Abstract
The protective mechanisms by which some obese individuals escape the detrimental metabolic consequences of obesity are not understood. This study examined differences in body fat distribution and adipocytokines in obese older persons with and without metabolic syndrome. Additionally, we examined whether adipocytokines mediate the association between body fat distribution and metabolic syndrome. Data were from 729 obese men and women (BMI≥30kg/m2), aged 70-79 participating in the Health, Aging and Body Composition (Health ABC) study. Thirty-one percent of these obese men and women did not have metabolic syndrome. Obese persons with metabolic syndrome had significantly more abdominal visceral fat (men:p=0.04; women:p<0.01) and less thigh subcutaneous fat (men:p=0.09; women:p<0.01) than those without metabolic syndrome. Additionally, those with metabolic syndrome had significantly higher levels of IL-6, TNF-α and PAI-1 than individuals without metabolic syndrome. Per standard deviation (SD) higher in visceral fat, the likelihood of metabolic syndrome significantly increased in women (odds ratio (OR):2.16, 95% confidence interval (CI):1.59-2.94). In contrast, the likelihood of metabolic syndrome decreased in both men (OR:0.56, 95%CI:0.39-0.80) and women (OR:0.49, 95%CI:0.34-0.69) with each SD higher in thigh subcutaneous fat. These associations were partly mediated by adipocytokines; the association between thigh subcutaneous fat and metabolic syndrome was no longer significant in men. In summary, metabolically healthy obese older persons had a more favorable fat distribution, characterized by lower visceral fat and greater thigh subcutaneous fat and a more favorable inflammatory profile compared to their metabolically unhealthy obese counterparts.
Introduction
Obesity is increasingly prevalent in older persons and is associated with physical disability and poor health (1,2) as well as metabolic and physiological abnormalities such as hypertension and dyslipidemia (3-5). However, it is still unclear whether obesity “per se” or rather the associated risk factors are linked to negative health outcomes. Not all obese persons show evidence of metabolic disturbances, a sizable subgroup of obese individuals is metabolically healthy that have normal to high levels of insulin sensitivity and a generally favorable cardiovascular profile exists (3,6,7). The factors that distinguish the metabolically healthy from the metabolically unhealthy obese are not understood.
One explanation why some obese individuals are protected against metabolic syndrome is through a more favorable body fat distribution. In particular, increased abdominal fat is more detrimental than higher total body fat. Studies have shown that increased visceral /abdominal fat is positively associated with metabolic disease (8,9), independent of overall adiposity (10-12). Similarly, high thigh intermuscular fat is associated with poorer glucose tolerance (9). On the contrary, subcutaneous thigh fat is associated with more favorable levels of glucose and lipids (13,14). Finally, some obese people may have a lower overall fat mass which may protect them from having metabolic abnormalities.
Another explanation for the more favorable metabolic profile of some obese people may be related to inflammatory status (15). Inflammatory markers, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and other adipokines such as resistin and adiponectin are associated with metabolic alterations (16,17). These adipocytokines are closely linked to abdominal obesity, particularly to visceral adipose tissue while some evidence suggests that thigh subcutaneous fat is related to more favorable inflammatory profiles (18-20).
Previous studies have confirmed the existence of metabolically healthy obese individuals (3,6,7) however, to our knowledge no study examined this in a large group of older obese men and women. The prevalence of metabolic alterations is higher in older persons (21) and body fat distribution alters with age (22). Further, studies that examined body composition differences between metabolically healthy and unhealthy obese individuals were limited by a lack of detailed measurement of multiple fat depots, especially depots outside of the abdomen. Thus, the present study examined 1) differences in body fat distribution characteristics of the abdomen and thigh measured by computed tomography (CT) and 2) differences in adipocytokines in obese older persons with and without metabolic syndrome. Additionally, we examined whether adipocytokines mediate the association between body fat distribution and metabolic syndrome.
Methods and Procedures
Study population
The Health, Aging and Body Composition (Health ABC) study is a longitudinal cohort study consisting of 3,075 initially well-functioning, 70- to 79-year old, black and white men and women. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible community-dwelling black residents in designated zip code areas surrounding Memphis, Tennessee, and Pittsburgh, Pennsylvania. Participants were eligible if they reported no difficulty in walking one quarter of a mile, going up 10 steps without resting, or performing basic activities of daily living. Participants were excluded if they reported a history of active treatment for cancer in the prior three years, planned to move out of the study area in the next three years, or were currently participating in a randomized trial of a lifestyle intervention. Baseline data, collected between April 1997 and June 1998, included an in-person interview and a clinic-based examination, with evaluation of body composition, clinical and sub-clinical diseases, and physical functioning. For the present analyses we included only obese individuals, defined as having a body mass index (BMI) greater than or equal to 30kg/m2 (n=784). Persons with missing data on metabolic syndrome or body composition were excluded (n=55), leaving 729 subjects for the present analyses. All participants signed informed written consent forms approved by the institutional review boards of the clinical sites.
Measures
Metabolic syndrome
The metabolic syndrome was defined according to the ATPIII guidelines (23) as meeting at least three of the following criteria:1) waist circumference ≥102cm in men and ≥88cm in women; 2) serum triglyceride level ≥150mg/dL or currently on drug treatment for high triglycerides; 3) high-density lipoprotein (HLD) cholesterol level <40mg/dL in men and <50mg/dL in women or currently on treatment for low HDL cholesterol; 4) diastolic blood pressure ≥85mmHg and/or systolic blood pressure ≥130mmHg or using antihypertensive medications; and 5) fasting glucose level ≥100mg/dL or using antidiabetic medication.
Body composition
Body weight was measured to the nearest 0.1kg with a standard balance beam scale. Body height was measured to the nearest 0.1cm using a wall-mounted stadiometer. Abdominal sagittal diameter was measured with a Holtain-Kahn abdominal caliper while the participant lay supine. The lower blade of the caliper was placed under the small of the back and the upper blade was lowered to a mark midway between the iliac crests. Total fat mass was acquired from total body scans using fan-beam DXA (Hologic QDR 4500A) with DXA software (Hologic, Bedford, MA). CT scans of the abdomen and thigh were obtained in Memphis using a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH) scanner and a 9800 Advantage scanner (General Electric, Milwaukee, WI) in Pittsburgh. The scans were obtained at 120kVp, 200 to 250mA seconds, at a slice thickness of 10 mm. Areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using ILD development software (RSI Systems, Boulder, CO). Scans of the abdomen were taken at the level of the space between the fourth and fifth lumbar vertebrae (L4–L5). The scan at mid-thigh level was performed at one half of the distance between the medial edge of the greater trochanter and the intercondyloid fossa. Visceral fat was manually distinguished from abdominal subcutaneous fat area by tracing along the fascial plane defining the internal abdominal wall. In the thighs, intermuscular and visible intramuscular fat tissue was separated from subcutaneous adipose tissue by drawing a line along the deep fascial plane surrounding the thigh muscles. Areas of the left and right thigh were added.
Adipocytokines
Measures for the cytokines IL-6 and TNF-α and for C-reactive protein (CRP) were obtained from frozen stored plasma or serum. Fasting blood samples were obtained in the morning, and after processing, the specimens were aliquoted into cryovials, frozen at -70°C and shipped to the Health ABC Core Laboratory at the University of Vermont. Cytokines were measured in duplicate by enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN). The detectable limit was 0.10pg/mL for IL-6 (by HS600 Quantikine Kit) and 0.18pg/mL for TNF-α (by HSTA50 kit). Serum levels of CRP were also measured in duplicate by ELISA based on purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). The CRP assay was standardized according to the World Health Organization First International Reference Standard with a sensitivity of 0.08μg/mL. Assays of blind duplicates collected for 150 participants showed an average interassay coefficient of variation of 10.3% for IL-6, 8.0% for CRP, and 15.8% for TNF-α. Plasma plasminogen activator inhibitor-1 (PAI-1) was measured by a two-site enzyme-linked immunosorbent assay (ELISA; Collen Laboratory, Belgium) with a coefficient of variation of 3.5%. Serum leptin and adiponectin concentrations were measured by radioimmunoassay in duplicate (Linco Research Inc., St. Charles, MO). The intra-assay coefficient of variation was 3.7%–7.5% for leptin and 1.8%–3.6% for adiponectin. Serum resistin concentration was measured using a sandwich enzyme-linked immunosorbent assay (ELISA; Linco Research Inc). Intra- and interassay coefficients of variation for this assay are 4.5% and 7.4%, respectively.
Covariates
Sociodemographic variables included age, race, study site (Memphis or Pittsburgh), and educational level (less than high school, high school graduate, postsecondary). Lifestyle factors included smoking (current, former, never), alcohol intake (never, current, former) and physical activity. Physical activity in the previous seven days was assessed at baseline; time and intensity level were reported for activities including gardening, heavy household chores, light house work, grocery shopping, laundry, climbing stairs, walking for exercise, walking for other purposes, aerobics, weight or circuit training, high-intensity exercise activities, and moderate-intensity exercise activities. Approximate metabolic equivalent unit (MET) values were assigned to each of the activity categories to calculate a weekly activity energy expenditure estimate in kcal/kg/wk (24). Three categories were created:exercise:≥1,000 kcal/wk exercise activities; lifestyle active <1,000 kcal/wk exercise activities and ≥2,719 kcal/wk total physical activity; and inactive <1,000 kcal/wk exercise and <2,719 kcal/wk total physical activity (25).
Statistical Analyses
Chi square test for categorical variables and t-tests for continuous variables were used to examine differences in baseline characteristics between obese persons with and without metabolic syndrome. Univariate analysis of variance was used to examine the association between metabolic syndrome status and body composition as well as between metabolic syndrome status and adipocytokines. All adipocytokines were log-transformed because they were not normally distributed. Adjusted means and standard errors are presented and analyses were adjusted for sociodemographics and lifestyle factors. Logistic regression analysis was performed to examine the relationship between abdominal visceral fat and thigh subcutaneous fat and metabolic syndrome. Two models were fitted: model 1 adjusted for sociodemographics, lifestyle factors, height, and total body fat; model 2 additionally adjusted for adipocytokines. Because of known differences in body composition and in the prevalence of metabolic syndrome between men and women, all results are shown for men and women separately. Interactions between fat depot and race, and adipocytokines and race were tested but were not statistically significant. Analyses were performed using SPSS, version 17.0 (SPSS Inc., Chicago, IL).
Results
Baseline characteristics according to metabolic syndrome status are shown in Table 1. Thirty-one percent of the obese men and women in the Health ABC study had no metabolic syndrome. There were no significant differences in sociodemographic and lifestyle factors between obese persons with and without metabolic syndrome. Only the number of current smokers was significantly higher in women with metabolic syndrome compared to women without metabolic syndrome (p=0.03). The prevalence of heart disease (p=0.02), peripheral arterial disease (p=0.04), and osteoarthritis (p-0.04) was significantly higher in men with metabolic syndrome. In both men and women, the prevalence of diabetes was higher in individuals with metabolic syndrome compared to those without metabolic syndrome (p<0.01).
Table 1. Baseline characteristics of obese men and women with and without metabolic syndrome.
| Men n=305 | Women n=424 | |||||
|---|---|---|---|---|---|---|
| No metabolic syndrome n=94 |
Metabolic syndrome n=211 |
p | No Metabolic syndrome n=130 |
Metabolic syndrome n=294 |
p | |
| Age, mean(SD) | 73.9(2.8) | 73.9(2.7) | 0.99 | 73.9(2.9) | 73.6(2.8) | 0.29 |
| Race, white,% | 52.1 | 58.3 | 0.32 | 26.2 | 35.4 | 0.06 |
| Site, Memphis,% | 55.3 | 46.0 | 0.13 | 43.1 | 47.3 | 0.42 |
| Education,% | ||||||
| Postsecondary | 37.2 | 46.2 | 0.06 | 20.2 | 27.1 | 0.29 |
| High school graduate | 24.5 | 29.0 | 45.0 | 39.0 | ||
| Less than high school | 38.3 | 24.8 | 34.9 | 33.9 | ||
| Physical activity,% | ||||||
| Exercise | 31.9 | 29.4 | 0.24 | 11.5 | 9.9 | 0.82 |
| Lifestyle | 46.8 | 55.9 | 66.9 | 67.7 | ||
| Inactive | 21.3 | 14.7 | 21.5 | 20.4 | ||
| Smoking,% | ||||||
| Never | 28.7 | 25.6 | 0.80 | 64.6 | 52.2 | 0.03 |
| Current | 7.4 | 6.6 | 3.1 | 7.5 | ||
| Former | 63.8 | 67.8 | 32.3 | 40.3 | ||
| Alcohol intake,% | ||||||
| Never | 19.1 | 10.9 | 0.10 | 48.1 | 38.4 | 0.13 |
| Current | 58.5 | 59.2 | 33.3 | 36.1 | ||
| Former | 22.3 | 29.9 | 18.6 | 25.5 | ||
| Heart disease,% | 14.9 | 27.0 | 0.02 | 10.0 | 15.3 | 0.14 |
| Cerebrovascular disease,% | 4.3 | 8.1 | 0.23 | 6.9 | 5.1 | 0.45 |
| Peripheral arterial disease,% | 1.1 | 6.6 | 0.04 | 3.8 | 4.1 | 0.91 |
| Diabetes mellitus,% | 9.9 | 44.7 | <0.01 | 2.4 | 37.3 | <0.01 |
| Lung disease,% | 23.4 | 25.1 | 0.75 | 10.8 | 15.3 | 0.21 |
| Osteoarthritis,% | 4.3 | 11.8 | 0.04 | 17.7 | 20.1 | 0.59 |
| Cancer,% | 25.5 | 22.7 | 0.60 | 10.0 | 16.0 | 0.10 |
| Depressive symptoms,% | 2.1 | 5.2 | 0.22 | 3.8 | 5.1 | 0.57 |
| Cognitively impaired,% | 7.4 | 7.1 | 0.92 | 5.4 | 10.2 | 0.11 |
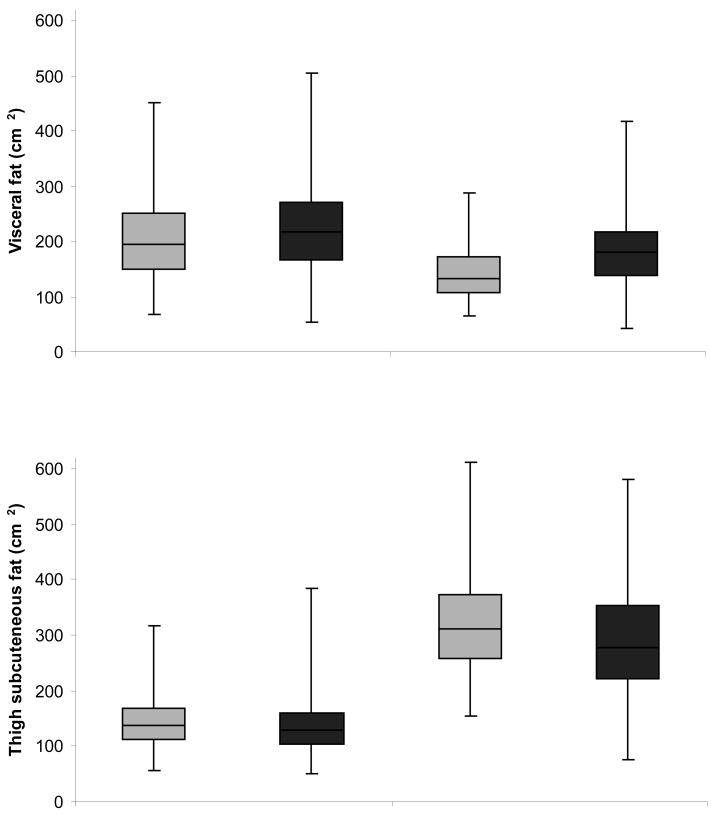
Table 2 presents adjusted means of body composition parameters by sex and metabolic syndrome status. Men with metabolic syndrome had a higher BMI and a larger waist circumference than men without metabolic syndrome. Sagittal diameter was greater in both men and women with metabolic syndrome. No significant differences in total fat mass were observed in men and women. In both men and women, those with metabolic syndrome had significantly more total abdominal fat and visceral fat area, but abdominal subcutaneous fat area did not differ. Men and women with metabolic syndrome had less thigh subcutaneous fat than those without metabolic syndrome. Figure 1 shows box plots of visceral fat and thigh subcutaneous fat for men and women with and without metabolic syndrome. In additional analysis we examined the ratio of visceral fat to thigh subcutaneous fat which was significantly greater in the obese with metabolic syndrome compared to those without metabolic syndrome in both men (mean(SE): 1.82(0.06) vs. 1.56(0.09), p=0.01) and women (mean(SE): 0.73(0.02) vs. 0.49(0.04), p<0.01). Figure 2 shows adjusted means of visceral fat and thigh subcutaneous fat area according to the number of metabolic abnormalities. We found a significant positive trend for visceral fat with those having 5 metabolic abnormalities have the greatest visceral fat area. The significant trend for thigh subcutaneous fat went in the opposite direction where the highest thigh subcutaneous fat areas were found in people with 0-1 metabolic abnormalities.
Table 2. Adjusted means of body composition in obese men and women with and without metabolic syndromea.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No metabolic syndrome | Metabolic syndrome | No metabolic syndrome | Metabolic syndrome | |||||||
| Mean | SE | Mean | SE | p | Mean | SE | Mean | SE | p | |
| BMI(kg/m2) | 32.2 | 0.3 | 32.9 | 0.2 | 0.02 | 33.6 | 0.3 | 34.2 | 0.2 | 0.08 |
| Waist circumference(cm) | 111.3 | 1.2 | 115.8 | 0.8 | <0.01 | 109.7 | 0.9 | 111.3 | 0.6 | 0.17 |
| Sagittal diameter(mm) | 272.5 | 2.4 | 281.0 | 1.6 | <0.01 | 264.3 | 2.0 | 272.7 | 1.4 | <0.01 |
| Total fat mass(kg) | 32.8 | 0.6 | 33.8 | 0.4 | 0.16 | 39.0 | 0.6 | 39.1 | 0.4 | 0.91 |
| Abdomen | ||||||||||
| Total area(cm2) | 846.4 | 12.2 | 890.0 | 8.1 | <0.01 | 871.8 | 11.3 | 909.1 | 7.5 | <0.01 |
| Total fat area(cm2) | 533.1 | 10.5 | 563.1 | 6.9 | 0.02 | 618.4 | 10.5 | 653.4 | 7.0 | <0.01 |
| Visceral fat area(cm2) | 205.2 | 7.1 | 223.0 | 4.7 | 0.04 | 146.9 | 4.9 | 182.5 | 3.3 | <0.01 |
| Subcutaneous fat area(cm2) | 327.9 | 8.2 | 340.1 | 5.4 | 0.22 | 471.4 | 9.3 | 471.0 | 6.1 | 0.97 |
| Thigh | ||||||||||
| Total area(cm2) | 504.8 | 6.3 | 505.4 | 4.2 | 0.94 | 579.5 | 8.7 | 561.6 | 5.7 | 0.09 |
| Total fat area(cm2) | 160.3 | 4.8 | 152.4 | 3.2 | 0.18 | 336.5 | 8.0 | 306.3 | 5.3 | <0.01 |
| Subcutaneous fat area(cm2) | 145.3 | 4.7 | 135.5 | 3.1 | 0.09 | 321.7 | 7.9 | 290.6 | 5.2 | <0.01 |
| Intermuscular fat area(cm2) | 15.0 | 0.9 | 16.9 | 0.6 | 0.08 | 14.9 | 0.6 | 15.7 | 0.4 | 0.26 |
Adjusted for age, race, site, education, physical activity, smoking, and alcohol intake
Figure 1.
Box plots a of visceral and thigh subcutaneous fat according in obese men and women with and without metabolic syndrome
a Dark line in the middle of the box is the median; bottom and top of the box represent the 25th and 75th percentile; end of the whiskers represent the minimum and maximum
Figure 2.
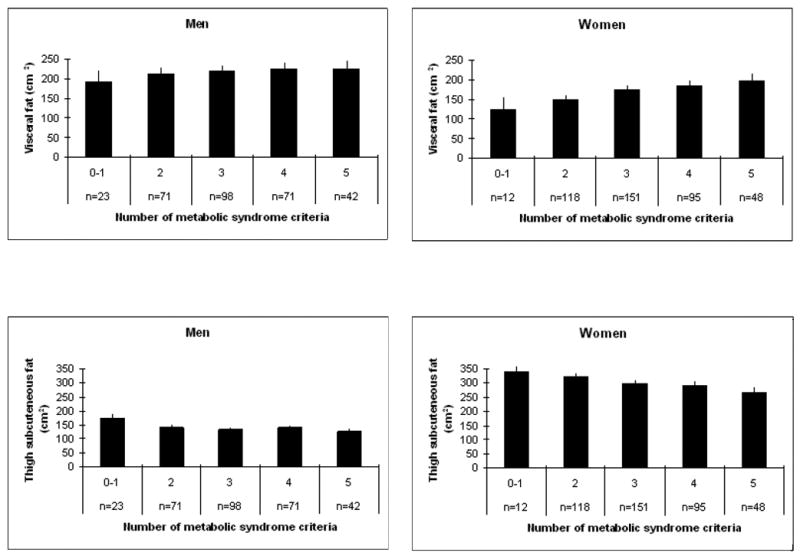
Adjusted mean values of visceral and thigh subcutaneous fat according the number of metabolic abnormalities a
aAdjusted for age, race, site, education, physical activity, smoking, alcohol intake, and total fat
As shown in Table 3, men and women with metabolic syndrome had significantly higher levels of TNF-α and PAI-1 than individuals without metabolic syndrome. IL-6 and CRP levels were significantly higher in women with metabolic syndrome but not in men. No significant differences were found for leptin, adiponectin, or resistin.
Table 3. Adjusted means of adipocytokines in obese men and women with and without metabolic syndromea.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No metabolic syndrome | Metabolic syndrome | No metabolic syndrome | Metabolic syndrome | |||||||
| Mean | SE | Mean | SE | p | Mean | SE | Mean | SE | p | |
| Log IL-6(pg/mL) | 0.73 | 0.06 | 0.85 | 0.04 | 0.08 | 0.70 | 0.05 | 0.89 | 0.04 | <0.01 |
| Log CRP(μg/mL) | 0.72 | 0.08 | 0.74 | 0.06 | 0.86 | 0.98 | 0.07 | 1.15 | 0.05 | 0.05 |
| Log TNF-α(pg/mL) | 1.04 | 0.04 | 1.27 | 0.03 | <0.01 | 1.06 | 0.04 | 1.21 | 0.02 | <0.01 |
| Log PAI-1(ng/mL), | 3.06 | 0.06 | 3.51 | 0.04 | <0.01 | 3.21 | 0.06 | 3.44 | 0.04 | <0.01 |
| Log Leptin(ng/mL) | 2.37 | 0.06 | 2.46 | 0.04 | 0.21 | 3.35 | 0.04 | 3.35 | 0.03 | 0.95 |
| Log Adiponectin(μg/mL) | 2.27 | 0.07 | 2.23 | 0.04 | 0.53 | 2.32 | 0.05 | 2.30 | 0.04 | 0.76 |
| Log Resistin(ng/mL) | 2.91 | 0.05 | 2.95 | 0.03 | 0.46 | 2.96 | 0.04 | 2.99 | 0.03 | 0.47 |
Adjusted for age, race, site, education, physical activity, smoking, alcohol intake, and total fat
The relationship between visceral fat and thigh subcutaneous fat with metabolic syndrome is examined in Table 4. Per standard deviation (SD) increase in visceral fat, the likelihood of metabolic syndrome significantly increased in women (model 1, odds ratio (OR):2.16, 95% confidence interval (CI):1.59-2.94). In contrast, the likelihood of metabolic syndrome decreased in both men (OR:0.56, 95%CI:0.39-0.80) and women (OR:0.49, 95%CI:0.34-0.69) with each SD increase in thigh subcutaneous fat. In a second model we additionally adjusted for the adipocytokines significantly associated with metabolic syndrome in table 3 (IL-6, CRP, TNF-α, and PAI-1). Including these adipocytokines attenuated the association between thigh subcutaneous fat and was no longer significant in men.
Table 4. Odds ratios (95%CI) of metabolic syndrome according to visceral fat and thigh subcutaneous fat.
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |||||||||
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Visceral fat (per SD increase)c | 1.26 | 0.92-1.71 | 0.15 | 1.17 | 0.83-1.65 | 0.37 | 2.16 | 1.59-2.94 | <0.01 | 1.95 | 1.42-2.68 | <0.01 |
| Thigh subcutaneous fat (per SD increase)d | 0.56 | 0.39-0.80 | <0.01 | 0.70 | 0.47-1.05 | 0.09 | 0.49 | 0.34-0.69 | <0.01 | 0.53 | 0.36-0.76 | <0.01 |
Adjusted for age, race, site, education, physical activity, smoking, alcohol intake, height, and total fat
Adjusted for age, race, site, education, physical activity, smoking, alcohol intake, height, total fat, IL-6, CRP, TNF-α, and PAI-1
SD men:73.3, SD women:61.6
SD men:44.2, SD women:90.6
In a model with both visceral fat and thigh subcutaneous fat adjusted for demographic and lifestyle factors, visceral fat was associated with an increased likelihood of metabolic syndrome (OR:2.13, 95%CI:1.59-2.84) and thigh subcutaneous fat with a decreased likelihood of metabolic syndrome (OR:0.78, 95%CI:0.61-0.99) in women (Figure 3). Visceral fat remained associated with a significantly increased likelihood of metabolic syndrome after adjustment for adipocytokines or total fat mass. In men visceral fat was associated with a higher likelihood of metabolic syndrome (OR:1.38, 95%CI:1.02-1.85) while thigh subcutaneous fat was borderline significant (OR:0.81, 95%CI:0.63-1.04). Results became non-significant after adding adipocytokines or total fat mass to the model.
Figure 3.
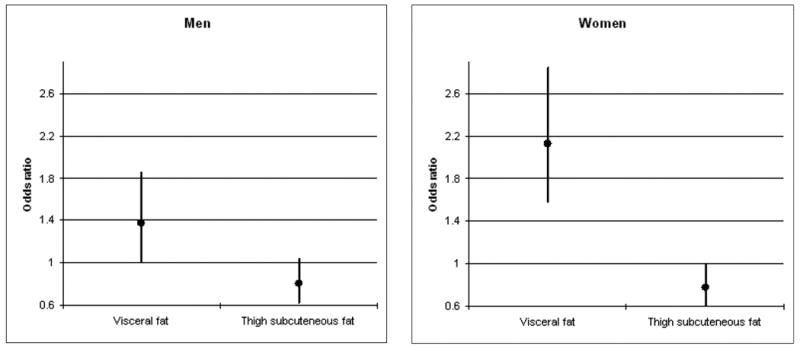
Odds ratios and 95% confidence intervals of metabolic syndrome according to visceral fat and thigh subcutaneous fat a
aModel includes both visceral and thigh subcutaneous fat and adjusted for age, race, site, education, physical activity, smoking, alcohol intake, and height
Discussion
The present study of older obese people shows that those with metabolic syndrome have more abdominal visceral fat and less thigh subcutaneous fat than people without metabolic syndrome while total fat mass did not differ between the two groups. Further, obese persons with metabolic syndrome had higher levels of IL6, CRP (only significant in women), TNF-α, and PAI-1 while there were no differences in levels of leptin, adiponectin, and resistin between the two groups. Increased visceral fat was associated with a significantly higher likelihood of metabolic syndrome in women while increased thigh subcutaneous thigh fat was associated with a significantly lower likelihood of metabolic syndrome in both men and women. These associations were partly mediated by inflammatory factors but the associations between visceral fat, thigh subcutaneous fat and metabolic syndrome remained significant in women.
It is unknown why some individuals defined as obese on the basis of total body weight relative to their height do not exhibit the deleterious metabolic consequences frequently associated with obesity. Even though total fat mass was similar in obese persons with and without metabolic alterations, fat distribution varied significantly which is not captured by using BMI to define obesity. Our results suggest that a higher visceral fat area and lower thigh subcutaneous fat area accompanied by higher levels of cytokines/inflammatory markers lead to an unhealthy metabolic phenotype in obese older persons. Duration of obesity might also be important and future studies should examine whether the metabolically unhealthy obese group mainly consist of obese individuals who have been obese for a longer time than the metabolically healthy obese group.
A few smaller studies among obese postmenopausal women examined differences in body composition and/or inflammatory profile between metabolically healthy and unhealthy obese women (5,6,26). A study among 43 obese postmenopausal women showed lower levels of visceral fat (6,26) and a more favorable inflammation profile (26) in the metabolically healthy obese women compared to the metabolically unhealthy women. In another study among 58 obese postmenopausal women, those with metabolic syndrome had more visceral adipose tissue but no differences in levels of inflammatory markers compared to those without metabolic syndrome were found (5). A recent study found no significant differences in visceral fat between the obese-insulin resistant and obese-insulin sensitive group but a significant difference in liver fat was observed (7). Liver fat has been shown to be important in the regulation of glucose and lipid metabolism and has been associated with metabolic syndrome (27-30). Future studies should examine differences in liver fat in metabolically healthy and unhealthy older adults and assess the relative importance of liver fat versus visceral fat. Fetuin-A, a hepatic secretary protein that is increased when there is fat accumulation in the liver, might also be important; it has been related to diabetes and visceral fat accumulation (31,32). Fetuin-A was measured in a small random subgroup of the Health ABC study; however, since our analysis was limited to obese individuals only we did not have enough statistical power for analysis with fetuin-A.
Unlike most previous studies, we also had the opportunity to examine differences in fat depots outside the abdomen. We found that high thigh subcutaneous fat was protective against the metabolic syndrome in obese men and women. Additionally, the number of metabolic alterations was associated with lower thigh subcutaneous fat area. In women, thigh subcutaneous fat was even associated with a lower likelihood of metabolic syndrome independent of visceral fat. This finding suggests that larger thigh subcutaneous fat is not just an indicator of lower visceral fat. The combined relations of thigh subcutaneous and abdominal visceral fat on metabolic risk should be examined in future research. Leg fat has previously been associated with a more favorable metabolic profile (13,33) and a more favorable inflammatory profile (20). Aging is associated with a redistribution of fat mass with an increase in abdominal fat, in particular visceral fat, combined with a decrease in lower body subcutaneous fat (22,34). Because of the protective role of thigh subcutaneous fat, understanding what factors may prevent the decline or what factors contribute to changes in this fat depot is important.
Adipocytokines have been associated with both body fat distribution and metabolic syndrome and we therefore hypothesized that these markers could, at least partially explain the link between body fat distribution and metabolic syndrome. Adipose tissue is a metabolically active endocrine organ which secretes adipocytokines (18). Visceral adipose tissue in particular has been associated with increased levels of inflammation (35-37). Beasley et al showed that visceral adiposity, and not abdominal subcutaneous fat, was most consistently associated with significantly higher levels of IL-6 and CRP levels in black and white men and women in the Health ABC study (20). Further, in women there was a trend toward lower inflammatory marker concentration with increasing thigh subcutaneous fat (20). In the present study we show that obese men and women with metabolic syndrome had significantly higher levels of inflammatory cytokines than obese persons without metabolic syndrome. These cytokines only partly explained the association between visceral fat and thigh subcutaneous fat with metabolic syndrome. No significant differences in levels of leptin, adiponectin and resistin were found between obese people with and without metabolic syndrome. The secretion of leptin and adiponectin is greater in subcutaneous than in visceral adipose tissue (18,38). We did not find significant differences in abdominal subcutaneous fat between obese persons with and without metabolic syndrome which might explain why we did not find any differences in leptin levels between the two groups. Our results with adiponectin are unexpected. Although there is some evidence that the secretion of adiponectin is greater in subcutaneous adipose tissue (18,38), other studies suggest an important role of visceral adipose tissue in the regulation of adiponectin secretion (39,40). In our study total circulating adiponectin was measured; high-molecular-weight adiponectin is, however, more strongly related to metabolic risk factors than total adiponectin (41) which may explain why we did not find any differences in adiponectin between the two obese groups.
Some limitations of the study need to be considered. This was a cross-sectional analysis which does not allow us to draw causal conclusions. Even though it is likely that an unfavorable fat distribution, characterized by high visceral fat and low thigh subcutaneous fat, contributes to metabolic syndrome, longitudinal studies are needed to confirm this. Further, Health ABC participants were well-functioning at baseline, so our findings may not be generalizable to other groups of older adults. Finally, there are different subcutaneous adipose tissue compartments in the abdomen with different metabolic characteristics (42). Examining superficial and deep subcutaneous adipose tissue separately may have resulted in different findings.
In summary, even though total fat mass was similar in obese persons with and without metabolic alterations, fat distribution varied significantly. A more favorable fat distribution, characterized by lower visceral fat and greater thigh subcutaneous fat and a more favorable inflammatory profile resulted in a metabolically healthy obesity phenotype in older adults. Since an unfavorable fat distribution, inflammation, and metabolic syndrome are all related to adverse health outcomes, such as heart disease, diabetes, and disability (23,43,44), it is likely that the risk of these conditions is different in metabolically healthy and unhealthy obese persons. Future studies are needed to confirm this. For clinical practice it is important to identify subgroups of obese individuals who are at especially high risk for adverse health outcomes.
Acknowledgments
This study was supported by National Institute on Aging contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. This research was supported (in part) by the Intramural Research Program of the NIH, National Institute on Aging. S.S was supported in part by a grant from the Finnish Academy (no. 125494 ).
Footnotes
Conflict of interest: None
References
- 1.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring) 2007;15:2855–65. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 3.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–75. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 5.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–22. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 6.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–5. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 7.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 8.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 11.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–9. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 12.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 13.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 14.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 15.Stenholm S, Koster A, Alley DE, et al. Adipocytokines and the metabolic syndrome among older persons with and without obesity - the InCHIANTI Study. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03742.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You T, Nicklas BJ, Ding J, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–9. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int J Obes (Lond) 2008;32:772–9. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 18.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 19.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 20.Beasley LE, Koster A, Newman AB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17:1062–9. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009:1–7. [PubMed] [Google Scholar]
- 22.Kuk JL, Saunders TJ, Davidson L, Ross R. Age-related Changes in Total and Regional Fat Distribution. Ageing Res Rev. 2009 doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–9. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 26.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–50. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 27.Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–65. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 28.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–7. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 29.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 30.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–60. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 31.Ix JH, Wassel CL, Chertow GM, et al. Fetuin-A and change in body composition in older persons. J Clin Endocrinol Metab. 2009;94:4492–8. doi: 10.1210/jc.2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-A and incident diabetes mellitus in older persons. Jama. 2008;300:182–8. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–7. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 34.Kotani K, Tokunaga K, Fujioka S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–2. [PubMed] [Google Scholar]
- 35.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 36.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 37.Lemieux I, Pascot A, Prud'homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–7. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 38.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 39.Kantartzis K, Rittig K, Balletshofer B, et al. The relationships of plasma adiponectin with a favorable lipid profile, decreased inflammation, and less ectopic fat accumulation depend on adiposity. Clin Chem. 2006;52:1934–42. doi: 10.1373/clinchem.2006.067397. [DOI] [PubMed] [Google Scholar]
- 40.Cote M, Mauriege P, Bergeron J, et al. Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90:1434–9. doi: 10.1210/jc.2004-1711. [DOI] [PubMed] [Google Scholar]
- 41.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 42.Koska J, Stefan N, Votruba SB, Smith SR, Krakoff J, Bunt JC. Distribution of subcutaneous fat predicts insulin action in obesity in sex-specific manner. Obesity (Silver Spring) 2008;16:2003–9. doi: 10.1038/oby.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64:96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicklas BJ, Cesari M, Penninx BW, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–20. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]