Abstract
Chronic interstitial lung disease in both adults and children is associated with mutations of the surfactant protein C (SP-C) proprotein. Among these, mutations within the distal COOH propeptide, known as the BRICHOS domain, are associated with a severe disease phenotype. We showed that prolonged expression of the BRICHOS mutants, SP-CΔexon4 and SP-CL188Q, destabilizes endoplasmic reticulum (ER) quality-control mechanisms (the unfolded protein response, or UPR), resulting in the induction of ER stress signaling, an inhibition of the ubiquitin/proteasome system, and the activation of apoptotic pathways. Based on recent observations that the UPR and ER stress can be linked to the induction of proinflammatory signaling, we hypothesized that the epithelial cell dysfunction mediated by SP-C BRICHOS mutants would activate proinflammatory signaling pathways. In a test of this hypothesis, A549 and human embryonic kidney epithelial (HEK293) cells, transiently transfected with either SP-CΔexon4 or SP-CL188Q mutants, each promoted the upregulation of multiple UPR response genes, including homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 (HERPUD1) and GRP78. Commensurate with these results, increases in IL-8 secretion occurred and were accompanied by the activation of c-Jun N-terminal kinase (JNK)/activating protein–1 signaling. The stimulation of IL-8 cytokine release was completely attenuated by treatment with the JNK-specific inhibitor, SP600125. In addition, SP-CΔexon4, but not SP-CL188Q, activated NFκB. The treatment of SP-CΔexon4 transfected cells with 4–phenylbutyric acid, a small molecule chaperone known to improve protein folding, blocked the activation of NFκB, but not the release of IL-8. Taken together, the results support the role of JNK signaling in mediating SP-C BRICHOS-induced cytokine release, and provide a link between SP-C BRICHOS mutants and proinflammatory cytokine signaling.
Keywords: surfactant proteins, unfolded protein response, ER stress, interleukin-8, interstitial lung disease
CLINICAL RELEVANCE.
Idiopathic pulmonary fibrosis (IPF) has been associated with the induction of ER stress signaling, apoptosis, and release of proinflammatory cytokines by epithelial cells of affected individuals. In this study, we show that expression of Surfactant Protein C (SP-C) mutations associated with IPF (i.e. SP-C BRICHOS) up-regulated multiple ER stress genes, increased IL-8 secretion, and activated c-Jun N-terminal kinase (JNK) signaling in vitro. Taken together, the results provide a link between ER stress and proinflammatory cytokine signaling, and highlight a potential new therapeutic target for IPF.
Interstitial lung disease (ILD) represents a broad classification of diffuse parenchymal lung diseases characterized by varying degrees of pulmonary inflammation and fibrosis. The pathophysiology of ILD reflects a mosaic of events, including epithelial injury, alveolitis, proinflammatory cytokine/chemokine elaboration, inflammatory cell infiltration, fibroblast activation, and matrix deposition, resulting in a final common pathway of lung remodeling and fibrotic scarring (1–3). Although many diseases incorporated into the classification are of unknown etiology, recent progress has allowed a further categorization of certain subgroups. This is based upon associations, or demonstrable cause-and-effect relationships, with environmental exposures, systemic diseases, or genetic factors. Based on a growing number of reports, abnormalities in various protein components of the pulmonary surfactant system can now be included within the genetic category of ILD (4).
Surfactant protein C (SP-C) is a hydrophobic protein component of pulmonary surfactant whose expression in the adult lung is restricted to alveolar Type II epithelial cells. Synthesized as a larger (191 or 197 amino acid) proprotein, the alveolar form of human SP-C is generated by proteolytic processing of the precursor form to a 3.7-kD mature protein deposited into the alveolar space by regulated exocytosis (5). More than 30 mutations were identified within the SP-C gene (SFTPC) linked to both familial and sporadic forms of ILD (6). SFTPC mutations can be classified into three subgroups: (1) those that reside within the Bri family (BRICHOS) domain, a homologous conserved C-terminal region of approximately 100 amino acids found in a number of proteins involved in degenerative or proliferative diseases, resulting in misfolding and aggregate formation; (2) mutations in the non-BRICHOS C-terminal domain that result in trafficking to the plasma membrane or endosomal system; or (3) mutations in the cytoplasmic (N-terminal) domain that result in retention of the endoplasmic reticulum (ER) (5).
To date, most reports that examined the relationship between SP-C mutations and chronic lung disease have focused on SP-C BRICHOS mutants, giving much attention to the splice-variant deletion of exon 4 (SP-CΔexon4), as well as a missense substitution at residue 188 (SP-CL188Q) (7–9). SFTPC BRICHOS mutations were shown to affect proper folding and trafficking of the proprotein, resulting in the formation of cytosolic aggregates (7). To preempt these events, the epithelial response to the expression of mutant SP-C isoforms can include an induction of the unfolded protein response (UPR) to resolve improper folding and limit new protein synthesis, and ER-associated degradation to enhance mutant protein disposal through utilization of the ubiquitin–proteasome system. However, prolonged or unregulated activation of these systems (i.e. ER stress) is associated with an induction of epithelial cell death through intricate apoptotic pathways that contribute to lung injury and remodeling (9).
In addition to the upregulation of compensatory pathways such as the UPR, a molecular link was documented between cellular stress responses and inflammation, ultimately augmenting the production of proinflammatory genes. Activation of one of several signaling cascades involving mitogen-activated protein kinases (MAPKs) such as c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), or p38 and/or NFκB was shown to integrate ER stress and inflammation in a number of pathologic conditions (10). To the best of our knowledge, the role of epithelial-based unfolded protein and ER stress responses in the integration of an inflammatory response in the distal lung was not previously addressed.
We reported that two different SP-C BRICHOS mutants (SPCΔexon4 and SPCL188Q) induce an ER stress response through the activation of a canonic UPR-sensing pathway using insulin response element 1 (IRE1) (9). The IRE1-mediated signaling cascade was shown to play a pivotal role in triggering inflammatory responses in other systems. Conformational changes in the cytosolic domain of IRE1, which allow for binding to TNFα receptor–associated factor 2 (TRAF2), can interface with both the NFκB and MAPK pathways. The IRE1–TRAF2 complex can recruit IκB kinase (IKK), which phosphorylates and removes the IκB inhibitory subunit of NFκB (11). The IRE1–TRAF2 complex can also activate JNK through phosphorylation, with phospho-JNK subsequently activating the transcription factor activating protein 1 (AP-1). The nuclear translocation of AP-1 or NFκB can each upregulate the transcription of a variety of target genes that include proinflammatory proteins such as cytokines, interferons, cell-adhesion molecules, and major histocompatibility (MHC) Class I molecules (12, 13).
Based on these data, we hypothesized that coupling of the UPR/ER stress response to inflammation could be induced in cells expressing SP-C BRICHOS mutants. We demonstrate here that mutant SFTPC expression promotes the release of the proinflammatory cytokine IL-8 by epithelial cells in vitro. Importantly, through the use of a variety of reporter techniques, whereas cytokine release occurs in association with activation of both JNK and NFκB signaling, only the pharmacologic inhibition of the JNK pathway blocks the release of IL-8. These results further underscore the emerging importance of the epithelial cell as a critical component in the pathogenesis of ILD through the selective induction of ER stress–mediated inflammatory pathways.
MATERIALS AND METHODS
Reagents
The pEGFP–C1 (enhanced green fluorescent protein) plasmid was originally purchased from Clontech, Inc. (Palo Alto, CA). The pcDNA3 eukaryotic expression plasmid was obtained from Invitrogen (San Diego, CA). The ER stress activated indicator (ERAI) (XBP-1/venus) expression plasmid for the detection of IRE1 activation (14) was a generous gift of Dr. Masayuki Miura (University of Tokyo, Tokyo, Japan).
Tissue culture medium was produced by the Cell Center Facility at the University of Pennsylvania. Precast gels and buffers for SDS-PAGE (Novex) were obtained from Invitrogen, Inc. Except where noted, all other reagents of analytical grade for electrophoresis and tissue culture were purchased from Sigma Chemical, Inc. (St. Louis, MO) or BioRad, Inc. (Melville, NY).
Rabbit monoclonal antisera included phospho-NFκB–p65 (3033), phospho-stress activated protein kinase (SAPK)/JNK (Cat# 4668), GFP (Cat# 632381) (Clontech, Mountain View, CA), and SAPK/JNK (Cat# 9258) (Cell Signaling Technologies, Beverly, MA). Polyclonal antisera included NFκB-p65 (Cat# 3034), phospho–c-Jun (Cat# 9261) (Cell Signaling Technologies), and β-actin (Sigma Chemical, Inc.). All primary antibodies were used at a dilution of 1:1,000. HRP-conjugated secondary antisera included goat anti-rabbit IgG or goat anti-mouse IgG, and were purchased from Jackson ImmunoResearch (West Grove, PA).
Cell Culture
Human lung epithelial (A549) and human embryonic kidney epithelial (HEK293) cell lines were obtained from ATCC (Manassas, VA), and grown as monolayers in Dulbecco's Modified Eagle Medium (DMEM) at 37°C, in humidified air equilibrated with 5% CO2. All media were supplemented with 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% FBS.
SP-C Construct Generation
The subcloning of human SP-C (SP-CWT), BRICHOS mutant SP-C (SP-CΔexon4 and SP-CL188Q), or non-BRICHOS mutant SP-C (SP-CI73T) constructs into the pEGFP-C1 and pcDNA3 expression vectors was previously described (7, 9, 15). The hemagglutinin (HA) tag (YPYDVPDYA) was added to the NH2 terminus of wild-type and mutant SP-C cDNAs by PCR, using a previously described method (9). For all constructs, the fidelity of PCR reactions and cloning was demonstrated by the automated DNA sequencing of cDNA coding regions, performed in both directions by the Core Facility of the Department of Genetics at the University of Pennsylvania.
Semiquantitative PCR
Total RNA was subjected to reverse transcription (RT) using a RETROscript Kit (Ambion, Inc., Austin, TX). Each RT reaction contained 2 μg of RNA, and was performed under conditions of heat denaturation at 80°C for 3 minutes, amplification for 1 hour at 44°C, and heat inactivation at 92°C for 10 minutes.
The resulting cDNAs were subjected to PCR using the PCR Master Mix (Promega Corporation, Madison, WI). Each reaction contained approximately 250 ng of cDNA template. DNA amplification was performed under conditions of denaturation at 94°C for 3 minutes, followed by the specified number of cycles at 94°C for 30 seconds, 62°C for 45 seconds, and 72°C for 1 minute. The products of reactions were then incubated at 72°C for 10 minutes to increase the yield of amplification. The homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 (HERPUD1) gene was amplified with the primers 5′-CTACTCCTCCCTGAGCAGATTC-3′ (forward) and 5′-GGTTGGGGTCTTCAGTTTCAGG-3′ (reverse) for 25 cycles. The Glucose Regulated Protein 78 (GRP78) gene was amplified with the primers 5′-GGAACACATGGTGCCTACCAA-3′ (forward) and 5′-GGAGCAGGAGGAATTCCAGTCA-3′ (reverse) for 20 cycles. The β-actin gene was amplified with the primers 5′-TGGGTCAGAAGGATTCCTATGT-3′ (forward) and 5′-CAGCCTGGATAGCAACGTACA-3′ (reverse) for 22 cycles. All primer sequences were derived from Donati and colleagues, and cycle parameters were individualized to each cell line (16). The relative mRNA content of each gene was normalized to mRNA for β-actin.
Cell Transfection and Pharmacologic Treatments
A549 or HEK293 cells grown to 75% confluence were transiently transfected with the indicated plasmid constructs, using either CaPO4 precipitation as previously described, or Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's recommendations (17). Where indicated, positive control samples were generated by the treatment of nontransfected cells 15 hours before harvest with either thapsigargin (2 μM) or tunicamycin (20 μg/ml), which were shown to elicit ER stress (9, 18), or with TNF-α (10 ng/ml), which was shown to elicit the maximal expression of IL-8 (19).
To harvest cells, media were removed from cells, and 100 μl of 1× sample loading buffer containing DTT was added to each well of a six-well dish. Cells were scraped with a “rubber policeman” and sonicated for 10 seconds. After incubation at 95°C for 5 minutes and centrifugation at 12,000 rpm for 5 minutes to pellet the cellular debris, the supernatants were used for SDS-PAGE. Consistent with our previous results (7, 9, 15, 18), using either Western blotting for protein expression or cell counting for GFP-positive cells, the transfection efficiencies of the wild-type and mutant SP-C isoforms were nearly equivalent in both HEK and A549 cells (as shown in Figure E1 in the online supplement).
Immunocytochemistry
Immunocytochemistry and fluorescence imaging were preformed according to a previously described protocol (9). After permeabilization, cells on coverslips were immunolabeled with primary anti-HA antibody (Roche, San Francisco, CA) for 1 hour at room temperature at a 1:100 dilution. Texas red–conjugated secondary goat anti-mouse IgG monoclonal antibody (Jackson ImmunoResearch Laboratories) was used for visualization at a 1:200 dilution.
Luciferase Reporter Gene Assays
The A549–NFκB–luciferase (luc) cell line was used to determine NFκB activity. This cell line stably expresses a Firefly luciferase reporter construct regulated by six copies of the NFκB response element (Panomics, Inc., Freemont, CA). SP-C and control plasmids were transiently introduced into the cell line, using Lipofectamine 2000 reagent (Invitrogen). The pRL-SV40 Renilla luciferase vector was used to normalize transfection efficiencies. Forty-eight hours after transfection, cells were lysed and assayed for Firefly and Renilla luciferase activities, using a dual luciferase kit (Promega Corporation).
SDS-PAGE and Western Blotting
Cell lysate protein was separated by SDS-PAGE, as described by Laemmli (20), using a 4% stacking gel and a 10% resolving gel, transferred to nitrocellulose (O/N, 20 V), and incubated with antibody in either 5% BSA in Tris-buffered saline (TBS) and 0.1% Tween-20 for phospho-specific antibodies, or 1% nonfat dry milk in TBS and 0.1% Tween-20 at 4°C overnight. After sequential incubation with primary and HRP-conjugated secondary antisera at the indicated dilutions, bands were detected by chemiluminescence, using X-Omat AR film (Kodak, Rochester, NY). Specific band intensities were quantitated using Kodak1D software, version 3.0.
ELISA for Human IL-8
A549 cells were transiently transfected with the indicated SP-C mutant construct using Lipofectamine 2000, or were treated with the indicated stimulator. Media collected 12–48 hours after transfection were used for the determination of released IL-8, measured using the DuoSet Human IL-8 ELISA Assay according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Intra-assay coefficients of variation (%CV) were accepted at a value of less than 10%, whereas interassay %CV were accepted at a value of less than 20%.
Statistical Analysis
Parametric data were analyzed with Instat, version 3.0 (Graph Pad Software, Inc., La Jolla, CA), and were expressed as mean ± SD. Experimental groups were compared according to an unpaired, two-tailed Student t test for comparing two samples, or ANOVA between sample groups using the Tukey–Kramer multiple-comparison post hoc test. Significance was accepted at P < 0.05.
RESULTS
Expression of SP-C BRICHOS Mutants Results in UPR Induction
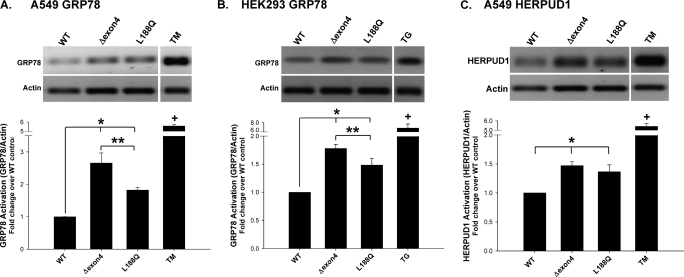
The expression of misfolded mutant proteins is associated with a generalized cell response aimed at promoting protein refolding and limiting cell toxicity. We assessed the ER stress response using semiquantitative RT-PCR for known UPR genes in our model system. Compared with cells transiently transfected with cDNA encoding EGFP–SP-CWT, EGFP fusion constructs containing the BRICHOS mutant SP-CΔexon4 induced a significant elevation of the well-recognized UPR gene product, GRP78/binding immunoglobulin protein (BiP), in both A549 and HEK293 cells (Figures 1A and 1B). Another BRICHOS mutant, SP-CL188Q, also increased the expression of GRP78 in each cell line, but to a lesser extent than SP-CΔexon4. Both SP-C BRICHOS mutants also increased the expression of HERPUD1, another UPR quality-control protein in A549 cells (Figure 1C). Taken in combination with previously reported data from our group and others (18, 21), these results support the notion that the expression of SP-C BRICHOS mutations induces a UPR response that is cell line–independent, and global in promoting the upregulation of a wide array of ER stress gene products.
Figure 1.
Unfolded protein response (UPR) gene induction in surfactant protein C (SP-C) BRICHOS mutant–expressing cells. A549 and human embryonic kidney epithelial (HEK293) cells, transiently transfected with EGFP–SP-C wild-type (WT), Δexon4, or L188Q constructs, were harvested 48 hours after transfection, and total RNA was prepared. Overnight treatments of 2 μM thapsigargin (TG) or 20 μg/ml tunicamycin (TM) were used for positive control samples. RT-PCR was performed with primers for GRP78/BiP (A and B) and homocysteine-inducible, endoplasmic reticulum stress–inducible, ubiquitin-like domain member 1 (HERPUD1) (C) expression, as described in Materials and Methods. Band intensities were quantified by densitometry, and the ratio of either GRP78 or HERPUD1 to β-actin was determined. Data are expressed as fold change over WT control samples, and represent the mean ± SD of three separate experiments. Representative agarose gels appear above each graph. *P < 0.05 versus WT control. **P < 0.05 versus Δexon4. +P < 0.001 versus WT control.
Expression of SP-C BRICHOS Mutants Results in IRE1 Activation
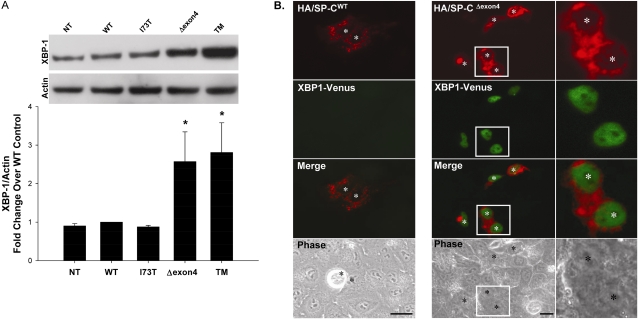
The response of the ER to a mutant or increased protein load can be sensed by at least three distinct transducers, IRE1, pancreatic ER kinase (PERK), and activating transcription factor 6 (ATF6). Of these, the IRE1–XBP-1 pathway was most closely linked with ER stress inflammatory signaling (13). The splicing and expression of the X-box binding protein 1 (XBP-1) transcription factor can be used as a surrogate for IRE1 activity. Therefore, the expression of XBP-1 protein was assessed by Western blotting of cell lysates, 48 hours after the transient expression of SP-C mutant constructs and controls. Significant increases in XBP-1 protein concentrations were evident in A549 epithelial cells expressing SP-CΔexon4, but not in cells expressing SP-CI73T, an ILD-associated SP-C mutation located outside the BRICHOS domain (Figure 2A). To demonstrate functional IRE-1 activity directly, A549 cells were cotransfected with HA–SP-C constructs and the ERA1 expression plasmid developed by Iwawaki and colleagues (14). ERA1 contains an insert encoding for a fusion protein of XBP-1 and venus, a GFP-like reporter protein. The XBP-1 cDNA contains a stop codon that prevents a full read-through unless spliced out by IRE1 endo-RNase activity, which occurs during IRE-1 dimerization/activation with ER stress. As shown in Figure 2B, cells staining for HA-SP-CΔexon4 co-expressed XBP-1/venus in a nuclear pattern consistent with the transcription, translation, and nuclear translocation of XBP-1.
Figure 2.
SP-C BRICHOS mutants activate the insulin response element 1 (IRE1) signaling pathway. (A) A549 cells, either nontransfected (NT) or transiently transfected with the indicated control or EGFP–SP-C constructs (WT, I73T, Δexon4 were harvested 48 hours after transfection. Overnight treatment with 10 μg/ml tunicamycin (TM) was used for a positive control sample. Cell lysates were analyzed for XBP-1 protein expression by Western blotting, as described in Materials and Methods. An anti–β-actin antibody was used as a loading control. A representative immunoblot appears above the graph. Band intensities were quantified by densitometry, and the ratio of XBP-1 to β-actin was determined. Data are expressed as fold change over WT control, and represent the mean ± SD of three separate experiments. *P < 0.05 versus WT control. (B) Hemagglutinin (HA)-tagged SP-CWT (left) or SP-CΔexon4 (right) were cotransfected with the ERAI plasmid (XBP-1/venus) developed by Iwawaki and colleagues (14) into A549 cells. Forty-eight hours after transfection, cells were fixed and stained with an anti-HA monoclonal antibody to label the HA–SP-C fusion protein, as described in Materials and Methods. Fluorescence microscopy shows XBP-1/venus expression in SP-CΔexon4 but not in SP-CWT transfected cells. Merged and phase contrast images are shown, and individual nuclei are identified by asterisks. Magnifications of selected areas are seen in the rightmost column.
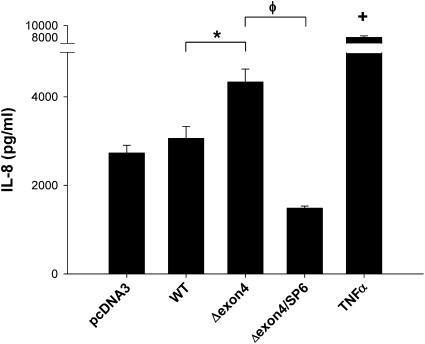
SP-C BRICHOS Mutants Induce Release of IL-8 by Epithelial Cells
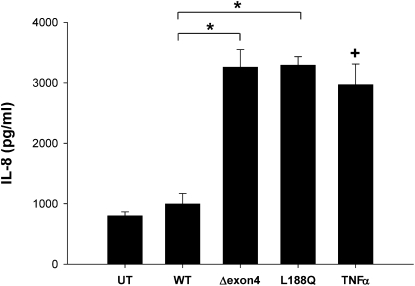
Exaggerated production of the proinflammatory CXC chemokine IL-8 was found in patients, mice, and epithelial cell lines expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) (22, 23). IL-8 is also significantly elevated in specimens from patients with idiopathic pulmonary fibrosis (IPF), and is associated with increased neutrophilia and poorer outcomes (24). To investigate whether the expression of SP-C BRICHOS mutants could trigger the release of IL-8 by epithelial cells, an IL-8–specific ELISA was performed on media collected from transiently transfected A549 cells expressing wild-type or mutant SP-C isoforms. The pharmacologic treatment of nontransfected cells with TNF-α served as a positive control, and resulted in a substantial increase in cytokine elaboration. Similarly, an increased release of IL-8 occurred in cells expressing either BRICHOS mutant (SP-CΔexon4 or SP-CL188Q), but not in cells transfected with wild-type SP-C (Figure 3), an effect that was time-dependent, reaching a maximum at 48 hours (Figure E2).
Figure 3.
SP-CΔexon4 and SP-CL188Q induce IL-8 cytokine release. A549 cells were transiently transfected with the indicated EGFP-tagged SP-C WT or mutant construct, using Lipofectamine 2000 reagent (Invitrogen). Untreated (UT) cells were used as a negative control sample, and overnight treatment of 10 ng/ml TNFα was used for a positive control sample. Media were collected 48 hours after transfection, and IL-8 was measured by ELISA, as described in Materials and Methods. Data are expressed as mean IL-8 concentration ± SD of triplicate samples, and are representative of three separate experiments. The intra-assay coefficients of variation (%CV) for the three experiments were 2.7%, 3.0%, and 2.1%, whereas the interassay %CV was 12.2%. *P < 0.05 and +P < 0.05 versus WT control.
SP-C BRICHOS Mutants Activate AP-1/JNK Signaling
A growing body of evidence suggests that the signaling pathways involved in ER stress induction and inflammation are interconnected. The activation of AP-1 was shown to occur in response to ER stress, when an increased protein load is sensed and transduced by the IRE1–XBP-1 signaling pathway. Because AP-1 is an important transcriptional activator for the induction of a number of inflammatory genes, we sought to assess the effects of SP-C BRICHOS mutations on these pathways.
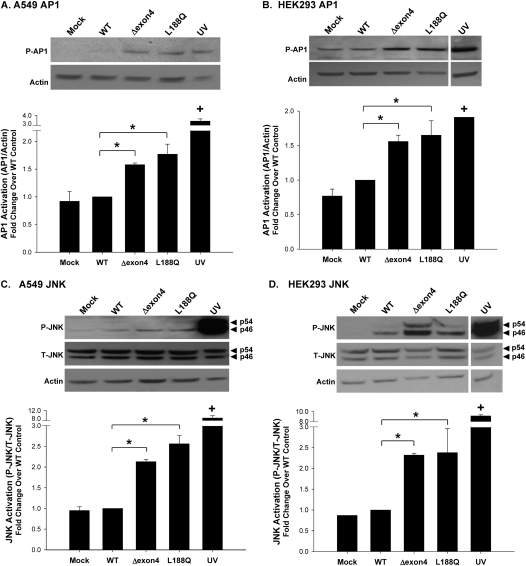
The phosphorylation of AP-1, shown to be necessary for its transcriptional activity, was evaluated via Western blotting. Cell lysates, harvested 48 hours after transfection, were immunoblotted with a phospho-specific c-Jun (subunit of the heterodimer complex of AP-1) antibody that recognizes the phosphorylation at Ser63. In both A549 and HEK293 cells, the expression of either the SP-CΔexon4 or SP-CL188Q mutant resulted in the increased phosphorylation of AP-1, compared with SP-CWT–expressing cells (Figures 4A and 4B).
Figure 4.
SP-C BRICHOS mutants upregulate activating protein 1 (AP1) and c-Jun N-terminal kinase (JNK) activation. A549 and HEK293 cells were transiently transfected in the absence (Mock) or presence of the indicated EGFP–SP-C constructs, and harvested 48 hours after transfection. Ultraviolet light (UV) treatment (40 mJ/cm2) was used for a positive control sample. Cell lysates were analyzed for phospho–c-Jun (A and B), phospho–SAPK/JNK, and total SAPK/JNK expression, using monoclonal antibodies (C and D). An anti–β-actin antibody was used as a loading control. Band intensities were quantified by densitometry, and the ratio of either c-Jun to β-actin or phospho-JNK to total JNK was determined. Data are expressed as fold change over WT control, and represent the mean ± SD of three separate experiments. Representative immunoblots appear above each graph. *P < 0.05 versus WT control. +P < 0.001 versus WT control.
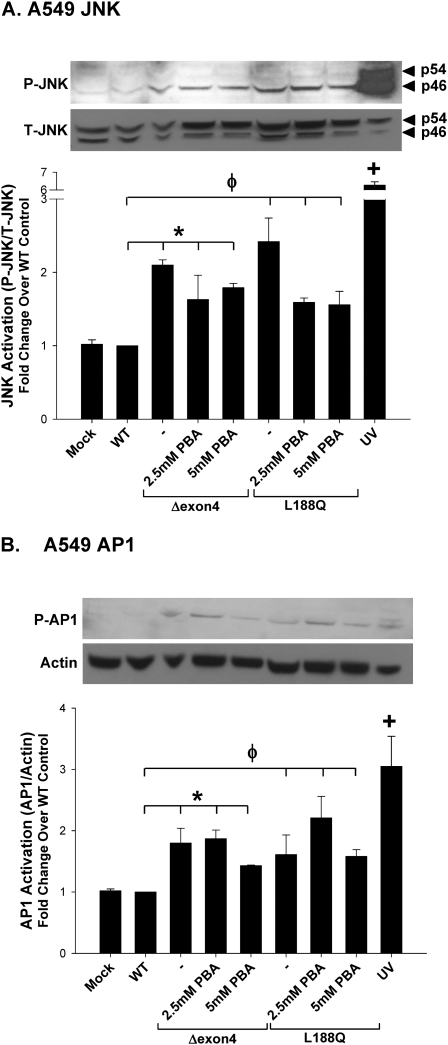
MAPK/JNK activity occurs upstream of AP-1 activation (25). Numerous JNK isoforms were identified, resulting from the alternative splicing of three JNK genes: JNK1, JNK2, and JNK3 (Mol. Wt. = 46, 54, and 57 kD, respectively) (26). To assess the involvement of the MAPK/JNK pathway in the induction of inflammation by SP-C BRICHOS mutants, the phosphorylation of JNK was measured in mutant-expressing A549 and HEK293 cells (Figures 4C and 4D). Using an antibody that recognizes the p46 and p54 isoforms of JNK, both the SP-CΔexon4 and SP-CL188Q mutants were shown to significantly increase phosphorylation of the p46 subunit in A549 cells, and of the p46 and p54 subunits in HEK293 cells, compared with SP-CWT–expressing cells.
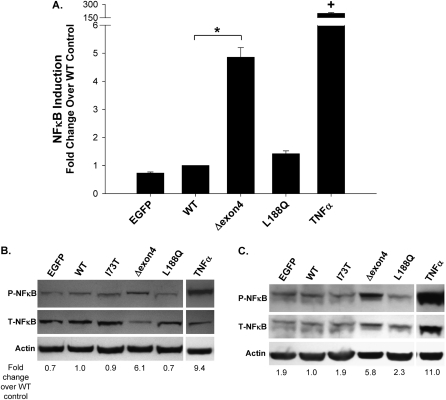
SP-C BRICHOS Mutants Differentially Affect NFκB Signaling
In some systems, UPR–inflammatory coupling was also linked to the activation of NFκB (10). To assess this phenomenon, the activation of NFκB in response to SP-C BRICHOS mutant expression was evaluated with several methodologies. We transiently transfected an A549–NFκB–luc reporter cell line with the indicated control or mutant SP-C constructs. The fidelity of the reporter assay was confirmed by treatment of the cells with the known NFκB trigger, TNF-α, which induced a robust increase in luciferase activity (Figure 5A). Similarly, a selective fivefold increase in luciferase activity occurred with the expression of the SP-CΔexon4 mutant, compared with either EGFP-C1 or SP-CWT transfected cells. The expression of SP-CL188Q did not activate NFκB signaling, with luciferase activity equivalent to that of control transfections.
Figure 5.
SP-CΔexon4 induces an NFκB response. NFκB activation induced by SP-C BRICHOS mutants was assessed in A549–NFκB–luciferase (luc), A549, and HEK293 cell lines. (A) A549–NFκB–luc cells were cotransfected with the indicated control (EGFP) or EGFP–SP-C constructs, with pRL-SV40 (expressing Renilla luciferase) to normalize transfection efficiencies. Overnight treatment with 10 ng/ml TNFα before harvesting was used for a positive control sample. Cells lysates were prepared 48 hours after transfection, and Firefly and Renilla luciferase activities were measured, using luciferin substrates provided by the Dual Luciferase Kit (Promega). The ratio of mean Firefly to Renilla luciferase was determined, and expressed as fold change over WT control. These data represent the mean ± SD of three separate experiments. *P < 0.05 versus WT control. +P < 0.001 versus WT control. (B and C) Cell lysates from A549 and HEK293 cells transfected with the indicated control (EGFP), WT, non-BRICHOS SP-C mutant (I73T), or BRICHOS mutant constructs (Δexon4 and L188Q) were prepared 48 hours after the introduction of plasmid cDNA. Representative immunoblots of the activated/phosphorylated P65 subunit of NFκB (P-NFκB) and total cellular P65 subunit of NFκB (T-NFκB) from A549 (B) and HEK293 (C) cells are shown. Cell lysates were analyzed for phospho–NFκB–P65 and total NFκB-P65 expression, using a monoclonal phospho-specific antibody and a polyclonal NFκB antibody, respectively. An anti–β-actin antibody was used as a loading control. Band intensities were quantified by densitometry, and the ratio of phosphorylated to total NFκB–P65/actin was determined. Data are expressed as fold change over WT control, and are representative of at least two separate experiments.
The differential effect of the two BRICHOS mutants on NFκB activation was then corroborated biochemically, using Western blotting for the detection of the phosphorylated p65 subunit of NFκB. Forty-eight hours after the transient expression of SP-C constructs and control samples, a significant increase in NFκB-p65 phosphorylation was observed in A549 cells expressing the SP-CΔexon4 mutant, in a pattern similar to that of nontransfected cells treated with TNF-α, but not with cells expressing other SP-C constructs (SP-CWT, SP-CL188Q, and SP-CI73T; Figure 5B). A selectively substantial increase in phospho–NFκB–p65 was also observed in HEK293 cells expressing SP-CΔexon4 or treated with TNF-α (Figure 5C). The increase in phospho-NFkB in HEK cells was accompanied by an increase in total NFkB, likely reflecting an additional effect on NFkB expression in this cell line. Taken together, these experiments indicate that the SP-CΔexon4 mutant selectively induces an NFκB response in both cell lines.
Inhibition of JNK Blocks the Release of IL-8
To determine the relative contribution of JNK/AP-1 signaling in SP-C BRICHOS–induced inflammation, A549 cells, transiently transfected with the indicated control or mutant SP-C constructs, were treated with SP600125, a reversible ATP-competitive inhibitor of JNK, and assessed for the production of IL-8. As shown in Figure 6, the selective blockade of JNK signaling led to a complete inhibition of the IL-8 release induced by SP-CΔexon4. This inhibition by SP600125 was specific to SP-CΔexon4 expression, because no change in IL-8 secretion was evident in A549 cells exposed to TNF-α, or in A549 cells transfected with SP-CWT constructs treated with the same dose of inhibitor (Figure E3).
Figure 6.
IL-8 release induced by SP-C BRICHOS mutants is blocked by the inhibition of JNK. A549 cells were transiently transfected with the indicated control or HA-tagged SP-C mutant construct, using Lipofectamine 2000 reagent. Twenty-four hours after transfection, 10 μM of the JNK-specific inhibitor SP600125 was added to the indicated samples. Overnight treatment with 10 ng/ml TNFα was used for a positive control sample. Media were collected 48 hours after transfection (18 hours after TNF-α treatment), and IL-8 was measured by ELISA, as described in Materials and Methods. Data are expressed as mean IL-8 concentration ± SD of triplicate samples, and are representative of three separate experiments. The intra-assay %CVs for the three experiments were 2.1%, 1.5%, and 1.6%, whereas the interassay %CV was 6.0%. *P < 0.05 versus WT control. φP < 0.05 versus SP-CΔexon4 sample. +P < 0.0001 versus WT control.
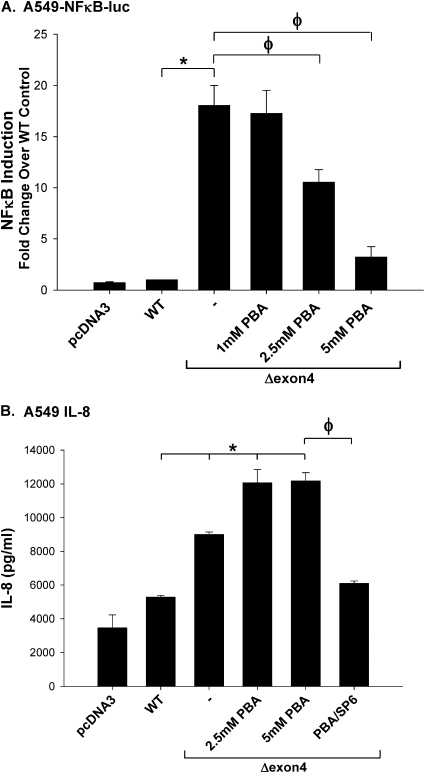
4–Phenylbutyric Acid, a Facilitator of Protein Folding, Blocks NFκB Activation, but Not IL-8 Release
We previously showed that the treatment of cells expressing the mutant SP-CΔexon4 with the small molecule chemical facilitator 4–phenylbutyric acid (4-PBA), a known modulator of protein folding, results in the redirection or dispersion of aggregated mutant SP-C protein (7). To determine if 4-PBA also corrected the proinflammatory signaling induced by SP-CΔexon4, the activation of NFκB and release of IL-8 were measured in transfected cells subjected to treatment with 4-PBA. As shown in Figure 7A, 4-PBA caused a dose-dependent decrease in luciferase activity in A549–NFκB–reporter cells transiently expressing SP-CΔexon4. Despite this complete inhibition of NFκB activation, 4-PBA treatment actually enhanced the release of IL-8 by A549 cells expressing SP-CΔexon4. When co-administered with 4-PBA, SP600125 completely antagonized the release of IL-8 induced by SP-CΔexon4 and 4-PBA (Figure 7B). Moreover, the effect of 4-PBA in modulating phospho-JNK and phospho–AP-1 concentrations was minimal in A549 cells transfected with either SP-CΔexon4 or SP-CL188Q (Figures 8A and 8B). When the same studies were performed using HEK293 cells, 4-PBA actually stimulated both JNK and AP1 phosphorylation in this cell line (Figure E4). Taken together, these data indicate that, in contrast to JNK MAPK, the attenuation of NFκB-based signaling had no effect on proinflammatory cytokine generation by epithelial cells expressing SP-C BRICHOS mutants.
Figure 7.
4–Phenylbutyric acid (4-PBA) blocks NFκB activation, but not the release of IL-8. (A) The A549–NFκB–luc cell line was transiently transfected with the indicated control or SP-C constructs. pRL-SV40 was transfected into all samples as an internal control to normalize transfection efficiencies. Eight hours after transfection, increasing concentrations of 4-PBA (PBA) were added to SP-CΔexon4–transfected cells, and overnight treatment with 10 ng/ml TNF-α was used for a positive control sample. Cells were harvested and lysed 48 hours after transfection. Firefly and Renilla luciferase activities were measured using luciferin substrates, as in Figure 5. The ratio of mean Firefly to Renilla luciferase was determined, and expressed as fold change over WT control. Graph represents the mean ± SD of triplicate experiments. (B) A549 cells were transiently transfected with the indicated control or EGFP–SP-C mutant constructs using Lipofectamine 2000 reagent. Eight hours after transfection, increasing concentrations of 4-PBA, or a combination of 5 mM PBA and 10 μM SP600125, was added to SP-CΔexon4 transfected cells, as indicated. Media were collected 48 hours after transfection, and IL-8 was measured by ELISA as described in Materials and Methods. Graph represents the mean ± SD of triplicate samples, and is representative of three separate experiments. The intra-assay %CVs for the three experiments were 1.4%, 3.4%, and 1.6%, whereas the interassay %CV was 2.3%. *P < 0.05 versus WT control. φP < 0.05 versus SP-CΔexon4 sample. +P < 0.0001 versus WT control.
Figure 8.
SP-C mutant induction of JNK and AP-1 is not altered by 4-PBA treatment. A549 cells were transiently transfected in the absence (Mock) or presence of the indicated EGFP–SP-C constructs, and harvested 48 hours after transfection. Eight hours after transfection, increasing concentrations of 4-PBA were added to SP-CΔexon4–transfected cells and harvested 48 hours after transfection. UV treatment (40 mJ/cm2) was used for a positive control sample. Cell lysates were analyzed for phospho-SAPK/JNK and total SAPK/JNK expression (A) and phospho–c-Jun (AP1) expression (B). An anti–β-actin antibody was used as a loading control. Band intensities were quantified by densitometry, and the ratio of either phospho-JNK to total JNK, or c-Jun to β-actin, was determined. Data are expressed as fold change over WT control, and represent the mean ± SD of three separate experiments. Representative immunoblots appear above each graph. *P < 0.05 for SP-CΔexon4 samples versus WT control. φP < 0.05 for SP-CL188Q samples versus WT control. +P < 0.01 for UV positive control versus WT control.
DISCUSSION
Recent observations indicate that pathologic conditions interfering with ER homeostasis result in the chronic activation of both the UPR and inflammation. We previously showed that the expression of mutant SP-C (BRICHOS domain mutants), associated with the development of pulmonary fibrosis in children and adults, is capable of inducing an ER stress response, caspase activation, and ultimately cell death (9, 18). The present study extends these observations through an examination of the proinflammatory signaling mediated by the UPR in lung epithelia expressing SP-C BRICHOS mutants. In addition to demonstrating the induction of IL-8 by the expression of SP-C BRICHOS mutants, our results establish the first mechanistic link between activation of the UPR and inflammation in pulmonary epithelia. Given that the BRICHOS domain in SP-C has marked structural homology with other Bri family members associated with neurodegeneration and dementia, these results have broader implications for understanding the pathogenesis of a variety of conformational diseases (27). In addition to Bri-induced encephalopathy, these diseases include α-1 antitrypsin proteinopathy, Huntington's disease, and Parkinson's disease, all of which were shown to result from the significant cellular dysfunction induced by protein misfolding (28–30).
The rationale for studying the relationship between the UPR and proinflammatory signaling was based on several lines of evidence. In addition to previous in vitro studies demonstrating the induction of the UPR and ultimately ER stress in epithelial cells by mutant SP-C (8, 9, 18, 21, 31), markers of both ER stress and apoptosis were documented in lung epithelia in vivo in patients with classic IPF (usual interstitial pneumonitis, or UIP) and in patients with UIP expressing the SP-C BRICHOS domain mutation, L188Q (8, 32). Similarly, lung biopsies from patients with idiopathic pulmonary fibrosis demonstrated enhanced levels of expression of the CXC chemokine, IL-8, compared with control tissue (24). Although a direct link between these two events was previously lacking, this study demonstrates that the expression of SP-C BRICHOS mutants in alveolar epithelial cells results in the significant expression of IL-8 (Figure 3). Thus, taken together, our results complement previous in vivo studies, and support the concept of the alveolar epithelial cell as an important effector regulating the local inflammatory milieu in pulmonary fibrosis.
Crosstalk between the UPR and inflammation was suggested to occur in several cell types, including hepatocytes, pancreatic β-islet cells, neurons, and macrophages, all of which have in common either enhanced metabolic or immune function or the expression of misfolded mutant protein (10). Mechanistically, data from these studies infer that two well-known signaling pathways involved in inflammation, JNK/AP-1 and NFκB, can be induced by prolonged activation of the UPR, and are capable of serving as molecular integrators between the two processes. In addition, the IL-8 promoter was shown to contain both AP-1 and NFκB transactivation elements (33). Results from the present study recapitulate this theme, and are summarized in Figure 9. The expression of two different SP-C BRICHOS mutants resulted in the phosphorylation of JNK and its downstream target, the transcription factor AP-1. The release of IL-8 by cells transfected with either SP-CΔexon4 or SP-CL188Q was completely blocked by treatment with a JNK-specific inhibitor, SP600125 (Figure 6). Coupled with the findings that the activation of NFκB was differentially induced only by SP-CΔexon4 (Figure 5), and that 4-PBA treatment blocked the activation of NFκB but not the release of IL-8 induced by SP-CΔexon4 in lung epithelia (Figure 7), JNK signaling appears to represent the major pathway mediating crosstalk between the ER and the nucleus, to generate a proinflammatory cytokine response. Although the inhibition of JNK was shown to modulate many other ER stress–related cellular responses such as insulin resistance (34), to our knowledge, this is the first report of JNK inhibition attenuating proinflammatory responses to misfolded proteins.
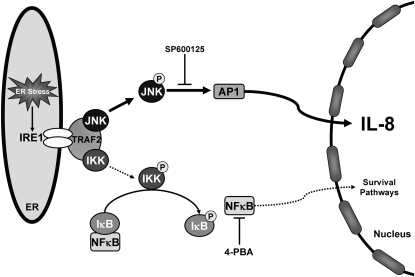
Figure 9.
Proinflammatory signaling pathways induced by SP-C BRICHOS mutants. Expression of SP-C BRICHOS mutants (SP-CΔexon4 and SP-CL188Q) induce an unfolded protein response, in part via activation of a canonical endoplasmic reticulum stress–sensing pathway, using insulin response element 1 (IRE1). Conformational changes in the cytosolic domain of IRE1, which allow for binding to TNF-α receptor–associated factor 2 (TRAF2), can interface with both NFκB and mitogen-activated protein kinase (MAPK) pathways. IRE1–TRAF2 activates JNK through phosphorylation, with phospho-JNK subsequently activating the transcription factor, activating protein 1 (AP1). Nuclear translocation of AP-1 leads to an upregulation of IL-8 release. Inhibition of IL-8 release by SP600125 treatment indicates that JNK signaling represents the major pathway mediating the proinflammatory cytokine response. With more severe misfolding, as observed with SP-CΔexon4, the IRE1–TRAF2 complex can also recruit IκB kinase (IKK), which phosphorylates and removes the IκB inhibitory subunit of NFκB. 4-PBA treatment downregulates NFκB, but not IL-8 release, and suggests that NFκB does not play a significant role in the inflammatory response of this system. P, phosphorylation modification.
Whereas previous studies established links between NFκB and the regulation of proinflammatory responses induced by cytokine stimulation (35), as well as between misfolded protein–induced ER stress and NFκB activation in vitro (36, 37), the upregulation of IL-8 by misfolded SP-C BRICHOS mutants could not be linked to the observed increase in NFκB activity. This finding suggests that the activation of NFκB in response to prolonged UPR or ER stress may play a different role. The constitutive activation of NFκB promotes survival by activating antiapoptotic genes in a wide range of cells, including malignant cells, B cells, and hepatocytes (38). Bridges and colleagues demonstrated that stably transfected HEK293 cells adapted to the chronic ER stress imposed by the constitutive expression of SP-CΔexon4 via an NFκB-dependent pathway (31). Using gene array analysis, this adaptation was associated with the upregulation of antiapoptotic gene programs, and the inhibition of NFκB resulted in cell death. A similar response has not yet been reported for SP-CL188Q. However, the differential response in the activation of NFκB by epithelial cells transfected with SP-CΔexon4 versus SP-CL188Q may reflect differences in the severity of mutations. Compared with SP-CL188Q, the G > A mutation that produces SP-CΔexon4 results in a 37 amino-acid deletion of the COOH flanking domain, resulting in a significant disruption of protein structure (39). Using macroaggregation as a biochemical readout, we previously reported that the Δexon4 mutation results in a more severe phenotype compared with the L188Q mutation (18), which is accompanied by an enhanced association with cytotoxicity in vitro (M.F.B., unpublished observations) and by a disruption of lung morphogenesis when SP-CΔexon4 is expressed in transgenic mice (21). Thus, the severity of protein misfolding imparted by SP-CΔexon4 may be more likely to trigger NFκB-mediated cytoprotective pathways than its SP-CL188Q counterpart.
The pathogenesis of chronic lung remodeling via the expression of proSP-C BRICHOS mutants can be thought of as part of a unified view of the cellular and molecular pathogenesis of a larger family of conformational diseases. The realization that conformational diseases are attributable to either aberrant intermolecular aggregation or transport to non-native compartments makes possible both general and specific approaches for the correction of defects. Recent data suggest that preserving or restoring ER function may be of therapeutic value. Small molecules that are classified as chemical facilitators modulate protein folding and the UPR, potentially mitigating ER stress. One of these, 4-PBA, has attracted a great deal of attention as a therapeutic agent because of its capacity to restore proper trafficking of mutant CFTR to the plasma membrane (40). Previously, the addition of 4-PBA to A549 cells expressing the SPCΔexon4 mutant was shown to prevent the formation of intracellular aggregates (7). Nonetheless, we show here that treatment with 4-PBA was characterized by a cell-dependent sustained or elevated release of IL-8, despite the attenuation of NFκB activation (Figure 7). Mechanistically, the lack of an anti-inflammatory effect of 4-PBA in our model system may have been attributable to off-target effects of 4-PBA, as described in other systems. These effects include histone deactylase inhibition (HDACi) (41–43) and enhanced MAPK (JNK) signaling (44). The treatment of cells with other inducers of HDACi in vitro (such as trichostatin A) was shown to modulate IL-8 production in both an NFκB-dependent and AP-1–dependent fashion, but this effect is highly cell-specific (33, 45). In addition, Roque and colleagues observed that 4-PBA enhances the production of IL-8 in lung epithelial cells expressing ΔF508–CFTR (44). The treatment of these cystic fibrosis cell lines with 4-PBA resulted in an activation of intracellular JNK signaling and the release of IL-8, despite a decrease in NFκB activation, a result similar to what we observed here in cells expressing BRICHOS mutants (Figures 7, 8, and E4).
Despite its effect on protein folding, it is unclear if 4-PBA exerts a consistent effect on ER stress. This conundrum was best described for Δ508 mutant CFTR, where a significant disparity also occurs in the effects of 4-PBA on folding and trafficking to the plasma membrane versus the UPR. Using IB3-1 cells expressing mutant Δ508 CFTR, 4-PBA actually increased the expression of ER stress markers such as GRP78 (ostensibly to promote folding) (46). In a preliminary report, 4-PBA treatment of HEK cells transiently expressing SP-C BRICHOS mutants was associated with the sustained expression of ER stress response proteins and cytotoxicity (47). Although we found no evidence of cytotoxicity from 4-PBA, we also observed in our models that GRP78/BiP concentrations were unaffected by treatment with 4-PBA (J.A.M., unpublished observations). Altogether, studies of CFTR and SP-C suggest that, for ER stress associated with mutant protein expression (versus chemically or metabolically induced ER stress), the beneficial effects seen with regard to protein folding mediated by 4-PBA are dissociated from the measured expression of key chaperones and UPR markers. Furthermore, the propensity for 4-PBA to decrease NFκB survival pathways, while promoting JNK-mediated inflammatory signaling, may obscure potential beneficial effects on protein folding and trafficking.
In conclusion, we demonstrate that SP-C BRICHOS mutants promote proinflammatory signaling in lung epithelial cells through the integration of UPR and inflammation via a JNK-dependent signaling cascade. Interestingly, in an emerging therapy aimed at improving protein folding, 4-PBA had deleterious effects on cytokine release in the same model system. Understanding these complex signaling pathways that integrate ER stress and inflammation may lead to effective new therapies for treating a previously untreatable, devastating disease. However, conformational diseases, as seen with mutant SP-C, will likely require combination therapies aimed at the restoration of protein folding, the enhanced clearance of protein aggregates, the blockade of inflammatory signaling, and the appropriate modulation of cytotoxicity and apoptosis. A number of studies showed that, in addition to 4-PBA, treatment with small molecule chaperones can restore proper protein folding and ER function. These chaperones include taurine-conjugated ursodeoxycholic acid, vaticanol B, and others, which were shown to decrease aggregate formation and prevent an ER stress response (48, 49). Although these treatments have shown promising results, they will likely have to be simultaneously evaluated for off-target effects on the many other cellular pathways that link ER stress and cellular dysfunction.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Susan Guttentag for helpful discussions, and to Dr. Audreesh Banerjee and Dr. Yassine Amrani for assistance with establishment of the IL-8 ELISA assay.
This work was supported by National Institutes of Health grants P30-ES013508 (M.F.B), HL 19737 (M.F.B.), HL 087177 (M.F.B.), and HL 090732 (S.M.). J.A.M. received support from National Institutes of Health grant 2T32 HL007748.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0382OC on May 12, 2010
Author Disclosure: M.F.B. received a sponsored grant from the National Institutes of Health for more than $100,001. J.A.M. received a sponsored grant from the National Institutes of Health for $10,001–$50,000. S.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 2002;19:794–796. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement: American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 3.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 2009;175:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantelidis P, Veeraraghavan S, du Bois RM. Surfactant gene polymorphisms and interstitial lung diseases. Respir Res 2002;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol 2005;67:663–696. [DOI] [PubMed] [Google Scholar]
- 6.Nogee LM, Dunbar AE III, Wert S, Askin F, Hamvas A, Whitsett JA. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest 2002;121:20S–21S. [DOI] [PubMed] [Google Scholar]
- 7.Wang WJ, Mulugeta S, Russo SJ, Beers MF. Deletion of exon 4 from human surfactant protein C results in aggresome formation and generation of a dominant negative. J Cell Sci 2003;116:683–692. [DOI] [PubMed] [Google Scholar]
- 8.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 2008;294:L1119–L1126. [DOI] [PubMed] [Google Scholar]
- 9.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol 2005;32:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008;454:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J 1995;14:2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994;12:141–179. [DOI] [PubMed] [Google Scholar]
- 13.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000;287:664–666. [DOI] [PubMed] [Google Scholar]
- 14.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med 2004;10:98–102. [DOI] [PubMed] [Google Scholar]
- 15.Wang WJ, Russo SJ, Mulugeta S, Beers MF. Biosynthesis of surfactant protein C (SP-C): sorting of SP-C proprotein involves homomeric association via a signal anchor domain. J Biol Chem 2002;277:19929–19937. [DOI] [PubMed] [Google Scholar]
- 16.Donati G, Imbriano C, Mantovani R. Dynamic recruitment of transcription factors and epigenetic changes on the ER stress response gene promoters. Nucleic Acids Res 2006;34:3116–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo SJ, Wang W-J, Lomax C, Beers MF. Structural requirements for intracellular targeting of surfactant protein C. Am J Physiol Lung Cell Mol Physiol 1999;277:L1034–L1044. [DOI] [PubMed] [Google Scholar]
- 18.Mulugeta S, Maguire JA, Newitt JL, Russo SJ, Kotorashvili A, Beers MF. Misfolded BRICHOS SP-C mutant proteins induce apoptosis via caspase 4 and cytochrome C related mechanisms. Am J Physiol Lung Cell Mol Physiol 2007;293:L720–L729. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda A, Stringer BK, Mijailovich SM, Rogers RA, Hamada K, Gray ML. Alveolar cell stretching in the presence of fibrous particles induces interleukin-8 responses. Am J Respir Cell Mol Biol 1999;21:455–462. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 21.Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human SP-C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem 2003;278:52739–52746. [DOI] [PubMed] [Google Scholar]
- 22.Berger M. Inflammatory mediators in cystic fibrosis lung disease. Allergy Asthma Proc 2002;23:19–25. [PubMed] [Google Scholar]
- 23.Jacquot J, Tabary O, Le Rouzic P, Clement A. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int J Biochem Cell Biol 2008;40:1703–1715. [DOI] [PubMed] [Google Scholar]
- 24.Keane MP, Arenberg DA, Lynch JP III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 1997;159:1437–1443. [PubMed] [Google Scholar]
- 25.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000;103:239–252. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Pulido L, Devos D, Valencia A. BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci 2002;27:329–332. [DOI] [PubMed] [Google Scholar]
- 28.Lomas DA. Genetic predisposition to chronic obstructive pulmonary disease: advances in alpha1-antitrypsin deficiency and the serpinopathies. Clin Med 2007;7:446–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margolis RL, Ross CA. Expansion explosion: new clues to the pathogenesis of repeat expansion neurodegenerative diseases. Trends Mol Med 2001;7:479–482. [DOI] [PubMed] [Google Scholar]
- 30.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science 2003;302:819–822. [DOI] [PubMed] [Google Scholar]
- 31.Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol 2006;172:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavey C, Muhlbauer M, Bossard C, Freund A, Durand S, Jorgensen C, Jobin C, Lazennec G. Interleukin-8 expression is regulated by histone deacetylases through the nuclear factor–kappaB pathway in breast cancer. Mol Pharmacol 2008;74:1359–1366. [DOI] [PubMed] [Google Scholar]
- 34.Yang R, Trevillyan JM. c-Jun N-terminal kinase pathways in diabetes. Int J Biochem Cell Biol 2008;40:2702–2706. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2002;2:725–734. [DOI] [PubMed] [Google Scholar]
- 36.Hidvegi T, Schmidt BZ, Hale P, Perlmutter DH. Accumulation of mutant {alpha}1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NF{kappa}B, and BAP31 but not the unfolded protein response. J Biol Chem 2005;280:39002–39015. [DOI] [PubMed] [Google Scholar]
- 37.Knorre A, Wagner M, Schaefer HE, Colledge WH, Pahl HL. DeltaF508-CFTR causes constitutive NF-kappaB activation through an ER-overload response in cystic fibrosis lungs. Biol Chem 2002;383:271–282. [DOI] [PubMed] [Google Scholar]
- 38.Burstein E, Duckett CS. Dying for NF-kappaB? Control of cell death by transcriptional regulation of the apoptotic machinery. Curr Opin Cell Biol 2003;15:732–737. [DOI] [PubMed] [Google Scholar]
- 39.Nogee LM, Dunbar AE, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 2001;344:573–579. [DOI] [PubMed] [Google Scholar]
- 40.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest 1997;100:2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br J Pharmacol 2007;150:829–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006;5:769–784. [DOI] [PubMed] [Google Scholar]
- 43.Liu T, Kuljaca S, Tee A, Marshall GM. Histone deacetylase inhibitors: multifunctional anticancer agents. Cancer Treat Rev 2006;32:157–165. [DOI] [PubMed] [Google Scholar]
- 44.Roque T, Boncoeur E, Saint-Criq V, Bonvin E, Clement A, Tabary O, Jacquot J. Proinflammatory effect of sodium 4–phenylbutyrate in deltaF508–cystic fibrosis transmembrane conductance regulator lung epithelial cells: involvement of extracellular signal–regulated protein kinase 1/2 and c-Jun–NH2–terminal kinase signaling. J Pharmacol Exp Ther 2008;326:949–956. [DOI] [PubMed] [Google Scholar]
- 45.Iwata K, Tomita K, Sano H, Fujii Y, Yamasaki A, Shimizu E. Trichostatin A, a histone deacetylase inhibitor, down-regulates interleukin-12 transcription in SV-40–transformed lung epithelial cells. Cell Immunol 2002;218:26–33. [DOI] [PubMed] [Google Scholar]
- 46.Singh OV, Vij N, Mogayzel PJ Jr, Jozwik C, Pollard HB, Zeitlin PL. Pharmacoproteomics of 4–phenylbutyrate-treated IB3-1 cystic fibrosis bronchial epithelial cells. J Proteome Res 2006;5:562–571. [DOI] [PubMed] [Google Scholar]
- 47.Stewart G, Weaver TE. Surfactant protein–C mutations and 4-phenylbutyrate (PBA) therapy: beneficial or deleterious? Am J Respir Crit Care Med 2009;179:A6262. [Google Scholar]
- 48.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of Type 2 diabetes. Science 2006;313:1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabata Y, Takano K, Ito T, Iinuma M, Yoshimoto T, Miura H, Kitao Y, Ogawa S, Hori O. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am J Physiol Cell Physiol 2007;293:C411–C418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.