FOXP3 expression failed to demonstrate significant adaptive Treg differentiation in any experimental group (Figure 4C). Experiments with LPS yielded similar results (Figures 4A–4C). These findings demonstrate that the tolerogenic influence of daily intranasal delivery of HDE and LPS on allergen-specific immunity is explained, at least in part, by the failed clonal expansion of naive allergen-specific CD4 cells, but not by their preferential differentiation into adaptive Tregs.
Figure 4.
Daily intranasal delivery of HDE and LPS inhibits antigen-specific CD4 cell proliferation, but does not promote the differentiation of naive allergen-specific CD4 cells into adaptive T-regulatory cells (Tregs). BALB/c mice received a single intranasal bolus or a week of intranasal daily HDE and LPS, as outlined in Figure 1. Mice also received CFSE-labeled or unlabeled DO.11.10 TG × RAG KO CD4 cells (DO.11.10), 1 day before a single intranasal vaccination of OVA with the final daily or single bolus dose of HDE and LPS. OVA alone was intranasally delivered 10 days later, and bronchial lymph nodes (BLNs) were harvested 24 hours afterward. (A) DO.11.10 TG × RAG-1 KO CD4 proliferation. (B) DO.11.10 TG × RAG-1 KO CD4 frequency. (C) FOXP3 expression. Bar graphs present mean values for four mice per group ± SEs (*P ≤ 0.01 for HDE/LPS–treated mice compared with mice receiving OVA alone). Similar results were obtained in two independent experiments.
Daily Intranasal Delivery of HDE and LPS Leads to the Local Recruitment and/or Expansion of Allergen-Nonspecific Natural Tregs
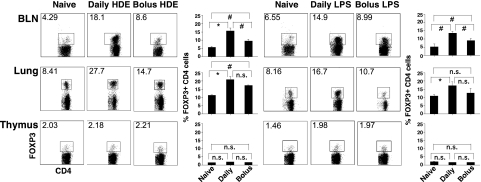
The results in Figure 4C demonstrate that daily intranasal delivery of HDE did not promote the development of allergen tolerance by selectively inducing the differentiation of FOXP3-negative naive CD4 cells into adaptive Tregs. However, because mice were assessed 11 days after intranasal delivery of HDE, these studies did not adequately consider the possibility that regular airway exposures to immunostimulants contained within the HDE might promote the local migration or proliferation of preexisting Tregs (i.e., natural Tregs) in an antigen-independent manner, or transiently induce FOXP3 expression by FOXP3-negative CD4 cells. To address these considerations further, BALB/c mice were treated with intranasal HDE alone, either as a single bolus or by daily low-dose delivery for 7 days. Twenty-four hours later, lungs and BLNs were harvested, cells were stained for CD4 and FOXP3 expression, and flow cytometric analyses were conducted.
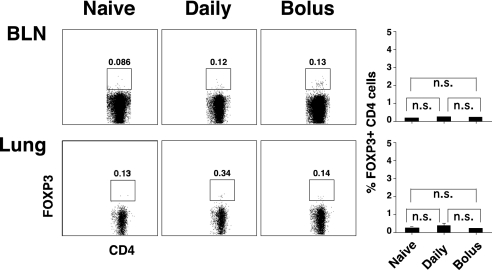
As shown in Figure 5, compared with untreated mice, clear increases in the percentages of BLN and lung CD4 cells expressing FOXP3 were evident in mice receiving intranasal HDE, but increases (particularly in BLNs) were greater with daily delivery than with bolus delivery. However, no changes in CD4 cell FOXP3 expression were found in the thymus (Figure 5) or spleen (data not shown) of HDE-treated mice. Similar results were obtained with LPS. To determine whether airway HDE delivery induced transient FOXP3 expression by FOXP3-negative CD4 cells, experiments (Figure 5) were repeated with DO11.10 TG × RAG-1 KO mice, which lack natural Tregs (13). In these experiments, neither bolus nor daily intranasal HDE delivery induced detectable FOXP3 expression by CD4 cells harvested from the BLNs and lungs of experimental mice (Figure 6). Taken together, these results indicate that local increases in CD4 cell expression of FOXP3 occur in wild-type mice receiving intranasal HDE as a consequence of local expansion or migration of preexisting Tregs, and are not attributable to the transient expression of FOXP3 by FOXP3-negative CD4 cells.
Figure 5.
Daily intranasal delivery of HDE and LPS promotes local increases in the frequency of FOXP3-positive CD4 cells. BALB/c mice were killed 24 hours after a single intranasal bolus or seven daily intranasal doses of HDE and LPS. BLNs, lungs, and thymuses were harvested, and cells were stained for CD4 and FOXP3 expression and analyzed via flow cytometry. Numeric values in dot blots represent the percentage of cells within the defined gate. Bar graphs present mean values for four mice per group ± SEs (#P ≤ 0.05, *P ≤ 0.01; n.s., P value not significant for the indicated comparison). Similar results were obtained in 2–3 independent experiments.
Figure 6.
Daily intranasal delivery of HDE does not induce FOXP3 expression by FOXP3-negative CD4 cells. DO.11.10 TG × RAG KO mice, which lack natural Tregs, were killed 24 hours after a single intranasal bolus or seven daily intranasal doses of HDE. BLNs and lungs were harvested, and cells were stained for CD4 and FOXP3 expression and analyzed via flow cytometry. Numeric values in dot blots represent the percentage of cells within the defined gate (n.s., P value not significant for the indicated comparison). Similar results were obtained for four mice per group.
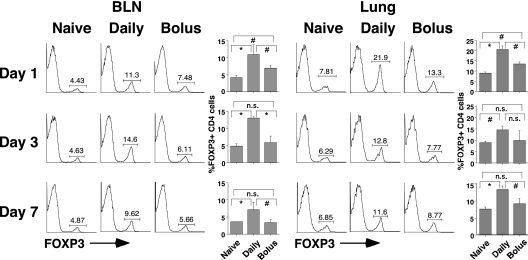
Local Increases in FOXP3-Positive CD4 Cells Last for at Least a Week after Intranasal Delivery of HDE
The experiments illustrated in Figure 5 clearly demonstrated that 24 hours after a week of daily low-dose intranasal delivery of HDE, substantially higher frequencies of FOXP3-positive CD4 cells in the BLNs and lungs of mice occurred than 24 hours after a single intranasal bolus of HDE. However, the possibility remained that these differences were attributable to differing kinetics of local Treg expansion in mice receiving a single intranasal HDE bolus or a week of daily low-dose intranasal delivery of HDE. To address this consideration, we replicated the studies presented in Figure 5, but killed the mice 1, 3, and 7 days after delivery of HDE. These studies were conducted with FOXP3egfp C57B/6 mice, which co-express FOXP3 and GFP. This simplified the staining of cells on harvest days. The experiments depicted in Figure 7 clearly demonstrate that BLN and lung frequencies of CD4 cells expressing FOXP3 were significantly higher in mice receiving daily intranasal HDE at all time points, compared with corresponding tissues harvested from mice receiving a single intranasal bolus of HDE. These results establish that daily low-dose airway exposures to HDE more effectively recruit Tregs to the respiratory tract than does a single high-dose bolus of HDE, and that the effect lasts for a week or longer.
Figure 7.
Local increases in the frequency of FOXP3-positive CD4 cells, associated with a daily intranasal delivery of HDE, last for a week or more. FOXP3egfp C57B/6 mice were killed 24 hours, 3 days, or 7 days after a single intranasal bolus or seven daily intranasal doses of HDE. BLN and lung cells were prepared, stained for CD4 expression, and characterized via flow cytometry. Numeric values in dot-blot analyses represent the percentage of cells within the defined gate. Bar graphs present mean values for four mice per group ± SEs (#P ≤ 0.05, *P ≤ 0.01; n.s., P value not significant for the indicated comparison). Similar results were obtained in two independent experiments.
The Tolerogenic Influence of Daily Intranasal Delivery of HDE Is Partly Dependent on Tregs
The findings in Figures 5 and 7 suggest that daily intranasal delivery of HDE might promote the development of allergen tolerance by selectively recruiting or expanding natural Treg numbers in the airways and their associated lymphoid tissues. Therefore, we determined whether Treg depletion with anti-CD25 mAb during the 10 immunization phase of experiments described in Figure 1 would influence the outcomes of OVA-vaccinated mice receiving daily intranasal HDE. As shown in Figure 8, mice receiving anti-CD25 mAb during 10 immunization with OVA and daily HDE developed airway hypersensitivity responses and immune profiles that were intermediate in magnitude between those of mice receiving an isotype control mAb during 10 immunization and those of mice that only received 20 sensitization. These results suggest that Tregs contribute to, but may not be exclusively responsible for, the tolerogenic influence of daily intranasal exposures to HDE on antigen-specific immunity.
DISCUSSION
Although little is known about the nature of real-world airway exposures to ambient immunostimulants, air and dust sampling studies generally report low but detectable endotoxin concentrations in almost all homes, with concentrations spanning a range of several logs (11, 14–16). Although assays for the detection and quantitation of other ambient immunostimulants are lacking, our investigations with TLR-deficient dendritic cells further demonstrated that immunostimulatory ligands for TLR2, TLR4, and TLR9 were present in dust samples collected from 10 of 10 homes tested (7). These findings imply that modeling the development of aeroallergen hypersensitivity and tolerance as a function of immunostimulant dose and dosing frequency has clinical relevance. In this regard, Figures 2 and 3 provide direct evidence that airway exposures to LPS and other stimulants of innate immunity contained in HDE not only have the potential to induce allergic hypersensitivities, but can also induce long-lived allergen-specific tolerance when delivered to the airways on a daily low-dose delivery schedule.
Several groups measured the endotoxin content of ambient air in homes. In one representative study of 162 homes, air samples had a geometric mean of 0.553 endotoxin units per cubic meter (EU/M3), but concentrations ranged from 0.018–14.82 EU/M3 (14). Assuming a child breathes 4 L per minute, and that 1 EU of endotoxin has the bioactivity of 0.1 ng of LPS, that study would suggest that on average, children inhale the equivalent of 0.3 ng of LPS each day (range, 0.01–8.5 ng/day). However, in selected environments (e.g., agricultural settings, slaughterhouses, or waste disposal plants), air concentrations as high as high as 69,360 EU/M3 were reported (17). This translates into the inhalation of roughly 1,700 ng of LPS each hour. In the present study, mice received daily and bolus doses of HDE containing the equivalent of 10 ng and 70 ng of LPS, respectively. Recognizing that models can only approximate reality and that mice are not humans, these calculations still suggest that the doses of HDE chosen for these in vivo experiments have real-world relevance.
The investigations depicted in Figure 4 demonstrate that the tolerogenic influence of daily intranasal delivery of HDE and LPS on allergen-specific immunity leads to failed clonal expansion of naive allergen-specific CD4 cells, but not their preferential differentiation into adaptive Tregs. However, daily and (to a lesser extent) bolus intranasal delivery of HDE or LPS was found to promote local increases in FOXP3-expressing CD4 cells in mice with preexisting Tregs (Figure 5). In contrast, neither the bolus nor daily intranasal delivery of HDE induced FOXP3 expression by CD4 cells isolated from the BLNs and lungs of mice that were completely deficient in natural FOXP3-positive CD4 cells (Figure 6). Taken together, these observations strongly suggest that airway exposures to the immunostimulants contained in HDE promoted the local migration or proliferation of preexisting natural Tregs, rather than de novo FOXP3 expression by FOXP3-negative CD4 cells. The experiments depicted in Figure 7 further establish that local increases in Tregs that developed after a week of low-dose intranasal delivery of HDE were significantly larger and longer-lived then those induced by bolus intranasal delivery of HDE. These findings are consistent with previous reports that Tregs proliferate in response to TLR ligand stimulation (18, 19) and selectively expand at sites of prolonged inflammation because of infection (20, 21).
Antigen-specific, FOXP3-negative CD4 cells were previously shown to differentiate into adaptive Tregs in mice receiving OVA in their drinking water and in mice immunized with antigen mixed in alum (22, 23). Likewise, antigen-specific Tregs were shown to expand at sites of chronic infection with Leishmania (24). Although inconsistent with those studies, our finding that intranasal delivery of immunostimulant led to local expansion of preexisting natural Tregs (Figure 5), rather than the de novo conversion of FOXP3-negative CD4 cells into adaptive Tregs (Figures 4 and 6), is consistent with the experimental findings of Haribhai and colleagues (13). In their study, an approximately threefold expansion of preexisting Tregs was evident in the draining lymph nodes of mice vaccinated with complete Freund's adjuvant. However, when mice received FOXP3-negative DO11.10 TG × RAG-1 KO CD4 cells before vaccination with OVA in complete Freund's adjuvant, only about 2% of the antigen-specific CD4 cells became FOXP3-positive. Experimental variables such as the frequency, route, and dose of immunostimulant and antigen exposure likely determined whether natural or adaptive (antigen-specific) Treg expansion was dominant in these vaccination studies. This issue clearly requires additional investigation. Nonetheless, the experimental findings presented here, along with those of other investigators, suggest that the T-cell receptor specificity of effector CD4 cells and Tregs expanding at sites of antigen and immunostimulant encounter may not overlap extensively (13, 25).
The results in Figure 8 demonstrate that mice depleted of Tregs with CD25 mAb during 10 OVA immunization with daily intranasal delivery of HDE developed higher levels of allergen-specific hypersensitivity after 20 OVA sensitization than mice receiving isotype control mAb. Therefore, Tregs are likely to play a functional role in mediating the tolerogenic influence of daily airway exposures to immunostimulant. However, mice that received 20 OVA sensitization alone developed more robust airway hypersensitivity than mice receiving CD25 mAb during 10 OVA immunization with a daily intranasal delivery of HDE. These results have two potential explanations. According to the more trivial explanation, CD25 mAb–mediated Treg depletion was not 100% efficient during 10 OVA immunization. However, the more intriguing possibility exists that along with regulating Treg activity, the tolerogenic effects of daily airway exposures to immunostimulant involve pathways that are Treg-independent. This issue is the focus of ongoing investigations in our laboratory.
Endotoxin (e.g., LPS) and other microbial products contained in ambient air have long been recognized as potent adjuvants (3, 4, 26). In juxtaposition with this observation, numerous investigators found that children raised in homes with a high endotoxin burden were more likely to develop allergen tolerance than hypersensitivity (15, 16, 27, 28). This raises the question of why daily exposures to ambient immunostimulants protect against the genesis of allergic diseases. Because LPS and other TLR ligands were initially described as Th1 adjuvants, these experimental findings were initially interpreted as evidence that ongoing exposures to LPS and other microbial products promote the development of “protective” Th1 responses to potential allergens. However, if this supposition is correct, one might predict that children raised in homes with a high microbial burden would be at increased risk for Th1-biased hypersensitivities rather than allergen tolerance. Moreover, we and others found that selected TLR ligands have the potential to promote the development of Th2-biased airway hypersensitivity in murine models (3, 4). The results presented here offer an alternative explanation for why regular exposures to LPS and other immunostimulants found in HDEs and the living environments they represent promote the development of allergen tolerance rather than hypersensitivity.
The present investigation provides direct evidence that daily airway exposures to HDE or LPS promote the development of long-lived aeroallergen tolerance. Tolerance in this model was associated with the failed expansion of allergen-specific CD4 cells, and was at least partly dependent on the outgrowth of allergen-nonspecific Tregs. Although important mechanistic issues remain to be resolved, these experiments provide a framework for understanding why regular airway exposures to immunostimulants in inspired air do not inherently promote the development antigen-specific hypersensitivity. Moreover, if our model of tolerance induction proves to recapitulate the development of physiologic aeroallergen tolerance in healthy children, these experimental results provide a rationale for investigating the use of daily low-dose airway immunostimulant therapy as a therapeutic approach to the prevention of treatment of aeroallergen hypersensitivities in clinical practice.
Supplementary Material
Acknowledgments
The authors thank Dr. Talal Chatila for kindly providing FOXP3egfp C57B/6 mice, and Dr. Leigh Courtney for her meticulous editorial review of the manuscript.
This work was supported by National Institutes of Health grant RO1-AI61772 and a University of California at San Diego Academic Senate Award (A.A.H.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0001OC on May 6, 2010
Author Disclosure: A.A.H. received a sponsored grant from the NIAID for $10,001–$50,000. N.D.P. received a sponsored grant from the NIAID for $10,001–$50,000. D.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; S.M.L does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; N.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; S.S.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; and G.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol 2006;117:1133–1140. [DOI] [PubMed] [Google Scholar]
- 2.Saxon A, Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat Immunol 2005;6:223–226. [DOI] [PubMed] [Google Scholar]
- 3.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4–dependent T helper cell Type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol 2004;113:448–454. [DOI] [PubMed] [Google Scholar]
- 5.Ohtani T, Nakagawa S, Kurosawa M, Mizuashi M, Ozawa M, Aiba S. Cellular basis of the role of diesel exhaust particles in inducing Th2-dominant response. J Immunol 2005;174:2412–2419. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Senechal S, de Nadai P, Chenivesse C, Gilet J, Vorng H, Legendre B, Tonnel AB, Wallaert B, Lassalle P, et al. Diesel exhaust exposure favors Th2 cell recruitment in nonatopic subjects by differentially regulating chemokine production. J Allergy Clin Immunol 2006;118:354–360. [DOI] [PubMed] [Google Scholar]
- 7.Boasen J, Chisholm D, Lebet L, Akira S, Horner AA. House dust extracts elicit Toll-like receptor–dependent dendritic cell responses. J Allergy Clin Immunol 2005;116:185–191. [DOI] [PubMed] [Google Scholar]
- 8.Ng N, Lam D, Paulus P, Batzer G, Horner AA. House dust extracts have both Th2 adjuvant and tolerogenic activities. J Allergy Clin Immunol 2006;117:1074–1081. [DOI] [PubMed] [Google Scholar]
- 9.Lam D, Ng N, Lee S, Batzer G, Horner AA. Airway house dust extract exposures modify allergen-induced airway hypersensitivity responses by TLR4-dependent and independent pathways. J Immunol 2008;181:2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 2006;117:1396–1403. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovitch N, Liu AH, Zhang L, Rodes CE, Foarde K, Dutton SJ, Murphy JR, Gelfand EW. Importance of the personal endotoxin cloud in school-age children with asthma. J Allergy Clin Immunol 2005;116:1053–1057. [DOI] [PubMed] [Google Scholar]
- 12.Takabayashi K, Libet L, Chisholm D, Zubeldia J, Horner AA. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in Th2-sensitized mice. J Immunol 2003;170:3898–3905. [DOI] [PubMed] [Google Scholar]
- 13.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol 2007;178:2961–2972. [DOI] [PubMed] [Google Scholar]
- 14.Dassonville C, Demattei C, Vacquier B, Bex-Capelle V, Seta N, Momas I. Indoor airborne endotoxin assessment in homes of paris newborn babies. Indoor Air 2008;18:480–487. [DOI] [PubMed] [Google Scholar]
- 15.Gehring U, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med 2002;166:939–944. [DOI] [PubMed] [Google Scholar]
- 16.Gereda JE, Klinnert MD, Price MR, Leung DY, Liu AH. Metropolitan home living conditions associated with indoor endotoxin levels. J Allergy Clin Immunol 2001;107:790–796. [DOI] [PubMed] [Google Scholar]
- 17.Liebers V, Bruning T, Raulf-Heimsoth M. Occupational endotoxin-exposure and possible health effects on humans. Am J Ind Med 2006;49:474–491. [DOI] [PubMed] [Google Scholar]
- 18.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest 2006;116:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med 2003;197:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demengeot J, Zelenay S, Moraes-Fontes MF, Caramalho I, Coutinho A. Regulatory T cells in microbial infection. Springer Semin Immunopathol 2006;28:41–50. [DOI] [PubMed] [Google Scholar]
- 21.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of FOXP3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J Immunol 2008;181:6456–6466. [DOI] [PubMed] [Google Scholar]
- 22.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive FOXP3+ regulatory T cell–dependent and–independent control of allergic inflammation. Immunity 2008;29:114–126. [DOI] [PubMed] [Google Scholar]
- 23.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring tregs. J Clin Invest 2005;115:1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted FOXP3+ natural regulatory T cells are specific for microbial antigens. J Exp Med 2006;203:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology 2008;125:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horner AA, Ronaghy A, Cheng PM, Nguyen MD, Cho HJ, Broide D, Raz E. Immunostimulatory DNA is a potent mucosal adjuvant. Cell Immunol 1998;190:77–82. [DOI] [PubMed] [Google Scholar]
- 27.Tse K, Horner AA. Defining a role for ambient TLR ligand exposures in the genesis and prevention of allergic diseases. Semin Immunopathol 2008;30:53–62. [DOI] [PubMed] [Google Scholar]
- 28.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002;347:869–877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.