Abstract
Angiopoietin (Ang)1 and Ang2 are ligands for Tie2 tyrosine kinase receptor (Tie2). Elevated levels of Ang1 and Ang2 in induced sputum of patients with asthma have been reported, with a positive correlation of Ang2 levels with the severity of airway occlusion. Although studies have shown Tie2-mediated regulation of nonvascular cells in some pathological conditions, current knowledge on Tie2 signaling in asthma is limited to the vasculature. We examined the expression pattern of Ang1, Ang2, vascular endothelial growth factor (VEGF), and Tie2 and their correlation with the degree of airway remodeling in the lung of ovalbumin (OVA)-sensitized and OVA-challenged mice with airway hyperresponsiveness. Lung tissues were isolated from Balb/c mice after OVA sensitization and challenge. Hematoxylin and eosin, periodic acid-Schiff, and trichrome staining were used to show the lung pathology. The expression of Ang1, Ang2, VEGF, and Tie2 was examined using immunofluorescence, Western blot, ELISA, and real-time PCR. In the lung of normal mice, Tie2 expression was detected only in the blood vessels. However, in the lung of OVA-sensitized and OVA-challenged mice, Tie2 was abundantly expressed in airway epithelial cells and in a subset of macrophages in addition to constitutive expression in pulmonary vessels. The increase in Tie2 expression correlated with the severity of airway remodeling. Macrophages and airway epithelial cells express Ang2 and VEGF only in allergic models. Ang1 was constitutively expressed, with a decrease in mRNA level in allergic models. In conclusion, increased expression of Tie2 and Ang2 in allergic airway epithelium and alveolar macrophages correlates with the severity of airway remodeling.
Keywords: airway epithelial cells, airway remodeling, angiopoietins, Tie2 tyrosine kinase receptor, vascular endothelial growth factor
CLINICAL RELEVANCE.
In patients with bronchial asthma, there is a widespread inflammation in the lung, which, under chronic conditions, causes airway remodeling. In this study, increased Tie2 and Ang2 expression was found in nonvascular cells in the lung tissue of ovalbumin-sensitized and ovalbumin-challenged mice. These findings suggest a broader role for Tie2 involving the direct regulation of nonvascular cells during the development of airway remodeling in asthma. This information should provide an opportunity to formulate superior therapeutic approaches in bronchial asthma.
Current therapies are unsatisfactory in effectively reversing airway remodeling (1, 2). The process of airway remodeling involves cross-talk between cells through the release of inflammatory mediators such as transforming growth factor (TGF)-β, IL-11, and IL-13 (3, 4). Features of airway remodeling include epithelial cell shedding, goblet cell hyperplasia/metaplasia, subepithelial fibrosis, smooth muscle cell hyperplasia, edema, and angiogenesis (3, 5). Edema and angiogenesis are regulated by angiogenic growth factors, primarily angiopoietins (Ang) and vascular endothelial growth factor (VEGF), through Tie2 receptor (Tie2) and VEGF receptors, respectively (6, 7).
Ang1, Ang2, and VEGF play complementary and coordinated roles in successful angiogenesis (7, 8). An increase in the number and permeability of blood vessels in asthmatic airways contributes to the thickness of the airway wall and consequently airway occlusion (8, 9). Ang2 and VEGF contribute to the proinflammatory nature of the blood vessels, whereas Ang1 elicits a net antiinflammatory effect (8, 10). Ang1, Ang2, and VEGF levels are increased in the induced sputum of patients with asthma, and this increase correlated with airway obstruction (6). There is also a positive correlation between Ang2 and VEGF levels and the vascular permeability index (6). The increase in Ang2 level also correlates with the severity of exercise-induced asthma (11). However, another study reported that Ang1 expression and Tie2 phosphorylation are reduced in the lung of ovalbumin (OVA)-treated mice (12). Ang1 and Ang2 bind to Tie2 with similar affinity; therefore, a net increase in Ang2 levels would favor Ang2-dominated Tie2 binding or vice versa (13, 14). Our current knowledge on Ang1 and Ang2 in asthma is limited to the microvasculature.
Angiogenic growth factors, including angiopoietins, contribute to the pathogenesis of various diseases by directly regulating the behavior of nonvascular cells (7, 8). A subset of monocytes expressing Tie2 has been found at the tumor sites (15). Ang2 has a chemotactic effect on Tie2-positive monocytes and may be responsible for recruiting this subset of monocytes to tumor sites (15). Ang2 activation of Tie2 on these cells inhibits the release of antiangiogenic cytokines, including IL-12 (15). Tie2-mediated signaling promotes neural outgrowth from dorsal root ganglion cells (16). Ang1 induces a chemotactic effect on fibroblastic synoviocytes in rheumatoid arthritis and fibroblast stably transfected with Tie2 (17). Thus, although Ang-induced, Tie2-mediated signaling plays a major role in the vasculature, studies done in pathological conditions suggest that their role outside the vasculature may be equally as important and therefore warrants further investigation.
We, for the first time, report here that there is increased expression of angiogenic growth factors and Tie2 receptors in the respiratory epithelium in allergic asthma and that their expression correlates with the severity of airway remodeling.
MATERIALS AND METHODS
Animals
We purchased BALB/c mice (aged 4–5 wk) from Harlan Laboratories (Indianapolis, IN). Mice were maintained in a pathogen-free environment at Creighton University. Food and water were provided ad libitum. The research protocol of this study was approved by the Institutional Animal Care and Use Committee of Creighton University.
Induction of Allergic Airway Disease
We sensitized mice with 20-μg intraperitoneal injections of OVA (Sigma-Aldrich, St. Louis, MO) emulsified in 2.25 mg of Imject alum (Pierce Biotechnology, Rockford, IL) on Days 0 and 14 (Figure 1A). Animals were challenged with 1% OVA for three consecutive days from Days 28 to 30 and with 5% OVA on Day 32. On Day 33, specific airway resistance was measured in randomly selected tracheostomized mice in response to aerosolized acetyl β-methylcholine (Figure 1B), and enhanced pause in response to aerosolized acetyl β-methylcholine (Sigma-Aldrich) was measured in the remaining animals using whole-body plethysmography (Buxco Electronics, Troy, NY). The remaining mice with established airway hyperresponsiveness (AHR) were challenged with 5% OVA by aerosol on Day 44. On Day 45, specific airway resistance was measured in randomly selected tracheostomized mice in response to aerosolized acetyl β-methylcholine (Figure 1B). Animals were challenged with 1% OVA weekly from Days 52 through 77 and with 5% OVA on Day 79. On Day 80, specific airway resistance was measured in the remaining mice in response to aerosolized acetyl β-methylcholine (Figure 1B).
Figure 1.
(A) Ovalbumin (OVA) antigen sensitization protocol. (B) Airway hyperresponsiveness analysis. Graph shows the specific airway resistance (RL) in tracheostomized mice in response to aerosolized acetyl β-methylcholine in phosphate-buffered saline (PBS)-treated and OVA-sensitized and OVA-challenged mice. (C) Total and differential inflammatory cell count in bronchoalveolar lavage fluid (BALF) of PBS-treated and OVA-sensitized and OVA-challenged mice. Data are shown as mean ± SEM of values of eight mice per group. *P < 0.05; **P < 0.001.
Bronchoalveolar Lavage Fluid and Cytokine Measurement
After mice were killed, lungs were gently lavaged with 1 ml of warm saline (37°C) via a tracheal cannula. Total cell counts were performed with a Coulter counter (Beckman and Coulter, Fullerton, CA). All samples were centrifuged at 400 × g for 10 minutes, and the supernatants were stored in at −80°C until ELISAs were performed. Mouse VEGF-A level was measured using an ELISA detection kit (cat# BMS 619/2; eBioscience, San Diego, CA) according to the manufacturer's protocol. Ang1 and Ang2 levels were measured according to the following protocol. Wells were coated with 100 μl of capture antibody (Ang1 [c-19] and Ang2 [c-19]; Santa Cruz Biotechnology, Santa Cruz, CA) and coating buffer (cat# 00-0044-59; eBioscience) and incubated overnight at 4°C. Wells were washed five times with 250 μl of PBS/0.05% Tween20 wash assay and blocked with 250 μl of assay diluent (cat# 00-4202-AD2; eBioscience) overnight. Wells were wash five times and incubated with 100 μl of samples in each well for 2 hours at room temperature (RT) and were washed again five times and incubated for 1 hour at RT with 100 μl of detection antibody (Ang1 [AB3120] and Ang2 [AB3121]; Millipore, Billerica, MA) and assay diluents in each well. Wells were incubated with HRP-conjugated secondary antibody (goat anti-rabbit; Novus, Littleton, CO) for 30 minutes at RT and washed seven times and incubated with 100 μl of substrate solution (cat# 00-4201-52; eBioscience) for 15 minutes. Stop solution (50 μl per well) was added, and absorbance was read at 450 nm.
Preparation and Staining of Lung Tissue
Lung lobes were fixed in 4% formalin and paraffin embedded. These lung sections were stained with hematoxylin and eosin following the manufacturer's standard protocol (Newcomer Supply, Middleton, WI). Mucus secretion was identified by periodic acid-Schiff (PAS) reaction using the standard protocol recommended by the manufacturer (Sigma-Aldrich). Trichrome staining was used to identify collagen following the standard protocol recommended by the manufacturer (IMEB Inc., San Marcos, CA).
Isolation of Airway Epithelial Cells
Mouse lung tissues were chopped and digested with collagenase D (1 mg/ml) in RPMI-1640 containing DNase (50 μg/ml) at 37°C for 1 hour. Single cell suspension was filtered through a 40-μm filter and centrifuged at 350 × g for 10 minutes. The supernatant was discarded, and the pellet was resuspended in ammonium Tris chloride solution and placed on ice for 7 minutes. We neutralized the ammonium Tris chloride solution with 10% FBS (1 ml FBS + 9 ml RPMI-1640) and centrifuged the solution at 350 × g for 10 minutes. The supernatant was discarded, and the pellet was resuspended in 10 ml MACS buffer and centrifuged at 350 × g for 10 minutes. The cells were counted with a Coulter counter. PE-conjugated EpCAM (eBioscience) microbeads (100 μl per 108 cells) were added, and cells were incubated on ice for 30 minutes and washed. Cells were incubated with MACS anti-PE microbeads, washed, and centrifuged at 1,500 × g for 10 minutes. The supernatant was discarded, and the pellet was resuspended in 1 ml of MACS buffer. The samples were run on AutoMACS (Miltenyi-Biotech, Bergisch Gladbach, Germany) under a double-sensitive (POSSELDS) separation program. The epithelial phenotype of the isolated cells was confirmed by EpCAM and cytokeratin staining. The purity of the cell isolate was 65 to 70%.
Immunofluorescence
We performed deparaffinization, rehydration, and antigen retrieval before immunostaining. The slides were incubated for 2 hours in block/permeabilizing solution containing 5% (vol/vol) of appropriate serum with 0.25% Triton X-100 and 0.1% BSA when goat serum was used. The slides were incubated with primary antibody and normal host IgG, including chromePure rat IgG, chromePure rabbit IgG, chromePure goat IgG (Jackson Immunoresearch, West Grove, PA), rat anti-EpCAM (eBioscience), rat anti-F4/80, rabbit anti–pan-cytokeratin (H-240), rabbit anti-Tie2 (C-20) and (H-176), rat anti-F4/80 (sc-59171), goat anti-integrin aX (CD11c, M-50), goat anti-Ang1 (N-18) and (C-19), and goat anti-Ang2 (C-19) and (F-18) (Santa Cruz Biotechnology). The sections were washed and incubated with Cyanine 3 or Cyanine 2 goat anti-rabbit IgG, goat anti-rat IgG, and donkey anti-goat IgG (Jackson Immunoresearch). Slides were incubated with goat serum after the first secondary antibody incubation, when Cyanine 3 donkey anti-goat was used as the first secondary and Cyanine 2 goat anti-rabbit was used as second secondary antibody for double staining. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Fluorescent analysis was performed with a confocal microscope.
Protein Extraction and Immunoblotting
Protein lysates were extracted with protein extraction RIPA buffer with 1% protease inhibitor (P8340; Sigma). The protein was quantified, and 50 μg of each protein sample was resolved by electrophoresis using 10 to 20% polyacryamide gels (Biorad, Hercules, CA) after mixing with Laemmli loading buffer with 10% mercaptoethanol. Separated proteins were transferred to Hybond–electrogenerated chemiluminescence nitrocellulose membrane (Biorad). The membrane was incubated in for 1 hour in blocking buffer (5% wt/vol nonfat dry milk, 0.05% Tween 20 in PBS) to minimize nonspecific binding. The membrane was incubated in rabbit anti-mouse Tie2 (C-20; Santa Cruz Biotechnology), mouse anti-Tie2 (clone Ab33, 05-584) (Millipore), anti-β actin (ACTBD11B7, sc-81178), goat anti-Ang1 (N-18), and goat anti-Ang2 (C-19) and (F-18) (Santa Cruz Biotechnology) diluted in blocking buffer (1:500–1:,1000) and incubated with the membrane at room temperature for 1 hour with gentle rocking. After three or four washes (10 min each) with washing buffer (0.05% Tween 20 in PBS), HRP-conjugated secondary antibodies (1:2,000) (NOVUS) were incubated with the membrane for 1 hour under the same conditions. The membrane was washed three times with washing buffer, and the immunoreactive bands were visualized using electrogenerated chemiluminescence detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ). The emission was detected in EpiChemi darkroom, and the image was captured with BioChemi CCD camera (UVP, Inc., Upland, CA).
RNA Isolation and Real-time PCR
Total RNA was isolated from EpCAM+ cells for analysis of angiopoietin and Tie2 expression. Real-time PCR analysis was performed for the identification of Ang1, Ang2, and Tie2 mRNA transcripts. The total RNA was isolated using the Trizol reagent (Sigma) method. The yield of RNA was quantified using Nanodrop (Thermo Scientific, Rockford, IL). First-strand cDNA synthesis was performed using 1 μg total RNA with oligo dT (1 μg), 5× reaction buffer, MgCl2, dNTP mix, and Improm II reverse transcriptase as per the Improm II reverse transcription kit (Promega, Madison, WI). After the first strand synthesis, real-time PCR was performed using 8 μl cDNA, 10 μl SYBR green PCR master mix (Biorad), and forward and reverse primers (10 pmol/μl) (Integrated DNA Technologies, San Diego, CA) using a real-time PCR system (CFX96; Biorad). The primers include the following: Tie2 forward, GAT TTT GGA TTG TCC CGA GGT CAA G; Tie2 reverse, CAC CAA TAT CTG GGC AAA TGA TGG; Ang1 forward, TGC AGC AAC CAG CGC CGA AA; Ang1 reverse, CAG GGC AGT TCC CGT CGT GT; Ang2 forward, GCT TCG GGA GCC CTC TGG GA; Ang2 reverse, CAG CGA ATG CGC CTC GTT GC. The specificity of the primers was analyzed by running a melting curve. The PCR cycling conditions used were 5 minutes at 95°C for initial denaturation, 40 cycles of 30 seconds at 95°C, 30 seconds at 52 to 58°C (depending upon the primer annealing temperatures), and 30 seconds at 72°C. Each real-time PCR was performed using four individual samples in duplicates, and the threshold cycle values were averaged. Calculations of relative gene expression were based on the differences in the threshold cycles. The fold change in expression between samples were calculated by fold change = 2 – ΔΔCt. The results were normalized against the housekeeping gene 18S.
Statistical Analysis
In all the studies, each group was represented by eight animals as determined by power of analysis. Data are presented as means ± SEM and were analyzed using GraphPad Prism. One-way ANOVA was used when one parameter was tested. In conditions that required the evaluation of two parameters simultaneously, two-way ANOVA was used. Multiple group comparisons were performed by Bonferroni's multiple comparison test. A value of P < 0.05 was considered significant.
RESULTS
Establishment of AHR to Methacholine and Airway Inflammation
OVA-sensitized and OVA-challenged mice (Figure 1A) demonstrated AHR to methacholine, with a marked increase in specific lung resistance on Days 33, 45, and 80 (Figure 1B). There was significant increase in total number of cells, eosinophils, and lymphocytes in the bronchoalveolar lavage fluid (BALF) of OVA-sensitized and challenged mice compared with the PBS-treated group (Figure 1C). Long-term exposure to OVA resulted in a significant increase in the number of neutrophils on Day 80 (Figure 1C). There were no lymphocytes in the BALF of the PBS-treated control group (Figure 1C).
Different Degrees of Airway Remodeling after OVA Sensitization and Challenge
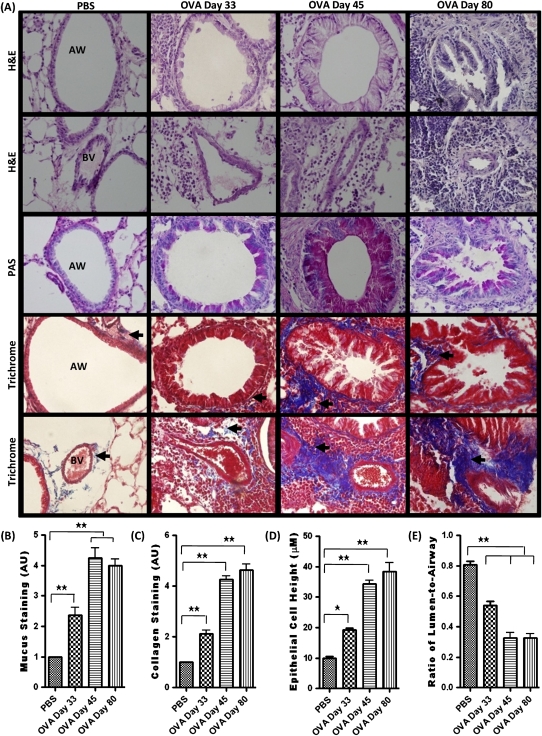
Airways of the control mice exhibited normal parenchyma, with no mucus staining and little collagen staining around the respiratory epithelium and vascular basement membrane (Figure 2A). On Day 33, the lungs of OVA-sensitized and OVA-challenged mice exhibited the signs of mild airway remodeling, showing airway epithelial cell hypertrophy, mild mucus staining, and sparse collagen deposition (Figure 2A). However, on Days 45 and 80, features of severe airway remodeling were present after additional antigen challenge (Figure 2A). The airway had marked epithelial cell hypertrophy indicated by an increase in epithelial cell height, airway occlusion measured by ratio of lumen to airway, mucus plugging, and collagen deposition (Figures 2B–2E).
Figure 2.
(A) Hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Trichrome staining in the airway (AW) and blood vessel (BV) of PBS-treated and of OVA-sensitized and challenged mice on Days 33, 45, and 80. (B and C) The severity of mucus and collagen staining was graded for quantification based on the following scale: 0, normal; 1, mild; 2, moderate; 3, strong; and 5, severe alterations. Mid to lower airway generations were typically used for analysis. (D and E) Zeiss imaging software was used to measure epithelial cell height and lumen area. Data are shown as mean ± SEM of values of 10 measurements per mouse. Eight mice were analyzed per group. *P < 0.05; **P < 0.001.
Expression of Tie2 in Respiratory Epithelium and Macrophages
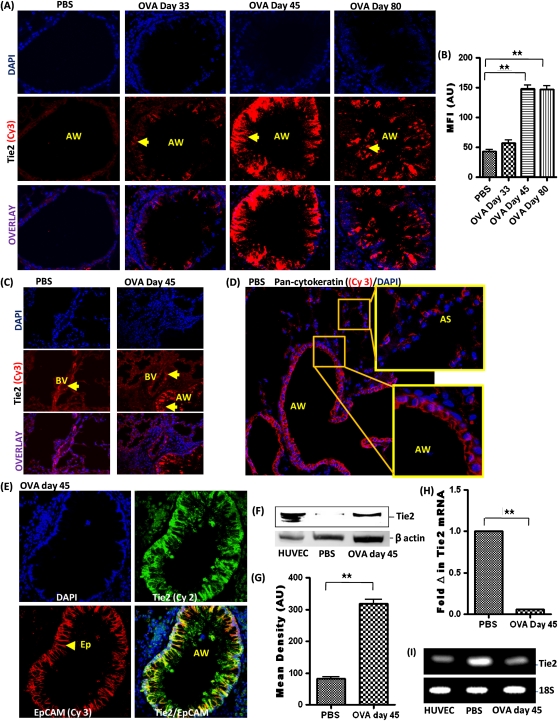
Tie2 expression was not detectable in the respiratory epithelium of PBS-treated control mice (Figure 3A). Tie2 expression was moderately increased in some of the airway epithelial cells of OVA-sensitized and OVA-challenged mice on Day 33. However, Tie2 expression was significantly increased in the airway epithelial cells of OVA-sensitized and OVA-challenged mice on Day 45. Tie2 expression in airway epithelial cells remained elevated in OVA-sensitized and OVA-challenged mice on Day 80 (Figures 3A and 3B). Pulmonary blood vessels of PBS-treated control and OVA-sensitized and OVA-challenged groups constitutively expressed Tie2 (Figure 3C). Pan-cytokeraton expression was detectable in airway epithelial cells and alveolar epithelial cells but not in alveolar macrophages (Figure 3D). EpCAM expression was detectable only in airway epithelial cells lining the airway wall (Figure 3E). Although some studies have reported the expression of EpCAM on some lymphocytes and alveolar epithelial cells (18), EpCAM expression was undetectable in these cell types under our described experimental conditions in this study (Figure 3E). Double staining with EpCAM and Tie2 further confirmed that Tie2 is expressed by airway epithelial cells (EpCAM+) after OVA sensitization and challenge.
Figure 3.
(A) Tie2 expression (arrowhead) in the AW. (B) Mean fluorescent intensity (MFI) of Tie2 immunostaining in AW measured in arbitrary units (AU) (using NIH Image J software). Data are shown as mean ± SEM of values of 10 measurements per mouse. Eight mice were analyzed per group. (C) Tie2 expression in BVs. (D) Pan-cytokeratin expression in the AW and alveolar sac (AS). (E) EpCAM and Tie2 double staining of the lung tissue. (F) Western blot analysis of Tie2 expression in isolated airway epithelial cells using human umbilical vein endothelial cell (HUVEC) as positive control. (G) Densitometric analysis. (H) Tie2 mRNA level in isolated airway epithelial cells by real-time PCR. (I) Agarose gel showing amplified DNA after real-time PCR. Data are shown as mean ± SEM of values of three measurements in each group. *P < 0.05; **P < 0.001.
Airway epithelial cells were isolated on the basis of their expression of EpCAM, previously shown to be expressed by this cell type (Figure 3E). Western blot protein analysis of these isolated airway epithelial cells further confirmed increased expression of Tie2 receptors in airway epithelial cells of OVA-sensitized and OVA-challenged mice on Day 45 (Figures 3F and 3G). There was a barely detectable level of Tie2 by Western blot in PBS-treated control airway epithelial cells. Western blot analysis of HUVEC whole-cell lysate confirmed Tie2 expression at the known 145 kD molecular weight, thereby validating the specificity of the antibodies chosen. Analysis of the Tie2 mRNA transcript by real-time PCR revealed a decrease in mRNA level in airway epithelial cells after OVA sensitization and challenge (Figures 3H and 3I).
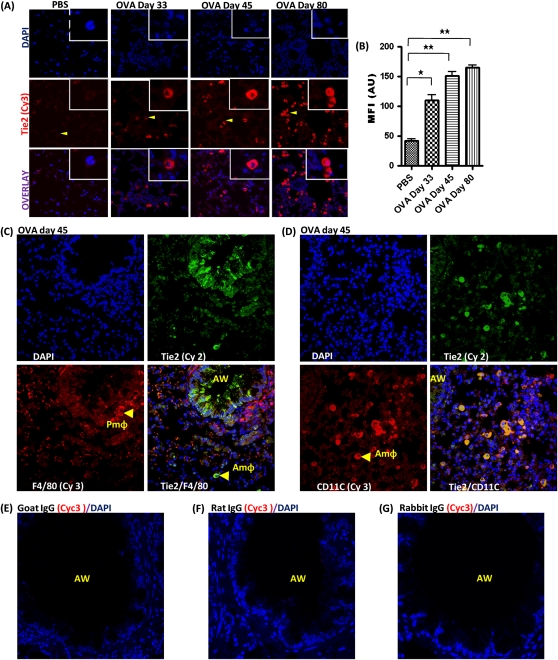
Although there was no detectable expression of Tie2 in macrophages of PBS-treated control mice, Tie2 expression was gradually increased in macrophages located in the alveolar sac in OVA-sensitized and OVA-challenged mice on Days 33, 45, and 80 (Figures 4A and 4B). F4/80 expression was heterogeneous in lung tissue macrophages, with particularly high expression in macrophages located in the perivascular and peribronchial areas but not in the alveolar sacs (Figure 4C). CD11c was highly expressed in macrophages located in the alveolar sacs (Figure 4D). Therefore, our subsequent identification of macrophages in the alveolar sac was based on CD11c expression. Our negative controls using IgG from primary antibody hosts chosen for the study revealed minimal nonspecific binding of nonimmunized antibody host IgG (Figures 4E and 4G).
Figure 4.
(A) Tie2 expression (arrowhead) in macrophage in the alveolar sac (AmΦ). (B) MFI of Tie2 immuno-staining in AmΦ, measured in AU (using NIH Image J software). (C) F4/80 and Tie2 double staining of macrophages in the peribronchial area (PmΦ) and alveolar sac. (D) CD11c and Tie2 double staining in AmΦ. (E) Immunofluorescent (IF) staining for normal goat IgG (control for Ang1 and Ang2 antibody). (F) Rat IgG (control for EpCAM and F4/80 antibody). (G) Rabbit IgG (control for Tie2, Pan-cytokeratin, and CD11c antibody). This is a representative immunostaining image for each group. Data are shown as mean ± SEM of values of 10 measurements in each group. *P < 0.05; **P < 0.001.
Expression of Ang1, Ang2, and VEGF in the Airways of PBS-Treated Mice and after OVA Sensitization and Challenge
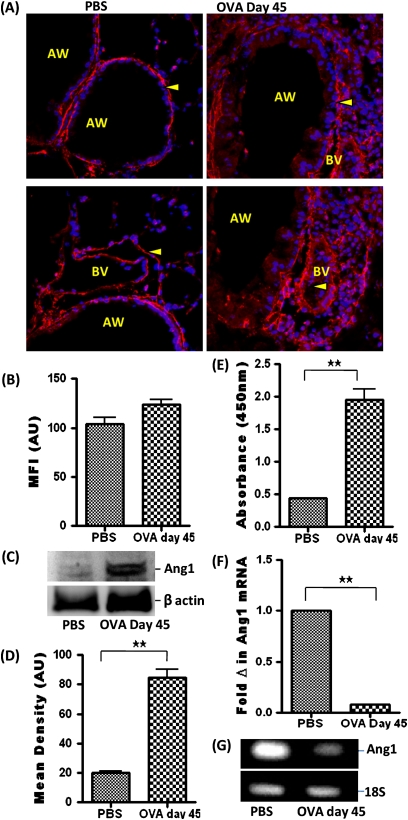
Ang1 was expressed in the lungs of PBS-treated control mice and increased in OVA-sensitized and OVA-challenged mice. Ang1 expression was localized to the pulmonary vascular smooth muscle cells and mesenchymal cells surrounding the airway epithelial layer (Figures 5A and 5B). This result was further confirmed by Western blot analysis of Ang1 in protein lysate of whole lung tissue (Figures 5C and 5D). Analysis of the BALF using ELISA revealed an increase in Ang1 after OVA sensitization and challenge (Figure 5E). Conversely, analysis of Ang1 mRNA transcript by real-time PCR revealed a decrease in mRNA level in airway epithelial cells after OVA sensitization and challenge (Figures 5F and 5G).
Figure 5.
(A) Ang1 (arrowhead) expression in the AW and BV of PBS-treated and OVA-treated mice. (B) MFI of Ang1 immunostaining, measured in AU (using NIH Image J software). Data are shown as mean ± SEM of values of 10 measurements per group. (C) Western blot analysis Ang1 expression in whole lung tissue protein lysate. (D) Densitometric analysis. (E) Ang1 level in BALF using ELISA. (F) Ang1 mRNA level in isolated epithelial cells by real-time PCR. (G) Agarose gel showing amplified DNA after real-time PCR. Data are shown as mean ± SEM of values of three measurements in each group. *P < 0.05; **P < 0.001.
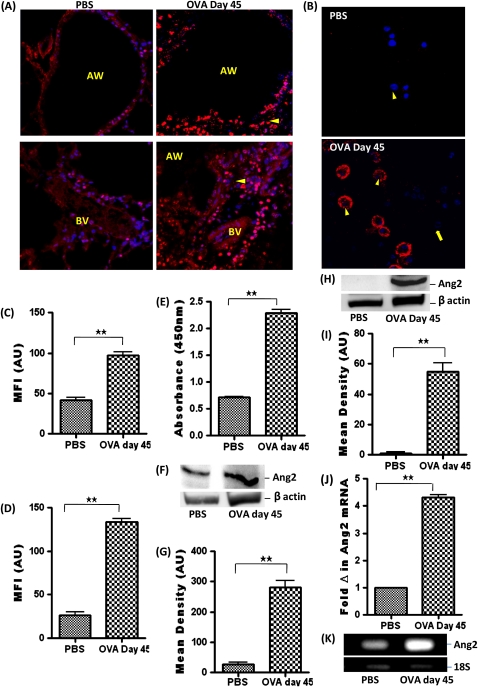
Ang2 expression was significantly increased in airway epithelial cells and macrophages in OVA-sensitized and OVA-challenged mice (Figures 6A and 6C). Ang2 expression in BALF cells was localized to the inflammatory macrophages in OVA-sensitized and OVA-challenged mice but was undetectable in residential macrophages from PBS-treated mice (Figures 6B and 6D). Analysis of the BALF using ELISA revealed an increase in Ang2 after OVA sensitization and challenge (Figure 6E). The increased level of Ang2 in the lung tissue was further confirmed by Western blot analysis of protein lysate of whole lung tissue (Figures 6F and 6G). Ang2 expression was undetectable by Western blot in isolated airway epithelial cells from PBS-treated mice but increased in OVA-treated mice (Figures 6H and 6I). Analysis of Ang2 mRNA transcript by real-time PCR also revealed an increase in Ang2 mRNA level in isolated airway epithelial cells after OVA sensitization and challenge (Figures 6J and 6K).
Figure 6.
(A) Ang2 (arrowhead) expression in the AW and BV of PBS and OVA-treated mice. (B) Ang2 expression and MFI measured in AU (using NIH Image J software) in (C) AW and (D) BALF cells, including macrophages (arrowhead) and eosinophils (arrow). Data are shown as mean ± SEM of values of 10 measurements in each group. (E) Ang2 level in BALF using ELISA. (F) Western blot analysis Ang2 expression in whole lung tissue protein lysate. (G) Densitometric analysis in AU. (H) Western-blot analysis of Ang2 expression in isolated airway epithelial cells. (I) Densitometric analysis. (J) Ang2 mRNA level in isolated airway epithelial cells by real-time PCR. (K) Agarose gel showing amplified DNA after real-time PCR. Data are shown as mean ± SEM of values of three measurements in each group. *P < 0.05; **P < 0.001.
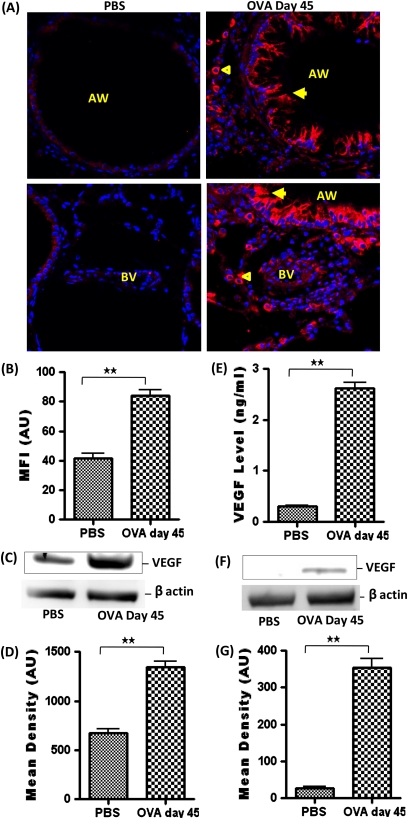
VEGF expression was significantly increased in airway epithelial cells and macrophages in the perivascular and peribronchial area in OVA-sensitized and OVA-challenged mice (Figures 7A and 7B). Analysis of the BALF using ELISA revealed an increase in VEGF level after OVA sensitization and challenge (Figure 7E). An increased level of VEGF in the lung tissue was further confirmed by Western blot analysis of whole lung tissue protein lysate (Figures 7C and 7D). VEGF expression was undetectable by Western blot in isolated airway epithelial cells from PBS-treated mice but was increased in OVA-treated mice (Figures 7F and 7G).
Figure 7.
(A) Vascular endothelial growth factor (VEGF) (arrowhead) expression in the AW and BV of PBS- and OVA-treated mice. (B) MFI of VEGF immunostaining, measured in AU (using NIH Image J software) in AW. Data are shown as mean ± SEM of values of 10 measurements in each group. (C) Western blot analysis and (D) densitometric analysis of VEGF expression in whole lung tissue protein lysate. (E) VEGF level in BALF using ELISA. (F) Western blot analysis and (G) densitometric analysis of VEGF expression in isolated airway epithelial cell protein lysate. Data are shown as mean ± SEM of values of three measurements in each group. *P < 0.05; **P < 0.001.
DISCUSSION
In this study, we found that Tie2 expression is increased in the respiratory epithelium and in a subset of macrophages in OVA-sensitized and OVA-challenged mice with airway remodeling and elevated AHR. This increase in Tie2 expression correlates positively with the severity of airway remodeling. There is constitutive expression of Tie2 in the pulmonary blood vessels. A decrease in mRNA expression of Tie2 suggests an imbalance between the rates of transcription and translation. This is consistent with reports from another study showing that, although Tie2 mRNA is down-regulated under hypoxic conditions, Tie2 translation is maintained (27). Understanding the regulatory mechanism involved in Tie2 expression would shed more light on this process. The Ang1 protein level is increased in the BALF of OVA-sensitized and OVA-challenged mice, consistent with the report from a previous human study (6). However, a decrease in Ang1 mRNA suggests that the Ang1 protein level could decrease, as reported in another allergic mice study (12). Ang2 and VEGF expression are increased in structural and inflammatory macrophages in OVA-sensitized and OVA-challenged mice. Constitutive expression of Ang1 and Tie2 in pulmonary blood vessels implies a physiological role for Tie2 signaling in maintaining the quiescent state of the microvasculature in the lungs, consistent with previous findings (8, 10). Increased expression of Tie2 in alveolar macrophages and airway epithelial cells of mice with mild and severe airway remodeling suggest that Tie2 activity in these cell types may be involved in the onset and exacerbation of the airway remodeling process.
Ang1 and Ang2 are expressed in the lungs and can bind to Tie2 with similar affinity. Therefore, they compete equally for binding to Tie2 receptors, and their bioavailability determines the dominant signaling pathway (13, 14). The findings in this study, including increased expression of Ang2 in the airway epithelial cells and alveolar macrophages of asthmatic mice, suggest that Ang2-induced, Tie2-mediated signaling would be the predominant signaling pathway. However, Ang1 and Ang2 could also have a complementary effect in regulating various cellular activities. Ang1-induced, Tie2-mediated signaling plays an important role in postnatal vascularization and maintenance of mature quiescent vasculature (14). Overexpression of Ang1 in the skin of a murine model results in leakage-resistant and enlarged vessels (19). Ang2 antagonistic action leads to vessel destabilization, a crucial process in the initiation of angiogenesis (13). A net gain of Ang2 over Ang1 would destabilize the blood vessels, making them more susceptible to other angiogenic growth factors such as VEGF, resulting in the initiation of the angiogenic process (13). Transgenic overexpression of Ang2 leads to vascular defect similar to Ang1 or Tie2 deficiency (13). Although several studies have reported that Ang2 does not activate Tie2 in endothelial cells, Ang2 can activate Tie2 in nonendothelial cells (8). Ang2 has a chemotactic effect on Tie2-positive monocytes and blocks the secretion of IL-12 from these cells at tumor sites (15). Because we have found an increased expression of Tie2 in alveolar macrophages in the lungs of asthmatic mice with mild and severe airway remodeling, it is possible that Ang2-induced activation of Tie2 may also block the release of pro-T helper (TH)1 immune response cytokines, such as IL-12, thereby contributing to a cytokine milieu that favors a TH2 response. Ang1-induced signaling promotes neural outgrowth from dorsal root ganglion cells. Tie2-mediated signal transduction can also elicit neuron-protective and mitogenic effect in vitro (16, 20). Thus, there is enough evidence to suggest that both beneficial and deleterious effects can be elicited through the activation of Tie2 in vascular and nonvascular cells. Any proposed effect of Tie2 signaling would be mere speculation at this point. It would be interesting to study the effect of Ang1 and Ang2 on epithelial–mesenchymal transition and airway epithelial cell apoptosis.
After secretion, Ang1 is incorporated into the extracellular matrix (21–23). Ang2, on the other hand, is not associated with the extracellular matrix but is stored in Weibel-palade bodies predominantly in the cytoplasm of endothelial cell and quickly secreted when needed. Ang2 is mainly expressed by endothelial cells and is located at the sites of vascular remodeling (21–23). In this study, we found that inflammatory macrophages also produce Ang2 in allergic asthmatic mice. Ang1 and Ang2 expressions are regulated by inflammatory mediators such as IL-1β, TNF-α, TGF-β, and hypoxia (8). However, the synthesis and release of Ang1 and Ang2 is a dynamic process and may depend on the microenvironment and pathological conditions.
Inflammatory mediators, such as IL-11, VEGF, and hypoxia, can induce and up-regulate Tie2 receptor expression (13, 14). Various transcriptional factors, including Ets-related factor-2, E74-like factor-1 (Elf-1), and endothelial PAS domain protein 1, can bind to the Tie2 promoter to induce transcription of Tie2 gene. Angiopoietins under hypoxic conditions can activate Ets-related factor 2 to increase Tie2 gene expression (13, 14, 24–26). In this study, we observed a significant increase in the Tie2 protein expression in the airway epithelial cells of allergic asthmatic mice with severe airway remodeling. In our preliminary studies, we observed an increased expression of Elf-1 transcription factor in the airway epithelial cells and macrophages of mice with severe airway remodeling (unpublished observations). Mediators such as VEGF and Ang2 can activate Elf-1, resulting in the up-regulation of Tie2 gene expression (8). However, the role of other transcription factors cannot be ruled out. Further studies are warranted to better understand the regulation of Tie2 expression in the lung of allergic asthmatic mice and their causative relationship with airway remodeling.
In summary, we have shown that Tie2 expression is increased in nonvascular cells in the lung tissue of OVA-sensitized and OVA-challenged mice. Increased expression of Ang2 suggests that the dominant pathway in the lung would be Ang2 induced but does not rule out a complementary relationship between Ang1- and Ang2-induced signaling. Our findings suggest a broader role for Tie2 involving the direct regulation of nonvascular cells during the development of airway remodeling in asthma, in addition to its role in the remodeling of microvasculature.
This work was supported by a grant from the Nebraska Cancer and Smoking-Related Diseases Program, Department of Health, Nebraska, and by NIH grant R01HL085680.
Originally Published in Press as DOI: 10.1165/rccm.2009-0330OC on May 12, 2010
Author Disclosure: T.O.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.K.A. received a sponsored grant from NIH for more than $100,001 and served on the advisory board for $1,001 to $5,000 and received sponsored grants from the state of Nebraska for more than $100,001 and $10,001 to $50,000.
References
- 1.Navarro RP, Schaecher KL, Rice GK. Asthma management guidelines: updates, advances, and new options. J Manag Care Pharm 2007;13(6 Suppl D):S3–S11; quiz S12–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogil J. Many asthma patients experience persistent symptoms despite appropriate clinical and guideline-based treatment with inhaled corticosteroids. J Am Acad Nurse Pract 2007;19:459–470. [DOI] [PubMed] [Google Scholar]
- 3.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol 2007;85:348–356. [DOI] [PubMed] [Google Scholar]
- 4.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol 2003;111:215–225, quiz 226. [DOI] [PubMed] [Google Scholar]
- 5.Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Curr Opin Pulm Med 2000;6:15–20. [DOI] [PubMed] [Google Scholar]
- 6.Kanazawa H, Nomura S, Asai K. Roles of angiopoietin-1 and angiopoietin-2 on airway microvascular permeability in asthmatic patients. Chest 2007;131:1035–1041. [DOI] [PubMed] [Google Scholar]
- 7.Makinde T, Murphy RF, Agrawal DK. Immunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodeling. Curr Mol Med 2006;6:831–841. [DOI] [PubMed] [Google Scholar]
- 8.Makinde T, Agrawal DK. Intra and extra-vascular trans-membrane signaling of angiopoietin-1-tie2 receptor in health and disease. J Cell Mol Med 2008;12:810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med 2001;164:S39–S45. [DOI] [PubMed] [Google Scholar]
- 10.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res 2000;87:603–607. [DOI] [PubMed] [Google Scholar]
- 11.Kanazawa H, Tochino Y, Asai K. Angiopoietin-2 as a contributing factor of exercise-induced bronchoconstriction in asthmatic patients receiving inhaled corticosteroid therapy. J Allergy Clin Immunol 2008;121:390–395. [DOI] [PubMed] [Google Scholar]
- 12.Simoes DC, Vassilakopoulos T, Toumpanakis D, Petrochilou K, Roussos C, Papapetropoulos A. Angiopoietin-1 protects against airway inflammation and hyperreactivity in asthma. Am J Respir Crit Care Med 2008;177:1314–1321. [DOI] [PubMed] [Google Scholar]
- 13.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science 1997;277:55–60. [DOI] [PubMed] [Google Scholar]
- 14.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000;6:460–463. [DOI] [PubMed] [Google Scholar]
- 15.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic tie2-expressing monocytes (tems) in human peripheral blood and cancer. Blood 2007;109:5276–5285. [DOI] [PubMed] [Google Scholar]
- 16.Ward NL, Putoczki T, Mearow K, Ivanco TL, Dumont DJ. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J Comp Neurol 2005;482:244–256. [DOI] [PubMed] [Google Scholar]
- 17.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase tie2. J Biol Chem 1998;273:18514–18521. [DOI] [PubMed] [Google Scholar]
- 18.Amann M, Friedrich M, Lutterbuese P, Vieser E, Lorenczewski G, Petersen L, Brischwein K, Kufer P, Kischel R, Baeuerle PA, et al. Therapeutic window of an epcam/cd3-specific bite antibody in mice is determined by a subpopulation of epcam-expressing lymphocytes that is absent in humans. Cancer Immunol Immunother 2009;58:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511–2514. [DOI] [PubMed] [Google Scholar]
- 20.Valable S, Bellail A, Lesne S, Liot G, Mackenzie ET, Vivien D, Bernaudin M, Petit E. Angiopoietin-1-induced pi3-kinase activation prevents neuronal apoptosis. FASEB J 2003;17:443–445. [DOI] [PubMed] [Google Scholar]
- 21.Sato A, Iwama A, Takakura N, Nishio H, Yancopoulos GD, Suda T. Characterization of tek receptor tyrosine kinase and its ligands, angiopoietins, in human hematopoietic progenitor cells. Int Immunol 1998;10:1217–1227. [DOI] [PubMed] [Google Scholar]
- 22.Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of tie1, tie2, and angiopoietins 1, 2, and 4 in kaposi's sarcoma and cutaneous angiosarcoma. Am J Pathol 2000;156:2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol 2003;10:38–44. [DOI] [PubMed] [Google Scholar]
- 24.Dube A, Akbarali Y, Sato TN, Libermann TA, Oettgen P. Role of the ets transcription factors in the regulation of the vascular-specific tie2 gene. Circ Res 1999;84:1177–1185. [DOI] [PubMed] [Google Scholar]
- 25.Dube A, Thai S, Gaspar J, Rudders S, Libermann TA, Iruela-Arispe L, Oettgen P. Elf-1 is a transcriptional regulator of the tie2 gene during vascular development. Circ Res 2001;88:237–244. [DOI] [PubMed] [Google Scholar]
- 26.Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, Manabe I, Nagai R. Endothelial pas domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, flt-1. Circ Res 2004;95:146–153. [DOI] [PubMed] [Google Scholar]
- 27.Park EH, Lee JM, Blais JD, Bell JC, Pelletier J. Internal translation initiation mediated by the angiogenic factor Tie2. J Biol Chem 2005;280:20945–20953. [DOI] [PubMed] [Google Scholar]