Abstract
Objective
A central function of the endothelium is to serve as a selective barrier that regulates fluid and solute exchange. While perturbation of barrier function can contribute to numerous disease states, our understanding of the molecular mechanisms regulating this aspect of endothelial biology remains incompletely understood. Accumulating evidence implicates the Kruppel-like factor 2 (KLF2) as a key regulator of endothelial function. However, its role in vascular barrier function is unknown.
Methods and Results
To assess the role of KLF2 in vascular barrier function in vivo, we measured the leakage of Evans Blue dye into interstitial tissues of the mouse ear after treatment with mustard oil. By comparison to KLF2+/+ mice, KLF2+/− mice exhibited a significantly higher degree of vascular leak. In accordance with our in vivo observation, adenoviral overexpression of KLF2 in HUVECs strongly attenuated the increase of endothelial leakage by thrombin and H2O2 as measured by FITC-Dextran passage. Conversely, KLF2 deficiency in HUVECs and primary endothelial cells derived from KLF2+/− mice exhibited a marked increase in thrombin and H2O2-induced permeability. Mechanistically, our studies indicate that KLF2 confers barrier-protection via differential effects on the expression of key junction protein occludin and modification of a signaling molecule (myosin light chain) that regulate endothelial barrier integrity.
Conclusions
These observations identify KLF2 as a novel transcriptional regulator of vascular barrier function.
Keywords: endothelium, KLF2, barrier function
Introduction
The endothelium forms the inner lining of all blood vessels and regulates vascular barrier function, blood coagulation, and homing of immune cells to specific sites of the body.1 Under normal physiological conditions, the endothelial monolayer serves as a barrier that maintains the integrity of the blood-fluid compartment and allows for the selective passage of liquid, solutes, and leukocytes.1 In contrast, under pathophysiologic conditions, the integrity of the endothelium can be perturbed resulting in leakage of fluids into the extravascular space.
Movement of fluid and solutes through the endothelium occurs either across cells (transcellular) or between cells (paracellular).2 While the former mechanism contributes to basal barrier function of the endothelium, paracellular flux has received increasing attention for its pathophysiologic importance in disease states such as ischemia-reperfusion injury, diabetic vasculopathy, and acute lung injury.2 Pro-permeability factor such as thrombin and hydrogen peroxide or inflammatory stimuli (e.g. LPS or TNFα) promote paracellular vascular leak by two predominant mechanisms.3,4-6 One mechanism is by the disruption of the cell-cell junctions between endothelial cells (EC), which involves the regulation of adhesive proteins that reside at the junctions between cells, termed tight junctions and adherens junctions.7,8, 9 The other mechanisms is cytoskeletal contraction leading to widened intercellular space, which involves activation of signaling molecules that converge on myosin light chain kinase (MLCK).10, 11,12 The resulting phosphorylation of myosin light chain (MLC) induces actin-myosin mediated endothelial cell contraction thereby pulling neighboring endothelial cells apart from each other.12 Collectively, these mechanisms enhance paracellular leak and thereby contribute to the pathology observed in disease states. Despite considerable effort, our understanding of the molecular mechanisms regulating endothelial barrier function remains incompletely understood. As such, the identification of factors that regulate endothelial barrier function is of considerable scientific interest.
Kruppel-like factors (KLF) are a subclass of the zinc finger family of DNA-binding transcription factors.13 Accumulating evidence from our group and others has led to the appreciation that members of this gene family critically regulate endothelial function and vascular homeostasis.14,15 Amongst this gene family, the Kruppel-like factor 2 (KLF2) has emerged as particularly important in endothelial biology. KLF2 is a shear stress-induced factor that confers anti-inflammatory and anti-thrombotic properties to the vascular endothelial cell. 15, 16,17,18,19-21 However, despite the acknowledged importance of the endothelium in determining vascular barrier function, studies assessing the role of KLF2 (or any other member of this gene family) have not been reported. Herein we provide in vitro and in vivo evidence implicating KLF2 as an essential regulator of endothelial barrier integrity.
Materials and Methods
An expanded Materials and Methods section can be found in the supplemental materials.
Measurement of endothelial leakage
A commercially available kit (Millipore, Temecula, CA) was used to measure EC monolayer leakage to high molecular weight proteins utilizing 2,000-kDa FITC-dextran based on the Transwell model. Briefly, a Transwell insert was coated with collagen for 1 h at room temperature, and ECs were then seeded at a density of 2×105/well in a final volume of 400 μl EGM-2 with supplements. The inserts were placed into 24-well plates containing 500 μl medium overnight. To measure agonist-induced EC leakage, 200 μl FITC-dextran was added into the insert and incubated for 2 h. The insert was then removed and 50 μl medium was collected from the bottom chamber. The fluorescence density of samples was analyzed on a Microplate Fluorometer at excitation and emission wavelengths of 485 nm and 530 nm respectively. Results are expressed as relative barrier function in which the control group was set to one. In experiments where adenoviruses were used, the control group was HUVECs infected with Ad-GFP; in leakage assays involving siRNA transfection, the control group was HUVECs transfected with non-specific siRNA; in experiments where murine primary ECs were used, ECs from KLF2+/+ mice without treatment was set as control.
Vascular barrier function assay
All animals were handled according to IACUC protocol (#2009-0108) approved by the Institutional Animal Care and Use Committee at Case Western Reserve University, which is certified by the American Association of Accreditation for Laboratory Animal Care. Evans blue dye (EBD- 30 mg/kg in 100 μl normal saline) was injected into the jugular vein of 7- to 8 week-old male mice. In some experiments, after 1 minute, mustard oil diluted to 5% in mineral oil was applied to the dorsal and ventral surfaces of the ear with a cotton swab; photographs were taken 15 minutes after EBD injection. After the mice were euthanized by CO2 inhalation, ears were removed, blotted dry, and weighed. The EBD was extracted from the ears with 1 ml of formamide overnight at 55°C and measured spectrophotometrically at 620 nm. The amount of EBD in each sample was calculated according to a standard curve generated from known amounts of EBD, and expressed as μg of dye/mg of ear tissue.22
Isolation of primary endothelial cells from mice
KLF2+/− mice (generously provided by J. Leiden) were generated as previously described.23, 24 Murine lungs were obtained from 4-6 week old KLF2+/+ and KLF2+/− mice on CD1 background. Murine ECs from these lungs were isolated through selection with intercellular adhesion molecule 2 (ICAM2) antibody (BD Biosciences) bound to Dynabeads (Invitrogen). Briefly, murine lungs were minced and digested with collagenase for 30 minutes, after which cells were treated with red blood cell lysis buffer, the remaining cells were plated on 0.1% gelatin coated plates. When the plate becomes confluent, endothelial cells were selected using ICAM-2 and then re-plated on collagen coated plates for further use. Purity of lung microvascular endothelial cell cultures was assessed by immonhistochemistry for the EC specific marker CD31 and was found to be greater than 80%.
Results
Hemizygous deficiency of KLF2 augments vascular leakage
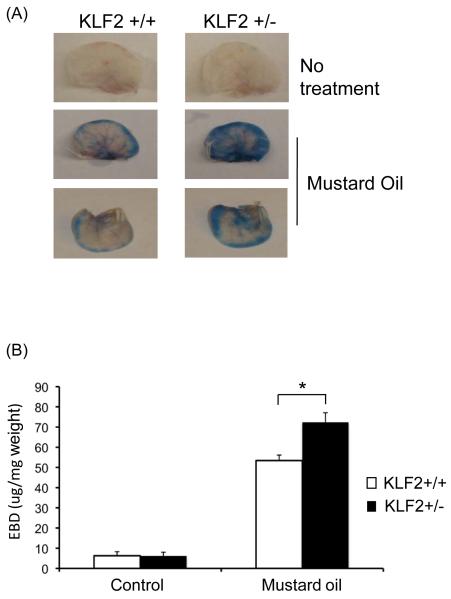
As a first step towards understanding the role of KLF2 in vascular barrier function, we performed in vivo assays to assess vascular leak in KLF2 heterozygous mice. These mice were used because both systemic and endothelial-specific deletion of KLF2 results in embryonic death.24, 25 Evans blue dye was injected intravenously into the jugular vein followed by treatment of the ear with mustard oil, a local inflammatory stimuli that induces inflammation and plasma leakage.22, 26 As expected, 15 minutes following application of mustard oil, a blue coloration developed in the ears of wild type mice (Figure 1A). However, the EBD staining was much more intense in the KLF2 heterozygous mice. Consistent with this observation, quantitative analysis of extravasation of dye from the vasculature in ears revealed a significantly greater increase of EBD in the heterozygous mice compared with the wild-type mice (53.4±2.68 μg/mg in KLF2+/+ versus 72.36 ±4.71μg/mg in KLF2+/−, P=0.042; Figure 1B). These data indicate that partial deficiency of KLF2 enhanced vascular leakage in response to an inflammatory stimuli.
Figure 1.
Hemizygous deficiency of KLF2 shows increased vascular permeability in response to Mustard oil. (A) Seven to eight weeks old male wild type and KLF2 heterozygous mice were used for this experiment. Evans blue dye was injected into the mice through internal jugular vein. Mustard oil was applied 1 minute later to the mice ear, photographs were taken after 15 minutes; then the mice were sacrificed and ear lobes harvested. Representative pictures are shown. (B) The ears were later cut into small pieces and the dye extracted using 1 ml of formamide, the EBD amount in each sample was quantified by spectrophotometer at a wavelength of 620nm and normalized to ear weight. In KLF2 heterozygous mice the amount of dye extravasated was statistically significant higher when compared to that of wild type (n=9, p<0.05).
KLF2 overexpression prevents endothelial leakage by diverse stimuli
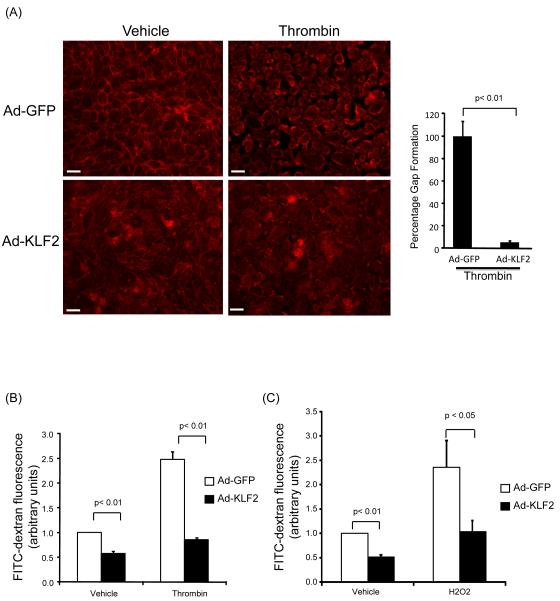
As discussed above, diverse inflammatory stimuli can induce intercellular gap formation by disrupting cell to cell adhesion proteins or by promoting cytoskeletal changes ultimately resulting in parcellular leak and an increase in endothelial leakage.7-12 In order to directly assess the role of KLF2 in regulating endothelial barrier function, we performed gain-of-function studies. A monolayer of HUVEC were infected with control (Ad-GFP) or KLF2 (Ad-KLF2) virus for 48 hours, challenged with thrombin (1U/ml), and then assessed for barrier function using three separate assessments: intercellular gap formation, FITC-Dextran passage, and change in transendothelial electrical resistance (TER). First, treatment with thrombin resulted in a dramatic intercellular gap formation in control virus treated HUVEC (Figure 2A). In contrast, HUVEC infected with KLF2 adenovirus were essentially resistant to intercellular gap formation. Next, we performed FITC-Dextran passage leakage assays using a transwell system. Stimulation of HUVEC with thrombin caused a significant increase in leakage as measured by the passage of FITC-dextran through the HUVEC monolayer (Figure 2B). However, in the presence of KLF2 overexpression, the passage of FITC-Dextran was markedly reduced. Similar findings were observed following treatment of HUVEC with hydrogen peroxide (Figure 2C) and histamine (data not shown). Finally, we analyzed TER change as a third assay for barrier integrity. Consistent with what we observed using the other two methods, KLF2 significantly attenuated thrombin and histamine-mediated reduction in TER (Supplemental Figures IA&IB). Collectively, these observations strongly implicate KLF2 as an important barrier protective factor in endothelial cells.
Figure 2.
Overexpression of KLF2 decreases endothelial leakage. (A) KLF2 overexpressed cells significantly attenuates thrombin mediated inter-endothelial gap formation when compared to Ad-GFP infected cells. Confluent HUVEC were infected with control (Ad-GFP) or KLF2 adenovirus for 48 hrs, exposed to thrombin (1U/ml for 15 minutes), and inter-endothelial gap formation assessed by actin staining. Percentage of exposed areas was quantified by NIH Image J software. The control group (Ad-GFP with thrombin) was normalized to 100%. A representative of three independent experiments is shown. Scale bar, 50 μm. (B) KLF2 decreases endothelial leakage when HUVEC were challenged with thrombin (1U/ml). HUVEC were infected as in (A), treated with thrombin (1U/ml), and leakage assay was performed to measure FITC-Dextran passage. N=6, p<0.01. (C) KLF2 decreases endothelial leakage when HUVEC were treated with 200uM of tert-butyl hydrogen peroxide. N=6, p<0.01.
KLF2 deficiency augments endothelial leakage increase
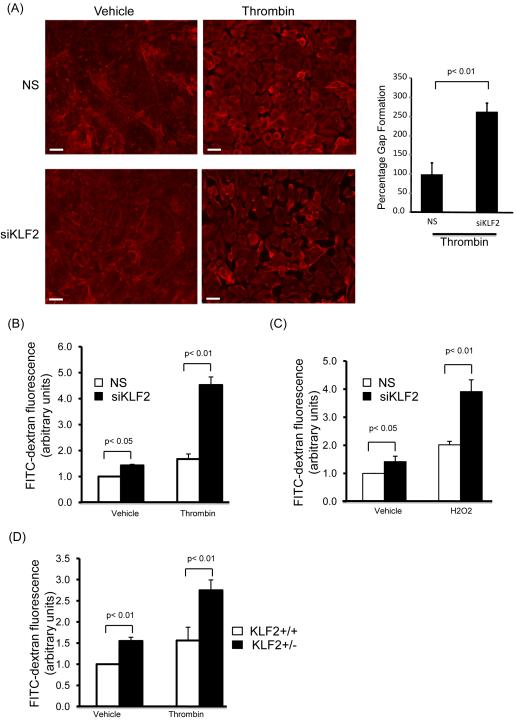
To further substantiate KLF2’s role in regulating endothelial barrier function, we carried out loss-of-function studies. First, we acutely knocked down KLF2 expression in HUVEC by siRNA mediated silencing and examined intercellular gap formation. HUVECs were transfected with non-specific (NS) or specific siRNA targeting for human KLF2 (siKLF2), treated with thrombin, and intercellular gap formation was assessed. Consistent with the overexpression studies above, upon stimulation with thrombin, KLF2 deficiency in HUVEC led to an enhancement of gap formation (Figure 3A). Next, we carried out endothelial leakage assays using HUVEC deficient in KLF2 as well as primary lung endothelial cells from KLF2 heterozygous mice. HUVEC were transfected with siKLF2 or NS siRNA for 48 hours, replated on the transwell, challenged with thrombin (or hydrogen peroxide) and assessed for FITC-Dextran passage. By comparison to NS group, knockdown of KLF2 augmented both basal and thrombin (or hydrogen peroxide) mediated increase of leakage (Figures 3B&C). We also examined the FITC-dextran passage using primary mouse lung microvascular endothelial cells. By comparison to primary lung endothelial cells from KLF2+/+ mice, KLF2+/− endothelial cells exhibited enhanced leak in response to thrombin (Figure 3D). Taken together, the results from the loss of function studies provided complementary evidence supporting the role of KLF2 as an endothelial barrier protector.
Figure 3.
KLF2 deficiency increases endothelial leakage. (A) KLF2 deficiency in HUVEC lead to an enhancement of inter-endothelial gap formation response to thrombin treatment. HUVEC were transfected with control siRNA and KLF2 siRNA, five days after transfection, cells were treated with thrombin (1U/ml) for gap formation assessment by actin staining. Percentage of exposed areas was quantified by NIH Image J software. The control group (NS with thrombin) was normalized to 100%. A representative of three independent experiments is shown. Scale bar, 50 μm. (B) Deficiency of KLF2 lead to increased endothelial leakage in response to thrombin. HUVEC were transfected with control siRNA and KLF2 siRNA for two days, then cells were replated onto transwell; permeability analysis was performed following treatment with thrombin. N=6, p<0.01. (C) KLF2 deficiency leads to increased endothelial leakage in response to hydrogen peroxide. HUVEC were transfected as above, and treated with hydrogen peroxide (200uM) followed by permeability assay. N=6, p<0.01. (D) Partial deficiency of KLF2 in primary ECs lead to a significant increase of endothelial leakage when treated with thrombin. Confluent lung microvascular endothelial cells from KLF2+/+ and KLF2+/− mice were treated with thrombin, then permeability assay performed. N=6, p<0.01.
KLF2 regulates key tight junction protein occludin in endothelial cells
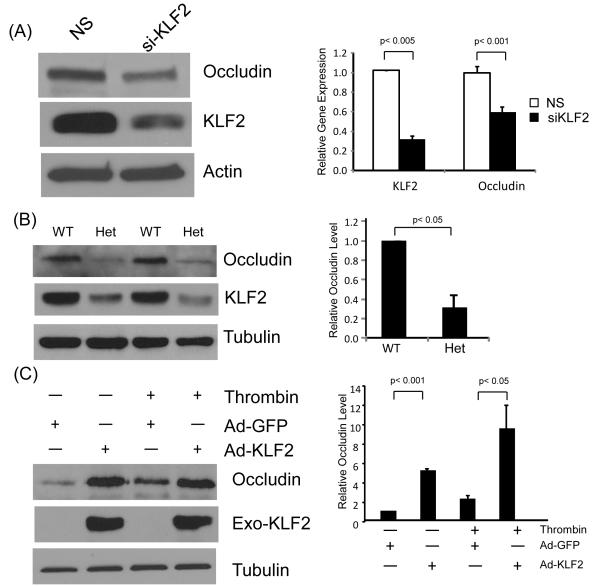
The observations from KLF2 overexpression and deficiency studies discussed above suggest that KLF2 regulates gap formation between endothelial cells. As noted earlier, intercellular junctions are composed of two main types of proteins - tight junctions and adherens junctions. To assess for effects on specific intercellular junction proteins, we performed gain and loss-of-function studies. Knockdown of KLF2 reduced one key tight junction protein, occludin (Figure 4A), however no significant effect was observed on zona-occludens -1 (ZO-1) and ZO-2 (data not shown). A similar reduction was seen in primary microvascular endothelial cells from KLF2+/− mice (Figure 4B). However, no significant effect was observed on the adherens proteins VE-cadherin and its associated actin-binding molecules α–, and, γ-catenin (data not shown). To determine if occludin is a direct target of KLF2, we performed overexpression studies. Forced expression of KLF2 potently induced the expression of occludin promoter (Supplemental Figure IIA), mRNA (Supplemental Figure IIB), and protein expression (Figure 4C). These results demonstrate that KLF2 is able to regulate one key tight junction protein occludin in ECs.
Figure 4.
KLF2 increases the expression of tight junction protein occludin. (A) siRNA-mediated KLF2 knockdown reduces occludin. HUVEC were transfected with non-specific siRNA and KLF2 siRNA for 48 hrs, and protein harvested for Western analysis. Representative blots of three independent experiments are shown. (B) Partial KLF2 deficiency in primary ECs results in decrease of occludin expression. Primary microvascular ECs were isolated from KLF2+/+ and KLF2+/− mice, extracted total protein were subjected to western blot. WT = KLF2+/+, Het = KLF2+/−. (C) Overexpression of KLF2 increases occludin expression. HUVEC were infected with Ad-GFP and KLF2 adenovirus for 24 hrs. Cells were treated with thrombin (1U/ml) for 15 and 30 minutes and proteins extracted for western blot analysis. Representative blots of three independent experiments are shown.
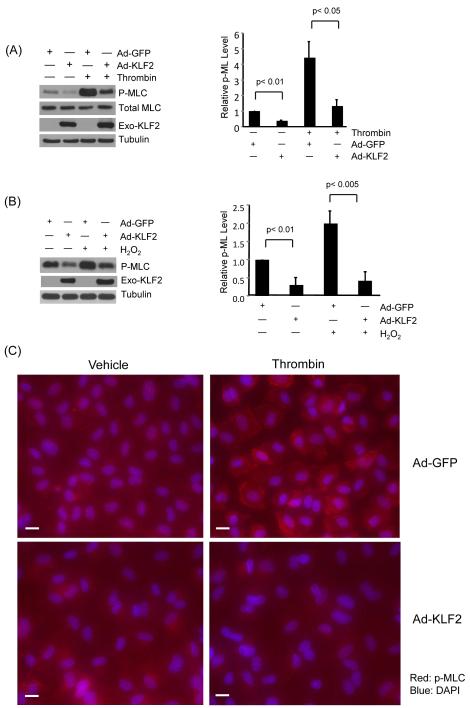
KLF2 inhibits phosphorylation of myosin light chain in endothelial cells
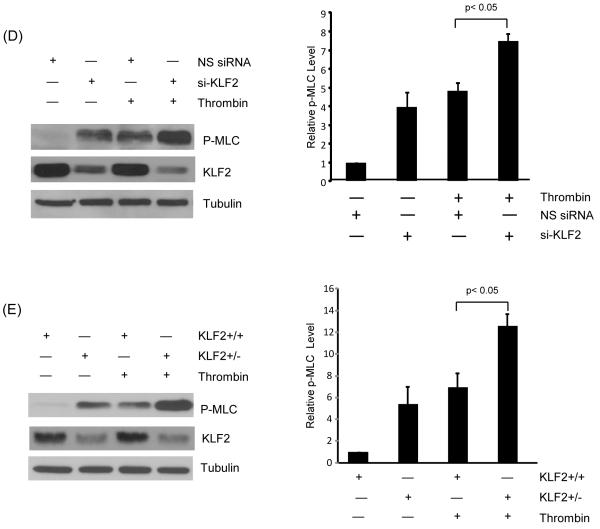
Most paracellular leak pathways induce actomyosin-based cell contractility, which augments intercellular gaps leading to increased leakage.12 Importantly, activation of paracellular leak by diverse stimuli all result in the phosphorylation of MLC, a key event in cell contraction.11, 27 In light of the observation that KLF2 prevents cellular contraction and gap formation following thrombin stimulation (Figure 2A), we hypothesized that KLF2 may alter MLC phosphorylation. HUVECs were infected with control (Ad-GFP) and Ad-KLF2 for 48 hours, stimulated with thrombin (1U/ml), and cell lysates assessed for phosphorylated MLC (serine 19 and threonine 18).11 As expected, following treatment with thrombin, a strong increase in MLC phosphorylation was observed (Figure 5A). Importantly, sustained expression of KLF2 strongly reduced the level of phospho-MLC (Figure 5A). A similar effect was observed with hydrogen peroxide treatment (Figure 5B). However, the expression of total MLC was not significantly altered by KLF2. We next investigated the spatially defined effects of KLF2 on MLC phosphorylation as assessed by p-MLC staining. Consistent with the western blot data, KLF2 overexpression markedly reduced phospho-MLC in endothelial cells (Figure 5C). Conversely, knockdown of KLF2 in HUVEC resulted in a hyperphosphorylation of MLC following thrombin treatment (Figure 5D). Concordant effects were seen in primary endothelial cells derived from wild type and KLF2 heterozygous mice (Figure 5E). Collectively, these studies clearly indicate that KLF2 regulates MLC phosphorylation.
Figure 5.
KLF2 inhibits phosphorylation of MLC in response to agonists. (A) HUVEC were infected with Ad-GFP and KLF2 adenovirus for 24 hrs, cells were then treated with thrombin (1U/ml) for 15 and 30 minutes, proteins were then extracted. Western blot analysis reveals attenuation of MLC phosphorylation in KLF2 overexpressed cells. Representative blot of three independent experiments is shown. (B) HUVEC were infected as above, followed by treatment with hydrogen peroxide for 30 minutes and 60 minute, total protein were isolated and subjected to western blot. Representative blots of three independent experiments are shown. (C) HUVEC infected with Ad-GFP and KLF2 adenovirus for 24 hrs were treated with thrombin (1U/ml) for 15 minutes, then immunostaining with phospho-MLC antibody was performed. Representative pictures of three independent experiments are shown. Scale bar, 50 μm. (D) HUVEC were transfected with non specific siRNA and siKLF2 using siPortAmine. After 48 hrs the cells were treated with thrombin (1U/ml) for 1 minute and total protein harvested using RIPA buffer. Western blot analysis revealed increased phosphorylation of MLC in siKLF2 transfected cells when compared to non specific siRNA transfected cells. (E) Primary lung microvascular endothelial cells were isolated from KLF2+/+ and KLF2+/− mice, they were then treated with mouse thrombin for 1 minute, proteins were extracted and western blot performed. A significant increase of MLC phosphorylation was observed in KLF2+/− ECs than that of KLF2+/+ ECs. A representative blot of three independent experiments is shown.
Discussion
This study is the first to implicate KLF2 as an essential regulator of vascular barrier function. This primary conclusion is supported by several key observations. First, hemizygous deficiency of KLF2 enhances vascular leak in response to an inflammatory stimulus. Second, in vitro gain and loss-of-function studies indicate that KLF2 regulates endothelial barrier function. Finally, mechanistic studies reveal that KLF2 differentially regulates expression of specific junction molecules and signaling molecules that mediate intercellular contact and cellular contraction. These findings build on previous observations and expand our understanding of KLF2’s role in vascular homeostasis.
Mediators such as thrombin, H2O2, and histamine activate very distinct mechanisms to exact their effects on endothelial barrier function. 5, 28,29,30,3, 31 The fact that KLF2 was able to ameliorate barrier function induced by such diverse stimuli was an important initial observation and suggested that its actions likely occur at some common convergence point. This line of reasoning led us to evaluate the two hallmark downstream events of paracellular leak – cellular contraction and interendothelial junctions. Indeed, our studies identify critical and specific roles for KLF2 in both processes.
A common downstream convergence point for diverse stimuli that induce endothelial barrier dysfunction (e.g. thrombin, histamine, and H2O2) is myosin light chain phosphorylation.3,28 This phosphorylation event leads to actin-myosin cross-bridge cycling, cellular contraction, and disruption of interendothelial junctions.12,27,32 Our studies indicate that KLF2 inhibits MLC-phosphorylation (Figure 5), an effect consistent with the observation that KLF2 prevents cell rounding and contraction (Figures 2&3). Of note, our observations regarding the effect of KLF2 on MLC phosphorylation is not unique to thrombin, as similar results were obtained when cells were challenged with histamine or hydrogen peroxide. The fact that KLF2 can block MLC phosphorylation and leakage in response to numerous stimuli render it an appealing therapeutic target given that multiple mediators contribute to vascular leak in most disease processes. We note that the precise molecular basis for how KLF2 alters MLC-phosphorylation remains incompletely understood. MLC can be phosphorylated by the endothelial form of MLC kinase (MLCK).11 This phosphorylation event can be further augmented by RhoA through its downstream effector Rho kinase (ROCK).33 Finally, KLF2 may alter expression and/or activity of MLC phosphatase (MLCP). Thus, additional studies are clearly needed to pinpoint the precise basis of KLF2 action.
Two major structures – tight junctions (TJs) and adherens junctions (AJs) – are critical in maintaining the endothelial barrier.1,2, 34 Our results indicate that KLF2 selectively affects TJs — an intriguing finding because, in contrast to AJs, our understanding of mechanisms regulating TJs is far less well understood.34 Endothelial TJs proteins such as occludin form homotypic interactions with neighboring cells.35,36 Occludin also requires co-expression of junctional proteins ZO-1 for cell surface expression and linkage to cortical actin.9 Our findings suggest that KLF2, by inducing the expression of occludin, may help coordinate TJ formation. These observations are likely to be important for specific vascular beds such as the blood-brain barrier and retinal microvasculature where TJs are abundant.37 We recognize that the role of occludin specifically and tight junctions in general has not been well established to participate significantly in microvascular barrier regulation. However our data in primary murine microvascular cells (Figure 3D and 4B) and the work of others38,35, 39, 40 demonstrate that occudin is present in microvascular endothelial cells and suggest that changes in occludin mass are associated with alterations in barrier function. Therefore, our data suggest that occludin might be important in the KLF2 effect on barrier function. Finally, while we did not observe significant effects on AJs, it should be noted that inhibition of actin-myosin cycling via effects on MLC-phosphorylation indirectly help maintain these junctions. As such, KLF2 effects on leakage likely result from direct and indirect effects on both the actomyosin complex and its intimate interaction with the interendothelial junction molecules.
There are several important limitations in our study. First, we have focused largely on the paracellular pathway of endothelial barrier function. This is principally because the preponderance of evidence suggests that paracellular leak is likely the dominant mechanism operative in most pathophysiologic states.1 However, we cannot exclude the possibility that KLF2’s effect may also be mediated, in part, via the transcellular pathway, which will be an interesting topic for future investigations. Second, because endothelial-specific deletion of KLF2 results in embryonic death,23-25 our in vivo barrier function studies were performed in KLF2+/− mice. While our in vitro studies in isolated KLF2+/− cells strongly support a critical role for KLF2 in the endothelium, one cannot exclude the possibility that other cell types (e.g. inflammatory cells) may contribute to the phenotype. Third, we note that several additional members of the KLF family are also expressed in endothelial cells.14 Amongst these, the work of Hamik et al. suggests that KLF4 may be particularly important in regulating key aspects of endothelial gene expression and function.41 Interestingly, KLF4 has been shown to regulate epithelial barrier function 42,43,44and thus future studies comparing and contrasting KLF2 and KLF4 function in endothelial barrier function are requisite.
Studies of the past decade strongly implicate KLF2 as a molecular switch that imparts an anti-inflammatory, anti-thrombotic, and anti-proliferative effects on endothelial cells. Remarkably, these conclusions were derived largely (if not exclusively) on studies in cultured endothelial cell lines. However, recent reports have begun to validate many of the predicted effects of KLF2 in vivo. Importantly, Atkins and colleagues recently reported that hemizygous deficiency of KLF2 augments experimental atherosclerosis.45 The observations provided in this study provide the first evidence that KLF2 plays an essential role in maintaining endothelial barrier integrity. These observations build on previous work and further substantiate the contention that KLF2 is an essential regulator of endothelial cell biology and vascular homeostasis. Finally, the increasing appreciation that KLF2 levels are induced by diverse pharmacologic agents (e.g. statins, resveratrol, and proteasome inhibitors) heightens enthusiasm that targeting of this factor may confer therapeutic benefit.46,47,48
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by NIH grants HL72952, HL75427, HL76754, HL086548, HL084154, and P01 HL48743 (to M.K.J.); HL087595 (to Z.L.); and HL088740 (to G.B.A.); HL097023 (to G.H.M); HL086668 (to J.F.); and a Robert Wood Johnson/Harold Amos Medical Faculty Development grant (to G.B.A.) and American Heart Association grants 0635579T (to Z.L.) and 0725297B (to D.K.).
Footnotes
Disclosure: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- 4.Siflinger-Birnboim A, Lum H, Del Vecchio PJ, Malik AB. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am J Physiol. 1996;270:L973–978. doi: 10.1152/ajplung.1996.270.6.L973. [DOI] [PubMed] [Google Scholar]
- 5.Moy AB, Blackwell K, Kamath A. Differential effects of histamine and thrombin on endothelial barrier function through actin-myosin tension. Am J Physiol Heart Circ Physiol. 2002;282:H21–29. doi: 10.1152/ajpheart.2002.282.1.H21. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 7.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- 8.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 9.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 10.Morel NM, Petruzzo PP, Hechtman HB, Shepro D. Inflammatory agonists that increase microvascular permeability in vivo stimulate cultured pulmonary microvessel endothelial cell contraction. Inflammation. 1990;14:571–583. doi: 10.1007/BF00914277. [DOI] [PubMed] [Google Scholar]
- 11.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moy AB, Van Engelenhoven J, Bodmer J, Kamath J, Keese C, Giaever I, Shasby S, Shasby DM. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J Clin Invest. 1996;97:1020–1027. doi: 10.1172/JCI118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieker JJ. Kruppel-like factors: three fingers in many pies. Journal of Biological Chemistry. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg MW, Lin Z, Fisch S, Jain MK. An emerging role for Kruppel-like factors in vascular biology. Trends Cardiovasc Med. 2004;14:241–246. doi: 10.1016/j.tcm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 16.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr., Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr., Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 18.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr., Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 20.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 21.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE., 3rd Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest. 2002;109:1057–1063. doi: 10.1172/JCI14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 28.Rivero-Vilches FJ, de Frutos S, Saura M, Rodriguez-Puyol D, Rodriguez-Puyol M. Differential relaxing responses to particulate or soluble guanylyl cyclase activation on endothelial cells: a mechanism dependent on PKG-I alpha activation by NO/cGMP. Am J Physiol Cell Physiol. 2003;285:C891–898. doi: 10.1152/ajpcell.00590.2002. [DOI] [PubMed] [Google Scholar]
- 29.Grand RJ, Turnell AS, Grabham PW. Cellular consequences of thrombin-receptor activation. Biochem J. 1996;313(Pt 2):353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol. 2000;279:C21–30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- 31.Clough GF, Bennett AR, Church MK. Effects of H1 antagonists on the cutaneous vascular response to histamine and bradykinin: a study using scanning laser Doppler imaging. Br J Dermatol. 1998;138:806–814. doi: 10.1046/j.1365-2133.1998.02217.x. [DOI] [PubMed] [Google Scholar]
- 32.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 33.Noda M, Yasuda-Fukazawa C, Moriishi K, Kato T, Okuda T, Kurokawa K, Takuwa Y. Involvement of rho in GTP gamma S-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Lett. 1995;367:246–250. doi: 10.1016/0014-5793(95)00573-r. [DOI] [PubMed] [Google Scholar]
- 34.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 35.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 36.Bazzoni G. Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost. 2006;95:36–42. [PubMed] [Google Scholar]
- 37.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 38.Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D’Amore PA. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4:e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110:58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280:H434–440. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- 41.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 42.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 43.Patel S, Xi ZF, Seo EY, McGaughey D, Segre JA. Klf4 and corticosteroids activate an overlapping set of transcriptional targets to accelerate in utero epidermal barrier acquisition. Proc Natl Acad Sci U S A. 2006;103:18668–18673. doi: 10.1073/pnas.0608658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaubert J, Cheng J, Segre JA. Ectopic expression of kruppel like factor 4 (Klf4) accelerates formation of the epidermal permeability barrier. Development. 2003;130:2767–2777. doi: 10.1242/dev.00477. [DOI] [PubMed] [Google Scholar]
- 45.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 47.Hiroi T, Deming CB, Zhao H, Hansen BS, Arkenbout EK, Myers TJ, McDevitt MA, Rade JJ. Proteasome inhibitors enhance endothelial thrombomodulin expression via induction of Kruppel-like transcription factors. Arterioscler Thromb Vasc Biol. 2009;29:1587–1593. doi: 10.1161/ATVBAHA.109.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gracia-Sancho J, Villarreal G, Jr., Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.