Abstract
Most prostate cancers (PCa) are critically reliant on functional androgen receptor (AR) signaling. At its onset, PCa is androgen-dependent and although temporarily halted by surgically or pharmacologically blocking the AR (androgen ablation), the disease ultimately recurs as an aggressive, fatal castration resistant prostate cancer (CRPC). FDA-approved treatments like docetaxel, a chemotherapeutic agent, and Provenge, a cancer vaccine, extend survival by a scant 3 and 4 months, respectively. It is clear that more effective drugs targeting CRPC are urgently needed. The ErbB family (EGFR/ErbB1, ErbB2/HER2/neu, ErbB3/HER3 and ErbB4/HER4) of receptor tyrosine kinases (RTKs) have long been implicated in PCa initiation and progression, but inhibitors of ErbB1 and ErbB2 (prototypic family members) fared poorly in PCa clinical trials. Recent research suggests that another family member ErbB3 abets emergence of the castration-resistant phenotype. Considerable efforts are being directed towards understanding ErbB3-mediated molecular mechanisms of castration resistance and searching for novel ways of inhibiting ErbB3 activity via rational drug design. Antibody-based therapy that prevents ligand binding to ErbB3 appears promising and fully-humanized antibodies that inhibit ligand-induced phosphorylation of ErbB3 are currently in early development. Small molecule tyrosine kinase inhibitors are also being vigorously pursued, as are siRNA-based approaches and combination treatment strategies- the simultaneous suppression of ErbB3 and its signaling partners or downstream effectors – with the primary purpose of undermining the resiliency of ErbB3-mediated signal transduction. This review summarizes the existing literature and reinforces the importance of ErbB3 as a therapeutic target in the clinical management of prostate cancer.
Keywords: ErbB3, Androgen Receptor, prostate cancer, castration resistance, EGFR, ErbB2, HER2, HER3, lapatinib, erlotinib, trastuzumab
1. INTRODUCTION
The prostate was first described in 1536 but prostate cancer (PCa) was not identified until 1853 [1]. At that time, it was considered a rare disease, likely due to shorter survival, since PCa does not affect men until they are older. At the present time, however, it is the most common type of cancer afflicting men in the Western world, with over 2 million currently living with the disease. It is the second leading cause of cancer death in American men after lung cancer. In 2010, at least 217,730 men were diagnosed with prostate cancer and 32,050 were expected to die from the disease (American Cancer Society – Facts and Figures, 2010).
The occurrence and progression of PCa have been linked to the age, race and family history of the patient. 65% of all PCa are diagnosed in men older than 65 [2]. African-American men are three times as likely as Caucasian men are to die from PCa, while Asian-American men are at the lowest risk of developing the disease [2, 3]. Men with a single first-degree relative with a history of prostate cancer are twice as likely to develop PCa, while those with two or more relatives are nearly four times as likely to be diagnosed. The risk increases if the affected family members were diagnosed at a young age and the most susceptible men are those whose family members were diagnosed before age 60.
Patients diagnosed with localized PCa undergo watchful waiting if they are at low-risk or undergo surgery or radiation therapy if they are considered high risk. Prostatectomy, or surgery to remove the prostate, is one of the most common treatments for localized prostate cancer [4]. Radiation therapy is also a common form of treatment for prostate cancer patients. External beam radiotherapy (EBT), co-administered with androgen-ablative treatment, results in improved relapse-free and survival rates and has become the standard-of-care for locally-advanced PCa. In recent years, brachytherapy has also become common in treating subsets of patients with localized PCa [5]. Seeds of radioactive material are implanted in the prostate gland and deliver radiation over a short distance, thereby minimizing damage to normal, non-cancerous tissues.
The majority of patients undergoing treatment for localized prostate cancer respond to these therapies. A small fraction of these patients (15~30%), however, experience tumor recurrence within 5 years following localized treatment, indicating the presence of disseminated disease. These patients are then treated by androgen withdrawal therapy (AW). In the early 1970s, Huggins and Hodges made the seminal observation that androgens played a key role in PCa development and that orchiectomy (removal of the testes) induced cancer regression [6]. Based on their observations, androgen withdrawal continues to be the therapeutic mainstay for disseminated PCa to date; although the majority of patients with metastatic PCa currently are treated with drugs that reduce testicular androgen production, rather than surgery to remove the testes [7].
Androgen withdrawal therapy (AW) is currently the primary, first line, and therapeutic intervention for recurrent prostate cancer [7]. Essentially, AW therapy blocks AR signaling and inhibits the receptor's transcriptional activity. Pharmacological ablation includes gonadotrophin-releasing hormone (GnRH) super-agonists luteinizing-hormone (LH)-releasing hormone (LHRH) analogues, which downregulate the GnRH receptor in pituitary gonadotropes, thus suppressing LH release and inhibiting testicular testosterone secretion [8]. Synthetic GnRH agonists include leuprolide (Lupron), goserelin (Zoladex), buserelin and nafarelin. GnRH antagonists, which inhibit hormone binding to the GnRH receptor, have also been developed as PCa treatments. Several of these antagonists, such as cetrorelix (Cetrotide), abarelix and orgalutran (Ganirelix) are as effective as GnRH agonists in lowering serum testosterone, without causing a testosterone flare associated with GnRH-agonist therapy [9].
The effect of the first line therapy, however, remains in the patient for only about 18-24 months on an average, after which they develop resistance to this therapy. Non-steroidal anti-androgens competitively inhibit the binding of DHT or testosterones to the AR. Examples within this category are flutamide, nilutamide and bicalutamide (Casodex). This method of treatment constitutes second line therapy and may be used upon failure of first line therapy, either alone, or together with LHRH modulators. Complete androgen blockade (CAB) combines an anti-androgen with a GnRH agonist [10]. This approach benefits about 25-35% of patients initially but does not confer any significant advantage in terms of survival for the majority of PCa sufferers.
Virtually all patients on AW or CAB eventually develop castration-resistant prostate cancer (CRPC) that is refractory to these treatments [7]. The current standard-of-care for CRPC is docetaxel-based chemotherapy, which offers a survival benefit of ~3 months [11], whereas the recently-FDA-approved PCa vaccine Sipuleucel-T (Provenge; Dendreon) extends patients’ lifespans by 4.1 months [12]. Hence neither treatment is permanently curative. Patients eventually succumb to the disease [7] and it is clear that more effective therapies are urgently required. A large number of clinical trials have been conducted to identify potential treatments that cure CRPC, but to no avail. Our laboratory has therefore taken the stand that it is more advantageous and feasible to prevent the progression of prostate cancer to CRPC than to cure CRPC after it has already developed. In this review, therefore, we will examine known causes for the development of CRPC and methods by which it could be prevented.
2. FACTORS AFFECTING THE DEVELOPMENT OF CASTRATION RESISTANCE
2.1. Cell Proliferation and Apoptosis in CRPC
In the normal prostate of a mature male, the rate of cellular proliferation (1–2% rate of growth) is balanced by the rate of apoptosis (1–2% per day). This is dependent upon an adequate supply of androgens which ensure that neither involution nor overgrowth of the glands occurs. In contrast, the cancerous prostate suffers from rampant cell growth and/or decreased apoptosis [13, 14]. As described above, PCa cells are initially dependent upon androgens for their sustenance and AW results in tumor regression. It was initially assumed that AW resulted in the apoptotic death of the majority of PCa cells, and that the few that remained were resistant and eventually returned as castration resistant tumors. The number of studies determining proliferation or apoptotic indices in human patients following AW treatment is limited since the majority of patients undergo prostatectomy prior to start of treatment. However in a few reported studies, the results differed widely. Some groups reported increased levels of apoptosis 3 months after AW [15-17], but other investigators found no increase in apoptotic indices in the majority of patients either shortly [18] or 3 months after AW [19]. The authors of the latter study observed that androgen-deprivation was not associated with degeneration or necrosis of neoplastic glands and surmised that AW ‘may be related more to suppression of tumor growth than to obliteration of tumor cells’. A similar concept had been put forth earlier [13], that both androgen-dependent and castration resistant human PCa tumors and cells altered their kinetic parameters (i.e., cell cycling status), rendering androgen ablative drugs utterly useless.
Attempts to test this hypothesis in animal models of prostate cancer have also yielded differing results. In the PC-82 and LuCaP xenograft models, increased apoptotic indices were observed following AW [20, 21], whereas in the Dunning R3327PAP rat model tumor growth and mitotic indices were reduced soon after AW but there were no signs of increased apoptosis and tumor cell numbers remained fairly constant throughout the study period [22, 23]. Earlier studies had determined that >80% of non-malignant rat ventral prostatic cells (taken from Sprague-Dawley or Copenhagen males) were lost within 10 days of castration [14, 24], and thus suggested that normal prostatic epithelial cell proliferation and death were differently controlled post-castration when compared to that in prostate tumors. Another study demonstrated that AW in mice bearing the androgen-dependent CWR22 human prostate tumor xenograft was associated with a decrease in the proliferative index [25], but cellular changes indicative of apoptosis were notably absent. The authors inferred that the tumor cells were growth-arrested in a G0/early G1 state. Later results from the same group corroborated that hypothesis, revealing that the emergence of a castrate-resistant phenotype was associated with release from cell cycle arrest [26].
2.2. AR Signaling and Molecular Mechanisms of Resistance in CRPC
PCa cells rely on the androgen receptor (AR) for proliferation and survival. The AR is activated by ligand-binding and nuclear translocation, dimerization of two AR molecules, and binding to specific androgen-responsive elements (AREs) of androgen-responsive genes and modulating their transcription [27]. The AR is expressed in the majority of prostate tumors, both before and after AW therapy, regardless of their hormone sensitivity [28]. High levels of phosphorylated AR are associated with aggressive clinicopathological features; while increases in AR mRNA and protein levels are necessary and sufficient for progression to CRPC. This in turn is dependent upon a functional AR DNA-binding domain, implying that AR activity and levels are the driving forces for CRPC [27, 28]. The prostate-specific antigen (PSA) gene is an androgen-responsive gene and PSA protein levels are detected in the majority of CRPC, indicating a functional AR-signaling pathway.
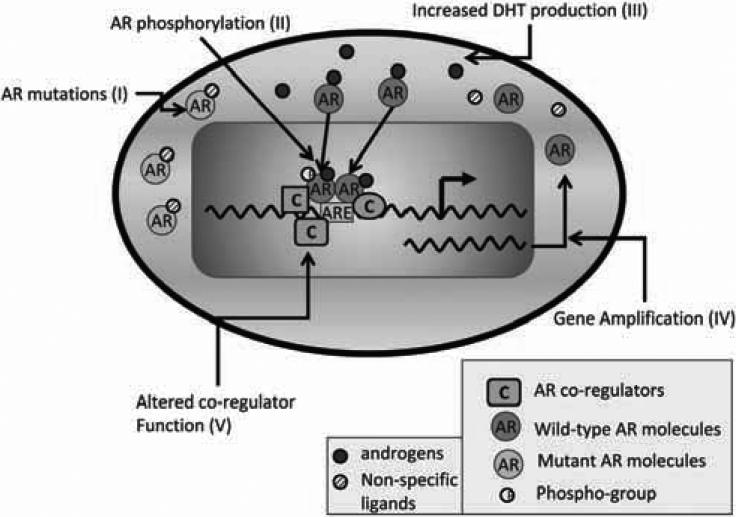
Various authors have concluded that there are multiple mechanisms responsible for castration resistance. Overall, there are five principal mechanisms which ultimately increase the AR's cell-growth-promoting functions (Fig. 1) (for a detailed review see [28] and references therein). (i) The androgen receptor is amplified in 25-30% of castrate resistant tumors. Increased AR levels result in increased sensitivity to residual low levels of androgens that are produced by the adrenal gland. (ii) Additionally, in some cases, there is evidence of enhanced rate of T (testosterone)→DHT (dihydrotestostereone) conversion by the enzyme 5α reductase. (iii) Further, the AR gene itself may be mutated, giving rise to a mutant protein which may be “promiscuous”, i.e. can be activated by other circulating steroid hormones (e.g. cortisol) and their metabolic by-products as well as by androgen antagonists like flutamide. These include expression of low molecular weight AR isoforms that are missing the ligand binding domain and are constitutively active allow for AR function in the absence of androgens. (iv) Co-regulator over-expression or co-repressor loss may also facilitate the conversion of anti-androgens into androgen agonists, or allow constitutive activation of the AR, despite the absence of significant levels of androgens in circulation. (v) Constitutive activation of the AR may also result from phosphorylation of the AR by various effectors which allow a configuration change in the AR, resulting in its enhanced transcriptional activity and transcription of target genes in CRPC cells at altered rates compared to castration sensitive cells. Further, altered co-repressor expression and binding and/or AR phosphorylation, also allows altered binding patterns of the AR in CRPC cells compared to its binding in castration sensitive cells [28].
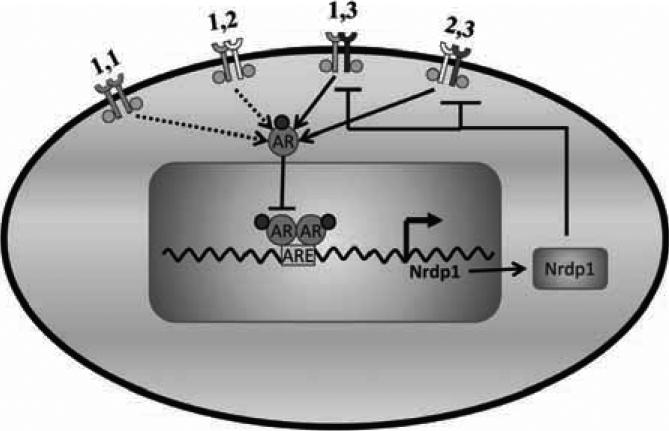
Fig. (1). Molecular mechanisms of castration resistance.
Normal prostate and ADPCa cells are dependent on ligand-driven AR activity for their growth and survival. The AR is activated by binding to its ligands, translocating to the nucleus, homodimer formation and binding to specific androgen-responsive elements (AREs) of androgen-responsive genes and modulating their transcription. On the other hand, CRPC cells activate mechanisms that enable their survival in an environment with castrate levels of androgen. These include (i) mutations in the AR, (ii) ligand-independent AR phosphorylation and activation, (iii) increased AR ligand production, (iv) AR gene amplification and (v) altered functions of AR co-regulatory proteins. Different shapes of the co-regulators (C) represent different types of coregulators that bind to the AR.
It is of interest to note that most castrate resistant PCa cells, nevertheless, are still androgen sensitive. Although these cells would not cease growth when treated with anti-androgens, they would proliferate at an enhanced rate when challenged by additional doses of androgens [29]. The expression of the AR, is also responsible for cell survival, and in multiple cases, it has been shown that loss of AR expression results in cell death, even in CRPC cells [30-32]. It is likely; therefore, that ligand-dependent AR transcriptional activity is mainly responsible for regulating cell cycle proliferation, while ligand-independent AR activity may additionally regulate cell survival. Hence, androgen withdrawal may result in cell cycle arrest but even in the absence of ligands, the AR may be activated by mechanisms that are independent of ligand binding, which keeps the cells alive. When alternate pathways that regulate cell cycle progression are activated in CRPC cells, this may result in a release from growth arrest and re-growth of the tumor.
2.3. Activation of Cell Signaling Pathways that Bypass AR Function in CRPC Cells
Studies from different laboratories indicate the existence of alternate pathways in CRPC cells that obviate the need for the AR in regulating the cell cycle pathways. Thus, the AR may be active and functional but cell survival may be regulated by parallel proliferation pathways, mediated, for example, by the serine/threonine kinase Akt [33]. Alternately the growth of the tumor may be facilitated by cancer stem or progenitor cells which do not express the AR but are selected by androgen-ablation therapy as the primary tumor cell type [34]. Alternately, the AR may be activated by a multitude of pathways that confer to it ligand-independent activation resulting in an ability to regulate cell survival, even in the absence of ligands. One of the major causes of re-activation of the cancer promoting pathways in cells that have undergone AW therapy is the phosphatidylinositol 3-kinase (PI3K) pathway. This pathway triggers a number of downstream targets such as Akt (reviewed by us earlier [35, 36]), which promotes cell survival pathways. The stimulation of these pathways prevents cell death during AW treatment [33, 37]. Since receptor tyrosine kinases (RTKs) of the ErbB family are known to turn on the PI3K pathway and regulate AR transcriptional activity in a ligand-independent manner, we will review in the following pages how ErbB receptors, regulate the progression to CRPC.
3. OVERVIEW OF ErbB RECEPTORS; STRUCTURE AND RECEPTOR ACTIVATION
The ErbB family consists of four closely related type 1 transmembrane tyrosine kinase receptors: the epidermal growth factor receptor (EGFR/HER1/ErbB1), ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4). Signaling by the ErbB family regulates many cellular activities important for cell survival and function including cell division, migration, adhesion, differentiation and apoptosis. EGFR and ErbB2 have been described in many excellent reviews [38, 39] and hence will be described here only briefly.
3.1. ErbB Receptors are Activated by Ligand Binding, Dimerization and Phosphorylation
The ErbB receptors are activated by mesen chymal ligands – including heregulins (HRG, human) and neuregulins (NRG, esp. mice) and other epidermal growth factor (EGF)-like ligands [40] (Fig. 2). The 4 ErbBs share an overall structure of two cysteine-rich domains in their extracellular region and an intracellular kinase domain, flanked by a carboxy-terminal tail with tyrosine autophosphorylation sites (Fig. 3). Although they have essentially the same domain structure, the functional activity of each varies. ErbB-1, -2 and -4 have active tyrosine kinase domains and ErbB-1, -3 and -4 possess known ligands. ErbB-2 has no known ligand but is constitutively available for dimerization [40]. ErbB-3 can bind several growth factors but until recently was thought to lack intrinsic tyrosine kinase ability (being devoid of the requisite ATP-binding amino acid residues). Recent work has disproved this notion and will be discussed later in this article.
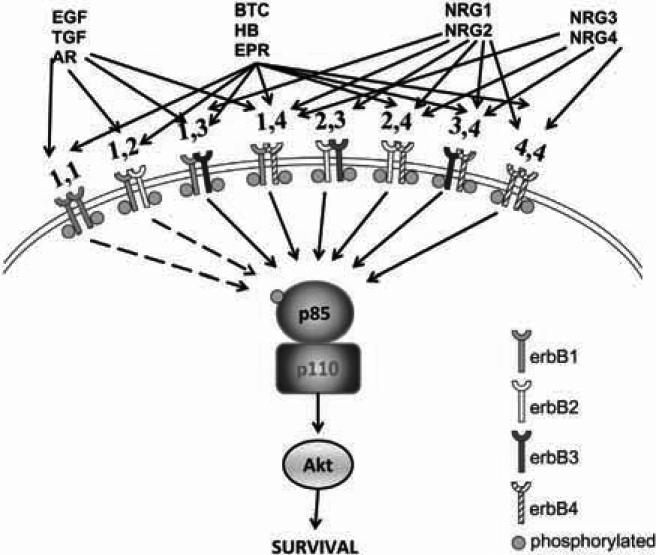
Fig. (2). ErbB family signaling.
Three groups of ligands bind to ErbB family receptors. EGF (epidermal growth factor), ARG (amphiregulin) and TGF-α (transforming growth factor alpha) bind to ErbB1; BTC (betacellulin), HB-EGF (heparin-binding EGF-like factor) and EPR (epiregulin) bind to ErbB1 and ErbB4; NRG-1 and NRG-2 (neuregulins 1, 2) bind to ErbB3 and ErbB4; NRG-3 and NRG-4 (neuregulins 3,4) bind only to ErbB4. Possible receptor pairings are shown (note that ErbB3 cannot homodimerize owing to its weak kinase activity and ErbB4 is absent in prostate cancer). ErbB dimers activate pro-survival pathways mediated by Akt (shown here) as well as other pathways not shown. ErbB3 is unique because it binds directly to PI3K which in turn associates directly with and activates Akt, which is directly known to stimulate cell survival.
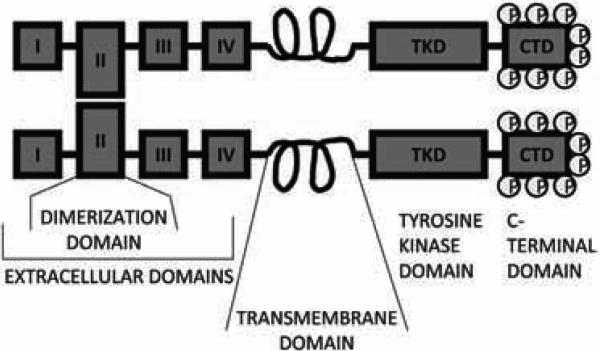
Fig. (3). Schematic of ErbB structure.
All members have a large extracellular ligand-binding region (consisting of subdomains I-IV), a single, small intracellular transmembrane-spanning region (which precedes the cytoplasmic tyrosine kinase domain) and a C-terminal tail, which houses the docking (ie phosphorylation) sites for phosphotyrosine-binding effector molecules. Subdomains I and III are leucine-rich repeats that function in ligand binding (also called L1 and L2), whereas subdomains II and IV are laminin-like, cysteine-rich domains (also called CR1 and CR2). The monomeric ErbB receptor is autoinhibited by the interaction of domain II with domain IV. This keeps subdomains I and III apart and prevents ligand binding by disrupting the ligand-binding pocket and burying the dimerization loop of domain II. Ligand binding relieves these inhibitory interactions and encourages dimerization by allowing the loop from domain II of one monomer to access the docking site on domain II of a second, ligand-bound monomer. The receptor dimer is thus stabilized, the kinase domain is activated and specific tyrosine residues within the cytoplasmic tail are phosphorylated. These phosphorylated residues serve as docking sites for a range of proteins and the subsequent activation of intracellular signalling pathways.
Receptor homo- or hetero-dimerization is imperative for ErbB function and signaling activity. ErbB receptors normally exist as inactive monomers with the homodimerization domains folded to prevent dimerization. Binding of a specific ligand induces a conformational change in the ErbB monomer and readies it for dimerization with a second, active ErbB monomer [40, 41]. The exception may be ErbB2, which is thought to be constitutively activated and readied for heterodimerization. Several different homodimer and heterodimer pairings are possible between the four receptors, with homodimers only weakly perpetuating signals compared to heterodimers (Fig. 2). This ligand-induced dimerization activates the intrinsic receptor tyrosine kinase activity and leads to trans-autophosphorylation of the monomeric partners [42]. Adapter proteins are recruited to these newly phosphorylated docking sites and a signaling cascade is initiated. It is important to note that ErbB2 and ErbB3 must heterodimerize with the other ErbBs if they are to transmit signals. ErbB2-containing heterodimers are the most potent complexes and the ErbB2-ErbB3 heterodimer is the most mitogenic and transforming of them all.
3.2. ErbB Function in Normal Tissue and in Tumorigenesis
The ErbB kinases are essential for development and tissue maintenance. Although these studies were conducted mostly in EGFR and ErbB2, it gives a broad overview of the functions of ErbB kinases in general. ErbB1 knockout mice die soon after birth, suffering defects in a large number of organs including skin, lung, the GI tract and the brain (reviewed in [43]). Basically, there is immature development in several epithelial organs. In normal mice, the ErbB2/ErbB4 heterodimer acts principally in the heart, whereas ErbB2/ErbB3 function is required for the development of the peripheral nervous system [43]. ErbB2 or ErbB3 knockout mice experience hypoplasia of the sympathetic ganglion chain, loss of cranial sensory ganglia and defective Schwann cell development, due to a loss of migratory ability of cells arising from the neural crest [44]. To circumvent the early lethality of ErbB2 knockout mice, conditional ErbB2 knockout mice have also been developed [45, 46]. Conditional knockdown of ErbB2 in various stages in the life of these mice demonstrated that lack of ErbB2 caused a development of cardiomyopathy, a lack of muscle spindles, defects in muscle regeneration, in effective neuromuscular synapses, abnormally thin myelin sheaths, movement abnormalities and a loss of motoneurons (reviewed in [43]). In the development of the mammary gland, the importance of ErbB1 in ductal growth and the contribution of ErbB2 and ErbB4 for lobulo-aveolar development and lactation has been demonstrated (reviewed in [47]). Based on these reports, it is fairly obvious that ErbB1 has major roles in epithelial cell development whereas ErbB2 plays an important role in cell migration and movement. While these receptors are essential in development, their malfunction later on in life may result in cancer development as well.
In the adult tissue, these receptors and their ligands are still present, but their function may be mainly to maintain the homeostasis of the organ. In cancer, on the other hand, the receptors are inappropriately activated resulting in increased proliferation, decreased survival and increased motility. Based on the existing literature to date, there are three main causes for the role of the ErbB receptors in tumorigenesis: (i) Increased receptor expression and/or gene amplification, (ii) increased ligand expression and (iii) activating mutation of the receptor. Increased expression of ErbB2 has been found to be a common cause for breast cancer [48]. ErbB2 overexpression in breast cancer is associated with poor prognosis, and resistance to hormonal therapy. ErbB2 overexpression has also been associated with metastasis in patients with breast and prostate cancer, especially to the bone [49]. On the other hand, the majority of tumors studied, not only those that are hormonally related, but also other solid tumors, do not exhibit any mutations in ErbB2, or for that matter, in ErbB3 or ErbB4. ErbB3 and ErbB4, when abnormally activated, is more likely to be due to increased availability of their ligands. The same is also true for ErbB1. In the normal prostate, the ligands for these receptors are produced in the stromal tissue, with receptor being expressed in the epithelial cells. In tumors, the epithelial cells themselves may start to produce the ligands, thereby maintaining the receptors in a constant state of activation. ErbB1 receptors, at least in some tumors, especially lung and head and neck, are also prone to mutations that keep these receptors in a constant state of activation [50, 51]. Comparison of the functions of EGFR and ErbB2 in normal development and in cancer indicates that these receptors continue to perform in cancer the tasks that they conducted in development, which is tissue generation and cell migration, expect that now these tasks are conducted to the detriment of the patient.
In prostate cancer, mutations of any of the erbB receptors have not been seen; however, a large number of studies indicate that EGFR (ErbB1) and ErbB2 (HER2) interact with the AR in the absence of AR ligand binding and stimulate cell survival. The AR was found to both regulate [52] and be regulated by ErbB1 and ErbB2 [53] in castration sensitive, but not in CRPC, human cell lines. In particular, AR expression was suppressed by the activation of ErbB1 [53]; while ectopic expression of ErbB2 was shown to stimulate ligand independent activation of the AR [54]. ErbB2 overexpression in an androgen dependent prostate cancer cell line enhanced AR activity and hormone-independent cell growth [55], whereas small interfering RNA (siRNA)–mediated ErbB2 knockdown impaired prostate cancer cell growth and AR activity [56]. Nevertheless, a large number of ErbB1 and ErbB2 inhibitors were identified which inhibited cell proliferation and survival and also prevented AR transcriptional activity (discussed below). Based on these reports, as well as the fact that ErbB2 regulated PI3K/Akt activation, which made them successful targets of therapy in a number of other solid cancers, ErbB1 and ErbB2 inhibitors were assumed to be the panacea that would kill prostate cancer cells, and prevent castrate resistant prostate cancer.
3.3. ErbB1 (EGFR) and ErbB2 (HER2) Inhibitors in Cancer Therapy
The ErbB family is an established therapeutic target for many human cancers. Anti-ErbB drugs include monoclonal antibodies (MAbs) that target the extracellular regions of the receptor (for example, Trastuzumab, which targets ErbB2), as well as small-molecule tyrosine kinase inhibitors (TKIs) that prevent signal transduction through the receptor's tyrosine kinase domain (for example, erlotinib, which targets ErbB1) (reviewed in [38]). ErbB1 and ErbB2 have been the major recipients of attention with much less consideration given to ErbB3 as a consequence of its impaired kinase activity and previously perceived subservient status compared to ErbB2, which was considered to be the “master positive regulator of the ErbB network” [57]. The anti-ErbB2 monoclonal antibody Trastuzumab was the first inhibitor of the ErbB family to be approved by the US Food and Drug Administration (FDA) in 1998 for the treatment of HER-2 positive breast cancer. Today it is in regular clinical use for the treatment of breast cancer alongside hormone-based therapy. The monoclonal antibody Cetuximab and the small molecule TKIs Gefitinib and Erlotinib target ErbB1 in several types of epithelial cancers and have also received regulatory approval – cetuximab (Erbitux) for metastatic colorectal cancer and squamous cell carcinoma of the head and neck, erlotinib (Tarceva) for metastatic pancreatic cancer and non-small-cell lung cancer (NSCLC) and gefitinib (Iressa) for advanced NSCLC [38]. A second-generation, irreversible, pan-ErbB inhibitor presently undergoing clinical trials in patients with advanced lung cancer is PF00299804 [58, 59]. This molecule is a potent inhibitor of ErbB1-activating mutations as well as the ErbB1 T790M resistance mutation both in vitro and in vivo. The drug also effectively inhibits wild-type ErbB2 and insertion ErbB2 mutations which are observed in the 20-30% of lung cancers that fail gefinitib or erlotinib therapy [58].
3.4. The Failure of ErbB1 and ErbB2 Inhibitors in Prostate Cancer
PCa cells express ErbB1, ErbB2, and ErbB3 receptors [60] so Trastuzumab, Gefitinib and Erlotinib were tested for single-agent therapeutic efficacy in clinical trials in patients with CRPC. No agent, however, displayed any meaningful activity in Phase II trials of men with PCa [61-65]. Preclinical studies had also used Pertuzumab (2C4) - a monoclonal antibody directed against ErbB2 but differed from Trastuzumab in that it prevented ErbB2 heterodimerization with other ErbB family members rather than obstructing ErbB2's ligand-binding domain [38]. Pertuzumab was used to inhibit the growth of CRPC xenografts, while Trastuzumab used in the same study showed minimal effectiveness in preventing CRPC xenograft growth [66].
In sharp contrast to the preclinical studies, phase II trials of Pertuzumab in patients with CRPC were wholly unsatisfactory - no patient achieved the primary endpoint of >50% decline in PSA [67]. The dual kinase inhibitor lapatinib fared somewhat better in phase II single-agent clinical trials, being fairly well-tolerated and resulting in stable disease for 12 weeks but evidencing no PSA responses [68]. These results challenged the significance of the ErbB1/ErbB2 axis in PCa.
3.5. ErbB3 Activation May Prevent ErbB1 and ErbB2 Inhibitors in PCa
It had been known that while ErbB kinase signals were required for optimal AR function at low levels of androgen, this signaling was mediated not by ErbB1 but by the heterodimerization of ErbB2 with ErbB3 [56]. Sergina et al. later demonstrated that ErbB3 was upregulated and provided compensatory signaling precisely in response to ErbB1/ErbB2-directed TKI treatment [69]. ErbB3 activity was characterized by increased membrane localization and phosphorylation. Indeed, ErbB3-directed siRNA duly restored the pro-apoptotic effects of TKIs [69]. These reports suggested that the failure of EGFR and ErbB2 inhibitors may be due to the activation of ErbB3 in these tumors.
Primary PCa cells frequently overexpress ErbB3, which is unaccompanied by increases in ErbB1 or ErbB2 protein [70]. In fact, a surge in the levels – and activation – of ErbB3 is seen when relatively small amounts of ErbB2 are present [71]. Recent work by Soler et al. demonstrates that ErbB3 is required for and promotes the invasive capacity of prostate epithelial cells [72]. It achieves this objective by ligand-specific transactivation with either ErbB1 or ErbB2. Castration resistant DU-145 PCa cells were reliant upon ErbB3 expression for optimal motility and clonogenicity in vitro and tumorigenicity in vivo in response to the NRG-1, EGF and fetal bovine serum [72]. Although MCF-7 breast cancer cells appeared to require ErbB3 as part of an autocrine response induced by EGF and FBS, the response of DU-145 prostate cancer cells to these stimuli, while requiring ErbB3, did not appear to involve autocrine stimulation of the receptor. In both cell types, clonogenicity and tumorigenicity were severely compromised after ErbB3 knockdown with siRNA [72].
ErbB3 has six binding sites for the p85 regulatory subunit of PI3K, as well as for activators of the Ras/mitogen activated protein kinase (MAPK) pathway, and ErbB3-mediated signaling may be responsible for oncogenic cell survival and the promotion of CRPC. As described earlier, AW results in cell cycle arrest whereas CRPC occurs because of release from that arrest. Recent work from our lab shows that in both castration sensitive and CRPC human PCa cell lines and xenografts, AW brought about a visible increase in the protein levels of ErbB3 [73]. This in turn augmented AR transcriptional activity and cell proliferation, signaling the reentry of growth-arrested tumor cells into an actively cycling state. Conversely, ErbB3 downregulation via siRNA suppressed cell viability and impeded CRPC growth [73]. These studies reveal the significant cross-talk between ErbB3 and the AR and indicate a mechanism by which cells may develop resistance to ErbB1 or ErbB2 inhibitors.
4. ErbB3 IN PROSTATE CANCER
4.1. Cellular Localization
The high expression of ErbB3 in certain human cancers suggested that it might be involved in tumor development and, if so, could be marked as a therapeutic target. The cancerous prostate, in comparison to its normal counterpart, overexpresses ErbB3 protein (by IHC visualization [73] and microarray analyses [70]), which indicate poor prognosis. A secreted isoform of ErbB3 – p45 sErbB3 - was found in PCa bone metastases, activated osteoblasts and new bone matrices but not in the epithelial cells of primary PCa [74]. This isoform stimulated the expression of osteonectin from bone cells which in turn enhanced the invasiveness of PCa cells [75]. It may be mentioned that a secreted, truncated form of ErbB3 – p85 sErbB3 - that acts as a negative regulator of ligand-stimulated ErbB-2, -3 and -4, was found to naturally occur in patients with metastatic breast cancer [76], but has not been studied in PCa patients.
Along with its plasma membranous and cytoplasmic locations, ErbB3, which has a nuclear localization sequence (NLS) near its C-terminal, has been observed in the nuclei of PCa tissues and cell lines. In human PCa tissues, nuclear levels of ErbB3 were low or absent in the benign prostate but increased as the cancer progressed to hormone resistance [77]. Surprisingly, in PCa cell lines, the trend was reversed, with nuclear ErbB3 levels being higher in hormone-sensitive rather than in CRPC cases [77]. As a result, the authors of that study initially associated nuclear ErbB3 staining with risk of disease progression, but in later work discovered that low nuclear localization of ErbB3 was a predictor of biochemical recurrence in patients with PCa and positive surgical margins after radical prostatectomy [78]. ErbB3 expression was also upregulated in the nuclei of PCa cells taken from lymph nodes and bone metastases of patients who had undergone AW therapy [79]. In subcutaneous xenograft tumors of MDA-PCa-2b and PC-3 cell lines, ErbB-3 was predominantly in the membrane/cytoplasm; however, it was present in the nuclei of the xenograft tumor cells implanted in the femur. Castration of mice bearing subcutaneous MDA PCa 2b tumors induced a transient nuclear translocation of ErbB-3, with relocalization to the membrane/cytoplasm upon tumor recurrence [79]. Based on these results, the authors speculate that nuclear localization of ErbB-3 may aid prostate cancer cell survival during androgen ablation and progression of prostate cancer in bone. Based on these results, one can conclude that nuclear localization of ErbB3 may reflect a response to cellular stress (in this case the blocking of AR signaling using an anti-androgen), regulation of RNA synthesis during growth arrest and release from nuclear sequestration in response to proliferation (i.e. when the anti-androgen is removed).
4.2. Ligand-Induced Activation of ErbB3
ErbB3 overexpression does not indicate its activation, since activation requires ligands, dimerization partners, the availability of phosphorylation sites and a variety of intracellular partners to enable signaling. In vitro studies suggest that overexpression of a normal receptor leads to transformation only when its appropriate ligand is present; therefore ErbB overexpression has to be accompanied by ligand upregulation (reviewed in [28]). For example, poor prognosis in CRPC directly correlates with overexpressed EGFR, ErbB2, and ErbB3 receptors (at mRNA and/or protein levels) and upregulation of ErbB ligands such as TGF-alpha, ARG, HB-EGF and EPG. mRNA levels for these ligands were increased 10-100 fold in CRPC as compared to castration sensitive PCa cells [80].
As mentioned earlier, the primary ligands for ErbB3 are members of the NRG family, a large group of isoforms possessing an EGF-like C-terminal and a variable N-terminal region [40]. NRG binding to ErbB3 is followed by ErbB3 heterodimerization, especially with ErbB2. ErbB3/ErbB2 dimerization is favored also by ErbB2 overexpression, which biases heterodimerization towards itself [40]. In the absence of ligand binding, ErbB3 exists in a self-associated, oligomeric, catalytically-inactive state, whereas NRG-bound ErbB3 undergoes a conformational change such that it is stabilized and it's extended form exposes the dimerization interface for interaction with ErbB2 [81] (Fig. 4). The extracellular domain of ErbB3 retains NRG-binding ability even at low acidic pH (owing to the absence of a critical, pH-sensitive histidine residue in domain III) indicating a mechanism of survival in the low pH tumor microenvironment [82]. Analysis of PCa cells reveals the existence of a paracrine loop involving NRG1 and the ErbB3-ErbB2 dimer [60]. The effects of ErbB3 activation by NRG likely depend upon the ratios of NRG isoforms present, their status as secreted (i.e. expressed but unprocessed or sequestered, hence inactive), and the relative amounts of other ErbB receptors.
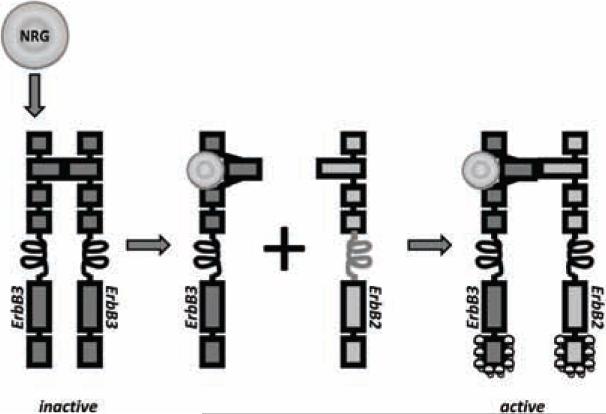
Fig. (4). NRG activation of ErbB3.
ErbB3 has a high affinity for NRG and this is greatly increased by dimerization with ERBB2. As with the other ErbBs, in the absence of ligand, a direct intramolecular interaction between domains II and IV keeps ErbB3 in a closed (locked or tethered) conformation that prevents interaction between domains I and III. This conformation disrupts the ligand-binding pocket and buries the dimerization arm of domain II. ErbB2 is inherently unable to dimerize because of a strong interaction between domains I and III which leads to a constitutively extended dimerization arm. ErbB2 is therefore constantly primed for interactions with ligand-bound receptors of the ErbB family. In the presence of NRG, the dimerization loop from domain II of ErbB3 extends to interact intramolecularly with a ligandless, primed ErbB2 monomer to form the oncogenic ErbB2-ErbB3 heterodimer.
NRG1 too is overexpressed in PCa and elicits different ErbB3/ErbB2 activation profiles depending upon the hormone-sensitivity of the cells [60]. For example, androgen-dependent LNCaP cells displayed ErbB3/ErbB2 activation, triggering several downstream cascades including PI3K in response to NRG addition [66]. In contrast, CRPC cell lines demonstrated highly variable outcomes – the AR-negative DU145 and PC-3 were unaffected by NRG, CWR22Rv1 demonstrated ErbB3/ErbB2 dimer formation and cell proliferation, and the recurrent PCa cell line CWR-R1 activated an autocrine pathway between NRG and low-level, constitutively-active ErbB3/ErbB2 that led to AR transactivation via the MAPK and PI3K/Akt routes [66, 83]. Significantly, the growth factors EGF and betacellulin, which are not canonical ErbB3 ligands (see Fig. 2), also showed increased binding to ErbB3 co-expressed with ErbB2 but other ErbB family ligands TGF-alpha, ARG and HB-EGF did not [84]. These reports indicate the ability of the cancerous cell to activate non-specific binding in ErbB3 although the mechanism of action in these cases is not fully known. Ligand-induced activation of ErbB3 is followed by physical association with other ErbB receptors (Fig. 4). It may be noted that ErbB4 expression is lost in most PCa patients, leaving only ErbB1 and ErbB2 available for heterodimerization with ErbB3 [60] (Fig. 5).
Fig. (5). AR controls ErbB3 levels via transcriptional control of the E3 ubiquitin ligase Nrdp1.
Activated AR enters the nucleus and binds to androgen response elements (ARE) in the Nrdp1 promoter region, initiating transcription of that molecule. Nrdp1 thus produced attaches ubiquitin to ErbB3 and marks it for proteasomal degradation, thereby regulating receptor levels. This regulation occurs in castration-sensitive PCa but is lost en route to castration-resistance. As a result, ErbB3 levels remain sufficiently high and continue to drive tumorigenic growth.
4.3. ErbB3 Phosphorylation and Downstream Signaling Partners
ErbB3 heterodimerization is followed by autophosphorylation on tyrosine residues and each receptor thus activates its partner (Fig. 4). Kinases other than ErbB family members can also phosphorylate ErbB3 and notable among these are Src and MET [85, 86]. Both kinases bind to ErbB3, increase its phosphorylation and enhance oncogenic signaling via the ErbB3/ErbB2 heterodimer. Additionally, ErbB3 is activated by the non-receptor Tec family tyrosine kinase Bmx/Etk [87]. In response to ligand stimulation, Bmx/Etk is activated by tyrosine phosphorylation downstream of Src and PI3K in PTEN-deficient PCa cells. Etk downregulation by siRNA markedly decreases PCa cell growth, implying potential validity as a therapeutic target. Other kinase activators of ErbB3 include CDK5 [88], the breast cancer associated BRK/PTK6 [89], transactivation by cellular stress and cytokines like TNF-alpha and Interferon-alpha [90, 91]. Janus tyrosine kinases JAK1 and TYK2 have also been implicated as ErbB3 interactors, though neither demonstrated physical association with ErbB3 [92]. The transphosphorylation events resulting from kinase activity create docking sites for adaptor protein binding. These phosphotyrosine binding proteins associate with the tail of each ErbB molecule after engagement into dimeric complexes and determine the specificity and potency of the ensuing intra-cellular signal.
An invariable target of activated ErbB3 heterodimeric complexes is the PI3K/AKT pathway. While ErbB1 and ErbB2 interact with and activate PI3K via adaptor proteins, ErbB3 possesses six binding sites for the p85 regulatory subunit of PI3K, enabling its direct activation [40]. Each of these p85 sites cooperatively contributed to ErbB3 signaling, as was demonstrated by sequential mutation and restoration. Indeed, ErbB3 seems to be the preferred partner when signaling occurs through the PI3K pathway [93]. Activated PI3K phosphorylates AKT which sets in motion the phosphorylation and activation of numerous downstream proteins, resulting in processes that represses apoptosis and promote survival. ErbB3/PI3K/AKT-induced survival and proliferation pathways have been implicated in numerous human cancers and AKT has been singled out for its regulation of CRPC cell proliferation by activating additional signal transduction pathways and stimulating ligand-independent AR activation [29, 35, 36]. Indeed, it has long been known that Akt phosphorylation increases during AW treatment of castration sensitive cells and remains high in CRPC, but for long, it was not known what factors contributed to this elevation. Our recent work implicates ErbB3 as a possible cause for the increase in Akt phosphorylation since ErbB3 also increased during AW and remained high in CRPC [73]. Therefore the increase in ErbB3 is likely a major cause for the inability of AW to induce cell death.
4.4. Interaction Between ErbB3 and the AR is Mediated by Ebp1
As mentioned in section 2.2, above, the AR is known to remain active in CRPC and continues to regulate signaling pathways that allow them to proliferate and differentiate. There is some evidence suggesting that ErbB3 may be responsible for this ligand-independent AR activation. It was observed that ErbB2/ErbB3 heterodimers, but not ErbB2/ErbB1 units, modulated AR transcriptional activity by stabilizing AR protein and enhancing binding to its cognate AREs [56]. Phosphorylated AR was correlated with activated ErbB3 in animal models and AR-mediated transactivation of reporter genes in human CWR-R1 PCa cells [83].
An intriguing mediator of AR-ErbB3 interaction is the ErbB3 binding protein-1 (Ebp1) [94]. First discovered in a yeast two-hybrid assay, it interacted with the first 15 amino acids of the juxtamembrane domain of unphosphorylated ErbB3, binding directly to ErbB3 only if that RTK was constitutively phosphorylated by PKC [95]. Ebp1 exists as two isoforms that differ in their abilities to bind ErbB3, localize intracellularly and affect cell survival and differentiation [96]. Ebp1 is also recognized as a nucleolar growth regulating factor and an inhibitor of eIF2α phosphorylation, an initiator of protein translation. Ebp1 is phosphorylated upon NRG stimulation, dissociates itself from ErbB3 and travels to the nucleus. There it interacts directly with the cell cycle regulator pRB, inhibiting transcription of E2F regulated genes by recruiting, among other factors, SIN3A and histone deacetylase (reviewed in [97]).
Ebp1 contains an LXXLL motif that allows it to interact with the AR. It is an AR corepressor which inhibits transcription from AR-responsive gene promoters, including transcription of the AR itself [98, 99]. Ebp1 mRNA and protein levels, therefore, decrease in PCa versus normal prostate tissue [100]. In vitro and in vivo data demonstrated that Ebp1 overexpression resulted in reduced incidence of LNCaP tumors and slower growth of remaining tumors while siRNA-mediated Ebp1 downregulation in LNCaP cells activated the AR despite absence of androgen [101]. Combined Ebp1 upregulation and cyclin D1 downregulation (Ebp1+/D1-) predicted PSA relapse, establishing Ebp1's correlation to PCa progression [102].
4.5. Regulation of ErbB3 Levels by the AR is Mediated by Nrdp1
Early work on the regulation of ErbB3 degradation by Nrdp1 was conducted in mammary tumor models and has only recently been applied to PCa. The proteasomal degradation of ErbB3 is regulated by the RING finger E3 ubiquitin ligase Nrdp1 (neuregulin receptor degradation protein 1), also known as RNF41 or FLRF. Like Ebp1, described above, Nrdp1 too was discovered as an ErbB3-interacting protein by yeast two-hybrid analyses and stimulated ErbB3 ubiquitination and degradation in a ligand-independent manner [103]. Thus it regulated the RTK's steady-state levels. Corepressor experiments indicated that Nrdp1 specifically bound to ErbB3 and ErbB4 but not to ErbB1 or ErbB2. The C-terminal domain (CTD) of Nrdp1 directly binds to ErbB3's cytoplasmic tail while the N-terminal RING finger domain is responsible for ErbB3 ubiquitination and turnover. Nrdp1 is itself highly labile, undergoing self-ubiquitination and proteasomal degradation via the deubiquitinating enzyme USP8 [104]. Both proteins – Nrdp1 and USP8 - thus contribute to the efficiency of ErbB3 downregulation by steering it away from the recycling pathway and towards the degradation route. Proteins th at target recep tors towards ligand-independent degradation potentially play a significant role in stifling tumor growth properties by suppressing receptor levels. In a transgenic murine model of ErbB2-induced mammary carcinogenesis, the ErbB2 transgene product is highly expressed in tumors but is scarcely detected in non-tumor tissue [105]. Similarly, ErbB3 protein is overexpressed only in tumors and not in uninvolved mammary tissues in these animals. This is not attributed to differences in transcript levels [105]. The same group reported the interesting observation that Nrdp1 protein was present in healthy mammary tissue from the ErbB2-transgenic mice but was completely lost in tumors [105], suggesting that Nrdp1 played the role of tumor suppressant by keeping ErbB3 levels – and signaling - in check.
Little however is known about the expression and function of Nrdp1 in PCa. Recent work from our lab has offered novel insight into one potential mechanism of Nrdp1-mediated CRPC development. We show that ErbB3 protein is negatively regulated by the AR in androgen dependent cells, but not in CRPC cells [73]. AW caused a sharp drop in AR protein levels and transcriptional activity, resulting in the growth arrest of castration sensitive cells. A simultaneous increase in ErbB3 levels was observed in the castration sensitive cells, persisting even after the cessation of AW treatment, which likely drove, at least partly, the eventual growth of the CRPC cells. Continued probe of the AR-ErbB3 relationship uncovered the involvement of Nrdp1, which was found to be under the positive transcriptional control of the AR in castration sensitive cells, and AR-mediated Nrdp1 expression resulted in the ubiquitination and degradation of ErbB3 in these cells. Significantly, CRPC cells, unlike castration sensitive ones, appeared to experience a proliferative advantage because the AR was no longer able to direct the transcription of Nrdp1 in CRPC. The differential regulation of ErbB receptors by the AR in castration sensitive, but not in CRPC cells have also been reported for EGFR and ErbB2 by two separate groups who demonstrated that the AR regulated and was regulated by ErbB1 and ErbB2 in castration sensitive, but not in CRPC, human cell lines [52, 53]. Steroid receptor control of the ErbB receptors likely indicates a mechanism by which the AR suppressed cell growth regulated by the ErbB receptors in castration sensitive cells, and loss of this control with PCa progression may be an important aspect of why and how castration resistance develops.
5. ErbB3 AND TKI RESISTANCE
It is apparent from the above discussion that ErbB3 is intimately involved in the transformative pathways that drive PCa from a castration sensitive to a castration resistant phenotype. Several experimental approaches are being developed using ErbB3 as a therapeutic target. Strategies to target this RTK can broadly be divided into two categories – targeting only the ErbB3 receptor or preventing the formation of ErbB2/ErbB3 oncogenic unit (see below). Among the classes of agents being developed, small molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (MAbs) have gone the farthest. The majority of small molecule TKIs interferes with ATP binding within the receptor's catalytic domain and obstructs trans-autophosphorylation whereas MAbs are raised such that they target the receptor's extracellular region and limit ligand binding. The exception is Pertuzumab which was developed to prevent the dimerization of ErbB2 with ErbB3 (discussed earlier). The end result is that ErbB signaling is inhibited. While we describe a myriad of methods, we note that not all of them have been applied specifically to a PCa model.
The principal signaling function of ErbB3 in cancers was thought to be its role as a binding partner of ErbB1 or ErbB2 and a scaffold for the recruitment of cytosolic signaling proteins. Targeting scaffold functions is difficult for currently available pharmaceutical technologies, and for a long time, ErbB3 lacked a specific inhibitor, particularly since ErbB3 was thought to lack kinase activity [106]. However, recent data from Shi et al. provide surprising evidence of ErbB3's ability to bind to ATP and promote autophosphorylation of the receptor's intracellular domain when clustered at a membrane surface [107]. While ErbB3's tyrosine kinase activity was ~1000-fold lower than that of ErbB1, this small amount of activity was clearly sufficient for the initial autophosphorylation steps. Full kinase activation – or activity that is 150-1000-fold greater – is required only for the receptor to phosphorylate downstream signaling or docking molecules [107]. The weakly-catalytic ErbB3 thus efficiently phosphorylates ErbB2 whose vastly superior kinase activity then takes up the task of phosphorylating downstream substrates, propagating the pro-survival signal in a rapid and robust manner. ErbB3 autophosphorylation in vitro is uninhibited by single inhibitors of ErbB1 or ErbB2, displaying the probable culpability of residual ErbB3 kinase signaling in promoting TKI resistance [107].
Despite the current finding of weak intrinsic kinase function in ErbB3, it is still difficult to target the function of this RTK because the overall role of the kinase function is relatively low-grade compared to its function in heterodimer formation and in scaffolding. To overcome this drawback, and yet recognizing the importance of ErbB3 in different cancers, pharmaceutical companies and other investigators have taken innovative approaches to inhibit this RTK. Below, we will discuss possible methods of inhibiting ErbB3 signaling, some intentional and some fortuitous (see Table 1).
Table 1.
List of ErbB Inhibitors Described in this Review
| Name of Drug | Class | Target | Current Status |
|---|---|---|---|
| MM-121 | Monoclonal humanized ErbB3 antibody | ligand-dependent ErbB3 activation | Phase II for triple-negative breast cancer, Phase I/II for advanced NSCLC, Phase I for gynaecological cancers |
| AMG-888 (U3-1287) | Monoclonal humanized ErbB3 antibody | ligand-induced phosphorylation of ErbB3 | Phase I for advanced NSCLC and advanced solid tumors |
| Canertinib (Cl-1033) | irreversible pan-ErbB TKI | ErbB tyrosine kinase domain | Phase II for refractory metastatic breast cancer and advanced NSCLC |
| MP-470 | pan-ErbB inhibitor (ErbB1, 2, 3) | ErbB phosphorylation | Phase I for advanced solid tumors |
| AZD8931 | reversible pan-ErbB inhibitor (ErbB1, 2, 3) | ErbB phosphorylation | Phase I for advanced solid tumors, Phase II for breast cancer |
| Trastuzumab (Herceptin) | monoclonal humanized ErbB2/ErbB3 antibody | ligand-dependent ErbB3 activation (prevents ErbB3/ErbB2 dimerisation) | FDA-approved for metastatic breast cancer |
| Erlotinib (Tarceva) | reversible ErbB 1 TKI | Prevents ATP binding to ErbB 1 TK domain | FDA-approved for metastatic NSCLC |
| Cetuximab (Erbitux) | monoclonal humanized ErbB1 antibody | ligand-dependent ErbB1 activation (prevents ErbB3/ErbB1 dimerisation) | FDA-approved for irinotecan-refractory colon cancer and advanced head-and-neck cancers |
| Lapatinib (Tykerb) | Dual TKI inhibitor (ErbB1, 2) | ErbB tyrosine kinase domain | FDA-approved for breast cancer (triple-positive) |
| PF00299804 | pan-ErbB inhibitor (ErbB1, 2, 4) | ErbB tyrosine kinase domain | Phase II for advanced NSCLC |
| Pertuzumab (Omnitarg/2C-4) | monoclonal humanized ErbB2 antibody | ligand-dependent ErbB2 activation (prevents ErbB3/ErbB2 dimerisation) | Phase II for advanced solid tumors |
| Gefitinib (Iressa) | reversible ErbB1 TKI | Prevents ATP binding to ErbB1 TK domain | FDA-approved for metastatic NSCLC |
5.1. Monoclonal Humanized Anti-ErbB3 Antibodies
ErbB3's signaling functions depend upon ligand binding to its extracellular domain and inhibitors are generated to disrupt this interaction. A recently-characterized, ErbB3-specific humanized antibody MM-121 blocked ligand-dependent ErbB3 activation induced by the ErbB1, ErbB2 or MET receptors [108]. This MAb was tested in a variety of human cancer cell lines and tumor xenograft models (lung, renal, gastric, breast and ovarian) and worked most efficiently in those cancers that overexpressed the ErbB3-specific ligand heregulin. The aggressive human prostate cancer cell line DU-145 also fell into this category, for it harbors a strongly-activating, ErbB3-heregulin autocrine loop. In contrast, the Ab fared poorly in cells with an amplified ErbB2 gene because their growth was likely driven by ligand-independent and not ligand-dependent mechanisms. MM-121 is currently in clinical development as a therapy against a variety of cancers [108].
Another ErbB3-targeted MAb is AMG-888 (U3-1287, NCT00730470) - in vitro studies showed that AMG-888 was able to inhibit the growth of multiple tumor cell lines (breast, lung, colorectal) that were resistant to other ErbB family inhibitors1. Additionally, AMG-888 demonstrated statistically significant growth inhibition of established xenograft tumors as a single agent and in combination with other ErbB family inhibitors. This fully-humanized MAb is currently in Phase I trials in patients with advanced solid tumors that have become refractory to standard therapy or for which no acceptable treatment currently exists. AMG-888 prevents ligand-induced phosphorylation of ErbB3, ErbB2, and downstream effector molecules including Akt, ERK1 and ERK2. In vivo studies show that colony formation in pancreatic cancer cells and tumor growth in pancreatic, non-small cell lung cancer, and colorectal xenograft models are both significantly decreased following treatment with this drug (see also [109]).
5.2. Dual- or Multi-ErbB Inhibitory Approach
It should be clear by now that the ErbB receptors cooperate with each other in driving signal transduction towards malignant transformation. The mutual interactions that exist between these receptors tend to compromise the success of drugs that target individual receptors in cancer treatment. Preclinical studies show that tumor cells can rescue themselves, in more ways than one, from the inhibitory effects of an agent directed toward one ErbB receptor. They may alter their activation ability by relying on the ligand for a different ErbB receptor [110], shifting their signaling profiles such that an untargeted receptor is made to drive cellular growth [69, 111] or co-opting an entirely different RTK into a pro-survival, heterotrimeric supercomplex [112]. In all cases, signaling is but temporarily halted, only to inevitably return stronger than before. On the other hand, both in vitro and in vivo models have shown that employing a dual- or multi-ErbB inhibitory approach demonstrates greater anti-tumor activity than agents targeting an individual ErbB receptor [113-117]. Strategies involve putting together two types of MAbs, combining TKIs with MAbs or administering single molecules that inhibit one or more ErbBs simultaneously (discussed later). In the case of ErbB3, MM-121 combined with the anti-ErbB1 MAb cetuximab led to prolonged RTK inhibition in a mouse lung cancer model when compared to MM-121 alone [108]. As an ErbB-targeted approach, the combination of a MAb and TKI uses two agents with different sites of action. For example, trastuzumab plus the dual ErbB1/ErbB2 inhibitor lapatinib given to patients with metastatic breast cancer increased progression-free survival rate [118]. Among the reasons proposed for their therapeutic synergy was the ability of lapatinib (but inability of trastuzumab) to bind to truncated ErbB2 [93], often overexpressed in metastatic breast cancer.
Multi-ErbB inhibitors are being pursued most vigorously and antagonize the actions of ErbB heterodimers or inhibit, at one time, more than one individual ErbB receptor. Implicit in the inhibition of the ErbB1/ErbB2 heterodimer is the notion that ErbB3 too will be deactivated for lack of available ErbB dimerisation partners, especially in diseases like PCa where the fourth member of this family, ErbB4, is lost [60] (Fig. 6). Of note is the fact that the newer pan-ErbB inhibitors also aim at directly disrupting ErbB3 activity.
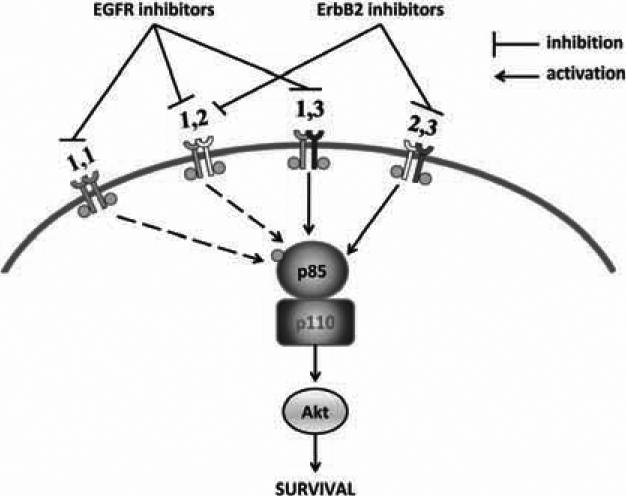
Fig. (6). Inhibition of ErbB3 signaling using a multi-receptor targeting approach.
The simultaneous inhibition of ErbB1 and ErbB2 in PCa will leave no dimerization partner for ErbB3 and halt its oncogenic signaling. The only possible ErbB dimers in PCa are EGFR homodimers and ErbB1-ErbB2, ErbB2-ErbB3, and ErbB1-ErbB3 heterodimers (see text). All these dimers would stimulate cell survival, for example, though the PI3K/Akt pathway (shown) as well as by other pathways (not shown). ErbB1 inhibitors would disrupt ErbB homodimers and ErbB1-ErbB2 and ErbB1-ErbB3 homodimers, but signaling would still continue through the ErbB2-ErbB3 heterodimers. Similarly, ErbB2 inhibitors would prevent signaling downstream of ErbB1-ErbB2 and ErbB2-ErbB3 heterodimers but allow signaling downstream of EGFR homodimers and ErbB1-ErbB3 heterodimers. However, dual inhibition of both EGFR and ErbB2 would inhibit all 4 dimers, thereby eliminating cell survival downstream of the ErbB receptors.
The first-generation, irreversible, pan-ErbB inhibitor canertinib (Cl-1033) inhibited TK activity of all the ErbB family members without affecting other RTKs (PDGFR, FGFR, IGFR) even when administered at high concentrations to a variety of human cancer cell lines, including PCa cell lines [119]. It is interesting to note that canertinib also induced G1 cell cycle arrest and apoptosis in an ErbB-independent manner in cell lines derived from human pre-myelocytes and histiocytic lymphomas [120]. While transcripts for all ErbBs were readily detected in these cell lines, protein expression was absent. This raises the possibility of canertinib exerting an off-target effect through an as-yet undetermined molecular mechanism, possibly involving the inhibition of mRNA translation of the ErbB receptors [120]. Canertinib is currently in Phase II clinical trials for the treatment of patients with advanced-stage non-small cell lung cancer (NSCLC) [121].
The pan-TKI MP470 was designed using a structure-based approach and inhibited cell proliferation in human castration resistant and CRPC cell lines [122]. When co-administered with erlotinib in the context of an LNCaP mouse xenograft model, the drugs not only completely abrogated ErbB1, ErbB2 and ErbB3 phosphorylation, but also prevented ErbB3 binding to PI3K and inhibited downstream Akt activity, even in androgen-depleted conditions. The safety and efficacy of the MP470-erlotinib combination is currently being evaluated in Phase 1 clinical trials for refractory solid tumors [122].
One of the most recently-documented pan-ErbB inhibitors is AstraZeneca's AZD8931 [123], shown to have activity as an equipotent TKI against ErbB1, ErbB2 and ErbB3 signaling in a variety of human head and neck, non-small-cell lung and breast cancer cell lines and murine xenograft models. The drug displayed greater inhibitory activity towards the ErbB3/ErbB2 oncodimer and was expected to be of particular use in solid tumors that did not contain amplified ErbB2 or mutated ErbB1 genes.
Another pan-ErbB inhibitor mentioned above is PF00299804, a potent inhibitor of EGFR-activating mutations as well as the EGFR T790M resistance mutation both in vitro and in vivo [58]. PF00299804 also inhibits both wild-type and gefitinib-resistant mutated ErbB2 identified in lung cancers [58]. Increased expression of ErbB3 was shown to induce resistance to PF00299804 [124]. This drug is an irreversible inhibitor of ErbB1 [58], which has been shown to inhibit the growth of various cell lines overexpressing ErbB3 [59].
One of the most successful pan-ErbB inhibitors have been lapatinib (GW275016) which has been mentioned throughout in this review. Tyrosine phosphorylation of ErbB2 and ErbB3, AR transactivation, and cell proliferation induced by heregulin were more potently inhibited by lapatinib than the EGFR-specific inhibitor gefitinib [83]. Basal proliferation in the absence of growth factors was also inhibited by lapatinib to a greater extent than gefitinib, suggesting that low level HER2/H ER3 activ ation perhaps by an autocrine pathway contributes to the proliferation signal [83, 125]. As mentioned earlier, a Phase II multicenter clinical trial to evaluate Lapatinib in early stage, hormonally untreated recurrent or metastatic prostate cancer was unsuccessful [68], but will be discussed further in the section below.
5.3. Effectiveness of Dual ErbB1/ErbB2 Inhibitors in Combination with AW Therapy
As mentioned earlier in this article, activation of the ErbB2/ErbB3 signaling cascade can lead to constitutive, ligand-independent activation of the AR and render PCa cells indifferent to AR inhibition [55, 56]. In fact, activation of the ErbB receptors, leading to stimulation of parallel signaling pathways that bypass the AR and regulate cell signaling and survival independent of the AR, is a major cause of the development of CRPC (see section 2.2). On the other hand, merely inhibiting ErbB2, or dual ErbB1/ErbB2 or even pan-ErbB inhibitors were insufficient to inhibit cell growth completely in patients with CRPC [67], given that this disease is associated with a large number of aberrations, many of which are associated with increased activation of the AR. Therefore, it is more reasonable to utilize the ErbB inhibitiors at an earlier stage in order to prevent the progression of the disease. Rather than apply these drugs to patients with CRPC, they may be better used in hormone-sensitive patients when combined with anti-androgens.
Indeed, applying an ErbB inhibitor alongside an AR inhibitor appears to be more efficacious, at least in initial studies. For example, in MDA PCa 2a prostate cancer cells, the AR antagonist hydroxyflutamide proved more efficacious when combined with cetuximab and trastuzumab [126]. Significantly, in androgen-dependent PCa cell lines, co-administration of gefitinib and bicalutamide resulted in concurrent inhibition of AR and ErbB1/ErbB2 pathways, causing a significant delay in the onset of ErbB-driven castration resistance [127]. The same principle has been suggested for PCa patients who have undergone radical prostatectomy and radiation therapy - lapatinib plus an anti-androgen appear to offer a better therapeutic option than lapatinib alone2.
The problem with anti-androgens is that the patients acquire resistant to this treatment fairly quickly. Acquisition of resistance employs multiple mechanisms including the failure of the drug to bind to its target. In that case, alternate mechanisms of action to decrease AR transcriptional activity are needed. Clinical resistance to TKI therapy is also associated with re-activation of PI3K signaling [69]. The combination of anti-ErbB/anti-PI3K therapeutics is effective in animal models and is undergoing extensive clinical testing [128]. There has been emphasis on the use of PI3K inhibitors in tumors that are resistant to the ErbB1 or ErbB2 inhibitors Erlotinib, Lapatinib, and Trastuzumab because the resurgence of PI3K signaling is largely due to the direct activation of upregulated ErbB3 [129-131].
6. CONCLUSIONS AND FUTURE DIRECTIONS
The preponderance of literature leads to the conclusion that CRPC arises because a few (or more) tumor cells survive first line AW therapy and then recur with an altered phenotype that no longer respond to this therapy. Hence, if the existing tumor cells are all eliminated completely, then the chances of the tumor recurring are reduced to a large extent, regardless of whether the tumor arises by alterations in existing tumor cells or whether cancer stem cells give rise to new tumors that are castration resistant. Activation of the PI3K pathway appears to be a major factor in the ability of the cells to survive, whether by apoptosis or by the triggering of autophagy. Therefore, disruption of the cell survival mechanism during AW seems to be a promising method by which CRPC can be prevented to a large extent.
Disruption of the PI3K/Akt pathway directly is of course possible, but Akt is such an important mechanism in the survival of all the cells in the body, that systemic inhibition of Akt phosphorylation is bound to have a tremendous impact on the survival of normal cells as well. Indeed, in Phase II clinical trials, the Akt inhibitor perifosine was shown to cause Grade 1-2 fatigue and gastrointestinal toxicities [132], and Grade 3 dose-limiting toxicities resulting in hyponatremia, arthritis, hyperuricemia, and photophobia [133]. Indeed, since the ErbB receptors are major activators of the PI3K/Akt pathway, it may be advantageous to inhibit the ErbB receptors directly. However, as has been shown above, inhibition of EGFR or ErbB2 individually did not seem to have a significant impact in clinical trials. We also offer proof that the failure of these single EGFR and ErbB2 inhibitors may result from the activation of ErbB3, and that dual inhibition of EGFR and ErbB2 may fare better, especially in patients undergoing AW therapy2. This observation is all the more significant because we have shown that AW therapy at the cellular level induces an increase in ErbB3 levels that may contribute to the induction of the CRPC phenotype [73].
Fig. (6) summarizes how the presence of ErbB3 prevents the effect of individual inhibitors of EGFR and ErbB2 on cell survival. Most prostate cancer cells do not express ErbB4 [60, 134], indeed, expression of ErbB4 appeared to disrupt the growth of prostate cancer cells [135, 136]. Therefore, the only possible ErbB dimers in PCa are EGFR homodimers and ErbB1-ErbB2, ErbB2-ErbB3, and ErbB1-ErbB3 heterodimers. Individual inhibition of EGFR using specific and selective inhibitors would disrupt the functioning of EGFR homodimers and ErbB1-ErbB2 and ErbB1-ErbB3 homodimers, but signaling would still continue through the ErbB2-ErbB3 heterodimers. Similarly, individual inhibition of ErbB2 would prevent signaling downstream of ErbB1-ErbB2 and ErbB2-ErbB3 heterodimers but allow signaling downstream of EGFR homodimers and ErbB1-ErbB3 heterodimers. However, dual inhibition of both EGFR and ErbB2 would inhibit all 4 dimers, thereby completely stopping the abnormal activation of downstream targets through the ErbB receptors.
Since it has become clear that ErbB3 occupies a prominent role in regulating cellular processes that promote CRPC future studies that explore in greater detail previously un-characterized aspects of ErbB3 biology are warrented. What roles do the truncated isoforms of ErbB3 play, given their opposing functions? Recent clinical findings indicate that p45 sErbB3 could be involved in the bone-forming pheno-type typical of bone metastases in PCa [137]. The novel ErbB3 isoform p85 sErbB3 may be an ideal candidate for cancer drug development, given its effectiveness at blocking HRG-induced cell growth [76]. What is the importance of ErbB3's nuclear and nucleolar localization? Recent work has revealed a vast array of interesting proteins - for example, Ras regulatory molecules and proteins involved in cell motility - that might bind to ErbB3 and promote ErbB3-mediated tumorigenesis [84]. The molecular basis of these interactions, as well as those involving ErbB3 regulation by the non-ErbB tyrosine kinases Src, MET and CDK5 (among others) remain unknown and merit further investigation. The widely-expressed Ebp1 has presented itself as a viable therapeutic target in CRPC and it would be interesting to learn of studies that advanced this premise. However, Fig. (6) also shows the limitations of single therapy using ErbB3 inhibitors. We conclude that ErbB3 inhibitors in combination with other related inhibitors may be of interest in the prevention of prostate cancer progression to CRPC.
ABBREVIATIONS
- AR
Androgen Receptor
- ARE
Androgen Response Element
- ARG
Ampiregulin
- AW
Androgen withdrawal
- BRK/PTK6
Breast tumour kinase/Tyrosine-protein kinase-6
- CAB
Complete androgen blockade
- CDK5
Cyclin-dependent kinase-5
- CRPC
Castration Resistant Prostate Cancer
- CTD
C-terminal domain
- DHT
Dihydrotestostereone
- Ebp1
ErbB3 binding protein-1
- EBT
External beam radiotherapy
- EGF
Epidermal Growth Factor
- EGFR
Epidermal Growth Factor Receptor
- eIF2α
Eukaryotic translation initiation factor 2
- EPG
Epiregulin
- ErbB
Erythroblastic Leukemia Viral Oncogene Homolog
- ERK
Extracellular signal-regulated kinase
- FDA
Food and Drug Admininstration
- FGFR
Fibroblast growth factor receptor
- FLRF
Fetal Liver Related Factor
- GnRH
Gonadotrophin-releasing hormone
- HB-EGF
Heparin-Binding Epidermal Growth Factor
- HER
Human Epidermal growth factor Receptor
- HRG
Heregulins
- IGFR
Insulin-like growth factor receptor
- IHC
Immunohistochemistry
- JAK
Janus kinase (“just another kinase”)
- LHRH
Luteinizing-hormone-releasing hormone
- MAb
Monoclonal antibody
- MAPK
Mitogen activated protein kinase
- MET
MNNG HOS Transforming gene
- NLS
Nuclear localization sequence
- Nrdp1
Neuregulin receptor degradation protein 1
- NRG
Neuregulin
- NSCLC
Non-small-cell lung cancer
- PCa
Prostate Cancer
- PDGFR
Platelet-derived growth factor receptor
- PI3K
Phosphatidylinositol 3-kinase
- PKC
Protein Kinase C
- pRB
Retinoblastoma gene product
- PSA
Prostate-specific antigen
- PTEN
Phosphatase with tensin homogy
- RING
Really interesting new gene
- RTK
Receptor tyrosine kinase
- siRNA
Small Interfering RNA
- TGF
Transforming Growth Factor
- TK
Tyrosine Kinase
- TKI
Tyrosine kinase inhibitor
- TNF
Tumor Necrosis Factor
- TYK2
Tyrosine kinase-2
Footnotes
Freeman, D., S. Ogbagabriel, M. Rothe, R. Radinsky, and M. Treder. Fully human anti-HER3 mAb U3-1287 (AMG 888) demonstrates unique in vitro and in vivo activities 309 versus other HER family inhibitors in NSCLC models. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research. 2008. San Diego, CA, USA.
Chen, Y., G. Wilding, J. Gee, R.P. DiPaola, M. Pins, M.A. Carducci, M.N. Stein, G. Bubley, and G. Liu; A phase II trial of lapatinib (GW572016) in patients with recurrent prostate cancer as evident by a rising PSA. J Clin Oncol, 2008. 26 (15), 5170-5170.
REFERENCES
- 1.Adams J. The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. Lancet. 1853;1(1):393–393. [Google Scholar]
- 2.Pisu M, Oliver JS, Kim YI, Elder K, Martin M, Richardson LC. Treatment for older prostate cancer patients: disparities in a southern state. Med. Care. 2010;48(10):915–922. doi: 10.1097/MLR.0b013e3181eb31a8. [DOI] [PubMed] [Google Scholar]
- 3.Williams H, Powell IJ. Epidemiology, pathology, and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol. Biol. 2009;472:439–453. doi: 10.1007/978-1-60327-492-0_21. [DOI] [PubMed] [Google Scholar]
- 4.Galvin DJ, Eastham JA. Critical appraisal of outcomes following open radical prostatectomy. Curr. Opin. Urol. 2009;19(3):297–302. doi: 10.1097/mou.0b013e328329eb13. [DOI] [PubMed] [Google Scholar]
- 5.Marcus DM, Jani AB, Godette K, Rossi PJ. A review of low-dose-rate prostate brachytherapy--techniques and outcomes. J. Natl. Med. Assoc. 2010;102(6):500–510. doi: 10.1016/s0027-9684(15)30559-9. [DOI] [PubMed] [Google Scholar]
- 6.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 7.Diaz M, Patterson SG. Management of androgen-independent prostate cancer. Cancer Control. 2004;11(6):364–373. doi: 10.1177/107327480401100604. [DOI] [PubMed] [Google Scholar]
- 8.Labrie F, Belanger A, Cusan L, Labrie C, Simard J, Luu-The V, Diamond P, Gomez J-L, Candas B. History of LHRH agonist and combination therapy in prostate cancer. Endocr. Relat. Cancer. 1996;3(3):243–278. [Google Scholar]
- 9.Thompson IM. Flare associated with LHRH-agonist therapy. Rev. Urol. 2001;3(Suppl. 3):S10–14. [PMC free article] [PubMed] [Google Scholar]
- 10.Akaza H. Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci. 2011;102(1):51–56. doi: 10.1111/j.1349-7006.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 11.Petrylak D. Therapeutic options in androgen-independent prostate cancer: building on docetaxel. BJU Int. 2005;96(Suppl. 2):41–46. doi: 10.1111/j.1464-410X.2005.05946.x. [DOI] [PubMed] [Google Scholar]
- 12.Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. Sipuleucel-T. Nat. Rev. Drug Discov. 2010;9(7):513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 13.Berges RR, Vukanovic J, Epstein JI, CarMichel M, Cisek L, Johnson DE, Veltri RW, Walsh PC, Isaacs JT. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin. Cancer Res. 1995;1(5):473–480. [PMC free article] [PubMed] [Google Scholar]
- 14.Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5(5):545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- 15.Armas OA, Aprikian AG, Melamed J, Cordon-Cardo C, Cohen DW, Erlandson R, Fair WR, Reuter VE. Clinical and pathobiological effects of neoadjuvant total androgen ablation therapy on clinically localized prostatic adenocarcinoma. Am. J. Surg. Pathol. 1994;18(10):979–991. doi: 10.1097/00000478-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima H, Goto T, Hosaka Y, Kitamura T, Kawabe K. Correlation between proliferation, apoptosis, and angiogenesis in prostate carcinoma and their relation to androgen ablation. Cancer. 1999;85(8):1822–1827. doi: 10.1002/(sici)1097-0142(19990415)85:8<1822::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Reuter VE. Pathological changes in benign and malignant prostatic tissue following androgen deprivation therapy. Urology. 1997;49(3A Suppl):16–22. doi: 10.1016/s0090-4295(97)00164-7. [DOI] [PubMed] [Google Scholar]
- 18.Westin P, Stattin P, Damber JE, Bergh A. Castration therapy rapidly induces apoptosis in a minority and decreases cell proliferation in a majority of human prostatic tumors. Am. J. Pathol. 1995;146(6):1368–1375. [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy WM, Soloway MS, Barrows GH. Pathologic changes associated with androgen deprivation therapy for prostate cancer. Cancer. 1991;68(4):821–828. doi: 10.1002/1097-0142(19910815)68:4<821::aid-cncr2820680426>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Bladou F, Vessella RL, Buhler KR, Ellis WJ, True LD, Lange PH. Cell proliferation and apoptosis during prostatic tumor xenograft involution and regrowth after castration. Int. J. Cancer. 1996;67(6):785–790. doi: 10.1002/(SICI)1097-0215(19960917)67:6<785::AID-IJC6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.van Weerden WM, van Kreuningen A, Elissen NM, Vermeij M, de Jong FH, van Steenbrugge GJ, Schröder FH. Castration-induced changes in morphology, androgen levels, and proliferative activity of human prostate cancer tissue grown in athymic nude mice. Prostate. 1993;23(2):149–164. doi: 10.1002/pros.2990230208. [DOI] [PubMed] [Google Scholar]
- 22.Brändström A, Westin P, Bergh A, Cajander S, Damber JE. Castration induces apoptosis in the ventral prostate but not in an androgen-sensitive prostatic adenocarcinoma in the rat. Cancer Res. 1994;54(13):3594–3601. [PubMed] [Google Scholar]
- 23.Westin P, Bergh A, Damber JE. Castration rapidly results in a major reduction in epithelial cell numbers in the rat prostate, but not in the highly differentiated Dunning R3327 prostatic adenocarcinoma. Prostate. 1993;22(1):65–74. doi: 10.1002/pros.2990220109. [DOI] [PubMed] [Google Scholar]
- 24.English HF, Kyprianou N, Isaacs JT. Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. Prostate. 1989;15(3):233–250. doi: 10.1002/pros.2990150304. [DOI] [PubMed] [Google Scholar]
- 25.Agus DB, Golde DW, Sgouros G, Ballangrud A, Cordon-Cardo C, Scher HI. Positron emission tomography of a human prostate cancer xenograft: association of changes in deoxyglucose accumulation with other measures of outcome following androgen withdrawal. Cancer Res. 1998;58(14):3009–3014. [PubMed] [Google Scholar]
- 26.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J. Natl. Cancer Inst. 1999;91(21):1869–1876. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 27.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int. 2005;95(9):1320–1326. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- 28.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh PM, Malik SN, Bedolla RG, Wang Y, Mikhailova M, Prihoda TJ, Troyer DA, Kreisberg JI. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr. Relat. Cancer. 2005;12(1):119–134. doi: 10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- 30.Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol. Cancer Ther. 2005;4(4):505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 31.Snoek R, Cheng H, Margiotti K, Wafa LA, Wong CA, Wong EC, Fazli L, Nelson CC, Gleave ME, Rennie PS. In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors. Clin. Cancer Res. 2009;15(1):39–47. doi: 10.1158/1078-0432.CCR-08-1726. [DOI] [PubMed] [Google Scholar]
- 32.Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66(21):10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 33.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60(24):6841–6845. [PubMed] [Google Scholar]
- 34.Lukacs RU, Lawson DA, Xin L, Zong Y, Garraway I, Goldstein AS, Memarzadeh S, Witte ON. Epithelial stem cells of the prostate and their role in cancer progression. Cold Spring Harb Symp. Quant. Biol. 2008;73:491–502. doi: 10.1101/sqb.2008.73.012. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh PM, Malik S, Bedolla R, Kreisberg JI. Akt in prostate cancer: possible role in androgen-independence. Curr. Drug Metab. 2003;4(6):487–496. doi: 10.2174/1389200033489226. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr. Cancer Drug Targets. 2007;7(6):591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 37.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by Mammalian target of rapamycin. Cancer Res. 2005;65(7):2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 38.Gross ME, Jo S, Agus DB. Update on HER-kinase-directed therapy in prostate cancer. Clin. Adv. Hematol. Oncol. 2004;2(1):53–56. 64. [PubMed] [Google Scholar]
- 39.Lorenzo GD, Bianco R, Tortora G, Ciardiello F. Involvement of growth factor receptors of the epidermal growth factor receptor family in prostate cancer development and progression to androgen independence. Clin. Prost Cancer. 2003;2(1):50–57. doi: 10.3816/cgc.2003.n.013. [DOI] [PubMed] [Google Scholar]
- 40.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO. J. 2000;19(13):3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 42.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 43.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu. Rev. Pharmacol. Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 44.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389(6652):725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 45.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr., Chien KR, Lee KFS. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002;8(5):459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 46.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2002;99(13):8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern DF. ErbBs in mammary development. Exp. Cell Res. 2003;284(1):89–98. doi: 10.1016/s0014-4827(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 48.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16(6):413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 49.Lu X, Kang Y. Epidermal growth factor signalling and bone metastasis. Br. J. Cancer. 2010;102(3):457–461. doi: 10.1038/sj.bjc.6605490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bronte G, Rizzo S, La Paglia L, Adamo V, Siragusa S, Ficorella C, Santini D, Bazan V, Colucci G, Gebbia N, Russo A. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat. Rev. 2010;36(Suppl. 3):S21–29. doi: 10.1016/S0305-7372(10)70016-5. [DOI] [PubMed] [Google Scholar]
- 51.Laurent-Puig P, Lievre A, Blons H. Mutations and response to epidermal growth factor receptor inhibitors. Clin. Cancer Res. 2009;15(4):1133–1139. doi: 10.1158/1078-0432.CCR-08-0905. [DOI] [PubMed] [Google Scholar]
- 52.Pignon J-C, Koopmansch B, Nolens G, Delacroix L, Waltregny D, Winkler R. Androgen receptor controls EGFR and ERBB2 gene expression at different levels in prostate cancer cell lines. Cancer Res. 2009;69(7):2941–2949. doi: 10.1158/0008-5472.CAN-08-3760. [DOI] [PubMed] [Google Scholar]
- 53.Cai C, Portnoy DC, Wang H, Jiang X, Chen S, Balk SP. Androgen receptor expression in prostate cancer cells is suppressed by activation of epidermal growth factor receptor and ErbB2. Cancer Res. 2009;69(12):5202–5209. doi: 10.1158/0008-5472.CAN-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Lorenzo G, Autorino R, De Laurentiis M, Cindolo L, D'Armiento M, Bianco AR, De Placido S. HER-2/neu receptor in prostate cancer development and progression to androgen independence. Tumori. 2004;90(2):163–170. doi: 10.1177/030089160409000201. [DOI] [PubMed] [Google Scholar]
- 55.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 1999;5(3):280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 56.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6(5):517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 57.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 58.Engelman JA, Zejnullahu K, Gale C-M, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong K-K, Jänne PA. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 59.Gonzales AJ, Hook KE, Althaus IW, Ellis PA, Trachet E, Delaney AM, Harvey PJ, Ellis TA, Amato DM, Nelson JM, Fry DW, Zhu T, Loi C-M, Fakhoury SA, Schlosser KM, Sexton KE, Winters RT, Reed JE, Bridges AJ, Lettiere DJ, Baker DA, Yang J, Lee HT, Tecle H, Vincent PW. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol. Cancer Ther. 2008;7(7):1880–1889. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 60.Grasso AW, Wen D, Miller CM, Rhim JS, Pretlow TG, Kung HJ. ErbB kinases and NDF signaling in human prostate cancer cells. Oncogene. 1997;15(22):2705–2716. doi: 10.1038/sj.onc.1201447. [DOI] [PubMed] [Google Scholar]
- 61.Salzberg M, Rochlitz C, Morant R, Thalmann G, Pedrazzini A, Roggero E, Schonenberger A, Knuth A, Borner M. An open-label, noncomparative phase II trial to evaluate the efficacy and safety of docetaxel in combination with gefitinib in patients with hormone-refractory metastatic prostate cancer. Onkologie. 2007;30(7):355–360. doi: 10.1159/000102452. [DOI] [PubMed] [Google Scholar]
- 62.Gross M, Higano C, Pantuck A, Castellanos O, Green E, Nguyen K, Agus DB. A phase II trial of docetaxel and erlotinib as first-line therapy for elderly patients with androgen-independent prostate cancer. BMC Cancer. 2007;7:142. doi: 10.1186/1471-2407-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris MJ, Reuter VE, Kelly WK, Slovin SF, Kenneson K, Verbel D, Osman I, Scher HI. HER-2 profiling and targeting in prostate carcinoma. Cancer. 2002;94(4):980–986. [PubMed] [Google Scholar]
- 64.Ziada A, Barqawi A, Glode LM, Varella-Garcia M, Crighton F, Majeski S, Rosenblum M, Kane M, Chen L, Crawford ED. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate. 2004;60(4):332–337. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 65.Lara PN, Jr., Chee KG, Longmate J, Ruel C, Meyers FJ, Gray CR, Edwards RG, Gumerlock PH, Twardowski P, Doroshow JH, Gandara DR. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer. 2004;100(10):2125–2131. doi: 10.1002/cncr.20228. [DOI] [PubMed] [Google Scholar]
- 66.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 67.de Bono JS, Bellmunt J, Attard G, Droz JP, Miller K, Flechon A, Sternberg C, Parker C, Zugmaier G, Hersberger-Gimenez V, Cockey L, Mason M, Graham J. Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-Refractory prostate cancer. J. Clin. Oncol. 2007;25(3):257–262. doi: 10.1200/JCO.2006.07.0888. [DOI] [PubMed] [Google Scholar]
- 68.Sridhar SS, Hotte SJ, Chin JL, Hudes GR, Gregg R, Trachtenberg J, Wang L, Tran-Thanh D, Pham N-A, Tsao M-S, Hedley D, Dancey JE, Moore MJ. A Multicenter phase II clinical trial of lapatinib (GW572016) in hormonally untreated advanced prostate cancer. Am. J. Clin. Oncol. 2009:1–1. doi: 10.1097/COC.0b013e3181beac33. [DOI] [PubMed] [Google Scholar]
- 69.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445(7126):437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lozano JJ, Soler M, Bermudo R, Abia D, Fernandez PL, Thomson TM, Ortiz AR. Dual activation of pathways regulated by steroid receptors and peptide growth factors in primary prostate cancer revealed by factor analysis of microarray data. BMC Genomics. 2005;6:109–109. doi: 10.1186/1471-2164-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith BL, Chin D, Maltzman W, Crosby K, Hortobagyi GN, Bacus SS. The efficacy of Herceptin therapies is influenced by the expression of other erbB receptors, their ligands and the activation of downstream signalling proteins. Br. J. Cancer. 2004;91(6):1190–1194. doi: 10.1038/sj.bjc.6602090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soler M, Mancini F, Meca-Cortés O, Sánchez-Cid L, Rubio N, López-Fernández S, Lozano JJ, Blanco J, Fernández PL, Thomson TM. HER3 is required for the maintenance of neuregulin-dependent and -independent attributes of malignant progression in prostate cancer cells. Int. J. Cancer. 2009;125(11):2565–2575. doi: 10.1002/ijc.24651. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, Siddiqui S, Bose S, Mooso B, Asuncion A, Bedolla RG, Vinall R, Tepper CG, Gandour-Edwards R, Shi X, Lu X-H, Siddiqui J, Chinnaiyan AM, Mehra R, deVere White R, Carraway KL, Ghosh PM. Nrdp1-mediated regulation of ErbB3 expression by the androgen receptor in androgen-dependent but not castrate-resistant prostate cancer cells. Cancer Res. 2010;70(14):5994–6003. doi: 10.1158/0008-5472.CAN-09-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vakar-Lopez F, Cheng C-J, Kim J, Shi GG, Troncoso P, Tu S-M, Yu-Lee L-Y, Lin S-H. Up-regulation of MDA-BF-1, a secreted isoform of ErbB3, in metastatic prostate cancer cells and activated osteoblasts in bone marrow. J. Pathol. 2004;203(2):688–695. doi: 10.1002/path.1568. [DOI] [PubMed] [Google Scholar]
- 75.Chen N, Ye X-C, Chu K, Navone NM, Sage EH, Yu-Lee L-Y, Logothetis CJ, Lin S-H. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 2007;67(14):6544–6548. doi: 10.1158/0008-5472.CAN-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee H, Akita RW, Sliwkowski MX, Maihle NJ. A Naturally Occurring Secreted Human ErbB3 Receptor Isoform Inhibits Heregulin-stimulated Activation of ErbB2, ErbB3, and ErbB4. Cancer Res. 2001;61(11):4467–4473. [PubMed] [Google Scholar]
- 77.Koumakpayi IH, Diallo J-S, Le Page C, Lessard L, Gleave M, Bégin LR, Mes-Masson A-M, Saad F. Expression and nuclear localization of ErbB3 in prostate cancer. Clin. Cancer Res. 2006;12(9):2730–2737. doi: 10.1158/1078-0432.CCR-05-2242. [DOI] [PubMed] [Google Scholar]
- 78.Koumakpayi IH, Diallo J-S, Le Page C, Lessard L, Filali-Mouhim A, Bégin LR, Mes-Masson A-M, Saad F. Low nuclear ErbB3 predicts biochemical recurrence in patients with prostate cancer. BJU Int. 2007;100(2):303–309. doi: 10.1111/j.1464-410X.2007.06992.x. [DOI] [PubMed] [Google Scholar]
- 79.Cheng C-J, Ye X-C, Vakar-Lopez F, Kim J, Tu S-M, Chen D-T, Navone NM, Yu-Lee L-Y, Lin S-H, Hu MCT. Bone microenvironment and androgen status modulate subcellular localization of ErbB3 in prostate cancer cells. Mol. Cancer Res. 2007;5(7):675–684. doi: 10.1158/1541-7786.MCR-06-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tørring N, Jørgensen PE, Sørensen BS, Nexø E. Increased expression of heparin binding EGF (HB-EGF), amphiregulin, TGF alpha and epiregulin in androgen-independent prostate cancer cell lines. Anticancer Res. 2000;20(1A):91–95. [PubMed] [Google Scholar]
- 81.Kani K, Warren CM, Kaddis CS, Loo JA, Landgraf R. Oligomers of ERBB3 have two distinct interfaces that differ in their sensitivity to disruption by heregulin. J. Biol. Chem. 2005;280(9):8238–8247. doi: 10.1074/jbc.M410944200. [DOI] [PubMed] [Google Scholar]
- 82.Kani K, Park E, Landgraf R. The extracellular domains of ErbB3 retain high ligand binding affinity at endosome pH and in the locked conformation. Biochemistry. 2005;44(48):15842–15857. doi: 10.1021/bi0515220. [DOI] [PubMed] [Google Scholar]
- 83.Gregory CW, Whang YE, McCall W, Fei X, Liu Y, Ponguta LA, French FS, Wilson EM, Earp HS. Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin. Cancer Res. 2005;11(5):1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- 84.Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447(2-3):227–231. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 85.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale C-M, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 86.Ishizawar RC, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene. 2007;26(24):3503–3510. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- 87.Jiang X, Borgesi RA, McKnight NC, Kaur R, Carpenter CL, Balk SP. Activation of nonreceptor tyrosine kinase Bmx/Etk mediated by phosphoinositide 3-kinase, epidermal growth factor receptor, and ErbB3 in prostate cancer cells. J. Biol. Chem. 2007;282(45):32689–32698. doi: 10.1074/jbc.M703412200. [DOI] [PubMed] [Google Scholar]
- 88.Fu AK, Fu WY, Cheung J, Tsim KW, Ip FC, Wang JH, Ip NY. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat, Neurosci. 2001;4(4):374–381. doi: 10.1038/86019. [DOI] [PubMed] [Google Scholar]
- 89.Kamalati T, Jolin HE, Fry MJ, Crompton MR. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene. 2000;19(48):5471–5476. doi: 10.1038/sj.onc.1203931. [DOI] [PubMed] [Google Scholar]
- 90.Hemi R, Paz K, Wertheim N, Karasik A, Zick Y, Kanety H. Transactivation of ErbB2 and ErbB3 by tumor necrosis factor-alpha and anisomycin leads to impaired insulin signaling through serine/threonine phosphorylation of IRS proteins. J. Biol. Chem. 2002;277(11):8961–8969. doi: 10.1074/jbc.M109391200. [DOI] [PubMed] [Google Scholar]
- 91.Walters DK, French JD, Arendt BK, Jelinek DF. Atypical expression of ErbB3 in myeloma cells: cross-talk between ErbB3 and the interferon-alpha signaling complex. Oncogene. 2003;22(23):3598–3607. doi: 10.1038/sj.onc.1206512. [DOI] [PubMed] [Google Scholar]
- 92.Walters DK, Jelinek DF. A role for Janus kinases in crosstalk between ErbB3 and the interferon-alpha signaling complex in myeloma cells. Oncogene. 2004;23(6):1197–1205. doi: 10.1038/sj.onc.1207203. [DOI] [PubMed] [Google Scholar]
- 93.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer. 2009;9(7):463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 94.Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, Hamburger AW. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br. J. Cancer. 2000;82(3):683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lessor TJ, Hamburger AW. Regulation of the ErbB3 binding protein Ebp1 by protein kinase C. Mol. Cell. Endocrinol. 2001;175(1-2):185–191. doi: 10.1016/s0303-7207(01)00387-2. [DOI] [PubMed] [Google Scholar]
- 96.Liu Z, Ahn J-Y, Liu X, Ye K. Ebp1 isoforms distinctively regulate cell survival and differentiation. Proc. Natl. Acad. Sci. USA. 2006;103(29):10917–10922. doi: 10.1073/pnas.0602923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15(7):413–448. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y, Fondell JD, Wang Q, Xia X, Cheng A, Lu ML, Hamburger AW. Repression of androgen receptor mediated transcription by the ErbB-3 binding protein, Ebp1. Oncogene. 2002;21(36):5609–5618. doi: 10.1038/sj.onc.1205638. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Hamburger AW. Specificity and heregulin regulation of Ebp1 (ErbB3 binding protein 1) mediated repression of androgen receptor signalling. Br. J. Cancer. 2005;92(1):140–146. doi: 10.1038/sj.bjc.6602257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Linn D, Liu Z, Melamed J, Tavora F, Young CY, Burger AM, Hamburger AW. EBP1, an ErbB3-binding protein, is decreased in prostate cancer and implicated in hormone resistance. Mol. Cancer Ther. 2008;7(10):3176–3186. doi: 10.1158/1535-7163.MCT-08-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Wang X-W, Jelovac D, Nakanishi T, Yu M-H, Akinmade D, Goloubeva O, Ross DD, Brodie A, Hamburger AW. The ErbB3-binding protein Ebp1 suppresses androgen receptor-mediated gene transcription and tumorigenesis of prostate cancer cells. Proc. Natl. Acad. Sci. USA. 2005;102(28):9890–9895. doi: 10.1073/pnas.0503829102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gannon PO, Koumakpayi IH, Le Page C, Karakiewicz PI, Mes-Masson A-M, Saad F. Ebp1 expression in benign and malignant prostate. Cancer Cell Int. 2008;8:18–18. doi: 10.1186/1475-2867-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diamonti AJ, Guy PM, Ivanof C, Wong K, Sweeney C, Carraway KL. An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proc. Nat. Acad. Sci. USA. 2002;99(5):2866–2871. doi: 10.1073/pnas.052709799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu X, Yen L, Irwin L, Sweeney C, Carraway KL. Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol. Cell. Biol. 2004;24(17):7748–7757. doi: 10.1128/MCB.24.17.7748-7757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yen L, Cao Z, Wu X, Ingalla ERQ, Baron C, Young LJT, Gregg JP, Cardiff RD, Borowsky AD, Sweeney C, Carraway KL. Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth. Cancer Res. 2006;66(23):11279–11286. doi: 10.1158/0008-5472.CAN-06-2319. [DOI] [PubMed] [Google Scholar]
- 106.Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br. J. Cancer. 2007;97(4):453–457. doi: 10.1038/sj.bjc.6603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc. Natl. Acad. Sci. USA. 2010;107(17):7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, Fulgham A, Song Y, Nielsen UB, Engelman JA, Wong KK. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70(6):2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van der Horst EH, Murgia M, Treder M, Ullrich A. Anti-HER-3 MAbs inhibit HER-3-mediated signaling in breast cancer cell lines resistant to anti-HER-2 antibodies. Int. J. Cancer. 2005;115(4):519–527. doi: 10.1002/ijc.20867. [DOI] [PubMed] [Google Scholar]
- 110.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62(11):3151–3158. [PubMed] [Google Scholar]
- 111.Garrett J, Olivares M, Rinehart C, Dave B, Cook R, Chang J, Arteaga C. Transcriptional and post-translational upregulation of HER3 (ErbB3) counteracts antitumor effect of HER2 tyrosine kinase inhibitors. Cancer Res. 2009;69(24 Supplement):63–63. [Google Scholar]
- 112.Huang X, Gao L, Wang S, McManaman JL, Thor AD, Yang X, Esteva FJ, Liu B. Heterotrimerization of the Growth Factor Receptors erbB2, erbB3, and Insulin-like Growth Factor-I Receptor in Breast Cancer Cells Resistant to Herceptin. Cancer Res. 2010;70(3):1204–1214. doi: 10.1158/0008-5472.CAN-09-3321. [DOI] [PubMed] [Google Scholar]
- 113.Konecny G, Finn R, Venkatesan N, Rusnak D, Gilmer T, Berger M, Chen J, Slamon DJ, Pegram M. The novel dual kinase inhibitor GW572016 is particularly active in HER2-positive and trastuzumab-conditioned breast cancer cells. Breast Cancer Res. Treat. 2003;82:S171–S171. [Google Scholar]
- 114.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith BR, Gilmer TM, Berger M, Podratz KC, Slamon DJ. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 115.Normanno N, Campiglio M, De Luca A, Somenzi G, Maiello M, Ciardiello F, Gianni L, Salomon DS, Menard S. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with trastuzumab (Herceptin) on human breast cancer cell growth. Ann. Oncol. 2002;13(1):65–72. doi: 10.1093/annonc/mdf020. [DOI] [PubMed] [Google Scholar]
- 116.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J, Baselga J. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 117.Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18(3):731–738. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 118.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 119.Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Semin. Oncol. 2001;28(5 Suppl. 16):80–85. doi: 10.1016/s0093-7754(01)90285-4. [DOI] [PubMed] [Google Scholar]
- 120.Trinks C, Djerf EA, Hallbeck A-L, Jönsson J-I, Walz TM. The pan-ErbB receptor tyrosine kinase inhibitor canertinib induces ErbB-independent apoptosis in human leukemia (HL-60 and U-937) cells. Biochem. Biophys. Res. Commun. 2010;393(1):6–10. doi: 10.1016/j.bbrc.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 121.Dewji MR. Early phase I data on an irreversible pan-erb inhibitor: CI-1033. What did we learn? J. Chemother. 2004;(16 Suppl. 4):44–48. doi: 10.1179/joc.2004.16.Supplement-1.44. [DOI] [PubMed] [Google Scholar]
- 122.Qi W, Cooke LS, Stejskal A, Riley C, Croce KD, Saldanha JW, Bearss D, Mahadevan D. MP470, a novel receptor tyrosine kinase inhibitor, in combination with Erlotinib inhibits the HER family/PI3K/Akt pathway and tumor growth in prostate cancer. BMC Cancer. 2009;9:142–142. doi: 10.1186/1471-2407-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, Beck S, Marshall G, Davenport S, Callis R, Mills E, Grosios K, Smith P, Barlaam B, Wilkinson RW, Ogilvie D. AZD8931, an Equipotent, Reversible Inhibitor of Signaling by Epidermal Growth Factor Receptor, ERBB2 (HER2), and ERBB3: A Unique Agent for Simultaneous ERBB Receptor Blockade in Cancer. Clin Cancer Res. 2010;16(4):1159–1169. doi: 10.1158/1078-0432.CCR-09-2353. [DOI] [PubMed] [Google Scholar]
- 124.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L, Lindeman NI, Murphy C, Akhavanfard S, Yeap BY, Xiao Y, Capelletti M, Iafrate AJ, Lee C, Christensen JG, Engelman JA, Janne PA. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Y, Majumder S, McCall W, Sartor CI, Mohler JL, Gregory CW, Earp HS, Whang YE. Inhibition of HER-2/neu kinase impairs androgen receptor recruitment to the androgen responsive enhancer. Cancer Res. 2005;65(8):3404–3409. doi: 10.1158/0008-5472.CAN-04-4292. [DOI] [PubMed] [Google Scholar]
- 126.Ye D, Mendelsohn J, Fan Z. Androgen and epidermal growth factor down-regulate cyclin-dependent kinase inhibitor p27Kip1 and costimulate proliferation of MDA PCa 2a and MDA PCa 2b prostate cancer cells. Clin. Cancer Res. 1999;5(8):2171–2177. [PubMed] [Google Scholar]
- 127.Festuccia C, Gravina GL, Muzi P, Biordi L, Ronchi P, Martella O, Vicentini C, Bologna M. Gefitinib and bicalutamide show synergistic effects in primary cultures of prostate cancer derived from androgen-dependent naive patients. Oncol. Rep. 2007;18(5):1321–1327. [PubMed] [Google Scholar]
- 128.Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer. 2010;10(2):130–137. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eichhorn PJA, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan Q-W, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67(17):7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 132.Posadas EM, Gulley J, Arlen PM, Trout A, Parnes HL, Wright J, Lee MJ, Chung EJ, Trepel JB, Sparreboom A, Chen C, Jones E, Steinberg SM, Daniels A, Figg WD, Dahut WL. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol. Ther. 2005;4(10):1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- 133.Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, Cambio A, Evans CP, Gandara DR, Lara PN., Jr. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin. Genitourin. Cancer. 2007;5(7):433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 134.Robinson D, He F, Pretlow T, Kung HJ. A tyrosine kinase profile of prostate carcinoma. Proc. Natl. Acad. Sci. USA. 1996;93(12):5958–5962. doi: 10.1073/pnas.93.12.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vidal GA, Clark DE, Marrero L, Jones FE. A constitutively active ERBB4/HER4 allele with enhanced transcriptional coactivation and cell-killing activities. Oncogene. 2007;26(3):462–466. doi: 10.1038/sj.onc.1209794. [DOI] [PubMed] [Google Scholar]
- 136.Williams EE, Trout LJ, Gallo RM, Pitfield SE, Bryant I, Penington DJ, Riese DJ., 2nd A constitutively active ErbB4 mutant inhibits drug-resistant colony formation by the DU-145 and PC-3 human prostate tumor cell lines. Cancer Lett. 2003;192(1):67–74. doi: 10.1016/s0304-3835(02)00690-0. [DOI] [PubMed] [Google Scholar]
- 137.Lin S-H, Lee Y-C, Choueiri MB, Wen S, Mathew P, Ye X, Do K-A, Navone NM, Kim J, Tu S-M, Yu-Lee L-Y, Logothetis CJ. Soluble ErbB3 levels in bone marrow and plasma of men with prostate cancer. Clin. Cancer Res. 2008;14(12):3729–3736. doi: 10.1158/1078-0432.CCR-08-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]